Abstract
The diet of the Neotropical otter Lontra longicaudis (Olfers, 1818) is one of the best known aspects of its biology throughout its distribution range. However, most dietary studies have been undertaken during short time periods, making it difficult to identify temporal patterns in the feeding behavior of the species. The present study aimed to describe the diet of L. longicaudis in the lower region of the Mambucaba Catchment, state of Rio de Janeiro, Brazil, during a three year period, based on analyses of spraints (feces). The results show fish as the main prey item (frequency of occurrence, FO = 85.78%), as already described in previous studies. Crustaceans were the second main prey (FO = 70.67%), occurring in the spraints during the whole year, however presenting a higher frequency of occurrence than fish in samples collected during some months. Anurans were the third most important prey item (FO = 9.56%) and mammals, birds and reptiles were preyed upon only rarely (less than 4%). Fish and crustaceans were present in the diet of the species throughout the year and frogs were important mostly from June to August (dry season). This higher rate of predation on amphibians during the drier months was probably related to the decrease of the main prey.
Atlantic Forest; diet; feeding ecology; Lutrinae; southeastern Brazil; spraint analysis
BIOLOGY
Seasonal and spatial differences in feeding habits of the Neotropical otter Lontra longicaudis (Carnivora: Mustelidae) in a coastal catchment of southeastern Brazil
Marcelo L. RheingantzI,** Corresponding author. E-mail: mlrheingantz@gmail.com. Editorial responsibility: Diego A. de Moraes ; Helen F. WaldemarinII; Lívia RodriguesII; Timothy P. MoultonIII
ILaboratório de Ecologia e Conservação de Populações, Departamento de Ecologia, Universidade Federal do Rio de Janeiro. Caixa Postal 68020, 21941-590 Rio de Janeiro, RJ, Brazil
IIAssociação Ecológica Ecomarapendi. Rua Paissandu 362, Laranjeiras, 22210-080 Rio de Janeiro, RJ, Brazil
IIIDepartamento de Ecologia, Universidade do Estado do Rio de Janeiro. Rua São Francisco Xavier 524, Maracanã, 20550-013 Rio de Janeiro, RJ, Brazil
ABSTRACT
The diet of the Neotropical otter Lontra longicaudis (Olfers, 1818) is one of the best known aspects of its biology throughout its distribution range. However, most dietary studies have been undertaken during short time periods, making it difficult to identify temporal patterns in the feeding behavior of the species. The present study aimed to describe the diet of L. longicaudis in the lower region of the Mambucaba Catchment, state of Rio de Janeiro, Brazil, during a three year period, based on analyses of spraints (feces). The results show fish as the main prey item (frequency of occurrence, FO = 85.78%), as already described in previous studies. Crustaceans were the second main prey (FO = 70.67%), occurring in the spraints during the whole year, however presenting a higher frequency of occurrence than fish in samples collected during some months. Anurans were the third most important prey item (FO = 9.56%) and mammals, birds and reptiles were preyed upon only rarely (less than 4%). Fish and crustaceans were present in the diet of the species throughout the year and frogs were important mostly from June to August (dry season). This higher rate of predation on amphibians during the drier months was probably related to the decrease of the main prey.
Key words: Atlantic Forest; diet; feeding ecology; Lutrinae; southeastern Brazil; spraint analysis.
Food habit studies are useful in determining how species deal with ecological changes in prey populations and habitat availability (ANOOP & HUSSAIN 2005). The feeding habits and availability of prey are essential features of ecological aspects of mammals and for the understanding of populations and communities (DEBLASE & MARTIN 1980, GALINDO-LEAL & KREBS 1998), and are one of the most important niche dimensions of Hutchinson's theory (HUTCHINSON 1957).
Otters have a very specialized diet when compared with other carnivores and their diet is one of the aspects of their ecology that has been most studied, especially as this is a relatively easy topic to study (KRUUK 1995). The use of spraints (feces) for studying otter diet is the most recommended for such an objective, because it is difficult to obtain stomach contents from dead animals or stomach contents from captured animals (WISE 1980, CARSS & PARKINSON 1996). The method is facilitated by the fact that otters defecate in conspicuous places, making it simple to collect fecal samples (FONSECA et al. 2008).
