Abstract
The identity of two insular populations of rodents of the nominal species, Zygodontomys brevicauda (Allen & Chapman, 1893), from the Veragua Archipelago was examined. The mitochondrial cytochrome-b gene was sequenced from specimens collected on Isla Coiba (n = 10), Isla Cébaco (n = 3) and on the nearby Peninsula Azuero (n = 3) in Panama and compared with sequences of Z. brevicauda and a number of other related species from GenBank. For Panama, phylogenetic analyses identified two clades within Zygodontomys Allen, 1897; one representing Isla Coiba and another clade composed of rats from Isla Cébaco and from the mainland on the Peninsula Azuero, as well as a GenBank sequence of Z. brevicauda from Venezuela. We suggest that the population from Isla Coiba may represent a previously undescribed species of sigmodontine rodent that is endemic to this Pacific Island.
Cébaco; island; mammal; Panamá
SHORT COMMUNICATION
Variation in cytochrome-b haplotypes suggests a new species of Zygodontomys (Rodentia: Cricetidae) endemic to Isla Coiba, Panama
Publio GonzálezI; Yadéeh E. SawyerII; Mario AvilaIII; Aníbal G. ArmiénIV; Blas ArmiénI; Joseph A. CookII,* * Corresponding Author. E-mail: cookjose@unm.edu
IInstituto Conmemorativo Gorgas de Estudios de la Salud. Apartado Postal 0816-02593, Ave. Justo Arosemena, Calle 35, Panamá, PANAMA
IIMuseum of Southwestern Biology, Department of Biology, MSC03 2020, University of New Mexico, Albuquerque, NM 87131-0001 USA
IIIMinisterio de Salud, Apartado Postal 06812, Ancón, Panamá, PANAMA
IVUniversity of Minnesota, 1333 Gorner Avenue, St. Paul, MN 55108, Minnesota, USA
ABSTRACT
The identity of two insular populations of rodents of the nominal species, Zygodontomys brevicauda (Allen & Chapman, 1893), from the Veragua Archipelago was examined. The mitochondrial cytochrome-b gene was sequenced from specimens collected on Isla Coiba (n = 10), Isla Cébaco (n = 3) and on the nearby Peninsula Azuero (n = 3) in Panama and compared with sequences of Z. brevicauda and a number of other related species from GenBank. For Panama, phylogenetic analyses identified two clades within Zygodontomys Allen, 1897; one representing Isla Coiba and another clade composed of rats from Isla Cébaco and from the mainland on the Peninsula Azuero, as well as a GenBank sequence of Z. brevicauda from Venezuela. We suggest that the population from Isla Coiba may represent a previously undescribed species of sigmodontine rodent that is endemic to this Pacific Island.
Keywords: Cébaco; island; mammal; Panamá.
Insular populations or species are often divergent from their mainland counterparts. This observation has led studies of island biota to play a central role in the development of the field of biology, specifically evolutionary theory and related empirical analyses (DARWIN 1859, RICKLEFS & BERMINGHAM 2008). The fauna of many Panamanian islands, especially those of the Pacific Coast, has not been extensively studied. The discovery that a number of historically important museum specimens purportedly from Isla Coiba were likely from other sites in Central America has further obscured our knowledge of the Pacific islands of Panama (OLSON 2008).
We sequenced a mitochondrial DNA gene from specimens recently collected on Isla Cébaco and Isla Coiba, and from mainland sites in Panama (Fig. 1). We then compared these to GenBank sequences of Zygodontomys Allen, 1897 from Colombia, Venezuela and French Guiana. Because the phylogenetic placement of Zygodontomys within the Sigmodontinae has also been controversial (CARLETON & MUSSER 2005) and the sister taxon is unknown, we included representatives of other related species based on previous studies of their phylogenetic relationships (STEPPAN et al. 2004, WEKSLER 2003, 2006, WEKSLER et al. 2006). This molecular framework provides an initial view of diversification of rodents of the genus Zygodontomys on two islands of the Veraguas archipelago.
As part of a multi-year survey of mammalian-borne pathogens (e.g., hantavirus), rodents were collected from a number of localities on the mainland in southern Veraguas Province on the Peninsula Azuero, and on the islands of Cébaco and Coiba (Fig. 1). Specimens were deposited at the Museum of Southwestern Biology in Albuquerque, New Mexico. We sequenced the mitochondrial cytochrome-b gene for 16 specimens of Zygodontomys and compared these sequences to related sequences on GenBank.
