Abstract
This study evaluated the seroprevalence of Toxoplasma gondii, Neospora caninum and Leptospira spp. in dogs from Foz do Iguaçu, Paraná, Brazil. Indirect immunofluorescent antibody test was used to detect antibodies anti-T.gondii and anti-N. caninum. Immunoenzymatic assay and microscopic serum agglutination were used for screening antibodies anti-T.gondii and anti-Leptospira spp., respectively. The results were: 67.02% of the samples reactive for T.gondii and 1.38% for N. caninum, both without statistically significant variables. For Leptospira spp. the results indicated seroprevalence of 23.11%. The analysis of the variables without distinction of serovar showed association for intrinsic characteristics as breed, age, nutritional status and dog category. The extrinsic variables as city region and access to the street presented association (p<0.05). The most prevalent serovars were: Canicola 59.47%; Bratislava 13.07% and Butembo 15.68%. Variables that make up the adjusted multiple analysis model using Leptospira spp. were: age, breed and nutritional status; serovar Canicola, sex, nutritional status and area (p<0.05); serovar Bratislava, lymphadenomegaly and presence of fleas (p<0.05). Given the results obtained, dogs can be used as sentinels for toxoplasmosis and leptospirosis in Foz do Iguaçu and other cities with similar outcomes. In addition, preventive measures should be taken by health authorities because they are zoonoses and humans are also at risk.
Keywords:
Spatial dispersion; toxoplasmosis; neosporosis; leptospirosis; Foz do Iguaçu
Resumo
Este estudo avaliou a soroprevalência de Toxoplasma gondii, Neospora caninum e Leptospira spp. em cães de Foz do Iguaçu, Paraná, Brasil. O teste de imunofluorescência indireta foi utilizado para detectar anticorpos anti-T. gondii e anti-N. caninum. Ensaio imunoenzimático e soroaglutinação microscópica foram utilizados para pesquisa de anticorpos anti-T. gondii e anti-Leptospira spp., respectivamente. Os resultados obtidos foram: 67,02% (435/649) das amostras reativas para T. gondii e, 1,38% (9/649) para N. caninum, ambas com ausência de variáveis significativas estatisticamente. Para Leptospira spp. os resultados indicaram soroprevalência de 23,11% (153/649). A análise das variáveis sem distinção de sorovar mostraram associação para caraterísticas intrísecas como raça, idade, estado nutricional e categoria de cães. Para as variáveis extrínsecas, a região da cidade e ter acesso à rua mostraram associação estatística (p<0,05). Os sorovares mais prevalentes foram: Canicola com 59,47% (91/153); Bratislava 13,07% (20/153) e Butembo 15,68% (24/153). As variáveis que compõem o modelo de análise multivariada ajustada usando como desfecho Leptospira spp. foram: idade, raça, estado nutricional e área. Para o sorovar Canicola, as variáveis significantes (p<0,05) foram sexo, estado nutricional e área; para o sorovar Bratislava, as variáveis significantes (p<0,05) foram linfadenomegalia e presença de pulgas. Dos resultados obtidos, cães podem ser usados como sentinelas para infecção por T. gondii e Leptospira spp. na cidade de Foz do Iguaçu e em outras cidades com desfechos similares. Além disso, medidas preventivas devem ser tomadas pelas autoridades de saúde, pois são zoonoses e os seres humanos também estão em risco.
Palavras-chave:
Distribuição espacial; toxoplasmose; neosporose; leptospirose; Foz do Iguaçu
Introduction
Foz do Iguaçu municipality is in the extreme west of the State of Paraná, on the triple border of Brazil, Argentina and Paraguay. Due to its location, it has sizeable migratory flow, and it is an area that is very vulnerable to diseases including the zoonoses. Due to low levels of maritime influence, the area has one of the largest annual thermal amplitudes, with an average temperature difference of approximately 11°C between winter and summer. Its characteristics are very peculiar. There are areas of interposition between rural areas, parks, ecological reserves and urban zones (ZIOBER & ZANIRATO, 2014Ziober BR, Zanirato SH. Actions to safeguard biodiversity during the building of the Itaipu Binacional Hydroelectric plant. Ambiente Soc 2014; 17(1): 59-78. http://dx.doi.org/10.1590/S1414-753X2014000100005.
http://dx.doi.org/10.1590/S1414-753X2014...
; IBGE, 2016Instituto Brasileiro de Geografia e Estatística – IBGE. Foz do Iguaçu – Infográficos: dados gerais do município [online]. Rio de Janeiro: IBGE; 2016 [cited 2017 jun 20]. Available from: https://cidades.ibge.gov.br/v4/brasil/pr/foz-do iguacu/panorama
https://cidades.ibge.gov.br/v4/brasil/pr...
). Climatic conditions, anthropic intervention in the ecosystems and the socio-cultural and economic characteristics of the city could facilitate the spread of infectious agents such as Toxoplasma gondii, Neospora caninum and Leptospira spp. The close relationship between humans and dogs makes these animals important flags and/or sentinels of microbiological and/or parasitic diseases (FAINE, 1982Faine S. Guidelines for the control of leptospirosis. WHO Offset Publ 1982;67: 1-171. PMid:7184280.; GARCIA et al., 1999Garcia JL, Navarro IT, Ogawa L, Oliveira RC. Soroepidemiology of toxoplasmosis in cats and dogs from rural properties of Jaguapitã county, Paraná State, Brazil. Cienc Rural 1999; 29(1): 99-104. http://dx.doi.org/10.1590/S0103-84781999000100018.
http://dx.doi.org/10.1590/S0103-84781999...
; MOURA et al., 2009Moura AB, Souza AP, Sartor AA, Bellato V, Teixeira EB, Pisetta GMP, et al. Occurrence of antibodies and risk factors for infection for Toxoplasma gondii in dogs in the cities of Lages and Balneário Camboriú, Santa Catarina State, Brazil. Rev Bras Parasitol Vet 2009; 18(3): 52-56. http://dx.doi.org/10.4322/rbpv.01803009. PMid:19772776.
http://dx.doi.org/10.4322/rbpv.01803009...
).
Toxoplasma gondii is one of the most studied parasites in the world due to its importance in the medical and veterinary fields. Felids are its final host, and all other endothermic animals (as dogs) are intermediate hosts. In humans this parasite can cause several problems in special in pregnant women because when acquired during pregnancy the toxoplasmosis can produce a severe congenital infection (DUBEY et al., 1970Dubey JP, Miller NL, Frenkel JK. Characterization of the new fecal form of Toxoplasma gondii. J Parasitol 1970; 56(3): 447-456. http://dx.doi.org/10.2307/3277601. PMid:5467864.
http://dx.doi.org/10.2307/3277601...
). Dogs have been used as sentinel animals for estimating T. gondii infection in the environment due their comportment and proximity with humans (LOPES et al., 2014Lopes AP, Dubey J, Dardé M, Cardoso L. Epidemiological review of Toxoplasma gondii infection in humans and animals in Portugal. Parasitology 2014; 141(13): 1699-1708. http://dx.doi.org/10.1017/S0031182014001413. PMid:25215422.
http://dx.doi.org/10.1017/S0031182014001...
).
Neospora caninum is a coccidian protozoan that affects mainly production animals and canids, which are the definitive hosts (DUBEY et al., 1988Dubey JP, Hattel AL, Lindsay DS, Topper MJ. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J Am Vet Med Assoc 1988; 193(10): 1259-1263. PMid:3144521.; MCALLISTER et al., 1998McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, McGuire AM. Rapid communication: dogs are definitive hosts of Neospora caninum. Int J Parasitol 1998; 28(9): 1473-1478. http://dx.doi.org/10.1016/S0020-7519(98)00138-6. PMid:9770635.
http://dx.doi.org/10.1016/S0020-7519(98)...
