Abstract
The intertidal sea-urchin Echinometra lucunter (Linnaeus, 1758) has been submitted to diluted sea water (SW) of salinity 25, or concentrated sea water of salinity 45. In addition, ionic challenges have been offered, supplementing 25 SW with Mg2+, Ca2+ or K+, until the concentration of each of these ions would reach the level of full-strength 35 SW (control). Perivisceral coelomic fluid has been sampled after six hours in these treatments for measurements of osmolality and concentrations of Na+, Cl-, Mg2+, Ca2+, and K+. Urchins have been further observed until five days. SW concentration (45) lead urchins to death after two days of exposure, while urchins tolerated five days in 25 SW without any sign of distress. Urchins displayed osmoconformation and ion-conformation for NaCl, and occasional small gradients with respect to the water for Mg2+ (~6% in full-strength SW), and K+ (8.5% in 25 SW), after six hours. These results are consistent with data compiled from the literature, for echinoderms, which frequently show positive gradients (often higher than 25%) for the most relevant ions, between the coelomic fluid and external SW. Supplemented cations have shown mutual interference, mostly affecting their own coelomic fluid concentrations. Under the protocol used here, urchins of the species E. lucunter held gradients for Mg2+, Ca2+, and K+, but not for Na+ or Cl- or osmolality. They were also able to tolerate at least a 30% reduction in sea water salinity for five days, showing reasonable euryhalinity. However, when compared to other echinoderms, E. lucunter is not especially capable of maintaining large ionic gradients with respect to external SW.
Calcium; ionic gradients; magnesium; osmoconformation; potassium; stenohalinity
MORPHOLOGY AND PHYSIOLOGY
Osmolality and ions of the perivisceral coelomic fluid of the intertidal sea urchin Echinometra lucunter (Echinodermata: Echinoidea) upon salinity and ionic challenges
Carolina A. Freire1 1 Corresponding Author. E-mail: cafreire@ufpr.br; carolina.a.freire@pq.cnpq.br ; Ivonete A. Santos; Denilton Vidolin
Departamento de Fisiologia, Setor de Ciências Biológicas, Centro Politécnico, Universidade Federal do Paraná. 81531-990 Curitiba, PR, Brazil
ABSTRACT
The intertidal sea-urchin Echinometra lucunter (Linnaeus, 1758) has been submitted to diluted sea water (SW) of salinity 25, or concentrated sea water of salinity 45. In addition, ionic challenges have been offered, supplementing 25 SW with Mg2+, Ca2+ or K+, until the concentration of each of these ions would reach the level of full-strength 35 SW (control). Perivisceral coelomic fluid has been sampled after six hours in these treatments for measurements of osmolality and concentrations of Na+, Cl-, Mg2+, Ca2+, and K+. Urchins have been further observed until five days. SW concentration (45) lead urchins to death after two days of exposure, while urchins tolerated five days in 25 SW without any sign of distress. Urchins displayed osmoconformation and ion-conformation for NaCl, and occasional small gradients with respect to the water for Mg2+ (~6% in full-strength SW), and K+ (8.5% in 25 SW), after six hours. These results are consistent with data compiled from the literature, for echinoderms, which frequently show positive gradients (often higher than 25%) for the most relevant ions, between the coelomic fluid and external SW. Supplemented cations have shown mutual interference, mostly affecting their own coelomic fluid concentrations. Under the protocol used here, urchins of the species E. lucunter held gradients for Mg2+, Ca2+, and K+, but not for Na+ or Cl- or osmolality. They were also able to tolerate at least a 30% reduction in sea water salinity for five days, showing reasonable euryhalinity. However, when compared to other echinoderms, E. lucunter is not especially capable of maintaining large ionic gradients with respect to external SW.
Key words: Calcium; ionic gradients; magnesium; osmoconformation; potassium; stenohalinity.
Echinoderm body fluids are osmotically and ionically very similar to full-strength sea water, their usual habitat. This does not mean that they have exactly the same ionic composition as sea water, or that some gradients cannot be established upon sea water dilution. Echinoderms are exclusively marine, and considered stenohaline, given their general lack of tolerance of significant modifications in sea water salinity (SABOURIN & STICKLE 1981, DIEHL 1986, STICKLE & DIEHL 1987). Nevertheless, some species inhabit reasonably diluted waters (<20), such as Luidia clathrata (Say, 1825) and Ophiophragmus filograneus (Lyman, 1875) in Florida, and Asterias rubens (Linnaeus, 1758) in the Baltic Sea (ELLINGTON & LAWRENCE 1974, HINTZ & LAWRENCE 1994, TALBOT & LAWRENCE 2002). Furthermore, they are frequent inhabitants of intertidal areas, and several studies report a moderate tolerance to salinity changes in some species/populations (STICKLE & AHOKAS 1974, STICKLE & DENOUX 1976, ROLLER & STICKLE 1985, 1993, 1994, VIDOLIN et al. 2007). Fairly more than "moderate" tolerance, starfishes of the species Patiriella regularis (Verrill, 1867) have been reported to survive up to four days in deionized water (BARKER & RUSSELL 2008), and urchins of the species Lytechinus variegatus (Lamarck, 1816), 32 days in salinity 20 (BISHOP et al. 1994). Thus, with respect to tolerance, most frequently to sea water dilution, echinoderms are not equally stenohaline, and show marked species differences (e.g. STICKLE & AHOKAS 1974, STICKLE & DENOUX 1976, SHUMWAY 1977, VIDOLIN et al. 2007, BARKER & RUSSELL 2008). This tolerance, contrary to the most common idea one has of these invertebrates, suggest that they may, at least to a certain degree, modify (or delay changes in) the osmotic and ionic concentrations of their extracellular fluid. This means the establishment of gradients between the extracellular fluid (coelomic fluid) and the external medium, at least for a few hours (STICKLE & AHOKAS 1974, MADRID et al. 1976, DIEHL 1986). The report of gradients is quite variable for different species and different experimental protocols. Thus, it appears that echinoderms actually cannot be considered uniformly "osmo- and ion-conformers".
