Abstract
This study aimed to identify the intestinal parasites of road-killed wild felines in the North Central and North, Paraná state, southern Brazil. The animals were monitored by sampling previously established transects. The places where the felines were run over were mapped, the animals were identified, and the gastrointestinal tract was evaluated. The feces were submitted to coproparasitological techniques of spontaneous sedimentation, floating in hypersaturated NaCl solution and centrifugal floating in zinc sulfate. All the parasitic structures detected were photomicrographed. In the coproparasitological analyses were identified oocysts of Cystoisospora spp., eggs of Ancylostomatidae, and Capillaria spp.; eggs of Aelurostrongylus spp., Toxocara spp., Physaloptera spp., Taenia spp., and Spirometra spp.; Aelurostrongylus abstrusus larvae; and eggs and adults of Ancylostoma cati and Taenia spp. One of the cats was parasitized by a flea of Ctenocephalides felis felis. Based on these results, the animals analyzed in this study supplied important samples for the evaluation of parasitic diversity of North of Paraná and suggested that this region may have conditions that allow the maintenance of these parasites life cycles in the environment and among wildlife.
Keywords:
Ecosystem; biodiversity; zoonosis; ecological corridors; Puma concolor; Leopardus guttulus
Resumo
O objetivo deste trabalho foi identificar os parasitas intestinais de felinos silvestres mortos em estradas nas regiões Norte Central e Norte, Paraná, sul do Brasil. Os animais foram monitorados por amostragem de transectos previamente estabelecidos. Os locais de atropelamento foram mapeados, os animais foram identificados e enviados para autópsias, durante as quais amostras de fezes foram coletadas e submetidas a técnicas coproparasitológicas de sedimentação espontânea, flutuação em solução hipersaturada de NaCl e flutuação por centrífugação em sulfato de zinco e fotografadas, quando estruturas parasitárias estavam presentes. Nas análises coproparasitológicas, foram identificados oocistos de Cystoisospora spp., ovos de Ancylostomatidae e Capillaria spp, Aelurostrongylus spp., Toxocara spp., Physaloptera spp., Taenia spp. e Spirometra spp.; larvas de Aelurostrongylus abstrusus; e ovos e adultos de Ancylostoma cati e Taenia spp. Um dos felídeos estava parasitado por Ctenocephalides felis felis. Com base nesses resultados, os animais analisados neste estudo forneceram amostras importantes para a avaliação da diversidade parasitária do Norte do Paraná e sugeriram que esta região pode apresentar condições que possibilitem a manutenção dos ciclos de vida desses parasitas no ambiente e entre a vida silvestre.
Palavras-chave:
Ecossistema; biodiversidade; zoonose; corredores ecológicos; Puma concolor; Leopardus guttulus
Introduction
The Brazilian wild felids comprise the Felidae family with three genus and nine species: Leopardus colocolo, Leopardus geoffroyi, Leopardus pardalis, Leopardus tigrinus, Leopardus guttulus, Leopardus wiedii, Puma concolor, Puma yagouaroundi, and Panthera onca (Cheida et al., 2011Cheida CC, Nakano-Oliveira E, Fusco-Costa R, Rocha-Mendes F, Quadro J. Ordem carnivora. In: Reis NR, Perachi AL, Pedro WA, Lima IP. Mamíferos do Brasil. 2. ed. Londrina: N R Reis; 2011. p. 235-288.; ICMBio, 2020Instituto Chico Mendes de Conservação e Biodiversidade – ICMBio. Carnívoros Brasileiros [online]. Brasília: ICMBio; 2020 [cited 2020 Aug 17]. Available from: https://www.icmbio.gov.br/cenap/carnivoros-brasileiros.html
https://www.icmbio.gov.br/cenap/carnivor...
). Most of the species have solitary and nocturn behavior and use large areas to feed and reproduction (Cheida et al., 2011Cheida CC, Nakano-Oliveira E, Fusco-Costa R, Rocha-Mendes F, Quadro J. Ordem carnivora. In: Reis NR, Perachi AL, Pedro WA, Lima IP. Mamíferos do Brasil. 2. ed. Londrina: N R Reis; 2011. p. 235-288.). These predators feed on a wide variety of animals, controlling populations and influencing the entire natural environment in which they live (Marchini et al., 2011Marchini S, Cavalcanti S, Cunha de Paula R. Predadores silvestres e animais domésticos guia prático de convivência. Brasília: ICMBio; 2011.).
According to Brazil Red Book of Threatened Species of Fauna (ICMBio, 2018Instituto Chico Mendes de Conservação e Biodiversidade – ICMBio. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção. Vol. 2 [online]. Brasília: ICMBio; 2018 [cited 2020 Aug 17]. Available from: https://www.icmbio.gov.br/portal/component/content/article/10187
https://www.icmbio.gov.br/portal/compone...
), seven species of Brazilian felids are considered vulnerable (L. geoffroyi, L. wiedii, L. colocolo, L. gutullus, P. concolor, P. yagouaroundi, and P. onca) and one considered endangered (L. tigrinus). Due to the felids are top predators of the food chain and might regulate the populations of other species, the decline of this group might pose a major threat to the entire ecosystem (Reis et al., 2006Reis NR, Peracchi AL, Pedro WA, Lima IP. Mamíferos do Brasil. In: Reis NR, Peracchi AL, Pedro WA, Lima IP, editores. Divisão de Processos Técnicos da Biblioteca Central da Universidade Estadual de Londrina. 2. ed. Londrina: EDUEL; 2006. p. 17-25.; ICMBio, 2020Instituto Chico Mendes de Conservação e Biodiversidade – ICMBio. Carnívoros Brasileiros [online]. Brasília: ICMBio; 2020 [cited 2020 Aug 17]. Available from: https://www.icmbio.gov.br/cenap/carnivoros-brasileiros.html
https://www.icmbio.gov.br/cenap/carnivor...
).
Although poaching, deforestation and pollution are included in the statistics of causes of death of wild animals, unfortunately, run over is now considered the main cause of death of these animals and is a significant threat to animal biodiversity (Seiler & Helldin, 2006Seiler A, Helldin J. Mortality in wildlife due to transportation. In: Davenport J, Davenport JL, editors. The ecology of transportation: managing mobility for the environment.Vol. 10. Dordrecht: Springer; 2006. p.165-189. https://doi.org/10.1007/1-4020-4504-2_8.
https://doi.org/10.1007/1-4020-4504-2_8...
; Ramos-Abrantes et al., 2018Ramos-Abrantes MM, Carreiro AN, Araújo DVF, Souza JG, Lima JPR, Cezar HRA, et al. Vertebrados silvestres atropelados na rodovia BR-230, Paraíba, Brasil. Pubvet 2018; 12(1): 139. http://dx.doi.org/10.22256/pubvet.v12n1a5.1-7.
http://dx.doi.org/10.22256/pubvet.v12n1a...
