Abstract
Parasitic diseases have caused significant problems to global aquaculture production. These studies will further our knowledge of this complex problem and help implement adequate prevention measures and control strategies. The present study aimed to investigate the presence of parasites in Megaleporinus obtusidens and to describe the epidemiology and pathology of parasitic infections in these fish. Five moribund fish were sent for parasitological examination. The integument and gills were scrapped off with a glass slide, and samples were examined under a light microscope. Parasitic crustaceans found in these specimens were submitted for scanning electron microscopy and histological analyses. The crustaceans Dolops carvalhoi and Lernaea cyprinacea and the Epistylis spp. were present in all fish examined. Epistylis spp. were also seen on the entire surface of the crustacean integument. Microscopic lesions observed in the parasitized gills included hyperplasia and hypertrophy of the lamellar epithelium, an inflammatory infiltrate, telangiectasia, foci of hemorrhage and necrosis, fusion of the secondary lamellae, and detachment of the lamellar epithelium. Crustacean parasites are important mechanical vectors of Epistylis infection and disseminate the disease in fish farming operations. Epistylis spp. infection affects the health of fish and has significant ecological and economical impact on aquaculture.
Keywords:
Aquaculture; epistyliasis, ectoparasites; epibionts; mechanical vector
Resumo
Doenças parasitárias causam problemas significativos a produção mundial de peixes. Esse estudo aprofundará nosso conhecimento neste complexo problema e ajudará implementar estratégias de prevenção e controle. O objetivo deste trabalho foi analisar os parasitas encontrados em Megaleporinus obtusidens de piscicultura extensiva e descrever as relações epidemiológicas e patológicas entre eles. Cinco peixes moribundos foram enviados para análise parasitológica. O tegumento e as brânquias foram raspados com lâminas de vidro e examinados em microscópio óptico. Os crustáceos parasitas foram processados para análises histologicas e de microscopia eletrônica de varredura. Todos os peixes analisados foram infestados pelos crustáceos Dolops carvalhoi, Lernaea cyprinacea e pelo Epistylis spp. Epistylis spp. foram também encontrados na superfície de todo tegumento dos crustáceos parasitas. As brânquias parasitadas apresentaram hiperplasia e hipertrofia do epitélio lamelar, infiltrado inflamatório, telangectasia, focos hemorrágicos e necróticos, extensas áreas com fusão de lamelas secundárias e desprendimento de epitélio lamelar. Os crustáceos parasitas são vetores mecânicos importantes da epistilíase, disseminando o microorganismo nas criações de peixes. A infestação por Epistylis spp. afeta a saúde dos peixes e tem impacto ecológico e econômico significativo na aquacultura.
Palavras-chave:
Aquicultura; epistilíase; ectoparasitas; epibiontes; vetor mecânico
Introduction
Farmed fish are often exposed to inadequate environmental factors including excessive organic matter and high population density which result in reduced dissolved oxygen ( GHIRALDELLI et al., 2006 Ghiraldelli L, Martins ML, Yamashita MM, Jerônimo GT. Hematologia de Oreochromis niloticus (Cichlidae) e Cyprinus carpio (Cyprinidae) mantidos em diferentes condições de manejo e alimentação no Estado de Santa Catarina, Brasil. Acta Sci Biol Sci 2006; 28(4): 319-325. ; ZANOLO & YAMAMURA, 2006 Zanolo R, Yamamura MH. Parasitas in tilapia of nile in fresh water net-tank system. Semina: Ciênc Agrár 2006; 27(2): 281-288. http://dx.doi.org/10.5433/1679-0359.2006v27n2p281.
http://dx.doi.org/10.5433/1679-0359.200...
). Unfavorable conditions induce a stress response in fish ( TEROVA et al., 2005 Terova G, Gornati R, Rimoldi S, Bernardini G, Saroglia M. Quantification of a glucocorticoid receptor in sea bass (Dicentrarchus labrax, L.) reared at high stocking density. Gene 2005; 357(2): 144-151. http://dx.doi.org/10.1016/j.gene.2005.06.016. PMid:16129569.
http://dx.doi.org/10.1016/j.gene.2005.0...
) which predisposes them to a number of diseases including parasitic infestations ( ENGERING et al., 2013 Engering A, Hogerwerf L, Slingenbergh J. Pathogen–host–environment interplay and disease emergence. Emerg Microbes Infect 2013; 2(2): e5. http://dx.doi.org/10.1038/emi.2013.5. PMid:26038452.
http://dx.doi.org/10.1038/emi.2013.5 ...
). High parasite loads can cause irreversible damage to both integument and gills of infected fish ( PAPERNA, 1991 Paperna I. Diseases caused by parasites in the aquaculture of warm water fish. Annu Rev Fish Dis 1991; 1: 155-194. http://dx.doi.org/10.1016/0959-8030(91)90028-I.
http://dx.doi.org/10.1016/0959-8030(91)...
).
Crustaceans are one of the most significant pathogens in aquaculture especially those of the subclasses Branchiura and Copepoda ( JITHENDRAN et al., 2008 Jithendran KP, Natarajan M, Azad IS. Crustacean parasites and their management in brackishwater finfish culture. Mar Finfish Aquat Network 2008; 13: 47-50. ). and occur in fish farms and in natural habitats of fish as well ( LUQUE et al., 2013 Luque JL, Vieira FM, Takemoto RM, Pavanelli GC, Eiras JC. Checklist of Crustacea parasitizing fishes from Brazil. Check List 2013; 9(6): 1449-1470. http://dx.doi.org/10.15560/9.6.1449.
http://dx.doi.org/10.15560/9.6.1449 ...
; TAVARES-DIAS et al., 2015 Tavares-Dias M, Dias-Júnior MBF, Florentino AC, Silva LMA, Cunha AC. Distribution pattern of crustacean ectoparasites of freshwater fish from Brazil. Rev Bras Parasitol Vet 2015; 24(2): 136-147. http://dx.doi.org/10.1590/S1984-29612015036. PMid:26154954.
http://dx.doi.org/10.1590/S1984-2961201...