The existence and maintenance of osmotic and ionic gradients for short times of exposure, between the perivisceral coelomic fluid and external sea water has been shown for those invertebrates. However, the possibility of differential treatment of different ions has not been so far sufficiently emphasized (STICKLE & DENOUX 1976, DIEHL 1986, STICKLE & DIEHL 1987). The basic rule is that when salinity is reduced, extracellular fluid (perivisceral coelomic fluid) ionic concentrations are "buffered", and positive gradients develop, with higher concentrations in the coelomic fluid. So, even with gradual dissipation of these gradients, the coelomic fluid is reported as hyper-ionic for at least some hours, in a way perfectly compatible with life under the rhythmic fluctuations of tidal cycles (STICKLE & AHOKAS 1974, STICKLE & DENOUX 1976, VIDOLIN et al. 2007).
Specific ions have certain notable effects on the tissues of echinoderms. Changes in magnesium, calcium or potassium concentrations affect the activity of relevant metabolic enzymes, and modify the viscosity of the dermis and body wall of holothurians, or of ligaments of ophiuroids, definitely affecting echinoderm connective tissues (e.g., EYLERS 1982, HIDAKA 1983, BYRNE 1985, LEVERONE et al. 1991, MOTOKAWA 1994, WILKIE et al. 1994, TROTTER & CHINO 1997, HAGEN 2003). Thus, it may well be that indeed echinoderm epithelia deal differently with different ions. And furthermore, it is also likely that different species display qualitative and quantitative differences in their capacity to show this incipient formation of gradients of specific ions.
The intertidal species chosen for this investigation was the sea-urchin Echinometra lucunter (Linnaeus, 1758). It is a widespread species along the Brazilian coast, on rocks and reefs, forming aggregates in intertidal and shallow subtidal zones (SÁNCHEZ-JÉREZ et al. 2001). These urchins occur down to 45 m deep and they carve individual spherical crevices in rocks, where they remain protected from predators and strong wave action, as it is typical of the family Echinometridae. (CABRAL DE OLIVEIRA 1991, CASTRO et al. 1995, HENDLER et al. 1995). They are grazers, feeding mainly at nighttime. When they leave their crevices to feed, they return to the same crevice after foraging in the nearby surroundings (MCPHERSON 1969).
The first hypothesis tested in this study was that intertidal sea urchins, selected to dwell in this unstable environment, and reported to tolerate sea water dilution better than sea water concentration (SANTOS-GOUVEA & FREIRE 2007), are able to sustain ionic gradients between their perivisceral coelomic fluid and external sea water, especially upon sea water dilution. The second hypothesis was that the selective supplementation of diluted sea water with the cations magnesium, calcium or potassium would reveal differential permeability to different ions.
MATERIAL AND METHODS
Animals and laboratory maintenance
Intertidal sea-urchins of the species E. lucunter have been manually obtained from the rocky coast of the beaches of Trapiche and Quilombo, city of Penha (26º46'S, 48º38'W), state of Santa Catarina, Brazil. Urchins have been obtained during low tide, in January and March of 2002 (Summer and early Autumn in the Southern hemisphere), thus outside the reproductive period (October-November) reported for E. lucunter from Southern Brazil (TAVARES et al. 2004, MARIANTE et al. 2009). Urchins have been immediately brought to the laboratory (threehour drive), where they were kept for two days in a holding tank (160 L) supplied with biological filtration, constant aeration, and containing full-strength sea water (salinity 35)at temperature 23ºC±1ºC and pH 7.5-8.0, under natural photoperiod (~13 h light/11 h dark). The test diameter of the specimens used (all adults) was 63.7 ± 2.5 mm (n = 36).
Salinity and ionic unbalance experiments
Three urchins were transferred to each of the six 30 L aquaria, containing: 1) control full-strength sea water (SW, 35), 2) diluted SW (25, hyposmotic conditions), 3) concentrated SW (45, hyperosmotic conditions), 4) diluted SW of 25 supplemented with MgCl2, 5) diluted SW of 25 supplemented with CaCl2, and 6) diluted SW of 25 supplemented with KCl. These ionic unbalance experiments have been performed in order to test for differences in the handling of those three physiologically-relevant cations, by the urchin epithelia. This whole procedure was then repeated, yielding a total of six urchins for each condition, and a total number of 36 urchins used for this study. These aquaria were maintained under natural photoperiod (~13 h light/11 h dark), temperature of 24 ± 1ºC, pH of 7.5-8.0, with constant aeration and biological filtration, for up to five days. Urchins were fed with green algae (Ulva sp.), on the second day of each experiment.