). In this context, road-killed animals can provide valuable samples to estimate the risk of exposure of these species to parasites and assist in assessing contamination of the environment by parasites with zoonotic potential (Nagamori et al., 2018Nagamori Y, Payton ME, Duncan-Decocq R, Johnson EM. Fecal survey of parasites in free-roaming cats in northcentral Oklahoma, United States. Vet Parasitol Reg Stud Reports 2018; 14: 50-53. http://dx.doi.org/10.1016/j.vprsr.2018.08.008. PMid:31014736.
http://dx.doi.org/10.1016/j.vprsr.2018.0...
).
As carnivores these animals participate effectively in the cycle of many parasites, having an important role in the maintenance of these agents and their transmission to other wild, domestic animals and even to man (Gressler et al., 2016Gressler LT, Noll JC, Freitas ÍB, Monteiro SG. Multiparasitism in a wild cat (Leopardus colocolo) (Carnivora: Felidae) in southern Brazil. Rev Bras Parasitol Vet 2016; 25(3): 374-377. http://dx.doi.org/10.1590/S1984-29612016047. PMid:27580395.
http://dx.doi.org/10.1590/S1984-29612016...
). Parasitism can affect your host's life in physiological, morphological and even reproductive aspects. In case of wild cats that occupy a prominent place in the food web, imbalances in its population are reflected in the entire ecological structure of its habitat (Marcogliese, 2005Marcogliese DJ. Parasites of the superorganism: are they indicators of ecosystem health? Int J Parasitol 2005; 35(7): 705-716. http://dx.doi.org/10.1016/j.ijpara.2005.01.015. PMid:15925594.
http://dx.doi.org/10.1016/j.ijpara.2005....
).
The dispersal areas of the wild animals might be evaluated by the studies with road-killed wild animals; since the vast majority are under some type of threat, generating data of biological and health interest makes it possible to carry out conservation actions (Beltrán-Saavedra et al., 2009Beltrán-Saavedra LF, Angulo S, Gonzales JL. Uso de metodologías de censos muestrales indirectos de fecas para evaluar endoparásitos en mamíferos silvestres: un ensayo en la Reserva Privada de San Miguelito, Santa Cruz, Bolivia. Ecol Boliv 2009; 44(1): 56-61.; Brandão et al., 2009Brandão ML, Chame M, Cordeiro JL, de Miranda Chaves SA. Diversidade de helmintos intestinais em mamíferos silvestres e domésticos na Caatinga do Parque Nacional Serra da Capivara, Sudeste do Piauí, Brasil. Rev Bras Parasitol Vet 2009;18(1 Suppl 1): 19-28. http://dx.doi.org/10.4322/rbpv.018e1004. PMid:20040186.
http://dx.doi.org/10.4322/rbpv.018e1004...
). In addition, coproparasitological research in wild felines has presented itself as an alternative for analyzing the health of the ecosystem and due to the direct action in the food chain, they are important bioindicators (Kusma et al., 2015Kusma SC, Wrublewski DM, Teixeira VN, Holdefer DR. Parasitos intestinais de Leopardus wiedii e Leopardus tigrinus (Felidae) da floresta nacional de três barras, SC. Luminária 2015; 17(1): 85-95.). The use of run over animals favors epidemiological and biological knowledge about these wild species, without the need for invasive collections in live animals. However, many animals are not collected because they are in an advanced decomposition process.
Given this scenario, this study aimed to identify the intestinal parasites of road-killed wild felines. It is noteworthy that the survey of information on parasitological fauna and the parasite-host interaction may foster a better understanding of the real conditions of the ecosystem and ecological corridors in the face of the adversities that affect the wild cats of the North Central and North of the Paraná regions.
Materials and Methods
Ethical issues
The project was approved by the Animal Use Ethics Committee (CEUA) of the State University of Londrina on March 10, 2017 under permit number 30/2017, and it was also approved by the Biodiversity Authorization and Information System (SISBIO) on December 19 October 2016 under permit number 55384-1.
Study site and animal collection
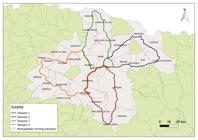
The work was carried out between 2016 and 2018 on the and roads that make up the North Central and North mesoregions in Paraná (Figure 1), which they were subdivided into transects including specifics municipalities (Tr): Tr1 - Londrina, Arapongas, Apucarana, Califórnia, Mauá da Serra, and Tamarana; Tr2 - Ibiporã, Jataizinho, Cornélio Procópio, Uraí, Sertaneja, and Sertanópolis; Tr3 Cambé, Rolândia, Prado Ferreira, Florestópolis, Alvorada do Sul, Bela Vista do Paraíso, and Londrina; and Tr4 - Arapongas, Mandaguari, Maringá, Astorga, Jaguapitã, Rolândia, Londrina (Figure 2). All transects have seasonal semideciduous forest vegetation and the biome is from the Atlantic Forest and interchangeable areas (Rossaneis, 2014Rossaneis BK. Medium and large-sized mammals in small forest remnants of the Atlantic Forest with anthropogenic influences in the North of Paraná. Semin Cienc Biol Saude 2014; 35(1): 15-24. http://dx.doi.org/10.5433/1679-0367.2014v35n1p15.
http://dx.doi.org/10.5433/1679-0367.2014...
). The four transects were travelled weekly by the project team, in a car at an average speed of 50km/hour for active search for road-killed animals. In addition, a partnership was signed with the 2nd Environmental Police Company and the 2nd Highway Police Company, which notified the team when animals were run over. All animals were collected dead, preferably in rigor mortis and had no evisceration.
Sample processing and coproparasitological analysis
The animals were sent to the Universidade Estadual de Londrina (UEL) for collection of ectoparasites and endoparasites. Stool samples were collected, refrigerated, and evaluated the presence of helminth eggs and protozoan at the Veterinary Parasitology and Parasitic Diseases Laboratory of the same institution.
Spontaneous sedimentation techniques (Hoffmann et al., 1934Hoffmann WA, Pons JA, Janer JL. The sedimentation concentration method in schistosomiasis. Puerto Rico J Public Health 1934; 9: 283-289.), floating in hypersaturated NaCl solution (Willis, 1921Willis HH. A simple levitation method for the detection of Hookworm Ova. Med J Aust 1921; 2(18): 375-376. http://dx.doi.org/10.5694/j.1326-5377.1921.tb60654.x.
http://dx.doi.org/10.5694/j.1326-5377.19...
) and centrifugal floating in zinc sulfate (Faust et al., 1939Faust EC, Sawitz W, Tobie J, Odom V, Peres C, Lincicome DR. Comparative efficiency of various technics for the diagnosis of protozoa and helminths in feces. J Parasitol 1939; 25(3): 241-262. http://dx.doi.org/10.2307/3272508.
http://dx.doi.org/10.2307/3272508...
) were performed. Two grams of feces were used for each of these techniques.