). Its zoonotic potential is still uncertain. The pathogenicity and severity of N. caninum varies with the host species. Cattle are the most susceptible, with infection resulting in great economic losses in the reproductive sphere (DUBEY et al., 2006Dubey JP, Buxton D, Wouda W. Pathogenesis of Bovine Neosporosis. J Comp Pathol 2006; 134(4): 267-289. http://dx.doi.org/10.1016/j.jcpa.2005.11.004. PMid:16712863.
http://dx.doi.org/10.1016/j.jcpa.2005.11...
).
Leptospira spp. is the bacteria that causes a zoonotic disease endemic in Brazil, has seasonality and occurs at higher rates in times of increased rainfall (FAINE, 1999Faine S. Leptospira and leptospirosis. Melbourne: MediSci; 1999.; LILENBAUM et al., 2005Lilenbaum W, Varges R, Moraes IA, Ferreira AMR, Pissinatti A. Leptospiral antibodies in captive lion tamarins (Leontopithecus sp) in Brazil. Vet J 2005; 169(3): 462-464. http://dx.doi.org/10.1016/j.tvjl.2004.03.015. PMid:15848790.
http://dx.doi.org/10.1016/j.tvjl.2004.03...
). Dogs are considered reservoirs for some serovars, such as Canicola, and are important for the maintenance of this agent in the environment (GREENE et al., 2006Greene CE, Sykes JE, Brown CA, Hartman K. Infectious diseases of the dog and cat. 4th ed. Saint Louis: Elsevier; 2006.).
Leptospira spp. and T. gondii dog infection is epidemiologically important because dogs share habitats with humans, which indicates that the environment represents an ecological niche for these pathogens and, consequently, a potential risk of infection for humans (FAINE, 1982Faine S. Guidelines for the control of leptospirosis. WHO Offset Publ 1982;67: 1-171. PMid:7184280.; HEATH & JOHNSON, 1994Heath SE, Johnson R. Leptospirosis. J Am Vet Med Assoc 1994; 205(11): 1518-1523. PMid:7730115.; GARCIA et al., 1999Garcia JL, Navarro IT, Ogawa L, Oliveira RC. Soroepidemiology of toxoplasmosis in cats and dogs from rural properties of Jaguapitã county, Paraná State, Brazil. Cienc Rural 1999; 29(1): 99-104. http://dx.doi.org/10.1590/S0103-84781999000100018.
http://dx.doi.org/10.1590/S0103-84781999...
; MOURA et al., 2009Moura AB, Souza AP, Sartor AA, Bellato V, Teixeira EB, Pisetta GMP, et al. Occurrence of antibodies and risk factors for infection for Toxoplasma gondii in dogs in the cities of Lages and Balneário Camboriú, Santa Catarina State, Brazil. Rev Bras Parasitol Vet 2009; 18(3): 52-56. http://dx.doi.org/10.4322/rbpv.01803009. PMid:19772776.
http://dx.doi.org/10.4322/rbpv.01803009...
).
Considering the importance of the T. gondii, N. caninum and Leptospira spp. for dogs and other species, the role of dogs as sentinels, as well as the peculiar characteristics of Foz do Iguaçu and the lack of previous studies, the objective of this study was to conduct a cross - sectional study on Toxoplasma gondii, Neospora caninum and Leptospira spp. seroprevalence in dogs in the city of Foz do Iguaçu, Brazil, to identify potential factors related to infection.
Materials and Methods
Study area and data collection
The work was approved by the Ethics Committee on the Use of Animals of the Sector of Agrarian Sciences of the Federal University of Paraná and was registered under number 044/14.
The municipality of Foz do Iguaçu has an estimated population of 263,782 inhabitants and 54,983 dogs. It is located at latitude 25°32’45”S and longitude 54°35’07”W. It has a humid subtropical climate, with hot summers, infrequent frosts, rain in all months of the year, and nine micro watersheds. Seven of the watersheds are circumscribed to the municipal perimeter. The main rivers are Paraná, Iguaçu, Tamanduá, São João, Almada, M'Boicy and Monjolo (IBGE, 2016Instituto Brasileiro de Geografia e Estatística – IBGE. Foz do Iguaçu – Infográficos: dados gerais do município [online]. Rio de Janeiro: IBGE; 2016 [cited 2017 jun 20]. Available from: https://cidades.ibge.gov.br/v4/brasil/pr/foz-do iguacu/panorama
https://cidades.ibge.gov.br/v4/brasil/pr...
). According to Instituto Brasileiro de Geografia e Estatística (IBGE, 2016Instituto Brasileiro de Geografia e Estatística – IBGE. Foz do Iguaçu – Infográficos: dados gerais do município [online]. Rio de Janeiro: IBGE; 2016 [cited 2017 jun 20]. Available from: https://cidades.ibge.gov.br/v4/brasil/pr/foz-do iguacu/panorama
https://cidades.ibge.gov.br/v4/brasil/pr...
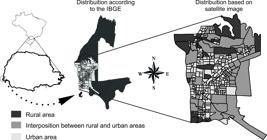
) it has a territorial area of 617,701 km2. Of this total area, 191.46 km2 are urban areas, 138.17 km2 are rural, 138.60 km2 are National Parks, 149.10 km2 make up Itaipu Lake, and 0.38 km2 make up Acaray Island. There are areas of interposition between rural and urban areas and there is great ecological biodiversity in the parks and lakes (Figure 1).
Schematic map with highlights for rural and urban areas, Foz do Iguaçu, Paraná, Brazil, 2014.
For this study, the city was divided into four areas (A to D) by using a stratified sampling strategy. The stratifications of the urban area were identified using a landscape approach. Thus, by recognizing the patterns of the landscape elements and their spatial distribution, the different landscape areas present in each area were considered to delineate the sampling strata. This method is related to the visual interpretation of the products of remote sensors. In this case, high-resolution images of the study area available from Google Earth were used, and the images were projected on an approximate scale of 1: 50,000. Four strata were identified in the city of Foz do Iguaçu, and each stratum was divided into areas of 400 × 400 m, and then quadrants (between 25 and 40 per area) were randomly selected, and the “best” scenario for the presence of rodents and placement of traps was determined. Five dogs were sampled (irrespective of their clinical status) from each patch according to methodology described in Thomaz-Soccol et al. (2017)Soccol VT, Pasquali AKS, Pozzolo EM, Leandro AS, Chiyo L, Baggio R, et al. More than the eyes can see: the worrying scenario of canine leishmaniasis in the Brazilian side of the triple border. PLoS One 2017; 12(12): e0189182. http://dx.doi.org/10.1371/journal.pone.0189182. PMid:29232388.
http://dx.doi.org/10.1371/journal.pone.0...
. When five dogs were not available in this house, dogs from the neighbourhood were included, up to five. Dogs from the houses were blood-sampled by veterinarians.
The city was divided into four areas: A, B, C and D (Figure 2). Area A: corresponds to the commercial region of the city with the highest density of buildings near the Paraná River. This area contains two forest remnants, which occupy a large part of this stratum. Area B: corresponds to one of the residential areas bordering the rural areas. It has medium density housing and is relatively uniform. Area C: located in the northern portion of the city, bordered to the west and north by the Paraná River and rural areas to the east. This area is discontinuous and is interrupted by large spaces of vegetation (e.g., sports fields, small cultivated areas). Area D: has buildings of high and intermediate densities, is at the mouth of the Rio Iguaçu and Rio Paraná, and has patches of vegetation around the coast of the mentioned rivers.
For all collections, the tutors completed the term of science and authorization, and an epidemiological questionnaire which contained information such breed (with defined or non-defined breed), sex (male or female), age, sleeping location, access to the street, immunizations (rabies vaccine and polyvalent vaccine), category of dog (company, guard, NGO - Non-governmental Organization or accompanying recyclable waste pickers), and origin (same neighborhood, another neighborhood, another city or other country) was given. Physical examination was carried out by the veterinarians to verify the general state of the animal, observing points such as presence of ectoparasites, body and nutritional status, besides mucosal staining and the presence of cutaneous wounds.
