Abstract
Natural products are ecofriendly agents that can be used against parasitic diseases. Eimeria species cause eimeriosis in many birds and mammals and resistance to available medications used in the treatment of eimeriosis is emerging. We investigated the in vitro and in vivo activity of Morus nigra leaf extracts (MNLE) against sporulation of oocysts and infection of mice with Eimeria papillata. Phytochemical analysis of MNLE showed the presence of seven compounds and the in vitro effects of MNLE, amprolium, DettolTM, formalin, ethanol, and phenol were studied after incubation with oocysts before sporulation. Furthermore, infection of mice with E. papillata induced an oocyst output of approximately 12 × 105 oocysts/g of feces. MNLE significantly decreased oocyst output to approximately 86% and the total number of parasitic stages in the jejunum by approximately 87%. In addition, the reduction in the number of goblet cells in the jejuna of mice was increased after treatment. These findings suggest that mulberry exhibited powerful anticoccidial activity.
Keywords:
Morus nigra extract; sporulation; eimeriosis; oocysts; mice; jejunum
Resumo
Os produtos naturais são agentes ecologicamente corretos que podem ser usados contra doenças parasitárias. As espécies de Eimeria causam eimeriose em muitas aves e mamíferos e a resistência aos medicamentos disponíveis usados no tratamento da eimeriose está emergindo. Foram investigadas as atividades in vitro e in vivo dos extratos de folhas de Morus nigra (MNLE) contra esporulação de oocistos e infecção de camundongos com Eimeria papillata. A análise fitoquímica do MNLE mostrou a presença de sete compostos e os efeitos in vitro do MNLE, amprolium, DettolTM, formalina, etanol e fenol foram estudados após incubação com oocistos antes da esporulação. Além disso, a infecção de camundongos com E. papillata induziu uma produção de oocistos de aproximadamente 12 × 105 oocistos / g de fezes. O MNLE reduziu significativamente a produção de oocistos para aproximadamente 86%, e o número total de estágios parasitários no jejuno em aproximadamente 87%. Além disso, a redução no número de células caliciformes no jejuno de camundongos aumentou após o tratamento. Esses achados sugerem que a amoreira exibia uma poderosa atividade anticoccidiana.
Palavras-chave:
Extrato de Morus nigra; esporulação; eimeriose; oocistos; camundongos; jejuno
Introduction
Coccidiosis, one of the most serious diseases affecting many animals (Mehlhorn, 2014Mehlhorn H. Encyclopedic reference of parasitology. 4th ed. Berlin: Springer; 2014.), is caused by infection with Eimeria spp. (Andrews et al., 2004Andrews AH, Blowey RW, Boyd H, Eddy RG. Bovine medicine disease and husbandry of cattle. 2nd ed. Oxford: Blackwell Science; 2004.) and leads to gastrointestinal problems characterized by diarrhea, poor growth performance, and in some cases death (Collier et al., 2008Collier CT, Hofacre CL, Payne AM, Anderson DB, Kaiser P, Mackie RI, et al. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet Immunol Immunopathol 2008; 122(1-2): 104-115. http://dx.doi.org/10.1016/j.vetimm.2007.10.014. PMid:18068809.
http://dx.doi.org/10.1016/j.vetimm.2007....
; Orengo et al., 2012Orengo J, Buendía AJ, Ruiz-Ibáñez MR, Madrid J, Del Río L, Catalá-Gregori P, et al. Evaluating the efficacy of cinnamaldehyde and Echinacea purpurea plant extract in broilers against Eimeria acervulina. Vet Parasitol 2012; 185(2-4): 158-163. http://dx.doi.org/10.1016/j.vetpar.2011.09.024. PMid:21996002.
http://dx.doi.org/10.1016/j.vetpar.2011....
). Furthermore, infections induced by Eimeria spp. can foster opportunistic infections with other pathogens such as bacteria (Collier et al., 2008Collier CT, Hofacre CL, Payne AM, Anderson DB, Kaiser P, Mackie RI, et al. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet Immunol Immunopathol 2008; 122(1-2): 104-115. http://dx.doi.org/10.1016/j.vetimm.2007.10.014. PMid:18068809.
http://dx.doi.org/10.1016/j.vetimm.2007....
). Consequently, this pathogen causes massive economic losses worldwide (Shirley et al., 2007Shirley MW, Smith AL, Blake DP. Challenges in the successful control of the avian coccidia. Vaccine 2007; 25(30): 5540-5547. http://dx.doi.org/10.1016/j.vaccine.2006.12.030. PMid:17224208.
http://dx.doi.org/10.1016/j.vaccine.2006...
; Chapman, 2014Chapman HD. Milestones in avian coccidiosis research: a review. Poult Sci 2014; 93(3): 501-511. http://dx.doi.org/10.3382/ps.2013-03634. PMid:24604841.
http://dx.doi.org/10.3382/ps.2013-03634...
).
Eimeria oocysts are relatively resistant to environment conditions, which makes control measures difficult (Stephen et al., 1997Stephen B, Rommel M, Daugschies A, Haberkorn A. Studies of resistance to anticoccidials in Eimeria field isolates and pure Eimeria strains. Vet Parasitol 1997; 69(1-2): 19-29. http://dx.doi.org/10.1016/S0304-4017(96)01096-5. PMid:9187026.
http://dx.doi.org/10.1016/S0304-4017(96)...
). Therefore, disruption of the sporulation process is a critical point where this parasite can be controlled (Mai et al., 2009Mai K, Sharman AP, Walker AR, Katrib M, Souza DD, McConville JM, et al. Oocyst wall formation and composition in coccidian parasites. Mem Inst Oswaldo Cruz 2009; 104(2): 281-289. http://dx.doi.org/10.1590/S0074-02762009000200022. PMid:19430654.
http://dx.doi.org/10.1590/S0074-02762009...
). In addition, the prevalent prophylactic use of anticoccidial feed additives has led to widespread resistance (Stephen et al., 1997Stephen B, Rommel M, Daugschies A, Haberkorn A. Studies of resistance to anticoccidials in Eimeria field isolates and pure Eimeria strains. Vet Parasitol 1997; 69(1-2): 19-29. http://dx.doi.org/10.1016/S0304-4017(96)01096-5. PMid:9187026.
http://dx.doi.org/10.1016/S0304-4017(96)...
), which has currently been reported against available drugs (Williams, 1999Williams RB. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int J Parasitol 1999; 29(8): 1209-1229. http://dx.doi.org/10.1016/S0020-7519(99)00086-7. PMid:10576573.
http://dx.doi.org/10.1016/S0020-7519(99)...