By the method of Hoffmann et al. (1934)Hoffmann WA, Pons JA, Janer JL. The sedimentation concentration method in schistosomiasis. Puerto Rico J Public Health 1934; 9: 283-289., the feces were mixed with 50 mL of water, filtered through gauze, and placed in a conical cup for spontaneous sedimentation of eggs and larvae. After 1 hour, the sediment was collected and a drop of it was placed on a slide and covered by a slide for reading under the microscope with a 100x objective.
For the Willis (1921)Willis HH. A simple levitation method for the detection of Hookworm Ova. Med J Aust 1921; 2(18): 375-376. http://dx.doi.org/10.5694/j.1326-5377.1921.tb60654.x.
http://dx.doi.org/10.5694/j.1326-5377.19...
technique feces were added to 15 mL of saturated NaCl solution, mixed until homogeneous, filtered through gauze and placed in the test tube until a meniscus was formed. A microscopy slide was placed over the meniscus, and after 15 minutes the slide was quickly inverted for removal, a coverslip was placed on the slide and a microscope reading was made using the 100 and 400x objective.
In the analyzes by the Faust et al. (1939)Faust EC, Sawitz W, Tobie J, Odom V, Peres C, Lincicome DR. Comparative efficiency of various technics for the diagnosis of protozoa and helminths in feces. J Parasitol 1939; 25(3): 241-262. http://dx.doi.org/10.2307/3272508.
http://dx.doi.org/10.2307/3272508...
method, 15 mL of zinc sulphate solution density 1,180 were added to the feces and this was mixed until homogeneous, this mixture was filtered through gauze, placed in a tube and centrifuged for 5 minutes at 2,000 rpm. With a platinum loop, a portion of the meniscus was removed, placed on a microscope slide, added with a drop of lugol and covered with a coverslip, then the microscope was read at 100 and 400x.
The parasitic structures observed during were photodocumented with an Olympus BX43 optical microscope (Shinjuku, Tokyo, Japan), 40x objective with an attached Olympus camera (QColor3). The analyses were performed with Olympus CellSens Standard® software, version 1.15 from 2016 (Olympus Scientific Solutions Americas Corp., US). The procedure for the identification of adults parasites was performed after the clarification, fixation and assembly of the slides as described in the literature (Amato & Amato, 2010Amato JFR, Amato SB. Técnicas gerais para coleta e preparação de helmintos endoparasitos de aves. In: Von Matter S, Straube FC, Accordi I, Piacetini V, Cândido-Jr JF. Ornitologia e conservação: ciência aplicada, técnicas de pesquisa e levantamento. Rio de Janeiro: Technical Books; 2010. p. 369-393.), as well as the identification of the eggs upon examination of the feces (Brandão et al., 2009Brandão ML, Chame M, Cordeiro JL, de Miranda Chaves SA. Diversidade de helmintos intestinais em mamíferos silvestres e domésticos na Caatinga do Parque Nacional Serra da Capivara, Sudeste do Piauí, Brasil. Rev Bras Parasitol Vet 2009;18(1 Suppl 1): 19-28. http://dx.doi.org/10.4322/rbpv.018e1004. PMid:20040186.
http://dx.doi.org/10.4322/rbpv.018e1004...
; Taylor et al., 2017Taylor MA, Coop RL, Wall RL. Helmintologia veterinária. In: Fagliari JJ, Rocha TG, editors. Parasitologia veterinária. 4th ed. Rio de Janeiro: Editora Guanabara Koogan; 2017. p. 65-478.). Ectoparasites were collected and identified, when present, in a binocular stereomicroscope based on the keys previously described (Bicho & Ribeiro, 1998Bicho CL, Ribeiro PB. Chave pictórica para as principais espécies de Siphonaptera de importância médica e veterinária, no Brasil. Rev Bras Parasitol Vet 1998; 7(1): 47-51.; Linardi & Santos, 2012Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): Some issues in correctly identify these species. Rev Bras Parasitol Vet 2012; 21(4): 345-354. http://dx.doi.org/10.1590/S1984-29612012000400002. PMid:23295817.
http://dx.doi.org/10.1590/S1984-29612012...
).
Georeferencing and statistical analysis
The collected wild felines were mapped using a Global Positioning System (GPS). The point distributions were plotted using QGIS 2.14 software (QGIS Development Team, 2016QGIS Development Team. QGIS Geographic Information System [online]. Chicago: Open Source Geospatial Foundation; 2016 [cited 2020 Dec 12]. Available from: https://www.qgis.org
https://www.qgis.org...
). The characteristics of the road-killed location, such as proximity to water courses and type of highway, and the animals conditions collected were tabulated and frequency analysis was performed using the software Epiinfo 3.5.4.
Results
Of the six felines run over, four were Leopardus guttulus, and two were Puma concolor (Tab. 1). Within the three transects covered, the animals were found in the municipalities of Apucarana, Faxinal, Ibiporã, Londrina, Sertaneja, and Tamarana (Figure 1). Among the animals included in the study,50.0% (3/6) of the animals were found close (100m) to a watercourse, and 66.7% (4/6) were run over on two-lane highways.
From the coproparasitological analyses, oocysts of Cystoisospora spp., eggs of Ancylostomatidae, Capillaria spp., Toxocara spp., Physaloptera spp., Taenia spp., and Spirometra spp., larvae of Aelurostrongylus abstrusus were identified. The adult forms of Toxocara cati and Taenia spp. were also observed macroscopically, as shown in Table 2. The pictures of parasitic structures observed on microscopic examination are shown in Figure 3.
Endoparasites identified in coproparasitological exams of road-killed wild cats from northern Paraná between 2017 and 2018 according to the technique used.
Parasitic structures observed in the microscopic examination of feces. (A) Cystoisospora spp.; (B) Ancylostomatidae; (C) Capillaria spp.; (D) Toxocara spp.; (E) Physaloptera spp.; (F) Taenia spp., (G) Spirometra spp.; H1. Anterior part of Aelurostrongylus abstrusus larva; H2. A. abstrusus larva tail.
Only Feline 2 (L. guttulus) was infested by an ectoparasite, which after identification was classified as a flea of the species Ctenocephalides felis felis (Bicho & Ribeiro 1998Bicho CL, Ribeiro PB. Chave pictórica para as principais espécies de Siphonaptera de importância médica e veterinária, no Brasil. Rev Bras Parasitol Vet 1998; 7(1): 47-51.; Linardi & Santos, 2012Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): Some issues in correctly identify these species. Rev Bras Parasitol Vet 2012; 21(4): 345-354. http://dx.doi.org/10.1590/S1984-29612012000400002. PMid:23295817.
http://dx.doi.org/10.1590/S1984-29612012...
).
Discussion
Linear infrastructure, such as highways, are characteristic of human action and they are the main causes of fragmentation of habitats, fauna deaths by run over, high predation around highways, and invasion of exotic and domestic species or humans in the wild environment (Forman & Alexander, 1998Forman RTT, Alexander LE. Roads and their major ecological effects. Annu Rev Ecol Syst 1998; 29(1): 207-231. http://dx.doi.org/10.1146/annurev.ecolsys.29.1.207.
http://dx.doi.org/10.1146/annurev.ecolsy...
