Abstract
Taunayia bifasciata (Eigenmann & Norris, 1900) is a small catfish that inhabits headwater streams of the Tietê and Paraíba do Sul river basins, southeastern Brazil, being restricted to the Atlantic rain forest. The species is found on lists of threatened species of Brazil and the state of São Paulo. Despite that, there is no literature information about the biology of the species. In the present study we endeavored to collect data on the biology of T. bifasciata. A total of 37 specimens were captured in two streams, Piracuama and Oliveiras. Of those, 22 were females, 14 were males and 1 specimen was immature. Adults with mature gonads were captured in all samples excepting the last, suggesting that T. bifasciata has a long reproductive season. The average fecundity was 319 oocytes/female (range 173-504), with diameters ranging from 0.183 to 2.135 mm. Seven different food items were found in the stomach contents of our subjects. Terrestrial insects and immature Plecoptera were the most frequent and most important food items in the diet of T. bifasciata
Diet; fecundity; population structure; reproductive season; eastern Serra da Mantiqueira
SHORT COMMUNICATION
Biological information of Taunayia bifasciata (Siluriformes: Heptapteridae): a threatened and unknown catfish
Giulianna RondineliI; Alberto Luciano CarmassiII; Francisco Manoel de Souza BragaII
IDepartamento de Produção Vegetal, Centro de Ciências Agrárias, Universidade Federal do Espírito Santo. Alto Universitário, Caixa Postal 16, Guararema, 29500-000 Alegre, ES, Brazil
IIDepartamento de Zoologia, Instituto de Biociências, Universidade Estadual Paulista "Júlio de Mesquita Filho". Avenida 24a, 1515, Caixa Postal 199, Bela Vista, 13506-900 Rio Claro, SP, Brazil. Corresponding author: E-mail: giulianna.rondineli@gmail.com
ABSTRACT
Taunayia bifasciata (Eigenmann & Norris, 1900) is a small catfish that inhabits headwater streams of the Tietê and Paraíba do Sul river basins, southeastern Brazil, being restricted to the Atlantic rain forest. The species is found on lists of threatened species of Brazil and the state of São Paulo. Despite that, there is no literature information about the biology of the species. In the present study we endeavored to collect data on the biology of T. bifasciata. A total of 37 specimens were captured in two streams, Piracuama and Oliveiras. Of those, 22 were females, 14 were males and 1 specimen was immature. Adults with mature gonads were captured in all samples excepting the last, suggesting that T. bifasciata has a long reproductive season. The average fecundity was 319 oocytes/female (range 173-504), with diameters ranging from 0.183 to 2.135 mm. Seven different food items were found in the stomach contents of our subjects. Terrestrial insects and immature Plecoptera were the most frequent and most important food items in the diet of T. bifasciata.
Key words: Diet; fecundity; population structure; reproductive season; eastern Serra da Mantiqueira.
Taunaya bifasciata (Eigenmann & Norris, 1900) is a small catfish that belongs to the family Heptapteridae. It has a colorful background ranging from brown to yellowish, and a broad transverse band on the pre-dorsal (OLIVEIRA & BRITSKI 2000). The species inhabits headwater streams of the Tietê and Paraíba do Sul river basins in southeastern Brazil, and is restricted to the Atlantic rain forest (TRAJANO & BOCKMANN 2000). Due the threats that this biome has suffered, and the fact that T. bifasciata populations are small in the few places where they occur, T. bifasciata is on the lists of endangered species of Brazil and state of São Paulo (ROSA & LIMA 2008, OYAKAWA et al. 2009). In streams of watersheds of eastern Serra da Mantiqueira, BRAGA & ANDRADE (2005) sampled forty-three specimens of T. bifasciata on a tributary of Ribeirão Grande, and Giulianna RONDINELI et al. (2011) sampled two specimens on a tributary of the Guaratinguetá River, all located in forested areas. As there is little scientific information on the biology and ecology of T. bifasciata, and given that this knowledge is fundamental for the development of conservation strategies, we endeavored to investigate the following biological aspects of T. bifasciata: sex-ratio, reproductive season, fecundity and diet.
Samples were obtained in two streams, Piracuama and Oliveiras during the months of February, April and July, 2009, and October, 2010. These streams are integrants of the Paraíba do Sul River and form the Piracuama watershed. Four collection points on the slope and piedmont were surveyed: site 1 (45º35'32"W, 22º49'43"S), site 2 (45º36'05"W, 22º50'56"S), site 3 (45º31'33"W, 22º48'29"S) and site 4 (45º32'21"W, 22º48'50"S). In each collection point, we evaluated the state of preservation of the riparian vegetation following an arbitrary scale: completely preserved, partially preserved and not preserved.
Fish were captured using electric fishing equipment which was dragged in the river water for a distance of 50 m, two times, without a contention net. All specimens collected were immediately preserved in 10% formalin, and later transferred to 70% ethanol. Voucher specimens were deposited in the icththyological collection of the Universidade Estadual Paulista, São José do Rio Preto, SP (DZSJRP 12.853).