), Argulus and Dolops are the genera of branchiuran crustaceans most frequently found in fish ( MARTINS et al., 2002 Martins ML, Onaka EM, Moraes FR, Bozzo FR, Paiva AMFC, Gonçalves A. Recent studies on parasitic infections of freshwater cultivated fish in the state of São Paulo, Brazil. Acta Scientiarum 2002; 24(4): 981-985. ; TAVARES-DIAS et al., 2007 Tavares-Dias M, Moraes FR, Onaka EM, Rezende PCB. Changes in blood parameters of hybrid tambacu fish parasitized by Dolops carvalhoi (Crustacea, Branchiura), a fish louse. Vet Arh 2007; 77(4): 355-363. ). These crustaceans are blood-sucking parasites and are capable of attaching to the integument and gills of fish ( THATCHER & BRITES-NETO, 1994 Thatcher VE, Brites-Neto J. Diagnóstico, prevenção e tratamento das enfermidades de peixes neotropicais de água doce. Rev Bras Med Vet 1994; 16(3): 111-128. ).These arthropods move rapidly, and break through the epithelium of the fish skin using their oral appendages ( CARVALHO et al., 2004 Carvalho LN, Arruda R, Del-Claro K. Host-parasite interactions between the piranha Pygocentrus nattereri (Characiformes: Characidae) and isopods and branchiurans (Crustacea) in the rio Araguaia basin, Brazil. Neotrop Ichthyol 2004; 2(2): 93-98. http://dx.doi.org/10.1590/S1679-62252004000200006.
http://dx.doi.org/10.1590/S1679-6225200...
). Lernaea and Lamproglena are the genera of copepods most often described in fish ( LUQUE & TAVARES, 2007 Luque JL, Tavares LE. Checklist of Copepoda associated with fishes from Brazil. Zootaxa 2007; 1579: 1-39. ). These crustaceans attach firmly to the integument and gills of their fish hosts causing marked epithelial damage and severe vascular injury ( INNAL et al., 2017 Innal D, Avenant-Oldewage A, Dogangil B, Stavrescu-Bedivan MM, Ozmen O, Mavruk S. Susceptibility of endemic and non-indigenous fish to Lernaea cyprinacea (Copepoda: Lernaeidae): a case study from Düger Spring Creek (Burdur-Turkey). Bull Eur Assoc Fish Pathol 2017; 37(3): 100-109. ).
Mortality outbreaks were reported in production fish parasitized by crustaceans ( CARNEVIA & SPERANZA, 2003 Carnevia D, Speranza G. First report of Lernaea cyprinacea L., 1758 in Uruguay, introduced by goldfish Carassius auratus (L., 1758) and affecting axolotl Ambystoma mexicanum. Bull Eur Assoc Fish Pathol 2003; 23(5): 255-256. ), such as reports of epizootics in Atlantic salmon infested by sea lice (Copepoda, Caligidae), in which they cause intense spoliation, loss of epithelium, hemorrhages, increased secretion of mucus, and necrosis of the affected tissue ( COSTELLO, 2006 Costello MJ. Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol 2006; 22(10): 475-483. http://dx.doi.org/10.1016/j.pt.2006.08.006. PMid:16920027.
http://dx.doi.org/10.1016/j.pt.2006.08....
). Disease outbreaks and mortality caused by crustaceans have been reported in farmed fish ( CARNEVIA & SPERANZA, 2003 Carnevia D, Speranza G. First report of Lernaea cyprinacea L., 1758 in Uruguay, introduced by goldfish Carassius auratus (L., 1758) and affecting axolotl Ambystoma mexicanum. Bull Eur Assoc Fish Pathol 2003; 23(5): 255-256. ). In epizootics of sea lice (Copepoda, Caligidae) infestation in Atlantic salmon, affected fish had severe spoliation, loss of the skin epithelium, hemorrhage, increased mucus secretion, and tissue necrosis ( COSTELLO, 2006 Costello MJ. Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol 2006; 22(10): 475-483. http://dx.doi.org/10.1016/j.pt.2006.08.006. PMid:16920027.
http://dx.doi.org/10.1016/j.pt.2006.08....
). In addition to the damage caused by branchiuran crustaceans and copepods on their fish hosts, these arthropods can also act as disease vectors. Caligus rogercresseyi (sea louse) was recently described as a mechanical vector of Infectious salmon anemia virus ( NYLUND et al., 1993 Nylund A, Wallace C, Hovland T. The possible role of Lepeophtheirus salmonis (Krøyer) in the transmission of infectious salmon anaemia. In: Boxshall GA, Defaye D. Pathogens of wild and farmed fish: sea lice. London: Ellis Horwood Limited; 1993. p. 367-373. ; OELCKERS et al., 2014 Oelckers K, Vike S, Duesund H, Gonzalez J, Wadsworth S, Nylund A. Caligus rogercresseyi is as a potential vector for transmission of Infectious Salmon Anaemia (ISA) virus in Chile. Aquaculture 2014; 420-421: 126-132. http://dx.doi.org/10.1016/j.aquaculture.2013.10.016.
http://dx.doi.org/10.1016/j.aquaculture...
).
Parasitic crustaceans may also serve as substrate for epibionts such as Epistylis spp. ( SILVA et al., 2011 Silva RJD, Azevedo RKD, Abdallah VD. First record of an epibiont protozoan Epistylis sp. (Ciliophora, Peritrichia) attached to Amplexibranchius bryconis Thatcher & Paredes, 1985 (Copepoda, Ergasilidae) from Peixe’s River, state of São Paulo, Brazil. Crustaceana 2011; 84(9): 1139-1144. http://dx.doi.org/10.1163/001121611X584352.
http://dx.doi.org/10.1163/001121611X584...
; AZEVEDO et al., 2014 Azevedo RK, Brandão H, Abdallah VD, Silva RJD. First record of an epibiont protozoan Epistylis sp. (Ciliophora, Peritrichia) attached to Ergasilus chelangulatus (Ergasilidae) in Brazil. Braz J Biol 2014; 74(2): 460-463. http://dx.doi.org/10.1590/1519-6984.10112. PMid:25166331.
http://dx.doi.org/10.1590/1519-6984.101...
; CÔRREA et al., 2016 Corrêa LL, Oliveira MSB, Prestes L, Tavares-Dias M. First record in Brazil of Epistylis sp. (Ciliophora) adhered to Argulus sp. (Argulidae), a parasite of Hoplias aimara (Eritrhinidae). Nat Resour 2016; 7(6): 331-336. ). These protozoa are often considered commensal organisms in fish farms. However, in adverse environmental conditions they can cause damage to the integument of Oreochromis niloticus (Nile tilapia) ( VALLADÃO et al., 2015 Valladão GMR, Levy-Pereira N, Viadanna PHO, Gallani SU, Farias THV, Pilarski F. Haematology and histopathology of Nile tilapia parasitised by Epistylis sp., an emerging pathogen in South America. Bull Eur Assoc Fish Pathol 2015; 35: 14-20. ) and Pseudoplatystoma reticulatum (barred sorubim)/Pseudoplatystoma corruscans (spotted sorubim) hybrids ( PÁDUA et al., 2016 Pádua SB, Martins ML, Valladão GMR, Utz L, Zara FJ, Ishikawa MM, et al. Host-parasite relationship during Epistylis sp. (Ciliophora: Epistylididae) infestation in farmed cichlid and pimelodid fish. Pesq Agropec Bras 2016; 51(5): 520-526. http://dx.doi.org/10.1590/S0100-204X2016000500012.
http://dx.doi.org/10.1590/S0100-204X201...