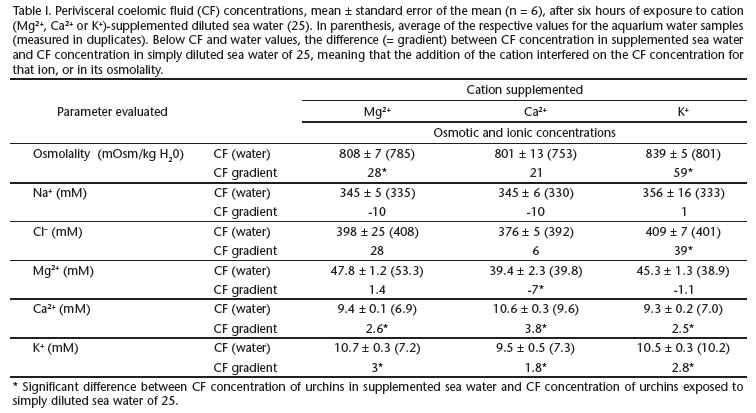
Diluted SW (salinity 25) was prepared adding filtered (cellulose and activated charcoal) tap water to full-strength natural SW; concentrated SW (salinity 45) was prepared adding commercially available marine salt to full-strength natural SW. Salinity was verified using a salinometer/refractometer (Shibuya S28). Diluted SW received chloride salts of Mg2+, Ca2+, or K+, to bring the cation concentration up to its level in full-strength SW, based on the levels indicated by PROSSER (1973) for standard SW (salinity 34.33). Osmolality and ionic concentrations measured on the supplemented diluted SW are displayed in Tab. I. The addition of Mg2+, Ca2+, or K+ as chlorides individually modified the concentration of the cation, as intended, but not SW osmolality or chloride concentrations (Tab. I, values in parenthesis). Addition of Mg2+ ions has elevated magnesium levels in diluted SW, from 38 mM to 54 mM de Mg2+; addition of Ca2+ ions has elevated calcium concentration from 7 to 10 mM, the same happening to K+ (Tab. I).
Coelomic fluid sampling and concentration assays
Coelomic fluid samples of approximately 500 ¼l were obtained from all urchins using disposable insulin syringes through peristomial membrane puncture, after six hours (to be within the time frame of a tidal cycle). Aquarium water samples have been obtained immediately after sampling the coelomic fluid, and again in the end of the observation period (up to five days).
Osmolality of undiluted samples has been assayed in a vapor pressure micro-osmometer (VAPRO 5520 Wescor, Logan, USA). Na+ concentration was determined through flame photometry (B462 Micronal, São Paulo, Brazil), with readings in duplicate, in appropriately diluted samples (1:2500). Coelomic fluid concentrations of Cl-, Mg2+, Ca2+, and K+ were assayed using commercially available colorimetric kits: Cl-, Mg2+, Ca2+ (Labtest, Lagoa Santa, Brazil), and K+ (Doles, Goiânia, Brazil). Samples have been appropriately diluted in deionized water, and absorbance was read using the Spectrophotometer (Ultrospec 2100 Amersham Pharmacia biotech, Uppsala, Sweden).
Statistics
Coelomic fluid concentrations were compared to water using the 95% confidence interval (CI). When the value of water did not fit inside the CI, we interpreted that CF concentration was significantly different from water and that the animal was able to establish a gradient. One-way ANOVAs followed by the post-hoc test of Tukey were performed to evaluate the effect of salinity (3 levels: 25, 35, 45) on the coelomic fluid concentrations. Unpaired Student's t-tests were employed to compare data of coelomic fluid concentrations from the ionic unbalance experiments (addition of either Mg2+, Ca2+, or K+ to 25 seawater) to respective controls in diluted sea water (plain 25 seawater), after six hours. Level of significance adopted was always of 0.05, and corresponding non-parametric tests were performed whenever data did not meet the requirements of normality and equality of variances.
RESULTS
General observations and behavioural data
During the experiments, the movement and position of the spines and ambulacral feet, and the displacement of the urchins in the aquaria have been observed. On the day of the start of the experiments, no behavioural alterations were observed in any of the experimental animals. After 24 hours, urchins submitted to concentrated seawater (SW) of salinity 45 displayed their spines bent down, limpy, with little movement of the ambulacral feet. Forty-eigth hours after the start of the experiment, all the urchins were apparently dead. Urchins submitted to diluted (salinity 25) or full-strength (salinity 35) SW did not show any sign of behavioural change or distress, even after five days.
