Abstract
Rickettsia rickettsii is the causative agent of Brazilian spotted fever (BSF), for which humans and dogs are both susceptible. Dogs are sentinels in serological surveys, however, canine disease is rarely reported. Therefore, we aimed to evaluate natural infection by spotted fever group (SFG) Rickettsia spp. in dogs and ticks collected from domiciles close to forest fragments, featuring domestic–wildlife interface areas. Samples from 115 dogs and 135 ixodids were assessed by polymerase chain reactions (PCR) targeting the gltA gene for Rickettsia spp. and the ompA gene for the SFG rickettsial species. One dog (0.87%; 1/115) was positive for R. rickettsii. This dog presented nonspecific laboratory and clinical abnormalities (thrombocytopenia, hyperproteinemia, lymph node enlargement, emaciation, anorexia, and lethargy). Rickettsia parkeri was identified in 2.96% (4/135) of the ticks (Amblyomma sculptum, A. aureolatum, and Rhipicephalus sanguineus). This study confirmed the presence of SFG bacteria in non-endemic and preserved locations, where domestic and wild populations interact. We reinforce the fact that the dog is susceptible to natural R. rickettsii infection. Although this is a rare finding, preventive measures should be taken against BSF in the studied areas. Finally, R. parkeri infection is possibly being demonstrated in A. sculptum for the first time.
Keywords:
Emerging infectious diseases; Brazilian spotted fever; Rickettsia rickettsii; Rickettsia parkeri; ixodids; Atlantic forest biome
Resumo
Rickettsia rickettsii é o agente causador da Febre Maculosa Brasileira (FMB), doença na qual humanos e cães são susceptíveis. Os cães são sentinelas nos inquéritos sorológicos, contudo, a doença canina é raramente descrita. Assim sendo, objetivou-se avaliar a infecção natural por Rickettsia spp. do Grupo da Febre Maculosa (GFM) em cães e carrapatos obtidos de domicílios próximos a fragmentos de mata, caracterizando áreas de interface doméstico–silvestre. Amostras de 115 cães e 135 ixodídeos foram avaliadas pela reação em cadeia da polimerase (PCR) tendo como alvo o gene gltA de Rickettsia spp. e o gene ompA das espécies do GFM. Um cão (0,87%; 1/115) foi positivo para R. rickettsii. Este animal apresentou alterações clínicas e laboratoriais inespecíficas (trombocitopenia, hiperproteinemia, linfonodos edemaciados, emagrecimento, anorexia e letargia). Rickettsia parkeri foi identificada em 2,96% (4/135) dos carrapatos (Amblyomma sculptum, A. aureolatum e Rhipicephalus sanguineus). Este estudo confirmou a presença de bactérias do GFM em locais preservados e não endêmicos, onde populações domésticas e silvestres interagem. Reforçamos o fato do cão ser susceptível à infecção natural por R. rickettsii. Embora este seja um achado raro, medidas preventivas devem ser tomadas contra a FMB nas áreas estudadas. Em última análise, a infecção por R. parkeri possivelmente está sendo demonstrada pela primeira vez em A. sculptum.
Palavras-chave:
Doenças infecciosas emergentes; febre maculosa brasileira; Rickettsia rickettsii; Rickettsia parkeri; Ixodídeos; bioma de Mata Atlântica
Introduction
Bacteria of the genus Rickettsia are obligate intracellular organisms. In Brazil, Rickettsia rickettsii and Rickettsia sp. str. Atlantic Rainforest are the main SFG rickettsiosis-causing bacteria that leads to human disease (Oliveira et al., 2016Oliveira SV, Guimarães JN, Reckziegel GC, Neves BMC, Araújo-Vilges KM, Fonseca LX, et al. An update on the epidemiological situation of spotted fever in Brazil. J Venom Anim Toxins Incl Trop Dis 2016; 22(1): 22. http://dx.doi.org/10.1186/s40409-016-0077-4. PMid:27555867.
http://dx.doi.org/10.1186/s40409-016-007...
). Rickettsia rickettsii is the etiological agent of the human Brazilian spotted fever (BSF), a severe tick-borne disease, endemic in southeastern Brazil, which can reach a mortality rate of 50% (Oliveira et al., 2016Oliveira SV, Guimarães JN, Reckziegel GC, Neves BMC, Araújo-Vilges KM, Fonseca LX, et al. An update on the epidemiological situation of spotted fever in Brazil. J Venom Anim Toxins Incl Trop Dis 2016; 22(1): 22. http://dx.doi.org/10.1186/s40409-016-0077-4. PMid:27555867.
http://dx.doi.org/10.1186/s40409-016-007...
). In this country, 1,574 human cases of BSF were reported between 2001 and 2017; 1,128 (71.66%) of those cases occurring in the southeastern region (Brasil, 2019Brasil. Ministério da Saúde. Secretaria de vigilância em saúde. Sistema de Informação de Agravos de Notificação. Febre Maculosa [online]. Brasília: SINAN; 2019 [cited 2019 Dec 06]. Available from: http://www.portalsinan.saude.gov.br/febre-maculosa
http://www.portalsinan.saude.gov.br/febr...
). Dogs are also susceptible to R. rickettsii infection, and although these are considered sentinels in serological surveys (based on the presence of antibodies), natural illness has rarely been reported in canines in Brazil (Labruna et al., 2009Labruna MB, Kamakura O, Moraes-Filho J, Horta MC, Pacheco RC. Rocky Mountain spotted fever in dogs, Brazil. Emerg Infect Dis 2009; 15(3): 458-460. http://dx.doi.org/10.3201/eid1503.081227. PMid:19239764.
http://dx.doi.org/10.3201/eid1503.081227...
).
The epidemiology of BSF is related to occupational and leisure activities, especially in rural areas, where there is a link between domestic and wild animal populations, which, in turn, maintain the main vector ticks (Amblyomma cajennense sensu lato and A. aureolatum) (Ogrzewalska et al., 2012Ogrzewalska M, Saraiva DG, Moraes-Filho J, Martins TF, Costa FB, Pinter A, et al. Epidemiology of Brazilian spotted fever in the Atlantic Forest, state of Sao Paulo, Brazil. Parasitology 2012; 139(10): 1283-1300. http://dx.doi.org/10.1017/S0031182012000546. PMid:22716923.
http://dx.doi.org/10.1017/S0031182012000...
; Szabó et al., 2013Szabó MPJ, Pinter A, Labruna MB. Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front Cell Infect Microbiol 2013; 3: 27. http://dx.doi.org/10.3389/fcimb.2013.00027. PMid:23875178.
http://dx.doi.org/10.3389/fcimb.2013.000...
; Oliveira et al., 2016Oliveira SV, Guimarães JN, Reckziegel GC, Neves BMC, Araújo-Vilges KM, Fonseca LX, et al. An update on the epidemiological situation of spotted fever in Brazil. J Venom Anim Toxins Incl Trop Dis 2016; 22(1): 22. http://dx.doi.org/10.1186/s40409-016-0077-4. PMid:27555867.
http://dx.doi.org/10.1186/s40409-016-007...
). Studies regarding ecological aspects of R. rickettsii suggest that this bacterium is a rare agent being found in few tick populations under natural conditions, which is probably due to the pathogenic effect of R. rickettsii on these vectors (Labruna, 2009Labruna MB. Ecology of rickettsia in South America. Ann N Y Acad Sci 2009; 1166(1): 156-166. http://dx.doi.org/10.1111/j.1749-6632.2009.04516.x. PMid:19538276.
http://dx.doi.org/10.1111/j.1749-6632.20...
).
On the other hand, the Atlantic Forest rickettsiosis, which has been associated with R. parkeri, causes a less severe human disease reported in the southern, southeastern and northeastern regions of Brazil, where A. ovale ticks have been implicated as vectors (Spolidorio et al., 2010Spolidorio MG, Labruna MB, Mantovani E, Brandão PE, Richtzenhain LJ, Yoshinari NH. Novel spotted fever group rickettsiosis, Brazil. Emerg Infect Dis 2010; 16(3): 521-523. https://dx.doi.org/10.3201%2Feid1603.091338
https://dx.doi.org/10.3201%2Feid1603.091...
; Silva et al., 2011Silva N, Eremeeva ME, Rozental T, Ribeiro GS, Paddock CD, Ramos EA, et al. Eschar-associated spotted fever rickettsiosis, Bahia, Brazil. Emerg Infect Dis 2011; 17(2): 275-278. http://dx.doi.org/10.3201/eid1702.100859. PMid:21291605.
http://dx.doi.org/10.3201/eid1702.100859...