; Laurance et al., 2009Laurance WF, Goosem M, Laurance SGW. Impacts of roads and linear clearings on tropical forests. Trends Ecol Evol 2009; 24(12): 659-669. http://dx.doi.org/10.1016/j.tree.2009.06.009. PMid:19748151.
http://dx.doi.org/10.1016/j.tree.2009.06...
). Currently, vehicle-induced mortality is one of the most studied effects, and the its vulnerability is associated with factors such as the type of locomotion, ecology and conduct and medium and large mammals, such as felids (Laurance et al., 2009Laurance WF, Goosem M, Laurance SGW. Impacts of roads and linear clearings on tropical forests. Trends Ecol Evol 2009; 24(12): 659-669. http://dx.doi.org/10.1016/j.tree.2009.06.009. PMid:19748151.
http://dx.doi.org/10.1016/j.tree.2009.06...
). However, in the region north of Paraná state there are no studies. In addition, the collection sites are poorly lit, which can favored the occurrence of accidents. Highways, therefore, may be increasing the risk of extinction of these mammals, especially when there is no warning of the presence of wild animals, even when close to the private natural heritage reserves; no radar to reduce speed in risk areas; or no presence of multiple lanes on the highway, making it difficult for drivers to respond quickly if animals are on the road. According to data from the Chico Mendes Institute for Biodiversity Conservation (ICMBio), the felines this study are at high risk of extinction in the wild and are classified as vulnerable (ICMBio, 2018Instituto Chico Mendes de Conservação e Biodiversidade – ICMBio. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção. Vol. 2 [online]. Brasília: ICMBio; 2018 [cited 2020 Aug 17]. Available from: https://www.icmbio.gov.br/portal/component/content/article/10187
https://www.icmbio.gov.br/portal/compone...
).
Dib et al. (2018)Dib LV, Cronemberger C, Pereira FDA, Bolais PF, Uchôa CMA, Bastos OMP, et al. Gastrointestinal parasites among felids inhabiting the Serra dos Órgãos National Park, Rio de Janeiro, Brazil. Brasil. Rev Bras Parasitol Vet 2018; 27(2): 131-140. http://dx.doi.org/10.1590/s1984-296120180016. PMid:29846454.
http://dx.doi.org/10.1590/s1984-29612018...
analyzed 82 fecal samples from felines in the Parque Nacional da Serra dos Órgãos, Rio de Janeiro. Most feces were collected from the ground in the environment. The authors detected eggs of the family Diphillobotriidae in 65.8% of the samples, eggs of the superfamily Ascaridoidea in 43.9%, and the nematode larvae and Strongylida eggs. The presence of other helminths, such as Capillaria spp., Trichuris sp., eggs of the order Spirurida and Platynosomum spp., and unsporulated coccid oocysts, were also recorded, differing from the results reported in this study, especially in relation to the variety of detected parasites. Napoli et al. (2016)Napoli E, Anile S, Arrabito C, Scornavacca D, Mazzamuto MV, Gaglio G, et al. Survey on parasitic infections in wildcat (Felis silvestris silvestris Schreber, 1777) by scat collection. Parasitol Res 2016; 115(1): 255-261. http://dx.doi.org/10.1007/s00436-015-4742-2. PMid:26377843.
http://dx.doi.org/10.1007/s00436-015-474...
conducted a study with 121 samples of wild felines from southern Italy, who collected feces from the ground and characterized them as being feces from felines by its shape and smell, finding 91% positivity, observed mainly Physaloptera spp., Taenia spp., Toxocara cati, Eucoleus aerophilus, Troglostrongylus brevior, and Cylicospirura sp., finding other parasites besides those we found in our study. It was possible that each region hosted a specific group of parasites.
Specimens of the genus Toxocara were found in three animals. Toxocara spp. it is usually transmitted by paratenic hosts in wild environments, moreover, this positive result may be related to the approach of these animals to urban areas or even the invasion of domestic animals into the wild environment (Carvalho & Rocha, 2011Carvalho EA, Rocha RL. Toxocariasis: visceral larva migrans in children. J Pediatr (Rio J) 2011; 87(2): 100-110. http://dx.doi.org/10.2223/JPED.2074. PMid:21503372.
http://dx.doi.org/10.2223/JPED.2074...
).
Kusma et al. (2015)Kusma SC, Wrublewski DM, Teixeira VN, Holdefer DR. Parasitos intestinais de Leopardus wiedii e Leopardus tigrinus (Felidae) da floresta nacional de três barras, SC. Luminária 2015; 17(1): 85-95. collected 35 samples from felids in Santa Catarina, of which 21 belonged to Leopardus wiedii, and found that 71.4% of the samples were positive for some form of parasite; the most abundant were Spirometra spp., with 85.7% prevalence, followed by eggs from Capillaria spp., with 71.4%, and larvae of Aelurostrongylus sp., with 38.1%. In the present study, Aelurostrongylus abstrusus was found in the feces of P. concolor. This parasite is a metastrongylid known to infect the lungs of domestic and wild felines. It has many paratenic hosts, which is why it is common in animals with the habit of hunting birds and rodents that may contain encysted larvae of the parasite (Di Cesare et al., 2014Di Cesare A, Frangipane di Regalbono A, Tessarin C, Seghetti M, Iorio R, Simonato G, et al. Mixed infection by Aelurostrongylus abstrusus and Troglostrongylus brevior in kittens from the same litter in Italy. Parasitol Res 2014; 113(2): 613-618. http://dx.doi.org/10.1007/s00436-013-3690-y. PMid:24271082.
http://dx.doi.org/10.1007/s00436-013-369...
). Therefore, it is likely that the sampled felids were infected by A. abstrusus when ingesting these paratenic hosts, which are common in forest areas and in agricultural areas, where all felines were found.
Spirometra spp. were found in 50.0% (3/6) of the animals, and all these animals, in turn, were found close to water courses. This fact is relevant since water plays a fundamental role in the hatching of eggs and release of coracidia, favoring dissemination and consequently maintenance of this protozoan in the environment. Parathenic hosts, such as fish, reptiles and amphibians also participate in the cycle when predated (Mueller, 1974Mueller JF. The biology of Spirometra. J Parasitol 1974; 60(1): 2-14. http://dx.doi.org/10.2307/3278670. PMid:4592501.
http://dx.doi.org/10.2307/3278670...
; Little & Ambrose, 2000Little S, Ambrose D. Spirometra infection in cats and dogs. Comp Cont Educ Pract 2000; 22(4): 299-305.), which can contain encysted plerocercoids in the musculature. Spirometra spp. are the causes of sparganosis, an infection with zoonotic potential resulting from the migration of plerocercoid larvae in tissues. In wild felines, this disease is associated with S. pretoriensis and S. theileri (Müller-Graf et al., 1999Müller-Graf CDM, Woolhouse MEJ, Packer C. Epidemiology of an intestinal parasite (Spirometra spp.) in two populations of African lions (Panthera leo). Parasitology 1999; 118(Pt 4): 407-415. http://dx.doi.org/10.1017/S0031182098003813. PMid:10340332.
http://dx.doi.org/10.1017/S0031182098003...
