Abstract
Flounders are commercially and economically important fish. A total of 120 specimens of flounders (60 Paralichthys isosceles, 30 Paralichthys patagonicus and 30 Xystreurys rasile) were collected off the coast of the state of Rio de Janeiro, Brazil. The fish were measured, necropsied and filleted, and then had their organs investigated for acanthocephalans. Taxonomic identification of the parasites was based on morphological, morphometric and genetic characters. Paralichthys isosceles and P. patagonicus were parasitized by juveniles of Serrasentis sagittifer, Bolbosoma turbinella, Corynosoma australe and C. cetaceum; Xystreurys rasile was parasitized by C. australe. Genetic characterization confirmed the identification of specimens of Bolbosoma turbinella and Corynosoma australe, as demonstrated by phylogenetic analyses using both ITS and cox1 molecular targets. Parasite indices of prevalence, intensity, mean intensity, abundance, mean abundance, and range of infection, as well as infection site, were evaluated for each parasite species. This is the first report of S. sagittifer parasitizing P. isosceles and P. patagonicus, and B. turbinella parasitizing P. patagonicus.
Keywords:
Acantocephalans; integrative taxonomy; Paralichthys isosceles; Paralichthys patagonicus; Xystreurys rasile; Brazil
Resumo
Os linguados são peixes comercial e economicamente importantes. Um total de 120 espécimes de linguados (60 Paralichthys isosceles, 30 P. patagonicus e 30 Xystreurys rasile) foram coletados no litoral do estado do Rio de Janeiro, Brasil. Os peixes foram medidos, necropsiados, filetados e tiveram seus órgãos investigados para a presença de acantocéfalos. A identificação taxonômica foi baseada em caracteres morfológicos, morfométricos e genéticos. Paralichthys isosceles e P. patagonicus estavam parasitados por acantocéfalos juvenis de Serrasentis sagittifer, Bolbosoma turbinella, Corynosoma australe e C. cetaceum; Xystreurys rasile estava parasitado com C. australe. A caracterização genética confirmou a identificação dos espécimes de Bolbosoma turbinella e Corynosoma australe, como demonstrado por análises filogenéticas usando ambos marcadores moleculares ITS e cox1. Foram analisados os índices parasitários: prevalência, intensidade, intensidade média, abundância, abundância média, amplitude de variação da infecção e sítio de infecção de cada espécie de parasito. Este é o primeiro registro de S. sagittifer parasitando P. isosceles e P. patagonicus, e de B. turbinella parasitando P. patagonicus.
Palavras-chave:
Acantocéfalos; taxonomia integrativa; Paralichthys isosceles; Paralichthys patagonicus; Xystreurys rasile; Brasil
Introduction
Flounders (Pleuronectiformes, Paralichthyidae) are known to be ravenous predators of fish, cephalopods and crustaceans (ARAÚJO & HAIMOVICE, 2000Araújo JN, Haimovice M. Determinação de idades e crescimento do linguado branco Paralichthys patagonicus (Jordan, 1889) no sul do Brasil. Rev Bras Oceanogr 2000; 48(1): 61-70. http://dx.doi.org/10.1590/S1413-77392000000100005.
http://dx.doi.org/10.1590/S1413-77392000...
). Species of the genera Paralichthys and Xystreurys (Paralichthyidae) occurring along the coast of the state of Rio de Janeiro, Brazil, have great commercial importance in both domestic and foreign markets (BERNARDES et al., 2005Bernardes RA, Figueiredo JL, Rodrigues AR, Fischer LG, Vooren CM, Haimovici M, et al. Peixes da zona econômica exclusiva da região sudeste-sul do Brasil: Levantamento com armadilhas, pargueiras e rede de arrasto de fundo. São Paulo: Editora da Universidade de São Paulo; 2005.). These fish are considered to be among the so-called “fine fish” because of the high quality of their meat and their high market value (FIGUEIREDO & MENEZES, 2000Figueiredo JL, Menezes NA. Manual de peixes marinhos do Sudeste do Brasil. São Paulo: Universidade de São Paulo, Museu de Zoologia; 2000. (VI Teleostei; no. 5).; MASSA et al., 2005Massa AE, Palacios DL, Paredi ME, Crupkin M. Postmortem changes in quality indices of ice-stored flounder (Paralichthys patagonicus). J Food Biochem 2005; 29(5): 570-590. http://dx.doi.org/10.1111/j.1745-4514.2005.00050.x.
http://dx.doi.org/10.1111/j.1745-4514.20...
).
Helminth parasites have been previously reported in flounders of the family Paralichthyidae in South America, including Brazil (SANTOS et al., 2008Santos CP, Gibson DI, Tavares LER, Luque JL. Checklist of acanthocephala associated with the fishes of Brazil. Zootaxa 2008; 1938: 1-22.; FELIZARDO et al., 2009aFelizardo NN, Knoff M, Pinto RM, Gomes DC. Larval anisakid nematodes of the flounder, Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) from Brazil. Neotrop Helminthol 2009a; 3(2): 57-64.,bFelizardo NN, Menezes RC, Tortelly R, Knoff M, Pinto RM, Gomes DC. Larvae of Hysterothylacium sp. (Nematoda: Anisakidae) in the sole fish Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) from the littoral of the state of Rio de Janeiro, Brazil. Vet Parasitol 2009b; 166(1-2): 175-177. http://dx.doi.org/10.1016/j.vetpar.2009.08.004. PMid:19713041.
http://dx.doi.org/10.1016/j.vetpar.2009....
, 2010Felizardo NN, Torres EJL, Fonseca MCG, Pinto RM, Gomes DC, Knoff M. Cestodes of the flounder Paralichthys isosceles Jordan, 1890 (Osteichthyes - Paralichthyidae) from the state of Rio de Janeiro, Brazil. Neotrop Helminthol 2010; 4(2): 113-126., 2011Felizardo NN, Justo MC, Knoff M, Fonseca MCG, Pinto RM, Gomes DC. Juvenile didymozoids of the types, Torticaecum and Neotorticaecum (Didymozoidae: Digenea), from new marine fish hosts (Pisces: Teleostei) in the Neotropical region of Brazil. J Helminthol 2011; 85(3): 270-275. http://dx.doi.org/10.1017/S0022149X10000507. PMid:20854704.
http://dx.doi.org/10.1017/S0022149X10000...
; ALARCOS & TIMI, 2012Alarcos AJ, Timi JT. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 2012; 110(6): 2155-2166. http://dx.doi.org/10.1007/s00436-011-2741-5. PMid:22167375.
http://dx.doi.org/10.1007/s00436-011-274...
; FONSECA et al., 2012Fonseca MCG, São Clemente SC, Felizardo NN, Gomes DC, Knoff M. Trypanorhyncha cestodes of hygienic-sanitary importance infecting flounders Paralichthys patagonicus Jordan, 1889 and Xystreurys rasile (Jordan, 1891) of the Neotropical region, Brazil. Parasitol Res 2012; 111(2): 865-874. http://dx.doi.org/10.1007/s00436-012-2912-z. PMid:22488201.
http://dx.doi.org/10.1007/s00436-012-291...
, 2016Fonseca MC, Knoff M, Felizardo NN, Di Azevedo MI, Torres EJ, Gomes DC, et al. Integrative taxonomy of Anisakidae and Raphidascarididae (Nematoda) in Paralichthys patagonicus and Xystreurys rasile (Pisces: Teleostei) from Brazil. Int J Food Microbiol 2016; 235(20): 113-124. http://dx.doi.org/10.1016/j.ijfoodmicro.2016.07.026. PMid:27491056.
http://dx.doi.org/10.1016/j.ijfoodmicro....
; KNOFF et al., 2012Knoff M, Felizardo NN, Iñiguez AM, Maldonado A Jr, Torres EJ, Pinto RM, et al. Genetic and morphological characterisation of a new species of the genus Hysterothylacium (Nematoda) from Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) of the Neotropical Region, State of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 2012; 107(2): 186-193. http://dx.doi.org/10.1590/S0074-02762012000200006. PMid:22415256.
http://dx.doi.org/10.1590/S0074-02762012...
; ALARCOS et al., 2016Alarcos AJ, Pereira AN, Taborda NL, Luque JL, Timi JT. Parasitological evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small and geographical scales in South American Atlantic coasts. Fish Res 2016; 173(3): 221-228. http://dx.doi.org/10.1016/j.fishres.2015.07.018.
http://dx.doi.org/10.1016/j.fishres.2015...
).
Thorny-headed worms, or acanthocephalans, are endoparasitic helminthes. There are approximately 1290 described species (AMIN, 2013Amin OM. Classification of the Acanthocephala. Folia Parasitol (Praha) 2013; 60(4): 273-305. http://dx.doi.org/10.14411/fp.2013.031. PMid:24261131.
http://dx.doi.org/10.14411/fp.2013.031...
), which can be found causing various pathological conditions in marine and freshwater fish worldwide. The life-cycles of acanthocephalans involve a fish definitive host and an arthropode intermediary such as amphipod, ostracod or copepod. A few cycles also incorporate paratenic or transport hosts (WILLIAMS & JONES, 1994Williams H, Jones A. Parasitic worms of fish. London: Taylor Francys Ltd.; 1994.).
