Abstract
Mean prevalence and intensity of infestations by epibionts were evaluated in Arenaeus cribrarius (Lamarck, 1818), based on monthly samplings (May/1991 to April/1993), in Ubatuba, Brazil. Bryozoans were quantified in terms of colony numbers and barnacles by the number of specimens. Prevalence of infestation was determined in 1,914 individuals of A. cribrarius and assessed with respect to sex, maturity stage and season. No significant interaction was detected between epibionts and biological host factors. Males showed a higher infestation rate by Chelonibia patula (Ranzani, 1818) when compared to mature non-ovigerous females, yet Octolasmis lowei Darwin, 1851 infestation was associated to adult crabs. The ovigerous females of A. cribrarius showed a higher prevalence of infestation than males and non-ovigerous females, with lower infestations being recorded during winter. A synchrony between the life cycle of the epibionts and their hosts was evident and promotes the continuity of the former in the area.
Bryozoan; Chelonibia patula; epibiosis; Octolasmis lowei
SYMBIOSIS
Universidade Estadual Paulista, Campus Experimental do Litoral Paulista, Research Group in Crustacean Biology. Praça Infante D. Henrique, 11330-900 São Vicente, São Paulo), Brazil. E-mail: costatm@csv.unesp.br
ABSTRACT
Mean prevalence and intensity of infestations by epibionts were evaluated in Arenaeus cribrarius (Lamarck, 1818), based on monthly samplings (May/1991 to April/1993), in Ubatuba, Brazil. Bryozoans were quantified in terms of colony numbers and barnacles by the number of specimens. Prevalence of infestation was determined in 1,914 individuals of A. cribrarius and assessed with respect to sex, maturity stage and season. No significant interaction was detected between epibionts and biological host factors. Males showed a higher infestation rate by Chelonibia patula (Ranzani, 1818) when compared to mature non-ovigerous females, yet Octolasmis lowei Darwin, 1851 infestation was associated to adult crabs. The ovigerous females of A. cribrarius showed a higher prevalence of infestation than males and non-ovigerous females, with lower infestations being recorded during winter. A synchrony between the life cycle of the epibionts and their hosts was evident and promotes the continuity of the former in the area.
Key words: Bryozoan; Chelonibia patula; epibiosis; Octolasmis lowei.
In unconsolidated benthic environments, the carapaces of decapod crustaceans are among the few solid surfaces that are available for colonization by the benthic invertebrates. These solid surfaces are utilized by specialized or facultative epibionts (ROSS 1983, ABELLÓ et al. 1990, GILI et al. 1993), and, to avoid the settlement of such epibionts, crustaceans employ anti-infestation behaviours, which include carapace cleaning with specific appendages and burying in the sediment (BAUER 1989, BECKER & WAHL 1996). In epibiosis studies, the terminology used varies according to the degree of association between the host and the infesting organism. WAHL (1989) defined epibiosis as the process of colonization of live surfaces by sessile organisms, whereas other authors have mentioned distinct terminologies according to the infestation position, distinguishing in ecto- and endosymbionts (ABELLÓ et al. 1990), or simply referring to these organisms as epizoonts (KEY et al. 1996). Due to the considerable variation in the terminology that refers to the host/infesting organism relationship, in this study, the term 'epibiosis' as defined by WAHL (1989) was adopted hereafter. The term describes only the organic interaction, without quantifying the degree of association (positive or negative).
In crustaceans, the degree of infestation by epibionts may be influenced by various factors (WAHL & LAFARGUE 1990), such as the pool of potential colonizers, the reproductive period of the infesting organism, and the amount of time the surface is exposed to the infestation. In addition, age, moult stage, sex, physiological condition, and the efficiency of anti-infestation defences from the host are relevant variables (BARNES & BAGENAL 1951, MALDONATO & URIZ 1992, DAVIS & WHITE 1994). In general, epibiosis is unfavourable to the host organism and explains the necessity for the development of mechanisms to avoid infestations (WAHL 1989, ABELLÓ et al. 1990). This has led the relationships between hosts and epibionts to be rarely species-specific (WAHL & MARK 1999). An ecdysis of the host, however, is potentially detrimental to the epibionts as they will be discarded along with the exuvia (ABELLÓ et al. 1990, ITANI et al. 2002).
The present study evaluated the mean prevalence and intensity of infestations on the speckeled swimming crab Arenaeus cribrarius (Lamarck, 1818) from the Ubatuba region, state of São Paulo, Brazil by several groups of epibionts, focusing more specifically on infestations (1) on the exoskeleton by the bryozoans and the barnacle Chelonibia patula (Ranzani, 1818) (Cheloni-biidae) and (2) in the branchial chambers by the stalked barnacle Octolasmis lowei Darwin, 1851 (Poecilasmatidae).