In the same day of the start of the experiment, urchins submitted to ionic unbalance through the addition of Mg2+ displayed limp, fairly motionless, and bent down spines and ambulacral feet, remaining this way after 24 hours, with two urchins dying after 48 hours of experiment. Those submitted to the addition of Ca2+ displayed no deviations from normality after 1 day, but two urchins died after 48 hours. Those exposed to the addition of K+ were the most affected, displaying bent down spines, flaccid ambulacral podia, and difficulty in remaining attached to the aquarium wall, immediately after the start of the experiment. Some individuals fell to the bottom, inverted (and were then manually turned right side up). After 24 hours, urchins submitted to the addition of K+ were already in bad condition, releasing a reddish fluid from their mouth. Four urchins were dead after 48 hours. In all cases, the smaller urchins were the ones that died first.
Effect of seawater dilution or concentration
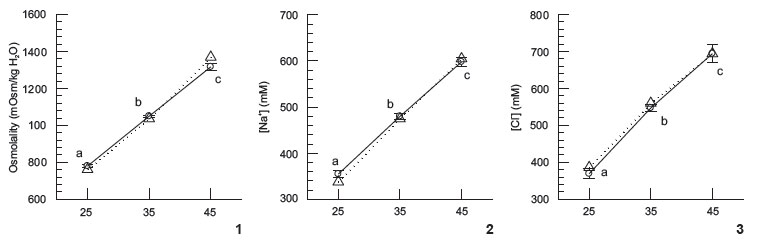
No difference was detected in osmolality, Na+, and Cl- values, between coelomic fluid (CF) of E. lucunter and external SW (Figs 1-3); urchins were always isosmotic and isoionic for NaCl, after six hours of exposure. For the ions Mg2+, Ca2+, and K+, occasional differences between the CF and SW have been detected: Mg2+ at 35, Ca2+ at 45, and K+ at 25 (Figs 4-6). Total conformation was observed (i.e., CF values in 25 were different from CF values in 35, which were also different from CF values in 45) at the three salinities tested, for all ions and osmolality, except for Mg2+ and Ca2+. For Mg2+ some stability in CF values was shown between 25 and 35, and for Ca2+, stability was shown between 35 and 45 (Figs 4 and 5).
Ionic unbalance experiments
Exposure of the urchins for six hours to diluted SW supplemented with MgCl2, CaCl2, or KCl resulted in differences in the CF osmolality and ionic concentrations between urchins submitted to ionic unbalance, and those exposed, also for six hours, to simply diluted SW of 25. Supplementation with Mg2+ resulted in increased CF osmolality, Ca2+, and K+; supplementation with Ca2+ resulted in decreased CF Mg2+, but increased Ca2+ and K+; supplementation with K+ resulted in increased CF osmolality, Cl-, Ca2+, and K+ (Tab. I).
DISCUSSION
Intertidal sea-urchins of the species E. lucunter have been shown to be iso-osmoconformers, sustaining no significant osmotic gradients between their extracellular fluid, the perivisceral coelomic fluid (CF), and external sea water (SW). Only occasionally has CF ionic composition differed from that of SW, basically only for Mg2+, Ca2+, and K+, after six hours of exposure to full-strength, diluted, or concentrated SW. Thus, despite being larger on average than L. variegatus, E. lucunter has not displayed significant ionic gradients such as was detected for L. variegatus in full-strength and diluted SW (35, 30 and 25), also after six hours of exposure (VIDOLIN et al. 2007): 6-8% for E. lucunter here, contrasting with 40-70% for L. variegatus (for K+, in salinities 25 to 35 SW). This result was quite surprising, in view of the fact that E. lucunter is an intertidal urchin, frequently exposed to the air during low tide, and given its average larger size with presumably larger surface-to-volume ratio (VIDOLIN et al. 2007). However, interestingly, air exposure in E. lucunter is not restricted to the larger specimens, it was also observed for the small younger individuals at our study site (Vidolin, personal observation).
Larger urchins have lower surface-to-volume ratios, and would thus be expected to 1) be more tolerant of salinity alterations, and/or 2) keep their CF concentrations stable for a longer time, when submitted to a salinity challenge. In other words, it takes longer for larger urchins to dissipate any gradient created upon a SW salinity change, in a so-called "damping effect" (STICKLE & AHOKAS 1974, STICKLE & DENOUX 1976, SHUMWAY 1977, HIMMELMAN et al. 1984, DIEHL 1986, VIDOLIN et al. 2007). Indeed, in the present study, when mortality was noted, smaller urchins died before the larger ones. As also previously noted (VIDOLIN et al. 2007), size is indeed relevant, but it is certainly not the single relevant issue with respect to salinity tolerance or maintenance of gradients between the extracellular fluid (perivisceral CF) and external SW. The seastar P. regularis is extremely tolerant to dilute SW, much more than its congener Patiriella mortenseni O'Loughlin, Waters & Roy, 2002 (BARKER & RUSSELL 2008), despite being of smaller size, with no size overlap between the two species: ~30 mm for P. mortenseni, and ~15 mm for P. regularis, measured from the center to the distal tip of longest arm (M. RUSSELL & M. BARKER, personal communication). Furthermore, STICKLE & DENOUX (1976) found smaller Strongylocentrotus droebachiensis (O.F. Müller, 1776) to be more tolerant of salinity reduction than larger specimens of the same species.