Cases of human acanthocephaliasis have been reported in association with the ingestion of raw fish, including Acanthocephalus rauschi Golvan, 1969 and Corynosoma strumosum (Rudolphi, 1802) Lühe, 1904, parasitizing Alaskan Eskimos (GOLVAN, 1969Golvan YJ. Systematique des Acanthocephales (Acanthocephala Rudolphi, 1801). L’ordre des Paleacanthocephala Meyer, 1931. La super-familie des Echinorhynchoidea (Cobbold, 1876) Golvan et Houin, 1963. Mem Mus Natl Hist Nat Paris Ser A Zool 1969; 57(single): 1-373.; SCHMIDT, 1971Schmidt GD. Acanthocephalan infection of man, with two new records. J Parasitol 1971; 57(3): 582-584. http://dx.doi.org/10.2307/3277920. PMid:5090967.
http://dx.doi.org/10.2307/3277920...
), and Bolbosoma Porta, 1908 and Corynosoma Lühe, 1904, in Japan (TADA et al., 1983Tada I, Otsuji Y, Kamiya H, Mimori T, Sakaguchi Y, Makizumi S. The first case of a human infected with an acanthocephalan parasite, Bolbosoma sp. J Parasitol 1983; 69(1): 205-208. http://dx.doi.org/10.2307/3281300. PMid:6827437.
http://dx.doi.org/10.2307/3281300...
; ACHA & SZYFRES, 2003Acha PN, Szyfres B. Zoonoses and communicable diseases common to man and animals. 3rd ed. Washington: PAHO; 2003. (Scientific and Technical Publication; no. 580; Parasitoses; vol. III).; ARIZONO et al., 2012Arizono N, Kuramochi T, Kagei N. Molecular and histological identification of the acanthocephalan Bolbosoma cf. capitatum from the human small intestine. Parasitol Int 2012; 61(4): 715-718. http://dx.doi.org/10.1016/j.parint.2012.05.011. PMid:22634485.
http://dx.doi.org/10.1016/j.parint.2012....
; FUJITA et al., 2016Fujita T, Waga E, Kitaoka K, Imagawa T, Komatsu Y, Takanashi K, et al. Human infection by acanthocephalan parasites belonging to the genus Corynosoma found from small bowel endoscopy. Parasitol Int 2016; 65(5 PtA): 491-493. http://dx.doi.org/10.1016/j.parint.2016.07.002. PMid:27396515.
http://dx.doi.org/10.1016/j.parint.2016....
).
In Brazil, there is only one study reporting acanthocephalans of flounder Paralichthys isosceles Jordan, 1890 (ALARCOS et al., 2016Alarcos AJ, Pereira AN, Taborda NL, Luque JL, Timi JT. Parasitological evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small and geographical scales in South American Atlantic coasts. Fish Res 2016; 173(3): 221-228. http://dx.doi.org/10.1016/j.fishres.2015.07.018.
http://dx.doi.org/10.1016/j.fishres.2015...
), which motivated new surveys on the acanthocephalans parasitizing flounders species from Brazilian coast.
The aim of this study was to assess the presence of juvenile acanthocephalans parasitizing P. isosceles, Paralichthys patagonicus Jordan, 1889 and Xystreurys rasile (Jordan, 1891) from off the coast of the state of Rio de Janeiro, in Brazil. The acanthocephalan species encountered were identified using morphological, morphometric and genetic characters, and their parasite indices calculated and infection sites reported.
Materials and Methods
One hundred and twenty flounder specimens, 60 P. isosceles with mean length 33.8 cm (25.0-39.5 cm) and mean weight 420.4 g (164-680 g); 30 P. patagonicus with mean length 40.8 cm (28.5-59 cm) and mean weight 820.4 g (280-2530 g); and 30 X. rasile with mean length 24.3 cm (11.5-31 cm) and mean weight 158.5 g (20-240 g), were collected from small markets selling only fish caught offshore of the municipalities of Cabo Frio (22°52’46” S; 42°01’07” W), Niterói (22°53’00” S; 43°06’13” W), Rio de Janeiro (22°54’13” S; 43°12’35” W), and Angra dos Reis (23º00'24” S; 44º19'05” W), in the state of Rio de Janeiro, Brazil. Fish species were identified in accordance with Nakamura et al. (1986)Nakamura I, Inada T, Takeda M, Hatanaka H. Important fishes trawled off Patagonia. Tokyo: Japan Marine Fishery Resource Research Center; 1986. and Figueiredo & Menezes (2000)Figueiredo JL, Menezes NA. Manual de peixes marinhos do Sudeste do Brasil. São Paulo: Universidade de São Paulo, Museu de Zoologia; 2000. (VI Teleostei; no. 5).. Internal organs and musculature were examined, with all acanthocephalans found being placed in Petri dishes with 0.65% NaCl solution. Specimens were fixed in AFA (ethanol, formalin, and acetic acid), preserved in 70% ethanol, stained in Langeron’s carmine, clarified in beechwood creosote, and preserved as whole mounts on Canada balsam according to Knoff & Gomes (2012)Knoff M, Gomes DC. Metodologia básica para coleta e o processamento de helmintos parasitos. In: Molinaro EM, Caputo LFG, Amendoeira MRR, editores. Conceitos e métodos para a formação de profissionais em laboratórios de saúde. vol. 5. Rio de Janeiro: EPSJV; 2012. p. 251-281.. Voucher specimens were deposited in the Helminthological Collection of the Oswaldo Cruz Institute (CHIOC), Rio de Janeiro, state of Rio de Janeiro, Brazil.
Taxonomic classification of the parasites followed Amin (2013)Amin OM. Classification of the Acanthocephala. Folia Parasitol (Praha) 2013; 60(4): 273-305. http://dx.doi.org/10.14411/fp.2013.031. PMid:24261131.
http://dx.doi.org/10.14411/fp.2013.031...
, while morphological identification relied on Travassos (1966)Travassos L. Serrasentis sagittifer (Linton, 1889) (Acantocephala). Mem Inst Oswaldo Cruz 1966; 64(1): 1-10., Salgado-Maldonado (1978)Salgado-Maldonado G. Acantocéfalos de peces V. Redescripción de cuatro especies de palaeacantocéfalos parásitos de peces de México. An Inst Biol Univ Nal Autón México Ser Zool 1978; 49(1): 49-70., Measures (1992)Measures LN. Bolbosoma turbinella (Acanthocephala) in a blue whale, Balaenoptera musculus, stranded in the St. Lawrence Estuary, Quebec. J Helminthol Soc Wash 1992; 59(2): 206-211., Pereira Junior & Neves (1993)Pereira Junior J, Neves LFM. Corynosoma australe Johnston, 1937 (Acanthocephala, Polymorphidae) em Micropogonias furnieri (Desmarest, 1823) (Perciformes, Sciaenidae) do litoral do Rio Grande do Sul. Comun Mus Cien PUCRS Ser Zool 1993; 6: 51-61. and Sardella et al. (2005)Sardella NH, Mattiucci S, Timi JT, Bastida RO, Rodríguez DH, Nascetti G. Corynosoma australe Johnston, 1937 and C. cetaceum Johnston & Best, 1942 (Acanthocephala: Polymorphidae) from marine mammals and fishes in Argentinian waters: allozyme markers and taxonomic status. Syst Parasitol 2005; 61(2): 143-156. http://dx.doi.org/10.1007/s11230-005-3131-0. PMid:15980967.
http://dx.doi.org/10.1007/s11230-005-313...
. Morphometric analysis was conducted using bright-field microscopy with an Olympus BX 41 microscope. Measurements were made in millimeters and are given as ranges followed by means in parentheses. Whole-mounted samples were photographed using an Axiophot Zeiss microscope with a micrographic system, while drawings were made with the aid of a drawing tube. Some specimens were processed for scanning electron microscopy (SEM) following Lopes Torres et al. (2013)Lopes Torres EJ, Souza W, Miranda K. Comparative analysis of Trichuris muris surface using conventional, low vacuum, environmental and field emission scanning electron microscopy. Vet Parasitol 2013; 196(3-4): 409-416. http://dx.doi.org/10.1016/j.vetpar.2013.02.026. PMid:23537947.
http://dx.doi.org/10.1016/j.vetpar.2013....
, which involved fixation in 70% ethanol, dehydration in an ethanol series (70% - 100%), critical-point drying in CO2, and coating in gold. The material was examined and photographed using a JEOL SM-25 SII SEM or a Zeiss 962 SEM, under an acceleration voltage of 15 kvolts.
Ecological terminology was according to Bush et al. (1997)Bush AO, Lafferty K, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 1997; 83(4): 575-583. http://dx.doi.org/10.2307/3284227. PMid:9267395.
http://dx.doi.org/10.2307/3284227...
, as follows: prevalence, intensity, mean intensty, abundance, mean abundance, and range of infection.
Since all acanthocephalans were fixed in AFA and preserved in ethanol 70º GL, the specimens used for genetic analysis were rinsed in a 0.65% NaCl solution, identified morphologically using a stereomicroscope and preserved at -20°C until DNA extraction. Serrasentis sp. (n=3), Bolbosoma sp. (n=20), and Corynosoma sp. (n=8) were submitted to genetic analysis. Samples were ground under the presence of liquid nitrogen prior to DNA extraction, which was performed using a QIAamp® DNA Mini Kit (QIAGEN, Hilden, Germany) (IÑIGUEZ et al., 2011Iñiguez AM, Carvalho VL, Motta MR, Pinheiro DC, Vicente AC. Genetic analysis of Anisakis typica (Nematoda: Anisakidae) from cetaceans of the northeast coast of Brazil: new data on its definitive hosts. Vet Parasitol 2011; 178(3-4): 293-299. http://dx.doi.org/10.1016/j.vetpar.2011.01.001. PMid:21324600.
http://dx.doi.org/10.1016/j.vetpar.2011....