Sampling effort consisted of 6300 trap-nights in October 2006 on Isla Coiba and 4800 trap-nights in May and July 2008 on Isla Cébaco. At each site, we used 100 Sherman traps placed 10 meters apart in square grids (ca. 1 ha.) located in a variety of habitats, including crop-fields, pastures, and various stages of secondary forest. Tissue samples from previous sampling efforts on the Peninsula Azuero were also used.
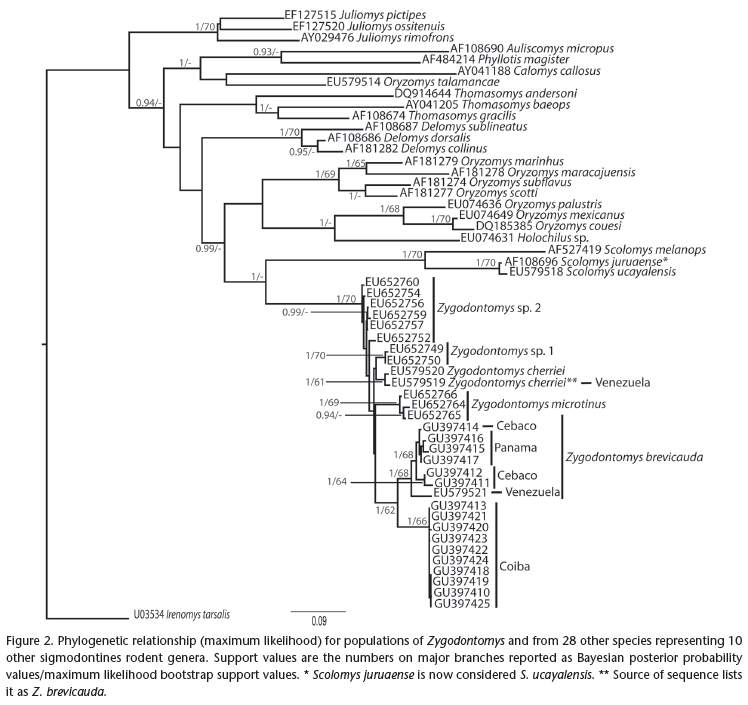
The mitochondrial cytochrome-b gene was sequenced for 16 specimens (Isla Coiba n = 10, Isla Cébaco n = 3, Peninsula Azuero n = 3) that were tentatively identified as Zygodontomys brevicauda (Allen & Chapman, 1893). DNA was extracted from frozen heart or liver tissues using a modified salt extraction (FLEMING & COOK 2002). The polymerase chain reaction (PCR) used the primers L14734 (IRWIN et al. 1991) and CytB Rev (ANDERSON & YATES 2000) to obtain the complete mtDNA cytochromeb sequence (1140bp). PCR conditions included 33 cycles with denaturation at 94ºC for 30 seconds, annealing at 51.7º for 25 seconds, and extension at 72º for 1 minute. PCR products were sequenced using the original primers and BigDye Terminator Cycle Sequencing Ready Reaction mix. V3.1 (Applied Biosystems). All sequences were deposited in GenBank (Accession numbers: GU397410-GU397425). Additionally, because Zygodontomys at various times was hypothesized to be affiliated with several different tribes within the Sigmodontinae (HERSHKOVITZ 1962, HOOPER & MUSSER 1964, GARDNER & PATTON 1976), we obtained sequences from GenBank from 28 species representing 10 other genera to further assess the phylogenetic placement of Zygodontomys among sigmodontine rodents. Irenomys Thomas, 1919 was designated the outgroup.
A maximum likelihood tree was created using GARLI (D.J. ZWICKL, University of Texas at Austin, unpublished data) with 1000 replicates and a Bayes tree was created using MrBayes 3.1 (HUELSENBECK & RONQUIST 2005) with codon position partitioning, and 4 Monte Carlo Markov Chains that proceeded for five million generations, with each sampled every 100 generations. Using ModelTest (POSADA & CRANDALL 1998), we choose the K81uf+I+G model with gamma = 0.8938. This model was used for the GARLI and a modified version (nst = 2 and rates = invgamma) for MrBayes. Nucleotide composition and genetic distance values were computed in MEGA (TAMURA et.al. 2007).