Rodent activity was estimated by 10 traps that were distributed in each quadrant for four consecutive days and were capable of detecting feces, urine and hair, as described by Cavia et al. (2012)Cavia R, Cueto GR, Suárez OV. Techniques to estimate abundance and monitoring rodent pests in urban environments. In: Soloneski S, Larramendy M, editors. Integrated pest management and pest control - current and future tactics. Rijeka: Intech; 2012. p. 147-172. http://dx.doi.org/10.5772/32541.
http://dx.doi.org/10.5772/32541...
.
Biological blood samples and serum
Blood collection from 649 dogs was performed in 2014 by puncturing the cephalic or jugular vein using a disposable syringe and needle (25 × 7 mm). The samples were placed in sterilized tubes without anticoagulants, identified and transported to the laboratory for centrifugation and serum separation. The serum was then aliquoted into polypropylene tubes (1.5 mL) and stored at negative 20 °C until the time of analysis.
Immunoenzymatic assay (ELISA) and Indirect immunofluorescence antibody test for T. gondii
Two tests were used for screening for antibodies anti-T. gondii, the indirect enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence antibody test (IFAT).
ELISA was performed as described by Garcia et al. (1999)Garcia JL, Navarro IT, Ogawa L, Oliveira RC. Soroepidemiology of toxoplasmosis in cats and dogs from rural properties of Jaguapitã county, Paraná State, Brazil. Cienc Rural 1999; 29(1): 99-104. http://dx.doi.org/10.1590/S0103-84781999000100018.
http://dx.doi.org/10.1590/S0103-84781999...
. Briefly, concentrations of T. gondii antigen, serum and peroxidase-conjugated protein A conjugate (Sigma Aldrich, Missouri, USA) were: 2.5 μg / mL, 1:100, and 1:2,500, respectively. All samples were tested in duplicate. The optimum conditions for the dilutions of antigen, serum and conjugate were estabilished by the greater ratio between the mean optical density of the positive samples and the mean of the negative samples. The cut-off point of each plate was obtained according to Garcia et al. (2006)Garcia JL, Navarro IT, Vidotto O, Gennari SM, Machado RZ, Pereira ABL, et al. Toxoplasma gondii: Comparison of a rhoptry-ELISA with IFAT and MAT for antibody detection in sera of experimentally infected pigs. Exp Parasitol 2006; 113(2): 100-105. http://dx.doi.org/10.1016/j.exppara.2005.12.011. PMid:16458299.
http://dx.doi.org/10.1016/j.exppara.2005...
. The general cut-off point was estimated using the ROC curve, which was constructed using the MedCalc Statistical Software program (SCHOONJANS et al., 1995Schoonjans F, Zalata A, Depuydt CE, Comhaire FH. MedCalc: a new computer program for medical statistics. Comput Methods Programs Biomed 1995; 48(3): 257-262. http://dx.doi.org/10.1016/0169-2607(95)01703-8. PMid:8925653.
http://dx.doi.org/10.1016/0169-2607(95)0...
).
To IFAT, the methodology was done according Camargo (1964)Camargo ME. Improved technique of indirect immunofluorescence for serological diagnosis of toxoplasmosis. Rev Inst Med Trop São Paulo 1964; 6: 117-118. PMid:14177810., where tachyzoites of RH strain were used as antigen, and in each slide positive and negative controls were used. Samples with titers ≥16 were considered positive (FERREIRA et al., 2016Ferreira FP, Miura AC, Mareze M, Garcia JL, Freire RL, Navarro IT. Frequency of anti-Toxoplasma gondii antibodies in dogs with clinical signs consistent with toxoplasmosis. Cienc Anim Bras 2016; 17(4): 640-646. http://dx.doi.org/10.1590/1089-6891v17i440999.
http://dx.doi.org/10.1590/1089-6891v17i4...
) and were diluted at a ratio four until the titer was negative. The results of the two tests were merged to analysis.
Indirect immunofluorescence for anti-Neospora spp. antibodies
For the titration of antibodies anti - N. caninum, an indirect immunofluorescent antibody test (IFAT) was used (CONRAD et al., 1993Conrad PA, Sverlow K, Anderson M, Rowe J, BonDurant R, Tuter G, et al. Detection of Serum Antibody Responses in Cattle with Natural or Experimental Neospora Infections. J Vet Diagn Invest 1993; 5(4): 572-578. http://dx.doi.org/10.1177/104063879300500412. PMid:8286457.
http://dx.doi.org/10.1177/10406387930050...
), using the tachyzoite forms of the NC-1 strain as antigen. During the analyses, positive and negative controls were included on each slide. Samples with titers ≥ 50 (DUBEY et al., 1988Dubey JP, Hattel AL, Lindsay DS, Topper MJ. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J Am Vet Med Assoc 1988; 193(10): 1259-1263. PMid:3144521.) were considered positive and diluted at a ratio of two until the titer was negative.
Microscopic soroagglutination forLeptospira spp.
Serum samples were submitted to microscopic serum agglutination for the detection of antibodies anti- Leptospira spp. In general, strains of the different serovars are reported (FAINE, 1999Faine S. Leptospira and leptospirosis. Melbourne: MediSci; 1999.) and for this reason 10 reference serovars were used: Bratislava, Butembo, Castellonis, Canicola, Grippotyphosa, Copenhageni, Icterohaemorrhagiae, Pomona, Pyrogenes and Hardjo. The antigens were maintained at 28 °C for five to ten days in EMJH medium prior to use (FAINE, 1982Faine S. Guidelines for the control of leptospirosis. WHO Offset Publ 1982;67: 1-171. PMid:7184280.). Serum samples that had at least 50% of Leptospira agglutinated at 1: 100 dilution were considered reactive, and they were then serially diluted 2-fold to determine the maximum positive dilution. The most likely serovar was the one with the highest titer (FAVERO et al., 2002Favero ACM, Pinheiro SR, Vasconcellos AS, Morais ZM, Ferreira F, Ferreira JS No. Sorovares de leptospiras predominates em exames sorológicos de bubalinos, ovinos, caprinos, equinos, suínos e cães de diversos estados brasileiros. Cienc Rural 2002; 32(4): 613-619. http://dx.doi.org/10.1590/S0103-84782002000400011.
http://dx.doi.org/10.1590/S0103-84782002...
).
Statistical analysis
The EpiInfo program 7.1.5.2 (DEAN et al., 1994Dean AG, Dean JA, Coulombier D, Brendel KA, Smith DC, Burton AH, et al. Epi Info: a word processing, database and statistics program for epidemiology on microcomputers. Version 6. Atlanta: CDC; 1994. 601 p.) was used to tabulate the epidemiological questionnaire variables, serological results and to determine statistical significance of the variables with a chi-square test or Fisher’s exact test, considering a significance level of 5%. Only the variables whose p value was <0.05 in the univariate analysis and which presented some biological meaning were included in the multiple logistic regression models, which were performed with program R 3.4 (TEAM R, 2011Team RDCR. A language and environment for statistical computing [online]. 2011 [cited 2003 Jan 10]. Available from: https://www.r-project.org
https://www.r-project.org...
). After adjustment, the model residues were checked. Residue analysis verified the presence or absence of systematic models of variation, in addition to the quality of fit. The odds ratio (OR) calculation with a 95% confidence interval (CI) was used as a measure of association.
Geoprocessing
Dog residences were mapped using the Global Positioning System (GPS). Point distribution, cluster analysis (EVERITT, 1974Everitt B. Cluster analysis. London: Wiley; 1974.), and kernel density were constructed using the Qgis software (TEAM, 2016Team QD. QGIS geographic information system. Open source geospatial foundation project [online] 2016 [cited 2019 May 17]. Available from: https://www.qgis.org
https://www.qgis.org...