MATERIAL AND METHODS
Collection of A. cribrarius were carried out monthly from May/1991 to April/1993, at Fortaleza Bay and Ubatuba Bay, in the city of Ubatuba, on the northern coast of the state of São Paulo (23°25'00"-23°35'00"S and 45°00'00"-45°12'00"W). Specimens were captured with an otter-trawl towed by a commercial shrimp fishing boat, and two tows (1.5 hour) were performed, for a total of three hours of effort/capture/month. Individuals collected were frozen for analysis in the laboratory.
Samples were defrosted and the specimens were sexed, measured with a calliper (CW = carapace width without lateral spines) and classified by the maturity stage following PINHEIRO & FRANSOZO (1998). The remaining epibionts occurring on the exoskeleton (bryozoans and the barnacle C. patula) were recorded and quantified. The bryozoans were quantified by the number of colonies present. To record the presence of the barnacle O. lowei, the cephalothorax of each specimen was removed and the branchial filaments in each side of the branchial chamber (left and right) were carefully inspected.
The prevalence of infestation, i.e. the proportion between infested hosts and the total number of crabs (KEY et al. 1997), was analyzed by log-linear models of contingency tables. The interaction amongst the following variables was examined: 1) season (spring, summer, fall, winter); 2) sex (males and non-ovigerous females); 3) maturity (juveniles and adults); and 4) frequency of infestation (present or absent). The G-test was applied to examine the obtained data. When significant interactions were observed, the analysis was decomposed into 2x2 frequency tables and re-evaluated using G-test or Tukey test for multiple proportions (ZAR 1999, SOKAL & ROHLF 2003). Similarly, this procedure was applied to examine the variability of the prevalence of infestation (frequency: present or absent) during the reproductive period of the females, and the following variables were investigated: 1) season (spring, summer, fall, winter); 2) reproductive condition (ovigerous and non-ovigerous adult females).
To investigate the intensity of infestation, which describes the number of epibionts present in each host (KEY et al. 1997), a two-way ANOVA was performed for the variables: sex (male and female) and season (spring, summer, fall, winter). Homocedasticity was tested with the Barlett test and when homogeneity of variance assumption was not met, the Mann-Whitney test (for variable 'sex') and the Median test (for variable 'season') were applied. The correlation between intensity of infestation of epibionts and host size was evaluated using the Spearman Rank Correlation test. All analyses were performed at 5% significance level.
RESULTS
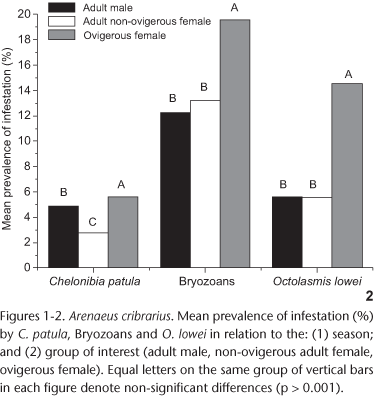
A total of 1,914 specimens of A. cribrarius was sampled during the study period, of which 1,398 were mature and 516 immature individuals, respectively. Infestation was found only on mature crabs, with bryozoans corresponding to main epibiont group (12.7% of infested mature animals), followed by C. patula and O. lowei (on 5.6% and 3.8% of mature crabs, respectively) (Tab. I). The prevalence of infestation varied significantly with the variables tested (sex, maturity and season); however, no significant interactions were found (Tabs II and III ). No significant seasonal variation was observed in the infestation of the crabs by the bryozoans (Tab. II) (Bartlett, χ2 = 21.34, p = 0.003; Median Test, χ2 = 1.59, p = 0.661), C. patula (ANOVA d.f. = 2, MS = 20.08, F = 1.32, p = 0.277) and O. lowei (Bartlett, χ2 = 57.50, p < 0.0001; Median Test, χ2 = 4.71, p = 0.203). Whilst a variation in the intensity of these taxa was not observed throughout the seasons, a significantly lower prevalence was noted during the winter (Fig. 1). In addition, the prevalence of infestation by C. patula was significantly lower during spring, and elevated rates of epibiosis were observed during the summer and the fall (Fig. 1).