), and the species S. proliferum migrates in felids and can result in ascites, splenomegaly, hepatomegaly, gastric ulcerations, and serous thickening of the abdominal organs (Buergelt et al., 1984Buergelt CD, Greiner EC, Senior DF. Proliferative sparganosis in a cat. J Parasitol 1984; 70(1): 121-125. http://dx.doi.org/10.2307/3281933. PMid:6737156.
http://dx.doi.org/10.2307/3281933...
). However, in the autopsied animals, in a study conducted by Prada (2004)Prada CS. Atropelamento de vertebrados silvestres em uma região fragmentada do Nordeste do Estado de São Paulo: quantificação do impacto e análise de fatores envolvidos [tese] São Carlos: Universidade Federal de São Carlos; 2004., none of these pathological changes were found, suggesting that the mortality was due to territorial habits developed after fragmentation of the environment for the construction of roads, which forces them to move through the generated segments, increasing the chances of collision with vehicles on the track, rather than due to parasitism.
Capillaria spp. is a nematode commonly found in the intestines of birds and mammals and found in two of the six animals studied. This parasite was reported mostly in rodents of the genus Rattus, which is one of the main carriers of Capillaria hepatica (Layne, 1968Layne JN. Host and ecological relationships of the parasitic helminth Capillaria hepatica in Florida mammals. Zoologica (NY) 1968; 53(4): 107-123.). This may explain the infection of wild felines, since due to human deforestation actions, wildlife is coming closer to the urban environment in search of resources for survival (Angarita-Yanes & Caceres-Martínez, 2019Angarita-Yanes CE, Cáceres-Martínez CH. Reportes de felinos silvestres (Carnívora: Felidae) en el área urbana de Cúcuta, Nororiente de Colombia. Mammal Notes 2019; 5(2): 2-5. http://dx.doi.org/10.47603/manovol5n2.2-5.
http://dx.doi.org/10.47603/manovol5n2.2-...
). Infection can occur when the animal consumes parasite eggs present in the environment or when the eggs and adults form in host animal tissues (Ruas et al., 2003Ruas JL, Soares MP, Farias NAR, Brum JGW. Infecção por Capillaria hepatica em carnívoros silvestres (Lycalopex gymnocercus e Cerdocyon thous) na região sul do Rio Grande do Sul. Arq Inst Biol (Sao Paulo) 2003; 70(2): 127-130.). After ingestion, the eggs can migrate to the organs, according to Quadros et al. (2009)Quadros RM, Pilati C, Marques SMT, Mazzolli M, Benedet RC. Capillaria hepatica in Puma concolor: first Report in Brazil. J Zoo Wildl Med 2009; 40(3): 586-587. http://dx.doi.org/10.1638/2008-0194.1. PMid:19746880.
http://dx.doi.org/10.1638/2008-0194.1...
. Species that actually parasitize felines, such as Calodium hepatica, Capillaria plica and Capillaria feliscati, does not release eggs in the feces, therefore the eggs of Capillaria spp. found in that study were probably due to pseudoparasitism (Inforzato et al., 2009Inforzato GR, Santos WRM, Neves MF. Capilariose em gatos. Rev Cient Elet Med Vet 2009; 7(12): 1-5.; Karawita et al., 2016Karawita AC, Perera VP, Perera S, de Silva DS, Jayaweera WR, Himsworth CG, et al. Calodium hepaticum in Jungle Cats ( Felis chaus ) in Sri Lanka. J Wildl Dis 2016; 52(4): 971-972. http://dx.doi.org/10.7589/2016-02-046. PMid:27434415.
http://dx.doi.org/10.7589/2016-02-046...
).
In the present study, eggs from Taenia sp. were identified in the feces of only one of the animals collected (Feline 1, Puma concolor). In the scientific literature, species of parasites of this genus have already been reported to parasitize cougar, with a record of T. oligarthra in Brazil (Travassos, 1965Travassos L. Contribuição para a inventario crítico da zoologia no Brasil. Rio de Janeiro: Museu Nacional; 1965.) and reports of T. omissa in Peru, Florida, and Canada (Foster et al., 2006Foster GW, Cunningham MW, Kinsella JM, McLaughlin G, Forrester DJ. Gastrointestinal Helminths of Free-ranging Florida Panthers (Puma concolor coryi) and the Efficacy of the Current Anthelmintic Treatment Protocol. J Wildl Dis 2006; 42(2): 402-406. http://dx.doi.org/10.7589/0090-3558-42.2.402. PMid:16870865.
http://dx.doi.org/10.7589/0090-3558-42.2...
; Dare & Watkins, 2012Dare OK, Watkins WG. First record of parasites from Cougars (Puma concolor) in Manitoba, Canada. Can Field Nat 2012; 126(4): 324-327. http://dx.doi.org/10.22621/cfn.v126i4.1378.
http://dx.doi.org/10.22621/cfn.v126i4.13...
; Gomez-Puerta et al., 2016Gomez-Puerta LA, Alarcon V, Pacheco J, Franco F, Lopez-Urbina MT, Gonzalez AE. Molecular and morphological evidence of Taenia omissa in pumas (Puma concolor) in the Peruvian Highlands. Rev Bras Parasitol Vet 2016; 25(3): 368-373. http://dx.doi.org/10.1590/S1984-29612016046. PMid:27580394.
http://dx.doi.org/10.1590/S1984-29612016...
). It is known that wild felids have an exclusively carnivorous diet, which can favor infection by this parasite. Feline 1 also presented eggs of the genus Physaloptera in the feces; this species is a nematode whose definitive hosts are felids and rarely dogs. The adults remain attached to the stomach mucosa, while the intermediate stage, infectious larval forms parasitize arthropods, such as beetles, crickets and cockroaches (Taylor et al., 2017Taylor MA, Coop RL, Wall RL. Helmintologia veterinária. In: Fagliari JJ, Rocha TG, editors. Parasitologia veterinária. 4th ed. Rio de Janeiro: Editora Guanabara Koogan; 2017. p. 65-478.). The adult forms are hematophagous and can cause bleeding, mucosal erosion and gastritis (Dos Santos, 1975Santos JA. Patologia especial dos animais domésticos (mamíferos e aves). Rio de Janeiro: Instituto Interamericano de Ciências Agrícolas; 1975.). Areas of forest or agricultural production where animals are found are favorable for the transmission of Physaloptera spp., as the presence of both hosts is necessary for the maintenance and dissemination of parasites.