). Kernel density is a statistical technique in which the distribution of events is transformed into a continuous surface, allowing the estimation of the density of the event in every area, identifying areas with the highest occurrence of the event (GATRELL et al., 1996Gatrell AC, Bailey TC, Diggle PJ, Rowlingson BS. Spatial point pattern analysis and its application in geographical epidemiology. Trans Inst Br Geogr 1996; 21(1): 256-274. http://dx.doi.org/10.2307/622936.
http://dx.doi.org/10.2307/622936...
). For cluster analysis, the plugin ClusterPoints from Qgis was used and after delimitation of the areas, the chi-square test was applied. Plugin Cluster Points conducts spatial clustering of points based on their mutual distance to each other. The user can select between the K-Means algorithm and (agglomerative) hierarchical clustering with several different link functions (TEAM, 2016Team QD. QGIS geographic information system. Open source geospatial foundation project [online] 2016 [cited 2019 May 17]. Available from: https://www.qgis.org
https://www.qgis.org...
).
Results
A total of 649 dogs were included in this study, their geographic distribution were 20.95% (136/649) from area A, 19.72% (128/649) from area B, 36.20% (235/649) from area C, and 23.11% (150/649) from area D (Figure 3). The intrinsic characters as sex were 54.39% female, and 86.44% adults. For the extrinsic characters we observed 73.18% as undefined breed, 92.91% sleep in the outside and 60.86% has street access (Table 1). The numerical differences in age, category and origin are due to the lack of information in the epidemiological questionnaires.
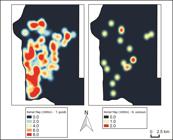
In the search for antibodies anti – T. gondii, the positivity varied according to the methodology employed; 51.30% (333/649) by ELISA approach and 40.83% (265/649) by IFAT. When considering both tests, the positivity was 67.02% (435/649) and varied from 60.15% in area B to 77.20% for the area A. We were unable to find any significant variables when using the results from both tests together (Table 2 and Figure 4).
Profile of dogs serologically tested for T. gondii and N. caninum, Foz do Iguaçu, Paraná, Brazil, 2014.
Kernel density analysis of the presence of antibodies anti-T. gondii and N. caninum in dogs, Foz do Iguaçu, Brazil, 2014.
For N. caninum, only 1.4% (9/649) were seropositive, with absence of significant variables (Table 2). The positivity varied according to the researched area from zero in area A to 2.62% in area C (Figure 4).
For Leptospira the microscopic serum agglutination showed 23.57% (153/649) of the dogs presenting antibodies anti-Leptospira spp. The most prevalent serovars were Canicola 59.47% (91/153), Bratislava 13.07% (20/153), Butembo 15.68% (24/153), Pyrogenes 2.61% (4/153), Pomona 1.96% (3/153), Copenhageni 1.96% (3/153), Grippotyphosa 2.61% (4/153), and Icterohaemorrhagiae 0.65% (1/153). No samples were seroreactive to the Hardjo and Castellonis serovars. Intrinsic variables as sex, age, breed, nutritional status, mood, presence of fleas and ticks, weight loss, presence of ocular secretion, alopecia and lymphadenomegaly and extrinsic variables as category, street access and origin area were statistically significant for the positivity to Leptospira in univariate analysis (Table 3). The adjusted models resulting from the multiple analysis are presented in Table 4. The variables that make up the model whose outcome is Leptospira spp. were area, age, breed and nutritional status; for serovar Canicola the adjusted model contained area, sex and nutritional status variables; when the outcome was serovar Bratislava, the variables were: presence of fleas and lymphadenomegaly. The area B presented higher prevalence of animals seropositive to Leptospira spp. and serovar Butembo, while area A presented higher prevalence of animals seropositive to serovar Canicola (Table 4). By spatial analysis, a significant cluster of points was observed for the dogs that were seropositive to Canicola serovar (p < 0.001), they were concentrated in a specific region of area D. Area B and C presented a lower occurrence (p<0.05) (Figure 5).
Significant variables in the univariate analysis for antibodies anti-Leptospira spp. and serovars analyzed in dogs, Foz do Iguaçu, Paraná, Brazil, 2014.
Significant variables in the multiple analysis for antibodies anti-Leptospira spp. and serovars analysed in dogs, Foz do Iguaçu, Paraná, Brazil, 2014.
Kernel density analysis (1000m) of the presence of antibodies anti-Leptospira spp. (A), anti-serovar Bratislava (B), anti-serovar Butembo (C) and anti-serovar Canicola (D), simple distribution and relative spatial risk for dogs with anti-serovar Canicola antibodies (E), Foz do Iguaçu, Paraná, Brazil, 2014.
With regard to the percentages of coinfection, 1.38% (9/649) of the animals presented antibodies anti-T. gondii and N. caninum; 15.56% (101/649), anti- anti-T. gondii and Leptospira spp.; 0.92% (6/649), anti-N. caninum and Leptospira spp.; 0.77% (5/649) presented antibodies against the three studied agents.
Rodent activity was observed in 70.87% (460/649) of the traps, with no higher concentration in a particular area and no statistical association to the presence of antibodies against the three pathogenic agents studied. It was observed that 70.34% (306/435) of the T. gondii seropositive dogs, 77.77% (7/9) of the N. caninum seropositive dogs and 73.20% of the Leptospira spp. seropositive dogs (112/153) had rodent activity in their homes.
Discussion
Toxoplasma gondii seroprevalence in dogs from Foz do Iguaçu county (67.02%) is close to the upper limit found in Paraná in the last decade: 10.3% and 70.85% (DREER et al., 2013Dreer MKP, Gonçalves DD, Caetano ICS, Gerônimo E, Menegas PH, Bergo D, et al. Toxoplasmosis, leptospirosis and brucellosis in stray dogs housed at the shelter in Umuarama municipality, Paraná, Brazil. J Venom Anim Toxins Incl Trop Dis 2013; 19(1): 23. http://dx.doi.org/10.1186/1678-9199-19-23. PMid:24066949.
http://dx.doi.org/10.1186/1678-9199-19-2...
; CALDART et al., 2015Caldart ET, Constantino C, Pasquali AKS, Benitez ADN, Hamada FN, Dias RCF, et al. Zoonosis in dogs and cats attended by the Birth Control Project: Toxoplasma gondii, Leishmania spp. and Leptospira spp., serodiagnosis and epidemiology. Semina: Ciênc Agrár 2015; 36(1): 253-265. http://dx.doi.org/10.5433/1679-0359.2015v36n1p253.
http://dx.doi.org/10.5433/1679-0359.2015...
; FERREIRA et al., 2016Ferreira FP, Miura AC, Mareze M, Garcia JL, Freire RL, Navarro IT. Frequency of anti-Toxoplasma gondii antibodies in dogs with clinical signs consistent with toxoplasmosis. Cienc Anim Bras 2016; 17(4): 640-646. http://dx.doi.org/10.1590/1089-6891v17i440999.
http://dx.doi.org/10.1590/1089-6891v17i4...
). Even though no epidemiological variables were statistically associated with this high prevalence, dogs that did not have access to the street, slept in the house and has a defined breed showed a higher frequency in the IFAT methodology. These characteristics show a greater affective contact of the tutors and that possibly the shared environment between them may offer conditions for infection by the pathogenic agent, demonstrating the important role of the dog as a sentinel for toxoplasmosis.
This study showed that dogs from Foz do Iguaçu had a low seroprevalence of antibodies anti- N. caninum (1.4%), and this was lower than that found in other regions of the country (TEIXEIRA et al., 2006Teixeira WC, Silva MIS, Pereira JG, Pinheiro AM, Almeida MAO, Gondim LFP. Frequency of Neospora caninum in dogs from São Luís, Maranhão. Arq Bras Med Vet Zootec 2006; 58(4): 685-687. http://dx.doi.org/10.1590/S0102-09352006000400038.
http://dx.doi.org/10.1590/S0102-09352006...
; ROMANELLI et al., 2007Romanelli PR, Freire RL, Vidotto O, Marana ERM, Ogawa L, De Paula VSO, et al. Prevalence of Neospora caninum and Toxoplasma gondii in sheep and dogs from Guarapuava farms, Parana State, Brazil. Res Vet Sci 2007; 82(2): 202-207. http://dx.doi.org/10.1016/j.rvsc.2006.04.001. PMid:17266999.
http://dx.doi.org/10.1016/j.rvsc.2006.04...