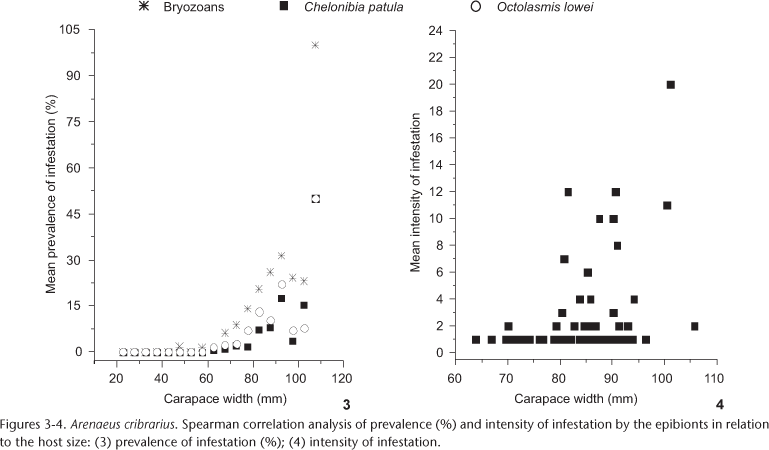
The intensity of infestation on males and non-ovigerous females did not vary significantly for bryozoans (U = 3567.00, p = 0.27), C. patula (d.f. = 1, MS = 9.065, F = 0.598, p = 0.4437) nor O. lowei (U = 743.00, p = 0.86). Nonetheless, males showed significantly higher prevalence of infestation by C. patula, when compared to non-ovigerous females (Fig. 2). A different pattern was observed for the bryozoans and O. lowei (Tab. II). However, the ovigerous condition of the females was proven as important for the establishment of all the analyzed groups of epibionts in the present study, which could be verified by their prevalence when compared with non-ovigerous condition and males, respectively (Tab. III , Fig. 2). Adults showed significantly higher prevalence of infestation than juveniles for all epibionts studied (Tab. II). Only for C. patula a significant correlation between the intensity of epibiont infestation and the host size was detected (Spearman r = 0.33, d.f. = 51, t = 2.50, p = 0.016, Figs 3 and 4). The remaining epibionts showed no correlation between the intensity of infestation and the size of the crabs (Spearman r = 0.058, d.f. = 176, t = 0.7661, p = 0.445, for bryozoans; and Spearman r = 0.15, d.f. = 76, t = 1.3271, p = 0.188, for O. lowei, Figs 3 and 4).
DISCUSSION
The presence of epibiont organisms on portunid crabs have been recorded by several authors, e.g. NEGREIROS-FRANSOZO et al. (1995) for Callinectes danae Smith, 1869 and C. ornatus Ordway, 1863, SANTOS (2002) for Portunus spinimanus Latreille, 1819, and MANTELATTO et al. (2003) for various portunid species sampled in the coast of the state of São Paulo, Brazil. In the species studied by those authors, the presence of the same epibionts registered in the present study could be verified. We detect the presence of Chelonibia patula in C. danae and C. ornatus; and Octolasmis lowei in A. cribrarius, C. danae, C. ornatus and P. spinimanus, indicating that these animals are generalists, due to wide diversity of hosts with which they are associated.
The interaction between bryozoans and the exoskeleton of portunids has been investigated previously, and studies have shown epibionts to cause variable effects. These varied from a negative impact to the host due to the increase in body weight, to benefits from the mimetic effects (INGLE 1983, KEY et al. 1999, OVERSTREET 1983, RASMUSSEN 1973, WAHL 1989), which is particularly important for the decorator crabs (PARAPAR et al. 1997, WICKSTEN 1980). Nonetheless, the infestations caused by bryozoans - Alcyonidium albescens Winston & Key, 1999, Membranipora arborescens (Canu & Bassler, 1928) and Triticella elongata (Osburn, 1912) - have a minimal negative impact towards the host, as verified in C. sapidus by KEY et al. (1999), where no special symbiosis was observed between the organisms. The pattern of the bryozoan colonies merges with the reticulated pattern of the carapace from A. cribrarius, and the bryozoans may benefit against predators by this association. Moreover, bryozoans benefit from the hosts through the additional substrates for settlement, feeding and reproduction. Despite the elevated infestation rate observed in the present study, the impact on the crab is low due to the negligible weight of the bryozoans.
Bryozoans are common infestants of sessile substrates and are also found as epibionts of mobile organisms, in both nektonic and benthonic hosts (KEY et al. 1996). Although there is a high diversity of described species (approximately 5,500 species according to ROCHA & D'HONDT 1999), a low diversity of bryozoans was observed as epibiont of mobile organisms as crabs, probably due the frequent ecdysis process of the host. Among the epibiont bryozoans, species of Triticella (Gymnolaemata: Ctenostomata: Triticellidae), as Triticella flava Dalyell, 1848, Triticella capsularis Gordon & Wear, 1999 and Triticella elongata (Osburn, 1912) were observed on crustacean decapods, mainly on portunid crabs (e.g. EGLESTON 1971, ABELLÓ & CORBERA 1996, KEY et al. 1999, GORDON & WEAR 1999 FERNANDEZ-LEBORANS 2003). In addition, other Ctenostomata, the species Alcyonidium mamillatum Alder, 1857, and species of Cheilostomata, as Acanthodesia tenuis (Desor, 1848), Membranipora arborescens (Canu & Bassler, 1928) and Membranipora membranacea (Linnaeus, 1767), were observed associated with brachyurans crabs (ABELLÓ & CORBERA 1996, KEY et al. 1999, MCGAW 2006, WINTER & MASUNARI 2006). Further studies about the identity of the bryozoans on A. cribrarius can bring important contributions to the knowledge about the interactions between both groups.
