Abstract
Toxoplasmosis is a parasitic disease caused by the protozoan Toxoplasma gondii. In cetaceans, T. gondii infection is a significant cause of morbidity and mortality. Despite the worldwide range and broad cetacean host record of T. gondii infection, there is limited information on toxoplasmosis in cetaceans from the Southern hemisphere. We investigated the occurrence of T. gondii by histopathology and immunohistochemistry in tissue samples of 185 animals comprising 20 different cetacean species from Brazil. Three out of 185 (1.6%) animals presented T. gondii-associated lesions: a captive killer whale Orcinus orca, a free-ranging common bottlenose dolphin Tursiops truncatus and a free-ranging Guiana dolphin Sotalia guianensis. The main lesions observed in these animals were necrotizing hepatitis, adrenalitis and lymphadenitis associated with protozoal cysts or extracellular tachyzoites presenting immunolabeling with anti-T. gondii antibodies. This study widens the spectrum of species and the geographic range of this agent in Brazil, and provides the first reports of T. gondii infection in a captive killer whale and in a free-ranging common bottlenose dolphin in South America.
Keywords:
Toxoplasmosis; mortality; stranding; South America; protozoan infection; marine mammal
Resumo
Toxoplasmose é uma doença parasitária causada pelo protozoário Toxoplasma gondii. A infecção por T. gondii é uma causa significativa de morbidade e mortalidade, nos cetáceos. Apesar da abrangência mundial e amplo registro de espécies de cetáceos infectadas por T. gondii, informações sobre toxoplasmose em cetáceos do hemisfério sul são limitadas. Neste estudo pesquisou-se por meio de histopatologia e imuno-histoquímica a ocorrência de T. gondii em amostras de tecido de 185 animais, compreendendo 20 diferentes espécies de cetáceos que ocorrem no Brasil. Três dos 185 (1,6%) animais apresentaram lesões associadas a T. gondii: uma orca Orcinus orca mantida em cativeiro, um golfinho-nariz-de-garrafa Tursiops truncatus e um boto-cinza Sotalia guianensis de vida livre. As principais lesões observadas nesses animais foram hepatite, adrenalite e linfadenite necrotizantes associadas a cistos protozoários ou taquizoítos extracelulares, marcados com anticorpos anti-T. gondii. O presente estudo amplia o espectro de espécies susceptíveis a esse agente e o seu alcance geográfico no Brasil, fornecendo o primeiro relato da infecção por T. gondii em uma orca mantida em cativeiro e em um golfinho-nariz-de-garrafa de vida livre na América do Sul.
Palavras-chave:
Toxoplasmose; mortalidade; encalhe; América do Sul; infecção por protozoários; mamífero marinho
Introduction
Toxoplasma gondii is a zoonotic intracellular coccidian protozoan of the phylum Apicomplexa (DUBEY, 2008Dubey JP. The history of Toxoplasma gondii - The first 100 years. J Eukaryot Microbiol 2008; 55(6): 467-475. http://dx.doi.org/10.1111/j.1550-7408.2008.00345.x. PMid:19120791.
http://dx.doi.org/10.1111/j.1550-7408.20...
). This protozoan is one of the most common parasites of warm-blooded animals (DUBEY et al., 2004Dubey JP, Lipscomb TP, Mense M. Toxoplasmosis in an elephant seal (Mirounga angustirostris). J Parasitol 2004; 90(2): 410-411. http://dx.doi.org/10.1645/GE-155R. PMid:15165069.
http://dx.doi.org/10.1645/GE-155R...
), and was described for the first time in Brazil and in Tunisia, in 1908 (NICOLLE & MANCEAUX, 2009Nicolle MC, Manceaux L. On a new protozoan in gundis (Toxoplasma N. Gen). Mem Inst Oswaldo Cruz 2009; 104: 1-3.; SPLENDORE, 2009Splendore A. A new protozoan parasite of rabbit found in histological lesions similar to human Kala-Azar. Mem Inst Oswaldo Cruz 2009; 104: 1-2.). The first record of toxoplasmosis in cetaceans was in a Guiana dolphin Sotalia guianensis from Rio de Janeiro state, Brazil, in the 1970’s (BANDOLI & OLIVEIRA, 1977Bandoli JG, Oliveira CAB. Toxoplasmose em Sotalia guianensis (Van Beneden, 1863, Cetacea-Delphinidae). Folha Med 1977; 75(4): 459-468.). Since then, the exposure and infection by this protozoan has been detected through serological, pathological and molecular techniques in a wide variety of captive and free-ranging cetaceans worldwide (INSKEEP et al., 1990Inskeep W 2nd, Gardiner CH, Harris RK, Dubey JP, Goldston RT. Toxoplasmosis in Atlantic bottle-nosed dolphins (Tursiops truncatus). J Wildl Dis 1990; 26(3): 377-382. http://dx.doi.org/10.7589/0090-3558-26.3.377. PMid:2388360.
http://dx.doi.org/10.7589/0090-3558-26.3...
; OMATA et al., 2006Omata Y, Umeshita Y, Watarai M, Tachibana M, Sasaki M, Murata K, et al. Investigation for presence of Neospora caninum, Toxoplasma gondii and Brucella-species infection in killer whales (Orcinus orca) mass-stranded on the coast of Shiretoko, Hokkaido, Japan. J Vet Med Sci 2006; 68(5): 523-526. http://dx.doi.org/10.1292/jvms.68.523. PMid:16757901.
http://dx.doi.org/10.1292/jvms.68.523...
; MAZZARIOL et al., 2012Mazzariol S, Marcer F, Mignone W, Serracca L, Goria M, Marsili L, et al. Dolphin morbillivirus and Toxoplasma gondii coinfection in a Mediterranean fin whale (Balaenoptera physalus). BMC Vet Res 2012; 8(1): 20. http://dx.doi.org/10.1186/1746-6148-8-20. PMid:22397492.
http://dx.doi.org/10.1186/1746-6148-8-20...
; GONZALES-VIERA et al., 2013Gonzales-Viera O, Marigo J, Ruoppolo V, Rosas FCW, Kanamura CT, Takakura C, et al. Toxoplasmosis in a Guiana dolphin (Sotalia guianensis) from Paraná, Brazil. Vet Parasitol 2013; 191(3-4): 358-362. http://dx.doi.org/10.1016/j.vetpar.2012.09.012. PMid:23063774.
http://dx.doi.org/10.1016/j.vetpar.2012....
; IQBAL et al., 2018Iqbal A, Measures L, Lair S, Dixon B. Toxoplasma gondii in stranded St. Lawrence Estuary beluga Delphinapterus leucas in Quebec, Canada. Dis Aquat Organ 2018; 130(3): 165-175. http://dx.doi.org/10.3354/dao03262. PMid:30259869.
http://dx.doi.org/10.3354/dao03262...
). Most of these studies have covered individual cases or small groups of cetaceans (DI GUARDO et al., 2010Di Guardo G, Proietto U, Di Francesco CE, Marsilio F, Zaccaroni A, Scaravelli D, et al. Cerebral toxoplasmosis in striped dolphins (Stenella coeruleoalba) stranded along the Ligurian Sea coast of Italy. Vet Pathol 2010; 47(2): 245-253. http://dx.doi.org/10.1177/0300985809358036. PMid:20118319.
http://dx.doi.org/10.1177/03009858093580...
; GONZALES-VIERA et al., 2013Gonzales-Viera O, Marigo J, Ruoppolo V, Rosas FCW, Kanamura CT, Takakura C, et al. Toxoplasmosis in a Guiana dolphin (Sotalia guianensis) from Paraná, Brazil. Vet Parasitol 2013; 191(3-4): 358-362. http://dx.doi.org/10.1016/j.vetpar.2012.09.012. PMid:23063774.
http://dx.doi.org/10.1016/j.vetpar.2012....
). Toxoplasmosis is a significant cause of morbidity and may lead to stranding and death, and is considered one of the most important emerging diseases in cetaceans worldwide (VAN BRESSEM et al., 2009Van Bressem MF, Raga JA, Di Guardo G, Jepson PD, Duignan PJ, Siebert U, et al. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis Aquat Organ 2009; 86(2): 143-157. http://dx.doi.org/10.3354/dao02101. PMid:19902843.
http://dx.doi.org/10.3354/dao02101...
; DI GUARDO et al., 2010Di Guardo G, Proietto U, Di Francesco CE, Marsilio F, Zaccaroni A, Scaravelli D, et al. Cerebral toxoplasmosis in striped dolphins (Stenella coeruleoalba) stranded along the Ligurian Sea coast of Italy. Vet Pathol 2010; 47(2): 245-253. http://dx.doi.org/10.1177/0300985809358036. PMid:20118319.
http://dx.doi.org/10.1177/03009858093580...