; Krawczak et al., 2016Krawczak FS, Muñoz-Leal S, Guztzazky AC, Oliveira SV, Santos FC, Angerami RN, et al. Rickettsia sp. Strain Atlantic rainforest infection in a patient from a spotted fever-endemic area in southern Brazil. Am J Trop Med Hyg 2016; 95(3): 551-553. https://doi.org/10.4269/ajtmh.16-0192.
https://doi.org/10.4269/ajtmh.16-0192...
; Faccini-Martínez et al., 2018Faccini-Martínez AA, Oliveira SV, Cerutti C Jr, Labruna MB. Febre Maculosa por Rickettsia parkeri no Brasil: condutas de vigilância epidemiológica, diagnóstico e tratamento. J Health Biol Sci 2018; 6(3): 299-312. http://dx.doi.org/10.12662/2317-3076jhbs.v6i3.1940.p299-312.2018Gehrke
http://dx.doi.org/10.12662/2317-3076jhbs...
).
Protected areas of Brazil, also known as conservation units (CUs), constitute domestic–wildlife interface areas, harboring a vast diversity of endemic fauna and flora and are important ecotourism areas. Unfortunately, some CUs have been damaged by inappropriate housing construction, an increase in the population of domestic animals, livestock, and deforestation. All these factors have allowed greater interactions between humans, domestic animals, and wildlife.
Against this background, it is possible that human and animal populations that inhabit (albeit improperly) and enjoy (leisure and ecotourism) Brazilian CUs are subject to exposure to various arthropod vectors and the pathogens potentially transmitted by these vectors. Considering the geographical location of the state of Rio de Janeiro (RJ); the lack of studies in environments protected by law; and in view of the ecological and epidemiological aspects of SFG rickettsiosis, this study aimed to evaluate the natural occurrence of SFG Rickettsia spp. in dogs and their ticks collected from CUs in the Atlantic Forest biome of RJ state and surrounding areas.
Materials and Methods
This study was approved by the Fluminense Federal University Ethics Committee on Animal Research (Reference no. 438/2013). At the time of sampling, the dog owners gave written informed consent authorizing the collection of data and biological samples.
Study areas
This study was conducted in and around four CUs managed by the Chico Mendes Institute for the Conservation of Biodiversity in the state of RJ, where a domestic–wildlife interface is present. We choose CUs in small regions where human BSF has not been frequently reported (Vale do Paraíba) and in the metropolitan region of the RJ. The following CUs were selected for study: Itatiaia National Park (22°20’02.15”S, 44°37’03.40”W) in the municipality of Itatiaia; Cicuta Forest region (22°33’20.34”S, 44°05’43.18”W) in the municipality of Barra Mansa; São Benedito Natural Farm (22°44’48.27”S, 44°05’41.21”W) in the municipality of Rio Claro; and Tijuca National Park (22°57’50.80”S, 43°17’10.86”W) in the city of RJ.
The studied areas were deemed as those showing increasing urbanization. At all sampling sites, humans and dogs coexisted with forest fragments, pastures, and water bodies, reinforcing that these were areas showing a domestic–wildlife interface.
Collection of samples
All dogs evaluated were included in this study regardless of age and sex, with only one visit per domicile. Samples were collected from healthy and sick dogs with a history of frequent ectoparasite infestation in households that typically maintained close contact with Atlantic Forest fragments and whose dogs lived unconstrained. Since the canine population within the CUs is low, the collection of samples was made by convenience, using all the animals visited, between September 2013 and November 2014. At the time of sampling, the dogs were examined by routine noninvasive procedures, and ticks were randomly collected via handpicking. Individual questionnaires were applied to collect animal data.
Ticks were stored in sterile 1.5 mL tubes containing isopropanol until identification based on previously described taxonomic keys (Barros-Battesti et al., 2006Barros-Battesti DM, Arzua M, Bechara GH. Carrapatos de importância médico-veterinária da região neotropical: Um guia ilustrado para identificação de espécies. São Paulo: Vox/ICTTD-3/Butantan; 2006.; Martins et al., 2010Martins TF, Onofrio VC, Barros-Battesti DM, Labruna MB. Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: descriptions, redescriptions, and identification key. Ticks Tick Borne Dis 2010; 1(2): 75-99. http://dx.doi.org/10.1016/j.ttbdis.2010.03.002. PMid:21771514.
http://dx.doi.org/10.1016/j.ttbdis.2010....
). The specimens initially identified as A. cajennense s. l. were later classified as A. sculptum, according to the reassessment and reinstatement of A. cajennense (Nava et al., 2014Nava S, Beati L, Labruna MB, Cáceres AG, Mangold AJ, Guglielmone AA. Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum Koch, 1844, and Amblyomma sculptum Berlese, 1888 (Ixodida: ixodidae). Ticks Tick Borne Dis 2014; 5(3): 252-276. http://dx.doi.org/10.1016/j.ttbdis.2013.11.004. PMid:24556273.
http://dx.doi.org/10.1016/j.ttbdis.2013....
). Later, the ticks were individually grounded under liquid nitrogen to prepare them for polymerase chain reactions (PCR).
Hematological and biochemical analysis
Complete blood counts were processed in an automated veterinary hematology analyzer (pocH-100iV Diff; Sysmex do Brasil Indústria e Comércio Ltda, São José dos Pinhais, Brazil). Blood smears were done after blood collection; they were stored in boxes and later fixed in methanol and stained with Giemsa (Merck®, Rio de Janeiro, Brazil). Serum was tested in an automated analyzer (Labmax 240 Premium; Labtest Diagnóstica AS, Lagoa Santa, Brazil) to determine alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase activity levels, as well as total protein and albumin levels. Globulins were obtained by calculating the difference between protein and albumin.
DNA extraction and PCR
The DNA extraction was performed using two commercial kits, one for whole blood (Illustra blood genomic Prep Mini Spin Kit; GE Healthcare Life Sciences, São Paulo, Brazil) and one for grounded ticks (Invisorb Spin Tissue Mini KitTM; Stratec Biomedical AG, Birkenfeld, Germany). The adult ticks and nymphs were processed individually.
Extracted DNA was quantified by fluorimetry and standardized for concentrations between 10 ng/μL (whole blood) and 25 ng/μL (ticks). In addition, samples from whole blood were randomly chosen to verify the presence of amplifiable DNA using the primers 5'-CCTTCATTGACCTCAACTACAT-3' and 5'-CCAAAGTTGTCATGGATGACC-3', resulting in amplification of a 399 bp fragment of the housekeeping gapdh gene, which encodes the glyceraldehyde-3-phosphatedehydrogenase and is present in all mammals. PCR mix was prepared containing 1× PCR buffer (Tris-HCl, KCl), 0.2 mM of dNTPs, 1.5 mM of magnesium chloride, 2.5 pmol of each of forward and reverse primers, 1 U of Taq DNA polymerase, 50 to 100 ng of genomic DNA, and the total reaction was made up to 25 µL using PCR-grade water. Amplifications were performed at 95°C for 5 min, followed by 30 cycles at 94°C for 45 s, 55°C for 45 s, 72°C for 45 s, and a final extension at 72°C for 2 min.
Conventional PCR based on Regnery et al. (1991)Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of Rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol 1991; 173(5): 1576-1589. http://dx.doi.org/10.1128/JB.173.5.1576-1589.1991. PMid:1671856.
http://dx.doi.org/10.1128/JB.173.5.1576-...
was used in all assays for the presence of rickettsial DNA. First, samples were screened via reactions targeting a fragment of the citrate synthase (gltA) gene, which is present in all members of the genus Rickettsia. For these reactions an optimization was performed in the forward primer (replacement with a degenerate primer and additional nucleotide bases in the 5′-end) and the annealing temperature was adjusted to 58°C after applying a temperature gradient. Amplifications for the gltA gene were carried out using 10 pmol of each primer (gltAFo 5′ TTGGGGRCCTGCTCACGG 3′ and RpCS.1258n 5′ ATTGCAAAAAGTACAGTGAACA 3′), 1.5 mM of magnesium chloride, 0.2 mM of dNTPs, 1 U of Taq DNA polymerase, and 50-100 ng of genomic DNA. Reactions were subjected to an initial denaturation at 94 °C for 5 min, followed by 35 cycles at 94°C for 20 s, 58°C for 30 s, 72°C for 1 min, and a final extension at 72 °C for 5 min.
Next, the samples were assessed for the SFG rickettsial outer membrane protein A (ompA) gene using primers Rr190.70p and Rr190.602n in accordance with Regnery et al. (1991)Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of Rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol 1991; 173(5): 1576-1589. http://dx.doi.org/10.1128/JB.173.5.1576-1589.1991. PMid:1671856.
http://dx.doi.org/10.1128/JB.173.5.1576-...