; COIRO et al., 2011Coiro CJ, Langoni H, Silva RC, Ullmann LS. Risk factors to leptospirosis, leishmaniasis, neosporosis and toxoplasmosis in domiciliated and peridomiciliated dogs in Botucatu - SP. Vet Zootec 2011; 18(3): 386-393.). The role of the dog in the epidemiology of neosporosis is evident, however, oocyst elimination is intermittent and short-term (LINDSAY et al., 2001Lindsay DS, Ritter DM, Brake D. Oocyst excretion in dogs fed mouse brains containing tissue cysts of a cloned line of Neospora caninum. J Parasitol 2001; 87(4): 909-911. http://dx.doi.org/10.1645/0022-3395(2001)087[0909:OEIDFM]2.0.CO;2. PMid:11534658.
http://dx.doi.org/10.1645/0022-3395(2001...
) and there may be no seroconversion (DIJKSTRA et al., 2001Dijkstra T, Barkema HW, Eysker M, Wouda W. Evidence of post-natal transmission of Neospora caninum in Dutch dairy herds. Int J Parasitol 2001; 31(2): 209-215. http://dx.doi.org/10.1016/S0020-7519(00)00160-0. PMid:11239942.
http://dx.doi.org/10.1016/S0020-7519(00)...
). On the other hand, the possibility of horizontal transmission among rural dogs is generally greater due to carnivorism and the ease of ingestion of rodents, birds and wild animals that may contain tissue cysts and serve as a source of parasite infection (WOUDA et al., 1999Wouda W, Dijkstra T, Kramer AMH, Van Maanen C, Brinkhof JMA. Seroepidemiological evidence for a relationship between Neospora caninum infections in dogs and cattle. Int J Parasitol 1999; 29(10): 1677-1682. http://dx.doi.org/10.1016/S0020-7519(99)00105-8. PMid:10608454.
http://dx.doi.org/10.1016/S0020-7519(99)...
). Fernandes et al. (2004)Fernandes BCTM, Gennari SM, Souza SLP, Carvalho JM, Oliveira WG, Cury MC. Prevalence of anti-Neospora caninum antibodies in dogs from urban, periurban and rural areas of the city of Uberlândia, Minas Gerais - Brazil. Vet Parasitol 2004; 123(1-2): 33-40. http://dx.doi.org/10.1016/j.vetpar.2004.05.016. PMid:15265569.
http://dx.doi.org/10.1016/j.vetpar.2004....
and Cunha et al. (2008)Cunha NA Fo, Lucas AS, Pappen FG, Ragozo AMA, Gennari SM, Lucia T Jr, et al. Fatores de risco e prevalência de anticorpos anti-Neospora caninum em cães urbanos e rurais do Rio Grande do Sul, Brasil. Rev Bras Parasitol Vet 2008; 17(Supl 1): 301-306. PMid:20059865., in studies carried out in Minas Gerais and Rio Grande do Sul, respectively, described a higher seropositivity to N. caninum in dogs from rural areas compared to dogs from urban areas. Rural dogs probably have increased contact with the main intermediate host in the transmission chain, the bovine. It is possible that the low seropositivity for N. caninum found in this study is due to the fact that the population studied came from an urban area.
The prevalence of Leptospira spp. in dogs, in Brazil, has been shown to vary from 2.66% to 59.25% (BENITEZ et al., 2012Benitez NA, Goncalves DD, Freire RL, Rodrigues WB, Souza VRA, Barbara JCA, et al. Seroepidemiology of leptospirosis in pet dogs in the urban area of the municipality of Jataizinho, Paraná. Semina: Ciênc Agrár 2012; 33(Supl 2): 3201-3210. http://dx.doi.org/10.5433/1679-0359.2012v33Supl2p3201.
http://dx.doi.org/10.5433/1679-0359.2012...
; CALDART et al., 2015Caldart ET, Constantino C, Pasquali AKS, Benitez ADN, Hamada FN, Dias RCF, et al. Zoonosis in dogs and cats attended by the Birth Control Project: Toxoplasma gondii, Leishmania spp. and Leptospira spp., serodiagnosis and epidemiology. Semina: Ciênc Agrár 2015; 36(1): 253-265. http://dx.doi.org/10.5433/1679-0359.2015v36n1p253.
http://dx.doi.org/10.5433/1679-0359.2015...
; SOUZA et al., 2016Souza MA, Castro JR, Moreira RQ, Bombonato NG, Soares PM, Lima AMC. Anti-Leptospira spp. antibodies in several animal species on the same farm. Biosci J 2016; 32(1): 202-207. http://dx.doi.org/10.14393/BJ-v32n1a2016-26605.
http://dx.doi.org/10.14393/BJ-v32n1a2016...
), the intermediate prevalence observed in this study (23.6%) demonstrates that infection by this agent is important in this region. The area B is a region of medium human population density with areas of interposition between rural and urban land. These environmental characteristics may be associated with the higher prevalence of animals that were seropositive to Leptospira spp., serovar Bratislava and serovar Butembo. The studied population has easy access to periurban areas, thus favoring contact between dogs and other possible reservoirs of the bacterium, including production or wild animals (AGUIAR et al., 2007Aguiar DM, Cavalcante GT, Marvulo MFV, Silva JCR, Pinter A, Vasconcellos AS, et al. Risk factors associated with anti-Leptospira spp. antibodies occurence in dogs from Monte Negro County, Rondônia, Brazilian Western Amazon. Arq Bras Med Vet Zootec 2007; 59(1): 70-76. http://dx.doi.org/10.1590/S0102-09352007000100012.
http://dx.doi.org/10.1590/S0102-09352007...
). Dogs are the main reservoirs of the serovar Canicola, the most prevalent in this study (60.00%), they can eliminate the agent in urine for long periods of time, even if they are asymptomatic (FAINE, 1999Faine S. Leptospira and leptospirosis. Melbourne: MediSci; 1999.). Area D, the most prevalent, includes the triple border and relatively uniform residential areas. A statistically significant cluster was found in this area, the specific site of the cluster is a fully residential area, probably with a greater number of dogs which can explain the concentration of seropositivity.
Recyclable garbage collectors have poor working conditions, and the garbage deposits are often in their own yards, which creates ideal conditions for the reproduction of rodents that serves as a source of transmission for various diseases, including leptospirosis. This explains the greater seroprevalence of Leptospira spp. (p<0.001) in the dogs that accompanies waste picker and share this environment. Of these animals, 100.00% were reactive to the Canicola serovar. Even the sampling being small the main importance is, that these animals accompany the scavengers, who have a greater contact with street dogs and their contaminated environment. Yasuda et al. (1980)Yasuda PH, Santa Rosa CA, Myers DM, Yanaguita RM. The isolation of leptospires from stray dogs in the city of São Paulo, Brazil. Int J Zoonoses 1980; 7(2): 131-134. PMid:7251258. carried out a study in São Paulo city and isolated this serovar from street dogs, showing the important role of these animals as a source of Leptospira spp. infection in urban centers for humans and dogs. Sex was another intrinsic variable associated with seropositivity to lepstospirosis, which may be due to the propensity of male dogs to travel long distances, following females in heat, and lick the genitalia of the females (BENITEZ et al., 2012Benitez NA, Goncalves DD, Freire RL, Rodrigues WB, Souza VRA, Barbara JCA, et al. Seroepidemiology of leptospirosis in pet dogs in the urban area of the municipality of Jataizinho, Paraná. Semina: Ciênc Agrár 2012; 33(Supl 2): 3201-3210. http://dx.doi.org/10.5433/1679-0359.2012v33Supl2p3201.
http://dx.doi.org/10.5433/1679-0359.2012...