; GONZALES-VIERA et al., 2013Gonzales-Viera O, Marigo J, Ruoppolo V, Rosas FCW, Kanamura CT, Takakura C, et al. Toxoplasmosis in a Guiana dolphin (Sotalia guianensis) from Paraná, Brazil. Vet Parasitol 2013; 191(3-4): 358-362. http://dx.doi.org/10.1016/j.vetpar.2012.09.012. PMid:23063774.
http://dx.doi.org/10.1016/j.vetpar.2012....
; BIGAL et al., 2018Bigal E, Morick D, Scheinin AP, Salant H, Berkowitz A, King R, et al. Detection of Toxoplasma gondii in three common bottlenose dolphins (Tursiops truncatus): a first description from the Eastern Mediterranean Sea. Vet Parasitol 2018; 258: 74-78. http://dx.doi.org/10.1016/j.vetpar.2018.06.009. PMid:30105982.
http://dx.doi.org/10.1016/j.vetpar.2018....
).
The epidemiology of T. gondii transmission has been established in several terrestrial animals (e.g., cats, livestock), and to a lesser extent, aquatic species (e.g., shellfish), including transplacental transmission in susceptible pregnant animals (TENTER et al., 2000Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol 2000; 30(12-13): 1217-1258. http://dx.doi.org/10.1016/S0020-7519(00)00124-7. PMid:11113252.
http://dx.doi.org/10.1016/S0020-7519(00)...
; CATÃO-DIAS et al., 2013Catão-Dias ZL, Epiphanio S, Kierulff MCM. Neotropical primates and their susceptibility to Toxoplasma gondii: new insights of an old problem. In: Brinkworth JF, Pechenkina K, editors. Primates, pathogens, and evolution. New York: Springer Press; 2013. p. 253-289. http://dx.doi.org/10.1007/978-1-4614-7181-3_9.
http://dx.doi.org/10.1007/978-1-4614-718...
; JARDINE & DUBEY, 2002Jardine JE, Dubey JP. Congenital toxoplasmosis in a Indo-Pacific bottlenose dolphin (Tursiops aduncus). J Parasitol 2002; 88(1): 197-199. http://dx.doi.org/10.1645/0022-3395(2002)088[0197:CTIAIP]2.0.CO;2. PMid:12053968.
http://dx.doi.org/10.1645/0022-3395(2002...
; MILLER et al., 2008Miller M, Conrad P, James ER, Packham A, Toy-Choutka S, Murray MJ, et al. Transplacental toxoplasmosis in a wild southern sea otter (Enhydra lutris nereis). Vet Parasitol 2008; 153(1-2): 12-18. http://dx.doi.org/10.1016/j.vetpar.2008.01.015. PMid:18304737.
http://dx.doi.org/10.1016/j.vetpar.2008....
). In terrestrial animals, infection also occurs through ingestion of tissue cysts or contaminated food and/or water containing infecting sporulated oocysts released in feline feces. However, the transmission of T. gondii in cetaceans has not been fully elucidated. One major source of transmission in cetaceans could be the polluted marine environments in proximity with the coast, e.g., run-off of cat feces or soil contaminated with T. gondii oocysts from rivers, polluted effluents and ballast water from ships (VAN BRESSEM et al., 2009Van Bressem MF, Raga JA, Di Guardo G, Jepson PD, Duignan PJ, Siebert U, et al. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis Aquat Organ 2009; 86(2): 143-157. http://dx.doi.org/10.3354/dao02101. PMid:19902843.
http://dx.doi.org/10.3354/dao02101...
). In utero transmission of T. gondii has been described in Risso's dolphin Grampus griseus and Indo-Pacific bottlenose dolphin Tursiops aduncus (JARDINE & DUBEY, 2002Jardine JE, Dubey JP. Congenital toxoplasmosis in a Indo-Pacific bottlenose dolphin (Tursiops aduncus). J Parasitol 2002; 88(1): 197-199. http://dx.doi.org/10.1645/0022-3395(2002)088[0197:CTIAIP]2.0.CO;2. PMid:12053968.
http://dx.doi.org/10.1645/0022-3395(2002...
; RESENDES et al., 2002Resendes AR, Almería S, Dubey JP, Obón E, Juan-Sallés C, Degollada E, et al. Disseminated toxoplasmosis in a Mediterranean pregnant Risso’s dolphin (Grampus griseus) with transplacental fetal infection. J Parasitol 2002; 88(5): 1029-1032. http://dx.doi.org/10.1645/0022-3395(2002)088[1029:DTIAMP]2.0.CO;2. PMid:12435153.
http://dx.doi.org/10.1645/0022-3395(2002...
). For offshore marine ecosystems, T. gondii transmission patterns are still unclear.
The occurrence of T. gondii in marine mammals remains a poorly understood phenomenon, particularly in Brazil, where very limited information exists. Exposure to T. gondii was reported in Amazon river dolphins Inia geoffrensis (SANTOS et al., 2011Santos PS, Albuquerque GR, Da Silva VMF, Martin AR, Marvullo MFV, Souza SLP, et al. Seroprevalence of Toxoplasma gondii in free-living Amazon River dolphins (Inia geoffrensis) from central Amazon, Brazil. Vet Parasitol 2011; 183(1-2): 171-173. http://dx.doi.org/10.1016/j.vetpar.2011.06.007. PMid:21764516.
http://dx.doi.org/10.1016/j.vetpar.2011....
), but to this date, only one cetacean species is known to be infected by T. gondii in Brazilian waters: the Guiana dolphin (BANDOLI & OLIVEIRA, 1977Bandoli JG, Oliveira CAB. Toxoplasmose em Sotalia guianensis (Van Beneden, 1863, Cetacea-Delphinidae). Folha Med 1977; 75(4): 459-468.; GONZALES-VIERA et al., 2013Gonzales-Viera O, Marigo J, Ruoppolo V, Rosas FCW, Kanamura CT, Takakura C, et al. Toxoplasmosis in a Guiana dolphin (Sotalia guianensis) from Paraná, Brazil. Vet Parasitol 2013; 191(3-4): 358-362. http://dx.doi.org/10.1016/j.vetpar.2012.09.012. PMid:23063774.
http://dx.doi.org/10.1016/j.vetpar.2012....
). We hypothesized that T. gondii infection could be present in a wider number of cetacean species in Brazil than previous studies have suggested. Thus, the aim of this study was to evaluate retrospectively the occurrence of T. gondii infection in captive and free-ranging cetaceans employing histopathological and immunohistochemical (IHC) analyses on tissue samples obtained from a large marine mammal tissue bank in Brazil.
Materials and Methods
We evaluated formalin-fixed paraffin-embedded tissue samples stored at the Marine Mammal Tissue Bank of the Laboratory of Wildlife Comparative Pathology, School of Veterinary Medicine and Animal Science, University of São Paulo, Brazil. The samples included in the study came from partial or complete standard necropsies conducted on odontocetes and mysticetes between 1988 and 2014, provided by partner institutions attending marine mammal strandings, rehabilitation and incidental captures along the Brazilian coast. Tissues from 185 individuals of 20 different cetacean species (Table 1) were evaluated. Among the evaluated samples, three were from originally wild animals kept in captivity in two different amusement parks of the São Paulo state (Brazil): a killer whale Orcinus orca from Iceland kept in captivity in the 1980’s, an Amazon river dolphin from the Amazon Basin, and a common bottlenose dolphin Tursiops truncatus of unknown origin. The remaining animals were all free-ranging species that stranded along the Brazilian coast, mostly from the southern region (43.9%; 80/182), followed by the southeastern (30.2%; 55/182), and northeastern (25.8%; 47/182) regions (Figure 1). The examined tissues were: liver, kidney, lung, spleen, skeletal muscle, lymph node, adrenal gland, brain, uterus, intestines, heart and eye.
Geographic distribution along the coastline of Brazil of Toxoplasma gondii- positive (green triangle) and -negative (red circle) animals, according to immunohistochemistry.
Formalin-fixed paraffin-embedded tissue sections from the aforementioned organs were sectioned at 5 µm thickness and stained with H&E. Additionally, special histochemical techniques - periodic acid-Schiff (PAS), Giemsa and Grocott’s methenamine silver stains - were used in selected tissue sections to improve visualization of protozoan structures compatible with T. gondii (LUNA, 1992Luna LG. Histopathologic methods and color atlas of special stains and tissue artifacts. Gaithersburg: America Histolabs; 1992.; POPPER et al., 1960Popper H, Paronetto F, Barka T. PAS-positive structures of nonglycogenic character in normal and abnormal liver. Arch Pathol 1960; 70: 300-313. PMid:14434186.).
