Abstract
This study investigated the effect of Folliculinum 6 cH on the oocyte meiosis resumption and viability rates, progesterone production and mitochondrial activity after in vitro maturation of cumulus-oocyte complexes (COCs) in sheep. Sheep ovaries were collected at a local slaughterhouse and COCs were recovered by slicing technique. The selected COCs were maturated in TCM199 (Control treatment), or control medium supplemented with 0.05% ethanol (v/v) (the vehicle of the homeopathic preparation – Ethanol treatment) or with Folliculinum 6 cH. After 24 h of in vitro maturation (IVM), oocytes were mechanically denuded and incubated with Hoechst 33342 and MitoTracker (0.5 μM) Orange CMTMRos for analysis of viability and chromatin configuration, and mitochondrial activity, respectively. The results showed that Folliculinum 6 cH addition increased oocyte degeneration and reduced meiotic resumption compared to the control (P < 0.05). Interestingly, the percentages meiotic resumption and oocyte maturation were lower in the Folliculinum 6 cH treatment compared to its vehicle (Ethanol treatment) (P < 0.05). On the other hand, when the treatments were compared, higher mitochondrial activity was observed in the Ethanol treatment (P < 0.05). In conclusion, contrary to its vehicle, the addition of Folliculinum 6 cH to the IVM medium promoted oocyte degeneration and affected negatively the mitochondrial distribution, impairing meiosis resumption.
Keywords:
culmulus-oocyte complex; homeopathy; in vitro maturation; sheep
Introduction
Homeopathy is a therapeutic method based on the use of drugs in a minimal dosage during treatment (Teixeira et al., 2008Teixeira M, Leal S, Ceschin V. Homeopathic practice in Intensive Care Units: objective semiology, symptom selection and a series of sepsis cases. Homeopathy. 2008;97(4):206-13. http://dx.doi.org/10.1016/j.homp.2008.08.002. PMid:19371570.
http://dx.doi.org/10.1016/j.homp.2008.08...
). In human reproduction, homeopathic preparations have been used in women who are intolerant to exogenous estrogen (Goswani and Conway, 2005Goswani D, Conway G. Premature ovariam failure. Hum Reprod. 2005;11:391-410.) to treat various reproductive disorders, such as hyperandrogenism, dysmenorrhea, endometriosis, uterine fibroids (Legros, 2010Legros M. Utilización de la homeopatía en endocrinologia ginecológica. Uso terapéutico de las hormonas diluidas y dinamizadas. Rev Med Homeopat. 2010;3(1):9-13. http://dx.doi.org/10.1016/S1888-8526(10)70047-3.
http://dx.doi.org/10.1016/S1888-8526(10)...
), among others. Among the homeopathic preparations it is possible to highlight Folliculinum 6 cH, which is a homeopathic medicine derived from estrone (Mareüil, 2016Mareüil EL. Déséquilibre œstrogénique et médicaments homéopathiques. La Revue d’Homéopathie. 2016;7(1):25-9. http://dx.doi.org/10.1016/j.revhom.2016.01.003.
http://dx.doi.org/10.1016/j.revhom.2016....
) and has been used in women to regulate the menstrual cycle, to treat reproductive diseases, such as polycystic ovary, secondary amenorrhea after childbirth (Legros, 2010Legros M. Utilización de la homeopatía en endocrinologia ginecológica. Uso terapéutico de las hormonas diluidas y dinamizadas. Rev Med Homeopat. 2010;3(1):9-13. http://dx.doi.org/10.1016/S1888-8526(10)70047-3.
http://dx.doi.org/10.1016/S1888-8526(10)...
), and breast cysts in patients with the hyperestrogenic syndrome (Pordes and Legru-Bertagne, 2012Pordes F, Legru-Bertagne P. Correction d’un syndrome hyperœstrogénique par Folliculinum 6 cH. Revhom. 2012;3:146-7. http://dx.doi.org/10.1016/j.revhom.2012.10.008.
http://dx.doi.org/10.1016/j.revhom.2012....
) as well as for infertility treatment (Legros, 2010Legros M. Utilización de la homeopatía en endocrinologia ginecológica. Uso terapéutico de las hormonas diluidas y dinamizadas. Rev Med Homeopat. 2010;3(1):9-13. http://dx.doi.org/10.1016/S1888-8526(10)70047-3.
http://dx.doi.org/10.1016/S1888-8526(10)...
). Although the mechanism of action of Folliculinum 6 cH is unknown, some studies suggested that Folliculinum 6 cH can regulate the production of estrogens and androgens (Legros, 2010Legros M. Utilización de la homeopatía en endocrinologia ginecológica. Uso terapéutico de las hormonas diluidas y dinamizadas. Rev Med Homeopat. 2010;3(1):9-13. http://dx.doi.org/10.1016/S1888-8526(10)70047-3.
http://dx.doi.org/10.1016/S1888-8526(10)...