, but with 2.0 mM magnesium chloride and at an annealing temperature of 55°C, both chosen after the application of the respective gradients. These reactions were subjected to an initial denaturation at 94°C for 5 min, followed by 35 cycles at 94°C for 40 s, 55°C for 30 s, 72°C for 1 min, and a final extension at 72°C for 10 min. For each reaction, we used a positive control (purified DNA of R. parkeri str. AT-24 extracted from cell cultures) and a negative control (nuclease-free water).
The amplicons were visualized on a 1.5% agarose gel stained with GelRed™ 1:20,000 (Uniscience, Miami, EUA). Positive samples were purified with a commercial kit (Illustra GFX PCR DNA and Gel Band Purification Kit; GE Healthcare Life Sciences), to prepare them for sequencing on a capillary-type Sanger platform (PDTIS/FIOCRUZ – RPT01A) at the Oswaldo Cruz Foundation. Partial nucleotide sequences were trimmed and assembled in a contig using Molecular Evolutionary Genetic Analysis program (MEGA) version 6. The resulting consensus sequence was used for comparison with available sequences from the GenBank® database, using the basic local alignment search tool (BLAST) to assess sequence identities. Phylograms (Figures 1 and 2) were generated from gltA or ompA multiple alignments using MEGA 6 software, with Neighbor-Joining method. The evolutionary models were defined by the lowest Bayesian Information Criterion (BIC) scores. Kimura 2-parameter model for ompA sequences, and Kimura 2-parameter model with Gamma distribution for gltA sequences. Bootstrap testing was performed with 10000 replicates. All sequence analyses were performed in CLC Main Workbench software, version 7.7.1 (QIAGEN Aarhus A/S, Aarhus, Denmark).
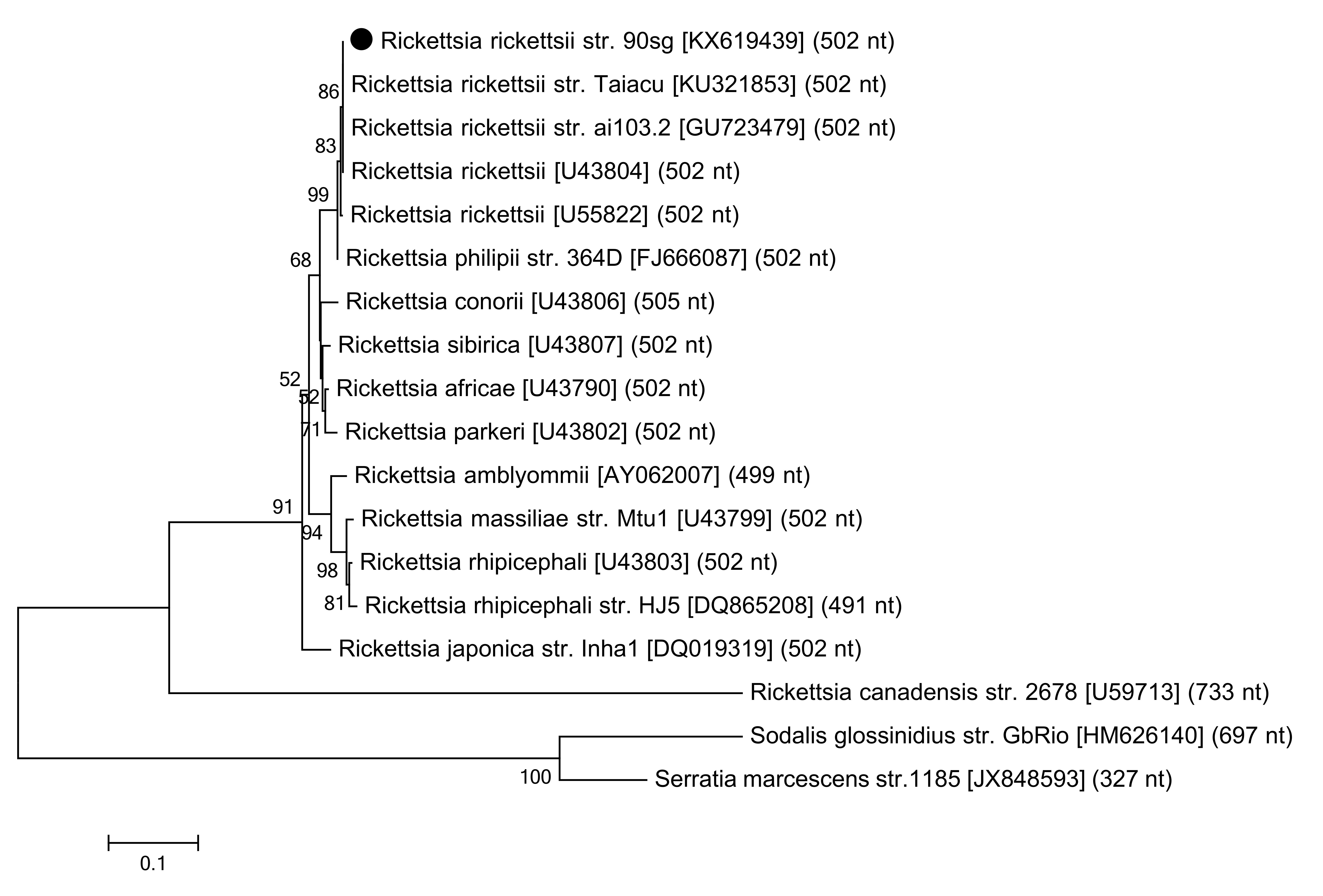
Phylogram of ompA partial sequences: the names of Rickettsia species from which ompA sequences were obtained are indicated, followed by the corresponding GenBank® accession numbers and nucleotide length used in multiple alignment. Distantly related ompA sequences are included as an outer group. The values on the phylogram nodes indicate bootstrap values. The dark circle indicates the R. rickettsii ompA sequence identified in the present study.
Phylogram of gltA partial sequences: the names of Rickettsia species from which gltA sequences were obtained are indicated, followed by the corresponding GenBank® accession numbers and nucleotide length used in multiple alignment. Distantly related gltA sequences are included as an outer group. The values on the cladogram nodes indicate bootstrap values. The dark diamonds indicate the gltA sequences identified in the present study.
Results
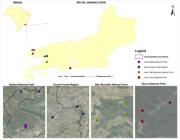
By visiting all households in the study areas (Figure 3), we obtained samples from 115 dogs and 135 ixodids. The largest number of dogs belonged to Itatiaia National Park, where 33.04% (38/115) of blood samples and 26.67% (36/135) of ticks were collected. In Tijuca National Park, 26.09% (30/115) of blood samples and 8.89% (12/135) of ticks were obtained, while in the Cicuta Forest region 22.61% (26/115) of blood samples and 28.15% (38/135) of the ticks were obtained. Finally, we collected 18.26% (21/115) of the blood samples and 36.29% (49/135) of the ticks in São Benedito Natural Farm. The distribution of dogs and ticks in CUs is shown in Table 1.
Spatial distribution of dogs sampled in and around four conservation units in the Rio de Janeiro state, southeastern Brazil. The black line represents the limit of municipalities. Image created by Jefferson Pereira Caldas dos Santos.
Frequency of dogs and ticks collected from dogs in human–wildlife interface areas in the state of Rio de Janeiro, Brazil, by Conservation Unit visited.
Of the 115 dogs studied, one (0.87%) presented rickettsial DNA detectable by PCR targeting the gltA and ompA genes in the whole blood samples. Blood films were negative on the search for hemoparasites. Nucleotide sequences reported here were deposited in the GenBank® database under the accession numbers KX619438 and KX619439. The ompA sequence demonstrated 100% identity and 100% query coverage with R. rickettsii obtained from Amblyomma tick egg masses from Mexico (accessions GU723478 and GU723479). ThegltA sequence was closely related to the R. rickettsii obtained from human cases in Brazil (accession KJ588069; 99.72% identity; 99% query coverage) and Colombia (accession MG206089; 99.44% identity; 100% query coverage).
The dog naturally infected by R. rickettsii was an adult mixed-breed male, which resided on a rural property inside São Benedito Natural Farm, had free access to forests and small collections of water, and had close contact with horses. At the time of sampling, this dog was not infested with ticks. However, its owner stated that it was a common practice to treat dogs for ectoparasites, which possibly contributed to the absence of ticks at that moment. This dog presented nonspecific clinical manifestations (lymphadenopathy, slight lethargy, weight loss, and anorexia). Laboratory abnormalities were severe thrombocytopenia (18 × 103 platelets/µL), increased serum proteins (9.0 g/dL), and increased globulins (6.65 g/dL). Albumin was within reference intervals (2.35 g/dL).