; Chapman, 2014Chapman HD. Milestones in avian coccidiosis research: a review. Poult Sci 2014; 93(3): 501-511. http://dx.doi.org/10.3382/ps.2013-03634. PMid:24604841.
http://dx.doi.org/10.3382/ps.2013-03634...
).
Medicinal plants are the major resources of all alternative or indigenous systems of medicine and are considered promising sources for discovery of new chemical compounds (Kalia, 2009Kalia AN. Textbook of industrial pharmacognosy. 1st ed. New Delhi: CBS Publishers and Distributers; 2009.). In addition to targeting parasites, these products also have organ-protecting properties in hosts infected with Eimeria (Wunderlich et al., 2014Wunderlich F, Al-Quraishy S, Steinbrenner H, Sies H, Dkhil MA. Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol Res 2014; 113(10): 3547-3556. http://dx.doi.org/10.1007/s00436-014-4101-8. PMid:25185667.
http://dx.doi.org/10.1007/s00436-014-410...
). Morus nigra (black mulberry) is a perennial woody plant (Pan & Lou, 2008Pan G, Lou CF. Isolation of an 1-aminocyclopropane-1-carboxylate oxidase gene from mulberry (Morus alba L.) and analysis of the function of this gene in plant development and stresses response. J Plant Physiol 2008; 165(11): 1204-1213. http://dx.doi.org/10.1016/j.jplph.2007.02.012. PMid:17997189.
http://dx.doi.org/10.1016/j.jplph.2007.0...
) that belongs to the family Moraceae (Yang et al., 2010Yang X, Yang L, Zheng H. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem Toxicol 2010; 48(8-9): 2374-2379. http://dx.doi.org/10.1016/j.fct.2010.05.074. PMid:20561945.
http://dx.doi.org/10.1016/j.fct.2010.05....
). The genus Morus is found in warm and temperate regions and subtropical regions of Asia, Africa, North America (Pérez-Gregorio et al., 2011Pérez-Gregorio M, Regueiro J, Alonso-González E, Pastrana-Castro L, Simal-Gándara J. Influence of alcoholic fermentation process on antioxidant activity and phenolic levels from mulberries (Morus nigra L.). Lebensm Wiss Technol 2011; 44(8): 1793-1801. http://dx.doi.org/10.1016/j.lwt.2011.03.007.
http://dx.doi.org/10.1016/j.lwt.2011.03....
) including the US (Abbasi et al., 2014Abbasi AM, Khan MA, Ahmad M, Munir M, Zafar M, Sultana S, et al. Ethnobotanical and taxonomic screening of genus Morus for wild edible fruits used by the inhabitants of Lesser Himalayas-Pakistan. J Med Plants Res 2014; 8(25): 889-898. http://dx.doi.org/10.5897/JMPR2010.733.
http://dx.doi.org/10.5897/JMPR2010.733...
), and South Europe. The fruits, bark, and leaves of black mulberry are used medicinally as analgesic, antipyretic and anti-diabetic (Rodrigues et al., 2019Rodrigues EL, Marcelino G, Silva GT, Figueiredo PS, Garcez WS, Corsino J, et al. Nutraceutical and medicinal potential of the Morus species in metabolic dysfunctions. Int J Mol Sci 2019; 20(2): 301. http://dx.doi.org/10.3390/ijms20020301. PMid:30646503.
http://dx.doi.org/10.3390/ijms20020301...
) and, in particular, the fruits are used against dysentery (Ercisli & Orhan, 2007Ercisli S, Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem 2007; 103(4): 1380-1384. http://dx.doi.org/10.1016/j.foodchem.2006.10.054.
http://dx.doi.org/10.1016/j.foodchem.200...
).
Additionally, the antiparasitic activity of Morus alba (Riffat et al., 1986Riffat S, Akhtar MS, Javed I, Shah BH. Antinematodal and anticestodal efficacy of Morus alba Linn. stem bark in sheep. Pak J Agric Sci 1986; 23(3-4): 122-129.; Nguyen-Pouplin et al., 2007Nguyen-Pouplin J, Tran H, Tran H, Phan TA, Dolecek C, Farrar J, et al. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J Ethnopharmacol 2007; 109(3): 417-427. http://dx.doi.org/10.1016/j.jep.2006.08.011. PMid:17010546.
http://dx.doi.org/10.1016/j.jep.2006.08....
) and anthelmintic activity of Morus indica (Mughal et al., 2013Mughal TA, Arshad S, Mahboob S. Evaluation of anthelmintic activity of some members of family Moraceae. J Med Plants Res 2013; 7(30): 2275-2279. http://dx.doi.org/10.5897/JMPR2013.4420.
http://dx.doi.org/10.5897/JMPR2013.4420...
) were reported. The present study was conducted to investigate the in vitro and in vivo effects of M. nigra leaf extracts on Eimeria papillata oocysts sporulation and viability.
Materials and Methods
Extract preparation of extract
The M. nigra leaf extract (MNLE) was prepared using leaves obtained from Riyadh, Saudi Arabia and the botanical identity of the plant was confirmed by a taxonomist at the Department of Botany, University of King Saud. The leaves (100 g) were air-dried at 40 °C, ground into a powder, and then extracted with methanol (70%) for 24 h at 4 °C. The obtained extract was concentrated and dried in a rotary vacuum evaporator (Yamato RE300, Japan) as previously reported by Dkhil et al. (2013)Dkhil MA, Al-Quraishy S, Abdel Moneim AE, Delic D. Protective effect of Azadirachta indica extract against Eimeria papillata-induced coccidiosis. Parasitol Res 2013; 112(1): 101-106. http://dx.doi.org/10.1007/s00436-012-3109-1. PMid:22972359.
http://dx.doi.org/10.1007/s00436-012-310...
. Distilled water was used to dissolve the powder for the various experiments.
Phytochemical analysis
The phytochemical analysis of MNLE was performed according to the recommended protocol of Kanthal et al. (2014)Kanthal LK, Dey A, Satyavathi K, Bhojaraju P. GC-MS analysis of bio-active compounds in methanolic extract of Lactuca runcinata DC. Pharmacognosy Res 2014; 6(1): 58-61. http://dx.doi.org/10.4103/0974-8490.122919. PMid:24497744.
http://dx.doi.org/10.4103/0974-8490.1229...
. The gas chromatography-mass spectrometry (GC-MS) analysis was performed using a Thermo Scientific, Trace GC Ultra and ISQ single quadruple MS (Miami, CA, USA).