Immunohistochemical analysis for T. gondii was performed on selected tissues for which T. gondii has demonstrated tropism in cetaceans (i.e., liver, kidney, lung, brain and lymph nodes) (INSKEEP et al., 1990Inskeep W 2nd, Gardiner CH, Harris RK, Dubey JP, Goldston RT. Toxoplasmosis in Atlantic bottle-nosed dolphins (Tursiops truncatus). J Wildl Dis 1990; 26(3): 377-382. http://dx.doi.org/10.7589/0090-3558-26.3.377. PMid:2388360.
http://dx.doi.org/10.7589/0090-3558-26.3...
; MIKAELIAN et al., 2000Mikaelian I, Boisclair J, Dubey JP, Kennedy S, Martineau D. Toxoplasmosis in beluga whales (Delphinapterus leucas) from St Lawrence Estuary: two case reports and a serological survey. J Comp Pathol 2000; 122(1): 73-76. http://dx.doi.org/10.1053/jcpa.1999.0341. PMid:10627393.
http://dx.doi.org/10.1053/jcpa.1999.0341...
; RESENDES et al., 2002Resendes AR, Almería S, Dubey JP, Obón E, Juan-Sallés C, Degollada E, et al. Disseminated toxoplasmosis in a Mediterranean pregnant Risso’s dolphin (Grampus griseus) with transplacental fetal infection. J Parasitol 2002; 88(5): 1029-1032. http://dx.doi.org/10.1645/0022-3395(2002)088[1029:DTIAMP]2.0.CO;2. PMid:12435153.
http://dx.doi.org/10.1645/0022-3395(2002...
; VAN BRESSEM et al., 2009Van Bressem MF, Raga JA, Di Guardo G, Jepson PD, Duignan PJ, Siebert U, et al. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis Aquat Organ 2009; 86(2): 143-157. http://dx.doi.org/10.3354/dao02101. PMid:19902843.
http://dx.doi.org/10.3354/dao02101...
; GONZALES-VIERA et al., 2013Gonzales-Viera O, Marigo J, Ruoppolo V, Rosas FCW, Kanamura CT, Takakura C, et al. Toxoplasmosis in a Guiana dolphin (Sotalia guianensis) from Paraná, Brazil. Vet Parasitol 2013; 191(3-4): 358-362. http://dx.doi.org/10.1016/j.vetpar.2012.09.012. PMid:23063774.
http://dx.doi.org/10.1016/j.vetpar.2012....
). Briefly, 3 μm -thick sections were deparaffinized and rehydrated through a series of graded alcohols. Toxoplasma gondii antigen was retrieved by heating tissue sections in citrate buffer (pH=7.0) solution for seven minutes at 90 °C. The sections were blocked with 1% normal rabbit serum in PBS for 30 minutes followed by overnight incubation with a polyclonal goat anti-T. gondii primary antibody (1 in 400 dilution; VMRD Inc; Pullman). This antibody does not cross-react with Neospora caninum (CORBELLINI et al., 2002Corbellini LG, Driemeier D, Cruz CFE, Gondim LFP, Wald V. Neosporosis as a cause of abortion in dairy cattle in Rio Grande do Sul, southern Brazil. Vet Parasitol 2002; 103(3): 195-202. http://dx.doi.org/10.1016/S0304-4017(01)00600-8. PMid:11750112.
http://dx.doi.org/10.1016/S0304-4017(01)...
). The sections were washed in PBS and incubated for 30 minutes with biotinylated polyclonal anti-goat secondary antibody (1 in 600 dilution; Dako) as previously described (DI GUARDO et al., 2010Di Guardo G, Proietto U, Di Francesco CE, Marsilio F, Zaccaroni A, Scaravelli D, et al. Cerebral toxoplasmosis in striped dolphins (Stenella coeruleoalba) stranded along the Ligurian Sea coast of Italy. Vet Pathol 2010; 47(2): 245-253. http://dx.doi.org/10.1177/0300985809358036. PMid:20118319.
http://dx.doi.org/10.1177/03009858093580...
). Amplification of the immunologic reaction was based on an avidin-biotin-peroxidase complex method (Elite ABC kit, Vector laboratories), following manufacturer’s instructions. Labeling was ‘visualized’ with 3-amino-9-ethyl-carbazole (Sigma) and/or diaminobenzidine (DAB D-5637; Sigma), and sections were counterstained with Mayer’s haematoxylin. Tissue sections in which the primary antibodies were replaced by phosphate buffered saline or nonimmune homologous serum served as negative controls. Sections of the adrenal gland of a Guiana dolphin infected by T. gondii (GONZALES-VIERA et al., 2013Gonzales-Viera O, Marigo J, Ruoppolo V, Rosas FCW, Kanamura CT, Takakura C, et al. Toxoplasmosis in a Guiana dolphin (Sotalia guianensis) from Paraná, Brazil. Vet Parasitol 2013; 191(3-4): 358-362. http://dx.doi.org/10.1016/j.vetpar.2012.09.012. PMid:23063774.
http://dx.doi.org/10.1016/j.vetpar.2012....
) were used as positive control. Morbillivirus immunohistochemistry was also performed on selected tissue sections of T. gondii positive cases to rule out potential coinfection, following a previously published protocol (SALIKI et al., 2002Saliki JT, Cooper EJ, Gustavson JP. Emerging morbillivirus infections of marine mammals: development of two diagnostic approaches. Ann N Y Acad Sci 2002; 969(1): 51-59. http://dx.doi.org/10.1111/j.1749-6632.2002.tb04350.x. PMid:12381563.
http://dx.doi.org/10.1111/j.1749-6632.20...
; GROCH et al., 2014Groch KR, Colosio AC, Marcondes MCC, Zucca D, Díaz-Delgado J, Niemeyer C, et al. Novel cetacean morbillivirus in Guiana dolphin, Brazil. Emerg Infect Dis 2014; 20(3): 511-513. http://dx.doi.org/10.3201/eid2003.131557. PMid:24565559.
http://dx.doi.org/10.3201/eid2003.131557...
), using a commercial monoclonal antibody against the nucleoprotein of Canine distemper virus (VMRD Inc.), known to cross-react with Cetacean morbillivirus. Positive tissue samples of an infected Guiana dolphin were selected as positive control (GROCH et al., 2014Groch KR, Colosio AC, Marcondes MCC, Zucca D, Díaz-Delgado J, Niemeyer C, et al. Novel cetacean morbillivirus in Guiana dolphin, Brazil. Emerg Infect Dis 2014; 20(3): 511-513. http://dx.doi.org/10.3201/eid2003.131557. PMid:24565559.
http://dx.doi.org/10.3201/eid2003.131557...
).
Results
Three of 185 (1.6%) animals were IHC-positive for T. gondii-: a captive killer whale, a free-ranging common bottlenose dolphin, and a free-ranging Guiana dolphin. These animals presented a variety of toxoplasmosis-compatible lesions, with positively labeled protozoal cysts and free tachyzoites. The occurrence of T. gondii in free-ranging species was 1.1% (2/182), whereas the occurrence in captive animals was 33.3% (1/3). A summary of T. gondii-associated lesions in these animals follows. Additional information regarding the microscopic findings of positive animals is available in Table 2. All three T. gondii positive animals were IHC-negative for morbillivirus. No PCR techniques were employed in these cases because frozen tissue samples were not available.
Case No 1 (MM#452) was a 5-6 years-old juvenile (2,500 kg) male killer whale captured in Iceland in 1983, and one year later brought into an oceanarium located in the city of São Paulo, São Paulo state, Brazil. The animal remained in this facility until its death, on March 7th, 1988. No clinical data were available. Samples of lungs, liver, spleen, kidneys, skeletal muscle, glandular stomach, large intestine, thyroid glands, and lymph nodes were collected upon necropsy. The only reported macroscopic finding was a renal cyst. Microscopically, the main lesions included moderate to marked, multifocal, acute fibrinosuppurative and hemorrhagic bronchopneumonia with protozoan cysts, moderate randomly multifocal to coalescent necrotizing hepatitis with protozoan cysts (compatible with T. gondii), marked necrosuppurative lymphadenitis, acute multifocal interstitial nephritis with mild-moderate mixed infiltrate, mild to moderate, multifocal, chronic membranous glomerulonephritis, and acute fibrinoid vasculitis.
Case No 2 (MM#178) was a juvenile male common bottlenose dolphin of 2.43 m total body length, that stranded alive and died shortly after stranding, on October 29th, 2001, in Lagos Region, Rio de Janeiro state, Brazil (22°56’S, 42°19’W). Samples of lungs, liver, kidneys, and large intestine were collected upon necropsy. No gross findings were reported. The main microscopic findings observed were moderate to severe, multifocal random to coalescing necrotizing hepatitis with protozoan cysts compatible with T. gondii and moderate to marked, multifocal, acute fibrinosuppurative bronchopneumonia.