To exclude concomitant tick-borne diseases, which could have similar symptoms, DNA extracted from the blood of the dog in question was also subjected to PCR using previously described protocols for organisms of the Anaplasmataceae family (Murphy et al., 1998Murphy GL, Ewing SA, Whitworth LC, Fox JC, Kocan AA. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet Parasitol 1998; 79(4): 325-339. http://dx.doi.org/10.1016/s0304-4017(98)00179-4. PMid:24556273.
http://dx.doi.org/10.1016/s0304-4017(98)...
) and piroplasms (Campos et al., 2018Campos SDE, Toma HK, Machado CSC, Assad RQ, Almosny NRP. Novel record of a tick Amblyomma sculptum with detection of piroplasm Rangelia vitalii DNA. Vet Parasitol Reg Stud Rep 2018; 13: 228-229. http://dx.doi.org/10.1016/j.vprsr.2018.07.007. PMid:31014880.
http://dx.doi.org/10.1016/j.vprsr.2018.0...
). There was no amplification for these reactions.
Out of 135 ixodid ticks were collected in this study (Table 1), four different species were identified: A. sculptum (45.93%; 60 nymphs and 2 adults); Rhipicephalus sanguineus (34.81%; 3 nymphs and 44 adults), A. aureolatum (17.78%; 24 adults), and A. ovale (1.48%; 2 adults). Most of the A. sculptum ticks (72.58%) were recovered from the region of São Benedito Natural Farm (where the R. rickettsii-positive dog was found). Of the ticks collected, four (2.96%) tested positively for Rickettsia spp. with regard to the gltA gene. Two of them (R. sanguineus) were collected from dogs in the surroundings of the Cicuta Forest, one (A. sculptum) was collected from a dog on São Benedito Natural Farm, and the last (A. aureolatum) was collected from a dog residing in Itatiaia National Park.
The Rickettsia sequences obtained from R. sanguineus (KY609326 and KY581579), A. aureolatum (KY498223), and A. sculptum (KX881763) showed high identity matches with R. parkeri isolate La Paloma (accession MG574939) obtained from human scab in Uruguai; R. parkeri str. At97ARG (accession KF782319) obtained from A. triste in Argentina; and R. parkeri str. Maculatum 20 (accession U59732). Percent of identity and query coverage found in this study were, respectively, 99.72% and 99% (KY581579); 99.44% and 99% (KY498223); 99.39% and 97% (KY609326); 98.33% and 99% (KX881763).
Discussion
Based on the presence of antibodies (exposure), dogs have been recognized as sentinels for BSF, presenting strong serological evidence of bacterial circulation (Poubel et al., 2018Poubel IT, Cunha NC, Fonseca ABM, Pinter A, Fonseca AH, Cordeiro MD, et al. Seroprevalence of Rickettsia rickettsii and Rickettsia parkeri in dogs during a Brazilian Spotted Fever outbreak in the State of Rio de Janeiro. Arq Bras Med Vet Zootec 2018; 70(3): 667-674. http://dx.doi.org/10.1590/1678-4162-9081.
http://dx.doi.org/10.1590/1678-4162-9081...
); however, the molecular detection of R. rickettsii in dogs’ blood samples is still considered very rare. The discrepancy between the amount of serological and molecular studies, in which dogs show the presence of rickettsial DNA in the bloodstream (rickettsemia), may be related to the fact that bacteria circulate in the blood for a brief period before they invade endothelial cells, or after being released back into the blood due to the disruption of infected cells. It is worth remembering that different tick-borne diseases in dogs can be similar to each other, with nonspecific clinical and laboratory manifestations, that cause confusion and misdiagnosis. Even with few reports, natural SFG infections of dogs are recognized in different countries, including infection with R. rickettsii in Brazil (Labruna et al., 2009Labruna MB, Kamakura O, Moraes-Filho J, Horta MC, Pacheco RC. Rocky Mountain spotted fever in dogs, Brazil. Emerg Infect Dis 2009; 15(3): 458-460. http://dx.doi.org/10.3201/eid1503.081227. PMid:19239764.
http://dx.doi.org/10.3201/eid1503.081227...
), R. parkeri in Bolivia (Tomassone et al., 2010Tomassone L, Conte V, Parrilla G, Meneghi DD. Rickettsia infection in dogs and Rickettsia parkeri in Amblyomma tigrinum Ticks, Cochabamba Department, Bolivia. Vector Borne Zoonotic Dis 2010; 10(10): 953-958. http://dx.doi.org/10.1089/vbz.2009.0126. PMid:20426684.
http://dx.doi.org/10.1089/vbz.2009.0126...
), and R. conorii in Italy (Solano-Gallego et al., 2006,Solano-Gallego L, Kidd L, Trotta M, Di Marco M, Caldin M, Furlanello T, et al. Febrile illness associated with Rickettsia conorii infection in dogs from Sicily. Emerg Infect Dis 2006; 12(12): 1985-1988. http://dx.doi.org/10.3201/eid1212.060326. PMid:17326960.
http://dx.doi.org/10.3201/eid1212.060326...
2015).
The Brazilian southeastern is known to be endemic to BSF, with 71.66% of all human reports of the disease (Brasil, 2019Brasil. Ministério da Saúde. Secretaria de vigilância em saúde. Sistema de Informação de Agravos de Notificação. Febre Maculosa [online]. Brasília: SINAN; 2019 [cited 2019 Dec 06]. Available from: http://www.portalsinan.saude.gov.br/febre-maculosa
http://www.portalsinan.saude.gov.br/febr...
). In this region, the state of São Paulo (SP) is the one with the most records, followed by the states of Minas Gerais (MG) and Rio de Janeiro (RJ) (Brasil, 2019Brasil. Ministério da Saúde. Secretaria de vigilância em saúde. Sistema de Informação de Agravos de Notificação. Febre Maculosa [online]. Brasília: SINAN; 2019 [cited 2019 Dec 06]. Available from: http://www.portalsinan.saude.gov.br/febre-maculosa
http://www.portalsinan.saude.gov.br/febr...
). Within the state of RJ not all areas are endemic and human cases appear to be concentrated in the northwestern area. In our study, rickettsial DNA was detected in the blood of one dog residing in a preserved area classified as nonendemic for BSF (Vale do Paraíba region). This analysis represents a new epidemiological profile. Since the keeping of domestic animals within CUs is prohibited, dogs in this study constitute a population that improperly inhabits preserved areas and can easily interact (and share vectors and pathogens) with wild animals, many of them with synanthropic habits. It is also possible that these dogs serve as a bridge in dispersion of the bacteria to human settlements.
Symptoms and laboratory findings of this study do not prove that the positively identified dog had BSF because the clinical signs are common to several febrile diseases transmitted by arthropods. However, no other hemoparasites were identified in the blood smear analysis or analyses using molecular techniques. Although rickettsial infection can be more severe than those observed in our study, similar symptoms have been described, in naturally and experimentally infected dogs by Rickettsia spp. (Solano-Gallego et al., 2006Solano-Gallego L, Kidd L, Trotta M, Di Marco M, Caldin M, Furlanello T, et al. Febrile illness associated with Rickettsia conorii infection in dogs from Sicily. Emerg Infect Dis 2006; 12(12): 1985-1988. http://dx.doi.org/10.3201/eid1212.060326. PMid:17326960.
http://dx.doi.org/10.3201/eid1212.060326...
; Piranda et al., 2008Piranda EM, Faccini JL, Pinter A, Saito TB, Pacheco RC, Hagiwara MK, et al. Experimental infection of dogs with a Brazilian strain of Rickettsia rickettsii: clinical and laboratory findings. Mem Inst Oswaldo Cruz 2008; 103(7): 696-701. http://dx.doi.org/10.1590/S0074-02762008000700012. PMid:19057821.
http://dx.doi.org/10.1590/S0074-02762008...
; Labruna et al., 2009Labruna MB, Kamakura O, Moraes-Filho J, Horta MC, Pacheco RC. Rocky Mountain spotted fever in dogs, Brazil. Emerg Infect Dis 2009; 15(3): 458-460. http://dx.doi.org/10.3201/eid1503.081227. PMid:19239764.
http://dx.doi.org/10.3201/eid1503.081227...
; Levin et al., 2014Levin ML, Killmaster LF, Zemtsova GE, Ritter JM, Langham G. Clinical presentation, convalescence, and relapse of Rocky Mountain spotted fever in dogs experimentally infected via tick bite. PLoS One 2014; 9(12): e115105. http://dx.doi.org/10.1371/journal.pone.0115105. PMid:25542001.
http://dx.doi.org/10.1371/journal.pone.0...