Standard length (Ls, mm) and total weight (Wt, g) were ascertained for each specimen. Sex and stages of gonadal maturity were identified considering texture, consistency, coloration, size and surface vascularization of the gonads (VAZZOLER 1996), through direct macroscopic observation. Four different gonadal mature states were considered: A, immature; B, in maturation or resting; C, mature; and D, spent. The following values were assigned to the different degrees of stomach repletion: 1 for stomachs considered empty, 2 for stomachs partially full, and 3 for stomachs completely full (BRAGA 1990).
Female fecundity was estimated as the total number of vitelogenic oocytes produced during the reproductive period. Mature ovaries were removed, immersed in Gilson solution (VAZZOLER 1996) for a complete detachment of ovarian membranes, washed and preserved in 70% alcohol. The type of spawning was determined by the frequency distribution of the oocyte diameter classes (0.1 mm intervals) taken randomly from 100 oocytes of each female (N= 9) (VAZZOLER 1996). The gonadossomatic relationship (weight ovary/weight female) was calculated for each female.
The DFP (degree of food preference) method (BRAGA 1999) was applied to stomachs considered completely full. In this method values are assigned to food items according to their relative participation in the stomach being analyzed. The value 4 is attributed when only one food item is found in the stomach; when more than one item is present, each item is given a value (3, 2 or 1), according to its relative participation in the stomach The value of DFP is obtained through the sum of the values attributed to each item, divided by the number of stomachs analyzed. Thus, food items can be classified as absolutely preferential (DFP = 4), preferential in high degree (3 < DFP < 4), preferential (2 < DFP < 3), secondary (1 < DFP < 2) and occasional (0 < DFP < 1).
A total of 37 specimens of T. bifasciata were captured, 14 in site 1, eight in site 2, 14 in site 3 and one in site 4. The highest capture rates (sites 1 and 3) happened on a slope, where the riparian vegetation is completely preserved. Sites 2 and 4, located on the piedmont, and with less preserved riparian vegetation (partially preserved in arbitrary scale), had lower capture rates, reflecting the relationship of T. bifasciata with the vegetation.
The sex ratio was 22 females: 14 males. One immature individual could not be sexed. The sex ratio did not differ from the expected (χ2 = 1.36, gl = 1, p > 0.05). The smaller specimen was 38 mm standard length (immature) and the largest was an adult male of 120 mm standard length.
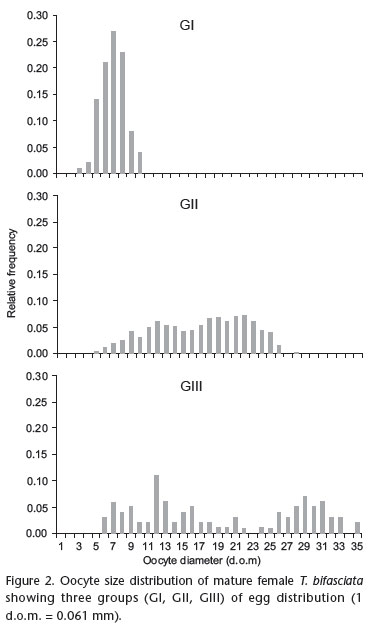
Mature specimens were captured in all samples, except the last, suggesting a long reproductive season (Fig. 1). Average fecundity for females was 319 oocytes ranging from 173 to 504 oocytes, with diameters ranging from 0.183 to 2.135 mm. It was possible to observe a progression of modes indicating the release of the most developed egg from group 1 to group 3 (Tab. I and Fig. 2). The gonadossomatic relationship, which is an efficient indicator of the functional state of the ovaries, followed this pattern: the values increased from groups GI to GIII, due the close relationship between the maturation progress of the oocytes and increase in volume, which implies an increase in the weight of the ovaries. The yolk oocytes are eliminated when the reach a diameter of 1.525 mm. Parceled spawning and a long reproductive season are the main reproductive characteristics of tropical and subtropical fishes, and correspond to adaptations for surviving in environments that have unfavorable abiotic conditions (NIKOLSKI 1963). Populations of T. bifasciata inhabit headwater areas, which impose variable and severe conditions on fish (ALLAN 1997). Parceled spawning enables the reproductive success through the rapid development of one or more egg batches have remained in the ovary. Furthermore, reduction in fecundity is compensated by larger oocytes, which give origin to larger immature individuals, with better capacity to explore the environment (WOOTTON 1992).