Mohsen & Hossein (2009)Mohsen A, Hossein H. Gastrointestinal parasites of stray cats in Kashan, Iran. Trop Biomed 2009; 26(1): 16-22. PMid:19696723. analyzed 113 fecal samples of stray cats in Iran and reported nematodes, cestoids and the sporozoa Isospora rivolta, Isospora felis, Sarcocystis spp., and Blastocystis spp. In our study, the only sporozoan found was of the genus Cystoisospora, which was present in the feces of the species P. concolor, which showed greater diversity of intestinal parasites among the analyzed samples. Isosporiasis is considered a parasitic disease that affects several animal species, and its pathogenicity depends on the animal's immune status; it commonly induces clinical changes that do not compromise the animal's well-being (Peixoto et al., 2019Peixoto TKF, Patrício IDF, Almeidas BV, Araújo Júnior VM, Viana IL, Borges Filho JA, et al. Isosporose em felino: relato de caso. MEDVEP Rev Cient Med Vet Pequenos Anim Anim Estim 2019; 16(32): 1-5.). Although P. concolor showed a greater diversity of intestinal parasites, it was most likely that the species in question did not die due to parasitism. Studies show that these species have cathemeral activity, which can vary according to the area in which they live due to factors such as food availability and the presence of threats (Albanesi et al., 2016Albanesi AS, Jayat JP, Brown AD. Patrones de actividad de mamíferos de medio y gran porte en el pedemonte de yungas del noroeste argentino. Mastozool Neotrop 2016; 23(2): 335-358.; Caceres-Martínez et al., 2016Caceres-Martínez CH, Acevedo Rincón AA, González-Maya JF. Terrestrial medium and large-sized mammal’s diversity and activity patterns from Tamá National Natural Park and buffer zone, Colombia. Therya 2016; 7(2): 285-298. http://dx.doi.org/10.12933/therya-16-397.
http://dx.doi.org/10.12933/therya-16-397...
), and these animals prefer to cross the highways at night, where there is less visibility with less chance of being observed and, at the same time, greater chance of being run over.
The fact that only one animal (Feline 2) was infested by Ctenocephalides felis felis may suggest that this low occurrence is linked to the fact that after death and the consequent drop in temperature of the host, these ectoparasites usually abandon the corpse, as occurs with fleas of species Tunga penetrans, Xenopsylla cheopis and Pulex irritans (Aragão, 1989Aragão MB. A propósito de “As duas parasitologias”. Cad Saude Publica 1989; 5(2): 234-235. http://dx.doi.org/10.1590/S0102-311X1989000200011.
http://dx.doi.org/10.1590/S0102-311X1989...
). C. felis felis is the most common flea species found in cats and dogs worldwide, and can be found in other domestic and wild mammals, it is the most prevalent species in wild cats (Rust & Dryden, 1997Rust MK, Dryden MW. The biology, ecology, and management of the cat flea. Annu Rev Entomol 1997; 42(1): 451-473. http://dx.doi.org/10.1146/annurev.ento.42.1.451. PMid:9017899.
http://dx.doi.org/10.1146/annurev.ento.4...
; Lawrence et al., 2014Lawrence AL, Brown GK, Peters B, Spielman DS, Morin-Adeline V, Šlapeta J. High phylogenetic diversity of the cat flea (Ctenocephalides felis) at two mitochondrial DNA markers. Med Vet Entomol 2014; 28(3): 330-336. http://dx.doi.org/10.1111/mve.12051. PMid:24548270.
http://dx.doi.org/10.1111/mve.12051...
; Clark et al., 2018Clark NJ, Seddon JM, Šlapeta J, Wells K. Parasite spread at the domestic animal - wildlife interface: anthropogenic habitat use, phylogeny and body mass drive risk of cat and dog flea (Ctenocephalides spp.) infestation in wild mammals. Parasit Vectors 2018; 11(1): 8. http://dx.doi.org/10.1186/s13071-017-2564-z. PMid:29307305.
http://dx.doi.org/10.1186/s13071-017-256...
).
Conclusion
In view of the parasite diversity found in our study, including species witch zoonotic potential, the importance of these animals for sampling to assess the parasitic diversity present in wild fauna was demonstrated. The results showed that northern Paraná, as well as the habits of these animals, offer conditions that allow the maintenance of parasites in the wild environment, and future studies should be considered in order to verify possible changes in this scenario.
-
How to cite: dos Santos Silva AC, Paschoal ATP, Bernardes JC, de Matos AMRN, Balbino LS, Santomauro RA, et al. Parasites in road-killed wild felines from North of Paraná state, Brazil. Braz J Vet Parasitol 2021; 30(1): e016320. https://doi.org/10.1590/S1984-296120201090
References
- Albanesi AS, Jayat JP, Brown AD. Patrones de actividad de mamíferos de medio y gran porte en el pedemonte de yungas del noroeste argentino. Mastozool Neotrop 2016; 23(2): 335-358.
- Amato JFR, Amato SB. Técnicas gerais para coleta e preparação de helmintos endoparasitos de aves. In: Von Matter S, Straube FC, Accordi I, Piacetini V, Cândido-Jr JF. Ornitologia e conservação: ciência aplicada, técnicas de pesquisa e levantamento Rio de Janeiro: Technical Books; 2010. p. 369-393.
- Angarita-Yanes CE, Cáceres-Martínez CH. Reportes de felinos silvestres (Carnívora: Felidae) en el área urbana de Cúcuta, Nororiente de Colombia. Mammal Notes 2019; 5(2): 2-5. http://dx.doi.org/10.47603/manovol5n2.2-5
» http://dx.doi.org/10.47603/manovol5n2.2-5 - Aragão MB. A propósito de “As duas parasitologias”. Cad Saude Publica 1989; 5(2): 234-235. http://dx.doi.org/10.1590/S0102-311X1989000200011
» http://dx.doi.org/10.1590/S0102-311X1989000200011 - Beltrán-Saavedra LF, Angulo S, Gonzales JL. Uso de metodologías de censos muestrales indirectos de fecas para evaluar endoparásitos en mamíferos silvestres: un ensayo en la Reserva Privada de San Miguelito, Santa Cruz, Bolivia. Ecol Boliv 2009; 44(1): 56-61.
- Bicho CL, Ribeiro PB. Chave pictórica para as principais espécies de Siphonaptera de importância médica e veterinária, no Brasil. Rev Bras Parasitol Vet 1998; 7(1): 47-51.
- Brandão ML, Chame M, Cordeiro JL, de Miranda Chaves SA. Diversidade de helmintos intestinais em mamíferos silvestres e domésticos na Caatinga do Parque Nacional Serra da Capivara, Sudeste do Piauí, Brasil. Rev Bras Parasitol Vet 2009;18(1 Suppl 1): 19-28. http://dx.doi.org/10.4322/rbpv.018e1004 PMid:20040186.
» http://dx.doi.org/10.4322/rbpv.018e1004 - Buergelt CD, Greiner EC, Senior DF. Proliferative sparganosis in a cat. J Parasitol 1984; 70(1): 121-125. http://dx.doi.org/10.2307/3281933 PMid:6737156.