).
The objectives of the present study are to report the association between Epistylis spp. and parasitic crustaceans in the headstander Megaleporinus obtusidens (known as piava or piapara in Brazil) and to describe the lesions produced by ciliate and crustacean co-infections in this fish host.
Materials and Methods
Fish and study area
Megaleporinus obtusidens (Characiformes: Anostomidae) (known as piava or piapara in Brazil) is an omnivore native fish with a wide geographical distribution, and occurs in the watersheds of the southern and southeastern regions of the country ( HARTZ et al., 2000 Hartz SM, Silveira CM, Carvalho S, Villamil C. Alimentação da piava, Leporinus obtusidens (Characiformes, Anostomidae), no lago Guaíba, Porto Alegre, Rio Grande do Sul, Brasil. Pesq Agropec Gaúch 2000; 6(1): 145-150. ).
This species of fish is ideal for intensive cultivation, and has good consumer acceptance due to the taste of its meat and its size as well; it can weigh up to 7.5 kg ( GLUSCZAK et al. 2006 Glusczak L, Santos Miron D, Crestani M, Braga da Fonseca M, Araújo Pedron F, Duarte MF, et al. Effect of glyphosate herbicide on acetylcholisnesterase activity and metabolic and hematological parameters in piava (Leporinus obtusidens). Ecotoxicol Environ Saf 2006; 65(2): 237-241. http://dx.doi.org/10.1016/j.ecoenv.2005.07.017. PMid:16174533.
http://dx.doi.org/10.1016/j.ecoenv.2005...
; ADORIAN et al. 2017 Adorian TJ, Mombach PI, Pianesso D, Uczay J, Decarlli J, Lazzari R. Utilization of vegetables oils in diets for piava juveniles (Leporinus obtusidens). Rev Ciênc Agrovet 2017; 16(2): 121-127. http://dx.doi.org/10.5965/223811711622017121.
http://dx.doi.org/10.5965/2238117116220...
).
In this study, fish examined for the presence of parasites originated from an extensive fishing establishment located in the county of Araraquara, State of São Paulo, southeast Brazil. They were cultivated in earth ponds and marketed to several fish-and-pay fishing camps in the area. The producer claimed that several fish from this farm died without any apparent cause, and that affected fish were coughing at the water entrance of the tanks.
Parasite analysis
Five moribund adult piavas were captured. In order to avoid detachment of parasites from the skin, fish were euthanized immediately after being captured; the spinal cord was severed with a knife or scalpel. Mucus was collected from the skin and gills of these fish by gently scrapping off their body and branchial surfaces with a glass slide. Mucus samples were examined under the light microscope. Parasitic crustaceans that were found in these specimens were gently removed from the surface of the glass slide with anatomical tweezers. Some parasites were stored in 70% alcohol while others were fixed in 2% glutaraldehyde for subsequent scanning electron microscopy analysis. Identification of parasitic crustaceans was based on the morphological characters of these arthropods and according to the information published by Robinson & Avenant-Oldewage (1996) Robinson J, Avenant-Oldewage A. Aspects of the morphology of the parasitic copepod Lernaea cyprinacea Linnaeus, 1758 and notes on its distribution in Africa. Crustaceana 1996; 69(5): 610-626. http://dx.doi.org/10.1163/156854096X00628.
http://dx.doi.org/10.1163/156854096X006...
and Thatcher (1991) Thatcher VE. Amazon fish parasites. Amazoniana 1991; 11(3-4): 263-572. . Images were captured using a Nikon microscope E200® equipped with a Motic 5.0 image capture system. The ecological descriptors are in accord those published by Bush et al. (1997) Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 1997; 83(4): 575-583. http://dx.doi.org/10.2307/3284227. PMid:9267395.
http://dx.doi.org/10.2307/3284227 ...
.
Histopathological analysis
Gill specimens were fixed in 10% formaldehyde and then preserved in 70% alcohol.
These tissue samples were dehydrated in alcoholic solutions of increasing concentrations and then embedded in paraffin. Sections of 5 μm were obtained using a microtome, and were stained with hematoxylin and eosin. All lesions were photographed under a light microscope.
Scanning electron microscopy analysis
Crustacean and gill samples were submitted for scanning electron microscopy analysis. Specimens were fixed in glutaraldehyde with 2% sodium cacodylate buffer and washed overnight in osmium tetroxide. Samples were dehydrated in alcoholic solutions of increasing concentrations. Critical point drying was used to remove water from these tissues. After that, samples were carefully placed in aluminum specimen stubs and bathed with gold. Images were captured using a JEOL JSM- 5410 electron microscope.
Results
Parasite identification
All fish were parasitized with the crustaceans Dolops carvalhoi ( YAMAGUTI, 1963 Yamaguti S. Parasitic Copepoda and Branchiura of fishes. New York: John Wiley; 1963. ) and Lernaea cyprinacea ( EIRAS et al., 2010 Eiras JC, Takemoto RM, Pavanelli GC. Diversidade de parasitas de água doce do Brasil. Maringá: Clichetec; 2010. ) and by the epibiont Epistylis spp. with prevalence rates of 100% for all three parasites. The mean parasite intensity of Dolops carvalhoi was 155.6 ± 61.31 (106-229). We were unable to count Lernaea cyprinacea since these copecods are fairly small and difficult to visualize with the naked eye, and fish were considered too large [average weight 2.22 kg ± 0.37 (1.8-2.7) and total length 60.6 cm ± 8.11 (54-74)] to be examined under the stereomicroscope.