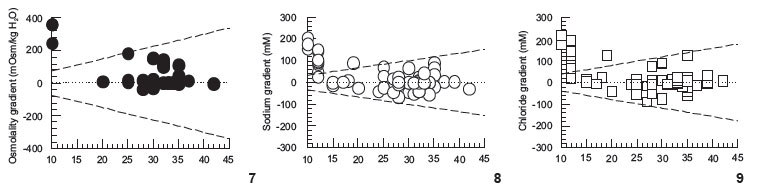
Thus, size seems to be relevant, but not the sole relevant factor, with respect to tolerance or maintenance of gradients between the extracellular fluid and external SW. So, irrespective of size, is there a pattern in the gradients sustained by echinoderms, between their CFs and external SW? The overall pattern detected for echinoderms in the data gathered from the literature and plotted in Figs 7-12 is of hyperionic and hyperosmotic CF with respect to ambient SW, especially in dilute SW. The "zero dotted line" means absence of gradient between CF and SW values. It is noticeable that many points are located above the +25% line, but none below the -25% line. Thus, echinoderms, despite being conformers, in general, are more often hyper- than hypo-ionic to their external medium. This is an adequate strategy for intertidal species, for example, as this way they avoid excess extracellular dilution during most of the tidal cycle, when salinity reduction is much more likely than salinity increase (STICKLE & DENOUX 1976, VIDOLIN et al. 2007). This "hyper-ionic" state is especially true for very low salinities (10-15), and for K+, Ca2+, and Mg2+, also for salinities close to full-strength SW (Figs 7-12), and is consistent with our data for E. lucunter. Coherently, low salinity values plotted in Figs 7-12 mostly refer to hours of exposure (3-10 hours), whereas data for higher salinities (i.e., around full-strength SW), refer to "habitat" values for the studied echinoderms. Exceptionally, data on very dilute SW refer to the habitat of the species A. rubens (BINYON 1966).
Sodium and chloride are the main contributors to CF osmolality, and both ions were kept by E. lucunter essentially equal in concentration to SW of 25, 35, or 45 salinities (i.e., zero gradient), after six hours. Thus, E. lucunter is indeed unable to sustain large gradients for these two ions, unlike other species depicted in Figs 8 and 9. The pattern observed for both ions, Na+ and Cl-in Figs 8 and 9, is essentially the same, with many symbols spreading between the +25% to -25% traced lines.
The subtle, however significant signal of Mg2+ and K+ gradients (6-8%) maintained by E. lucunter may be related to the physiological relevance of both cations, and are well supported by the literature, that has quite often highlighted the maintenance of even much steeper gradients for these two cations, and also for Ca2+ (STICKLE & AHOKAS 1974, STICKLE & DENOUX 1976, SHUMWAY 1977, PAGETT 1980, ROBERTSON 1980, DIEHL 1986, STICKLE & DIEHL 1987, BISHOP et al. 1994, VIDOLIN et al. 2007). Besides its general widespread action as a cofactor for various enzymes, and in several other intracellular roles (SARIS et al. 2000), Mg2+ is a major constituent of the sea urchin test, as aragonite (MgCO3) (MILES et al. 2007). However, under a similar protocol, the sea-urchins L. variegatus and Arbacia lixula (Linnaeus, 1758) did not show evidence for the maintenance of Mg2+ gradients (VIDOLIN et al. 2007). Under a protocol of progressive but gradual salinity alteration, the sea-urchin S. droebachiensis has sustained a huge gradient for Mg2+ (27 mM in SW of salinity 10, or 170%) when exposed to the simulation of a steep tidal cycle, essentially showing extracellular Mg2+ regulation (STICKLE & AHOKAS 1974). Different rates of salinity changes (different experimental protocols) may account for these different results. In this same quoted study, Pisaster ochraceus (Brandt, 1835) showed evidence of extracellular K+ regulation (gradient of 7.6 mM, or 260% of K+ concentration in salinity 10), and Cucumaria miniata (Brandt, 1835) showed evidence of Ca2+ regulation. These three cations show higher positive gradients in echinoderm CFs, than sodium or chloride (Figs 7-12). In summary, different ions are treated differently, and different species of echinoderms respond differently to a same salinity challenge (e.g., ELLINGTON & LAWRENCE 1974, STICKLE & AHOKAS 1974, STICKLE & DENOUX 1976, SHUMWAY 1977, VIDOLIN et al. 2007, BARKER & RUSSELL 2008). Most ionic gradients are indeed already dissipated after 6-8 hours in echinoderms (MADRID et al. 1976, DIEHL 1986, STICKLE & DIEHL 1987), but there are species that maintain significant ionic differences, as clearly shown in Figs 7-12. Ionic differences among distinct fluid compartments of echinoderms are also repeatedly reported. Ambulacral fluids of sea stars and intestinal fluids of urchins have higher K+ concentrations than SW or respective CFs (PRUSH 1977, STICKLE & DIEHL 1987, BISHOP et al. 1994). Importantly, in the intestinal fluid this may relate to digested materials.