; Danno et al., 2013Danno K, Colas A, Terzan L, Bordet MF. Homeopathic treatment of premenstrual syndrome: a case series. Homeopathy. 2013;102(1):59-65. http://dx.doi.org/10.1016/j.homp.2012.10.004. PMid:23290881.
http://dx.doi.org/10.1016/j.homp.2012.10...
). Shirazi and Moalemian (2007)Shirazi A, Moalemian Z. Ovine cumulus cells estradiol-17Beta production in the presence or absence of oocyte. Anim Reprod Sci. 2007;101(1-2):125-33. http://dx.doi.org/10.1016/j.anireprosci.2006.09.002. PMid:17045430.
http://dx.doi.org/10.1016/j.anireprosci....
reported that the sheep COCs and cumulus cells are capable to produce estradiol in detectable amounts in a steroid-free maturation medium. Also, it is common to add commercial estradiol or serum containing estradiol to the culture medium to improve in vitro maturation (Brevini et al., 2005Brevini TA, Vassena R, Francisci C, Gandolfi F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes. Biol Reprod. 2005;72(5):1218-23. http://dx.doi.org/10.1095/biolreprod.104.038141. PMid:15659704.
http://dx.doi.org/10.1095/biolreprod.104...
; Fang et al., 2016Fang Y, Zhang X, Zhang J, Zhong R, Zhou D. Global DNA methylation and related mRNA profiles in sheep oocytes and early embryos derived from pre-pubertal and adult donors. Anim Reprod Sci. 2016;164:144-51. http://dx.doi.org/10.1016/j.anireprosci.2015.11.022. PMid:26686460.
http://dx.doi.org/10.1016/j.anireprosci....
; Wang et al., 2018Wang DH, Ren J, Zhou CJ, Han Z, Wang L, Liang CG. Supplementation with CTGF, SDF1, NGF, and HGF promotes ovine in vitro oocyte maturation and early embryo development. Domest Anim Endocrinol. 2018;65:38-48. http://dx.doi.org/10.1016/j.domaniend.2018.05.003. PMid:29890304.
http://dx.doi.org/10.1016/j.domaniend.20...
). Therefore, the use of Folliculinum 6 cH in in vitro procedures, such as in vitro maturation (IVM), could be an alternative to improve oocyte maturation in many species. Moreover, the advantages of using homeopathic substances including Folliculinum 6 cH are due to the much lower cost and low toxicity of homeopathic medicines.
Despite been used for more than 200 hundred years, homeopathy is still a controversial topic. For instance, it has been suggested that researchers have not been able to develop objective measures that show the effects of extremely dilute products in the human body. Others argued that the supposed effect of homeopathic products is due to their vehicles, such as ethanol or even to a possible placebo effect (Moffett et al., 2006Moffett J, Arun P, Namboodiri M. Laboratory Research in Homeopathy: con. Integr Cancer Ther. 2006;5(4):333-42. http://dx.doi.org/10.1177/1534735406294795. PMid:17101762.
http://dx.doi.org/10.1177/15347354062947...
). In this sense, the in vitro models, for example the IVM, could represent an excellent tool to solve such intriguing issues. Concerning reproduction, few studies investigated the effects of homeopathic medicinal products on the in vitro preantral follicle survival and development (sheep – Lima et al., 2016Lima L, Rocha R, Alves A, Carvalho A, Chaves R, Lopes C, Báo S, Campello C, Rodrigues A, Figueiredo J. Comparison between the additive effects of diluted (rFSH) and diluted/dynamized (FSH 6 cH) recombinant follicle-stimulating hormone on the in vitro culture of ovine preantral follicles enclosed in ovarian tissue. Complement Ther Med. 2016;25:39-44. http://dx.doi.org/10.1016/j.ctim.2015.12.016. PMid:27062946.
http://dx.doi.org/10.1016/j.ctim.2015.12...
and pig - Lima et al., 2017Lima L, Rubessa M, Rocha R, Winters R, Milner D, Campello C, Figueiredo J, Wheeler M. High diluted and dynamised follicle stimulating hormone modulates steroid production in isolated porcine preantral follicles cultured in vitro. Homeopathy. 2017;106(2):87-92. http://dx.doi.org/10.1016/j.homp.2017.03.004. PMid:28552178.
http://dx.doi.org/10.1016/j.homp.2017.03...
). However, to the best of our knowledge, there are no reports on the in vitro effects of those products on the in vitro maturation of oocytes. Therefore, the originality of the present paper is to investigate for the first time the effect of Folliculinum 6 cH and its vehicle (ethanol) on the in vitro maturation, viability and mitochondrial activity of ovine oocytes.