Host-parasite relationship
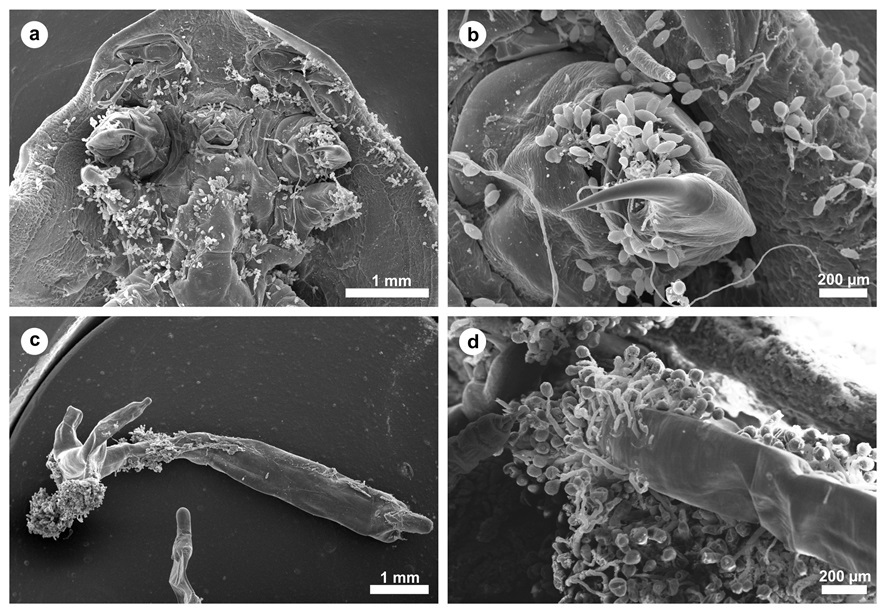
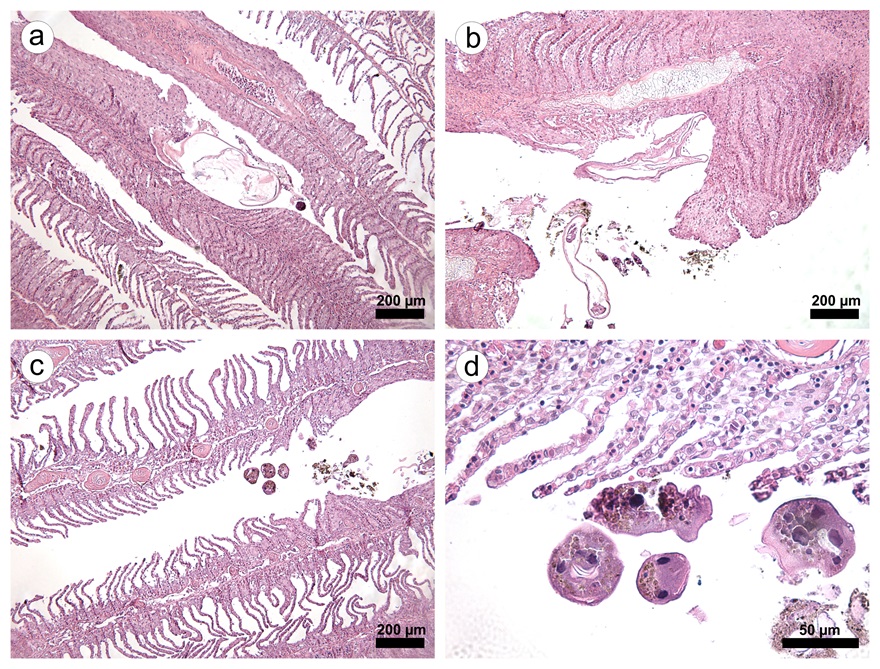
Large numbers of the ciliate Epistylis spp. were found attached to the integumentary surface of both D. carvalhoi and L. cyprinacea ( Figure 1 ). Epistylis spp. was also attached to the secondary lamellae of each gill filament from these fish. Tissue damage of varying severity was present in all fish infested with parasites. Microscopic lesions observed in the parasitized gills included hyperplasia and hypertrophy of the lamellar epithelium, an inflammatory infiltrate composed of eosinophils, telangiectasia, foci of hemorrhage and necrosis, extensive fusion of the secondary lamellae, and detachment of the lamellar epithelium (epitheilai lifting) ( Figure 2 ).
a) Dolops carvalhoi in ventral position with the intense presence of the adhesion of the protozoan Epistylis spp., scale bar: 1 mm. b) In detail a hook of the crustacean Dolops carvalhoi demonstrating the presence of Epistylis spp., scale bar: 200 µm. c) Panoramic view of a Lernaea cyprinacea, scale bar: 1 mm. d) Adhesion of Epistylis spp. in the cephalothorax of the crustacean Lernaea cyprinacea , scale bar: 200 µm.
a) Lernaea cyprinacea adhered to a branchial filament, causing intense hypertrophy and hyperplasia epithelial, scale bar: 200 μm. b) Crustacean parasite and Epistylis spp. causing lamellar damage, with areas of hemorrhage and necrosis, and severe inflammatory infiltrate, scale bar: 200 μm. c) Epistylis spp. near areas of epithelial hyperplasia and scaling, scale bar: 200 μm. d) In detail, Epistylis spp. parasitizing branchial filament, scale bar: 50 μm.
Discussion
Fish ectoparasites are important mechanical vectors in aquatic environments, and are capable of transferring pathogens from infected fish to healthy fish or between wild fish and farmed fish ( XU et al., 2007 Xu DH, Shoemaker CA, Klesius PH. Evaluation of the link between gyrodactylosis and streptococcosis of Nile tilapia, Oreochromis niloticus (L.). J Fish Dis 2007; 30(4): 233-238. http://dx.doi.org/10.1111/j.1365-2761.2007.00806.x. PMid:17394525.
http://dx.doi.org/10.1111/j.1365-2761.2...
). Crustaceans are mechanical vectors of a number of pathogens in aquatic ecosystems including viruses, bacteria, and parasites. Parasitic crustaceans can have a major economic impact on commercial fish culture, and can pose a significant threat to species diversity within an aquatic habitat ( OVERSTREET et al., 2009 Overstreet RM, Jovonovich J, Ma H. Parasitic crustaceans as vectors of viruses, with an emphasis on three penaeid viruses. Integr Comp Biol 2009; 49(2): 127-141. http://dx.doi.org/10.1093/icb/icp033. PMid:21669853.
http://dx.doi.org/10.1093/icb/icp033 ...
). Gnathiid isopods, for example, are vectors of haemoggregarines which are haemoprotozoans that can cause mild chronic infection in fish from coral reefs ( DAVIES & SMIT, 2001 Davies AJ, Smit NJ. The life cycle of Haemogregarina bigemina (Adeleina: Haemogregarinidae) in South African hosts. Folia Parasitol (Praha) 2001; 48(3): 169-177. http://dx.doi.org/10.14411/fp.2001.029. PMid:11699651.
http://dx.doi.org/10.14411/fp.2001.029 ...
). The pathology of hemogreggarine infections in fish, albeit largely unknown, may have marked deleterious effects on fish farms ( SMIT et al., 2006 Smit NJ, Grutter AS, Adlard RD, Davies AJ. Hematozoa of teleosts from Lizard Island, Australia, with some comments on their possible mode of transmission and the description of a new hemogregarine species. J Parasitol 2006; 92(4): 778-788. http://dx.doi.org/10.1645/GE-756R.1. PMid:16995396.
http://dx.doi.org/10.1645/GE-756R.1 ...