The Neotropical otter Lontra longicaudis (Olfers, 1818) has a wide distribution in Latin America, occurring from Mexico to the north of Argentina, and it is present throughout Brazil. The species can be found in almost all freshwater habitats, marine shores associated with coastal lagoons and habitats associated with water bodies, such as forests (BLACHER 1987, EMMONS & FEER 1990, MASON 1990, ROSAS et al. 1991, LARIVIÈRE 1999).
As with other otter species, most studies undertaken with Neotropical otters have focused on their diets. In general, these studies have shown that the species feeds mainly on fish, besides other aquatic animals such as crustaceans, mollusks and insects, plus amphibians, reptiles, birds and small mammals (JOSÉ & KER DE ANDRADE 1997, PARDINI 1998, COLARES & WALDEMARIN 2000, QUADROS & MONTEIRO-FILHO 2001, GORI et al. 2003, ALARCON & SIMÕES-LOPES 2004, KASPER et al. 2004, JOSEF et al. 2008, CARVALHOJUNIOR et al. 2010). However, only the study by CARVALHO-JUNIOR et al. (2010), which was carried out in a Lagoon environment, was done over a long period of time. Other studies were carried out for short periods of time, which did not permit the evaluation of possible differences in the diet of the species in different years. Also, most of the studies did not include continuous collections during the year, and testing for seasonal patterns in the feeding of the species was not possible.
The present study aimed to identify the diet of the Neotropical otter in the lower Mambucaba River basin (state of Rio de Janeiro, Brazil), and includes seasonal and annual differences of the feeding habits of the species.
MATERIAL AND METHODS
The present study was conducted in the Angra dos Reis municipality, including the 13 final kilometers of the Mambucaba River, 1 km of the Perequê River (tributary of the first river) and 1 km of swamp channels near to the mouth of the Mambucaba River into Ilha Grande Bay (between 23º01'40.61"S, 44º31'12.02"W and 22º59'16.50"S, 44º32' 33.88"W). The entire study area is a typical Atlantic Forest coastal environment (Fig. 1).
Mambucaba River begins in the Serra da Bocaina, state of São Paulo, and flows towards Ilha Grande Bay, state of Rio de Janeiro, Southeastern Brazil. The lower part of Mambucaba watershed, which includes the study area, has a slow water flow, high anthropogenic influence, including a national highway crossing the river, deforestation of the riparian forests, houses, sand extraction, and sewage flowing into the river (NATRONTEC 1998).
The stretch of the Perequê River under study has sewage discharge and no riparian vegetation in its lower part. The mangrove channels, on the other hand, present a good mangrove vegetation and no sewage input.
Rainfall is abundant and well distributed through the year (2240 mm), with a more humid period in the summer (between October and March; maximum in January of 350 mm) and a drier period in the winter (between April and September; minimum in July of 75 mm) (Rainfall data from the Meteorology System of Central Nuclear Almirante Álvaro Alberto - CNAAA). There is a reduction in precipitation and river flow during the colder period, as compared to the warmer period (NATRONTEC 1998).
We divided the study area into three stretches (5 km each) along the river, in a coast-continent direction (Fig. 1): 1) Stretch I - this diversified stretch includes the lower stretch of the Mambucaba River (0-3 km from river mouth), of the Perequê River (1 km), a tributary of Mambucaba River, and the mangrove channels (1 km). The channels are close to the coast and have conductivity between 417.2 and 1142 μS/cm, depth between 1.3 and 2.5 m, transparency around 0.3 m, water flow between 0.02 and 0.37 km/h and tidal amplitude of 50 to 100 cm; the bottom is composed of mud and sand and the water has the highest temperatures (21.5-24.1ºC). 2) Stretch II (3-8 km from the river's mouth) - does not have tidal influence; has a higher water flow, with values between 0.17 and 1.5 km/h, depth between 3.2 and 5.5 m, water transparency around 1 m, and a lower conductivity, between 36.9 and 132 ½S/cm, and water temperature between18.5 and 22.1ºC. The riverbed is dominated by sand, with few rocks and some fallen trees. 3) Stretch III - located in the upper part of the study area (8-13 km from the river's mouth), has large rocks and many fallen trees, with a gravel-sand riverbed. This stretch has the lowest values of water conductivity, with values between 27.8 and 67.1 ½S/cm and water temperature between 17.5 and 21.3ºC. It also has the highest water flow, between 0.3 and 2.5 km/h, depth, between 3.7 and 6.1 m and transparency around 1.2 m.