Amongst the various groups of invertebrate epibionts, barnacles are one of the most common, either by internal or external infestation (CHRISTIE & DALLEY 1987). The barnacles associated with brachyurans are represented by the Coronulidae and Balanidae families, and particularly, by the Chelonibia Leach, 1817 (Chelonibiidae) and Amphibalanus Darwin, 1854 (Balanidae) genera, respectively (PHILIPS & CANNON 1978, HAEFNER 1985, VAN ENGEL 1987, NEGREIROS-FRANSOZO et al. 1995). Barnacles of Chelonibia occur on the carapaces and appendages of pelagic crabs, on sea turtles and on other floating objects (KEY et al. 1997, PASTERNAK et al. 2002). According to OVERSTREET (1983), the barnacle C. patula may infest the portunid crabs of Callinectes (STUBBINGS 1967, NEGREIROS-FRANSOZO et al. 1995, KEY et al. 1997) and Portunus (SHIELDS 1992), and has also been recorded in a number of majids of Libinia Leach, 1815 (Pisinae) (PEARSE 1952). The distribution of C. patula on the host carapace is better correlated to larval settlement (PASTERNAK et al. 2002) than to the abrasion processes of the exoskeleton during burying (KEY et al. 1997). For PASTERNAK et al. (2002), cypris of C. patula chose the orientation and the location of settlement, and settled preferentially on the most elevated and central region of the carapace where water flow is more intense. Also, the role of chemical cues on larvae settlement has been described (MOLENOCK & GOMEZ 1972) influencing the dynamics of barnacle recruitment (ZARDUS & HADFIELD 2004) but never directly tested in the settlement of commensal barnacles.
The prevalence of infestation by the barnacle C. patula has also been recorded in males and females of other portunid crabs. In C. sapidus, infestation rate by this barnacle was higher in females than males, occurring in 70% of the females (KEY et al. 1997), corroborating earlier studies showing infestation by C. patula in females that had previously spawned two or three times (TAGATZ 1968, CRISP 1983). Hence, many authors have concluded that the higher infestation rate observed in females of C. sapidus could be explained by females entering anecdysis soon after puberty while males continue to grow (TRUITT 1939, VAN ENGEL 1958, TAGATZ 1968, PERRY 1975, OVERSTREET 1983, CRISP 1983). In the present study, males of A. cribrarius showed higher a infestation rate by C. patula compared to non-ovigerous females. This pattern could be the result of the significant correlation between host size and infestation intensity. As males reach larger sizes than females (PINHEIRO & HATTORI 2006), the former becomes more susceptible to settlement of cypris of C. patula.
Several species of brachyurans host stalked barnacles of Octolasmis (Gray, 1825) in their branchial chamber. A few authors have registered the occurrence of this barnacle in crabs (Dorippidae, Calappidae, Leucosiidae, Majidae, Portunidae and Xanthidae) and lobsters (Scyllaridae and Palinuridae) (JEFFRIES & VORIS 1983, JEFFRIES et al. 1984, 1991). In Brazil, YOUNG (1990), SANTOS (2002), SANTOS & BUENO (2002) and MANTELATTO et al. (2003) reported the infestation of the brachyurans by O. lowei. The association of the species from Octolasmis is due to the facilitated nutrient acquisition and the protection provided by the branchial chambers of the crabs, where the development of the epibiont is completed. These epibionts tend to select preferentially hosts in intermoult (SANTOS 2002) and settlement tends to increase after sexual maturity is reached. Moreover, according to JEFFRIES et al. (1992), in the crab Scylla serrata (Forskal, 1775), individuals having less than 12 instars are not infested by octolasmids because of the short intermoult period, which would prevent the growth and reproduction of the epibiont. The results from the present investigation concur with the above studies because adults of A. cribrarius showed higher prevalence of infestation by O. lowei, and the intermoult period in the adults would be sufficient for settlement, growth and reproduction of the epibiont to occur before the moulting of the host.