; Solano-Gallego et al., 2015Solano-Gallego L, Caprì A, Pennisi MG, Caldin M, Furlanello T, Trotta M. Acute febrile illness is associated with Rickettsia spp infection in dogs. Parasit Vectors 2015; 8(1): 216. http://dx.doi.org/10.1186/s13071-015-0824-3. PMid:25886403.
http://dx.doi.org/10.1186/s13071-015-082...
). Once again, tick-borne diseases present a wide range of clinical and laboratory signs, which show variable severity, according to the parasitic load and the patient's immune status.
A large decrease in the platelet count of the infected dog was observed. In rickettsial infection, thrombocytopenia appears to be a constant abnormality and can vary from mild to severe (Piranda et al., 2008Piranda EM, Faccini JL, Pinter A, Saito TB, Pacheco RC, Hagiwara MK, et al. Experimental infection of dogs with a Brazilian strain of Rickettsia rickettsii: clinical and laboratory findings. Mem Inst Oswaldo Cruz 2008; 103(7): 696-701. http://dx.doi.org/10.1590/S0074-02762008000700012. PMid:19057821.
http://dx.doi.org/10.1590/S0074-02762008...
; Labruna et al., 2009Labruna MB, Kamakura O, Moraes-Filho J, Horta MC, Pacheco RC. Rocky Mountain spotted fever in dogs, Brazil. Emerg Infect Dis 2009; 15(3): 458-460. http://dx.doi.org/10.3201/eid1503.081227. PMid:19239764.
http://dx.doi.org/10.3201/eid1503.081227...
; Levin et al., 2014Levin ML, Killmaster LF, Zemtsova GE, Ritter JM, Langham G. Clinical presentation, convalescence, and relapse of Rocky Mountain spotted fever in dogs experimentally infected via tick bite. PLoS One 2014; 9(12): e115105. http://dx.doi.org/10.1371/journal.pone.0115105. PMid:25542001.
http://dx.doi.org/10.1371/journal.pone.0...
; Solano-Gallego et al., 2015Solano-Gallego L, Caprì A, Pennisi MG, Caldin M, Furlanello T, Trotta M. Acute febrile illness is associated with Rickettsia spp infection in dogs. Parasit Vectors 2015; 8(1): 216. http://dx.doi.org/10.1186/s13071-015-0824-3. PMid:25886403.
http://dx.doi.org/10.1186/s13071-015-082...
). Although less common, an increase in globulins has been reported in dogs infected with R. conorii (Solano-Gallego et al., 2006Solano-Gallego L, Kidd L, Trotta M, Di Marco M, Caldin M, Furlanello T, et al. Febrile illness associated with Rickettsia conorii infection in dogs from Sicily. Emerg Infect Dis 2006; 12(12): 1985-1988. http://dx.doi.org/10.3201/eid1212.060326. PMid:17326960.
http://dx.doi.org/10.3201/eid1212.060326...
, 2015Solano-Gallego L, Caprì A, Pennisi MG, Caldin M, Furlanello T, Trotta M. Acute febrile illness is associated with Rickettsia spp infection in dogs. Parasit Vectors 2015; 8(1): 216. http://dx.doi.org/10.1186/s13071-015-0824-3. PMid:25886403.
http://dx.doi.org/10.1186/s13071-015-082...
). In our study, hyperglobulinemia was also present and led to hyperproteinemia.
It should be noted that in the region of São Benedito Natural Farm, area where we identified a dog with PCR-detectable rickettsemia, an epidemiological study based on the indirect immunofluorescence assay (IFA) using antigens of R. rickettsii str. Taiaçu and R. parkeri str. AT-24, observed that the frequency of seroreactive dogs was 23.8%, with titers ranging from 1:64 to 1:512 (Campos et al., 2017Campos SDE, Cunha NC, Machado CSC, Souza TVT, Fonseca ABM, Pinter A, et al. Circulação de Rickettsias do Grupo da Febre Maculosa em cães no entorno de Unidades de Conservação Federais do estado do Rio de Janeiro: evidência sorológica e fatores associados. Pesq Vet Bras 2017; 37(11): 1307-1312. http://dx.doi.org/10.1590/s0100-736x2017001100018.
http://dx.doi.org/10.1590/s0100-736x2017...
). These findings corroborate the presence of SFG organisms in this region of the Atlantic Forest biome.
With regard to ectoparasites, the high frequency of A. sculptum in the study sample can be explained by the low specificity of this tick (Szabó et al., 2013Szabó MPJ, Pinter A, Labruna MB. Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front Cell Infect Microbiol 2013; 3: 27. http://dx.doi.org/10.3389/fcimb.2013.00027. PMid:23875178.
http://dx.doi.org/10.3389/fcimb.2013.000...
). The frequency of R. sanguineus was also high, corroborating the assertion that this is one of the major tick species of domestic dogs (Szabó et al., 2013Szabó MPJ, Pinter A, Labruna MB. Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front Cell Infect Microbiol 2013; 3: 27. http://dx.doi.org/10.3389/fcimb.2013.00027. PMid:23875178.
http://dx.doi.org/10.3389/fcimb.2013.000...
). Notably, 95.93% of the A. aureolatum ticks were found in Itatiaia National Park, which can be explained by the biological characteristics of this tick. While R. sanguineus is an urban tick well adapted to inhabited areas, A. aureolatum is a neotropical tick, found mainly parasitizing dogs with free access to forests in areas with high humidity and low temperatures, which, within the Atlantic forest biome, is represented by the high altitude regions of the Brazilian southeastern (Pinter et al., 2004Pinter A, Dias RA, Gennari SM, Labruna MB. Study of the seasonal dynamics, life cycle, and host specificity of Amblyomma aureolatum (Acari: ixodidae). J Med Entomol 2004; 41(3): 324-332. http://dx.doi.org/10.1603/0022-2585-41.3.324. PMid:15185932.
http://dx.doi.org/10.1603/0022-2585-41.3...
). These eco-epidemiological features are consistent with the scenario found in the Itatiaia National Park and its surroundings, especially in the sampling places, where altitude varied approximately between 600 m and 1450 m. Regarding the species A. ovale, only two adult ticks were collected. This number could have been higher considering that this species has an enormous distribution capacity among Brazilian biomes, being reported from dogs kept unrestrained in rural areas (Szabó et al., 2013Szabó MPJ, Pinter A, Labruna MB. Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front Cell Infect Microbiol 2013; 3: 27. http://dx.doi.org/10.3389/fcimb.2013.00027. PMid:23875178.
http://dx.doi.org/10.3389/fcimb.2013.000...
). However, adults of A. ovale use many species of mammals (particularly of the Order Carnivora, canids and felids), in addition to dogs, as primary hosts (Guglielmone et al., 2003Guglielmone AA, Estrada-Peña A, Mangold AJ, Barros-Battesti DM, Labruna MB, Martins JR, et al. Amblyomma aureolatum (Pallas, 1772) and Amblyomma ovale Koch, 1844 (Acari: Ixodidae): Hosts, distribution and 16S rDNA sequences. Vet Parasitol 2003; 113(3-4): 273-288. https://doi.org/10.1016/s0304-4017(03)00083-9
https://doi.org/10.1016/s0304-4017(03)00...
).
The rate of infection of ticks with Rickettsia spp. (2.96%) was higher than expected, especially because studies regarding ecological aspects of R. rickettsii suggest that this bacterium is a rare agent among tick populations in nature (Labruna, 2009Labruna MB. Ecology of rickettsia in South America. Ann N Y Acad Sci 2009; 1166(1): 156-166. http://dx.doi.org/10.1111/j.1749-6632.2009.04516.x. PMid:19538276.
http://dx.doi.org/10.1111/j.1749-6632.20...
). For example, we have studies in the state of RJ where the prevalence of Rickettsia spp. in various tick species was between 0 and 0.5% (Cunha et al., 2009Cunha NC, Fonseca AH, Rezende J, Rozental T, Favacho ARM, Barreira JD, et al. First identification of natural infection of Rickettsia rickettsii in the Rhipicephalus sanguineus tick, in the State of Rio de Janeiro. Pesq Vet Bras 2009; 29(2): 105-108. http://dx.doi.org/10.1590/S0100-736X2009000200003.
http://dx.doi.org/10.1590/S0100-736X2009...
; Gehrke et al, 2009Gehrke FS, Gazeta GS, Souza ER, Ribeiro A, Marrelli MT, Schumaker TT. Rickettsia rickettsii, Rickettsia felis and Rickettsia sp. TwKM03 infecting Rhipicephalus sanguineus and Ctenocephalides felis collected from dogs in Brazilian spotted fever focus in the State of Rio de Janeiro/ Brazil. Clin Microbiol Infect 2009; 15(Suppl. 2): 267-268. https://doi.org/10.1111/j.1469-0691.2008.02229.x.
https://doi.org/10.1111/j.1469-0691.2008...