Case No 3 (MM#67) was a 1.86 m-long adult, female Guiana dolphin that stranded dead on March 3th, 1998, in Paranaguá Bay, Paraná state, Brazil (25°31’S, 48°30’W). No necropsy records were available. Samples of eye and adrenal glands were evaluated. Microscopically, the main finding was marked, multifocal, acute necrotizing adrenalitis with numerous protozoal cysts and extracellular tachyzoites compatible with T. gondii, and further highlighted with PAS and Giemsa stains.
Discussion
Despite the widespread geographical range of T. gondii infection, in Brazil, the current knowledge regarding cetacean toxoplasmosis is limited to few species and studies (BANDOLI & OLIVEIRA, 1977Bandoli JG, Oliveira CAB. Toxoplasmose em Sotalia guianensis (Van Beneden, 1863, Cetacea-Delphinidae). Folha Med 1977; 75(4): 459-468.; SANTOS et al., 2011Santos PS, Albuquerque GR, Da Silva VMF, Martin AR, Marvullo MFV, Souza SLP, et al. Seroprevalence of Toxoplasma gondii in free-living Amazon River dolphins (Inia geoffrensis) from central Amazon, Brazil. Vet Parasitol 2011; 183(1-2): 171-173. http://dx.doi.org/10.1016/j.vetpar.2011.06.007. PMid:21764516.
http://dx.doi.org/10.1016/j.vetpar.2011....
; GONZALES-VIERA et al., 2013Gonzales-Viera O, Marigo J, Ruoppolo V, Rosas FCW, Kanamura CT, Takakura C, et al. Toxoplasmosis in a Guiana dolphin (Sotalia guianensis) from Paraná, Brazil. Vet Parasitol 2013; 191(3-4): 358-362. http://dx.doi.org/10.1016/j.vetpar.2012.09.012. PMid:23063774.
http://dx.doi.org/10.1016/j.vetpar.2012....
). Based on microscopical and immunohistochemical examinations, we provide the first description of T. gondii in a common bottlenose dolphin of South America, and in a captive killer whale. Furthermore, the identification of toxoplasmosis in a Guiana dolphin corroborates with previous observations regarding this species and geographic area, the Paranaguá Bay. On the other hand, in spite of the high prevalence of T. gondii antibodies reported in free-ranging Amazon river dolphins (SANTOS et al., 2011Santos PS, Albuquerque GR, Da Silva VMF, Martin AR, Marvullo MFV, Souza SLP, et al. Seroprevalence of Toxoplasma gondii in free-living Amazon River dolphins (Inia geoffrensis) from central Amazon, Brazil. Vet Parasitol 2011; 183(1-2): 171-173. http://dx.doi.org/10.1016/j.vetpar.2011.06.007. PMid:21764516.
http://dx.doi.org/10.1016/j.vetpar.2011....
), our sampled captive Amazon river dolphin was T. gondii- negative.
We detected protozoal cysts and free tachyzoites of T. gondii by IHC and observed histological changes within multiorgan necroinflammatory foci, e.g., necrotizing hepatitis, necrosuppurative lymphadenitis (killer whale), necrotizing hepatitis (common bottlenose dolphin), and necrotizing adrenalitis (Guiana dolphin). These lesions are in agreement with previous reports of T. gondii infection in odontocetes (CRUICKSHANK et al., 1990Cruickshank JJ, Haines DM, Palmer NC, St Aubin DJ. Cysts of a Toxoplasma-like organism in an Atlantic bottlenose dolphin. Can Vet J 1990; 31(3): 213-215. PMid:17423539.; RESENDES et al., 2002Resendes AR, Almería S, Dubey JP, Obón E, Juan-Sallés C, Degollada E, et al. Disseminated toxoplasmosis in a Mediterranean pregnant Risso’s dolphin (Grampus griseus) with transplacental fetal infection. J Parasitol 2002; 88(5): 1029-1032. http://dx.doi.org/10.1645/0022-3395(2002)088[1029:DTIAMP]2.0.CO;2. PMid:12435153.
http://dx.doi.org/10.1645/0022-3395(2002...
; DI GUARDO & MAZZARIOL, 2013Di Guardo G, Mazzariol S. Toxoplasma gondii: clues from stranded dolphins. Vet Pathol 2013; 50(5): 737. http://dx.doi.org/10.1177/0300985813486816. PMid:24014612.
http://dx.doi.org/10.1177/03009858134868...
) and mysticetes (MAZZARIOL et al., 2012Mazzariol S, Marcer F, Mignone W, Serracca L, Goria M, Marsili L, et al. Dolphin morbillivirus and Toxoplasma gondii coinfection in a Mediterranean fin whale (Balaenoptera physalus). BMC Vet Res 2012; 8(1): 20. http://dx.doi.org/10.1186/1746-6148-8-20. PMid:22397492.
http://dx.doi.org/10.1186/1746-6148-8-20...
). The main lesions observed in cetacean cases of toxoplasmosis are necrotizing hepatitis, lymphadenitis and lymphoid necrosis, interstitial pneumonia, adrenal necrosis, and non-suppurative encephalitis and meningoencephalitis with bradyzoites and free tachyzoites (INSKEEP et al., 1990Inskeep W 2nd, Gardiner CH, Harris RK, Dubey JP, Goldston RT. Toxoplasmosis in Atlantic bottle-nosed dolphins (Tursiops truncatus). J Wildl Dis 1990; 26(3): 377-382. http://dx.doi.org/10.7589/0090-3558-26.3.377. PMid:2388360.
http://dx.doi.org/10.7589/0090-3558-26.3...
; MIGAKI et al., 1990Migaki G, Sawa TR, Dubey JP. Fatal disseminated toxoplasmosis in a Spinner dolphin (Stenella longirostris). Vet Pathol 1990; 27(6): 463-464. http://dx.doi.org/10.1177/030098589902700615. PMid:2278137.
http://dx.doi.org/10.1177/03009858990270...
; MIKAELIAN et al., 2000Mikaelian I, Boisclair J, Dubey JP, Kennedy S, Martineau D. Toxoplasmosis in beluga whales (Delphinapterus leucas) from St Lawrence Estuary: two case reports and a serological survey. J Comp Pathol 2000; 122(1): 73-76. http://dx.doi.org/10.1053/jcpa.1999.0341. PMid:10627393.
http://dx.doi.org/10.1053/jcpa.1999.0341...
; RESENDES et al., 2002Resendes AR, Almería S, Dubey JP, Obón E, Juan-Sallés C, Degollada E, et al. Disseminated toxoplasmosis in a Mediterranean pregnant Risso’s dolphin (Grampus griseus) with transplacental fetal infection. J Parasitol 2002; 88(5): 1029-1032. http://dx.doi.org/10.1645/0022-3395(2002)088[1029:DTIAMP]2.0.CO;2. PMid:12435153.
http://dx.doi.org/10.1645/0022-3395(2002...
; VAN BRESSEM et al., 2009Van Bressem MF, Raga JA, Di Guardo G, Jepson PD, Duignan PJ, Siebert U, et al. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis Aquat Organ 2009; 86(2): 143-157. http://dx.doi.org/10.3354/dao02101. PMid:19902843.
http://dx.doi.org/10.3354/dao02101...
; GONZALES-VIERA et al., 2013Gonzales-Viera O, Marigo J, Ruoppolo V, Rosas FCW, Kanamura CT, Takakura C, et al. Toxoplasmosis in a Guiana dolphin (Sotalia guianensis) from Paraná, Brazil. Vet Parasitol 2013; 191(3-4): 358-362. http://dx.doi.org/10.1016/j.vetpar.2012.09.012. PMid:23063774.
http://dx.doi.org/10.1016/j.vetpar.2012....
; ROE et al., 2013Roe WD, Howe L, Baker EJ, Burrows L, Hunter SA. An atypical genotype of Toxoplasma gondii as a cause of mortality in Hector’s dolphins (Cephalorhynchus hectori). Vet Parasitol 2013; 192(1-3): 67-74. http://dx.doi.org/10.1016/j.vetpar.2012.11.001. PMid:23207018.
http://dx.doi.org/10.1016/j.vetpar.2012....
). In T. gondii-positive cases, all tested tissues were negative for morbillivirus, suggesting that T. gondii possibly acted as a primary agent, as previously observed (DI GUARDO et al., 2010Di Guardo G, Proietto U, Di Francesco CE, Marsilio F, Zaccaroni A, Scaravelli D, et al. Cerebral toxoplasmosis in striped dolphins (Stenella coeruleoalba) stranded along the Ligurian Sea coast of Italy. Vet Pathol 2010; 47(2): 245-253. http://dx.doi.org/10.1177/0300985809358036. PMid:20118319.
http://dx.doi.org/10.1177/03009858093580...