The duration of the intermoult period, the habitat distribution, and the burying behaviour of the host are determinant factors in the distribution of the species of Octolasmis (ROSS 1983, JEFFRIES et al. 1992), and explain the clear differences in the prevalence amongst the host species (SANTOS & BUENO 2002). Furthermore, the epibionts can explore only a portion of the host population, affecting specific age, sex and even the environment (VORIS et al. 1994). The presence of Octolasmis species in the branchial chamber can affect the host in many ways: 1) reducing the water circulation and the gas exchange of the gills due to the fixation of the barnacle; 2) consuming the oxygen in the ventilatory water of the crab; and 3) obstructing of the ventilatory current in the host, which would elevate the energy spent in ventilation. However, according to GANNON & WHEATLY (1992), Octolasmis muelleri Coker, 1902 (Poecilasmatidae) depends on the ventilatory current from C. sapidus to survive, and those authors have not observed any significant perturbation caused by the presence of the epibiont. This barnacle may be detrimental to the host only during intense infestation, which is contrary to evidence from most of the brachyurans studied, where the interaction between the species of Octolasmis and the host is common.
Seasonal analysis is one of the methods to evaluate the interaction between the epibionts and the hosts, where it is possible to determine the compatibility of their life cycles. In the present study, there was a significant seasonal difference in the prevalence of infestation for C. patula, bryozoans and O. lowei, with lower infestation occurring during the winter. These results suggest that the epibionts have a synchronous life cycle with their hosts. As ovigerous females (along with males and non-ovigerous females) are less abundant during the coldest months (PINHEIRO & FRANSOZO 2002), therefore occur a subsequent reduction of the hosts available for epibiont settlement. Moreover, considering that the epibionts are available equally throughout the year, we should expect an increase in epibiont settlement per host. As the hosts showed lower proportion of infestation during the winter months suggesting that epibiont are also in lower availability during this season.
The quantitative analysis of the epibionts investigated has shown a higher prevalence of infestation in ovigerous females when compared to the remaining reproductive groups studied. Similar results were obtained in other portunid species (SHIELDS 1992, KEY et al. 1999, SANTOS & BUENO 2002), where the different infestation rates observed were regarded as the result of the differential behaviour of the ovigerous females. Studying the infestation of bryozoans in C. sapidus, KEY et al. (1999), suggested that the higher infestation on ovigerous females, following the puberty moult, could be explained by the lower frequency of moults in females in relation to males. Thus, the infestation on ovigerous females in the present study may be caused by other factors, such as: 1) greater age and longer susceptibility to the settlement of bryozoan larvae; 2) the higher attractiveness to larval settlement; and 3) a longer period spent in high salinity waters. For the host species investigated in the present study, PINHEIRO & FRANSOZO (2002) have demonstrated that ovigerous females of A. cribrarius prefer deeper waters, higher salinities and sediment composed by coarser sand, which is utilized to shape their egg mass. During this period, females excavate a small depression in the sediment and use it as an incubation chamber, while their carapace stays partially uncovered and susceptible to colonization by epibionts (PINHEIRO & FRANSOZO 1999). In general, this species remains buried in the sediment, exposing only the rostral region and the upper surface of the chelipeds, where most of the epibiosis have been recorded. In addition, when females are carrying the egg mass, their natatory activity is slowed and cryptic behaviours are observed, which might enhance the settlement of the epibionts.
In crustaceans, ecdysis is the most limiting and relevant biological process affecting the epibiont/host interaction because the occurrence of ecdysis is directly related to the intermoult period. Therefore, the succession pattern of epibionts in crabs can be analysed, not only according to size, but also by the moult stage of the host. Accordingly, this pattern could provide evidence for the existence of a terminal moult in the host, which is unknown in many species of crustaceans (ABELLÓ et al. 1990, SHIELDS 1992, NEGREIROS-FRANSOZO et al. 1995). One possible explanation to this pattern is that epibiosis is correlated with the maturity size of the host because the epibionts are not relatively fast to establish and develop in juvenile crabs, since these age-classes undergo ecdysis more frequently before puberty. On the other hand, the epibionts can develop continuously in adult crabs, where the intermoult period is longer (ABELLÓ et al. 1990). The prevalence of infestation was positively correlated to the host size in all groups examined, possibly, due to the larger area available for larval settlement. Conversely, larger crabs did not present infestation rates close to 100%, which would be evidence for the existence of a terminal moult, as suggested by ABELLÓ et al. (1990). Thus, in agreement with other portunids, A. cribrarius do not undergo a terminal moult after reaching sexual maturity.
ACKNOWLEDGEMENTS
We thank FAPESP for the PhD studentship awarded to Marcelo A.A. Pinheiro (Proc. # 1992/01752-8) and Adilson Fran-sozo for the access to the research infrastructure of the Depar-tamento de Zoologia - IB/UNESP Botucatu. The experiments carried out in this study comply with the current laws of Brazil.
LITERATURE CITED
Submitted: 16.VI.2009; Accepted: 14.II.2010.
Editorial responsibility: Marcus V. Domingues
- ABELLÓ, P. & J. CORBERA. 1996. Epibiont bryozoans (Bryozoa, Ctenostomatida) of the crab Goneplax rhomboides (Brachyura, Goneplacidae) off the Ebro delta (western Mediterranean). Miscelania Zoologica 19: 43-52.