, 2012Iñiguez AM, Leles D, Jaeger LH, Carvalho-Costa FA, Araújo A, Amazonas Research Group. Genetic characterisation and molecular epidemiology of Ascaris spp. from humans and pigs in Brazil. Trans R Soc Trop Med Hyg 2012; 106(10): 604-612. http://dx.doi.org/10.1016/j.trstmh.2012.06.009. PMid:22944771.
http://dx.doi.org/10.1016/j.trstmh.2012....
). PCR was performed targeting the complete nuclear internal transcribed spacer region (ITS), including ITS-1, the 5.8S rDNA gene, and ITS-2, and the cytocrome c oxidase subunit I (cox1) gene. PCR primers for ITS and the conditions for amplification of ~800bp are the same as those described in Zhu et al. (1999)Zhu X, Chilton NB, Jacobs DE, Boes J, Gasser RB. Characterisation of Ascaris from human and pig hosts by nuclear ribosomal DNA sequences. Int J Parasitol 1999; 29(3): 469-478. http://dx.doi.org/10.1016/S0020-7519(98)00226-4. PMid:10333331.
http://dx.doi.org/10.1016/S0020-7519(98)...
and Knoff et al. (2012)Knoff M, Felizardo NN, Iñiguez AM, Maldonado A Jr, Torres EJ, Pinto RM, et al. Genetic and morphological characterisation of a new species of the genus Hysterothylacium (Nematoda) from Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) of the Neotropical Region, State of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 2012; 107(2): 186-193. http://dx.doi.org/10.1590/S0074-02762012000200006. PMid:22415256.
http://dx.doi.org/10.1590/S0074-02762012...
, respectively. PCR of cox1 was performed targeting a 655bp fragment using the primers LCO1490 (5’-GGTCAACAAATCATAAAGATATTGG-3’) and HCO2198 (5’-TAAACTTCAGGGT GACCAAAAAATCA-3’), as described in Folmer et al. (1994)Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 1994; 3(5): 294-299. PMid:7881515.. Reactions were performed with a total volume of 50µl, comprising 10mM Tris–HCl (pH 8.0), 50mM KCl, 1.5mM MgCl2, 0.2mM of each dNTP, 400 ng of each primer, 1.5U Platinum Taq polymerase (Invitrogen), and 50 ng of genomic DNA. The PCR reactions were subjected to an initial cycle of 3min at 96º C, followed by 35 cycles at 96 °C for 1min, 56 °C for 1min, and 72 °C for 1min in a programmable thermal controller (PTC100 60v, MJ Research, Inc). PCR products were analyzed by electrophoresis, in 1.2% agarose gels for ITS and 1.8% agarose gels for cox1, and then visualized under UV light after staining with ethidium bromide. The amplicons were directly sequenced using Big Dye Terminator v 3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems) in a 3100 Automated DNA Sequencer, as recommended by the suppliers. Sequencing analysis was performed using the global Basic Local Alignment Search Tool (National Center for Biotechnology Information database) and BioEdit v7.0.4.1 (Department of Microbiology, North Carolina State University, USA) (HALL, 1999Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 1999; 41: 95-98.). Sequences obtained in this study were compared with all acanthocephalan sequences available in GenBank for both targets. ITS datasets were constructed with all reference sequences available in Genbank (10/2018) for the genera Bolbosoma, Corynosoma and Polymorphus. The cox1 dataset contained all available sequences in Genbank (10/2018) for the genera Bolbosoma and Corynosoma. Hexaglandula corynosoma was used as an outgroup. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 7 (TAMURA et al., 2013Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 2013; 30(12): 2725-2729. http://dx.doi.org/10.1093/molbev/mst197. PMid:24132122.
http://dx.doi.org/10.1093/molbev/mst197...
). Phylogenetic trees were constructed using the Neighbor-joining (NJ) method, the Kimura-2-Parameters (K2P) model, and following the CBOL barcoding protocol (http://www.barcodeoflife.org/content/resources/ standards-and-guidelines). The pairwise deletion parameter was applied with 1000 bootstrap replicates. Maximum likelihood (ML) trees were constructed using the best-fitting model of DNA substitution, as determined by the Bayesian information criterion (BIC).
Results
A total of 134 juvenile acanthocephalans were collected: 47 from P. isosceles, 81 from P. patagonicus and 6 from X. rasile. Paralichthys isosceles and P. patagonicus were parasitized by a species of Serrasentis, a species of Bolbosoma, and two species of Corynosoma. Xystreurys rasile was parasitized by a species of Corynosoma. The prevalence of acanthocephalans found in each species of flounders was: 48.3% for P. isosceles, 40.0% for P. patagonicus, and 10.0% for X. rasile.
Some juveniles were alive and exhibited limited motility. Acanthocephalans were taxonomically identified as below.
Rhadinorhynchidae Lühe, 1912
Serrasentis Van Cleave, 1923
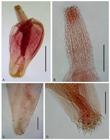
Serrasentis sagittifer (Linton, 1889) Van Cleave, 1923 (Figures 1, 2 -3)
Juveniles of Serrasentis sagittifer in Paralichthys isosceles. A: entire female in lateral view; B: detail of vagina in ventral view; C: hooks of proboscis and sensory papilla; D: posterior portion of male in lateral view. Abreviations: at = anterior testis, bc = bursa copulatrix, c = cirrus, cg = cement glands, fc = first comb, l = leminisci, lc = last comb, n = neck, p = proboscis, pr = proboscis receptacle, pt = posterior testis, rc = receptacle cement, sp = sensory papilla, Sp = Saefftigen’s pouch, sv = seminal vesicle, ts = trunk spines, u = uterus, v = vagina, vb = vagina bulb, vf = vagina funnel, vs = vagina sphincther. Bars A and D = 0.4 mm, B = 0.1 mm, and C = 0.2 mm.
Juvenile of Serrasentis sagittifer in Paralichthys patagonicus. A: entire male in lateral view; B: detail of proboscis and neck. Bars A and B = 0.4 mm.
Juveniles of Serrasentis sagittifer in Paralichthys isosceles under SEM. A: proboscis, neck and anterior trunk spines. B: detail of trunk combs. Bars: A = 200 µm and B = 100 µm.
Features observed in 19 juveniles, 8 from P. isosceles, and 11 from P. patagonicus. Body elongate and cylindrical. Anterior extremity with hooks forming longitudinally-curved U-shaped combs on the ventral and lateral surfaces. Proboscis claviform, armed with 22-24 longitudinal hook rows each with 16-17 hooks. Neck short, conical. Proboscis hooks thicker ventrally than dorsally, decreasing in size from apex to base. Proboscis receptacle with thick double wall. Pair of extremely long leminisci extend for half the length of the trunk. Small sensory papilla present on each side of the base of proboscis. Trunk elongate, with 24-28 longitudinal rows of 8-9 spines on anterior extremity, followed posteriorly by transversal rows of combs of spines (18-20 in males and 18-22 in females) occupying half of the length of the trunk on the ventral surface. Male genital apparatus: two ovoid testes in tandem; four tubular cement glands; cement receptacle linked to elongate Saefftigen’s pouch; pyriform seminal vesicle; and spiny cirrus inside bursa copulatrix. Female genital apparatus: ovarium balls; uterine bell; uterus straight; and muscular vagina divided into funnel, sphincter, and bulb.
Morphometric parameters are shown in Table 1.
Morphometric data of juveniles of Serrasentis sagittifer collected from Paralichthys isosceles and Paralichthys patagonicus in the state of Rio de Janeiro, Brazil.
Hosts: P. isosceles and P. patagonicus.
Parasitic indices for P. isosceles (Pi): prevalence = 20%, mean intensity = 1.67 ± 0.77, mean abundance = 0.33 ± 1.41 and range of infection = 1-3 and for P. patagonicus (Pp): prevalence = 26.7%, mean intensity = 4.37 ± 3.25, mean abundance = 1.17 ± 4.94 and range of infection = 1-11.
Infection sites for P. isosceles: intestine and for P. patagonicus: stomach and intestine.
Collected specimens for P. isosceles: 20 and for P. patagonicus: 35.
Deposited specimens: CHIOC 37828, 37829, 37830, 37831(Pi); CHIOC 38079a, b, 38080, 38081a-c, 38082, 38083 (Pp).