; Cordeiro et al., 2015Cordeiro MD, Raia VA, Pinter A, Cunha NC, Souza CE, Fonseca AH. Seroprevalence of Rickettsia spp. and a study of the tick fauna in dogs from the municipality of Seropédica, State of Rio de Janeiro. Semina: Ciênc Agrár 2015; 36(6): 3787-3794. http://dx.doi.org/10.5433/1679-0359.2015v36n6p3787.
http://dx.doi.org/10.5433/1679-0359.2015...
). Although positive results have been obtained for the gltA gene, no amplification was seen in case of the ticks for the ompA gene, which could be related to the low rickettsial load achieved when using conventional PCR protocols, which are less sensitive. This limitation was also verified by Temoche et al. (2018)Temoche LFC, Seabra ES Jr, Cordeiro MD, Fonseca AH, Cunha NC, Almosny NRP. Molecular detection of Rickettsia spp. in ticks collected from dogs from the Department of Piura, Peru. Semina: Ciênc Agrár 2018; 39(2): 605-612. http://dx.doi.org/10.5433/1679-0359.2018v39n2p605.
http://dx.doi.org/10.5433/1679-0359.2018...
.
In Brazil, disease caused by R. parkeri in humans is predominantly mild, and occurs in the Atlantic Forest biome, where A. ovale ticks have been implicated as vectors (Faccini-Martínez et al., 2018Faccini-Martínez AA, Oliveira SV, Cerutti C Jr, Labruna MB. Febre Maculosa por Rickettsia parkeri no Brasil: condutas de vigilância epidemiológica, diagnóstico e tratamento. J Health Biol Sci 2018; 6(3): 299-312. http://dx.doi.org/10.12662/2317-3076jhbs.v6i3.1940.p299-312.2018Gehrke
http://dx.doi.org/10.12662/2317-3076jhbs...
). Although the prevalence of R. parkeri-infected ticks in Brazil is not fully elucidated, this pathogen has also been reported in A. aureolatum and R. sanguineus (Medeiros et al., 2011Medeiros AP, Souza AP, Moura AB, Lavina MS, Bellato V, Sartor AA, et al. Spotted fever group Rickettsia infecting ticks (Acari: Ixodidae) in the state of Santa Catarina, Brazil. Mem Inst Oswaldo Cruz 2011; 106(8): 926-930. http://dx.doi.org/10.1590/S0074-02762011000800005. PMid:22241112.
http://dx.doi.org/10.1590/S0074-02762011...
), which is similar to the results of the present study. To the best of the authors knowledge, this was the first time that detection of R. parkeri has been naturally identified in A. sculptum, although previous studies have already proved experimental infection in A. cajennense s.l. (syn. A. sculptum) (Sangioni et al., 2005Sangioni LA, Horta MC, Vianna MC, Gennari SM, Soares RM, Galvão MA, et al. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg Infect Dis 2005; 11(2): 265-270. http://dx.doi.org/10.3201/eid1102.040656. PMid:15752445.
http://dx.doi.org/10.3201/eid1102.040656...
; Horta et al., 2010Horta MC, Sabatini GS, Moraes-Filho J, Ogrzewalska M, Canal RB, Pacheco RC, et al. Experimental infection of the opossum Didelphis aurita by Rickettsia felis, Rickettsia bellii, and Rickettsia parkeri and evaluation of the transmission of the infection to ticks Amblyomma cajennense and Amblyomma dubitatum. Vector Borne Zoonotic Dis 2010; 10(10): 959-967. https://doi.org/10.1089/vbz.2009.0149.
https://doi.org/10.1089/vbz.2009.0149...
).
Until now, it has not been possible to prove the participation of A. sculptum as a competent vector in the transmission of R. parkeri to other veterbrate hosts. However, Horta et al. (2010)Horta MC, Sabatini GS, Moraes-Filho J, Ogrzewalska M, Canal RB, Pacheco RC, et al. Experimental infection of the opossum Didelphis aurita by Rickettsia felis, Rickettsia bellii, and Rickettsia parkeri and evaluation of the transmission of the infection to ticks Amblyomma cajennense and Amblyomma dubitatum. Vector Borne Zoonotic Dis 2010; 10(10): 959-967. https://doi.org/10.1089/vbz.2009.0149.
https://doi.org/10.1089/vbz.2009.0149...
experimentally demonstrated that the A. sculptum tick can acquire R. parkeri after feeding on an animal with rickettsemia, corroborating what may have happened in our study. Additionally, the authors were able to prove the transstadial transmission of R. parkeri between larvae and nymphs of A. sculptum. Further studies are needed in order to fully elucidate the role of A. sculptum ticks in the R. parkeri epidemiological cycle.
The results found in the present study reinforce the need for permanent surveillance and monitoring of rickettsiosis in humans and animals, since the tick species found infected by R. parkeri are among those that most often parasitize humans (Reck et al., 2018Reck J, Souza U, Souza G, Kieling E, Dall’Agnol B, Webster A, et al. Records of ticks on humans in Rio Grande do Sul state, Brazil. Ticks Tick Borne Dis 2018; 9(5): 1296-1301. http://dx.doi.org/10.1016/j.ttbdis.2018.05.010. PMid:29803756.
http://dx.doi.org/10.1016/j.ttbdis.2018....
). Given that the dogs in the present study had free access to forest and urban areas, they could be parasitized by ticks from both environments and, under such circumstances, a tick feeding on a dog concurrently harboring infected ticks, could acquire the bacteria during the dog rickettsemia. Rickettsia-infected ticks may also be carried into or near human dwellings, where they will possibly detach from the dog and bite humans.
The detection of R. parkeri in A. aureolatum and R. sanguineus ticks was of particular concern, because these species proved to be more susceptible to infection and maintenance of rickettsiae than A. sculptum (Labruna et al., 2008Labruna MB, Ogrzewalska M, Martins TF, Pinter A, Horta MC. Comparative susceptibility of larval stages of Amblyomma aureolatum, Amblyomma cajennense, and Rhipicephalus sanguineus to infection by Rickettsia rickettsii. J Med Entomol 2008; 45(6): 1156-1159. http://dx.doi.org/10.1603/0022-2585(2008)45[1156:CSOLSO]2.0.CO;2. PMid:19058642.
http://dx.doi.org/10.1603/0022-2585(2008...
). The participation of R. sanguineus as a rickettsial vector in Brazil is speculative; however, this topic demands awareness, since it is known that this tick is associated with transmission of R. conorii in the Mediterranean Basin and R. rickettsii in the USA and Mexico (Demma et al., 2005Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, et al. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 2005; 353(6): 587-594. http://dx.doi.org/10.1056/NEJMoa050043. PMid:16093467.
http://dx.doi.org/10.1056/NEJMoa050043...
; Solano-Gallego et al., 2015Solano-Gallego L, Caprì A, Pennisi MG, Caldin M, Furlanello T, Trotta M. Acute febrile illness is associated with Rickettsia spp infection in dogs. Parasit Vectors 2015; 8(1): 216. http://dx.doi.org/10.1186/s13071-015-0824-3. PMid:25886403.
http://dx.doi.org/10.1186/s13071-015-082...
; Tinoco-Gracia et al., 2018Tinoco-Gracia L, Lomelí MR, Hori-Oshima S, Stephenson N, Foley J. Molecular Confirmation of Rocky Mountain Spotted Fever Epidemic Agent in Mexicali, Mexico. Emerg Infect Dis 2018; 24(9): 1723-1725. http://dx.doi.org/10.3201/eid2409.171523. PMid:30124418.
http://dx.doi.org/10.3201/eid2409.171523...
). In addition, human parasitism by the brown dog tick has been established before, particularly under conditions of high population density (Reck et al., 2018Reck J, Souza U, Souza G, Kieling E, Dall’Agnol B, Webster A, et al. Records of ticks on humans in Rio Grande do Sul state, Brazil. Ticks Tick Borne Dis 2018; 9(5): 1296-1301. http://dx.doi.org/10.1016/j.ttbdis.2018.05.010. PMid:29803756.
http://dx.doi.org/10.1016/j.ttbdis.2018....
). Even though A. ovale ticks sampled in the present study did not contain Rickettsia DNA, the importance of this tick as a vector for R. parkeri in the Atlantic forest biome should not be neglected. In fact, A. ovale ticks can bite humans more easily than A. aureolatum (Guglielmone et al., 2003Guglielmone AA, Estrada-Peña A, Mangold AJ, Barros-Battesti DM, Labruna MB, Martins JR, et al. Amblyomma aureolatum (Pallas, 1772) and Amblyomma ovale Koch, 1844 (Acari: Ixodidae): Hosts, distribution and 16S rDNA sequences. Vet Parasitol 2003; 113(3-4): 273-288. https://doi.org/10.1016/s0304-4017(03)00083-9
https://doi.org/10.1016/s0304-4017(03)00...