A total of 36 field trips to the study area were carried out monthly from April 2001 to March 2004, but only data from 30 trips were analyzed. During each field trip, the river banks were checked and all the otter spraints found were collected, stored in plastic bags and labeled. In the laboratory, the samples were washed in running water, using a 1 mm mesh sieve, and dried for 48 hours at 40ºC.
After drying, the remaining hard parts (scales, bones, fur and exoskeletons) were sorted. The fragments were further classified into the following taxa: insects, crustaceans, amphibians, reptiles, fish, birds and mammals. The items which could not be classified, due to intense fragmentation, were classified as "other".
The data are presented as Frequency of Occurrence (FO) which consists of the percentage of samples among the total number of samples that contained each of the taxa.
We used Monotonic Multi-Dimensional Scaling (MMDS) ordination to reduce the dimensionality of the prey items found in each spraint, from a Jaccard similarity matrix. Prey composition was defined as the presence or absence of each prey category in each spraint. We checked whether the spraint composition (MMDS dimension - dependent variable) varied depending on the stretch and monthly accumulated rainfall (factors), using multivariate analysis of variance (MANOVA) (ZAR 1999). For this analysis, we assumed that the prey items found in otter spraints in a certain stretch represented the diet of the Neotropical otter in that stretch. Thus, the MMDS standardized dimensions were used as dependent variables in the MANOVA model.
RESULTS
During the field work, a total of 450 fecal samples were analyzed. In 2002 we collected and did not analyze spraints of the months of January, April, July, November, and December, and in 2003 we did not analyze spraints of January. Of the total, 182 samples analyzed were collected during the first year (April 2001 to March 2002), 102 samples in the second year (April 2002 to March 2003) and 166 in the third year (April 2003 to March 2004).
Considering the total number of the analyzed samples, fish were present in 86% of the samples, crustaceans 71%, frogs 10%, mammals 3%, birds 0.6%, reptiles 0.2% and 1.5% contained unidentified material. Of the 450 samples, 105 (23.3%) presented only fish, 47 (10.4%) presented only crustaceans and two (0.4%) presented only frogs. Among the 450 samples, only three (0.7%) did not contain fish or crustaceans as preyed taxa. The monthly frequencies of taxa occurrence are presented in Table I.
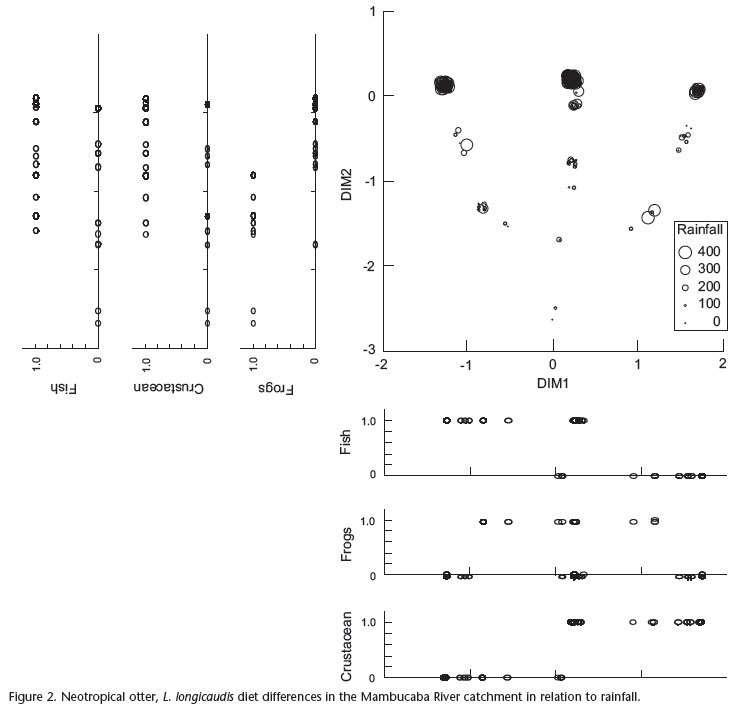
One MMDS dimension recovered 97% of the diet composition indicated in the Jaccard Correlation Matrix (stress = 0.10) and two dimensions recovered 99% (stress = 0.05). Although one dimension recovered the majority of the variation, the two dimensions were used because only one dimension only cannot explain frog variation (Fig. 2).