Sixteen full stomachs were analyzed. Seven different food items were found in the stomach contents: Annelida (Oligochaeta), terrestrial insects (Coleoptera) and immature forms of insects (Coleoptera, Trichoptera, Ephemeroptera and Plecoptera) (Tab. II). The food items more frequent and more important in the diet of T. bifasciata were terrestrial insects and immature Plecoptera, classified as secondary items. Thus we can classify T. bifasciata as an insectivorous species, which consumes autochthonous and allochthonous items. Due to the little information available about the diet of this species, it has been classified by different authors as omnivorous (ROSA & LIMA 2008, OYAKAWA et al. 2009), benthic insectivore (BRAGA & GOMIERO 2009) and insectivore (Giulianna Rondineli, unpubl. data). The riparian vegetation provides a wide variety of food items, which have great importance in the food chain and survival of tropical freshwater fishes (LOWE-MCCONNELL 1999). Both autochthonous andallochthonous items are dependent on the riparian forest, hence the importance of forest conservation to maintain the ecological balance of a river (ALVIM & PERET 2004).
ACKNOWLEDGMENTS
We are grateful to CNPq for financial support, Francisco Langeani Neto for fish identification, "Núcleo de Educação Ambiental do Ribeirão Grande" for the logistic support and the anonymous reviewers for comments on the manuscript.
LITERATURE CITED
Submitted: 07.XII.2010; Accepted: 12.V.2011.
Editorial responsibility: Wolmar B. Wosiacki
- ALLAN, J.D. 1997. Stream Ecology: structure and function of running water. New York, Chapman & Hall, 338p.
- ALVIM, M.C.C. & A.C. PERET. 2004. Food resources sustaining the fish fauna in a section of the upper São Francisco River in Três Marias, MG, Brazil. Brazilian Journal of Biology 64(2):195-202.
- BRAGA, F.M.S. 1990. Aspectos da reprodução e alimentação de peixes comuns em um trecho do rio Tocantins entre Imperatriz e Estreito, Estado do Maranhão e Tocantins, Brasil. Revista Brasileira de Biologia 50(3):547-558.
- BRAGA, F.M.S. 1999. O grau de preferência alimentar: um método qualitativo e quantitativo para o estudo do conteúdo estomacal de peixes. Acta Scientiarum 21(2):291-295.
- BRAGA, F.M.S. & P.M. ANDRADE. 2005. Distribuição de peixes na microbacia do Ribeirão Grande, serra da Mantiqueira Oriental, São Paulo, Brasil. Iheringia, Série Zoologia, 95(2):121-126.
- BRAGA, F.M.S. & L.M. GOMIERO. 2009. Alimentação de peixes na microbacia do Ribeirão Grande, Serra da Mantiqueira oriental, SP. Biota Neotropica 9(3):1-6.
- LOWE-MCCONNELL, R.H. 1999. Estudos ecológicos de comunidades de peixes tropicais. São Paulo, Editora da Universidade de São Paulo, 535p.
- NIKOLSKI, G.V. 1963. The ecology of fishes. London, Academic Press, 325p.
- OLIVEIRA, J.C. & H.A. BRITSKI. 2000. Redescrição de Taunayia bifasciata (Eigenmann & Norris, 1900), comb. nova, um bagre enigmático do estado de São Paulo (Siluriformes, Pimelodidae, Heptapteridae). Papéis Avulsos de Zoologia 41(8):119-1332.
- OYAKAWA, O.T.; N.A. MENEZES; O.A. SHIBATTA; F.T. LIMA; F. LANGEANI; C.S. PAVANELLI & D.T.B. NIELSEN. 2009. Peixes, p. 383 In: M. BRESSAN, M.C.M. KIERULFF & A.M. SUGIEDA (Eds). Fauna ameaçada de extinção no estado de São Paulo. São Paulo, Fundação Parque Zoológico de São Paulo, Secretaria do Meio Ambiente, 645p.
- RONDINELI, G.R.; A.L. CARMASSI & F.SM.S. BRAGA. 2011. Pisces, Buenos and Guaratinguetá watersheds, eastern Serra da Mantiqueira, São Paulo, Brazil. Check List 7(1):71-74.
- ROSA, R.S. & F.C.T. LIMA. 2008. Os peixes ameaçados de extinção, p. 210-211. In: A.B.M. Machado; G.M. Drummond & A.P. Paglia (Eds). Livro vermelho da fauna brasileira ameaçada de extinção. Belo Horizonte, Ministério do Meio Ambiente, Fundação Biodiversitas, vol. 2, 1420p.
- TRAJANO, E. & F.A. BOCKMANN. 2000. Ecology and behavior of a new cave catfish of the genus Taunayia from northeastern Brazil (Siluriformes: Pimelodidadae). Ichthyological Explorations of Freshwater 11(3):207-216.
- VAZZOLER, A.E.A.M. 1996. Biologia da reprodução de peixes teleósteos: Teoria e Prática Maringá, EDUEM, SBI, São Paulo, 169p.
- WOOTTON, R.J. 1992. Fish Ecology New York, Chapman and Hall, 212p.
Publication Dates
-
Publication in this collection
16 Sept 2011 -
Date of issue
Aug 2011
History
-
Accepted
12 May 2011 -
Received
07 Dec 2010