The Isla Coiba and Isla Cébaco sequences exhibited a base and amino acid composition expected for genuine mammalian cytochrome-b sequences (IRWIN et al. 1991). Nucleotide composition of the Isla Coiba sequences was 27.3% adenine, 31.9% thymine, 28.9% cytosine, and had an overall deficit of guanine (12.3%). The average genetic distance value within the Isla Coiba haplotypes (4 locations) was minimal (0.7%) with similar low levels of variation found within Panamanian mainland samples (0.9%) and within Isla Cebaco (0.7%). Distance values between Isla Coiba specimens and mainland representatives of Z. brevicauda and other species of Zygodontomys ranged from 7.5% to 9.2% (Tab. I), while Isla Cebaco and the nearby mainland (0.3%) was much lower (Tab. I). The higher values separating Isla Coiba from the others are similar to species-level differences for this gene for sister-species comparisons of mammals (BAKER & BRADLEY 2006). Scolomys Anthony, 1924 fell out as the sister genus to Zygodontomys, with an approximate genetic divergence of 30%.
ML and Bayes trees showed nearly identical topologies (Fig. 2). The 10 Isla Coiba specimens formed a monophyletic clade that was supported in all analyses (Bayes = 1, ML = 66). The Isla Coiba clade associated most closely with the Z. brevicauda clade, while Isla Cébaco haplotypes formed a clade with mainland Z. brevicauda from Panama.
Mammals found on oceanic islands are often genetically isolated from other populations due to reduced migration. Among insular mammals, rodents are notorious for their devastating introductions worldwide. Rodents, however, also have a relatively high degree of endemism in insular faunas (AMORI et al. 2009). Worldwide, islands support an enormous and largely irreplaceable heritage of rodent biological diversity and much of it is poorly studied (CEBALLOS & BROWN 1995, CARLETON & OLSON 1999, SALVADOR et al. 2008).
For Panama, a number of rodent species need careful taxonomic and phylogeographic study based on expanded geographic sampling (MENDEZ 1993). Isla Coiba, which is about 18 km from the mainland, is 53,528 ha in size. This molecular perspective on the Coiba population of Zygodontomys indicates that these rats likely represent a distinct species based on the degree of sequence divergence relative to other distinct species of Zygodontomys. Careful morphological comparisons are now needed to explore the taxonomic status of this population. Another endemic rodent from this island (REID 2009) is the Coiba Island agouti, Dasyprocta coibae Thomas, 1902, an island form that is currently listed as Endangered by the IUCN. That taxon, however, should be reviewed through a wider study of geographic variation among various populations of Neotropical agoutis (CARLETON & MUSSER 2005). A number of endemic birds have also been associated with Isla Coiba (WETMORE 1957, OLSON 2007). In contrast, Isla Cébaco is much smaller (8500 ha) and only about 8 km from the mainland. This island population is less divergent from other Zygodontomys populations with haplotypes more closely related to those on the nearby mainland. Geologic history of the region indicates that Isla Coiba and Isla Cébaco were united with the mainland on multiple occasions in the Late Cenozoic, most recently when sea levels dropped during the Last Glacial Maximum that ended about 10,000 years ago (CASTROVIEJO et al. 1997). Because the nearby lowlands of the Peninsula Azuero have been highly populated by humans and deforested since the pre-Columbian period (COATES 2003), the islands of the Veragua Archipelago are important components of efforts to maintain the native fauna and flora of Panama.
The number of extant species of Zygodontomys has been the subject of debate (CARLETON & MUSSER 2005) and our phylogeny suggests that this issue is far from resolved. VOSS (1991) assessed morphological variation across a large number of specimens representing populations from Costa Rica, Panama, Colombia, and Venezuela and concluded that the genus consisted of two extant species. He recognized Z. brevicauda as a widely distributed species that occurs from central Costa Rica south and east through northern South America to northern Brasil. The second species is Zygodontomys brunneus Thomas, 1898, a rat that is limited to the intermontane valleys of northern Colombia. CARLETON & MUSSER (2005) followed that taxonomic arrangement, but BOVICINO et al. (2008) used chromosomal and cytochrome-b sequence variation to suggest the possibility of two new species in Brazil and Guiana. Those populations (Fig. 2, Sp 1 and Sp 2) show limited divergence in our analysis, while specimens from Isla Coiba are among the most distinctive identified with this assessment of cytochrome-b sequence variation.