The species diet was significantly different according to the stretch (Pillay-Trace = 1.131, F12, 258 = 13.010, p < 0.001), probably due to the presence of frogs in the spraints, which occurred more in the spraints from Stretches II and III (Figs 2-5).
Furthermore, the diet was also influenced by rainfall (Pillay-Trace = 0.135, F3,84 = 4.380, p = 0.006). The frequency of occurrence of crustaceans in the otter diet did not vary during the year, but the frequency of frogs in the diet presented a strong seasonality and seemed to be inversely related to the area's rainfall pattern. Fish and crustaceans were more frequent, while reptiles and birds were only preyed, during the rainy season (Figs 2-5).
DISCUSSION
Otters are semi-aquatic carnivores that acquired, through evolution, several morphological and physiological adaptations that allowed them to efficiently occupy aquatic environments (ESTES 1989). In spite of this, otters maintain a strong dependence on the terrestrial environment for some activities such as reproduction, parental care and resting (CHANIN 1985, ESTES 1989, KRUUK 1995). For these reasons, most otter activities are carried out in the water and on banks along water bodies. Thus, it is not surprising that the diet of the otters is composed mainly of aquatic (fish and crustaceans) or semi-aquatic items (frogs) (CHANIN 1985, ESTES 1989, KRUUK 1995).
The high frequencies of occurrence of fish (86%) and crustaceans (71%) observed in the Neotropical otter spraint samples analyzed here are similar to what has been previously described by several authors (OLÍMPIO 1992, JOSÉ & KER DE ANDRADE 1997, PARDINI 1998, SPINOLA & VAUGHAN 1998, COLARES & WALDEMARIN 2000, QUADROS & MONTEIRO-FILHO 2001, GORI et al. 2003, CARVALHOJUNIOR et al. 2010) as well as what was observed in Lutra lutra (Linnaeus, 1758) (ADRIAN & DELIBES 1987, CLAVERO et al. 2003, 2006) and in Lontra canadensis (Schreber, 1777) (COTE et al. 2008). Freshwater otter diet usually shows seasonal patterns (PARDINI 1998), and in our study we observed that frogs occurred more in Neotropical otter spraints collected during the dry periods. Differently to what was carried out by PARDINI (1998), KRUUK et al. (1988, 1993), COTE et al. (2008), and CARVALHO-JUNIOR et al. (2010), we compared the rainfall level, and not the whole aggregated season. By doing this, we could estimate the real influence of rainfall in the otters' diet. Excluding fish and crustaceans, the remaining important items of the Neotropical otter diet varied considerably among different studies. While in some areas invertebrates like insects and mollusks were more important (PARDINI 1998, UTRERAS et al. 1998, COLARES & WALDEMARIN 2000, GORI et al. 2003, KASPER et al. 2004), in other studies vertebrates such as amphibians, reptiles, mammals and birds assumed a larger importance in the diet of the species (OLIMPIO 1992, JOSÉ & KER DE ANDRADE 1997, COLARES & WALDEMARIN 2000, QUADROS & MONTEIRO-FILHO 2001, ALARCON & SIMÕES-LOPES 2004).
Frogs assumed considerable importance in the diet of the Neotropical otter, occurring in approximately 30% of the samples collected in July. When compared with other studies, preying upon frogs by L. longicaudis in this study was higher, with frequency of occurrence varying from 0.35% to 4.5% (OLIMPIO 1992, JOSÉ & KER DE ANDRADE 1997, PARDINI 1998, GORI et al. 2003, KASPER et al. 2004). Even when considering the frequency of total occurrence of this group in the samples analyzed in the present study (FO = 9.56%), frogs occurred more than previously observed for the species. For other otter species, such as Lutra lutra, frogs occurred in higher frequencies, reaching 40% of the frequency of occurrence in the spraints (JENKINS & HARPER 1980, LANSZKI & KORMENDI 1996, ROCHE 1998, JEDRZEJEWSKA et al. 2001).