Oocyst sporulation
Fresh E. papillata unsporulated oocysts were originally obtained from Prof. Mehlhorn at Duesseldorf University (Duesseldorf, Germany) and the parasite was maintained by passaging in mice (Dkhil et al., 2011Dkhil MA, Abdel-Baki AS, Wunderlich F, Sies H, Al-Quraishy S. Anticoccidial and antiinflammatory activity of garlic in murine Eimeria papillata infections. Vet Parasitol 2011; 175(1-2): 66-72. http://dx.doi.org/10.1016/j.vetpar.2010.09.009. PMid:20943319.
http://dx.doi.org/10.1016/j.vetpar.2010....
). The unsporulated oocysts (1×105) were incubated in 5 mL potassium dichromate containing one of the following: MNLE (100, 200, and 300 mg/mL), 8.3 mg amprolium (Veterinary Agriculture Products Company [VAPCO], Jordan), 109 μL DettolTM, or 25 μL phenol while 5 mL potassium dichromate (2.5%) alone was used as the control. In addition, unsporulated oocysts (1×105) were incubated in 5 mL distilled water, ethanol (70%), and formalin (5%). We used three replicates for each treatment and all Petri dishes used incubated for 48 and 90 h at 25-29 °C (Gadelhaq et al., 2018Gadelhaq SM, Arafa WM, Abolhadid SM. In vitro activity of natural and chemical products on sporulation of Eimeria species oocysts of chickens. Vet Parasitol 2018; 251: 12-16. http://dx.doi.org/10.1016/j.vetpar.2017.12.020. PMid:29426468.
http://dx.doi.org/10.1016/j.vetpar.2017....
). The sporulated oocysts were counted using a Mcmaster chamber and the percentage sporulation was calculated using the following equation:
Infection of animals
Adult male C57BL/6 mice (9-11 weeks old) were used. The experiments were approved by the institutional review board of the Princess Nourah Bint Abdulrahman University (IRB Approval Number: 19-0259). Fresh faecal pellets were collected and weighed for each mouse once every 24 h and the bedding was changed to eliminate reinfection. The output of oocysts was calculated as mentioned previously by Schito et al. (1996)Schito ML, Barta JR, Chobotar B. Comparison of four murine Eimeria species in immunocompetent and immunodeficient mice. J Parasitol 1996; 82(2): 255-262. http://dx.doi.org/10.2307/3284157. PMid:8604093.
http://dx.doi.org/10.2307/3284157...
.
For oocyst flotation, the fecal pellets from each individual mouse were suspended and diluted using 2.5% (w/v) potassium dichromate in saturated sodium chloride (NaCl). Each mouse was inoculated orally with 100 μL sterile saline solution containing 103 E. papillata sporulated oocysts. A McMaster chamber was used to count the oocysts, and the results are expressed as the number per gram of wet feces (Dkhil et al., 2011Dkhil MA, Abdel-Baki AS, Wunderlich F, Sies H, Al-Quraishy S. Anticoccidial and antiinflammatory activity of garlic in murine Eimeria papillata infections. Vet Parasitol 2011; 175(1-2): 66-72. http://dx.doi.org/10.1016/j.vetpar.2010.09.009. PMid:20943319.
http://dx.doi.org/10.1016/j.vetpar.2010....
).
Experimental design
The mice were divided into six groups of eight animals each group. The first group (control) was daily gavaged with 100 µL 0.9% NaCl for 5 days. The second group was daily treated with 100 μL MNLE (200 mg/kg) by oral gavage, while the third, fourth, fifth, and sixth group were orally infected with 103 E. papillata oocysts. The last three groups were daily treated for 5 days with 200, 400, and 800 mg/kg of MNLE, respectively.
Sample collection
Fresh feces samples were collected from the mice on day 5 after infection, where each mouse was separated in this day in a small cage to collect its faeces and the oocysts shed per gram of feces was calculated (Schito et al., 1996Schito ML, Barta JR, Chobotar B. Comparison of four murine Eimeria species in immunocompetent and immunodeficient mice. J Parasitol 1996; 82(2): 255-262. http://dx.doi.org/10.2307/3284157. PMid:8604093.
http://dx.doi.org/10.2307/3284157...
). Then, all the mice were euthanized and parts of the jejunum were collected and fixed in formalin (10%) for the histological and histochemical examination.
Number of oocysts in jejunum
Pieces of the jejunum were fixed in 10% neutral formalin buffered, dehydrated in ethanol, embedded in paraffin wax, and then cut into 5-μm thick sections. The sections were stained with hematoxylin and eosin (H&E) (Drury & Wallington, 1980Drury RAB, Wallington EA. Carleton’s histological technique. 5th ed. Oxford: Oxford University Press; 1980.). Oocysts were counted in 10 well-oriented villous-crypt units (VCU) for each animal using Olympus BX61 light microscope (Tokyo, Japan).
Number of goblet cells
The number of goblet cells was determined in Alcian blue-stained sections. The number was expressed as mean goblet cells per 10 VCU (Allen et al., 1986Allen A, Hutton DA, Leonard AJ, Pearson JP, Sellers LA. The role of mucus in the protection of the gastroduodenal mucosa. Scand J Gastroenterol Suppl 1986;21(Suppl 125): 71-78. http://dx.doi.org/10.3109/00365528609093820. PMid:3103205.
http://dx.doi.org/10.3109/00365528609093...
).
Statistical analysis
Analysis of ANOVA was carried out in one way, and statistical comparisons between groups were made using the Duncan method. Values have been expressed as mean ± SD, at a significance level of ≤ 0.05. SigmaPlot software (version 11) was used for statistical analysis.
Results
Figure 1 shows the GC-MS chromatogram of MNLE. The phytochemical components identified were 1,3-benzenediamine, 2,4-dinitro-N3, N3-dipropyl-6-(trifluoromethyl)-, ß-carotene, gamabufotalin, ricinoleic acid, cholesteryl benzoate, tetradecanoic acid, and methotrexate (Table 1). After a 48 h incubation with MNLE (200 and 300 mg/mL), formalin, or ethanol, the E. papillata unsporulated oocysts showed no sporulation. However, oocysts incubated with potassium dichromate (2.5%), MNLE (100 mg/mL), amprolium, and DettolTM showed different levels of sporulation (Table 2).
Identification of phytochemical compounds by GC-Mass in Morus nigra leaf extracts. RT: Retention time, M-H: Protonated molecuole, MS: Mass acquired range.
Effect of Morus nigra on sporulation of E. papillata oocysts. MNLE: Morus nigra leaf extracts, P: Probability.