» http://dx.doi.org/10.2307/3281933 - Caceres-Martínez CH, Acevedo Rincón AA, González-Maya JF. Terrestrial medium and large-sized mammal’s diversity and activity patterns from Tamá National Natural Park and buffer zone, Colombia. Therya 2016; 7(2): 285-298. http://dx.doi.org/10.12933/therya-16-397
» http://dx.doi.org/10.12933/therya-16-397 - Carvalho EA, Rocha RL. Toxocariasis: visceral larva migrans in children. J Pediatr (Rio J) 2011; 87(2): 100-110. http://dx.doi.org/10.2223/JPED.2074 PMid:21503372.
» http://dx.doi.org/10.2223/JPED.2074 - Cheida CC, Nakano-Oliveira E, Fusco-Costa R, Rocha-Mendes F, Quadro J. Ordem carnivora. In: Reis NR, Perachi AL, Pedro WA, Lima IP. Mamíferos do Brasil 2. ed. Londrina: N R Reis; 2011. p. 235-288.
- Clark NJ, Seddon JM, Šlapeta J, Wells K. Parasite spread at the domestic animal - wildlife interface: anthropogenic habitat use, phylogeny and body mass drive risk of cat and dog flea (Ctenocephalides spp.) infestation in wild mammals. Parasit Vectors 2018; 11(1): 8. http://dx.doi.org/10.1186/s13071-017-2564-z PMid:29307305.
» http://dx.doi.org/10.1186/s13071-017-2564-z - Dare OK, Watkins WG. First record of parasites from Cougars (Puma concolor) in Manitoba, Canada. Can Field Nat 2012; 126(4): 324-327. http://dx.doi.org/10.22621/cfn.v126i4.1378
» http://dx.doi.org/10.22621/cfn.v126i4.1378 - Di Cesare A, Frangipane di Regalbono A, Tessarin C, Seghetti M, Iorio R, Simonato G, et al. Mixed infection by Aelurostrongylus abstrusus and Troglostrongylus brevior in kittens from the same litter in Italy. Parasitol Res 2014; 113(2): 613-618. http://dx.doi.org/10.1007/s00436-013-3690-y PMid:24271082.
» http://dx.doi.org/10.1007/s00436-013-3690-y - Dib LV, Cronemberger C, Pereira FDA, Bolais PF, Uchôa CMA, Bastos OMP, et al. Gastrointestinal parasites among felids inhabiting the Serra dos Órgãos National Park, Rio de Janeiro, Brazil. Brasil. Rev Bras Parasitol Vet 2018; 27(2): 131-140. http://dx.doi.org/10.1590/s1984-296120180016 PMid:29846454.
» http://dx.doi.org/10.1590/s1984-296120180016 - Faust EC, Sawitz W, Tobie J, Odom V, Peres C, Lincicome DR. Comparative efficiency of various technics for the diagnosis of protozoa and helminths in feces. J Parasitol 1939; 25(3): 241-262. http://dx.doi.org/10.2307/3272508
» http://dx.doi.org/10.2307/3272508 - Forman RTT, Alexander LE. Roads and their major ecological effects. Annu Rev Ecol Syst 1998; 29(1): 207-231. http://dx.doi.org/10.1146/annurev.ecolsys.29.1.207
» http://dx.doi.org/10.1146/annurev.ecolsys.29.1.207 - Foster GW, Cunningham MW, Kinsella JM, McLaughlin G, Forrester DJ. Gastrointestinal Helminths of Free-ranging Florida Panthers (Puma concolor coryi) and the Efficacy of the Current Anthelmintic Treatment Protocol. J Wildl Dis 2006; 42(2): 402-406. http://dx.doi.org/10.7589/0090-3558-42.2.402 PMid:16870865.
» http://dx.doi.org/10.7589/0090-3558-42.2.402 - Gomez-Puerta LA, Alarcon V, Pacheco J, Franco F, Lopez-Urbina MT, Gonzalez AE. Molecular and morphological evidence of Taenia omissa in pumas (Puma concolor) in the Peruvian Highlands. Rev Bras Parasitol Vet 2016; 25(3): 368-373. http://dx.doi.org/10.1590/S1984-29612016046 PMid:27580394.
» http://dx.doi.org/10.1590/S1984-29612016046 - Gressler LT, Noll JC, Freitas ÍB, Monteiro SG. Multiparasitism in a wild cat (Leopardus colocolo) (Carnivora: Felidae) in southern Brazil. Rev Bras Parasitol Vet 2016; 25(3): 374-377. http://dx.doi.org/10.1590/S1984-29612016047 PMid:27580395.
» http://dx.doi.org/10.1590/S1984-29612016047 - Hoffmann WA, Pons JA, Janer JL. The sedimentation concentration method in schistosomiasis. Puerto Rico J Public Health 1934; 9: 283-289.
- Inforzato GR, Santos WRM, Neves MF. Capilariose em gatos. Rev Cient Elet Med Vet 2009; 7(12): 1-5.
- Instituto Chico Mendes de Conservação e Biodiversidade – ICMBio. Carnívoros Brasileiros [online]. Brasília: ICMBio; 2020 [cited 2020 Aug 17]. Available from: https://www.icmbio.gov.br/cenap/carnivoros-brasileiros.html
» https://www.icmbio.gov.br/cenap/carnivoros-brasileiros.html - Instituto Chico Mendes de Conservação e Biodiversidade – ICMBio. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção Vol. 2 [online]. Brasília: ICMBio; 2018 [cited 2020 Aug 17]. Available from: https://www.icmbio.gov.br/portal/component/content/article/10187
» https://www.icmbio.gov.br/portal/component/content/article/10187 - Karawita AC, Perera VP, Perera S, de Silva DS, Jayaweera WR, Himsworth CG, et al. Calodium hepaticum in Jungle Cats ( Felis chaus ) in Sri Lanka. J Wildl Dis 2016; 52(4): 971-972. http://dx.doi.org/10.7589/2016-02-046 PMid:27434415.
» http://dx.doi.org/10.7589/2016-02-046 - Kusma SC, Wrublewski DM, Teixeira VN, Holdefer DR. Parasitos intestinais de Leopardus wiedii e Leopardus tigrinus (Felidae) da floresta nacional de três barras, SC. Luminária 2015; 17(1): 85-95.
- Laurance WF, Goosem M, Laurance SGW. Impacts of roads and linear clearings on tropical forests. Trends Ecol Evol 2009; 24(12): 659-669. http://dx.doi.org/10.1016/j.tree.2009.06.009 PMid:19748151.
» http://dx.doi.org/10.1016/j.tree.2009.06.009 - Lawrence AL, Brown GK, Peters B, Spielman DS, Morin-Adeline V, Šlapeta J. High phylogenetic diversity of the cat flea (Ctenocephalides felis) at two mitochondrial DNA markers. Med Vet Entomol 2014; 28(3): 330-336. http://dx.doi.org/10.1111/mve.12051 PMid:24548270.