; GONZALES-VIERA et al., 2013Gonzales-Viera O, Marigo J, Ruoppolo V, Rosas FCW, Kanamura CT, Takakura C, et al. Toxoplasmosis in a Guiana dolphin (Sotalia guianensis) from Paraná, Brazil. Vet Parasitol 2013; 191(3-4): 358-362. http://dx.doi.org/10.1016/j.vetpar.2012.09.012. PMid:23063774.
http://dx.doi.org/10.1016/j.vetpar.2012....
; ROE et al., 2013Roe WD, Howe L, Baker EJ, Burrows L, Hunter SA. An atypical genotype of Toxoplasma gondii as a cause of mortality in Hector’s dolphins (Cephalorhynchus hectori). Vet Parasitol 2013; 192(1-3): 67-74. http://dx.doi.org/10.1016/j.vetpar.2012.11.001. PMid:23207018.
http://dx.doi.org/10.1016/j.vetpar.2012....
). The scarce number of brain samples available for this study (n = 4) probably prevented the identification of T. gondii-associated lesions in nervous tissue, which is commonly involved in cetacean toxoplasmosis cases (DI GUARDO et al., 2010Di Guardo G, Proietto U, Di Francesco CE, Marsilio F, Zaccaroni A, Scaravelli D, et al. Cerebral toxoplasmosis in striped dolphins (Stenella coeruleoalba) stranded along the Ligurian Sea coast of Italy. Vet Pathol 2010; 47(2): 245-253. http://dx.doi.org/10.1177/0300985809358036. PMid:20118319.
http://dx.doi.org/10.1177/03009858093580...
; SIERRA et al., 2014Sierra E, Sánchez S, Saliki JT, Blas-Machado U, Arbelo M, Zucca D, et al. Retrospective study of etiologic agents associated with nonsuppurative meningoencephalitis in stranded cetaceans in the Canary Islands. J Clin Microbiol 2014; 52(7): 2390-2397. http://dx.doi.org/10.1128/JCM.02906-13. PMid:24759718.
http://dx.doi.org/10.1128/JCM.02906-13...
).
In this study, we evaluated cetacean samples from geographic areas that correspond to a large portion of the Brazilian coastline, most of them coastal species, mainly franciscanas (Pontoporia blainvillei) (55.0%) and Guiana dolphins (14.5%). Coastal cetaceans, considered suitable sentinels of the marine environmental ecosystem and important indicators of marine pollution, are usually more exposed to anthropogenic activities (WELLS et al., 2004Wells RS, Rhinehart HL, Hansen LJ, Sweeney JC, Townsend FI, Stone R, et al. Bottlenose dolphins as marine ecosystem sentinels: developing health monitoring system. EcoHealth 2004; 1(3): 246-254. http://dx.doi.org/10.1007/s10393-004-0094-6.
http://dx.doi.org/10.1007/s10393-004-009...
; BOSSART, 2011Bossart GD. Marine mammals as sentinel species for oceans and human health. Vet Pathol 2011; 48(3): 676-690. http://dx.doi.org/10.1177/0300985810388525. PMid:21160025.
http://dx.doi.org/10.1177/03009858103885...
; MOURA et al., 2014Moura JF, Hauser-Davis RA, Lemos L, Emin-Lima R, Siciliano S. Guiana dolphins (Sotalia guianensis) as marine ecosystem sentinels: ecotoxicology and emerging diseases. Rev Environ Contam Toxicol 2014; 228: 1-29. http://dx.doi.org/10.1007/978-3-319-01619-1_1. PMid:24162090.
http://dx.doi.org/10.1007/978-3-319-0161...
). From these, we found histological and immunohistochemical evidences of toxoplasmosis in one Guiana dolphin. This is the third report of toxoplasmosis in this species (BANDOLI & OLIVEIRA, 1977Bandoli JG, Oliveira CAB. Toxoplasmose em Sotalia guianensis (Van Beneden, 1863, Cetacea-Delphinidae). Folha Med 1977; 75(4): 459-468.; GONZALES-VIERA et al., 2013Gonzales-Viera O, Marigo J, Ruoppolo V, Rosas FCW, Kanamura CT, Takakura C, et al. Toxoplasmosis in a Guiana dolphin (Sotalia guianensis) from Paraná, Brazil. Vet Parasitol 2013; 191(3-4): 358-362. http://dx.doi.org/10.1016/j.vetpar.2012.09.012. PMid:23063774.
http://dx.doi.org/10.1016/j.vetpar.2012....
). This animal presented severe adrenalitis, similar to what was reported by Gonzales-Viera et al. (2013)Gonzales-Viera O, Marigo J, Ruoppolo V, Rosas FCW, Kanamura CT, Takakura C, et al. Toxoplasmosis in a Guiana dolphin (Sotalia guianensis) from Paraná, Brazil. Vet Parasitol 2013; 191(3-4): 358-362. http://dx.doi.org/10.1016/j.vetpar.2012.09.012. PMid:23063774.
http://dx.doi.org/10.1016/j.vetpar.2012....
in another Guiana dolphin from the same area (Paranaguá Bay) that died in the same year (1998). Given the marked pattern of site fidelity presented by Guiana dolphins (MOURA et al., 2014Moura JF, Hauser-Davis RA, Lemos L, Emin-Lima R, Siciliano S. Guiana dolphins (Sotalia guianensis) as marine ecosystem sentinels: ecotoxicology and emerging diseases. Rev Environ Contam Toxicol 2014; 228: 1-29. http://dx.doi.org/10.1007/978-3-319-01619-1_1. PMid:24162090.
http://dx.doi.org/10.1007/978-3-319-0161...
), this result suggests a common source of exposure to T. gondii and a possible negative impact of toxoplasmosis on this population. Although the low occurrence of T. gondii infection found in the present study does not allow us to infer further on this protozoan’s geographic distribution, the location of the Guiana dolphin case and previous reports suggest that infection may occur at higher rates in coastal areas of Brazil. Coastal areas usually receive freshwater run-offs and terrestrial biologic pollutants, contributing to the presence of T. gondii (SHAPIRO et al., 2015Shapiro K, VanWormer E, Aguilar B, Conrad PA. Surveillance for Toxoplasma gondii in California mussels (Mytilus californianus) reveals transmission of atypical genotypes from land to sea. Environ Microbiol 2015; 17(11): 4177-4188. http://dx.doi.org/10.1111/1462-2920.12685. PMid:25367256.
http://dx.doi.org/10.1111/1462-2920.1268...
). These results also suggest areas in which future research efforts should be concentrated.
Concerning killer whales, positive serology for T. gondii was reported in a killer whale calf that stranded alive in Japan, in 1988 (MURATA et al., 2004Murata K, Mizuta K, Imazu K, Terasawa F, Taki M, Endoh T. The prevalence of Toxoplasma gondii antibodies in wild and captive cetaceans from Japan. J Parasitol 2004; 90(4): 896-898. http://dx.doi.org/10.1645/GE-197R. PMid:15357097.
http://dx.doi.org/10.1645/GE-197R...
), and T. gondii infection was identified by molecular techniques in a stranded killer whale from the Northeastern Pacific, in California (GIBSON et al., 2011Gibson AK, Raverty S, Lambourn DM, Huggins J, Magargal SL, Grigg ME. Polyparasitism is associated with increased disease severity in Toxoplasma gondii-infected marine sentinel species. PLoS Negl Trop Dis 2011; 5(5): e1142. http://dx.doi.org/10.1371/journal.pntd.0001142. PMid:21629726.
http://dx.doi.org/10.1371/journal.pntd.0...
). However, these reports lacked detailed pathological examinations. In the present study, we provide the first histopathological and immunohistochemical evidences of toxoplasmosis in this species. Based on the severity and extent of characteristic multiorganic necroinflammatory foci, we believe that T. gondii played a major role in the death of the evaluated killer whale. In this case, potential environmental sources of T. gondii infectious forms may include water and/or food contamination in one or more of the following scenarios: (1) the facility’s water system (e.g., used to clean fish and the enclosure, and fill the tank), especially considering that sporulated oocysts of T. gondii are viable in saltwater for at least two years (AUBERT & VILLENA, 2009Aubert D, Villena I. Detection of Toxoplasma gondii oocysts in water: proposition of a strategy and evaluation in Champagne-Ardenne region, France. Mem Inst Oswaldo Cruz 2009; 104(2): 290-295. http://dx.doi.org/10.1590/S0074-02762009000200023. PMid:19430655.
http://dx.doi.org/10.1590/S0074-02762009...