Specimens from Isla Cébaco were included in the monograph of VOSS (1991). His morphological analysis (Fig. 26 of VOSS 1991) clustered these specimens with other island (e.g., San Miguel Island, Panama) or distant mainland (e.g., French Guiana) populations, but distinct from nearby mainland populations. In contrast, the mitochondrial data suggest a close affinity between populations of Zygodontomys on Isla Cébaco and the nearby Panamanian mainland. Further exploration of these findings will be required to interpret the taxonomic status of the Isla Cébaco population. At higher taxonomic levels, WEKSLER's (2006) morphological and molecular assessment indicated that Zygodontomys was related to the oryzomine clade (WEKSLER 2006). Furthermore, Scolomys is considered to be sister taxon to Zygodontomys (WEKSLER 2006) and our expanded mitochondrial analysis is consistent with both of those views.
In summary, cytochrome-b haplotypes found in rodents of the genus Zygodontomys on Isla Coiba are not shared with other populations and form a distinctive clade that is divergent at a level comparable to other sister-species comparisons in sigmodontine rodents (Tab. I). Further assessment of molecular and morphological variation is required to understand the taxonomic placement of these endemic rodents which could be useful indicators of overall island conservation status (AMORI et al. 2009). Because Isla Coiba was a penal colony for more than 70 years beginning in 1919, the island has been relatively undisturbed by humans. In 1991, Isla Coiba was declared a National Park (CASTROVIEJO et al. 1997). Protective status should help ensure the persistence of endemic forms so far identified from this Pacific island, but substantial inventory and systematic work remains to document and fully understand the extent of endemism on islands in Central America.
ACKNOWLEDGMENTS
This study was part of a collaborative project managed by the Instituto Conmemorativo Gorgas de Estudios de la Salud in Panama and financed by the Secretaria Nacional de Ciencia y Tecnologia, Innovation and Technology Program (FTD06-089) in Panama, US Public Health Service (NIH) grants to the University of New Mexico: Infectious Diseases Research (ICIDR) program of the National Institutes of Health, Ministry of Health U19 AI 45452 and Fogarty International Center Research Grant D43 TW007131, Centers for Disease Control, and the Yates Field Fund of the Museum of Southwestern Biology. We thank the National Authority of the Environment (ANAM), residents and landowners of local communities, and D. Vargas, R. Rodriguez, H. Rivera, D. González, J. González and E. Calderon for support during fieldwork. A. Hope, B. Barker, J. Malaney, J. Dunnum, and F. Torres-Perez helped with technical aspects of this paper. We dedicate this paper to the memory of David Omar Vargas.
LITERATURE CITED
Submitted: 15.I.2010; Accepted: 27.VI.2010.
Editorial responsibility: Marcio R. Pie
- AMORI, G.; S. GIPPOLITI & K.M. HELGEN. 2009. Diversity, distribution, and conservation of endemic island rodents. Quaternary International 182: 6-15.
- ANDERSON, S. & T.L. YATES. 2000. A new genus and species of Phyllotine rodent from Bolivia. Journal of Mammalogy 81: 18-36.
- BAKER, R.J. & R. BRADLEY. 2006. Speciation in mammals and the genetic species concept. Journal of Mammalogy 87: 643-662.
- BONVICINO, C.R.; P.R. GONCALVES; J.A. DE OLIVEIRA; L.F.B. DE OLIVIEIRA & M.S. MATTEVI. 2008. Divergence in Zygodontomys (Rodentia: Sigmodontinae) and distribution of Amazonian savannas. Journal of Heredity 100 (3): 322-328.
- CARLETON, M.D. & G.G. MUSSER. 2005. Order Rodentia, p. 745-1600. In: D.E. WILSON & D.M. REEDER (Eds). Mammal species of the world: a taxonomic and geographic reference. Baltimore, Johns Hopkins University Press, XXXII+1242p.
- CARLETON, M.D. & S.L. OLSON. 1999. Amerigo Vespucci and the rat of Fernando de Noronha: a new genus and species of Rodentia (Muridae: Sigmodontinae) from a volcanic island off Brazil's continental shelf. American Museum Novitates 3256: 1-59.
- CASTROVIEJO, S. 1997. Flora y Fauna del Parque Nacional de Coiba (Panamá) Inventario Preliminar. Madrid, AECI, 534p.