» http://dx.doi.org/10.1111/mve.12051 - Layne JN. Host and ecological relationships of the parasitic helminth Capillaria hepatica in Florida mammals. Zoologica (NY) 1968; 53(4): 107-123.
- Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): Some issues in correctly identify these species. Rev Bras Parasitol Vet 2012; 21(4): 345-354. http://dx.doi.org/10.1590/S1984-29612012000400002 PMid:23295817.
» http://dx.doi.org/10.1590/S1984-29612012000400002 - Little S, Ambrose D. Spirometra infection in cats and dogs. Comp Cont Educ Pract 2000; 22(4): 299-305.
- Marchini S, Cavalcanti S, Cunha de Paula R. Predadores silvestres e animais domésticos guia prático de convivência Brasília: ICMBio; 2011.
- Marcogliese DJ. Parasites of the superorganism: are they indicators of ecosystem health? Int J Parasitol 2005; 35(7): 705-716. http://dx.doi.org/10.1016/j.ijpara.2005.01.015 PMid:15925594.
» http://dx.doi.org/10.1016/j.ijpara.2005.01.015 - Mohsen A, Hossein H. Gastrointestinal parasites of stray cats in Kashan, Iran. Trop Biomed 2009; 26(1): 16-22. PMid:19696723.
- Mueller JF. The biology of Spirometra. J Parasitol 1974; 60(1): 2-14. http://dx.doi.org/10.2307/3278670 PMid:4592501.
» http://dx.doi.org/10.2307/3278670 - Müller-Graf CDM, Woolhouse MEJ, Packer C. Epidemiology of an intestinal parasite (Spirometra spp.) in two populations of African lions (Panthera leo). Parasitology 1999; 118(Pt 4): 407-415. http://dx.doi.org/10.1017/S0031182098003813 PMid:10340332.
» http://dx.doi.org/10.1017/S0031182098003813 - Nagamori Y, Payton ME, Duncan-Decocq R, Johnson EM. Fecal survey of parasites in free-roaming cats in northcentral Oklahoma, United States. Vet Parasitol Reg Stud Reports 2018; 14: 50-53. http://dx.doi.org/10.1016/j.vprsr.2018.08.008 PMid:31014736.
» http://dx.doi.org/10.1016/j.vprsr.2018.08.008 - Napoli E, Anile S, Arrabito C, Scornavacca D, Mazzamuto MV, Gaglio G, et al. Survey on parasitic infections in wildcat (Felis silvestris silvestris Schreber, 1777) by scat collection. Parasitol Res 2016; 115(1): 255-261. http://dx.doi.org/10.1007/s00436-015-4742-2 PMid:26377843.
» http://dx.doi.org/10.1007/s00436-015-4742-2 - Peixoto TKF, Patrício IDF, Almeidas BV, Araújo Júnior VM, Viana IL, Borges Filho JA, et al. Isosporose em felino: relato de caso. MEDVEP Rev Cient Med Vet Pequenos Anim Anim Estim 2019; 16(32): 1-5.
- Prada CS. Atropelamento de vertebrados silvestres em uma região fragmentada do Nordeste do Estado de São Paulo: quantificação do impacto e análise de fatores envolvidos [tese] São Carlos: Universidade Federal de São Carlos; 2004.
- QGIS Development Team. QGIS Geographic Information System [online]. Chicago: Open Source Geospatial Foundation; 2016 [cited 2020 Dec 12]. Available from: https://www.qgis.org
» https://www.qgis.org - Quadros RM, Pilati C, Marques SMT, Mazzolli M, Benedet RC. Capillaria hepatica in Puma concolor: first Report in Brazil. J Zoo Wildl Med 2009; 40(3): 586-587. http://dx.doi.org/10.1638/2008-0194.1 PMid:19746880.
» http://dx.doi.org/10.1638/2008-0194.1 - Ramos-Abrantes MM, Carreiro AN, Araújo DVF, Souza JG, Lima JPR, Cezar HRA, et al. Vertebrados silvestres atropelados na rodovia BR-230, Paraíba, Brasil. Pubvet 2018; 12(1): 139. http://dx.doi.org/10.22256/pubvet.v12n1a5.1-7
» http://dx.doi.org/10.22256/pubvet.v12n1a5.1-7 - Reis NR, Peracchi AL, Pedro WA, Lima IP. Mamíferos do Brasil. In: Reis NR, Peracchi AL, Pedro WA, Lima IP, editores. Divisão de Processos Técnicos da Biblioteca Central da Universidade Estadual de Londrina 2. ed. Londrina: EDUEL; 2006. p. 17-25.
- Rossaneis BK. Medium and large-sized mammals in small forest remnants of the Atlantic Forest with anthropogenic influences in the North of Paraná. Semin Cienc Biol Saude 2014; 35(1): 15-24. http://dx.doi.org/10.5433/1679-0367.2014v35n1p15
» http://dx.doi.org/10.5433/1679-0367.2014v35n1p15 - Ruas JL, Soares MP, Farias NAR, Brum JGW. Infecção por Capillaria hepatica em carnívoros silvestres (Lycalopex gymnocercus e Cerdocyon thous) na região sul do Rio Grande do Sul. Arq Inst Biol (Sao Paulo) 2003; 70(2): 127-130.
- Rust MK, Dryden MW. The biology, ecology, and management of the cat flea. Annu Rev Entomol 1997; 42(1): 451-473. http://dx.doi.org/10.1146/annurev.ento.42.1.451 PMid:9017899.
» http://dx.doi.org/10.1146/annurev.ento.42.1.451 - Santos JA. Patologia especial dos animais domésticos (mamíferos e aves) Rio de Janeiro: Instituto Interamericano de Ciências Agrícolas; 1975.
- Seiler A, Helldin J. Mortality in wildlife due to transportation. In: Davenport J, Davenport JL, editors. The ecology of transportation: managing mobility for the environmentVol. 10. Dordrecht: Springer; 2006. p.165-189. https://doi.org/10.1007/1-4020-4504-2_8
» https://doi.org/10.1007/1-4020-4504-2_8 - Taylor MA, Coop RL, Wall RL. Helmintologia veterinária. In: Fagliari JJ, Rocha TG, editors. Parasitologia veterinária 4th ed. Rio de Janeiro: Editora Guanabara Koogan; 2017. p. 65-478.
- Travassos L. Contribuição para a inventario crítico da zoologia no Brasil Rio de Janeiro: Museu Nacional; 1965.
- Willis HH. A simple levitation method for the detection of Hookworm Ova. Med J Aust 1921; 2(18): 375-376. http://dx.doi.org/10.5694/j.1326-5377.1921.tb60654.x
» http://dx.doi.org/10.5694/j.1326-5377.1921.tb60654.x
Publication Dates
-
Publication in this collection
12 Feb 2021 -
Date of issue
2021
History
-
Received
14 July 2020 -
Accepted
24 Nov 2020