); (2) contaminated food (e.g., fish); and (3) contaminated enclosure (e.g., contaminated fomites, personnel and/or presence of roaming cats). These findings highlight the importance of proper hygiene and husbandry in captive cetaceans, including properly treated water and reliable food sources free of T. gondii sporulated oocysts. Although unlikely, considering the physiopathology of toxoplasmosis in highly susceptible or ‘less adapted’ hosts (CATÃO-DIAS et al., 2013Catão-Dias ZL, Epiphanio S, Kierulff MCM. Neotropical primates and their susceptibility to Toxoplasma gondii: new insights of an old problem. In: Brinkworth JF, Pechenkina K, editors. Primates, pathogens, and evolution. New York: Springer Press; 2013. p. 253-289. http://dx.doi.org/10.1007/978-1-4614-7181-3_9.
http://dx.doi.org/10.1007/978-1-4614-718...
) - which could be the case for the killer whale - one should also consider the possibility of infection prior to the specimen’s capture in the wild (e.g., transplacental or transmammary) or initial period in captivity (1984-1988). In that case, the infection would have been quiescent until being activated by the stress of captivity and/or other predisposing factors (e.g., concomitant diseases) (LINDSAY et al., 2003Lindsay DS, Collins MV, Mitchell SM, Cole RA, Flick GJ, Wetch CN, et al. Sporulation and survival of Toxoplasma gondii oocysts in seawater. J Eukaryot Microbiol 2003;50(s1 Suppl): 687-688. http://dx.doi.org/10.1111/j.1550-7408.2003.tb00688.x. PMid:14736220.
http://dx.doi.org/10.1111/j.1550-7408.20...
; AUBERT & VILLENA, 2009Aubert D, Villena I. Detection of Toxoplasma gondii oocysts in water: proposition of a strategy and evaluation in Champagne-Ardenne region, France. Mem Inst Oswaldo Cruz 2009; 104(2): 290-295. http://dx.doi.org/10.1590/S0074-02762009000200023. PMid:19430655.
http://dx.doi.org/10.1590/S0074-02762009...
; MAZZARIOL et al., 2012Mazzariol S, Marcer F, Mignone W, Serracca L, Goria M, Marsili L, et al. Dolphin morbillivirus and Toxoplasma gondii coinfection in a Mediterranean fin whale (Balaenoptera physalus). BMC Vet Res 2012; 8(1): 20. http://dx.doi.org/10.1186/1746-6148-8-20. PMid:22397492.
http://dx.doi.org/10.1186/1746-6148-8-20...
).
Toxoplasma gondii infections in common bottlenose dolphins have been observed worldwide; in the Mediterranean Sea and the Atlantic Ocean (CRUICKSHANK et al., 1990Cruickshank JJ, Haines DM, Palmer NC, St Aubin DJ. Cysts of a Toxoplasma-like organism in an Atlantic bottlenose dolphin. Can Vet J 1990; 31(3): 213-215. PMid:17423539.; INSKEEP et al., 1990Inskeep W 2nd, Gardiner CH, Harris RK, Dubey JP, Goldston RT. Toxoplasmosis in Atlantic bottle-nosed dolphins (Tursiops truncatus). J Wildl Dis 1990; 26(3): 377-382. http://dx.doi.org/10.7589/0090-3558-26.3.377. PMid:2388360.
http://dx.doi.org/10.7589/0090-3558-26.3...
; DI GUARDO et al., 1995Di Guardo G, Agrimi U, Morelli L, Cardeti G, Terracciano S, Kennedy S. Post mortem investigations on cetaceans found stranded on the coasts of Italy between 1990 and 1993. Vet Rec 1995; 136(17): 439-442. http://dx.doi.org/10.1136/vr.136.17.439. PMid:7631479.
http://dx.doi.org/10.1136/vr.136.17.439...
; DUBEY et al., 2008Dubey JP, Fair PA, Sundar N, Velmurugan G, Kwok OC, McFee WE, et al. Isolation of Toxoplasma gondii from bottlenose dolphins (Tursiops truncatus). J Parasitol 2008; 94(4): 821-823. http://dx.doi.org/10.1645/GE-1444.1. PMid:18576793.
http://dx.doi.org/10.1645/GE-1444.1...
; PRETTI et al., 2010Pretti C, Mancianti F, Nardoni S, Ariti G, Monni G, Di Bello D, et al. Detection of Toxoplasma gondii infection in dolphins stranded along the Tuscan coast, Italy. Rev Med Vet 2010; 161(10): 428-431.; PROFETA et al., 2015Profeta F, Di Francesco CE, Marsilio F, Mignone W, Di Nocera F, De Carlo E, et al. Retrospective seroepidemiological investigations against morbillivirus, Toxoplasma gondii and Brucella spp. in cetaceans stranded along the Italian coastline (1998-12014). Res Vet Sci 2015; 101: 89-92. http://dx.doi.org/10.1016/j.rvsc.2015.06.008. PMid:26267096.
http://dx.doi.org/10.1016/j.rvsc.2015.06...
; BIGAL et al., 2018Bigal E, Morick D, Scheinin AP, Salant H, Berkowitz A, King R, et al. Detection of Toxoplasma gondii in three common bottlenose dolphins (Tursiops truncatus): a first description from the Eastern Mediterranean Sea. Vet Parasitol 2018; 258: 74-78. http://dx.doi.org/10.1016/j.vetpar.2018.06.009. PMid:30105982.
http://dx.doi.org/10.1016/j.vetpar.2018....
), and also in captive specimens (DUBEY et al., 2009Dubey JP, Mergl J, Gehring E, Sundar N, Velmurugan GV, Kwok OC, et al. Toxoplasmosis in captive dolphins (Tursiops truncatus) and walrus (Odobenus rosmarus). J Parasitol 2009; 95(1): 82-85. http://dx.doi.org/10.1645/GE-1764.1. PMid:19245284.
http://dx.doi.org/10.1645/GE-1764.1...
). Furthermore, it has also been detected in a related species from the Pacific Ocean - the Indo-Pacific bottlenose dolphin (JARDINE & DUBEY, 2002Jardine JE, Dubey JP. Congenital toxoplasmosis in a Indo-Pacific bottlenose dolphin (Tursiops aduncus). J Parasitol 2002; 88(1): 197-199. http://dx.doi.org/10.1645/0022-3395(2002)088[0197:CTIAIP]2.0.CO;2. PMid:12053968.
http://dx.doi.org/10.1645/0022-3395(2002...
). However, this is the first report of T. gondii in an Atlantic bottlenose dolphin from South America (Figure 2), widening the geographic range of T. gondii occurrence for this species.
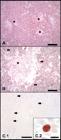
Microscopic lesions and immunohistochemical findings in Toxoplasma gondiipositive animals. (A) Atlantic bottlenose dolphin (Tursiops truncatus). Liver, necrotizing hepatitis (asterisk), H&E, scale bar = 200 μm; (B) Killer whale (Orcinus orca). Lung, bronchopneumonia (arrows) with alveolar infiltrate (asterisk), H&E, scale bar = 200 μm; (C.1) Guiana dolphin (Sotalia guianensis). Adrenal gland, there is focally extensive lytic necrosis with multiple protozoal cysts (arrows). IHC forT. gondii, Mayer’s hematoxylin counterstaining, scale bar = 500 μm; (C.2) Detailed view ofT. gondiiprotozoal cyst containing numerous bradyzoites. IHC for T. gondii, Mayer’s hematoxylin counterstaining, scale bar = 25 μm.
Because cetacean species’ susceptibility to T. gondii infection is unknown, non-exposure and natural resistance to this agent should be considered in negative cases (DI GUARDO & MAZZARIOL, 2013Di Guardo G, Mazzariol S. Toxoplasma gondii: clues from stranded dolphins. Vet Pathol 2013; 50(5): 737. http://dx.doi.org/10.1177/0300985813486816. PMid:24014612.
http://dx.doi.org/10.1177/03009858134868...
). Omata et al. (2006)Omata Y, Umeshita Y, Watarai M, Tachibana M, Sasaki M, Murata K, et al. Investigation for presence of Neospora caninum, Toxoplasma gondii and Brucella-species infection in killer whales (Orcinus orca) mass-stranded on the coast of Shiretoko, Hokkaido, Japan. J Vet Med Sci 2006; 68(5): 523-526. http://dx.doi.org/10.1292/jvms.68.523. PMid:16757901.
http://dx.doi.org/10.1292/jvms.68.523...
proposed that killer whales from Japan might be resistant to T. gondii; however, the evidence provided seemed slight because none of these animals presented antibodies against the agent.
Finally, this is the first report of a large-scale T. gondii survey based on histopathological and immunohistochemical examinations in cetaceans of Brazil. This study widens the spectrum of T. gondii-susceptible species and geographic range of this agent in Brazil and presents the first report of T. gondii-infection in a captive killer whale, and in a free-ranging Atlantic bottlenose dolphin from South America. Furthermore, it corroborates with previous observations of toxoplasmosis in Guiana dolphin and the geographic area of Paranaguá Bay. Further studies are warranted to characterize the circulating strains and clarify the distribution of this protozoan, its transmission route(s), unsolved pathogenetic mechanisms, cetacean host-specific susceptibilities and potential implications to the conservation of cetacean species of Brazil and mechanisms involved in human exposure.