In order to further examine the suggestion of some permeability control or even putative transport of those physiologically relevant cations, we devised a protocol to test the effects of ionic unbalance. We diluted SW, but kept the concentration of one cation (Mg2+, Ca2+, or K+) individually high, equal to its own concentration in full-strength SW, thus potentially interfering on Donnan equilibrium (DIEHL 1986, STICKLE & DIEHL 1987). Importantly, the addition of chloride salts of Mg2+, Ca2+, or K+ to diluted SW (25) did not affect the chloride concentration measured in the aquaria (Tab. I, values in parenthesis), thus not affecting chloride gradients. A loss of tonus of spines and ambulacral feet was noted in urchins submitted to diluted SW with the addition of Mg2+, which acts as a muscle relaxant, depressing neuromuscular transmission, and also in urchins submitted to diluted SW with the addition of K+. The addition of K+ may have also impaired neuromuscular transmission, leading to paralysis (HAGEN 2003). Actually, the lack of fixation to the glass wall of the aquaria, of E. lucunter submitted to K+ addition in the water, is entirely compatible with the suggestion of the use of KCl in the water as a "detachment agent" in echinoculture (HAGEN 2003). The addition of either Mg2+, Ca2+, or K+ to diluted SW led to sharp interference on the CF concentrations of these same ions (Tab. I). Thus, there seems to be some kind of interaction between these cations, an idea that further supports the hypothesis of differential treatment of different ions by echinoderm epithelia.
Another interesting aspect is the quite frequent observation and report that SW dilution is less harmful or better tolerated than SW concentration (DIEHL & LAWRENCE 1985, DIEHL 1986, LEVERONE et al. 1991, SANTOS-GOUVEA & FREIRE 2007). Actually, most studies report data on dilute SW, as also apparent in Figs 7-12. The sea urchin E. lucunter has tolerated the hypo-saline challenge (25) much better than the hyper-saline challenge (45), albeit both were of the same magnitude (±10 salinity units). This result may reflect a better capacity to regulate cell volume when dealing with water entry rather than water loss, or with the fact that excess salt is deleterious (SABOURIN & STICKLE 1981, DIEHL & LAWRENCE 1985, DIEHL 1986, SANTOS-GOUVEA & FREIRE 2007), causing for example a decrease in the activity of metabolic enzymes (LEVERONE et al. 1991). Moreover, SW dilution is a much more likely event than SW concentration in coastal waters, so this may even have to do with evolutionary constraints (ELLINGTON & LAWRENCE 1974, FREIRE et al. 2008). But interestingly, even species that naturally occur in estuaries such as the ophiuroid Ophiophragmus filograneus (Lyman, 1875), perform better at higher salinities, close to full-strength SW (TAL-BOT & LAWRENCE 2002).
The present results thus confirm previous studies, in that sea water salinity reduction for a few hours in the intertidal habitat will likely lead to no serious damage, as morbidity and mortality were only observed after more than 24 hours of exposure to hyper-saline sea water, or to intense ionic unbalance. Thus, even with the dissipation of any gradients between the perivisceral coelomic fluid and external sea water, echinoderms can easily survive when trapped in tide pools challenged with heavy rainfall or intense sunshine leading to salinity fluctuations. This survival, in typical osmoconforming behaviour, may reflect some capacity for cell volume regulation upon water influx or efflux (LANGE 1964, ELLINGTON & LAWRENCE 1974, DIEHL 1986, STICKLE & DIEHL 1987, LEVERONE et al. 1991, HINTZ & LAWRENCE 1994), or even a broad tolerance to cell volume changes, typically taking days to fully recover from the salinity challenges (LANGE 1964, ELLINGTON & LAWRENCE 1974). In conclusion, the intertidal urchin E. lucunter, in accordance with the general echinoderm pattern, exhibits some capacity to maintain gradients, especially of the cations potassium, calcium, and magnesium. However, the gradients sustained by E. lucunter are of small magnitude, when compared to other species, thus indicating that this capacity is not so tightly associated to the intertidal habitat where the species is found. Further, they tolerate sea water dilution by 30% for at least five days, without signs of distress, thus being far more euryhaline than their habitat demands. The analysis performed here has strengthened previous reports in the literature, that echinoderms are not uniformly and equally stenohaline and ion conformers. There are clear species and ionic differences that warrant further investigation on the underlying mechanisms responsible for ionic gradient maintenance in echinoderms.
ACKNOWLEDGMENTS
Authors wish to gratefully acknowledge the very kind supply by Michael Russell, of size data of the specimens of P. mortenseni and P. regularis used in the article BARKER & RUSSELL (2008). Authors also thank TECPAR for the use of the flame photometer. Authors wish to acknowledge the donation of lab equipment to the laboratory of CAF, by the German Academic Exchange Service (DAAD). DV has been awarded with a Masters' stipend from CAPES/Brazil.
LITERATURE CITED
Submitted: 10.VI.2010; Accepted: 26.IV.2011.
Editorial responsibility: Rosana M. da Rocha
- BARKER, M.F. & M.P. RUSSELL. 2008. The distribution and behaviour of Patiriella mortenseni and P. regularis in the extreme hyposaline conditions of the Southern New Zealand Fiords. Journal of Experimental Marine Biology and Ecology 355:76-84.
- BINYON, J. 1966. Salinity tolerance and ionic regulation, p. 359-377. In: R.A. BOOLOOTIAN (Ed.). Physiology of Echinodermata. New York, Interscience Publishers.