- ABELLÓ, P.; R. VILLANEUVA & J.M. GILI. 1990. Epibiosis in deep-sea crab populations as indicator of biological and behavioural characteristics of the host. Journal of the Marine Biological Association of the United Kingdom 70: 687-695.
- BARNES, H. & T.B. BAGENAL. 1951. Observation on Nephrops norvegicus (L.) and on an epizoic population of Balanus crenatus Brug. Journal of the Marine Biological Association of the United Kingdom 30: 369-380.
- BAUER, R.T. 1989. Decapod crustacean grooming: functional morphology, adaptive value and phylogenetic significance, p. 49-74. In: B.E. FELGENHAUER & L. WATLING & A.B. THISTLE (Eds). Functional morphology of feeding and grooming in Crustacea. Rotterdam, Crustacean Issues, vol. 6, 320p.
- BECKER, K. & M. WAHL. 1996. Behaviour patterns as natural antifouling mechanisms of tropical marine crabs. Journal of Experimental Marine Biology and Ecology 203: 245-258.
- CHRISTIE, A.O. & R. DALLEY. 1987. Barnacle fouling and its prevention, p. 419-433. In: A.J. SOUTHWARD (Ed). Barnacle Biology. Rotterdam, Crustacean Issues, vol. 5, 496.
- CRISP, D.J. 1983. Chelonibia patula (Ranzani), a pointer to the evolution of the complemental male. Marine Biology Letters 4 (5): 281-294.
- DAVIS, A.R. & G.A. WHITE. 1994. Epibiosis in a guild of sessile subtidal invertebrates in southeastern Australia: a quantitative survey. Journal of Experimental Marine Biology and Ecology 177: 1-14.
- EGGLESTON, D. 1971. Synchronization between moulting in Calocaris macandrea (Decapoda) and reproduction in its epibiont Triticella (sic) koreni Journal of the Marine Biological Association of the United Kingdom 51: 409-410.
- FERNANDEZ-LEBORANS, G. 2003. Protist-bryozoan-crustacean hyperepibiosis on Goneplax rhomboides (Linnaeus, 1758) (Decapoda, Brachyura) from the NW Mediterranean coast. Crustaceana 76 (4): 479-497.
- GANON, A.T. & M.G. WHEATLY. 1992. Physiological effects of an ectocommensal gill barnacle, Octolasmis muelleri on gas exchange in the blue crab Callinectes sapidus Journal of Crustacean Biology 12 (1): 11-18.
- GILI, J.M.; P. ABELLÓ & R. VILLANUEVA. 1993. Epibionts and intermoult duration in the crab Bathynectes piperitus Marine Ecology Progress Series 98: 107-113.
- GORDON, D.P. & R.G. WEAR. 1999. A new ctenostome bryozoans ectosymbiotic with terminal-moult paddle crabs (Portunidae) in New Zealand. New Zealand Journal of Zoology 26: 373-380.
- HAEFNER JR, P.A. 1985. Morphometry, reproduction, diet, and epizoites of Ovalipes stephensoni Williams, 1976 (Decapoda, Brachyura). Journal of Crustacean Biology 5 (4): 658-672.
- INGLE, R.W. 1983. Shallow-water crabs. Synopses of the British Fauna New Series 25: 1-206.
- ITANI, G.; M. KATO & Y. SHIRAYAMA. 2002. Behaviour of the shrimp ectosymbionts, Peregrinamor ohshimai (Mollusca: Bivalvia) and Phyllodurus sp. (Crustacea: Isopoda) through host ecdyses. Journal of the Marine Biological Association of the United Kingdom 82: 69-78.
- JEFFRIES, W.B. & H.K. VORIS. 1983. The distribution, size, and reproduction of the pedunculate barnacle, Octolasmis mülleri (Coker, 1902), on the blue crab, Callinectes sapidus (Rathbun, 1896). Fieldiana Zoology 16: 1-10.
- JEFFRIES, W.B.; H.K. VORIS & C.M. YANG. 1984. Diversity and distribution of the pedunculate barnacle Octolasmis Gray, 1825, epizoic on the scyllarid lobster, Thenus orientalis Crustaceana 46 (3): 300-308.
- JEFFRIES, W.B.; H.K. VORIS & C.M. YANG. 1991. Species recognition among the pedunculate barnacles (Cirripedia: Thoracica) on the mangrove crabs, Scylla serrata Raffles Bulletin of Zoology 40 (1): 83-92.
- JEFFRIES, W.B.; H.K. VORIS & S. POOVACHIRANON. 1992. Age of the mangrove crab Scylla serrata at colonization by stalked barnacles of the genus Octolasmis Biological Bulletin 182: 188-194.