Acknowledgements
We thank Leonardo L. Wedekin, Jorge Oyakawa and Sândara Sguario for their technical support. We also thank the Aquário de Santos, Associação de Pesquisa e Preservação de Ecossistemas Aquáticos (AQUASIS), Instituto Baleia Jubarte, Instituto de Pesquisas de Cananéia (IPeC), Grupo de Estudos de Mamíferos Aquáticos do Rio Grande do Sul (GEMARS) Laboratório de Mamíferos Aquáticos e Bioindicadores ‘Profa. Izabel Gurgel’ - Faculdade de Oceanografia - Universidade do Estado do Rio de Janeiro (MAQUA), Museu Oceanográfico da FURG, Playcenter, Projeto Biopesca - Universidade Estadual Paulista-UNESP, and Projeto Baleia Franca/Instituto Australis for providing the samples and necropsy records. This study was co-financed by CAPES and FAPESP. The financial sources had no influence over the project’s development. All procedures were performed in accordance with the Ethical Committee of the School of Veterinary Medicine and Animal Sciences, University of São Paulo (process number: 2551070144). JDD is the recipient of a post-doctoral fellowship by FAPESP (grant #2017/02223-8), and CNPq provided scholarship to J.L.C.-D (grant #305349/2015-5).
References
- Aubert D, Villena I. Detection of Toxoplasma gondii oocysts in water: proposition of a strategy and evaluation in Champagne-Ardenne region, France. Mem Inst Oswaldo Cruz 2009; 104(2): 290-295. http://dx.doi.org/10.1590/S0074-02762009000200023 PMid:19430655.
» http://dx.doi.org/10.1590/S0074-02762009000200023 - Bandoli JG, Oliveira CAB. Toxoplasmose em Sotalia guianensis (Van Beneden, 1863, Cetacea-Delphinidae). Folha Med 1977; 75(4): 459-468.
- Bigal E, Morick D, Scheinin AP, Salant H, Berkowitz A, King R, et al. Detection of Toxoplasma gondii in three common bottlenose dolphins (Tursiops truncatus): a first description from the Eastern Mediterranean Sea. Vet Parasitol 2018; 258: 74-78. http://dx.doi.org/10.1016/j.vetpar.2018.06.009 PMid:30105982.
» http://dx.doi.org/10.1016/j.vetpar.2018.06.009 - Bossart GD. Marine mammals as sentinel species for oceans and human health. Vet Pathol 2011; 48(3): 676-690. http://dx.doi.org/10.1177/0300985810388525 PMid:21160025.
» http://dx.doi.org/10.1177/0300985810388525 - Catão-Dias ZL, Epiphanio S, Kierulff MCM. Neotropical primates and their susceptibility to Toxoplasma gondii: new insights of an old problem. In: Brinkworth JF, Pechenkina K, editors. Primates, pathogens, and evolution New York: Springer Press; 2013. p. 253-289. http://dx.doi.org/10.1007/978-1-4614-7181-3_9
» http://dx.doi.org/10.1007/978-1-4614-7181-3_9 - Corbellini LG, Driemeier D, Cruz CFE, Gondim LFP, Wald V. Neosporosis as a cause of abortion in dairy cattle in Rio Grande do Sul, southern Brazil. Vet Parasitol 2002; 103(3): 195-202. http://dx.doi.org/10.1016/S0304-4017(01)00600-8 PMid:11750112.
» http://dx.doi.org/10.1016/S0304-4017(01)00600-8 - Cruickshank JJ, Haines DM, Palmer NC, St Aubin DJ. Cysts of a Toxoplasma-like organism in an Atlantic bottlenose dolphin. Can Vet J 1990; 31(3): 213-215. PMid:17423539.
- Di Guardo G, Agrimi U, Morelli L, Cardeti G, Terracciano S, Kennedy S. Post mortem investigations on cetaceans found stranded on the coasts of Italy between 1990 and 1993. Vet Rec 1995; 136(17): 439-442. http://dx.doi.org/10.1136/vr.136.17.439 PMid:7631479.
» http://dx.doi.org/10.1136/vr.136.17.439 - Di Guardo G, Mazzariol S. Toxoplasma gondii: clues from stranded dolphins. Vet Pathol 2013; 50(5): 737. http://dx.doi.org/10.1177/0300985813486816 PMid:24014612.
» http://dx.doi.org/10.1177/0300985813486816 - Di Guardo G, Proietto U, Di Francesco CE, Marsilio F, Zaccaroni A, Scaravelli D, et al. Cerebral toxoplasmosis in striped dolphins (Stenella coeruleoalba) stranded along the Ligurian Sea coast of Italy. Vet Pathol 2010; 47(2): 245-253. http://dx.doi.org/10.1177/0300985809358036 PMid:20118319.
» http://dx.doi.org/10.1177/0300985809358036 - Dubey JP, Fair PA, Sundar N, Velmurugan G, Kwok OC, McFee WE, et al. Isolation of Toxoplasma gondii from bottlenose dolphins (Tursiops truncatus). J Parasitol 2008; 94(4): 821-823. http://dx.doi.org/10.1645/GE-1444.1 PMid:18576793.
» http://dx.doi.org/10.1645/GE-1444.1 - Dubey JP, Lipscomb TP, Mense M. Toxoplasmosis in an elephant seal (Mirounga angustirostris). J Parasitol 2004; 90(2): 410-411. http://dx.doi.org/10.1645/GE-155R PMid:15165069.
» http://dx.doi.org/10.1645/GE-155R - Dubey JP, Mergl J, Gehring E, Sundar N, Velmurugan GV, Kwok OC, et al. Toxoplasmosis in captive dolphins (Tursiops truncatus) and walrus (Odobenus rosmarus). J Parasitol 2009; 95(1): 82-85. http://dx.doi.org/10.1645/GE-1764.1 PMid:19245284.
» http://dx.doi.org/10.1645/GE-1764.1 - Dubey JP. The history of Toxoplasma gondii - The first 100 years. J Eukaryot Microbiol 2008; 55(6): 467-475. http://dx.doi.org/10.1111/j.1550-7408.2008.00345.x PMid:19120791.
» http://dx.doi.org/10.1111/j.1550-7408.2008.00345.x - Gibson AK, Raverty S, Lambourn DM, Huggins J, Magargal SL, Grigg ME. Polyparasitism is associated with increased disease severity in Toxoplasma gondii-infected marine sentinel species. PLoS Negl Trop Dis 2011; 5(5): e1142. http://dx.doi.org/10.1371/journal.pntd.0001142 PMid:21629726.
» http://dx.doi.org/10.1371/journal.pntd.0001142 - Gonzales-Viera O, Marigo J, Ruoppolo V, Rosas FCW, Kanamura CT, Takakura C, et al. Toxoplasmosis in a Guiana dolphin (Sotalia guianensis) from Paraná, Brazil. Vet Parasitol 2013; 191(3-4): 358-362. http://dx.doi.org/10.1016/j.vetpar.2012.09.012 PMid:23063774.
» http://dx.doi.org/10.1016/j.vetpar.2012.09.012 - Groch KR, Colosio AC, Marcondes MCC, Zucca D, Díaz-Delgado J, Niemeyer C, et al. Novel cetacean morbillivirus in Guiana dolphin, Brazil. Emerg Infect Dis 2014; 20(3): 511-513. http://dx.doi.org/10.3201/eid2003.131557 PMid:24565559.
» http://dx.doi.org/10.3201/eid2003.131557 - Inskeep W 2nd, Gardiner CH, Harris RK, Dubey JP, Goldston RT. Toxoplasmosis in Atlantic bottle-nosed dolphins (Tursiops truncatus). J Wildl Dis 1990; 26(3): 377-382. http://dx.doi.org/10.7589/0090-3558-26.3.377 PMid:2388360.
» http://dx.doi.org/10.7589/0090-3558-26.3.377 - Iqbal A, Measures L, Lair S, Dixon B. Toxoplasma gondii in stranded St. Lawrence Estuary beluga Delphinapterus leucas in Quebec, Canada. Dis Aquat Organ 2018; 130(3): 165-175. http://dx.doi.org/10.3354/dao03262 PMid:30259869.
» http://dx.doi.org/10.3354/dao03262 - Jardine JE, Dubey JP. Congenital toxoplasmosis in a Indo-Pacific bottlenose dolphin (Tursiops aduncus). J Parasitol 2002; 88(1): 197-199. http://dx.doi.org/10.1645/0022-3395(2002)088[0197:CTIAIP]2.0.CO;2 PMid:12053968.