). The interaction between the branchiurian Argulus foliaceus (fish louse) and Rhabdovirus carpio, which causes spring viraemia of carp, is another example of a crustacean acting as a vector for diseases in fish. Crustaceans carrying Rhabdovirus carpio attach to the body surface of Cyprinus carpio (common carp) and transmit the virus to these fish ( AHNE, 1985 Ahne W. Argulus foliaceus L. and Piscicola geometra L. as mechanical vectors of spring viraemia of carp virus (SVCV). J Fish Dis 1985; 8(2): 241-242. http://dx.doi.org/10.1111/j.1365-2761.1985.tb01220.x.
http://dx.doi.org/10.1111/j.1365-2761.1...
). High mortality rates have been recorded for spring viremia of carp ( DIKKEBOOM et al., 2004 Dikkeboom A, Radi C, Toohey-Kurth K, Marcquenski S, Engel M, Goodwin AE, et al. First report of spring viremia of carp virus (SVCV) in wild common carp in North America. J Aquat Health 2004; 16(4): 169-178. http://dx.doi.org/10.1577/H03-064.1.
http://dx.doi.org/10.1577/H03-064.1 ...
).
To date, there are no publications reporting that the crustacean D. carvalhoi is capable of transmitting pathogens to fish even though other parasitic crustaceans may act as basibionts for Epistylis spp. including Ergasilus chelangulatus ( AZEVEDO et al., 2014 Azevedo RK, Brandão H, Abdallah VD, Silva RJD. First record of an epibiont protozoan Epistylis sp. (Ciliophora, Peritrichia) attached to Ergasilus chelangulatus (Ergasilidae) in Brazil. Braz J Biol 2014; 74(2): 460-463. http://dx.doi.org/10.1590/1519-6984.10112. PMid:25166331.
http://dx.doi.org/10.1590/1519-6984.101...
). In the present study, L. cyprinacea was found in fish skin mucus samples examined by light microscopy. Free-living crustaceans may also act as basibionts for ciliates. An example of such interaction is the association between the shrimp Macrobrachium rosenbergii ( MANDAL et al., 2015 Mandal B, Dubey SK, Ghosh AK, Dash G. Parasitic occurrence in the giant freshwater prawn Macrobrachium rosenbergii from coastal West Bengal, India. J Parasitol Vector Biol 2015; 7(6): 115-119. ) and the planktonic copepods Pseudodiaptomus stuhlmanni ( JONES et al., 2016 Jones S, Carrasco NK, Perissinotto R, Vosloo A. Association of the epibiont Epistylis sp. with a calanoid copepod in the St Lucia Estuary, South Africa. J Plankton Res 2016; 38(6): 1404-1411. ), Thermocyclops minutus, and Notodiaptomus amazonicus ( CABRAL et al., 2016 Cabral AF, Utz LRP, Velho LFM. First report of an epibiotic relationship between ciliates and planktonic copepods in a Brazilian floodplain. Rev Bras Zoociênc 2016; 17(1): 124-131. ).
The ciliate epibionts Epistylis spp. are considered commensal protozoan organisms ( VALLADÃO et al., 2015 Valladão GMR, Levy-Pereira N, Viadanna PHO, Gallani SU, Farias THV, Pilarski F. Haematology and histopathology of Nile tilapia parasitised by Epistylis sp., an emerging pathogen in South America. Bull Eur Assoc Fish Pathol 2015; 35: 14-20. ) but have been associated with a decrease in food conversion efficiency and increased susceptibility to predation in crustaceans ( VISSE, 2007 Visse M. Detrimental effect of peritrich ciliates (Epistylis sp.) as epibionts on the survival of the copepod Acartia bifilosa. Proc Estonian Acad Sci Biol Ecol 2007; 56(3): 173-178. ). Furthermore, Epistylis spp. contributed to mortality of the copepod Boeckella triarticulata in an environment with limited food availability ( XU & BURNS, 1991 Xu Z, Burns CW. Effects of the epizoic ciliate, Epistylis daphniae, on growth, reproduction and mortality of Boeckella triarticulata (Thomson) (Copepoda: Calanoida). Hydrobiologia 1991; 209(3): 183-189. http://dx.doi.org/10.1007/BF00015341.
http://dx.doi.org/10.1007/BF00015341 ...
). Fish parasitized by crustaceans for prolonged periods of time may develop chronic stress. Fish are more susceptible to infections, including epistiliasis, due to stress-induced immunosuppression ( PICKERING & POTTINGER, 1989 Pickering AD, Pottinger TG. Stress responses and disease resistance in salmonid fish: effects of chronic elevation of plasma cortisol. Fish Physiol Biochem 1989; 7(1-6): 253-258. http://dx.doi.org/10.1007/BF00004714. PMid:24221779.
http://dx.doi.org/10.1007/BF00004714 ...
; TORT, 2011 Tort L. Stress and immune modulation in fish. Dev Comp Immunol 2011; 35(12): 1366-1375. http://dx.doi.org/10.1016/j.dci.2011.07.002. PMid:21782845.
http://dx.doi.org/10.1016/j.dci.2011.07...
; WALKER et al., 2004 Walker PD, Flik G, Bonga SW. The biology of parasites from the genus Argulus and a review of the interactions with its host. Symp Soc Exp Biol 2004; 55(55): 107-129. PMid:15446447. ). Parasitic crustaceans act as carriers for Epistylis spp., and disseminate this protozoan organism in fish farms.
This ciliate can cause a variety of lesions in fish such as damage to the scale epithelium including hemorrhage and necrosis ( WELICKY et al., 2017 Welicky RL, De Swardt J, Gerber R, Netherlands EC, Smit NJ. Drought-associated absence of alien invasive anchorworm, Lernaea cyprinacea (Copepoda: Lernaeidae), is related to changes in fish health. Int J Parasitol Parasites Wildl 2017; 6(3): 430-438. http://dx.doi.org/10.1016/j.ijppaw.2017.01.004.
http://dx.doi.org/10.1016/j.ijppaw.2017...