). In addition, the territorial behavior of urine marking and/or the urine smell of other dogs are behaviors that favor both direct contact between healthy dogs and asymptomatic carriers and the transmission of leptospires, mainly of the Canicola serovar.
In dogs, leptospirosis is characterized by a variety of clinical signs, ranging from asymptomatic to superacute, acute, subacute or chronic. The presence of lymphoadenomegaly (serovar Bratislava; p = 0.04), alopecia (Leptospira spp.; p = 0.01), ocular secretion (Leptospira spp.; <0.01) and weight loss (Leptospira spp.; p = 0.03) observed in the seropositive dogs in this study have already been described in case reports of canine leptospirosis, which allows us to suggest that dogs with these clinical signs and positivity to microscopic seroagglutination were infected. The intensity of the clinical signs may vary depending on the vaccination status of the dog, age, serovar virulence, degree of exposure to the disease and host immune response (FREIRE et al., 2007Freire IMA, Varges RG, Gomes YNP, Pombo CR, Lilenbaum W. Leptospira serovars in clinically ill dogs from Rio de Janeiro, Brazil. Rev Bras Cienc Vet 2007; 14(2): 83-85. http://dx.doi.org/10.4322/rbcv.2014.238.
http://dx.doi.org/10.4322/rbcv.2014.238...
; MAELE et al., 2008Maele I, Claus A, Haesebrouck F, Daminet S. Leptospirosis in dogs: a review with emphasis on clinical aspects. Vet Rec 2008; 163(14): 409-413. http://dx.doi.org/10.1136/vr.163.14.409. PMid:18836154.
http://dx.doi.org/10.1136/vr.163.14.409...
; TOCHETTO et al., 2012Tochetto C, Flores MM, Kommers GD, Barros CSL, Fighera RA. Pathological aspects of leptospirosis in dogs: 53 cases (1965-2011). Pesq Vet Bras 2012; 32(5): 430-443. http://dx.doi.org/10.1590/S0100-736X2012000500012.
http://dx.doi.org/10.1590/S0100-736X2012...
).
The presence of ectoparasites and nutritional status are variables that may indirectly demonstrate a lack of purchasing power or concern for the dog, important points that reflect on the animal's health and may be associated with a lack of vaccination, inappropriate diet, access to the street, and an inadequate immune response. These factors alone do not lead to Leptospira spp. infection but increase the chance of infections in general. In this study, these variables were associated with seropositivity to Leptospira spp. and serovar Bratislava in particular.
The city of Foz do Iguaçu underwent a major transformation during the 1970s, the construction of a hydroelectric plant in the north of the municipality with a dam between Paraguay and Brazil (ZIOBER & ZANIRATO, 2014Ziober BR, Zanirato SH. Actions to safeguard biodiversity during the building of the Itaipu Binacional Hydroelectric plant. Ambiente Soc 2014; 17(1): 59-78. http://dx.doi.org/10.1590/S1414-753X2014000100005.
http://dx.doi.org/10.1590/S1414-753X2014...
). The migratory process is an important factor related to the construction of a hydroelectric plant, Foz do Iguaçu had a population increase from 28,212 in 1960 to 263,782 to the present day (IBGE, 2016Instituto Brasileiro de Geografia e Estatística – IBGE. Foz do Iguaçu – Infográficos: dados gerais do município [online]. Rio de Janeiro: IBGE; 2016 [cited 2017 jun 20]. Available from: https://cidades.ibge.gov.br/v4/brasil/pr/foz-do iguacu/panorama
https://cidades.ibge.gov.br/v4/brasil/pr...
), which may have favored the marginalization of part of the population. The area D, near Argentina, is the poorest and has a set of substandard housing, many dogs wandering and frequent floods, which favors the increase of the population of rodents and the transmission of leptospirosis (SILVA & MOREIRA, 2013Silva PL, Moreira SM. Leptospirose: fatores ambientais que favorecem a sua ocorrência em humanos. Acervo da Iniciação Científica 2013; 1: 1-14.). The city has a very different composition and customs, either by the construction of the hydroelectric plant or by the proximity between two other countries or by the ecological tourism and intense shopping that collaborates for the installation and spread of diseases.
Rodent activity was found in 70.87% of the evaluated sites. These sites, even without a significant association, had a higher prevalence of antibodies against the three agents studied. The high occurrence of antibodies anti-Leptospira spp. in dogs at these sites is easily explained by the fact that rodents are the main reservoirs of some serovars and disseminators of Leptospira spp. (FAINE, 1999Faine S. Leptospira and leptospirosis. Melbourne: MediSci; 1999.). For T. gondii, rodents are highly susceptible to infection (INNES, 1997Innes EA. Toxoplasmosis: comparative species susceptibility and host immune response. Comp Immunol Microbiol Infect Dis 1997; 20(2): 131-138. http://dx.doi.org/10.1016/S0147-9571(96)00038-0. PMid:9208198.
http://dx.doi.org/10.1016/S0147-9571(96)...
) and are thus considered important prey for felines, the definitive host, and for some intermediate hosts, such as dogs (DUBEY et al., 1970Dubey JP, Miller NL, Frenkel JK. Characterization of the new fecal form of Toxoplasma gondii. J Parasitol 1970; 56(3): 447-456. http://dx.doi.org/10.2307/3277601. PMid:5467864.
http://dx.doi.org/10.2307/3277601...
). Romanelli et al. (2007)Romanelli PR, Freire RL, Vidotto O, Marana ERM, Ogawa L, De Paula VSO, et al. Prevalence of Neospora caninum and Toxoplasma gondii in sheep and dogs from Guarapuava farms, Parana State, Brazil. Res Vet Sci 2007; 82(2): 202-207. http://dx.doi.org/10.1016/j.rvsc.2006.04.001. PMid:17266999.
http://dx.doi.org/10.1016/j.rvsc.2006.04...
, in Guarapuava, PR, observed the presence of rodents as an important risk factor for canine toxoplasmosis. With respect to N. caninum, Dantas et al. (2013)Dantas SBA, Fernandes ARF, Souza OL No, Mota RA, Alves CJ, Azevedo SS. Occurrence and risk factors associated with Toxoplasma gondii and Neospora caninum infections in dogs in the county of Natal, Rio Grande do Norte state, Northeastern Brazil. Cienc Rural 2013; 43(11): 2042-2048. http://dx.doi.org/10.1590/S0103-84782013001100020.
http://dx.doi.org/10.1590/S0103-84782013...
reported that dogs that cohabitated with rodents were 2.34 times more likely to be seropositive to N. caninum, the authors also stated that in the wild, naturally infected rodents can function as reservoirs and play an important role in the maintenance and dissemination of the agent and that infection of the dog can occur through hunting. The lack of statistical association between the diseases studied and the presence of rodents in the present study may have occurred due to the wide and homogeneous distribution of rodents throughout the city.
This is the first study that shows the prevalence of three pathogens that cause infectious-parasitic diseases in dogs at Foz do Iguaçu. We suggest that dogs can be used as sentinels for toxoplasmosis and leptospirosis in Foz do Iguaçu city, preventive measures should be taken by health officials because they are zoonoses and humans are also at risk because high prevalence rates have been observed.
Conclusion
The results indicate that T. gondii and Leptospira spp. are common in the studied canine population. Regarding N. caninum, although the parasite is circulating, a low seroprevalence was observed. This article shows that the use of dogs as sentinels for knowledge of the circulation of pathogens responsible for zoonoses is essential, since health is unique and multidisciplinary factors are responsible for installation and maintenance of the diseases. This research generates information that allows health managers to make decisions based on health rather than disease.
Acknowledgements
This project was funded by the International Development Research Center (IDRC) n° 107577-002 and we would like to acknowledge the IDRC (International Development Research Center) for funding. Thanks to CAPES (Coordination for the Improvement of Higher Education Personnel) for the master’s scholarship and Cristiane da Silva from State University of Londrina by the help with samples processing.