Polymorphidae Meyer, 1931
Bolbosoma Porta, 1908
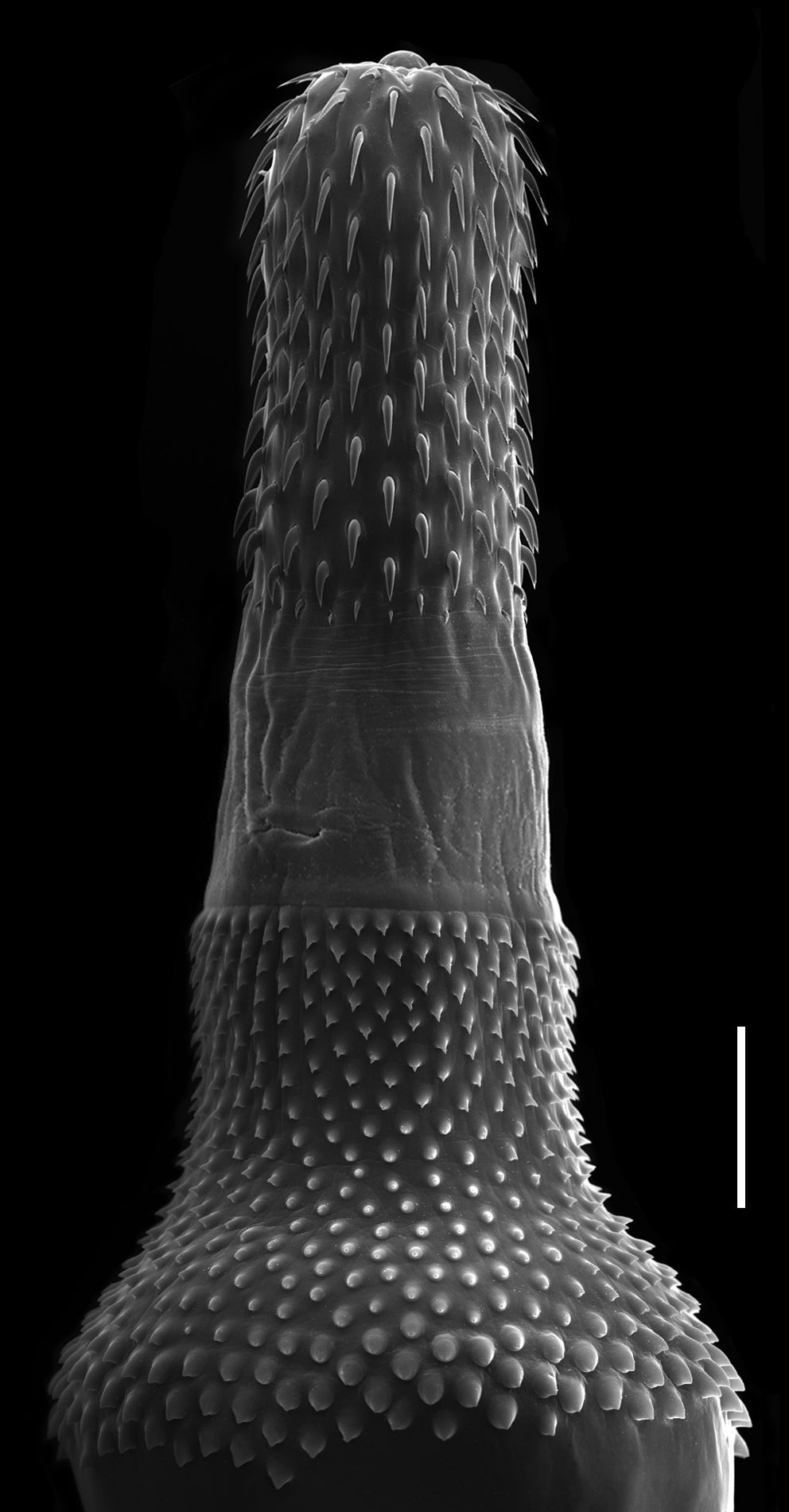
Bolbosoma turbinella (Diesing, 1851) (Figures 4, 5-6)
Juveniles of Bolbosoma turbinella in Paralichthys isosceles. A: entire female in lateral view; B: detail of vagina in ventral view; C: hooks of proboscis; D: posterior portion of male in lateral view. Abreviations: at = anterior testis, bc = bursa copulatrix, c = cirrus, cg = cement glands, l = leminisci, n = neck, ob = ovarian balls, p = proboscis, pr = proboscis receptacle, pt = posterior testis, Sp = Saefftigen’s pouch, sv = seminal vesicle, ts = trunk spines, u = uterus, ub = uterine bell, v = vagina, vb = vagina bulb, vf = vagina funnel, vs = vagina sphincter. Bars A = 0.8 mm, B and D = 0.4 mm, and C = 0.2 mm.
Juvenile female of Bolbosoma turbinella in Paralichthys patagonicus. Detail of vagina in ventral view. Abreviations: vb = vagina bulb, vf = vagina funnel, vs = vagina sphincter. Bar = 0.1 mm.
Juvenile of Bolbosoma turbinella in Paralichthys isosceles under SEM. Proboscis, neck and trunk spines. Bar = 200 µm.
Features observed in 12 juveniles, 8 from P. isosceles, and 4 from P. patagonicus. Body elongate, anterior region dilated, proboscis cylindrical and armed with 20-22 longitudinal rows of 7-8 hooks each. Neck short, wider at posterior end, without hooks. Bulb flattened with trunk spines arranged in 38-40 longitudinal rows with 21-22 hooks each, extending dorsally and ventrally covering about half of the proboscis receptacle; spines smaller near base of neck. Pair of slightly convoluted leminisci of similar length present. Male genital apparatus: testes ovoid, in line; four tubular cement glands followed by elongate Saefftigen’s pouch; seminal vesicle ovoid; and cirrus inside bursa copulatrix. Female genital apparatus: ovarian balls; uterine bell elongate; uterus straight; and muscular vagina divided into funnel, sphincter, and bulb.
Morphometric parameters are shown in Table 2.
Morphometric data of juveniles of Bolbosoma turbinella collected from Paralichthys isosceles and Paralichthys patagonicus in the state of Rio de Janeiro, Brazil.
Hosts: P. isosceles and P. patagonicus.
Parasitic indices for P. isosceles (Pi): prevalence = 13.3%, mean intensity = 2.00 ± 1.21, mean abundance = 0.27 ± 2.12 and range of infection = 1-4 and for P. patagonicus (Pp): prevalence = 13.3%, mean intensity = 2.50 ± 1.29, mean abundance = 0.33 ± 2.12 and range of infection = 1-4.
Infection sites for P. isosceles: stomach and intestine and for P. patagonicus: intestine.
Collected specimens for P. isosceles: 16 and for P. patagonicus: 10.
Deposited specimens: CHIOC 37832, 37833, 37834, 37835 (Pi); CHIOC 38084, 37988, 37989, 37990 (Pp).
Corynosoma Lühe, 1904
Corynosoma australe Johnston, 1937 (Figures 7-8)
Juveniles of Corynosoma australe in Paralichthys patagonicus. A: entire female in lateral view; B: detail of female proboscis; C: posterior end of female in lateral view; D: posterior end of male in ventral view. Bars A = 1 mm, B = 0.25 mm, C = 0.2 mm, and D = 0.1 mm.
Juvenile of Corynosoma australe in Paralichthys patagonicus under SEM. A: entire male in dorsolateral view; B: detail of male proboscis in dorsolateral view; C: posterior portion of male with detail of genital spines in dorsolateral view. Bars A = 200 µm, B = 100 µm, C = 40 µm.
Features observed in 14 juveniles, 1 from P. isosceles, 11 from P. patagonicus and 2 from X. rasile. Body pyriform, anterior region dilated and proboscis cylindrical. Proboscis wider at posterior end, armed with 18 longitudinal rows of 12-14 hooks each; 9-11 anterior hooks with well developed posteriorly directed roots and three small basal hooks with small anteriorly directed roots. Neck short, wider at posterior end. Anterior portion of trunk swollen and flattened in form of a bulb. Proboscis receptacle double-walled. Tegumental spines covering bulb dorsally at fore-trunk, extending to the ventral surface at the posterior end. Pair of moderately short, lobe-like leminisci reaching half the length of proboscis receptacle. In some female specimens, genital and tegumental spines are contiguous in the ventral region, but clearly distinguishable. Genital opening surrounded by triangular genital spines that are larger than tegumental spines. Male genital apparatus: testes rounded, contiguous; six claviform cement glands, followed by elongate Saefftigen’s pouch; elongate seminal vesicle; and bursa copulatrix. Female genital apparatus: ovarian balls; uterine bell elongate; uterus straight; and muscular vagina divided into funnel, sphincter, and bulb. Male genital spines show a distinctive radial distribution pattern with 14 spines on each side of the opening of the bursa copulatrix, and one central spine, called “c”, on the dorsal surface. Some spines are bifid, such as observed in 1 and 1’. Female genital spines are located on the ventral side and are smaller than those of males.
Morphometric parameters are shown in Table 3.
Morphometric data of juveniles of Corynosoma australe collected from Paralichthys isosceles, Paralichthys patagonicus and Xystreurys rasile in the state of Rio de Janeiro, Brazil.
Hosts: P. isosceles, P. patagonicus and X. rasile.
Parasitic indices for P. isosceles (Pi): prevalence = 5%, mean intensity = 3.33 ± 1.52, mean abundance = 0.17 ± 2.82, range of infection = 1-5; for P. patagonicus (Pp): prevalence = 16.7%, mean intensity = 3.60 ± 2.56, mean abundance = 0.60 ± 3.53 and range of infection = 1-7 and for X. rasile (Xr): prevalence = 10%, mean intensity = 2.00 ± 0.74, mean abundance = 0.20 ± 1.41 and range of infection = 1-4.
Infection sites for P. isosceles: stomach and intestine; and for P. patagonicus and X. rasile: intestine.
Collected specimens for P. isosceles: 10, for P. patagonicus: 18 and for X. rasile: 6.
Deposited specimens: CHIOC 37836b (Pi); CHIOC 37172, 37837, 37964, 38048a-b, 38049a-b, 38050a-c (Pp); 38055, 38056 (Xr).
Corynosoma cetaceum (Johnston & Best, 1942) (Figure 9)
Juveniles of Corynosoma cetaceum in Paralichthys patagonicus. A: entire male in lateral view; B: proboscis, neck and trunk spines in lateral view; C: posterior end of female in lateral view. Brackets indicate the two ventral trunk folds that are devoid of spines, in lateral view; D: posterior end of male with detail of genital spine in lateral view. Bars A = 1 mm, B = 0.5 mm, C = 0.4 mm, and D = 0.1 mm.