). Epistylis spp. causes injury to the integument of the Nile tilapia Oreochromis niloticus which is characterized by the presence of a subepithelial infiltrate of lymphocytes and mast cells and epithelial hyperplasia and hypertrophy ( VALLADÃO et al., 2015 Valladão GMR, Levy-Pereira N, Viadanna PHO, Gallani SU, Farias THV, Pilarski F. Haematology and histopathology of Nile tilapia parasitised by Epistylis sp., an emerging pathogen in South America. Bull Eur Assoc Fish Pathol 2015; 35: 14-20. ). Microscopic lesions that are seen in the integument of cultured cichlid fish and siluriformes (catfish) in association with the cilitate Epistylis include hydropic degeneration, multifocal necrosis, proliferation of skin mucous (globet) cells, and the presence of a mixed inflammatory infiltrates composed of granulocytes and mononuclear cells ( PÁDUA et al., 2016 Pádua SB, Martins ML, Valladão GMR, Utz L, Zara FJ, Ishikawa MM, et al. Host-parasite relationship during Epistylis sp. (Ciliophora: Epistylididae) infestation in farmed cichlid and pimelodid fish. Pesq Agropec Bras 2016; 51(5): 520-526. http://dx.doi.org/10.1590/S0100-204X2016000500012.
http://dx.doi.org/10.1590/S0100-204X201...
). Studies have shown that Epistylis spp. is involved in severe outbreaks of Aeromonas hydrophila infection in farmed fish with high mortality. The ciliate is capable of propagating bacteria which adhere to their peduncles ( HAZEN et al., 1978 Hazen TC, Raker ML, Esch GW, Fliermans CB. Ultrastructure of red -sore lesions on largemouth bass (Micropterus salmoides): association of the ciliate Epistylis sp. and the bacterium Aeromonas hydrophila. J Protozool 1978; 25(3): 351-355. http://dx.doi.org/10.1111/j.1550-7408.1978.tb03901.x. PMid:102785.
http://dx.doi.org/10.1111/j.1550-7408.1...
; MILLER & CHAPMAN, 1976 Miller RW, Chapman WR. Epistylis and Aeromonas hydrophila infections in fishes from North Carolina reservoirs. Prog Fish-Cult 1976; 38(3): 165-168. http://dx.doi.org/10.1577/1548-8659(1976)38[165:EAAHII]2.0.CO;2.
http://dx.doi.org/10.1577/1548-8659(197...
).
The gill lesions found in our study are consistent with those previously described by other researchers in cases of macroparasite infection of fish ( BYRNE et al., 2002 Byrne CJ, Holland CV, Poole R, Kennedy CR. Comparison of the macroparasite communities of wild and stocked brown trout (Salmo trutta L.) in the west of Ireland. Parasitology 2002; 124(4): 435-445. http://dx.doi.org/10.1017/S0031182001001330. PMid:12003067.
http://dx.doi.org/10.1017/S003118200100...
; MARQUES & CABRAL, 2007 Marques JF, Cabral HN. Effects of sample size on fish parasite prevalence, mean abundance and mean intensity estimates. J Appl Ichthyology 2007; 23(2): 158-162. http://dx.doi.org/10.1111/j.1439-0426.2006.00823.x.
http://dx.doi.org/10.1111/j.1439-0426.2...
). Branchial lesions in which the parasite is present are severe whereas those in adjacent areas without the parasites are mild. Since branchial lesions are usually focal, most of the unaffected gill surface area is still functional which allows fish to survive. Death ultimately occurs only in those cases of heavy parasite infestation in which extensive and severe gill lesions are present.
It is imperative to actively monitor parasitic diseases in farmed fish as they can cause significant problems to global aquaculture production resulting in major economic losses. In farmed fish, parasitic crustaceans cause erosions on the integument and gills and can also spread many pathogens including Epistylis spp. Further studies are needed to assess the impact of Epistylis spp. on fish health. The mechanisms involved in the attachment of parasitic crustaceans on the integument of fish should be investigated in order to further our understanding of the interactions between these arthropods and their fish hosts. Crustaceans parasites are important mechanical vectors of Epistylis spp. infection and Epistylis-Dolops-Lernaea complex affects the health of fish.
References
- Adorian TJ, Mombach PI, Pianesso D, Uczay J, Decarlli J, Lazzari R. Utilization of vegetables oils in diets for piava juveniles (Leporinus obtusidens). Rev Ciênc Agrovet 2017; 16(2): 121-127. http://dx.doi.org/10.5965/223811711622017121.
» http://dx.doi.org/10.5965/223811711622017121 - Ahne W. Argulus foliaceus L. and Piscicola geometra L. as mechanical vectors of spring viraemia of carp virus (SVCV). J Fish Dis 1985; 8(2): 241-242. http://dx.doi.org/10.1111/j.1365-2761.1985.tb01220.x.
» http://dx.doi.org/10.1111/j.1365-2761.1985.tb01220.x - Azevedo RK, Brandão H, Abdallah VD, Silva RJD. First record of an epibiont protozoan Epistylis sp. (Ciliophora, Peritrichia) attached to Ergasilus chelangulatus (Ergasilidae) in Brazil. Braz J Biol 2014; 74(2): 460-463. http://dx.doi.org/10.1590/1519-6984.10112. PMid:25166331.
» http://dx.doi.org/10.1590/1519-6984.10112 - Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 1997; 83(4): 575-583. http://dx.doi.org/10.2307/3284227. PMid:9267395.
» http://dx.doi.org/10.2307/3284227 - Byrne CJ, Holland CV, Poole R, Kennedy CR. Comparison of the macroparasite communities of wild and stocked brown trout (Salmo trutta L.) in the west of Ireland. Parasitology 2002; 124(4): 435-445. http://dx.doi.org/10.1017/S0031182001001330. PMid:12003067.
» http://dx.doi.org/10.1017/S0031182001001330 - Cabral AF, Utz LRP, Velho LFM. First report of an epibiotic relationship between ciliates and planktonic copepods in a Brazilian floodplain. Rev Bras Zoociênc 2016; 17(1): 124-131.
- Carnevia D, Speranza G. First report of Lernaea cyprinacea L., 1758 in Uruguay, introduced by goldfish Carassius auratus (L., 1758) and affecting axolotl Ambystoma mexicanum. Bull Eur Assoc Fish Pathol 2003; 23(5): 255-256.
- Carvalho LN, Arruda R, Del-Claro K. Host-parasite interactions between the piranha Pygocentrus nattereri (Characiformes: Characidae) and isopods and branchiurans (Crustacea) in the rio Araguaia basin, Brazil. Neotrop Ichthyol 2004; 2(2): 93-98. http://dx.doi.org/10.1590/S1679-62252004000200006.
» http://dx.doi.org/10.1590/S1679-62252004000200006 - Corrêa LL, Oliveira MSB, Prestes L, Tavares-Dias M. First record in Brazil of Epistylis sp. (Ciliophora) adhered to Argulus sp. (Argulidae), a parasite of Hoplias aimara (Eritrhinidae). Nat Resour 2016; 7(6): 331-336.