Materials and methods
Unless mentioned otherwise, the reagents and chemicals used in the present study were purchased from Sigma Chemical Co. (St. Louis. MO. USA). The preparation of Folliculinum 6 cH was performed in Pharmacy Homeovitae (Campo Grande, MS).
Research ethics
One of the major alternatives to in vivo animal testing is in vitro cell culture. In line with this ethical issue, the present study aimed to evaluate the effects of the tested substances (ethanol and Folliculinum 6 cH preparations) on in vitro folliculogenesis using sheep follicles recovered from slaughterhouse ovaries. This source of ovarian material represents a by-product of the food industry and is more readily acceptable than euthanasia of animals specifically for scientific purposes.
Oocyte collection and in vitro maturation (IVM)
Ovaries were collected at a local slaughterhouse and transported within 1 to 2 h to the laboratory in Minimum Essential Medium (MEM) supplemented with HEPES and antibiotics (100 µg/mL penicillin-streptomycin) at 33 to 35 °C. In the laboratory, the COCs were recovered from sheep ovary by slicing and only oocytes with homogeneous cytoplasm and surrounded by at least three compact layers of cumulus cells were selected and 20 to 35 oocytes were cultured together (Davashi et al., 2014Davashi ND, Shahneh AZ, Kohram H, Zhandi M, Dashti S, Shamsi H, Moghadam R. In vitro ovine embryo production: the study of seasonal and oocyte recovery method effects. Iran Red Crescent Med J. 2014;16(9):e20749. http://dx.doi.org/10.5812/ircmj.20749. PMid:25593733.
http://dx.doi.org/10.5812/ircmj.20749...
). The basic in vitro maturation medium consisted of TCM199 plus sodium bicarbonate (TCM 199 B - supplemented with 0.5 µg/mL of recombinant bovine FSH (Nanocore, Brazil), 5 µg/mL of LH, 1 µg/mL of 17 β-estradiol, 10 ng/mL of murine EGF (Sigma - E4127), 0.911 mM/L of pyruvate, 100 µM/L of cysteamine, 50 ng/mL of recombinant human IGF-1 (Sigma - I3769), and 1% of BSA - Luz et al., 2013Luz VB, Araújo VR, Duarte ABG, Silva GM, Chaves RN, Brito IR, Serafim MKB, Campello CC, Feltrin C, Bertolini M, Almeida AP, Santos RR, Figueiredo JR. Kit ligand and insulin-like growth factor I affect the in vitro development of ovine preantral follicles. Small Rumin Res. 2013;115(1-3):99-102. http://dx.doi.org/10.1016/j.smallrumres.2013.09.003.
http://dx.doi.org/10.1016/j.smallrumres....
) which was referred to as TCM 199 (Control medium). Groups of 20 to 35 oocytes were cultured for 24 hours under 5% CO2 in air in 200-350 μL (10 µL per COC) in TCM199 (Control treatment), or control medium supplemented with 0.05% ethanol (v/v) (the vehicle of the homeopathic preparation- Ethanol treatment) or with Folliculinum 6 cH (Figure 1).
Experimental design to assess the effect Folliculinum 6 cH on the oocyte chromatin configuration, mitochondrial activity and progesterone production after 24 h of IVM. Control treatment- control medium alone; Ethanol - control medium supplemented with 0.05% ethanol (v/v) (the vehicle of the homeopathic preparation- Ethanol treatment); Folliculinum 6 cH - control medium supplemented with Folliculinum 6 cH.