Features observed in 13 juveniles, 1 from P. isosceles, and 12 from P. patagonicus. Body pyriform, anterior region dilated and proboscis cylindrical. Proboscis wider at posterior end, armed with 18-20 longitudinal rows of 15-16 hooks each; 12-13 anterior hooks with well-developed posteriorly-directed roots and 2-3 (usually 2) small basal hooks with small anteriorly directed roots. Neck short, wider at posterior end. Anterior portion of trunk swollen and flattened in form of a bulb. In females, fore-trunk and hind-trunk delimited by a typical ventral fold; in males, fold delimiting fore-trunk and hind-trunk is more superficial and not always discernible. Proboscis receptacle double-walled. Tegumental spines covering bulb dorsally for the length of the fore-trunk; in males, these spines extend ventrally covering 60-75% of trunk length; in females, these spines extend ventrally covering 97-98% of trunk length, and the trunk possesses two ventral transverse folds, that are devoid of spines, and delimit a blunt lobe between fore- and hind-trunk. A pair of moderately short, lobe-like leminisci extend over half of the proboscis receptacle. Genital spines absent in both sexes; less frequently males possess a unique, clearly distinguishable genital spine. Male genital apparatus: testes round, contiguous; six claviform cement glands, followed by elongate Saefftigen’s pouch; seminal vesicle elongate; and bursa copulatrix. Female genital apparatus: ovarian balls; uterine bell elongate; uterus straight; and a muscular vagina divided into funnel, sphincter, and bulb.
Morphometric parameters are shown in Table 4.
Morphometric data of juveniles of Corynosoma cetaceum, collected from Paralichthys isosceles and Paralichthys patagonicus in the state of Rio de Janeiro, Brazil.
Hosts: P. isosceles and P. patagonicus.
Parasitic indices for P. isosceles (Pi): prevalece = 1.67%, intensity = 1.00, abundance = 0.02 and for P. patagonicus (Pp): prevalence = 26.7%, mean intensity = 2.25 ± 1.76, mean abundance = 0.60 ± 5.65 and range of infection = 1-6.
Infection sites for P. isosceles and P. patagonicus: intestine.
Collected specimens for P. isosceles: 1 and for P. patagonicus: 18.
Deposited specimens: CHIOC 37836a (Pi); CHIOC 38051, 38052a-f, 38053, 38054 (Pp).
Genetic analysis. Of the acanthocephalan specimens submitted to genetic analysis, four specimens of Bolbosoma sp. and one specimen of Corynosoma sp. yielded DNA sequences: three specimens of Bolbosoma sp. and the one of Corynosoma sp. for the ITS region and two of Bolbosoma sp. and the one of Corynosoma sp. for the cox1 gene.
An ITS dataset (843bp) was constructed with all the reference sequences available for the genera Bolbosoma, Corynosoma and Polymorphus, and the Bolbosoma sp. samples HE14, HE23, and HE25 (ITS Dataset I). In order to include the short sequence recovered from Corynosoma sp. (the HE38 sample; 574pb) in the ITS phylogenetic analysis, the dataset was shortened and named ITS Dataset II. The cox1 dataset contained reference sequences and the sequences from Bolbosoma sp. and Corynosoma sp. (samples HE25, HE38 and HE43). The best-fitting model of DNA substitution using BIC was the Tamura 3-parameter model (TN92) with the gamma distribution (+G) for ITS Dataset I; K2P+G for ITS Dataset II; and Hasegawa-Kishino-Yano model (HKY) plus gamma distribution and invariable sites (+G+I) for the cox1 dataset. Nucleotide sequences were deposited in GenBank with the accession numbers KU314817-KU314823.
Using the ITS Dataset I, phylogenetic trees based on NJ K2P and ML TN92+G (Figure 10A) confirmed the identification of Bolbosoma sp. The three specimens grouped with the two available ITS sequences for Bolbosoma capitatum and Bolbosa nipponicum with high bootstrap values (98% and 99%, for NJ and ML analyses, respectively). Using the ITS Dataset II (NJ K2P and ML K2P), one specimen (HE38) of Corynosoma sp. (Figure 10B), was included in the genus-specific cluster (NJ = 73%, ML = 76%), as closely related to the C. australe and C. bullosum subcluster (NJ = 88%, ML = 93%). Both datasets, and both mehtods used, agreed in showing the genera Bolbosoma and Corynosoma as monophyletic and closely related (NJ = 100%, ML = 99/98%). The only exception was when the ML method was applied since C. cetaceum (AF286310) did not group within the Corynosoma genus cluster, but instead was placed as an outgroup basal to Bolbosoma + Corynosoma. The phylogenetic trees produced by NJ K2P and ML HKY+G+I using the cox1 data set revealed the same topology (Figure 11). The specimen HE38 grouped within the C. australe cluster with maximun bootstrap values for both NJ and ML. In addition, two Bolbosoma sp. sequences (HE25 and HE43) clustered with the two Bolbosoma sp. cox1 sequences available (NJ = 94%, ML= 97%), and robustly with B. turbinella with maximun bootstrap value (NJ/ML = 100%) (Figure 11).
Maximum likelihood (ML) phylogenetic analyses of ITS sequence data. A: ITS Dataset I including sequences HE14, HE23 and HE25 from this study and GenBank reference sequences (species and accession numbers are shown) using the TN92+G model; B: ITS Dataset II including sequence HE14, HE23, HE25, HE38 from this study and GenBank reference sequences using the K2P+G model. Numbers at nodes are bootstrap support values, when higher than 50%. Regular numbers correspond to ML while italicized numbers correspond to Neighbor-joining bootstraps. Bars represent the expected number of substitutions per nucleotide.
Maximum likelihood (ML) phylogenetic analysis of cox1 gene sequences, including sequences HE25, HE38 and HE43 from this study and GenBank reference sequences. Numbers at nodes are bootstrap support values, when higher than 50%. Regular numbers correspond to ML analysis with the HKY model and G+I parameters, while italicized numbers correspond to Neighbor-joining analysis with the K2P model. Bar represents the expected number of substitutions per nucleotide.
Discussion
The acanthocephans identified as S. sagittifer in the present study, morphologically resemble those previously recorded as immature and adult specimens of this parasite from several teleost species from various regions of the world (AL-ZUBAIDY & MHAISEN, 2012Al-Zubaidy AB, Mhaisen FT. A record of two species of Acanthocephala (Echinorhynchida: Rhadinorhynchidae) from Red Sea fishes, Yemeni coastal waters. Mesop J Mar Sci 2012; 27(1): 15-28.; ABDEL-GHAFFAR et al., 2014Abdel-Ghaffar F, Morsy K, Abdel-Gaber R, Mehlhorn H, Al Quraishy S, Mohammed S. Prevalence, morphology, and molecular analysis of Serrasentis sagittifer (Acanthocephala: Palaeacanthocephala: Rhadinorhynchidae), a parasite of gilthead Sea bream Sparus aurata (Sparidae). Parasitol Res 2014; 113(7): 2445-2454. http://dx.doi.org/10.1007/s00436-014-3889-6. PMid:24828344.
http://dx.doi.org/10.1007/s00436-014-388...
; MOHAMADAIN & ADEL, 2015Mohamadain HS, Adel A. Light and scanning electron microscopy on Serrasentis sagittifer Linton, 1889 (Acanthocephala): Palaeacanthocephala: Rhadinorhynchidae) infecting the common sea bream in Egypt. J Egypt Soc Parasitol 2015; 45(1): 23-28. http://dx.doi.org/10.12816/0010846. PMid:26012215.
http://dx.doi.org/10.12816/0010846...
). The difference in size found by the present study is likely due to the developmental stage of the parasite, while the differences found in the number of combs is likely due to intraspecific variation, as reported by Al-Zubaidy & Mhaisen (2012)Al-Zubaidy AB, Mhaisen FT. A record of two species of Acanthocephala (Echinorhynchida: Rhadinorhynchidae) from Red Sea fishes, Yemeni coastal waters. Mesop J Mar Sci 2012; 27(1): 15-28.. Therefore, as only juvenile specimens of S. sagittifer were found in the present study, the flounders are acting as paratenic hosts.
The juvenile of B. turbinella collected in the present study were identified according to previous morphological descriptions of adults collected from cetaceans, and mainly involved characters relating to proboscis hooks and the distribution of trunk spines (MEASURES, 1992Measures LN. Bolbosoma turbinella (Acanthocephala) in a blue whale, Balaenoptera musculus, stranded in the St. Lawrence Estuary, Quebec. J Helminthol Soc Wash 1992; 59(2): 206-211.). Morphometrically, these specimens were smaller then those of adult collected from Balaenoptera borealis off the coast of Rio de Janeiro, Brazil, by Machado Filho (1964)Machado Filho DA. Contribuição para o conhecimento do gênero “Bolbosoma” Porta, 1908 (Palaeacanthocephala, Polymorphidae). Rev Bras Biol 1964; 24(3): 341-348. PMid:14275360., which can be explained by them being of different ontogenetic stages.
The morphological and morphometric characters of the C. australe specimens collected in the present study are in accordance with previous descriptions reported for specimens from Brazilian and Argentinean fish hosts (KNOFF et al., 2001Knoff M, São Clemente SC, Pinto RM, Gomes DC. Digenea and Acanthocephala of elasmobranch fishes from the Southern Coast of Brazil. Mem Inst Oswaldo Cruz 2001; 96(8): 1095-1101. http://dx.doi.org/10.1590/S0074-02762001000800012. PMid:11784929.
http://dx.doi.org/10.1590/S0074-02762001...