Incubation with MNLE (300 mg/mL), formalin, and ethanol for 90 h inhibited sporulation by approximately 100% (Table 2). MNLE (100 and 200 mg/mL), amprolium, DettolTM, and phenol induced average sporulation levels of 90.4%, 31%, 81.1%, 87% and 28%, respectively. On day 5 post-infection, the highest oocyst output was level was 12.1 ± 6.2 × 105 oocysts/g of feces in infected mice. Treatment with different doses of MNLE (200, 400, and 800 mg/kg) significantly (P < 0.01) reduced the oocyst output to 86.8%, 86%, and 93.8%, respectively (Figure 2). For further investigations, we have chosen the dose 200 mg/kg as a lower dose that can help to avoid adverse side effects, drug diversion, and toxicity where there was no significant difference in effect between the other treated groups.
Effect of M. nigra extract on oocyst output on day 5 postinfection with E. papillata oocysts. *Significance against the infected group (P < 0.01).
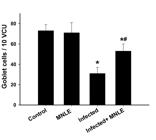
MNLE (200 mg/kg) significantly (P < 0.01) decrease the oocyst number in the mouse jejuna by approximately 86% (Figure 3). Finally, examination of Alcian blue-stained sections showed that the infection significantly reduced the number of goblet cells in the jejunum villi (Figure 4). Compared to the infected group, mice treated with 200 mg/kg MNLE showed a significant (P < 0.01) increase in the number of goblet cells by approximately 71% (Figure 5).
Effect of M. nigra extract on the number of parasitic stages in ten well-orientated villous-crypt units (VCU) for each mouse, on the fifth day of infection with E. papillata. *Significant at p < 0.01. MNLE: Morus nigra leaf extracts.
Effect of M. nigra extract on mice jejunal goblet cells (arrow head) infected with E. papillata. (A) non-infected control group (B) MNLE treated group (C) E. papillata infected group with decreased number of goblet cells (D) infected-MNLE treated group. Sections were stained with Alcian blue. Bar = 50 μm. MNLE: Morus nigra leaf extracts.
Effect of M. nigra extract on the number of goblet cells on day 5 p.i. with E. papillata. *Significance against non-infected control group (p ≤ 0.01). #Significance against E. papillata-infected group (p < 0.01). MNLE: Morus nigra leaf extracts.
Discussion
Eimeriosis affects most animal species, causing considerable economic loss in many countries (Andrews et al., 2004Andrews AH, Blowey RW, Boyd H, Eddy RG. Bovine medicine disease and husbandry of cattle. 2nd ed. Oxford: Blackwell Science; 2004.). E. papillata infections occur when sporozoites of ingested sporulated oocysts are released, invade the jejunal epithelial cells, and rapidly multiply before oocysts are formed. Within the oocysts, outside the host sporogony occurs and they become infectious (Pakandl, 2005Pakandl M. Selection of precocious line of the rabbit coccidium Eimeria flavescens Marotel and Guilhon (1941) and characterisation of its endogenous cycle. Parasitol Res 2005; 97(2): 150-155. http://dx.doi.org/10.1007/s00436-005-1411-x. PMid:15986244.
http://dx.doi.org/10.1007/s00436-005-141...
). Drug resistant eimeriosis has been reported (Williams, 1999Williams RB. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int J Parasitol 1999; 29(8): 1209-1229. http://dx.doi.org/10.1016/S0020-7519(99)00086-7. PMid:10576573.
http://dx.doi.org/10.1016/S0020-7519(99)...
; Chapman, 2014Chapman HD. Milestones in avian coccidiosis research: a review. Poult Sci 2014; 93(3): 501-511. http://dx.doi.org/10.3382/ps.2013-03634. PMid:24604841.
http://dx.doi.org/10.3382/ps.2013-03634...
) and, therefore, research into the use natural of products as antiparasitic agents has been the focus of attention because these products are more effective, less harmful, and exhibit fewer less side effects than conventional chemical agents (Wunderlich et al., 2014Wunderlich F, Al-Quraishy S, Steinbrenner H, Sies H, Dkhil MA. Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol Res 2014; 113(10): 3547-3556. http://dx.doi.org/10.1007/s00436-014-4101-8. PMid:25185667.
http://dx.doi.org/10.1007/s00436-014-410...
).
Several studies have reported the effects of mulberry in treating many diseases (Ody, 2000Ody P. The complete guide medicinal herbal. 2nd ed. London: Dorling Kindersley; 2000.; Naderi et al., 2004Naderi GA, Asgary S, Sarraf-Zadegan N, Oroojy H, Afshin-Nia F. Antioxidant activity of three extracts of Mores nigra. Phytother Res 2004; 18(5): 365-369. http://dx.doi.org/10.1002/ptr.1400. PMid:15173994.
http://dx.doi.org/10.1002/ptr.1400...
; Wang et al., 2009Wang L, Gong T, Chen RY. Two new prenylflavonoids from Morus nigra L. Chin Chem Lett 2009; 20(12): 1469-1471. http://dx.doi.org/10.1016/j.cclet.2009.06.035.
http://dx.doi.org/10.1016/j.cclet.2009.0...
) and its potential effectiveness as an antiparasitic agent (Riffat et al., 1986Riffat S, Akhtar MS, Javed I, Shah BH. Antinematodal and anticestodal efficacy of Morus alba Linn. stem bark in sheep. Pak J Agric Sci 1986; 23(3-4): 122-129.; Ercisli & Orhan 2007Ercisli S, Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem 2007; 103(4): 1380-1384. http://dx.doi.org/10.1016/j.foodchem.2006.10.054.
http://dx.doi.org/10.1016/j.foodchem.200...
; Nguyen-Pouplin et al., 2007Nguyen-Pouplin J, Tran H, Tran H, Phan TA, Dolecek C, Farrar J, et al. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J Ethnopharmacol 2007; 109(3): 417-427. http://dx.doi.org/10.1016/j.jep.2006.08.011. PMid:17010546.
http://dx.doi.org/10.1016/j.jep.2006.08....