Viability, chromatin configuration, and mitochondrial activity in in vitro matured oocytes
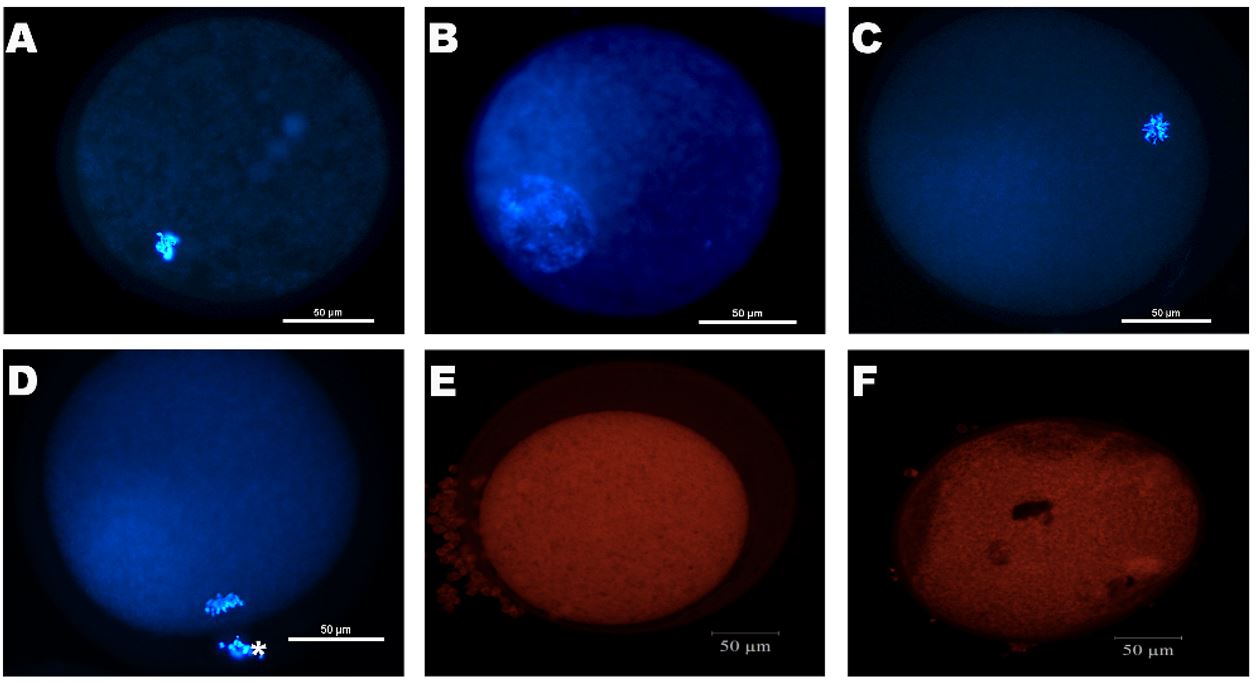
After IVM, oocytes were mechanically denuded and fixed in 1% glutaraldehyde for viability and chromatin configuration, and mitochondrial activity assays. Oocytes were stained by Hoechst 33342 (emission at 483 nm) and the oocyte viability and chromatin configuration were assessed by fluorescence microscopy (Eclipse 80 i, Nikon, Tokyo, Japan). The oocytes were classified as degenerated (DEG), germinal vesicle (GV), germinal vesicle breakdown (GVBD), and metaphase II (MII – Luz et al., 2013Luz VB, Araújo VR, Duarte ABG, Silva GM, Chaves RN, Brito IR, Serafim MKB, Campello CC, Feltrin C, Bertolini M, Almeida AP, Santos RR, Figueiredo JR. Kit ligand and insulin-like growth factor I affect the in vitro development of ovine preantral follicles. Small Rumin Res. 2013;115(1-3):99-102. http://dx.doi.org/10.1016/j.smallrumres.2013.09.003.
http://dx.doi.org/10.1016/j.smallrumres....
; Figure 2AD).
Representative images of fluorescent ovine oocytes after 24 hours of in vitro maturation. Oocytes staining with Hoescht 33342 (blue): degenerated (A) and normal oocytes in germinal vesicle (GV - B), germinal vesicle break down (GVBD - C), and metaphase II (MII - D) state; oocyte staining with Mitotracker probe with a high (E) and low (F) fluorescence intensity (A-F: 50μm bar).
To evaluate the mitochondrial activity after IVM, the oocytes were incubated for 30 minutes in MitoTracker (0.5 μM) Orange CMTMRos (M7510, 38.5 °C and 5% CO2). Then, oocytes were mechanically denuded and fixed in 1% glutaraldehyde and were assessed by LM710 confocal microscope (Zeiss, Germany). The mitochondrial activity in each oocyte was evaluated by the fluorescence intensity, using Zen lite 2.3 SP1 software (Brevini et al., 2005Brevini TA, Vassena R, Francisci C, Gandolfi F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes. Biol Reprod. 2005;72(5):1218-23. http://dx.doi.org/10.1095/biolreprod.104.038141. PMid:15659704.
http://dx.doi.org/10.1095/biolreprod.104...
; Figure 22F).
Levels of progesterone
The spent media after maturation were collected and stored at -80 °C for progesterone assay. The concentrations of progesterone were measured from standard aliquots (200 µL), using the enzyme-linked fluorescent assay (ELFA), according to the manufacturer’s instructions (VIDAS® Progesterone, ref 30409).
Statistical analyses
All statistical analyses were carried out using Sigma Plot version 11.0 (Systat Software Inc., USA). Data were reported as mean (±SEM) and percentage, and the results were considered significant when P<0.05. Comparisons of means were performed by Kruskal-Wallis test, whereas variables expressed as percentages were analyzed by chi-square or Fisher´s exact tests.