; SARDELLA et al., 2005Sardella NH, Mattiucci S, Timi JT, Bastida RO, Rodríguez DH, Nascetti G. Corynosoma australe Johnston, 1937 and C. cetaceum Johnston & Best, 1942 (Acanthocephala: Polymorphidae) from marine mammals and fishes in Argentinian waters: allozyme markers and taxonomic status. Syst Parasitol 2005; 61(2): 143-156. http://dx.doi.org/10.1007/s11230-005-3131-0. PMid:15980967.
http://dx.doi.org/10.1007/s11230-005-313...
; AZNAR et al., 2016Aznar FJ, Crespo EA, Raga JA, Hernández-Orts J. Trunk spines in cystacanths and adults of Corynosoma spp. (Acanthocephala): Corynosoma cetaceum as an exceptional case of phenotypic variability. Zoomorphology 2016; 135(1): 19-31. http://dx.doi.org/10.1007/s00435-015-0290-7.
http://dx.doi.org/10.1007/s00435-015-029...
). According to Sardella et al. (2005)Sardella NH, Mattiucci S, Timi JT, Bastida RO, Rodríguez DH, Nascetti G. Corynosoma australe Johnston, 1937 and C. cetaceum Johnston & Best, 1942 (Acanthocephala: Polymorphidae) from marine mammals and fishes in Argentinian waters: allozyme markers and taxonomic status. Syst Parasitol 2005; 61(2): 143-156. http://dx.doi.org/10.1007/s11230-005-3131-0. PMid:15980967.
http://dx.doi.org/10.1007/s11230-005-313...
, the male genital opening is surrounded by three irregular rows of 18-34 triangular spines, while the specimens of the present study had 29 spines distributed around the male genital opening (Figure 8).
The specimens of C. cetaceum of the present study exhibited morphological and morphometric characters in agreement with the original descriptions, as well as other descriptive studies (AZNAR et al., 2002Aznar FJ, Berón-Vera B, Crespo EA, Raga JA. Presence of genital spines in a male Corynosoma cetaceum Johnston and Best, 1942 (Acanthocephala). J Parasitol 2002; 88(2): 403-404. http://dx.doi.org/10.1645/0022-3395(2002)088[0403:POGSIA]2.0.CO;2. PMid:12054020.
http://dx.doi.org/10.1645/0022-3395(2002...
, 2016Aznar FJ, Crespo EA, Raga JA, Hernández-Orts J. Trunk spines in cystacanths and adults of Corynosoma spp. (Acanthocephala): Corynosoma cetaceum as an exceptional case of phenotypic variability. Zoomorphology 2016; 135(1): 19-31. http://dx.doi.org/10.1007/s00435-015-0290-7.
http://dx.doi.org/10.1007/s00435-015-029...
; SARDELLA et al., 2005Sardella NH, Mattiucci S, Timi JT, Bastida RO, Rodríguez DH, Nascetti G. Corynosoma australe Johnston, 1937 and C. cetaceum Johnston & Best, 1942 (Acanthocephala: Polymorphidae) from marine mammals and fishes in Argentinian waters: allozyme markers and taxonomic status. Syst Parasitol 2005; 61(2): 143-156. http://dx.doi.org/10.1007/s11230-005-3131-0. PMid:15980967.
http://dx.doi.org/10.1007/s11230-005-313...
).
Alarcos & Timi (2012)Alarcos AJ, Timi JT. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 2012; 110(6): 2155-2166. http://dx.doi.org/10.1007/s00436-011-2741-5. PMid:22167375.
http://dx.doi.org/10.1007/s00436-011-274...
reported juvenile acanthocephalans from the same hosts as investigated in the present study, but from Argentina, and found only the species C. australe and C. cetaceum. In comparison with our results, C. australe of that study had higher parasite indices in P. patagonicus with prevalence of 94.12% and mean abundance of 6.35, in P. isosceles with prevalence of 92.16% and mean abundance of 14.69 and in X. rasile with prevalence of 89.58% and mean abundance of 9.23. Alarcos & Timi (2012)Alarcos AJ, Timi JT. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 2012; 110(6): 2155-2166. http://dx.doi.org/10.1007/s00436-011-2741-5. PMid:22167375.
http://dx.doi.org/10.1007/s00436-011-274...
also found C. cetaceum occurring in P. patagonicus with higher parasite indices (prevalence of 74.51% and mean abundance of 2.55). Furthermore, they found it present in X. rasile. Subsequently, Alarcos et al. (2016)Alarcos AJ, Pereira AN, Taborda NL, Luque JL, Timi JT. Parasitological evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small and geographical scales in South American Atlantic coasts. Fish Res 2016; 173(3): 221-228. http://dx.doi.org/10.1016/j.fishres.2015.07.018.
http://dx.doi.org/10.1016/j.fishres.2015...
reported juvenile acanthocephalans parasitizing P. isosceles in Rio de Janeiro, Brazil. In contrast to our results, these authors only found two acanthocephalan species: C. australe with similar parasite indices (prevalence of 7.9% and mean abundance of 0.1), and B. turbinella with higher indices for hosts collected in Cabo Frio (prevalence of 36.8% and mean abundance of 0.5) and in Niterói, state of Rio de Janeiro (prevalence of 25% and mean abundance of 0.6). The present study differed by having found the additional acanthocephalan species S. sagittifer and C. cetaceum in P. isosceles.
According to Spalding et al. (2007)Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 2007; 57(7): 573-583. http://dx.doi.org/10.1641/B570707.
http://dx.doi.org/10.1641/B570707...
, marine ecoregions have relatively homogeneous species compositions that are clearly distinct from adjacent systems. Species composition is likely determined by the predominance of a small number of ecosystems and/or a distinct suite of oceanographic or topographic features. The dominant biogeographic agents that define ecoregions vary from location to location but may include physico-chemical and biological agents. In ecological terms, these ecoregions are strongly cohesive units that are sufficiently large to encompass ecological or life history processes for most sedentary species. Although some marine ecoregions may have important levels of endemism, this is not a key determinant in ecoregion identification, as it has been for terrestrial ecoregions. Therefore, features observed among and within ecoregions can influence their fish parasite communities, and thus explain the parasite compositions of the flounder species from waters off Necochea, Argentina, by Alarcos & Timi (2012)Alarcos AJ, Timi JT. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 2012; 110(6): 2155-2166. http://dx.doi.org/10.1007/s00436-011-2741-5. PMid:22167375.
http://dx.doi.org/10.1007/s00436-011-274...
, and from waters off Rio de Janeiro, Brazil, by Alarcos et al. (2016)Alarcos AJ, Pereira AN, Taborda NL, Luque JL, Timi JT. Parasitological evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small and geographical scales in South American Atlantic coasts. Fish Res 2016; 173(3): 221-228. http://dx.doi.org/10.1016/j.fishres.2015.07.018.
http://dx.doi.org/10.1016/j.fishres.2015...
and the present study.
Serrasentis sagittifer and C. cetaceum were recently reported from paralichthyid fish at higher prevalences than those found in the present study. Barton & Smales (2015)Barton DP, Smales LR. Acanthocephalan cystacanths from flatfish (Order Pleuronectiformes) in Tropical Australian Waters. J Parasitol 2015; 101(4): 429-435. http://dx.doi.org/10.1645/15-731.1. PMid:25807200.
http://dx.doi.org/10.1645/15-731.1...
investigated six species of the genus Pseudorhombus Bleeker, 1862, from Australian waters, and found cystacants of S. cf. sagittifer (prevalecne of 36.32%) in fish from the western Gulf of Carpentaria, and S. cf. sagittifer (prevalence of 25%) and Corynosoma cetaceum (prevalence of 21.25%) in fish off the central coast of Queensland, showing that these acanthocephalan species occur in other paralichthyid fish in other parts of the world. Thus, P. isosceles and P. patagonicus are new host records for S. sagittifer, and P. patagonicus is a new host record for B. turbinella.
The parasitization of paralichthyid fish by species of Corynosoma and Bolbosoma turbinella recorded in the present study indicates that these fish are positioned at an intermediate trophic level of the marine food web where they act as paratenic hosts, as has been reported for other species of this family (FUJITA et al., 2016Fujita T, Waga E, Kitaoka K, Imagawa T, Komatsu Y, Takanashi K, et al. Human infection by acanthocephalan parasites belonging to the genus Corynosoma found from small bowel endoscopy. Parasitol Int 2016; 65(5 PtA): 491-493. http://dx.doi.org/10.1016/j.parint.2016.07.002. PMid:27396515.
http://dx.doi.org/10.1016/j.parint.2016....
), while marine mammals and birds are final hosts (HERNÁNDEZ-ORTS et al., 2017Hernández-Orts JS, Brandão M, Georgieva S, Raga JA, Crespo EA, Luque JL, et al. From mammals back to birds: Host-switch of the acanthocephalan Corynosoma australe from pinnipeds to the Magellanic penguin Spheniscus magellanicus. PLoS One 2017; 12(10): e0183809. http://dx.doi.org/10.1371/journal.pone.0183809. PMid:28981550.
http://dx.doi.org/10.1371/journal.pone.0...
).