- Costello MJ. Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol 2006; 22(10): 475-483. http://dx.doi.org/10.1016/j.pt.2006.08.006. PMid:16920027.
» http://dx.doi.org/10.1016/j.pt.2006.08.006 - Davies AJ, Smit NJ. The life cycle of Haemogregarina bigemina (Adeleina: Haemogregarinidae) in South African hosts. Folia Parasitol (Praha) 2001; 48(3): 169-177. http://dx.doi.org/10.14411/fp.2001.029. PMid:11699651.
» http://dx.doi.org/10.14411/fp.2001.029 - Dikkeboom A, Radi C, Toohey-Kurth K, Marcquenski S, Engel M, Goodwin AE, et al. First report of spring viremia of carp virus (SVCV) in wild common carp in North America. J Aquat Health 2004; 16(4): 169-178. http://dx.doi.org/10.1577/H03-064.1.
» http://dx.doi.org/10.1577/H03-064.1 - Eiras JC, Takemoto RM, Pavanelli GC. Diversidade de parasitas de água doce do Brasil Maringá: Clichetec; 2010.
- Engering A, Hogerwerf L, Slingenbergh J. Pathogen–host–environment interplay and disease emergence. Emerg Microbes Infect 2013; 2(2): e5. http://dx.doi.org/10.1038/emi.2013.5. PMid:26038452.
» http://dx.doi.org/10.1038/emi.2013.5 - Ghiraldelli L, Martins ML, Yamashita MM, Jerônimo GT. Hematologia de Oreochromis niloticus (Cichlidae) e Cyprinus carpio (Cyprinidae) mantidos em diferentes condições de manejo e alimentação no Estado de Santa Catarina, Brasil. Acta Sci Biol Sci 2006; 28(4): 319-325.
- Glusczak L, Santos Miron D, Crestani M, Braga da Fonseca M, Araújo Pedron F, Duarte MF, et al. Effect of glyphosate herbicide on acetylcholisnesterase activity and metabolic and hematological parameters in piava (Leporinus obtusidens). Ecotoxicol Environ Saf 2006; 65(2): 237-241. http://dx.doi.org/10.1016/j.ecoenv.2005.07.017. PMid:16174533.
» http://dx.doi.org/10.1016/j.ecoenv.2005.07.017 - Hartz SM, Silveira CM, Carvalho S, Villamil C. Alimentação da piava, Leporinus obtusidens (Characiformes, Anostomidae), no lago Guaíba, Porto Alegre, Rio Grande do Sul, Brasil. Pesq Agropec Gaúch 2000; 6(1): 145-150.
- Hazen TC, Raker ML, Esch GW, Fliermans CB. Ultrastructure of red -sore lesions on largemouth bass (Micropterus salmoides): association of the ciliate Epistylis sp. and the bacterium Aeromonas hydrophila. J Protozool 1978; 25(3): 351-355. http://dx.doi.org/10.1111/j.1550-7408.1978.tb03901.x. PMid:102785.
» http://dx.doi.org/10.1111/j.1550-7408.1978.tb03901.x - Innal D, Avenant-Oldewage A, Dogangil B, Stavrescu-Bedivan MM, Ozmen O, Mavruk S. Susceptibility of endemic and non-indigenous fish to Lernaea cyprinacea (Copepoda: Lernaeidae): a case study from Düger Spring Creek (Burdur-Turkey). Bull Eur Assoc Fish Pathol 2017; 37(3): 100-109.
- Jithendran KP, Natarajan M, Azad IS. Crustacean parasites and their management in brackishwater finfish culture. Mar Finfish Aquat Network 2008; 13: 47-50.
- Jones S, Carrasco NK, Perissinotto R, Vosloo A. Association of the epibiont Epistylis sp. with a calanoid copepod in the St Lucia Estuary, South Africa. J Plankton Res 2016; 38(6): 1404-1411.
- Luque JL, Tavares LE. Checklist of Copepoda associated with fishes from Brazil. Zootaxa 2007; 1579: 1-39.
- Luque JL, Vieira FM, Takemoto RM, Pavanelli GC, Eiras JC. Checklist of Crustacea parasitizing fishes from Brazil. Check List 2013; 9(6): 1449-1470. http://dx.doi.org/10.15560/9.6.1449.
» http://dx.doi.org/10.15560/9.6.1449 - Mandal B, Dubey SK, Ghosh AK, Dash G. Parasitic occurrence in the giant freshwater prawn Macrobrachium rosenbergii from coastal West Bengal, India. J Parasitol Vector Biol 2015; 7(6): 115-119.
- Marques JF, Cabral HN. Effects of sample size on fish parasite prevalence, mean abundance and mean intensity estimates. J Appl Ichthyology 2007; 23(2): 158-162. http://dx.doi.org/10.1111/j.1439-0426.2006.00823.x.
» http://dx.doi.org/10.1111/j.1439-0426.2006.00823.x - Martins ML, Onaka EM, Moraes FR, Bozzo FR, Paiva AMFC, Gonçalves A. Recent studies on parasitic infections of freshwater cultivated fish in the state of São Paulo, Brazil. Acta Scientiarum 2002; 24(4): 981-985.
- Miller RW, Chapman WR. Epistylis and Aeromonas hydrophila infections in fishes from North Carolina reservoirs. Prog Fish-Cult 1976; 38(3): 165-168. http://dx.doi.org/10.1577/1548-8659(1976)38[165:EAAHII]2.0.CO;2.
» http://dx.doi.org/10.1577/1548-8659(1976)38[165:EAAHII]2.0.CO;2 - Nylund A, Wallace C, Hovland T. The possible role of Lepeophtheirus salmonis (Krøyer) in the transmission of infectious salmon anaemia. In: Boxshall GA, Defaye D. Pathogens of wild and farmed fish: sea lice London: Ellis Horwood Limited; 1993. p. 367-373.
- Oelckers K, Vike S, Duesund H, Gonzalez J, Wadsworth S, Nylund A. Caligus rogercresseyi is as a potential vector for transmission of Infectious Salmon Anaemia (ISA) virus in Chile. Aquaculture 2014; 420-421: 126-132. http://dx.doi.org/10.1016/j.aquaculture.2013.10.016.
» http://dx.doi.org/10.1016/j.aquaculture.2013.10.016 - Overstreet RM, Jovonovich J, Ma H. Parasitic crustaceans as vectors of viruses, with an emphasis on three penaeid viruses. Integr Comp Biol 2009; 49(2): 127-141. http://dx.doi.org/10.1093/icb/icp033. PMid:21669853.