Results
A total of 453 oocytes were distributed in three treatments: control, ethanol and Folliculinum 6 cH. The addition of ethanol (the vehicle of the homeopathic preparation) to the control IVM medium did not affect the percentage of degenerate, GV and GVBD and MII oocytes after IVM (Table 1). Moreover, no statistical difference was observed in the progesterone production among the treatments after IVM. In contrast, when compared to the control, Folliculinum 6 cH addition increased oocyte degeneration and reduced meiotic resumption rates (P < 0.05). Interestingly, the percentages of meiotic resumption and MII-oocytes were lower in the Folliculinum 6 cH treatment compared to its vehicle (ethanol treatment) (P < 0.05 - Table 1). Finally, when the treatments were compared, higher mitochondrial activity was observed in the ethanol treatment (P < 0.05 - Table 1).
Chromatin configuration, mitochondrial activity and progesterone production (mean ± SEM) of sheep COCs after 24h of in vitro maturation in the absence (control) or presence of Folliculinum 6 cH or ethanol (homeopathic vehicle).
Discussion
This study shows for the first time the effect of a homeopathic medicine, Folliculinum 6 cH, on oocyte in vitro maturation. The results clearly showed that for some endpoints Folliculinum 6 cH did differ from the controls including its vehicle (ethanol treatment).
Folliculinum 6 cH addition increased oocyte degeneration and reduced meiotic resumption (P < 0.05) when compared to the control treatment. The substance used to prepare Folliculinum 6 cH is the estrone, which is an estradiol precursor (Demarque et al., 2009Demarque D, Jouanny J, Poitevin B, Saint-Jean Y. Pharmacologie et matière médicale homéopatique. France: Organon; 2009.). Some studies reported that Folliculinum 6 cH treats hormonal imbalance by stimulating estradiol secretion (Demarque et al., 2009Demarque D, Jouanny J, Poitevin B, Saint-Jean Y. Pharmacologie et matière médicale homéopatique. France: Organon; 2009.; Legros, 2010Legros M. Utilización de la homeopatía en endocrinologia ginecológica. Uso terapéutico de las hormonas diluidas y dinamizadas. Rev Med Homeopat. 2010;3(1):9-13. http://dx.doi.org/10.1016/S1888-8526(10)70047-3.
http://dx.doi.org/10.1016/S1888-8526(10)...
). However, the results of the present study suggest that Folliculinum 6 cH supplementation to the control maturation medium might stimulate estradiol production, which in turn overstimulates the oocyte resulting in high rates of degeneration. However, the mechanism of action of homeopathic medicines, like Folliculinum 6 cH, remains to be elucidated.
Considering that, homeopathy is still a controversial topic, in the present study care was taken to avoid researcher bias by using a double-blind approach along with an in vitro culture technique (oocyte in vitro maturation). The in vitro maturation technique is an outstanding tool that allows objective analysis of oocyte chromatin configuration. In the present study, the efficiency of Folliculinum 6 cH was evaluated in the in vitro maturation using two controls, i.e., maturation medium (TCM199) and ethanol, which was the vehicle used for the preparation of Folliculinum 6 cH. Surprisingly, Folliculinum 6 cH treatment yielded lower mature oocyte rates compared to its vehicle (ethanol treatment). In agreement with previous results from our team (Lima et al., 2016Lima L, Rocha R, Alves A, Carvalho A, Chaves R, Lopes C, Báo S, Campello C, Rodrigues A, Figueiredo J. Comparison between the additive effects of diluted (rFSH) and diluted/dynamized (FSH 6 cH) recombinant follicle-stimulating hormone on the in vitro culture of ovine preantral follicles enclosed in ovarian tissue. Complement Ther Med. 2016;25:39-44. http://dx.doi.org/10.1016/j.ctim.2015.12.016. PMid:27062946.
http://dx.doi.org/10.1016/j.ctim.2015.12...
), these results clearly showed that the effect of Folliculinum 6 cH was not due to its vehicle suggesting a different mechanism of action (Rughinis et al., 2018Rughiniş C, Ciocanel A, Vasile S. Homeopathy as boundary object and distributed therapeutic agency: a discussion on the homeopathic placebo response. Am J Ther. 2018;25(4):447-52. http://dx.doi.org/10.1097/MJT.0000000000000607. PMid:28984633.
http://dx.doi.org/10.1097/MJT.0000000000...
). It is well known that medium supplements, including hormones, growth factors, and antioxidants affect the efficiency of the in vitro culture of oocytes (Li et al., 2016Li Y, Liu Q, Chen Q, Yan X, Li N. Insulin-like growth factor 1 promotes cumulus cell expansion and nuclear maturation of oocytes via Pi3K/Akt pathway. Int J Clin Exp Pathol. 2016;9(11):11436-43.; Veshkini et al., 2018Veshkini A, Mohammadi-Sangcheshmeh A, Ghanem N, Abazari-kia AH, Mottaghi E, Kamaledini R, Deldar H, Ozturk I, Gastal EL. Oocyte maturation with royal jelly increases embryo development and reduces apoptosis in goats. Anim Reprod. 2018;15(2):124-34. http://dx.doi.org/10.21451/1984-3143-2017-AR986.
http://dx.doi.org/10.21451/1984-3143-201...