The species of Corynosoma and Bolbosoma turbinella were found alive in the present study, which reinforces the importance of hygienic-sanitary practices because some species of these two genera are involved with zoonoses. This fact was reported by Fujita et al. (2016)Fujita T, Waga E, Kitaoka K, Imagawa T, Komatsu Y, Takanashi K, et al. Human infection by acanthocephalan parasites belonging to the genus Corynosoma found from small bowel endoscopy. Parasitol Int 2016; 65(5 PtA): 491-493. http://dx.doi.org/10.1016/j.parint.2016.07.002. PMid:27396515.
http://dx.doi.org/10.1016/j.parint.2016....
, who also commented that such infections are closely associated with eating uncooked food, and are mostly reported from Japan because of the traditional food culture there (i.e., sushi and sashimi). Even though the polymorphid acanthocephalan specimens of the present study were not found in the musculature, they can migrate there via inadequated fish cleanning, with the rupture of the walls of the intestine and stomach, and stay available for ingestion, potentially infecting consumers. As suggested by FAO/WHO (2014)Food and Agriculture Organization – FAO, World Health Organisation – WHO. Multicriteria-bases ranking for risk management of food-borne parasites. Geneva: WHO Press; 2014. (Microbiological Risk Assessment Series; no. 23). and Fujita et al. (2016)Fujita T, Waga E, Kitaoka K, Imagawa T, Komatsu Y, Takanashi K, et al. Human infection by acanthocephalan parasites belonging to the genus Corynosoma found from small bowel endoscopy. Parasitol Int 2016; 65(5 PtA): 491-493. http://dx.doi.org/10.1016/j.parint.2016.07.002. PMid:27396515.
http://dx.doi.org/10.1016/j.parint.2016....
, treatments of heating or freezing are desired for the prevention of parasite infections by these species, as is the usual case for other food-borne parasites, and recently in Brazil (PORTO ALEGRE, 2016Porto Alegre. Portaria SMS nº 1109 de 23/08/2016. Norma Municipal - Porto Alegre – RS. Aprova as exigências mínimas para produção, preparo e comercialização de sushis e sashimis no Município de Porto Alegre. Normas Brasil [online], ago. 2016 [cited 2019 March 13]. Available from: http://www.normasbrasil.com.br/norma/ portaria-1109-2016-porto-alegre-rs_327789.html
http://www.normasbrasil.com.br/norma/ ...
), the minimum requirements for the production, preparation and commercialization of sushis and sashimis in the city of Porto Alegre (Brazil) were published. Therefore, future studies should be conducted to evaluate the zoonotic potential of the polymorphid species analyzed in the present study.
Acknowledgements
The authors would like to thank Heloisa Nogueira Diniz and Ricardo Baptista Schmidt (Serviço de Produção e Tratamento de Imagens do Instituto Oswaldo Cruz/FIOCRUZ) for processing the figures. This work was supported by CNPq fellowships (AMI: 307932/2014-1 and 312934/2017-3; SCSC: 308048/2013-0; MCGF: 140093/2012-5 and 150140/2018-5); a FAPERJ grant (AMI: 26/202.945/2016) and a CAPES grant (MINDA: ECM09/2009).
References
- Abdel-Ghaffar F, Morsy K, Abdel-Gaber R, Mehlhorn H, Al Quraishy S, Mohammed S. Prevalence, morphology, and molecular analysis of Serrasentis sagittifer (Acanthocephala: Palaeacanthocephala: Rhadinorhynchidae), a parasite of gilthead Sea bream Sparus aurata (Sparidae). Parasitol Res 2014; 113(7): 2445-2454. http://dx.doi.org/10.1007/s00436-014-3889-6 PMid:24828344.
» http://dx.doi.org/10.1007/s00436-014-3889-6 - Acha PN, Szyfres B. Zoonoses and communicable diseases common to man and animals 3rd ed. Washington: PAHO; 2003. (Scientific and Technical Publication; no. 580; Parasitoses; vol. III).
- Alarcos AJ, Pereira AN, Taborda NL, Luque JL, Timi JT. Parasitological evidence of stocks of Paralichthys isosceles (Pleuronectiformes: Paralichthyidae) at small and geographical scales in South American Atlantic coasts. Fish Res 2016; 173(3): 221-228. http://dx.doi.org/10.1016/j.fishres.2015.07.018
» http://dx.doi.org/10.1016/j.fishres.2015.07.018 - Alarcos AJ, Timi JT. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 2012; 110(6): 2155-2166. http://dx.doi.org/10.1007/s00436-011-2741-5 PMid:22167375.
» http://dx.doi.org/10.1007/s00436-011-2741-5 - Al-Zubaidy AB, Mhaisen FT. A record of two species of Acanthocephala (Echinorhynchida: Rhadinorhynchidae) from Red Sea fishes, Yemeni coastal waters. Mesop J Mar Sci 2012; 27(1): 15-28.
- Amin OM. Classification of the Acanthocephala. Folia Parasitol (Praha) 2013; 60(4): 273-305. http://dx.doi.org/10.14411/fp.2013.031 PMid:24261131.
» http://dx.doi.org/10.14411/fp.2013.031 - Araújo JN, Haimovice M. Determinação de idades e crescimento do linguado branco Paralichthys patagonicus (Jordan, 1889) no sul do Brasil. Rev Bras Oceanogr 2000; 48(1): 61-70. http://dx.doi.org/10.1590/S1413-77392000000100005
» http://dx.doi.org/10.1590/S1413-77392000000100005 - Arizono N, Kuramochi T, Kagei N. Molecular and histological identification of the acanthocephalan Bolbosoma cf. capitatum from the human small intestine. Parasitol Int 2012; 61(4): 715-718. http://dx.doi.org/10.1016/j.parint.2012.05.011 PMid:22634485.
» http://dx.doi.org/10.1016/j.parint.2012.05.011 - Aznar FJ, Berón-Vera B, Crespo EA, Raga JA. Presence of genital spines in a male Corynosoma cetaceum Johnston and Best, 1942 (Acanthocephala). J Parasitol 2002; 88(2): 403-404. http://dx.doi.org/10.1645/0022-3395(2002)088[0403:POGSIA]2.0.CO;2 PMid:12054020.
» http://dx.doi.org/10.1645/0022-3395(2002)088[0403:POGSIA]2.0.CO;2 - Aznar FJ, Crespo EA, Raga JA, Hernández-Orts J. Trunk spines in cystacanths and adults of Corynosoma spp. (Acanthocephala): Corynosoma cetaceum as an exceptional case of phenotypic variability. Zoomorphology 2016; 135(1): 19-31. http://dx.doi.org/10.1007/s00435-015-0290-7
» http://dx.doi.org/10.1007/s00435-015-0290-7 - Barton DP, Smales LR. Acanthocephalan cystacanths from flatfish (Order Pleuronectiformes) in Tropical Australian Waters. J Parasitol 2015; 101(4): 429-435. http://dx.doi.org/10.1645/15-731.1 PMid:25807200.
» http://dx.doi.org/10.1645/15-731.1 - Bernardes RA, Figueiredo JL, Rodrigues AR, Fischer LG, Vooren CM, Haimovici M, et al. Peixes da zona econômica exclusiva da região sudeste-sul do Brasil: Levantamento com armadilhas, pargueiras e rede de arrasto de fundo São Paulo: Editora da Universidade de São Paulo; 2005.
- Bush AO, Lafferty K, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 1997; 83(4): 575-583. http://dx.doi.org/10.2307/3284227 PMid:9267395.
» http://dx.doi.org/10.2307/3284227 - Felizardo NN, Justo MC, Knoff M, Fonseca MCG, Pinto RM, Gomes DC. Juvenile didymozoids of the types, Torticaecum and Neotorticaecum (Didymozoidae: Digenea), from new marine fish hosts (Pisces: Teleostei) in the Neotropical region of Brazil. J Helminthol 2011; 85(3): 270-275. http://dx.doi.org/10.1017/S0022149X10000507 PMid:20854704.
» http://dx.doi.org/10.1017/S0022149X10000507 - Felizardo NN, Knoff M, Pinto RM, Gomes DC. Larval anisakid nematodes of the flounder, Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) from Brazil. Neotrop Helminthol 2009a; 3(2): 57-64.
- Felizardo NN, Menezes RC, Tortelly R, Knoff M, Pinto RM, Gomes DC. Larvae of Hysterothylacium sp. (Nematoda: Anisakidae) in the sole fish Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) from the littoral of the state of Rio de Janeiro, Brazil. Vet Parasitol 2009b; 166(1-2): 175-177. http://dx.doi.org/10.1016/j.vetpar.2009.08.004 PMid:19713041.
» http://dx.doi.org/10.1016/j.vetpar.2009.08.004 - Felizardo NN, Torres EJL, Fonseca MCG, Pinto RM, Gomes DC, Knoff M. Cestodes of the flounder Paralichthys isosceles Jordan, 1890 (Osteichthyes - Paralichthyidae) from the state of Rio de Janeiro, Brazil. Neotrop Helminthol 2010; 4(2): 113-126.
- Figueiredo JL, Menezes NA. Manual de peixes marinhos do Sudeste do Brasil São Paulo: Universidade de São Paulo, Museu de Zoologia; 2000. (VI Teleostei; no. 5).
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 1994; 3(5): 294-299. PMid:7881515.
- Fonseca MC, Knoff M, Felizardo NN, Di Azevedo MI, Torres EJ, Gomes DC, et al. Integrative taxonomy of Anisakidae and Raphidascarididae (Nematoda) in Paralichthys patagonicus and Xystreurys rasile (Pisces: Teleostei) from Brazil. Int J Food Microbiol 2016; 235(20): 113-124. http://dx.doi.org/10.1016/j.ijfoodmicro.2016.07.026 PMid:27491056.