; Faccini-Martínez et al., 2018Faccini-Martínez AA, Oliveira SV, Cerutti C Jr, Labruna MB. Febre Maculosa por Rickettsia parkeri no Brasil: condutas de vigilância epidemiológica, diagnóstico e tratamento. J Health Biol Sci 2018; 6(3): 299-312. http://dx.doi.org/10.12662/2317-3076jhbs.v6i3.1940.p299-312.2018Gehrke
http://dx.doi.org/10.12662/2317-3076jhbs...
). Finally, we highlight the fact that this study relied only on the aid of molecular diagnostic techniques and does not exclude the need for serological surveys on dogs and other animals.
Conclusions
This study confirmed the natural R. rickettsii infection of one dog resident in non-endemic and preserved Atlantic Forest area. Clinical and laboratory abnormalities may be nonspecific, although thrombocytopenia remains a constant finding. The infection rate by R. parkeri in tick populations may differ from that in case of R. rickettsii; however, further studies are needed to elucidate the true prevalence of R. parkeri in nature and its potential vectors. Rickettsia parkeri is possibly being demonstrated in A. sculptum for the first time, and in addition to R. sanguineus and A. aureolatum, A. sculptum can be involved in the R. parkeri cycle. Finally, this study emphasizes the importance of epidemiological approaches and preventive measures, especially in areas of human–domestic–wildlife interface, where dogs serve as sentinels and may act as a bridge between human settlements, vectors, and arthropod-borne pathogens.
Acknowledgements
We are grateful to the Chico Mendes Institute for the Conservation of Biodiversity and to the non-governmental organization Connecta, for allowing the sampling of animals, as well as to the Oswaldo Cruz Foundation, for providing access to the PDTIS/FIOCRUZ Genomic Platform for DNA Sequencing - RPT01A. We would like to thank Editage (www.editage.com) for English language editing.
-
Funding: The Brazilian funding agency Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) allowed the development of this work through the granting of scholarships and support to the laboratory. The International Mobility of Researchers at Charles University (number CZ.02.2.69/0.0/0.0/16_027/0008495) supported Erich L. Telleria.
-
How to cite: Campos SDE, Cunha NC, Machado CSC, Nadal NV, Seabra Junior ES, Telleria EL, et al. Spotted fever group rickettsial infection in dogs and their ticks from domestic–wildlife interface areas in southeastern Brazil. Braz J Vet Parasitol 2020; 29(1): e020219. http://doi.org/10.1590/S1984-29612020012
References
- Barros-Battesti DM, Arzua M, Bechara GH. Carrapatos de importância médico-veterinária da região neotropical: Um guia ilustrado para identificação de espécies São Paulo: Vox/ICTTD-3/Butantan; 2006.
- Brasil. Ministério da Saúde. Secretaria de vigilância em saúde. Sistema de Informação de Agravos de Notificação. Febre Maculosa [online]. Brasília: SINAN; 2019 [cited 2019 Dec 06]. Available from: http://www.portalsinan.saude.gov.br/febre-maculosa
» http://www.portalsinan.saude.gov.br/febre-maculosa - Campos SDE, Cunha NC, Machado CSC, Souza TVT, Fonseca ABM, Pinter A, et al. Circulação de Rickettsias do Grupo da Febre Maculosa em cães no entorno de Unidades de Conservação Federais do estado do Rio de Janeiro: evidência sorológica e fatores associados. Pesq Vet Bras 2017; 37(11): 1307-1312. http://dx.doi.org/10.1590/s0100-736x2017001100018
» http://dx.doi.org/10.1590/s0100-736x2017001100018 - Campos SDE, Toma HK, Machado CSC, Assad RQ, Almosny NRP. Novel record of a tick Amblyomma sculptum with detection of piroplasm Rangelia vitalii DNA. Vet Parasitol Reg Stud Rep 2018; 13: 228-229. http://dx.doi.org/10.1016/j.vprsr.2018.07.007 PMid:31014880.
» http://dx.doi.org/10.1016/j.vprsr.2018.07.007 - Cordeiro MD, Raia VA, Pinter A, Cunha NC, Souza CE, Fonseca AH. Seroprevalence of Rickettsia spp. and a study of the tick fauna in dogs from the municipality of Seropédica, State of Rio de Janeiro. Semina: Ciênc Agrár 2015; 36(6): 3787-3794. http://dx.doi.org/10.5433/1679-0359.2015v36n6p3787
» http://dx.doi.org/10.5433/1679-0359.2015v36n6p3787 - Cunha NC, Fonseca AH, Rezende J, Rozental T, Favacho ARM, Barreira JD, et al. First identification of natural infection of Rickettsia rickettsii in the Rhipicephalus sanguineus tick, in the State of Rio de Janeiro. Pesq Vet Bras 2009; 29(2): 105-108. http://dx.doi.org/10.1590/S0100-736X2009000200003
» http://dx.doi.org/10.1590/S0100-736X2009000200003 - Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, et al. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 2005; 353(6): 587-594. http://dx.doi.org/10.1056/NEJMoa050043 PMid:16093467.
» http://dx.doi.org/10.1056/NEJMoa050043 - Faccini-Martínez AA, Oliveira SV, Cerutti C Jr, Labruna MB. Febre Maculosa por Rickettsia parkeri no Brasil: condutas de vigilância epidemiológica, diagnóstico e tratamento. J Health Biol Sci 2018; 6(3): 299-312. http://dx.doi.org/10.12662/2317-3076jhbs.v6i3.1940.p299-312.2018Gehrke
» http://dx.doi.org/10.12662/2317-3076jhbs.v6i3.1940.p299-312.2018Gehrke - Gehrke FS, Gazeta GS, Souza ER, Ribeiro A, Marrelli MT, Schumaker TT. Rickettsia rickettsii, Rickettsia felis and Rickettsia sp. TwKM03 infecting Rhipicephalus sanguineus and Ctenocephalides felis collected from dogs in Brazilian spotted fever focus in the State of Rio de Janeiro/ Brazil. Clin Microbiol Infect 2009; 15(Suppl. 2): 267-268. https://doi.org/10.1111/j.1469-0691.2008.02229.x
» https://doi.org/10.1111/j.1469-0691.2008.02229.x - Guglielmone AA, Estrada-Peña A, Mangold AJ, Barros-Battesti DM, Labruna MB, Martins JR, et al. Amblyomma aureolatum (Pallas, 1772) and Amblyomma ovale Koch, 1844 (Acari: Ixodidae): Hosts, distribution and 16S rDNA sequences. Vet Parasitol 2003; 113(3-4): 273-288. https://doi.org/10.1016/s0304-4017(03)00083-9
» https://doi.org/10.1016/s0304-4017(03)00083-9 - Horta MC, Sabatini GS, Moraes-Filho J, Ogrzewalska M, Canal RB, Pacheco RC, et al. Experimental infection of the opossum Didelphis aurita by Rickettsia felis, Rickettsia bellii, and Rickettsia parkeri and evaluation of the transmission of the infection to ticks Amblyomma cajennense and Amblyomma dubitatum. Vector Borne Zoonotic Dis 2010; 10(10): 959-967. https://doi.org/10.1089/vbz.2009.0149
» https://doi.org/10.1089/vbz.2009.0149 - Krawczak FS, Muñoz-Leal S, Guztzazky AC, Oliveira SV, Santos FC, Angerami RN, et al. Rickettsia sp. Strain Atlantic rainforest infection in a patient from a spotted fever-endemic area in southern Brazil. Am J Trop Med Hyg 2016; 95(3): 551-553. https://doi.org/10.4269/ajtmh.16-0192
» https://doi.org/10.4269/ajtmh.16-0192 - Labruna MB, Kamakura O, Moraes-Filho J, Horta MC, Pacheco RC. Rocky Mountain spotted fever in dogs, Brazil. Emerg Infect Dis 2009; 15(3): 458-460. http://dx.doi.org/10.3201/eid1503.081227 PMid:19239764.
» http://dx.doi.org/10.3201/eid1503.081227 - Labruna MB. Ecology of rickettsia in South America. Ann N Y Acad Sci 2009; 1166(1): 156-166. http://dx.doi.org/10.1111/j.1749-6632.2009.04516.x PMid:19538276.
» http://dx.doi.org/10.1111/j.1749-6632.2009.04516.x - Labruna MB, Ogrzewalska M, Martins TF, Pinter A, Horta MC. Comparative susceptibility of larval stages of Amblyomma aureolatum, Amblyomma cajennense, and Rhipicephalus sanguineus to infection by Rickettsia rickettsii. J Med Entomol 2008; 45(6): 1156-1159. http://dx.doi.org/10.1603/0022-2585(2008)45[1156:CSOLSO]2.0.CO;2 PMid:19058642.