- CEBALLOS, G. & J.H. BROWN. 1995. Global patterns of mammalian diversity, endemism and endangerment. Conservation Biology 9: 559-568.
- COATES, A.G. 2003. Una Historia de la Naturaleza y Cultura de Centroamérica Paseo Pantera. Washington, D.C., Smithsonian Books, 302p.
- DARWIN, C. 1859. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London, John Murray.
- FLEMING, M.A. & J.A. COOK. 2002. Phylogeography of endemic ermine (Mustela erminea) in Southeast Alaska. Molecular Ecology 11: 795-807.
- GARDNER, A.L. & J.L. PATTON. 1976. Karyotypic variation in oryzomyine rodents (Cricetidae) with comments on chromosomal evolution in the Neotropical cricetinae complex. Occasional Papers, Louisiana State University Museum of Zoology 49: 1-48.
- HERSHKOVITZ, P. 1962. Evolution of Neotropical cricetine rodents (Muridae) with special reference to the phyllotine group. Fieldiana Zoology 46: 1-524.
- HOOPER, E.T. & G.G. MUSSER. 1964. The glans penis in Neotropical cricetines (family Muridae) with comments on classification of muroid rodents. Miscellaneous Publication, Museum of Zoology, University of Michigan 123: 1-57.
- IRWIN, D.M.; T.D. KOCHER & A.C. WILSON.1991. Evolution of the cytochrome b gene of mammals. Journal of Molecular Evolution 32: 128-144.
- MENDEZ, E. 1993. Los Roedores de Panamá. Panama, Privately Printed, 9th ed., 345p.
- OLSON, S.L. 2007. A new subspecies of Sporophila angolensis from Isla de Coiba and other additions to the Veragua Archipelago, Panama. Proceedings of the Biological Society of Washington 120: 377-381.
- OLSON, S.L. 2008. Falsified data associated with specimens of birds, mammals, and insects from the Veragua Archipelago, Panama, collected by J. A. Batty. American Museum Novitates 3620: 1-37.
- POSADA, D. & K.A.CRANDALL. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14: 817-818.
- REID, F.A. 2009. A field guide to the Mammals of Central America and Southeast Mexico. New York, Oxford University Press, 2nd ed., 346p.
- RICKLEFS, R. & E. BERMINGHAM. 2008. The West Indies as a laboratory of biogeography and evolution. Philosophical Transactions of the Royal Society B 363: 2393-2413
- HUELSENBECK, J.P. & F. RONQUIST. 2005. Bayesian analysis of molecular evolution using MrBayes, p. 183-232. In: R. NIELSEN (Ed.). Statistical Methods in Molecular Evolution. New York, Springer.
- SALVADOR, C.H. & F.A.S. FERNANDEZ. 2008. Population dynamics and conservation status of the insular cavy Cavia intermedia (Rodentia: Caviidae). Journal of Mammalogy 89:721-729.
- STEPPAN, S.J.; R. ADKINS & J. ANDERSON. 2004. Phylogeny and divergence-date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Systematic Biology 53: 533-553.
- TAMURA, K.; J. DUDLEY; M. NEI & S. KUMAR. 2007. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596-1599.
- VOSS, R.S. 1991. An introduction to the Neotropical muroid rodent genus Zygodontomys Bulletin of the American Museum of Natural History 210: 1-113.
- WEKSLER, M. 2003. Phylogeny of Neotropical oryzomyine rodents (Muridae: Sigmodontinae) based on the nuclear IRBP exon. Molecular Phylogenetics and Evolution 29: 331-349.
- WEKSLER, M. 2006. Phylogenetic relationships of oryzomyine rodents (Muroidea: Sigmodontinae): separate and combined analyses of morphological and molecular data. Bulletin of the American Museum of Natural History 296: 1-149.
- WEKSLER, M.; A.R. PERCEQUILLO & R.S. VOSS. 2006. Ten new genera of oryzomine rodents (Cricetidae: Sigmodontinae). American Museum Novitates 3537: 1-29.
- WETMORE, A. 1957. The birds of Isla Coiba, Panama. Smithsonian Miscellaneous Collections 134 (9): 1-105.
Publication Dates
-
Publication in this collection
27 Sept 2010 -
Date of issue
Aug 2010
History
-
Received
15 Jan 2010 -
Accepted
27 June 2010