» http://dx.doi.org/10.1016/j.ijfoodmicro.2016.07.026 - Fonseca MCG, São Clemente SC, Felizardo NN, Gomes DC, Knoff M. Trypanorhyncha cestodes of hygienic-sanitary importance infecting flounders Paralichthys patagonicus Jordan, 1889 and Xystreurys rasile (Jordan, 1891) of the Neotropical region, Brazil. Parasitol Res 2012; 111(2): 865-874. http://dx.doi.org/10.1007/s00436-012-2912-z PMid:22488201.
» http://dx.doi.org/10.1007/s00436-012-2912-z - Food and Agriculture Organization – FAO, World Health Organisation – WHO. Multicriteria-bases ranking for risk management of food-borne parasites Geneva: WHO Press; 2014. (Microbiological Risk Assessment Series; no. 23).
- Fujita T, Waga E, Kitaoka K, Imagawa T, Komatsu Y, Takanashi K, et al. Human infection by acanthocephalan parasites belonging to the genus Corynosoma found from small bowel endoscopy. Parasitol Int 2016; 65(5 PtA): 491-493. http://dx.doi.org/10.1016/j.parint.2016.07.002 PMid:27396515.
» http://dx.doi.org/10.1016/j.parint.2016.07.002 - Golvan YJ. Systematique des Acanthocephales (Acanthocephala Rudolphi, 1801). L’ordre des Paleacanthocephala Meyer, 1931. La super-familie des Echinorhynchoidea (Cobbold, 1876) Golvan et Houin, 1963. Mem Mus Natl Hist Nat Paris Ser A Zool 1969; 57(single): 1-373.
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 1999; 41: 95-98.
- Hernández-Orts JS, Brandão M, Georgieva S, Raga JA, Crespo EA, Luque JL, et al. From mammals back to birds: Host-switch of the acanthocephalan Corynosoma australe from pinnipeds to the Magellanic penguin Spheniscus magellanicus. PLoS One 2017; 12(10): e0183809. http://dx.doi.org/10.1371/journal.pone.0183809 PMid:28981550.
» http://dx.doi.org/10.1371/journal.pone.0183809 - Iñiguez AM, Carvalho VL, Motta MR, Pinheiro DC, Vicente AC. Genetic analysis of Anisakis typica (Nematoda: Anisakidae) from cetaceans of the northeast coast of Brazil: new data on its definitive hosts. Vet Parasitol 2011; 178(3-4): 293-299. http://dx.doi.org/10.1016/j.vetpar.2011.01.001 PMid:21324600.
» http://dx.doi.org/10.1016/j.vetpar.2011.01.001 - Iñiguez AM, Leles D, Jaeger LH, Carvalho-Costa FA, Araújo A, Amazonas Research Group. Genetic characterisation and molecular epidemiology of Ascaris spp. from humans and pigs in Brazil. Trans R Soc Trop Med Hyg 2012; 106(10): 604-612. http://dx.doi.org/10.1016/j.trstmh.2012.06.009 PMid:22944771.
» http://dx.doi.org/10.1016/j.trstmh.2012.06.009 - Knoff M, Felizardo NN, Iñiguez AM, Maldonado A Jr, Torres EJ, Pinto RM, et al. Genetic and morphological characterisation of a new species of the genus Hysterothylacium (Nematoda) from Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) of the Neotropical Region, State of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 2012; 107(2): 186-193. http://dx.doi.org/10.1590/S0074-02762012000200006 PMid:22415256.
» http://dx.doi.org/10.1590/S0074-02762012000200006 - Knoff M, Gomes DC. Metodologia básica para coleta e o processamento de helmintos parasitos. In: Molinaro EM, Caputo LFG, Amendoeira MRR, editores. Conceitos e métodos para a formação de profissionais em laboratórios de saúde vol. 5. Rio de Janeiro: EPSJV; 2012. p. 251-281.
- Knoff M, São Clemente SC, Pinto RM, Gomes DC. Digenea and Acanthocephala of elasmobranch fishes from the Southern Coast of Brazil. Mem Inst Oswaldo Cruz 2001; 96(8): 1095-1101. http://dx.doi.org/10.1590/S0074-02762001000800012 PMid:11784929.
» http://dx.doi.org/10.1590/S0074-02762001000800012 - Lopes Torres EJ, Souza W, Miranda K. Comparative analysis of Trichuris muris surface using conventional, low vacuum, environmental and field emission scanning electron microscopy. Vet Parasitol 2013; 196(3-4): 409-416. http://dx.doi.org/10.1016/j.vetpar.2013.02.026 PMid:23537947.
» http://dx.doi.org/10.1016/j.vetpar.2013.02.026 - Machado Filho DA. Contribuição para o conhecimento do gênero “Bolbosoma” Porta, 1908 (Palaeacanthocephala, Polymorphidae). Rev Bras Biol 1964; 24(3): 341-348. PMid:14275360.
- Massa AE, Palacios DL, Paredi ME, Crupkin M. Postmortem changes in quality indices of ice-stored flounder (Paralichthys patagonicus). J Food Biochem 2005; 29(5): 570-590. http://dx.doi.org/10.1111/j.1745-4514.2005.00050.x
» http://dx.doi.org/10.1111/j.1745-4514.2005.00050.x - Measures LN. Bolbosoma turbinella (Acanthocephala) in a blue whale, Balaenoptera musculus, stranded in the St. Lawrence Estuary, Quebec. J Helminthol Soc Wash 1992; 59(2): 206-211.
- Mohamadain HS, Adel A. Light and scanning electron microscopy on Serrasentis sagittifer Linton, 1889 (Acanthocephala): Palaeacanthocephala: Rhadinorhynchidae) infecting the common sea bream in Egypt. J Egypt Soc Parasitol 2015; 45(1): 23-28. http://dx.doi.org/10.12816/0010846 PMid:26012215.
» http://dx.doi.org/10.12816/0010846 - Nakamura I, Inada T, Takeda M, Hatanaka H. Important fishes trawled off Patagonia Tokyo: Japan Marine Fishery Resource Research Center; 1986.
- Pereira Junior J, Neves LFM. Corynosoma australe Johnston, 1937 (Acanthocephala, Polymorphidae) em Micropogonias furnieri (Desmarest, 1823) (Perciformes, Sciaenidae) do litoral do Rio Grande do Sul. Comun Mus Cien PUCRS Ser Zool 1993; 6: 51-61.
- Porto Alegre. Portaria SMS nº 1109 de 23/08/2016. Norma Municipal - Porto Alegre – RS. Aprova as exigências mínimas para produção, preparo e comercialização de sushis e sashimis no Município de Porto Alegre. Normas Brasil [online], ago. 2016 [cited 2019 March 13]. Available from: http://www.normasbrasil.com.br/norma/ portaria-1109-2016-porto-alegre-rs_327789.html
» http://www.normasbrasil.com.br/norma/ - Salgado-Maldonado G. Acantocéfalos de peces V. Redescripción de cuatro especies de palaeacantocéfalos parásitos de peces de México. An Inst Biol Univ Nal Autón México Ser Zool 1978; 49(1): 49-70.
- Santos CP, Gibson DI, Tavares LER, Luque JL. Checklist of acanthocephala associated with the fishes of Brazil. Zootaxa 2008; 1938: 1-22.
- Sardella NH, Mattiucci S, Timi JT, Bastida RO, Rodríguez DH, Nascetti G. Corynosoma australe Johnston, 1937 and C. cetaceum Johnston & Best, 1942 (Acanthocephala: Polymorphidae) from marine mammals and fishes in Argentinian waters: allozyme markers and taxonomic status. Syst Parasitol 2005; 61(2): 143-156. http://dx.doi.org/10.1007/s11230-005-3131-0 PMid:15980967.
» http://dx.doi.org/10.1007/s11230-005-3131-0 - Schmidt GD. Acanthocephalan infection of man, with two new records. J Parasitol 1971; 57(3): 582-584. http://dx.doi.org/10.2307/3277920 PMid:5090967.
» http://dx.doi.org/10.2307/3277920 - Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 2007; 57(7): 573-583. http://dx.doi.org/10.1641/B570707
» http://dx.doi.org/10.1641/B570707 - Tada I, Otsuji Y, Kamiya H, Mimori T, Sakaguchi Y, Makizumi S. The first case of a human infected with an acanthocephalan parasite, Bolbosoma sp. J Parasitol 1983; 69(1): 205-208. http://dx.doi.org/10.2307/3281300 PMid:6827437.
» http://dx.doi.org/10.2307/3281300 - Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 2013; 30(12): 2725-2729. http://dx.doi.org/10.1093/molbev/mst197 PMid:24132122.
» http://dx.doi.org/10.1093/molbev/mst197 - Travassos L. Serrasentis sagittifer (Linton, 1889) (Acantocephala). Mem Inst Oswaldo Cruz 1966; 64(1): 1-10.
- Williams H, Jones A. Parasitic worms of fish London: Taylor Francys Ltd.; 1994.
- Zhu X, Chilton NB, Jacobs DE, Boes J, Gasser RB. Characterisation of Ascaris from human and pig hosts by nuclear ribosomal DNA sequences. Int J Parasitol 1999; 29(3): 469-478. http://dx.doi.org/10.1016/S0020-7519(98)00226-4 PMid:10333331.
» http://dx.doi.org/10.1016/S0020-7519(98)00226-4
Publication Dates
-
Publication in this collection
13 June 2019 -
Date of issue
Jul-Sep 2019
History
-
Received
04 Jan 2019 -
Accepted
22 Apr 2019