). In this study, MNLE (300 mg/mL) affected the oocysts sporulation, which is attributable to the presence of numerous bioactive phytochemical constituents (Sharma et al., 2010Sharma SB, Tanwar RS, Rini A, Singh UR, Gupta S, Shukla SK. Protective effect of Morus rubra L. leaf extract on diet-induced atherosclerosis in diabetic rats. Indian J Biochem Biophys 2010; 47(1): 26-31. PMid:21086751.). In addition, our data showed that formalin (5%) as a hazard chemical completely inhibited sporulation of E. papillata and Gadelhaq et al. (2018)Gadelhaq SM, Arafa WM, Abolhadid SM. In vitro activity of natural and chemical products on sporulation of Eimeria species oocysts of chickens. Vet Parasitol 2018; 251: 12-16. http://dx.doi.org/10.1016/j.vetpar.2017.12.020. PMid:29426468.
http://dx.doi.org/10.1016/j.vetpar.2017....
reported that formalin (10%) completely inhibited sporulation of Eimeria tenella. In addition, Chroustová & Pinka (1987)Chroustová E, Pinka K. The efficiency of disinfectants on the oocysts of Eimeria tenella. Acta Vet Brno 1987; 56(1-2): 141-149. http://dx.doi.org/10.2754/avb198756010141.
http://dx.doi.org/10.2754/avb19875601014...
reported that formalin (2%) significantly affected sporulation of E. tenella oocyst. Formalin contains a highly reactive chemical (40% formaldehyde in water) (Power, 1995Power EGM. Aldehydes as biocides. Prog Med Chem 1995; 34: 149-201. http://dx.doi.org/10.1016/S0079-6468(08)70107-3.
http://dx.doi.org/10.1016/S0079-6468(08)...
) that interacts with proteins in vitro (Fraenkel-Conrat et al., 1945Fraenkel-Conrat H, Cooper M, Olcott HS. The reaction of formaldehyde with proteins. J Am Chem Soc 1945; 67(6): 950-954. http://dx.doi.org/10.1021/ja01222a023.
http://dx.doi.org/10.1021/ja01222a023...
) to inhibit sporulation.
Ethanol (70%) inhibited sporulation and oocyst wall deterioration, considering that the most potent concentration of antimicrobial alcohol is between 60 to 90% (McDonnell & Russell, 1999McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 1999; 12(1): 147-179. http://dx.doi.org/10.1128/CMR.12.1.147. PMid:9880479.
http://dx.doi.org/10.1128/CMR.12.1.147...
). Ethanol causes rapid denaturation of proteins, disrupts membranes, and causes subsequent interference with metabolism and cell lysis (Morton, 1983Morton HE. Alcohols. In: Bloch SS, editor. Disinfection, sterilization, and preservation. 3rd ed. Philadelphia: Lea & Febiger; 1983. p. 225-239.; Larson & Morton, 1991Larson EL, Morton HE. Alcohols. In: Bloch SS, editor. Disinfection, sterilization, and preservation. 4th ed. Philadelphia: Lea & Febiger; 1991. p. 191-203.). In addition, phenol (10%) has been reported to inhibit sporulation (Samaha et al., 2013Samaha HA, Haggag YN, Nossair MA, Habib HM. Assessment efficiency of some chemical disinfectants commonly used Against Coccidia in poultry farms. Alex J Vet Sci 2013; 39: 82-90.) whereas, in contrast, DettolTM had no effect on oocyst sporulation. These findings may be because the oocyst wall is impermeable to water-soluble substances and resistant to proteolysis (Kuticic & Wikerhauser, 1996Kuticic V, Wikerhauser T. Studies of the effect of various treatments on the viability of Toxoplasma gondii tissue cysts and Oocysts. In: Gross U, editor. Toxoplasma gondii: current topics in microbiology and immunology. Berlin: Springer; 1996. (vol. 219). http://dx.doi.org/10.1007/978-3-642-51014-4_23.
http://dx.doi.org/10.1007/978-3-642-5101...
; Mai et al., 2009Mai K, Sharman AP, Walker AR, Katrib M, Souza DD, McConville JM, et al. Oocyst wall formation and composition in coccidian parasites. Mem Inst Oswaldo Cruz 2009; 104(2): 281-289. http://dx.doi.org/10.1590/S0074-02762009000200022. PMid:19430654.
http://dx.doi.org/10.1590/S0074-02762009...
).
Jejunal infection with E. papillata in mice results in oxidative damage and serious local and systemic inflammatory responses (Dkhil et al., 2013Dkhil MA, Al-Quraishy S, Abdel Moneim AE, Delic D. Protective effect of Azadirachta indica extract against Eimeria papillata-induced coccidiosis. Parasitol Res 2013; 112(1): 101-106. http://dx.doi.org/10.1007/s00436-012-3109-1. PMid:22972359.
http://dx.doi.org/10.1007/s00436-012-310...
). M. nigra is a plant that possesses anti-inflammatory (Yildirim et al., 2019Yildirim TT, Ozan G, Dundar S, Bozoglan A, Karaman T, Dildes N, et al. The effects of morus nigra on the alveolar bone loss in experimentally-induced periodontitis. Eur Oral Res 2019; 53(3): 99-105. http://dx.doi.org/10.26650/eor.20190021. PMid:31579889.
http://dx.doi.org/10.26650/eor.20190021...
), antioxidant (Lee et al., 2018Lee J, Lee HJ, Lee JJ. Coating rice with mulberry leaves rich in deoxynojirimycin ameliorates hyperglycemia and dyslipidemia in C57BL/KsJ db/db mice. Nutr Res Pract 2018; 12(6): 469-478. http://dx.doi.org/10.4162/nrp.2018.12.6.469. PMid:30515274.
http://dx.doi.org/10.4162/nrp.2018.12.6....
), and antiparasitic (Ercisli & Orhan, 2007Ercisli S, Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem 2007; 103(4): 1380-1384. http://dx.doi.org/10.1016/j.foodchem.2006.10.054.
http://dx.doi.org/10.1016/j.foodchem.200...
) activity. The leaves of black mulberry contain flavonoids, ascorbic, and phenolics (Iqbal et al., 2012Iqbal S, Younas U, Sirajuddin U, Chan KW, Sarfraz RA, Uddin K. Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): a comparative study. Int J Mol Sci 2012; 13(6): 6651-6664. http://dx.doi.org/10.3390/ijms13066651. PMid:22837655.
http://dx.doi.org/10.3390/ijms13066651...
; Chen et al., 2016Chen H, Pu J, Liu D, Yu W, Shao Y, Yang G, et al. Anti-inflammatory and antinociceptive properties of flavonoids from the fruits of black mulberry (Morus nigra L.). PLoS One 2016; 11(4): e0153080. http://dx.doi.org/10.1371/journal.pone.0153080. PMid:27046026.
http://dx.doi.org/10.1371/journal.pone.0...