- BISHOP, C.D.; K.J. LEE & S.A. WATTS. 1994. A comparison of osmolality and specific ion concentrations in the fluid compartments of the regular sea urchin Lytechinus variegatus Lamarck (Echinodermata: Echinoidea) in varying salinities. Comparative Biochemistry and Physiology 108A:497-502.
- BYRNE, M. 1985. The mechanical properties of the autotomy tissues of the holothurian Eupentacta quinquesemita and the effects of certain physico-chemical agents. Journal of Experimental Biology 117:69-86.
- CABRAL DE OLIVEIRA, M. 1991. Survival of seaweeds ingested by three species of tropical sea urchins from Brazil. Hydrobiologia 222:13-17.
- CASTRO, C.B.; C.A. ECHEVERRIA; D.O. PIRES; B.J.A. MASCARENHAS & S.G. FREITAS. 1995. Distribuição de Cnidaria e Echinodermata no infralitoral de costões rochosos de Arraial do Cabo, Rio de Janeiro, Brasil. Revista Brasileira de Biologia 55:471-480.
- DIEHL, W.J. 1986. Osmorregulation in echinoderms. Comparative Biochemistry and Physiology 84A:199-205.
- DIEHL, W.J. & J.M. LAWRENCE. 1985. Effect of salinity on the intracellular osmolytes in the pyloric caeca and tube feet of Luidia clathrata (Say) (Echinodermata: Asteroidea). Comparative Biochemistry and Physiology 82A:559-566.
- ELLINGTON, W.R. & J.M. LAWRENCE. 1974. Coelomic fluid volume regulation and isosmotic intracellular regulation by Luidia clathrata (Echinodermata: Asteroidea) in response to hyposmotic stress. Biological Bulletin 146:20-31.
- EYLERS, J.P. 1982. Ion-dependent viscosity of holothurian body wall and its implications for the functional morphology of echinoderms. Journal of Experimental Biology 99:1-8.
- FREIRE, C.A.; E.M. AMADO; L.R. SOUZA; M.P.T. VEIGA; J.R.S. VITULE; M.M. SOUZA & V. PRODOCIMO. 2008. Muscle water control in crustaceans and fishes as a function of habitat, osmoregulatory capacity, and degree of euryhalinity. Comparative Biochemistry and Physiology 149A:435-446.
- HAGEN, N.T. 2003. KCl induced paralysis facilitates detachment of hatchery reared juvenile green sea urchins, Strongylocentrotus droebachiensis Aquaculture 216:155-164.
- HENDLER, G.; J.E. MILLER; D.L. PAWSON & P.M. KIER. 1995. Echinoderms of Florida and the Caribbean: Sea Stars, Sea Urchins and Allies. Washington: Smithsonian Institution Press, ix+390p.
- HIDAKA, M. 1983. Effects of certain physico-chemical agents on the mechanical properties of the catch apparatus of the seaurchin spine. Journal of Experimental Biology 103:15-29.
- HIMMELMAN, J.H.; H. GUDERLEY; G. VIGNAULT; G. DROUIN & P.G. WELLS. 1984. Response of the sea urchin, Strongylocentrotus droebachiensis, to reduced salinities: importance of size, acclimation, and interpopulation differences. Canadian Journal of Zoology 62:1015-1021.
- HINTZ, J.L. & J.M. LAWRENCE. 1994. Acclimation of gametes to reduced salinity prior to spawning in Luidia clathrata (Echinodermata: Asteroidea). Marine Biology 120:443-446.
- LANGE, R. 1964. The osmotic adjustment in the echinoderm, Strongylocentrotus droebachiensis Comparative Biochemistry and Physiology 13:205-216.
- LEVERONE, J.R.; C.A. LUER & J.M. LAWRENCE. 1991. The effect of cations on the specific activities of pyruvate kinase and glucose-6-phosphate dehydrogenase of Luidia clathrata (Say) (Echinodermata: Asteroidea). Comparative Biochemistry and Physiology 99B:259-264.
- MADRID, E.; I.P. ZANDERS & F.C. HERRERA. 1976. Changes in coelomic fluid and intracellular ionic composition in holothurians exposed to diverse sea water concentrations. Comparative Biochemistry and Physiology 54A:167-174.
- MARIANTE, F.L.F.; G.B. LEMOS; F.J. EUTRÓPIO & L.C. GOMES. 2009. Biologia reprodutiva de Echinometra lucunter (Echinodermata: Echinoidea) na Praia da Costa, Vila Velha, Espírito Santo. Zoologia 26(4):641-646.
- MCPHERSON, B.F. 1969. Studies on the biology of the tropical sea urchins, Echinometra lucunter and Echinometra viridis Bulletin of Marine Science 19:194-213.
- MILES, H.; S. WIDDICOMBE; J.I. SPICER & J. HALL-SPENCER. 2007. Effects of anthropogenic sea water acidification on acid-base balance in the sea urchin Psammechinus miliaris Marine Pollution Bulletin 54:89-96.