) and embryos (Marques et al., 2010Marques A, Santos P, Antunes G, Chaveiro A, Silva FM. Effect of alpha-tocopherol on bovine in vitro fertilization. Reprod Domest Anim. 2010;45(1):81-5. http://dx.doi.org/10.1111/j.1439-0531.2008.01245.x. PMid:20137061.
http://dx.doi.org/10.1111/j.1439-0531.20...
; Ashkar et al., 2010Ashkar FA, Semple E, Schmidt CH, St. John E, Bartlewski PM, King WA. Thyroid hormone supplementation improves bovine embryo development in vitro. Hum Reprod. 2010;25(2):334-44. http://dx.doi.org/10.1093/humrep/dep394. PMid:19920067.
http://dx.doi.org/10.1093/humrep/dep394...
; Thongkittidilok et al., 2015Thongkittidilok C, Tharasanit T, Songsasen N, Sananmuang T, Buarpung S, Techakumphu M. Epidermal growth factor improves developmental competence and embryonic quality of singly cultured domestic cat embryos. J Reprod Dev. 2015;61(4):269-76. http://dx.doi.org/10.1262/jrd.2014-167. PMid:25985792.
http://dx.doi.org/10.1262/jrd.2014-167...
) in a concentration-dependent manner. Even though the dynamization of folliculinum, i.e. 6cH used in the present study was not suitable for oocyte meiotic resumption, studies aiming to find out optimal concentrations of this component as well as other homeopathic products would be of great importance. Therefore, the use of homeopathy medicine in in vitro procedures, such as IVM, could be an alternative to improve oocyte maturation in many species. Moreover, the advantages of using homeopathic substances are due to the much lower cost and low toxicity of homeopathic medicines.
In the ethanol treatment, the oocytes presented the highest mitochondrial activity. The ethanol increases the concentration of cytoplasmic calcium ions (Ca+2 – Liu et al., 1998Liu L, Ju J, Yang X. Parthenogenetic development and protein patterns of newly matured bovine oocytes after chemical activation. Mol Reprod Dev. 1998;49(3):298-307. http://dx.doi.org/10.1002/(SICI)1098-2795(199803)49:3<298::AID-MRD10>3.0.CO;2-T. PMid:9491382.
http://dx.doi.org/10.1002/(SICI)1098-279...
) and Ca+2 inside the cell acts as a second messenger, regulating important cellular events. Moreover, a single calcium increase can induce early oocyte activation events, such as resumption of meiosis and MII arrest, but not late events, such as pronuclear formation, and cleavage (Liu et al., 1998Liu L, Ju J, Yang X. Parthenogenetic development and protein patterns of newly matured bovine oocytes after chemical activation. Mol Reprod Dev. 1998;49(3):298-307. http://dx.doi.org/10.1002/(SICI)1098-2795(199803)49:3<298::AID-MRD10>3.0.CO;2-T. PMid:9491382.
http://dx.doi.org/10.1002/(SICI)1098-279...
). Then, we suggest that increase of cytoplasmic Ca+2 concentrations by the ethanol, may increase the mitochondrial activity and the ATP production (Brookes et al., 2004Brookes P, Yoon Y, Robotham J, Anders M, Sheu S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):817-33. http://dx.doi.org/10.1152/ajpcell.00139.2004. PMid:15355853.
http://dx.doi.org/10.1152/ajpcell.00139....
), promoting meiosis resumption (Yu et al., 2010Yu Y, Dumollard R, Rossbach A, Lai F, Swann K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J Cell Physiol. 2010;224(3):672-80. http://dx.doi.org/10.1002/jcp.22171. PMid:20578238.
http://dx.doi.org/10.1002/jcp.22171...
).
Conclusion
In conclusion, Folliculinum 6 cH promoted oocyte degeneration and affect negatively the mitochondrial distribution, impairing meiosis resumption. Taken together, these results suggest that, at least for metaphase II rate and mitochondrial activity, the mechanism of action of Folliculinum 6 cH differs from its vehicle. Thus, this study opens new perspectives for the use of other homeopathic substances in in vitro maturation protocols.
Acknowledgements
This work was supported by CNPq (Brazil, Grant 554812/ 2006-1-RENORBIO). K. N. M Damasceno is a recipient of a scholarship from Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP - Brazil).
-
Financial support: This work was supported by CNPq (Brazil, Grant 554812/ 2006-1-RENORBIO). K. N. M Damasceno is a recipient of a scholarship from Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP - Brazil).