» http://dx.doi.org/10.1603/0022-2585(2008)45[1156:CSOLSO]2.0.CO;2 - Levin ML, Killmaster LF, Zemtsova GE, Ritter JM, Langham G. Clinical presentation, convalescence, and relapse of Rocky Mountain spotted fever in dogs experimentally infected via tick bite. PLoS One 2014; 9(12): e115105. http://dx.doi.org/10.1371/journal.pone.0115105 PMid:25542001.
» http://dx.doi.org/10.1371/journal.pone.0115105 - Martins TF, Onofrio VC, Barros-Battesti DM, Labruna MB. Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: descriptions, redescriptions, and identification key. Ticks Tick Borne Dis 2010; 1(2): 75-99. http://dx.doi.org/10.1016/j.ttbdis.2010.03.002 PMid:21771514.
» http://dx.doi.org/10.1016/j.ttbdis.2010.03.002 - Medeiros AP, Souza AP, Moura AB, Lavina MS, Bellato V, Sartor AA, et al. Spotted fever group Rickettsia infecting ticks (Acari: Ixodidae) in the state of Santa Catarina, Brazil. Mem Inst Oswaldo Cruz 2011; 106(8): 926-930. http://dx.doi.org/10.1590/S0074-02762011000800005 PMid:22241112.
» http://dx.doi.org/10.1590/S0074-02762011000800005 - Murphy GL, Ewing SA, Whitworth LC, Fox JC, Kocan AA. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet Parasitol 1998; 79(4): 325-339. http://dx.doi.org/10.1016/s0304-4017(98)00179-4 PMid:24556273.
» http://dx.doi.org/10.1016/s0304-4017(98)00179-4 - Nava S, Beati L, Labruna MB, Cáceres AG, Mangold AJ, Guglielmone AA. Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum Koch, 1844, and Amblyomma sculptum Berlese, 1888 (Ixodida: ixodidae). Ticks Tick Borne Dis 2014; 5(3): 252-276. http://dx.doi.org/10.1016/j.ttbdis.2013.11.004 PMid:24556273.
» http://dx.doi.org/10.1016/j.ttbdis.2013.11.004 - Ogrzewalska M, Saraiva DG, Moraes-Filho J, Martins TF, Costa FB, Pinter A, et al. Epidemiology of Brazilian spotted fever in the Atlantic Forest, state of Sao Paulo, Brazil. Parasitology 2012; 139(10): 1283-1300. http://dx.doi.org/10.1017/S0031182012000546 PMid:22716923.
» http://dx.doi.org/10.1017/S0031182012000546 - Oliveira SV, Guimarães JN, Reckziegel GC, Neves BMC, Araújo-Vilges KM, Fonseca LX, et al. An update on the epidemiological situation of spotted fever in Brazil. J Venom Anim Toxins Incl Trop Dis 2016; 22(1): 22. http://dx.doi.org/10.1186/s40409-016-0077-4 PMid:27555867.
» http://dx.doi.org/10.1186/s40409-016-0077-4 - Pinter A, Dias RA, Gennari SM, Labruna MB. Study of the seasonal dynamics, life cycle, and host specificity of Amblyomma aureolatum (Acari: ixodidae). J Med Entomol 2004; 41(3): 324-332. http://dx.doi.org/10.1603/0022-2585-41.3.324 PMid:15185932.
» http://dx.doi.org/10.1603/0022-2585-41.3.324 - Piranda EM, Faccini JL, Pinter A, Saito TB, Pacheco RC, Hagiwara MK, et al. Experimental infection of dogs with a Brazilian strain of Rickettsia rickettsii: clinical and laboratory findings. Mem Inst Oswaldo Cruz 2008; 103(7): 696-701. http://dx.doi.org/10.1590/S0074-02762008000700012 PMid:19057821.
» http://dx.doi.org/10.1590/S0074-02762008000700012 - Poubel IT, Cunha NC, Fonseca ABM, Pinter A, Fonseca AH, Cordeiro MD, et al. Seroprevalence of Rickettsia rickettsii and Rickettsia parkeri in dogs during a Brazilian Spotted Fever outbreak in the State of Rio de Janeiro. Arq Bras Med Vet Zootec 2018; 70(3): 667-674. http://dx.doi.org/10.1590/1678-4162-9081
» http://dx.doi.org/10.1590/1678-4162-9081 - Reck J, Souza U, Souza G, Kieling E, Dall’Agnol B, Webster A, et al. Records of ticks on humans in Rio Grande do Sul state, Brazil. Ticks Tick Borne Dis 2018; 9(5): 1296-1301. http://dx.doi.org/10.1016/j.ttbdis.2018.05.010 PMid:29803756.
» http://dx.doi.org/10.1016/j.ttbdis.2018.05.010 - Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of Rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol 1991; 173(5): 1576-1589. http://dx.doi.org/10.1128/JB.173.5.1576-1589.1991 PMid:1671856.
» http://dx.doi.org/10.1128/JB.173.5.1576-1589.1991 - Sangioni LA, Horta MC, Vianna MC, Gennari SM, Soares RM, Galvão MA, et al. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg Infect Dis 2005; 11(2): 265-270. http://dx.doi.org/10.3201/eid1102.040656 PMid:15752445.
» http://dx.doi.org/10.3201/eid1102.040656 - Silva N, Eremeeva ME, Rozental T, Ribeiro GS, Paddock CD, Ramos EA, et al. Eschar-associated spotted fever rickettsiosis, Bahia, Brazil. Emerg Infect Dis 2011; 17(2): 275-278. http://dx.doi.org/10.3201/eid1702.100859 PMid:21291605.
» http://dx.doi.org/10.3201/eid1702.100859 - Spolidorio MG, Labruna MB, Mantovani E, Brandão PE, Richtzenhain LJ, Yoshinari NH. Novel spotted fever group rickettsiosis, Brazil. Emerg Infect Dis 2010; 16(3): 521-523. https://dx.doi.org/10.3201%2Feid1603.091338
» https://dx.doi.org/10.3201%2Feid1603.091338 - Solano-Gallego L, Caprì A, Pennisi MG, Caldin M, Furlanello T, Trotta M. Acute febrile illness is associated with Rickettsia spp infection in dogs. Parasit Vectors 2015; 8(1): 216. http://dx.doi.org/10.1186/s13071-015-0824-3 PMid:25886403.
» http://dx.doi.org/10.1186/s13071-015-0824-3 - Solano-Gallego L, Kidd L, Trotta M, Di Marco M, Caldin M, Furlanello T, et al. Febrile illness associated with Rickettsia conorii infection in dogs from Sicily. Emerg Infect Dis 2006; 12(12): 1985-1988. http://dx.doi.org/10.3201/eid1212.060326 PMid:17326960.
» http://dx.doi.org/10.3201/eid1212.060326 - Szabó MPJ, Pinter A, Labruna MB. Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front Cell Infect Microbiol 2013; 3: 27. http://dx.doi.org/10.3389/fcimb.2013.00027 PMid:23875178.
» http://dx.doi.org/10.3389/fcimb.2013.00027 - Temoche LFC, Seabra ES Jr, Cordeiro MD, Fonseca AH, Cunha NC, Almosny NRP. Molecular detection of Rickettsia spp. in ticks collected from dogs from the Department of Piura, Peru. Semina: Ciênc Agrár 2018; 39(2): 605-612. http://dx.doi.org/10.5433/1679-0359.2018v39n2p605
» http://dx.doi.org/10.5433/1679-0359.2018v39n2p605 - Tinoco-Gracia L, Lomelí MR, Hori-Oshima S, Stephenson N, Foley J. Molecular Confirmation of Rocky Mountain Spotted Fever Epidemic Agent in Mexicali, Mexico. Emerg Infect Dis 2018; 24(9): 1723-1725. http://dx.doi.org/10.3201/eid2409.171523 PMid:30124418.
» http://dx.doi.org/10.3201/eid2409.171523 - Tomassone L, Conte V, Parrilla G, Meneghi DD. Rickettsia infection in dogs and Rickettsia parkeri in Amblyomma tigrinum Ticks, Cochabamba Department, Bolivia. Vector Borne Zoonotic Dis 2010; 10(10): 953-958. http://dx.doi.org/10.1089/vbz.2009.0126 PMid:20426684.
» http://dx.doi.org/10.1089/vbz.2009.0126
Publication Dates
-
Publication in this collection
06 Apr 2020 -
Date of issue
2020
History
-
Received
30 Oct 2019 -
Accepted
31 Jan 2020