The seasonality verified in the predation of frogs observed in the present study has already been documented for other otter species. The predation on anurans of the genus Bufo has been documented for Lutra lutra (SLATER 2002). The time of reproduction of B. crucifer and other frog species coincides with their high frequencies in otter spraints, probably because this is the period when the anurans are more exposed for sexual activities, making them easier prey for otters (LANSZKI & KORMENDI 1996, ROCHE 1998, JEDRZEJEWSKA et al. 2001, SLATER 2002).
Although we cannot be sure what anuran species were consumed by the otters during this study, the fact that anuran consumption increased in the dry season, during which most anuran species have decreased densities, may indicate Bufo crucifer as a probable anuran species consumed by the otters in the study area. This species reproduces in the dry season, between May and August, with a consequent aggregation, due to the reproductive activities, of individuals on the river margins (TOLEDO et al. 2003, PRADO & POMBAL-JR 2005).
The lower Mambucaba River catchment is an estuarine area, with a high availability of crustaceans, including freshwater, marine and estuarine species. This was probably the reason for the high importance of crustaceans in the Neotropical otter diet in the present study.
Although we did not analyze prey availability for this study, we can suggest that the longitudinal differences in frequencies of occurrence of prey in the spraints are related to differences in prey availability, as other studies showed that availability influences otter diet (PARDINI 1998). This river has longitudinal differences in fish and crustacean availability, with marine and estuarine species occurring in the lower stretch and river species occurring in upper stretches.
This study describes Neotropical otter diet during three years based on otter spraints, and shows variations in the diet of this species between years, which was previously poorly known, thus increasing the knowledge of the species' feeding behavior. Studies based on diet are important for otter conservation, establishing the Neotropical otter food requirements and, therefore, understanding one of the factors that determine the occurrence of this species, its reproductive strategies and its dispersion pattern. The seasonal variation in the diet of L. longicaudis observed in this study shows that the species feeds mainly on fish and crustaceans, with higher occurrence of frogs when occurrence of fish and/or crustaceans decreased.
ACKNOWLEDGMENTS
This work was part of the PhD thesis of H.Waldemarin in the Post-graduate Program in Biology at the Universidade do Estado do Rio de Janeiro, based on the ecology of the Neotropical otter (graduate scholarship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico). We would like to thank the Associação Ecológica Ecomarapendi and the Hotel do Bosque for logistic support which allowed this work to take place. Ideawild provided equipment. This study would not have been possible without the work of many people, such as Manoel Muanis and Victor Dill, who helped us in the field and in the lab, and our boat pilot.
LITERATURE CITED
Submitted: 01.VI.2010; Accepted: 28.XI.2010.
- ADRIAN, M.I. & M. DELIBES. 1987. Food habits of the otter (Lutra lutra) in two habitats of the Doñana National Park, SW Spain. Journal of Zoology London 212: 399-406.
- ALARCON, G.G. & P.C. SIMÕES-LOPES. 2004. The Neotropical otter Lontra longicaudis feeding habits in a marine coastal area, Southern Brazil. IUCN Otter Specialist Group Bulletin 21 (1): 17-20.
- ANOOP, K.R. & S.A. HUSSAIN. 2005. Food and feeding habits of smooth-coated otters (Lutra perspicillata) and their significance to the fish population of Kerala, India. Journal of Zoology London 266: 15-23.
- BLACHER, C. 1987. Ocorrência e preservação de Lutra longicaudis (Mammalia: Mustelidae) no litoral de Santa Catarina. Boletim FBCN 22: 105-17.
- CARSS, D.N. & S.G. PARKINSON. 1996. Errors associated with otter Lutra lutra faecal analysis. I. Assessing general diet from spraints. Journal of Zoology 238: 301-317.
- CARVALHO-JUNIOR, O.; A.B. BIROLO & L.C.P. MACEDO-SOARES. 2010. Ecological Aspects of Neotropical Otter (Lontra longicaudis) in Peri Lagoon, South Brazil. IUCN Otter Specialist Group Bulletin 27 (2): 105-115.
- CHANIN, P. 1985. The Natural History of Otters. London, Croom Helm, 179p.