-
How to cite: Damasceno KNM, Sá NAR, Tetaping GM, Paes VM, Lima LF, Monreal ACD, Sousa FGC, Alves BG, Figueiredo JR, Araújo VR. Ultra-diluted Folliculinum 6 cH impairs ovine oocyte viability and maturation after in vitro culture. Anim Reprod. 2020;17(2):e20190100.
References
- Ashkar FA, Semple E, Schmidt CH, St. John E, Bartlewski PM, King WA. Thyroid hormone supplementation improves bovine embryo development in vitro Hum Reprod. 2010;25(2):334-44. http://dx.doi.org/10.1093/humrep/dep394 PMid:19920067.
» http://dx.doi.org/10.1093/humrep/dep394 - Brevini TA, Vassena R, Francisci C, Gandolfi F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes. Biol Reprod. 2005;72(5):1218-23. http://dx.doi.org/10.1095/biolreprod.104.038141 PMid:15659704.
» http://dx.doi.org/10.1095/biolreprod.104.038141 - Brookes P, Yoon Y, Robotham J, Anders M, Sheu S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):817-33. http://dx.doi.org/10.1152/ajpcell.00139.2004 PMid:15355853.
» http://dx.doi.org/10.1152/ajpcell.00139.2004 - Danno K, Colas A, Terzan L, Bordet MF. Homeopathic treatment of premenstrual syndrome: a case series. Homeopathy. 2013;102(1):59-65. http://dx.doi.org/10.1016/j.homp.2012.10.004 PMid:23290881.
» http://dx.doi.org/10.1016/j.homp.2012.10.004 - Davashi ND, Shahneh AZ, Kohram H, Zhandi M, Dashti S, Shamsi H, Moghadam R. In vitro ovine embryo production: the study of seasonal and oocyte recovery method effects. Iran Red Crescent Med J. 2014;16(9):e20749. http://dx.doi.org/10.5812/ircmj.20749 PMid:25593733.
» http://dx.doi.org/10.5812/ircmj.20749 - Demarque D, Jouanny J, Poitevin B, Saint-Jean Y. Pharmacologie et matière médicale homéopatique. France: Organon; 2009.
- Fang Y, Zhang X, Zhang J, Zhong R, Zhou D. Global DNA methylation and related mRNA profiles in sheep oocytes and early embryos derived from pre-pubertal and adult donors. Anim Reprod Sci. 2016;164:144-51. http://dx.doi.org/10.1016/j.anireprosci.2015.11.022 PMid:26686460.
» http://dx.doi.org/10.1016/j.anireprosci.2015.11.022 - Goswani D, Conway G. Premature ovariam failure. Hum Reprod. 2005;11:391-410.
- Legros M. Utilización de la homeopatía en endocrinologia ginecológica. Uso terapéutico de las hormonas diluidas y dinamizadas. Rev Med Homeopat. 2010;3(1):9-13. http://dx.doi.org/10.1016/S1888-8526(10)70047-3
» http://dx.doi.org/10.1016/S1888-8526(10)70047-3 - Li Y, Liu Q, Chen Q, Yan X, Li N. Insulin-like growth factor 1 promotes cumulus cell expansion and nuclear maturation of oocytes via Pi3K/Akt pathway. Int J Clin Exp Pathol. 2016;9(11):11436-43.
- Lima L, Rocha R, Alves A, Carvalho A, Chaves R, Lopes C, Báo S, Campello C, Rodrigues A, Figueiredo J. Comparison between the additive effects of diluted (rFSH) and diluted/dynamized (FSH 6 cH) recombinant follicle-stimulating hormone on the in vitro culture of ovine preantral follicles enclosed in ovarian tissue. Complement Ther Med. 2016;25:39-44. http://dx.doi.org/10.1016/j.ctim.2015.12.016 PMid:27062946.
» http://dx.doi.org/10.1016/j.ctim.2015.12.016 - Lima L, Rubessa M, Rocha R, Winters R, Milner D, Campello C, Figueiredo J, Wheeler M. High diluted and dynamised follicle stimulating hormone modulates steroid production in isolated porcine preantral follicles cultured in vitro Homeopathy. 2017;106(2):87-92. http://dx.doi.org/10.1016/j.homp.2017.03.004 PMid:28552178.
» http://dx.doi.org/10.1016/j.homp.2017.03.004 - Liu L, Ju J, Yang X. Parthenogenetic development and protein patterns of newly matured bovine oocytes after chemical activation. Mol Reprod Dev. 1998;49(3):298-307. http://dx.doi.org/10.1002/(SICI)1098-2795(199803)49:3<298::AID-MRD10>3.0.CO;2-T PMid:9491382.