- KEY JR, M.M.; J.E. WINSTON; J.W. VOLPE; W.B. JEFRIES & H.K. VORIS. 1999. Bryozoan fouling of the blue crab Callinectes sapidus at Beaufort North Carolina. Bulletin of Marine Science 64 (3):513-533.
- KEY JR, M.M.; J.W. VOLPE; W.B. JEFFRIES & H.K, VORIS. 1997. Barnacles fouling of the blue crab Callinectes sapidus at Beaufort, North Carolina. Journal of Crustacean Biology 17 (3): 424-439.
- KEY JR, M.M.; W.B. JEFFRIES; H.K. VORIS & C.M. YANG. 1996. Epizoic bryozoans, horseshoe crabs, and other mobile benthic substrates. Bulletin of Marine Science 58 (2): 368-384.
- MALDONATO, M. & M.J. URIZ. 1992. Relationship between sponges and crabs: patterns of epibiosis on Inachus aguiarii (Decapoda: Majidae). Marine Biology 113: 281-286.
- MANTELATTO, F.L.; J.J. O'BRIEN & R. BIAGI. 2003. Parasites and symbionts of crabs from Ubatuba Bay, São Paulo State, Brazil. Comparative Parasitology 70 (2): 211-214.
- MCGAW, I.J. 2006. Epibionts of sumpatric species of Cancer crabs in Barkley Sound, British Columbia. Journal of Crustacean Biology 26 (1): 85-93.
- MOLENOCK, J. & E.D. GOMEZ. 1972. Larval stages and settlement of the barnacle Balanus (Conopea) galeatus (L.) (Cirripedia Thoracica). Crustaceana 23: 100-108.
- NEGREIROS-FRANSOZO, M.L.; T.M. COSTA & A. FRANSOZO. 1995. Epibiosis and molting in two species of Callinectes (Decapoda: Portunidae) from Brazil. Revista Biologia Tropical 43 (1-3): 257-264.
- OVERSTREET, R.M. 1983. Metazoan Symbionts of Crustraceans, p. 155-250. In: A.J. PROVENZANO JR (Ed). The Biology of Crustacea: Pathobiology. New York, Academic Press, vol. 6, 290p.
- PARAPAR, J.; L. FERNANDEZ; E. GONZALEZ-GURRIARAN & R. MUIÑO. 1997. Epibiosis and masking material in the spider crab Maja squinado (Decapoda: Majidae) in the Ria de Arousa (Galicia, NW Spain). Cahiers de Biologie Marine 38 (4): 221-234.
- PASTERNAK, Z.; A. ABELSON & Y. ACHITUV. 2002. Orientation of Chelonibia patula (Crustacea: Cirripedia) on the carapace of its crab host is determined by the feeding mechanism of the adult barnacles. Journal of the Marine Biological Association of the United Kingdom 82: 583-588.
- PEARSE, A.S. 1952. Parasitic Crustacea from the Texas coast. Publication Institute of Marine Science, University of Texas 2 (2): 5-42.
- PERRY, H.M. 1975. The blue crab fishery in Mississippi. Gulf Research Reports 5: 39-57.
- PHILIPS, W.J. & L.R.G. CANNON. 1978. Ecological observations on the commercial sand crab, Portunus pelagicus (L.), and its parasite, Sacculina granifera Boschma, 1973 (Cirripedia: Rhizocephala). Journal of Fish Diseases 1: 137-149.
- PINHEIRO, M.A.A. & A. FRANSOZO. 1998. Sexual maturity of the speckled swimming crab Arenaeus cribrarius (Lamarck, 1818) (Decapoda, Brachyura, Portunidae), in the Ubatuba litoral, São Paulo state, Brazil. Crustaceana 71 (4): 434-452.
- PINHEIRO, M.A.A. & A. FRANSOZO. 1999. Reproductive behavior of the swimming crab Arenaeus cribrarius (Lamarck, 1818) (Crustacea, Brachyura, Portunidae) in captivity. Bulletin of Marine Science 64 (2): 243-253.
- PINHEIRO, M.A.A. & A. FRANSOZO. 2002. Reproductive of the speckled swimming crab Arenaeus cribrarius (Lamarck, 1818) (Brachyura, Portunidae), on the north coast of São Paulo State, Brazil. Journal of Crustacean Biology 22 (2): 416-428.
- PINHEIRO, M.A.A. & G.Y. HATTORI. 2006. Growth of the speckled swimming crab, Arenaeus cribrarius (Lamarck, 1818) (Crustacea, Brachyura, Portunidae), in Ubatuba (SP), Brazil. Journal of Natural History 40 (21-22): 1331-1341.
- RASMUSSEN, E. 1973. Systematic and ecology of the is fjord marine fauna (Denmark). Ophelia 11: 1-495.