- Convention on Biological Diversity 1992. Available online at: http//www.biodiv.org [Accessed: 29/06/2004]
» link - CLAVERO, M.; J. PRENDA & M. DELIBES. 2003. Trophic diversity of the otter (Lutra lutra L.) in temperate and Mediterranean freshwater habitats. Journal of Biogeography 30: 761-769.
- CLAVERO, M.; J. PRENDA & M. DELIBES. 2006. Seasonal use of coastal resources by otters: Comparing sandy and rocky stretches. Estuarine, Coastal and Shelf Science 66: 387-394.
- COLARES, E.P. & H.F. WALDEMARIN. 2000. Utilization of resting sites and dens by the Neotropical river otter (Lutra longicaudis) in the south of Rio Grande do Sul state, southern Brazil IUCN Otter Specialist Group Bulletin 17 (1): 14-19.
- COTE, D.; H.M.J. STEWART; R.S. GREGORY; J. GOSSE; J.J. REYNOLDS; G.B. STENSON & E.H. MILEER. 2008. Prey selection by marine-coastal river otters (Lontra canadensis) in Newfoundland, Canada. Journal of Mammalogy 89 (4): 1001-1011.
- DEBLASE, A.F. & R.E. MARTIN.1980. A Manual of Mammalogy With Keys to Families of the World. Dubuque, Wm. C. Brown Company Publishers, 352p.
- EMMONS, L.H. & F. FEER. 1990. Neotropical Rainforest Mammals: a field guide. Chicago, The University of Chicago Press, 396p.
- ESTES, J.A. 1989. Adaptations for aquatic living by carnivores, p. 242-282. In: J.L. GITTLEMAN (Ed.). Carnivore behavior, ecology and evolution. New York, Cornell University Press, vol. 1, 620 p.
- FONSECA, V.C.; M.L. RHEINGANTZ & F.A.S. FERNANDEZ. 2008. A Comparison of Two Different Methods for Estimating the Diet of the Neotropical Otter, Lontra Longicaudis, with the Proposal of a New Index for Dietary Studies. IUCN Otter Specialist Group Bulletin 25 (1): 6-12.
- GALINDO-LEAL, C. & C.J. KREBS. 1998. Effects of food abundance on individuals and populations of the rock mouse (Peromyscus difficilis). Journal of Mammalogy 79: 1131-1142.
- GORI, M.; G.M. CARPANETO & P. OTTINO. 2003. Spatial distribution and diet of the Neotropical otter Lontra longicaudis in the Ibera Lake (northern Argentina). Acta Theriologica 48: 495-504.
- HUTCHINSON, G.E. 1957. Concluding Remarks. Cold Spring Harbor Symposia on Quantitative Biology 22: 415-17.
- JEDRZEJEWSKA, B.; V.E. SIDOROVICH; M.M. PIKULIK & W. JEDRZEWSKI. 2001. Feeding habitats of the otter and the American mink in Bialowieza Primeval Forest (Poland) compared to other Eurasian populations. Ecography 24: 165-180.
- JENKINS, D. & R.J. HARPER. 1980. Ecology of otters in northern Scotland II. Analysis of otter (Lutra lutra) and mink (Mustela vison) faeces from Deeside, N. E. Scotland, in 1977-78. Journal of Animal Ecology 49: 737-54.
- JOSÉ, H. & H. KER DE ANDRADE. 1997. Food and feeding habitats of the neotropical river otter Lontra longicaudis (Carnivora: Mustelidae). Mammalia 61 (2): 193-203.
- JOSEF, C.F.; L.R. ADRIANO; E.J. FRANÇA; G.G.A. CARVALHO & J.R. FERREIRA. 2008. Determination of Hg and diet identification in otter (Lontra longicaudis) feces. Environmental Pollution 152 (3): 592-596.
- KASPER, C.B.; M.J. FELDENS; J. SALVI & H.C.Z. GRILLO. 2004. Estudo Preliminar sobre a ecologia de Lontra longicaudis (Olfers) (Carnívora:Mustelidae) no Vale do Taquari, Sul do Brasil. Revista Brasileira de Zoologia 21 (1): 65-72.
- KRUUK, H. 1995. Wild Otters: Predation and Populations. Oxford, Oxford University Press, 290p.