). MNLE showed anticoccidial activity following treatment of mice, as evidenced by a significant reduction in the production of E. papillata oocysts in infected mouse feces and oocysts in the jejunal villi. These results are in agreement with those of other studies that investigated the Punica granatum plant (Amer et al., 2015Amer OS, Dkhil MA, Hikal WM, Al-Quraishy S. Antioxidant and anti-inflammatory activities of pomegranate (Punica granatum) on Eimeria papillata-induced infection in mice. BioMed Res Int 2015; 2015: 219670. http://dx.doi.org/10.1155/2015/219670. PMid:25654088.
http://dx.doi.org/10.1155/2015/219670...
) and Azadirachta indica (Dkhil et al., 2013Dkhil MA, Al-Quraishy S, Abdel Moneim AE, Delic D. Protective effect of Azadirachta indica extract against Eimeria papillata-induced coccidiosis. Parasitol Res 2013; 112(1): 101-106. http://dx.doi.org/10.1007/s00436-012-3109-1. PMid:22972359.
http://dx.doi.org/10.1007/s00436-012-310...
) as potential sources of anticoccidial agents.
Parasitic infections cause a decrease in the number of goblet cells, which are known to be significant immunocompetent intestinal cells that secrete mucus (Linh et al., 2009Linh BK, Hayashi T, Horii Y. Eimeria vermiformis infection reduces goblet cells by multiplication in the crypt cells of the small intestine of C57BL/6 mice. Parasitol Res 2009; 104(4): 789-794. http://dx.doi.org/10.1007/s00436-008-1256-1. PMid:19005680.
http://dx.doi.org/10.1007/s00436-008-125...
). These cells may be reduced by the parasite-induced damage to stem cells (Cheng, 1974Cheng H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. II Mucous cells. Am J Anat 1974; 141(4): 481-501. http://dx.doi.org/10.1002/aja.1001410404. PMid:4440633.
http://dx.doi.org/10.1002/aja.1001410404...
). A decrease in the number of goblet cells encourages an increase in opportunistic pathogens or their penetration of the local epithelium (Yunus et al., 2005Yunus M, Horii Y, Makimura S, Smith AL. Murine goblet cell hypoplasia during Eimeria pragensis infection is ameliorated by clindamycin treatment. J Vet Med Sci 2005; 67(3): 311-315. http://dx.doi.org/10.1292/jvms.67.311. PMid:15805736.
http://dx.doi.org/10.1292/jvms.67.311...
). MNLE significantly increased the number of goblet cells, which was likely mediated by the numerous bioactive phytochemical constituents present in mulberry leaves (Sharma et al., 2010Sharma SB, Tanwar RS, Rini A, Singh UR, Gupta S, Shukla SK. Protective effect of Morus rubra L. leaf extract on diet-induced atherosclerosis in diabetic rats. Indian J Biochem Biophys 2010; 47(1): 26-31. PMid:21086751.).
Different plant or herbal extracts are widely used as poultry diets to promote growth rates and animal health, particularly when health challenges are required. Quite enough research has confirmed the beneficial effects of plant extract on poultry productivity (Alçiçek et al., 2004Alçiçek A, Bozkurt M, Çabuk M. The effect of a mixture of herbal essential oils, an organic acid or a probiotic on broiler performance. S Afr J Anim Sci 2004; 34(4): 217-222.; Gracia et al., 2016Gracia MI, Millán C, Sánchez J, Guyard-Nicodème M, Mayot J, Carre Y, et al. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period: part B. Poult Sci 2016; 95(4): 886-892. http://dx.doi.org/10.3382/ps/pev346. PMid:26706354.
http://dx.doi.org/10.3382/ps/pev346...
). Due to the anticoccidial activity of MNLE, we assumed that it could be used as a food additive in poultry feed.
Our results indicate that MNLE possessed a powerful antiparasitic and anti-sporulation activity. Additional studies are needed to elucidate the histological and molecular mechanism of sporulation inhibition by MNLE and its protective effects against E. Papillata-induced intestinal injury.
Acknowledgements
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the young researcher funding program (Grant no# YR-1440-7).
-
How to cite: Thagfan FA, Al-Megrin WA, Al-Quraishy S, Dkhil MAM. Mulberry extract as an ecofriendly anticoccidial agent: in vitro and in vivo application. Braz J Vet Parasitol 2020; 29(4): e009820. https://doi.org/10.1590/S1984-29612020072
References
- Abbasi AM, Khan MA, Ahmad M, Munir M, Zafar M, Sultana S, et al. Ethnobotanical and taxonomic screening of genus Morus for wild edible fruits used by the inhabitants of Lesser Himalayas-Pakistan. J Med Plants Res 2014; 8(25): 889-898. http://dx.doi.org/10.5897/JMPR2010.733
» http://dx.doi.org/10.5897/JMPR2010.733 - Alçiçek A, Bozkurt M, Çabuk M. The effect of a mixture of herbal essential oils, an organic acid or a probiotic on broiler performance. S Afr J Anim Sci 2004; 34(4): 217-222.
- Allen A, Hutton DA, Leonard AJ, Pearson JP, Sellers LA. The role of mucus in the protection of the gastroduodenal mucosa. Scand J Gastroenterol Suppl 1986;21(Suppl 125): 71-78. http://dx.doi.org/10.3109/00365528609093820 PMid:3103205.
» http://dx.doi.org/10.3109/00365528609093820 - Amer OS, Dkhil MA, Hikal WM, Al-Quraishy S. Antioxidant and anti-inflammatory activities of pomegranate (Punica granatum) on Eimeria papillata-induced infection in mice. BioMed Res Int 2015; 2015: 219670. http://dx.doi.org/10.1155/2015/219670 PMid:25654088.
» http://dx.doi.org/10.1155/2015/219670 - Andrews AH, Blowey RW, Boyd H, Eddy RG. Bovine medicine disease and husbandry of cattle 2nd ed. Oxford: Blackwell Science; 2004.
- Chapman HD. Milestones in avian coccidiosis research: a review. Poult Sci 2014; 93(3): 501-511. http://dx.doi.org/10.3382/ps.2013-03634 PMid:24604841.
» http://dx.doi.org/10.3382/ps.2013-03634 - Chen H, Pu J, Liu D, Yu W, Shao Y, Yang G, et al. Anti-inflammatory and antinociceptive properties of flavonoids from the fruits of black mulberry (Morus nigra L.). PLoS One 2016; 11(4): e0153080. http://dx.doi.org/10.1371/journal.pone.0153080 PMid:27046026.
» http://dx.doi.org/10.1371/journal.pone.0153080 - Cheng H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. II Mucous cells. Am J Anat 1974; 141(4): 481-501. http://dx.doi.org/10.1002/aja.1001410404 PMid:4440633.