» http://dx.doi.org/10.1093/icb/icp033 - Pádua SB, Martins ML, Valladão GMR, Utz L, Zara FJ, Ishikawa MM, et al. Host-parasite relationship during Epistylis sp. (Ciliophora: Epistylididae) infestation in farmed cichlid and pimelodid fish. Pesq Agropec Bras 2016; 51(5): 520-526. http://dx.doi.org/10.1590/S0100-204X2016000500012.
» http://dx.doi.org/10.1590/S0100-204X2016000500012 - Paperna I. Diseases caused by parasites in the aquaculture of warm water fish. Annu Rev Fish Dis 1991; 1: 155-194. http://dx.doi.org/10.1016/0959-8030(91)90028-I.
» http://dx.doi.org/10.1016/0959-8030(91)90028-I - Pickering AD, Pottinger TG. Stress responses and disease resistance in salmonid fish: effects of chronic elevation of plasma cortisol. Fish Physiol Biochem 1989; 7(1-6): 253-258. http://dx.doi.org/10.1007/BF00004714. PMid:24221779.
» http://dx.doi.org/10.1007/BF00004714 - Robinson J, Avenant-Oldewage A. Aspects of the morphology of the parasitic copepod Lernaea cyprinacea Linnaeus, 1758 and notes on its distribution in Africa. Crustaceana 1996; 69(5): 610-626. http://dx.doi.org/10.1163/156854096X00628.
» http://dx.doi.org/10.1163/156854096X00628 - Smit NJ, Grutter AS, Adlard RD, Davies AJ. Hematozoa of teleosts from Lizard Island, Australia, with some comments on their possible mode of transmission and the description of a new hemogregarine species. J Parasitol 2006; 92(4): 778-788. http://dx.doi.org/10.1645/GE-756R.1. PMid:16995396.
» http://dx.doi.org/10.1645/GE-756R.1 - Silva RJD, Azevedo RKD, Abdallah VD. First record of an epibiont protozoan Epistylis sp. (Ciliophora, Peritrichia) attached to Amplexibranchius bryconis Thatcher & Paredes, 1985 (Copepoda, Ergasilidae) from Peixe’s River, state of São Paulo, Brazil. Crustaceana 2011; 84(9): 1139-1144. http://dx.doi.org/10.1163/001121611X584352.
» http://dx.doi.org/10.1163/001121611X584352 - Tavares-Dias M, Dias-Júnior MBF, Florentino AC, Silva LMA, Cunha AC. Distribution pattern of crustacean ectoparasites of freshwater fish from Brazil. Rev Bras Parasitol Vet 2015; 24(2): 136-147. http://dx.doi.org/10.1590/S1984-29612015036. PMid:26154954.
» http://dx.doi.org/10.1590/S1984-29612015036 - Tavares-Dias M, Moraes FR, Onaka EM, Rezende PCB. Changes in blood parameters of hybrid tambacu fish parasitized by Dolops carvalhoi (Crustacea, Branchiura), a fish louse. Vet Arh 2007; 77(4): 355-363.
- Terova G, Gornati R, Rimoldi S, Bernardini G, Saroglia M. Quantification of a glucocorticoid receptor in sea bass (Dicentrarchus labrax, L.) reared at high stocking density. Gene 2005; 357(2): 144-151. http://dx.doi.org/10.1016/j.gene.2005.06.016. PMid:16129569.
» http://dx.doi.org/10.1016/j.gene.2005.06.016 - Thatcher VE, Brites-Neto J. Diagnóstico, prevenção e tratamento das enfermidades de peixes neotropicais de água doce. Rev Bras Med Vet 1994; 16(3): 111-128.
- Thatcher VE. Amazon fish parasites. Amazoniana 1991; 11(3-4): 263-572.
- Tort L. Stress and immune modulation in fish. Dev Comp Immunol 2011; 35(12): 1366-1375. http://dx.doi.org/10.1016/j.dci.2011.07.002. PMid:21782845.
» http://dx.doi.org/10.1016/j.dci.2011.07.002 - Valladão GMR, Levy-Pereira N, Viadanna PHO, Gallani SU, Farias THV, Pilarski F. Haematology and histopathology of Nile tilapia parasitised by Epistylis sp., an emerging pathogen in South America. Bull Eur Assoc Fish Pathol 2015; 35: 14-20.
- Visse M. Detrimental effect of peritrich ciliates (Epistylis sp.) as epibionts on the survival of the copepod Acartia bifilosa. Proc Estonian Acad Sci Biol Ecol 2007; 56(3): 173-178.
- Walker PD, Flik G, Bonga SW. The biology of parasites from the genus Argulus and a review of the interactions with its host. Symp Soc Exp Biol 2004; 55(55): 107-129. PMid:15446447.
- Welicky RL, De Swardt J, Gerber R, Netherlands EC, Smit NJ. Drought-associated absence of alien invasive anchorworm, Lernaea cyprinacea (Copepoda: Lernaeidae), is related to changes in fish health. Int J Parasitol Parasites Wildl 2017; 6(3): 430-438. http://dx.doi.org/10.1016/j.ijppaw.2017.01.004.
» http://dx.doi.org/10.1016/j.ijppaw.2017.01.004 - Xu DH, Shoemaker CA, Klesius PH. Evaluation of the link between gyrodactylosis and streptococcosis of Nile tilapia, Oreochromis niloticus (L.). J Fish Dis 2007; 30(4): 233-238. http://dx.doi.org/10.1111/j.1365-2761.2007.00806.x. PMid:17394525.
» http://dx.doi.org/10.1111/j.1365-2761.2007.00806.x - Xu Z, Burns CW. Effects of the epizoic ciliate, Epistylis daphniae, on growth, reproduction and mortality of Boeckella triarticulata (Thomson) (Copepoda: Calanoida). Hydrobiologia 1991; 209(3): 183-189. http://dx.doi.org/10.1007/BF00015341.
» http://dx.doi.org/10.1007/BF00015341 - Yamaguti S. Parasitic Copepoda and Branchiura of fishes New York: John Wiley; 1963.
- Zanolo R, Yamamura MH. Parasitas in tilapia of nile in fresh water net-tank system. Semina: Ciênc Agrár 2006; 27(2): 281-288. http://dx.doi.org/10.5433/1679-0359.2006v27n2p281.
» http://dx.doi.org/10.5433/1679-0359.2006v27n2p281
Publication Dates
-
Publication in this collection
30 Aug 2018 -
Date of issue
Jul-Sep 2018
History
-
Received
19 Apr 2018 -
Accepted
18 May 2018