» http://dx.doi.org/10.1002/(SICI)1098-2795(199803)49:3<298::AID-MRD10>3.0.CO;2-T - Luz VB, Araújo VR, Duarte ABG, Silva GM, Chaves RN, Brito IR, Serafim MKB, Campello CC, Feltrin C, Bertolini M, Almeida AP, Santos RR, Figueiredo JR. Kit ligand and insulin-like growth factor I affect the in vitro development of ovine preantral follicles. Small Rumin Res. 2013;115(1-3):99-102. http://dx.doi.org/10.1016/j.smallrumres.2013.09.003
» http://dx.doi.org/10.1016/j.smallrumres.2013.09.003 - Mareüil EL. Déséquilibre œstrogénique et médicaments homéopathiques. La Revue d’Homéopathie. 2016;7(1):25-9. http://dx.doi.org/10.1016/j.revhom.2016.01.003
» http://dx.doi.org/10.1016/j.revhom.2016.01.003 - Marques A, Santos P, Antunes G, Chaveiro A, Silva FM. Effect of alpha-tocopherol on bovine in vitro fertilization. Reprod Domest Anim. 2010;45(1):81-5. http://dx.doi.org/10.1111/j.1439-0531.2008.01245.x PMid:20137061.
» http://dx.doi.org/10.1111/j.1439-0531.2008.01245.x - Moffett J, Arun P, Namboodiri M. Laboratory Research in Homeopathy: con. Integr Cancer Ther. 2006;5(4):333-42. http://dx.doi.org/10.1177/1534735406294795 PMid:17101762.
» http://dx.doi.org/10.1177/1534735406294795 - Pordes F, Legru-Bertagne P. Correction d’un syndrome hyperœstrogénique par Folliculinum 6 cH. Revhom. 2012;3:146-7. http://dx.doi.org/10.1016/j.revhom.2012.10.008
» http://dx.doi.org/10.1016/j.revhom.2012.10.008 - Rughiniş C, Ciocanel A, Vasile S. Homeopathy as boundary object and distributed therapeutic agency: a discussion on the homeopathic placebo response. Am J Ther. 2018;25(4):447-52. http://dx.doi.org/10.1097/MJT.0000000000000607 PMid:28984633.
» http://dx.doi.org/10.1097/MJT.0000000000000607 - Shirazi A, Moalemian Z. Ovine cumulus cells estradiol-17Beta production in the presence or absence of oocyte. Anim Reprod Sci. 2007;101(1-2):125-33. http://dx.doi.org/10.1016/j.anireprosci.2006.09.002 PMid:17045430.
» http://dx.doi.org/10.1016/j.anireprosci.2006.09.002 - Teixeira M, Leal S, Ceschin V. Homeopathic practice in Intensive Care Units: objective semiology, symptom selection and a series of sepsis cases. Homeopathy. 2008;97(4):206-13. http://dx.doi.org/10.1016/j.homp.2008.08.002 PMid:19371570.
» http://dx.doi.org/10.1016/j.homp.2008.08.002 - Thongkittidilok C, Tharasanit T, Songsasen N, Sananmuang T, Buarpung S, Techakumphu M. Epidermal growth factor improves developmental competence and embryonic quality of singly cultured domestic cat embryos. J Reprod Dev. 2015;61(4):269-76. http://dx.doi.org/10.1262/jrd.2014-167 PMid:25985792.
» http://dx.doi.org/10.1262/jrd.2014-167 - Veshkini A, Mohammadi-Sangcheshmeh A, Ghanem N, Abazari-kia AH, Mottaghi E, Kamaledini R, Deldar H, Ozturk I, Gastal EL. Oocyte maturation with royal jelly increases embryo development and reduces apoptosis in goats. Anim Reprod. 2018;15(2):124-34. http://dx.doi.org/10.21451/1984-3143-2017-AR986
» http://dx.doi.org/10.21451/1984-3143-2017-AR986 - Wang DH, Ren J, Zhou CJ, Han Z, Wang L, Liang CG. Supplementation with CTGF, SDF1, NGF, and HGF promotes ovine in vitro oocyte maturation and early embryo development. Domest Anim Endocrinol. 2018;65:38-48. http://dx.doi.org/10.1016/j.domaniend.2018.05.003 PMid:29890304.
» http://dx.doi.org/10.1016/j.domaniend.2018.05.003 - Yu Y, Dumollard R, Rossbach A, Lai F, Swann K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J Cell Physiol. 2010;224(3):672-80. http://dx.doi.org/10.1002/jcp.22171 PMid:20578238.
» http://dx.doi.org/10.1002/jcp.22171
Publication Dates
-
Publication in this collection
26 June 2020 -
Date of issue
2020
History
-
Received
12 Sept 2019 -
Accepted
05 May 2020