- MOTOKAWA, T. 1994. Effects of ionic environment on viscosity of triton-extracted catch connective tissue of a sea cucumber body wall. Comparative Biochemistry and Physiology 109B:613-622.
- PAGETT, R.M. 1980. Distribution of sodium, potassium and chloride in the ophiuroid, Ophiocomina nigra (Abildgaard). Journal of the Marine Biological Association of the UK 60:163-170.
- PROSSER, C.L. 1973. Comparative Animal Physiology. Philadelphia, W.B. Saunders Co, XIII+966p.
- PRUSCH, R.D. 1977. Solute secretion by the tube foot epithelium in the starfish Asterias forbesi Journal of Experimental Biology 68:35-43.
- ROBERTSON, J.D. 1980. Osmotic constituents of some echinoderm muscles. Comparative Biochemistry and Physiology 67A:535-543.
- ROLLER, R.A & W.B. STICKLE. 1985. Effects of salinity on larval tolerance and early developmental rates of four species of echinoderms. Canadian Journal of Zoology 63:1531-1538.
- ROLLER, R.A & W.B. STICKLE. 1993. Effects of temperature and salinity acclimation of adults on larval survival, physiology, and early development of Lytechinus variegatus (Echinodermata: Echinoidea). Marine Biology 116:583-591.
- ROLLER, R.A. & W.B. STICKLE. 1994. Effects of adult salinity acclimation on larval survival and early development of Strongylocentrotus droebachiensis and Strongylocentrotus pallidus (Echinodermata: Echinoidea). Canadian Journal of Zoology 72:1931-1939.
- SABOURIN, T.D. & W.B. STICKLE. 1981. Effects of salinity on respiration and nitrogen excretion in two species of echinoderms. Marine Biology 65:91-99.
- SÁNCHEZ-JÉREZ, P.; A. CESAR; F.S. CORTEZ; C.D.S. PEREIRA & S.L.R. SILVA. 2001. Distribución espacial de las poblaciones de erizos más abundantes de la costa sudeste del litoral de São Paulo (Brasil). Ciencia Marina 27:139-153.
- SANTOS-GOUVEA, I.A. & C.A. FREIRE. 2007. Effects of hypo- and hypersaline seawater on the microanatomy and ultrastructure of epithelial tissues of Echinometra lucunter (Echinodermata: Echinoidea) of intertidal and subtidal populations. Zoological Studies 46:203-215.
- SARIS, N.E.; E. MERVAALA; H. KARPPANEN; J.A. KHAWAJA & A. LEWENSTAM. 2000. Magnesium: An update on physiological, clinical and analytical aspects. Clinica Chimica Acta 294:1-26.
- SHUMWAY, S.E. 1977. The effects of fluctuating salinities on four species of asteroid echinoderms. Comparative Biochemistry and Physiology 58A:177-179.
- STICKLE, W.B. & R. AHOKAS. 1974. The effects of tidal fluctuation of salinity on the perivisceral fluid composition of several echinoderms. Comparative Biochemistry and Physiology 47A:469-476.
- STICKLE, W.B. & G.J. DENOUX. 1976. Effects of in situ tidal fluctuations on osmotic and ionic composition of body fluid in Southeastern Alaska rocky intertidal fauna. Marine Biology 37:125-135.
- STICKLE, W.B. & W.J. DIEHL. 1987. Effects of salinity on echinoderms, p. 235-285. In: M. JANGOUX & J.M. LAWRENCE (Eds). Echinoderm Studies 2. Rotterdam, AA Balkema.
- TALBOT, T.D. & J.M. LAWRENCE. 2002. The effect of salinity on respiration, excretion, regeneration and production in Ophiophragmus filograneus (Echinodermata: Ophiuroidea). Journal of Experimental Marine Biology and Ecology 275:1-14.
- TAVARES, Y.A.G.; H.G. KAWALL & C.A. BORZONE. 2004. Biochemical changes in the gonad in relation to the reproductive cycle of Echinometra lucunter and Arbacia lixula in southern Brazil, p. 147-155. In: M. LAWRENCE & O. GUZMAN (Eds). Sea urchin fisheries and ecology. Lancaster, DESTec Publications Inc.
- TROTTER, J.A. & K. CHINO. 1997. Regulation of cell-dependent viscosity in the dermis of the sea cucumber Actinopyga agassizi Comparative Biochemistry and Physiology 118A: 805-811.
- VIDOLIN, D.; I.A. SANTOS-GOUVEA & C.A. FREIRE. 2007. Differences in ion regulation in the sea urchins Lytechinus variegatus and Arbacia lixula (Echinodermata: Echinoidea). Journal of the Marine Biological Association of the UK 87:769-775.
- WILKIE, I.C.; R.H. EMSON & C.M. YOUNG. 1994. Variable tensility of the ligaments in the stalk of a sea-lily. Comparative Biochemistry and Physiology 109A:633-641.
- ZANDERS, I.P. & F.C. HERRERA. 1974. Ionic distribution and fluxes in holothurian tissues. Comparative Biochemistry and Physiology 47A:1153-1170.
Publication Dates
-
Publication in this collection
16 Sept 2011 -
Date of issue
Aug 2011
History
-
Received
10 June 2010 -
Accepted
26 Apr 2011