» http://dx.doi.org/10.1002/aja.1001410404 - Chroustová E, Pinka K. The efficiency of disinfectants on the oocysts of Eimeria tenella. Acta Vet Brno 1987; 56(1-2): 141-149. http://dx.doi.org/10.2754/avb198756010141
» http://dx.doi.org/10.2754/avb198756010141 - Collier CT, Hofacre CL, Payne AM, Anderson DB, Kaiser P, Mackie RI, et al. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet Immunol Immunopathol 2008; 122(1-2): 104-115. http://dx.doi.org/10.1016/j.vetimm.2007.10.014 PMid:18068809.
» http://dx.doi.org/10.1016/j.vetimm.2007.10.014 - Dkhil MA, Abdel-Baki AS, Wunderlich F, Sies H, Al-Quraishy S. Anticoccidial and antiinflammatory activity of garlic in murine Eimeria papillata infections. Vet Parasitol 2011; 175(1-2): 66-72. http://dx.doi.org/10.1016/j.vetpar.2010.09.009 PMid:20943319.
» http://dx.doi.org/10.1016/j.vetpar.2010.09.009 - Dkhil MA, Al-Quraishy S, Abdel Moneim AE, Delic D. Protective effect of Azadirachta indica extract against Eimeria papillata-induced coccidiosis. Parasitol Res 2013; 112(1): 101-106. http://dx.doi.org/10.1007/s00436-012-3109-1 PMid:22972359.
» http://dx.doi.org/10.1007/s00436-012-3109-1 - Drury RAB, Wallington EA. Carleton’s histological technique 5th ed. Oxford: Oxford University Press; 1980.
- Ercisli S, Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem 2007; 103(4): 1380-1384. http://dx.doi.org/10.1016/j.foodchem.2006.10.054
» http://dx.doi.org/10.1016/j.foodchem.2006.10.054 - Fraenkel-Conrat H, Cooper M, Olcott HS. The reaction of formaldehyde with proteins. J Am Chem Soc 1945; 67(6): 950-954. http://dx.doi.org/10.1021/ja01222a023
» http://dx.doi.org/10.1021/ja01222a023 - Gadelhaq SM, Arafa WM, Abolhadid SM. In vitro activity of natural and chemical products on sporulation of Eimeria species oocysts of chickens. Vet Parasitol 2018; 251: 12-16. http://dx.doi.org/10.1016/j.vetpar.2017.12.020 PMid:29426468.
» http://dx.doi.org/10.1016/j.vetpar.2017.12.020 - Gracia MI, Millán C, Sánchez J, Guyard-Nicodème M, Mayot J, Carre Y, et al. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period: part B. Poult Sci 2016; 95(4): 886-892. http://dx.doi.org/10.3382/ps/pev346 PMid:26706354.
» http://dx.doi.org/10.3382/ps/pev346 - Iqbal S, Younas U, Sirajuddin U, Chan KW, Sarfraz RA, Uddin K. Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): a comparative study. Int J Mol Sci 2012; 13(6): 6651-6664. http://dx.doi.org/10.3390/ijms13066651 PMid:22837655.
» http://dx.doi.org/10.3390/ijms13066651 - Kalia AN. Textbook of industrial pharmacognosy 1st ed. New Delhi: CBS Publishers and Distributers; 2009.
- Kanthal LK, Dey A, Satyavathi K, Bhojaraju P. GC-MS analysis of bio-active compounds in methanolic extract of Lactuca runcinata DC. Pharmacognosy Res 2014; 6(1): 58-61. http://dx.doi.org/10.4103/0974-8490.122919 PMid:24497744.
» http://dx.doi.org/10.4103/0974-8490.122919 - Kuticic V, Wikerhauser T. Studies of the effect of various treatments on the viability of Toxoplasma gondii tissue cysts and Oocysts. In: Gross U, editor. Toxoplasma gondii: current topics in microbiology and immunology Berlin: Springer; 1996. (vol. 219). http://dx.doi.org/10.1007/978-3-642-51014-4_23
» http://dx.doi.org/10.1007/978-3-642-51014-4_23 - Larson EL, Morton HE. Alcohols. In: Bloch SS, editor. Disinfection, sterilization, and preservation 4th ed. Philadelphia: Lea & Febiger; 1991. p. 191-203.
- Lee J, Lee HJ, Lee JJ. Coating rice with mulberry leaves rich in deoxynojirimycin ameliorates hyperglycemia and dyslipidemia in C57BL/KsJ db/db mice. Nutr Res Pract 2018; 12(6): 469-478. http://dx.doi.org/10.4162/nrp.2018.12.6.469 PMid:30515274.
» http://dx.doi.org/10.4162/nrp.2018.12.6.469 - Linh BK, Hayashi T, Horii Y. Eimeria vermiformis infection reduces goblet cells by multiplication in the crypt cells of the small intestine of C57BL/6 mice. Parasitol Res 2009; 104(4): 789-794. http://dx.doi.org/10.1007/s00436-008-1256-1 PMid:19005680.
» http://dx.doi.org/10.1007/s00436-008-1256-1 - Mai K, Sharman AP, Walker AR, Katrib M, Souza DD, McConville JM, et al. Oocyst wall formation and composition in coccidian parasites. Mem Inst Oswaldo Cruz 2009; 104(2): 281-289. http://dx.doi.org/10.1590/S0074-02762009000200022 PMid:19430654.
» http://dx.doi.org/10.1590/S0074-02762009000200022 - McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 1999; 12(1): 147-179. http://dx.doi.org/10.1128/CMR.12.1.147 PMid:9880479.
» http://dx.doi.org/10.1128/CMR.12.1.147 - Mehlhorn H. Encyclopedic reference of parasitology 4th ed. Berlin: Springer; 2014.
- Morton HE. Alcohols. In: Bloch SS, editor. Disinfection, sterilization, and preservation 3rd ed. Philadelphia: Lea & Febiger; 1983. p. 225-239.
- Mughal TA, Arshad S, Mahboob S. Evaluation of anthelmintic activity of some members of family Moraceae. J Med Plants Res 2013; 7(30): 2275-2279. http://dx.doi.org/10.5897/JMPR2013.4420
» http://dx.doi.org/10.5897/JMPR2013.4420 - Naderi GA, Asgary S, Sarraf-Zadegan N, Oroojy H, Afshin-Nia F. Antioxidant activity of three extracts of Mores nigra. Phytother Res 2004; 18(5): 365-369. http://dx.doi.org/10.1002/ptr.1400 PMid:15173994.