References
- Aguiar DM, Cavalcante GT, Marvulo MFV, Silva JCR, Pinter A, Vasconcellos AS, et al. Risk factors associated with anti-Leptospira spp. antibodies occurence in dogs from Monte Negro County, Rondônia, Brazilian Western Amazon. Arq Bras Med Vet Zootec 2007; 59(1): 70-76. http://dx.doi.org/10.1590/S0102-09352007000100012
» http://dx.doi.org/10.1590/S0102-09352007000100012 - Benitez NA, Goncalves DD, Freire RL, Rodrigues WB, Souza VRA, Barbara JCA, et al. Seroepidemiology of leptospirosis in pet dogs in the urban area of the municipality of Jataizinho, Paraná. Semina: Ciênc Agrár 2012; 33(Supl 2): 3201-3210. http://dx.doi.org/10.5433/1679-0359.2012v33Supl2p3201
» http://dx.doi.org/10.5433/1679-0359.2012v33Supl2p3201 - Caldart ET, Constantino C, Pasquali AKS, Benitez ADN, Hamada FN, Dias RCF, et al. Zoonosis in dogs and cats attended by the Birth Control Project: Toxoplasma gondii, Leishmania spp. and Leptospira spp., serodiagnosis and epidemiology. Semina: Ciênc Agrár 2015; 36(1): 253-265. http://dx.doi.org/10.5433/1679-0359.2015v36n1p253
» http://dx.doi.org/10.5433/1679-0359.2015v36n1p253 - Camargo ME. Improved technique of indirect immunofluorescence for serological diagnosis of toxoplasmosis. Rev Inst Med Trop São Paulo 1964; 6: 117-118. PMid:14177810.
- Cavia R, Cueto GR, Suárez OV. Techniques to estimate abundance and monitoring rodent pests in urban environments. In: Soloneski S, Larramendy M, editors. Integrated pest management and pest control - current and future tactics Rijeka: Intech; 2012. p. 147-172. http://dx.doi.org/10.5772/32541
» http://dx.doi.org/10.5772/32541 - Coiro CJ, Langoni H, Silva RC, Ullmann LS. Risk factors to leptospirosis, leishmaniasis, neosporosis and toxoplasmosis in domiciliated and peridomiciliated dogs in Botucatu - SP. Vet Zootec 2011; 18(3): 386-393.
- Conrad PA, Sverlow K, Anderson M, Rowe J, BonDurant R, Tuter G, et al. Detection of Serum Antibody Responses in Cattle with Natural or Experimental Neospora Infections. J Vet Diagn Invest 1993; 5(4): 572-578. http://dx.doi.org/10.1177/104063879300500412 PMid:8286457.
» http://dx.doi.org/10.1177/104063879300500412 - Cunha NA Fo, Lucas AS, Pappen FG, Ragozo AMA, Gennari SM, Lucia T Jr, et al. Fatores de risco e prevalência de anticorpos anti-Neospora caninum em cães urbanos e rurais do Rio Grande do Sul, Brasil. Rev Bras Parasitol Vet 2008; 17(Supl 1): 301-306. PMid:20059865.
- Dantas SBA, Fernandes ARF, Souza OL No, Mota RA, Alves CJ, Azevedo SS. Occurrence and risk factors associated with Toxoplasma gondii and Neospora caninum infections in dogs in the county of Natal, Rio Grande do Norte state, Northeastern Brazil. Cienc Rural 2013; 43(11): 2042-2048. http://dx.doi.org/10.1590/S0103-84782013001100020
» http://dx.doi.org/10.1590/S0103-84782013001100020 - Dean AG, Dean JA, Coulombier D, Brendel KA, Smith DC, Burton AH, et al. Epi Info: a word processing, database and statistics program for epidemiology on microcomputers Version 6. Atlanta: CDC; 1994. 601 p.
- Dijkstra T, Barkema HW, Eysker M, Wouda W. Evidence of post-natal transmission of Neospora caninum in Dutch dairy herds. Int J Parasitol 2001; 31(2): 209-215. http://dx.doi.org/10.1016/S0020-7519(00)00160-0 PMid:11239942.
» http://dx.doi.org/10.1016/S0020-7519(00)00160-0 - Dreer MKP, Gonçalves DD, Caetano ICS, Gerônimo E, Menegas PH, Bergo D, et al. Toxoplasmosis, leptospirosis and brucellosis in stray dogs housed at the shelter in Umuarama municipality, Paraná, Brazil. J Venom Anim Toxins Incl Trop Dis 2013; 19(1): 23. http://dx.doi.org/10.1186/1678-9199-19-23 PMid:24066949.
» http://dx.doi.org/10.1186/1678-9199-19-23 - Dubey JP, Buxton D, Wouda W. Pathogenesis of Bovine Neosporosis. J Comp Pathol 2006; 134(4): 267-289. http://dx.doi.org/10.1016/j.jcpa.2005.11.004 PMid:16712863.
» http://dx.doi.org/10.1016/j.jcpa.2005.11.004 - Dubey JP, Hattel AL, Lindsay DS, Topper MJ. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J Am Vet Med Assoc 1988; 193(10): 1259-1263. PMid:3144521.
- Dubey JP, Miller NL, Frenkel JK. Characterization of the new fecal form of Toxoplasma gondii. J Parasitol 1970; 56(3): 447-456. http://dx.doi.org/10.2307/3277601 PMid:5467864.
» http://dx.doi.org/10.2307/3277601 - Everitt B. Cluster analysis London: Wiley; 1974.
- Faine S. Guidelines for the control of leptospirosis. WHO Offset Publ 1982;67: 1-171. PMid:7184280.
- Faine S. Leptospira and leptospirosis Melbourne: MediSci; 1999.
- Favero ACM, Pinheiro SR, Vasconcellos AS, Morais ZM, Ferreira F, Ferreira JS No. Sorovares de leptospiras predominates em exames sorológicos de bubalinos, ovinos, caprinos, equinos, suínos e cães de diversos estados brasileiros. Cienc Rural 2002; 32(4): 613-619. http://dx.doi.org/10.1590/S0103-84782002000400011
» http://dx.doi.org/10.1590/S0103-84782002000400011 - Fernandes BCTM, Gennari SM, Souza SLP, Carvalho JM, Oliveira WG, Cury MC. Prevalence of anti-Neospora caninum antibodies in dogs from urban, periurban and rural areas of the city of Uberlândia, Minas Gerais - Brazil. Vet Parasitol 2004; 123(1-2): 33-40. http://dx.doi.org/10.1016/j.vetpar.2004.05.016 PMid:15265569.
» http://dx.doi.org/10.1016/j.vetpar.2004.05.016 - Ferreira FP, Miura AC, Mareze M, Garcia JL, Freire RL, Navarro IT. Frequency of anti-Toxoplasma gondii antibodies in dogs with clinical signs consistent with toxoplasmosis. Cienc Anim Bras 2016; 17(4): 640-646. http://dx.doi.org/10.1590/1089-6891v17i440999
» http://dx.doi.org/10.1590/1089-6891v17i440999 - Freire IMA, Varges RG, Gomes YNP, Pombo CR, Lilenbaum W. Leptospira serovars in clinically ill dogs from Rio de Janeiro, Brazil. Rev Bras Cienc Vet 2007; 14(2): 83-85. http://dx.doi.org/10.4322/rbcv.2014.238
» http://dx.doi.org/10.4322/rbcv.2014.238 - Garcia JL, Navarro IT, Ogawa L, Oliveira RC. Soroepidemiology of toxoplasmosis in cats and dogs from rural properties of Jaguapitã county, Paraná State, Brazil. Cienc Rural 1999; 29(1): 99-104. http://dx.doi.org/10.1590/S0103-84781999000100018
» http://dx.doi.org/10.1590/S0103-84781999000100018 - Garcia JL, Navarro IT, Vidotto O, Gennari SM, Machado RZ, Pereira ABL, et al. Toxoplasma gondii: Comparison of a rhoptry-ELISA with IFAT and MAT for antibody detection in sera of experimentally infected pigs. Exp Parasitol 2006; 113(2): 100-105. http://dx.doi.org/10.1016/j.exppara.2005.12.011 PMid:16458299.