» http://dx.doi.org/10.1645/0022-3395(2002)088[0197:CTIAIP]2.0.CO;2 - Lindsay DS, Collins MV, Mitchell SM, Cole RA, Flick GJ, Wetch CN, et al. Sporulation and survival of Toxoplasma gondii oocysts in seawater. J Eukaryot Microbiol 2003;50(s1 Suppl): 687-688. http://dx.doi.org/10.1111/j.1550-7408.2003.tb00688.x PMid:14736220.
» http://dx.doi.org/10.1111/j.1550-7408.2003.tb00688.x - Luna LG. Histopathologic methods and color atlas of special stains and tissue artifacts Gaithersburg: America Histolabs; 1992.
- Mazzariol S, Marcer F, Mignone W, Serracca L, Goria M, Marsili L, et al. Dolphin morbillivirus and Toxoplasma gondii coinfection in a Mediterranean fin whale (Balaenoptera physalus). BMC Vet Res 2012; 8(1): 20. http://dx.doi.org/10.1186/1746-6148-8-20 PMid:22397492.
» http://dx.doi.org/10.1186/1746-6148-8-20 - Migaki G, Sawa TR, Dubey JP. Fatal disseminated toxoplasmosis in a Spinner dolphin (Stenella longirostris). Vet Pathol 1990; 27(6): 463-464. http://dx.doi.org/10.1177/030098589902700615 PMid:2278137.
» http://dx.doi.org/10.1177/030098589902700615 - Mikaelian I, Boisclair J, Dubey JP, Kennedy S, Martineau D. Toxoplasmosis in beluga whales (Delphinapterus leucas) from St Lawrence Estuary: two case reports and a serological survey. J Comp Pathol 2000; 122(1): 73-76. http://dx.doi.org/10.1053/jcpa.1999.0341 PMid:10627393.
» http://dx.doi.org/10.1053/jcpa.1999.0341 - Miller M, Conrad P, James ER, Packham A, Toy-Choutka S, Murray MJ, et al. Transplacental toxoplasmosis in a wild southern sea otter (Enhydra lutris nereis). Vet Parasitol 2008; 153(1-2): 12-18. http://dx.doi.org/10.1016/j.vetpar.2008.01.015 PMid:18304737.
» http://dx.doi.org/10.1016/j.vetpar.2008.01.015 - Moura JF, Hauser-Davis RA, Lemos L, Emin-Lima R, Siciliano S. Guiana dolphins (Sotalia guianensis) as marine ecosystem sentinels: ecotoxicology and emerging diseases. Rev Environ Contam Toxicol 2014; 228: 1-29. http://dx.doi.org/10.1007/978-3-319-01619-1_1 PMid:24162090.
» http://dx.doi.org/10.1007/978-3-319-01619-1_1 - Murata K, Mizuta K, Imazu K, Terasawa F, Taki M, Endoh T. The prevalence of Toxoplasma gondii antibodies in wild and captive cetaceans from Japan. J Parasitol 2004; 90(4): 896-898. http://dx.doi.org/10.1645/GE-197R PMid:15357097.
» http://dx.doi.org/10.1645/GE-197R - Nicolle MC, Manceaux L. On a new protozoan in gundis (Toxoplasma N. Gen). Mem Inst Oswaldo Cruz 2009; 104: 1-3.
- Omata Y, Umeshita Y, Watarai M, Tachibana M, Sasaki M, Murata K, et al. Investigation for presence of Neospora caninum, Toxoplasma gondii and Brucella-species infection in killer whales (Orcinus orca) mass-stranded on the coast of Shiretoko, Hokkaido, Japan. J Vet Med Sci 2006; 68(5): 523-526. http://dx.doi.org/10.1292/jvms.68.523 PMid:16757901.
» http://dx.doi.org/10.1292/jvms.68.523 - Popper H, Paronetto F, Barka T. PAS-positive structures of nonglycogenic character in normal and abnormal liver. Arch Pathol 1960; 70: 300-313. PMid:14434186.
- Pretti C, Mancianti F, Nardoni S, Ariti G, Monni G, Di Bello D, et al. Detection of Toxoplasma gondii infection in dolphins stranded along the Tuscan coast, Italy. Rev Med Vet 2010; 161(10): 428-431.
- Profeta F, Di Francesco CE, Marsilio F, Mignone W, Di Nocera F, De Carlo E, et al. Retrospective seroepidemiological investigations against morbillivirus, Toxoplasma gondii and Brucella spp. in cetaceans stranded along the Italian coastline (1998-12014). Res Vet Sci 2015; 101: 89-92. http://dx.doi.org/10.1016/j.rvsc.2015.06.008 PMid:26267096.
» http://dx.doi.org/10.1016/j.rvsc.2015.06.008 - Resendes AR, Almería S, Dubey JP, Obón E, Juan-Sallés C, Degollada E, et al. Disseminated toxoplasmosis in a Mediterranean pregnant Risso’s dolphin (Grampus griseus) with transplacental fetal infection. J Parasitol 2002; 88(5): 1029-1032. http://dx.doi.org/10.1645/0022-3395(2002)088[1029:DTIAMP]2.0.CO;2 PMid:12435153.
» http://dx.doi.org/10.1645/0022-3395(2002)088[1029:DTIAMP]2.0.CO;2 - Roe WD, Howe L, Baker EJ, Burrows L, Hunter SA. An atypical genotype of Toxoplasma gondii as a cause of mortality in Hector’s dolphins (Cephalorhynchus hectori). Vet Parasitol 2013; 192(1-3): 67-74. http://dx.doi.org/10.1016/j.vetpar.2012.11.001 PMid:23207018.
» http://dx.doi.org/10.1016/j.vetpar.2012.11.001 - Saliki JT, Cooper EJ, Gustavson JP. Emerging morbillivirus infections of marine mammals: development of two diagnostic approaches. Ann N Y Acad Sci 2002; 969(1): 51-59. http://dx.doi.org/10.1111/j.1749-6632.2002.tb04350.x PMid:12381563.
» http://dx.doi.org/10.1111/j.1749-6632.2002.tb04350.x - Santos PS, Albuquerque GR, Da Silva VMF, Martin AR, Marvullo MFV, Souza SLP, et al. Seroprevalence of Toxoplasma gondii in free-living Amazon River dolphins (Inia geoffrensis) from central Amazon, Brazil. Vet Parasitol 2011; 183(1-2): 171-173. http://dx.doi.org/10.1016/j.vetpar.2011.06.007 PMid:21764516.
» http://dx.doi.org/10.1016/j.vetpar.2011.06.007 - Shapiro K, VanWormer E, Aguilar B, Conrad PA. Surveillance for Toxoplasma gondii in California mussels (Mytilus californianus) reveals transmission of atypical genotypes from land to sea. Environ Microbiol 2015; 17(11): 4177-4188. http://dx.doi.org/10.1111/1462-2920.12685 PMid:25367256.
» http://dx.doi.org/10.1111/1462-2920.12685 - Sierra E, Sánchez S, Saliki JT, Blas-Machado U, Arbelo M, Zucca D, et al. Retrospective study of etiologic agents associated with nonsuppurative meningoencephalitis in stranded cetaceans in the Canary Islands. J Clin Microbiol 2014; 52(7): 2390-2397. http://dx.doi.org/10.1128/JCM.02906-13 PMid:24759718.
» http://dx.doi.org/10.1128/JCM.02906-13 - Splendore A. A new protozoan parasite of rabbit found in histological lesions similar to human Kala-Azar. Mem Inst Oswaldo Cruz 2009; 104: 1-2.
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol 2000; 30(12-13): 1217-1258. http://dx.doi.org/10.1016/S0020-7519(00)00124-7 PMid:11113252.
» http://dx.doi.org/10.1016/S0020-7519(00)00124-7 - Van Bressem MF, Raga JA, Di Guardo G, Jepson PD, Duignan PJ, Siebert U, et al. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis Aquat Organ 2009; 86(2): 143-157. http://dx.doi.org/10.3354/dao02101 PMid:19902843.
» http://dx.doi.org/10.3354/dao02101 - Wells RS, Rhinehart HL, Hansen LJ, Sweeney JC, Townsend FI, Stone R, et al. Bottlenose dolphins as marine ecosystem sentinels: developing health monitoring system. EcoHealth 2004; 1(3): 246-254. http://dx.doi.org/10.1007/s10393-004-0094-6
» http://dx.doi.org/10.1007/s10393-004-0094-6
Publication Dates
-
Publication in this collection
12 Aug 2019 -
Date of issue
Jul-Sep 2019
History
-
Received
14 Feb 2019 -
Accepted
24 May 2019