» http://dx.doi.org/10.1002/ptr.1400 - Nguyen-Pouplin J, Tran H, Tran H, Phan TA, Dolecek C, Farrar J, et al. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J Ethnopharmacol 2007; 109(3): 417-427. http://dx.doi.org/10.1016/j.jep.2006.08.011 PMid:17010546.
» http://dx.doi.org/10.1016/j.jep.2006.08.011 - Ody P. The complete guide medicinal herbal 2nd ed. London: Dorling Kindersley; 2000.
- Orengo J, Buendía AJ, Ruiz-Ibáñez MR, Madrid J, Del Río L, Catalá-Gregori P, et al. Evaluating the efficacy of cinnamaldehyde and Echinacea purpurea plant extract in broilers against Eimeria acervulina. Vet Parasitol 2012; 185(2-4): 158-163. http://dx.doi.org/10.1016/j.vetpar.2011.09.024 PMid:21996002.
» http://dx.doi.org/10.1016/j.vetpar.2011.09.024 - Pakandl M. Selection of precocious line of the rabbit coccidium Eimeria flavescens Marotel and Guilhon (1941) and characterisation of its endogenous cycle. Parasitol Res 2005; 97(2): 150-155. http://dx.doi.org/10.1007/s00436-005-1411-x PMid:15986244.
» http://dx.doi.org/10.1007/s00436-005-1411-x - Pan G, Lou CF. Isolation of an 1-aminocyclopropane-1-carboxylate oxidase gene from mulberry (Morus alba L.) and analysis of the function of this gene in plant development and stresses response. J Plant Physiol 2008; 165(11): 1204-1213. http://dx.doi.org/10.1016/j.jplph.2007.02.012 PMid:17997189.
» http://dx.doi.org/10.1016/j.jplph.2007.02.012 - Pérez-Gregorio M, Regueiro J, Alonso-González E, Pastrana-Castro L, Simal-Gándara J. Influence of alcoholic fermentation process on antioxidant activity and phenolic levels from mulberries (Morus nigra L.). Lebensm Wiss Technol 2011; 44(8): 1793-1801. http://dx.doi.org/10.1016/j.lwt.2011.03.007
» http://dx.doi.org/10.1016/j.lwt.2011.03.007 - Power EGM. Aldehydes as biocides. Prog Med Chem 1995; 34: 149-201. http://dx.doi.org/10.1016/S0079-6468(08)70107-3
» http://dx.doi.org/10.1016/S0079-6468(08)70107-3 - Riffat S, Akhtar MS, Javed I, Shah BH. Antinematodal and anticestodal efficacy of Morus alba Linn stem bark in sheep. Pak J Agric Sci 1986; 23(3-4): 122-129.
- Rodrigues EL, Marcelino G, Silva GT, Figueiredo PS, Garcez WS, Corsino J, et al. Nutraceutical and medicinal potential of the Morus species in metabolic dysfunctions. Int J Mol Sci 2019; 20(2): 301. http://dx.doi.org/10.3390/ijms20020301 PMid:30646503.
» http://dx.doi.org/10.3390/ijms20020301 - Samaha HA, Haggag YN, Nossair MA, Habib HM. Assessment efficiency of some chemical disinfectants commonly used Against Coccidia in poultry farms. Alex J Vet Sci 2013; 39: 82-90.
- Schito ML, Barta JR, Chobotar B. Comparison of four murine Eimeria species in immunocompetent and immunodeficient mice. J Parasitol 1996; 82(2): 255-262. http://dx.doi.org/10.2307/3284157 PMid:8604093.
» http://dx.doi.org/10.2307/3284157 - Sharma SB, Tanwar RS, Rini A, Singh UR, Gupta S, Shukla SK. Protective effect of Morus rubra L. leaf extract on diet-induced atherosclerosis in diabetic rats. Indian J Biochem Biophys 2010; 47(1): 26-31. PMid:21086751.
- Shirley MW, Smith AL, Blake DP. Challenges in the successful control of the avian coccidia. Vaccine 2007; 25(30): 5540-5547. http://dx.doi.org/10.1016/j.vaccine.2006.12.030 PMid:17224208.
» http://dx.doi.org/10.1016/j.vaccine.2006.12.030 - Stephen B, Rommel M, Daugschies A, Haberkorn A. Studies of resistance to anticoccidials in Eimeria field isolates and pure Eimeria strains. Vet Parasitol 1997; 69(1-2): 19-29. http://dx.doi.org/10.1016/S0304-4017(96)01096-5 PMid:9187026.
» http://dx.doi.org/10.1016/S0304-4017(96)01096-5 - Wang L, Gong T, Chen RY. Two new prenylflavonoids from Morus nigra L. Chin Chem Lett 2009; 20(12): 1469-1471. http://dx.doi.org/10.1016/j.cclet.2009.06.035
» http://dx.doi.org/10.1016/j.cclet.2009.06.035 - Williams RB. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int J Parasitol 1999; 29(8): 1209-1229. http://dx.doi.org/10.1016/S0020-7519(99)00086-7 PMid:10576573.
» http://dx.doi.org/10.1016/S0020-7519(99)00086-7 - Wunderlich F, Al-Quraishy S, Steinbrenner H, Sies H, Dkhil MA. Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol Res 2014; 113(10): 3547-3556. http://dx.doi.org/10.1007/s00436-014-4101-8 PMid:25185667.
» http://dx.doi.org/10.1007/s00436-014-4101-8 - Yang X, Yang L, Zheng H. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem Toxicol 2010; 48(8-9): 2374-2379. http://dx.doi.org/10.1016/j.fct.2010.05.074 PMid:20561945.
» http://dx.doi.org/10.1016/j.fct.2010.05.074 - Yildirim TT, Ozan G, Dundar S, Bozoglan A, Karaman T, Dildes N, et al. The effects of morus nigra on the alveolar bone loss in experimentally-induced periodontitis. Eur Oral Res 2019; 53(3): 99-105. http://dx.doi.org/10.26650/eor.20190021 PMid:31579889.
» http://dx.doi.org/10.26650/eor.20190021 - Yunus M, Horii Y, Makimura S, Smith AL. Murine goblet cell hypoplasia during Eimeria pragensis infection is ameliorated by clindamycin treatment. J Vet Med Sci 2005; 67(3): 311-315. http://dx.doi.org/10.1292/jvms.67.311 PMid:15805736.
» http://dx.doi.org/10.1292/jvms.67.311
Publication Dates
-
Publication in this collection
21 Oct 2020 -
Date of issue
2020
History
-
Received
28 Apr 2020 -
Accepted
08 July 2020