» http://dx.doi.org/10.1016/j.exppara.2005.12.011 - Gatrell AC, Bailey TC, Diggle PJ, Rowlingson BS. Spatial point pattern analysis and its application in geographical epidemiology. Trans Inst Br Geogr 1996; 21(1): 256-274. http://dx.doi.org/10.2307/622936
» http://dx.doi.org/10.2307/622936 - Greene CE, Sykes JE, Brown CA, Hartman K. Infectious diseases of the dog and cat 4th ed. Saint Louis: Elsevier; 2006.
- Heath SE, Johnson R. Leptospirosis. J Am Vet Med Assoc 1994; 205(11): 1518-1523. PMid:7730115.
- Innes EA. Toxoplasmosis: comparative species susceptibility and host immune response. Comp Immunol Microbiol Infect Dis 1997; 20(2): 131-138. http://dx.doi.org/10.1016/S0147-9571(96)00038-0 PMid:9208198.
» http://dx.doi.org/10.1016/S0147-9571(96)00038-0 - Instituto Brasileiro de Geografia e Estatística – IBGE. Foz do Iguaçu – Infográficos: dados gerais do município [online]. Rio de Janeiro: IBGE; 2016 [cited 2017 jun 20]. Available from: https://cidades.ibge.gov.br/v4/brasil/pr/foz-do iguacu/panorama
» https://cidades.ibge.gov.br/v4/brasil/pr/foz-do - Lilenbaum W, Varges R, Moraes IA, Ferreira AMR, Pissinatti A. Leptospiral antibodies in captive lion tamarins (Leontopithecus sp) in Brazil. Vet J 2005; 169(3): 462-464. http://dx.doi.org/10.1016/j.tvjl.2004.03.015 PMid:15848790.
» http://dx.doi.org/10.1016/j.tvjl.2004.03.015 - Lindsay DS, Ritter DM, Brake D. Oocyst excretion in dogs fed mouse brains containing tissue cysts of a cloned line of Neospora caninum. J Parasitol 2001; 87(4): 909-911. http://dx.doi.org/10.1645/0022-3395(2001)087[0909:OEIDFM]2.0.CO;2 PMid:11534658.
» http://dx.doi.org/10.1645/0022-3395(2001)087[0909:OEIDFM]2.0.CO;2 - Lopes AP, Dubey J, Dardé M, Cardoso L. Epidemiological review of Toxoplasma gondii infection in humans and animals in Portugal. Parasitology 2014; 141(13): 1699-1708. http://dx.doi.org/10.1017/S0031182014001413 PMid:25215422.
» http://dx.doi.org/10.1017/S0031182014001413 - Maele I, Claus A, Haesebrouck F, Daminet S. Leptospirosis in dogs: a review with emphasis on clinical aspects. Vet Rec 2008; 163(14): 409-413. http://dx.doi.org/10.1136/vr.163.14.409 PMid:18836154.
» http://dx.doi.org/10.1136/vr.163.14.409 - McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, McGuire AM. Rapid communication: dogs are definitive hosts of Neospora caninum. Int J Parasitol 1998; 28(9): 1473-1478. http://dx.doi.org/10.1016/S0020-7519(98)00138-6 PMid:9770635.
» http://dx.doi.org/10.1016/S0020-7519(98)00138-6 - Moura AB, Souza AP, Sartor AA, Bellato V, Teixeira EB, Pisetta GMP, et al. Occurrence of antibodies and risk factors for infection for Toxoplasma gondii in dogs in the cities of Lages and Balneário Camboriú, Santa Catarina State, Brazil. Rev Bras Parasitol Vet 2009; 18(3): 52-56. http://dx.doi.org/10.4322/rbpv.01803009 PMid:19772776.
» http://dx.doi.org/10.4322/rbpv.01803009 - Romanelli PR, Freire RL, Vidotto O, Marana ERM, Ogawa L, De Paula VSO, et al. Prevalence of Neospora caninum and Toxoplasma gondii in sheep and dogs from Guarapuava farms, Parana State, Brazil. Res Vet Sci 2007; 82(2): 202-207. http://dx.doi.org/10.1016/j.rvsc.2006.04.001 PMid:17266999.
» http://dx.doi.org/10.1016/j.rvsc.2006.04.001 - Schoonjans F, Zalata A, Depuydt CE, Comhaire FH. MedCalc: a new computer program for medical statistics. Comput Methods Programs Biomed 1995; 48(3): 257-262. http://dx.doi.org/10.1016/0169-2607(95)01703-8 PMid:8925653.
» http://dx.doi.org/10.1016/0169-2607(95)01703-8 - Silva PL, Moreira SM. Leptospirose: fatores ambientais que favorecem a sua ocorrência em humanos. Acervo da Iniciação Científica 2013; 1: 1-14.
- Soccol VT, Pasquali AKS, Pozzolo EM, Leandro AS, Chiyo L, Baggio R, et al. More than the eyes can see: the worrying scenario of canine leishmaniasis in the Brazilian side of the triple border. PLoS One 2017; 12(12): e0189182. http://dx.doi.org/10.1371/journal.pone.0189182 PMid:29232388.
» http://dx.doi.org/10.1371/journal.pone.0189182 - Souza MA, Castro JR, Moreira RQ, Bombonato NG, Soares PM, Lima AMC. Anti-Leptospira spp. antibodies in several animal species on the same farm. Biosci J 2016; 32(1): 202-207. http://dx.doi.org/10.14393/BJ-v32n1a2016-26605
» http://dx.doi.org/10.14393/BJ-v32n1a2016-26605 - Team QD. QGIS geographic information system. Open source geospatial foundation project [online] 2016 [cited 2019 May 17]. Available from: https://www.qgis.org
» https://www.qgis.org - Team RDCR. A language and environment for statistical computing [online]. 2011 [cited 2003 Jan 10]. Available from: https://www.r-project.org
» https://www.r-project.org - Teixeira WC, Silva MIS, Pereira JG, Pinheiro AM, Almeida MAO, Gondim LFP. Frequency of Neospora caninum in dogs from São Luís, Maranhão. Arq Bras Med Vet Zootec 2006; 58(4): 685-687. http://dx.doi.org/10.1590/S0102-09352006000400038
» http://dx.doi.org/10.1590/S0102-09352006000400038 - Tochetto C, Flores MM, Kommers GD, Barros CSL, Fighera RA. Pathological aspects of leptospirosis in dogs: 53 cases (1965-2011). Pesq Vet Bras 2012; 32(5): 430-443. http://dx.doi.org/10.1590/S0100-736X2012000500012
» http://dx.doi.org/10.1590/S0100-736X2012000500012 - Wouda W, Dijkstra T, Kramer AMH, Van Maanen C, Brinkhof JMA. Seroepidemiological evidence for a relationship between Neospora caninum infections in dogs and cattle. Int J Parasitol 1999; 29(10): 1677-1682. http://dx.doi.org/10.1016/S0020-7519(99)00105-8 PMid:10608454.
» http://dx.doi.org/10.1016/S0020-7519(99)00105-8 - Yasuda PH, Santa Rosa CA, Myers DM, Yanaguita RM. The isolation of leptospires from stray dogs in the city of São Paulo, Brazil. Int J Zoonoses 1980; 7(2): 131-134. PMid:7251258.
- Ziober BR, Zanirato SH. Actions to safeguard biodiversity during the building of the Itaipu Binacional Hydroelectric plant. Ambiente Soc 2014; 17(1): 59-78. http://dx.doi.org/10.1590/S1414-753X2014000100005
» http://dx.doi.org/10.1590/S1414-753X2014000100005
Publication Dates
-
Publication in this collection
01 Aug 2019 -
Date of issue
Jul-Sep 2019
History
-
Received
14 Feb 2019 -
Accepted
16 May 2019