- ROCHA, R.M. & J.-L. D'HONDT. 1999. Filo Ectoprocta ou Bryozoa, p. 241-249. In: A.E. MIGOTTO & C.G. TIAGO (Eds). Biodiversi-dade do Estado de São Paulo, Brasil: síntese do conhecimento ao final do século XX. São Paulo, FAPESP, vol. 3, 310p.
- ROSS, D.M. 1983. Symbiotic relations, p. 163-212. In: F.J. VERNBERG & W.B. VERNBERG (Eds). The biology of Crustacea: behavior and ecology. New York, Academic Press, vol. 7, 383p.
- SANTOS, C. & S.L.S. BUENO. 2002. Infestation by Octolasmis lowei (Cirripedia: Poecilasmatidae) in Callinectes danae (Decapoda: Portunidae) from São Sebastião, Brazil. Journal of Crustacean Biology 22 (2): 241-248.
- SANTOS, S. 2002. Symbiosis between Portunus spinimanus Latreille, 1819 (Decapoda, Portunidae) and Octolasmis lowei (Darwin, 1852) (Thoracica, Poecilasmatidae) from Ubatuba, São Paulo, Brazil, p. 205-209. In: E. ESCOBAR-BRIONES & F. ALVAREZ (Eds). Modern Approaches to the study of Crustacea. New York, Kluwer Academic Publishers, 376p.
- SHIELDS, J.D. 1992. Parasites and symbionts of the crab Portunus pelagicus from Moreton Bay, Eastern Australia. Journal of Crustacean Biology 12 (1): 94-100.
- SOKAL, R.R. & F.J. ROHLF. 2003. Biometry: the principles and pratice of statistics in biological research. New York, W.H. Freeman, 3rd ed., 887p.
- STUBBINGS, H.G. 1967. Cirriped fauna of tropical West Africa. Bulletin of the British Museum Natural History. Zoology 15: 1-39.
- TAGATZ, M.E. 1968. Biology of the blue crab, Callinectes sapidus Rathbun, in the St. Johns River, Florida. Fishery Bulletin-NOAA 67: 17-33.
- TRUITT, R.V. 1939. Our water resources and their conservation. Solomons, Chesapeake Biological Laboratory, Contribution 27, 103p.
- VAN ENGEL, W.A. 1958. The blue crab and its fishery in Chesapeake Bay. Part 1. Reproduction, early development, growth, and migration. Commercial Fisheries Review 20: 6-17.
- VAN ENGEL, W.A. 1987. Factors affecting the distribution and abundance of the blue crab in Chesapeake Bay, p. 178-209. In: S.K. MAJUMDAR; L.W. HALL JR & H.M. AUSTIN (Eds). Contaminant problems and management of living Chesapeake Bay resources, Pennylvania. Philadelphia, Academy of Sciences, 573p.
- VORIS, H.K.; W.B. JEFFRIES & S. POOVACHIRANON. 1994. Patterns of distribution of two barnacle species on the mangrove crab, Scylla serrata Biological Bulletin 187: 346-354.
- WAHL, M. 1989. Marine epibiosis. I. Fouling and antifouling: some basic aspects. Marine Ecology Progress Series 58: 175-189.
- WAHL, M. & F. LAFARGUE. 1990. Marine Epibiosis II. Reduced fouling on Polysyncraton lacazei (Didemnidae, Tunicata) and proposal of an antifouling potential index. Oecologia 82: 275-282.
- WAHL, M. & O. MARK. 1999. The predominantly facultative nature of epibiosis: experimental and observational evidence. Marine Ecology Progress Series 187: 59-66.
- WICKSTEN, M.K. 1980. Decorator crabs. Scientific American 242: 146-154.
- WINTER, V.C. & S. MASUNARI. 2006. Macroepizoísmo em Libinia ferreirae (Crustacea, Brachyura, Majidae). Iheringia, Série Zoologia, 96 (2): 135-140.
- YOUNG, P.S. 1990. Lepadomorph cirripeds from the Brazilian coast. I - Families Lepadidae, Poecilasmatidade and Heteralepadidae. Bulletin of Marine Science 47: 641-655.
- ZAR, J.H. 1999. Biostatistical Analysis. New Jersey, Prentice Hall, 4th ed., 121p.
- ZARDUS, J.D. & HAEDFIELD, M.G. 2004. Larval development and complemental males in Chelonibia testudinaria, a barnacle commensal with sea turtles. Journal of Crustacean Biology 24 (3): 409-421.
Epibionts on Arenaeus cribrarius (Brachyura: Portunidae) from Brazil
Publication Dates
-
Publication in this collection
12 July 2010 -
Date of issue
June 2010
History
-
Received
16 June 2009 -
Accepted
14 Feb 2010