- KRUUK, H.; B. NOLET & D. FRENCH. 1988. Fluctuations in numbers and activity of inshore demersal fishes in Shetland. Journal of Marine Biology 68: 601-617.
- KRUUK, H.; D.N. CARSS; J.W.H. CONROY & L. DURBIN. 1993. Otter (Lutra lutra L.) numbers and fish productivity in rivers in north-east Scotland. Symposia of the Zoological Society of London 65: 171-191.
- LANSZKI, J. & S. KORMENDI. 1996. Otter diet in relation to fish availability in a fish pond in Hungary. Acta Theriologica 41 (2): 127-136.
- LARIVIÈRE, S. 1999. Lontra longicaudis Mammalian Species 609: 1-5.
- MASON, C. 1990. An introduction to the Otters, p. 4-7. In: P. FOSTER-TURLEY; S. MACDONALD & C. MASON (Eds). Otters: An Action Plan for their Conservation. Gland, IUCN/SSC Otter Specialist Group, 126p.
- NATRONTEC. 1998. Relatório de impacto ambiental (RIMA) da Usina de Angra II. Angra dos Reis, Ibama, Technical report, 379p.
- OLIMPIO, J. 1992. Considerações preliminares sobre os hábitos alimentares de Lutra longicaudis (Olfers, 1818) (Carnivora: Mustelidae) na Lagoa do Peri, Ilha de Santa Catarina, p. 36-42. In: Proceedings of the III Reunião de Trabalhos de Especialistas em Mamíferos Aquáticos da América do Sul. Valdivia, Central de Publicações Universidade Austral do Chile.
- PARDINI, R. 1998. Feeding ecology on neotropical river otter Lontra longicaudis in an Atlantic Forest stream, south-eastern Brazil. Journal of Zoology London 245: 385-391.
- PRADO, G.M. & J.P. POMBAL-JR. 2005. Distribuição espacial e temporal dos anuros em um brejo da Reserva Biológica de Duas Bocas, Sudeste do Brasil. Arquivos do Museu Nacional 63 (4): 685-705.
- QUADROS, J. & E.L.A. MONTEIRO-FILHO. 2001. Diet of the neotropical otter, Lontra longicaudis, in Atlantic Forest area, Santa Catarina State, Southern Brazil. Studies on Neotropical Fauna and Environment 36 (1): 15-21.
- ROCHE, K. 1998. The diet of otters. Nature and Environment 93: 57-71.
- ROSAS, F.C.W.; E.P. COLARES; I.G. COLARES & V.M.F.M. DA SILVA. 1991. Mamíferos aquáticos da Amazônia brasileira, p. 405-11. In: A.L. VAL; R. FIGLIUOLO & E. FELDSBERG (Eds). Bases científicas para o estabelecimento de estratégias de preservação e desenvolvimento da Amazônia: fatos e perspectivas. Manaus, INPA, vol. 1, 440p.
- SLATER, F. 2002. Progressive skinning of toads (Bufo bufo) by the Eurasian otter (Lutra lutra). IUCN Otter Specialist Group Bulletin 19 (1): 25-29.
- SPINOLA, R.M. & C. VAUGHAN. 1995. Abundancia relativa y actividad de marcaje de la nutria Neotropical (Lutra longicaudis) en Costa Rica. Vida Silvestre Neotropical 4 (1): 38-45.
- TOLEDO, L.F.; J. ZINA & C.F.B. HADDAD. 2003. Temporal and Spatial Distribution in an Anuran Community in Municipality of Rio Claro, São Paulo, Brazil. Holos Environment 3 (2): 136-149.
- UTRERAS, V.; M. RODRÍGUEZ & I. ARAYA. 1998. Preliminary Study on the Diet of the Neotropical Otter Lutra longicaudis in the Tiputini Park, Ecuadorian Amazonia. IUCN Otter Specialist Group Bulletin 19A: 370-373.
- WISE, M.H. 1980. The use of fish vertebrae in scats for estimating prey size of otters and mink. Journal of Zoology London 192: 25-31.
- ZAR, J.H. 1999. Biostatistical Analysis. Upper Saddle River, Prentice Hall, 4th ed., 929p.
Publication Dates
-
Publication in this collection
21 Mar 2011 -
Date of issue
Feb 2011
History
-
Received
01 June 2010 -
Accepted
28 Nov 2010