Abstract
In this work we describe the gonad morphology and spawning season of Hypostomus affinis (Steindachner, 1877) in a tropical reservoir based on 55 males and 125 females. Our aim was to assess eventual adaptations in reproductive tactics developed by this riverine species inhabiting an oligotrophic reservoir with low habitat complexity, few rocks and few other preferred consolidated substrata. We described the stages of cells of reproductive lineage, gonadal development and some reproductive traits which were compared with information in the available literature. Cells from the spermatogenic lineage were spermatogonia, spermatocytes, spermatids and spermatozoa, and cells from the oocytarian lineage were primary oocytes, previtelogenic oocytes, cortical vesicle oocytes and yolk globules or vitellogenic. Five stages were described for the males/females according to the distribution of oocytes and spermatogenic lineage cells: resting; initial maturation; advanced maturation; partially spent/spawned; totally spent/spawn. Females outnumbered males and reached larger size. Synchronic ovary development in two groups was found with diameter of mature oocytes ranging from 2 to 3.35 mm. Indication of early maturation, a longer reproductive period and the production of smaller eggs in small clutches seems to be features of the reservoir population not found in riverine systems. Such changes in tactics may indicate a shift to an opportunistic strategy, helping the population to withstand environmental constraints and to succeed in this oligotrophic and poorly structured reservoir.
Brazil; fish reproduction; reproductive biology; reservoirs; tactics
BIOLOGY
Reproductive plasticity of Hypostomus affinis (Siluriformes: Loricariidae) as a mechanism to adapt to a reservoir with poor habitat complexity
Silvana DuarteI; Francisco Gerson AraújoI, 1; Nilo BazzoliII
ILaboratório de Ecologia de Peixes, Universidade Federal Rural do Rio de Janeiro. Rodovia BR 465, km 7, 23890-000 Seropédica, RJ, Brazil
IIDepartamento de Morfologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais. Caixa Postal 486, 30161-970 Belo Horizonte, MG, Brazil
ABSTRACT
In this work we describe the gonad morphology and spawning season of Hypostomus affinis (Steindachner, 1877) in a tropical reservoir based on 55 males and 125 females. Our aim was to assess eventual adaptations in reproductive tactics developed by this riverine species inhabiting an oligotrophic reservoir with low habitat complexity, few rocks and few other preferred consolidated substrata. We described the stages of cells of reproductive lineage, gonadal development and some reproductive traits which were compared with information in the available literature. Cells from the spermatogenic lineage were spermatogonia, spermatocytes, spermatids and spermatozoa, and cells from the oocytarian lineage were primary oocytes, previtelogenic oocytes, cortical vesicle oocytes and yolk globules or vitellogenic. Five stages were described for the males/females according to the distribution of oocytes and spermatogenic lineage cells: resting; initial maturation; advanced maturation; partially spent/spawned; totally spent/spawn. Females outnumbered males and reached larger size. Synchronic ovary development in two groups was found with diameter of mature oocytes ranging from 2 to 3.35 mm. Indication of early maturation, a longer reproductive period and the production of smaller eggs in small clutches seems to be features of the reservoir population not found in riverine systems. Such changes in tactics may indicate a shift to an opportunistic strategy, helping the population to withstand environmental constraints and to succeed in this oligotrophic and poorly structured reservoir.
Key words: Brazil; fish reproduction; reproductive biology; reservoirs; tactics.
Knowledge on the reproductive strategy and tactics such as sex ratio, gonadal development and breeding season are essential in order to obtain a comprehensive understanding of the population dynamics of any fish species (WINEMILLER 1989, WOOTTON 1989, LASSALA & RENESTO 2007, TAMADA 2009). The balance between the advantages and disadvantages in the allocation of energy dictates the reproductive strategy and its variations (tactics). Some studies have demonstrated the plasticity of reproductive strategies in fishes confronted with changing environmental conditions, food availability and predation pressure (SUZUKI et al. 2000, HARDIE et al. 2007). Therefore, species widely distributed in different types of changeable habitats need to be investigated in order to assess eventual change in their reproductive traits.
Hypostomus affinis (Steindachner, 1877) is a detritivorous species from the Paraiba do Sul River basin that has successfully adapted to the Lajes Reservoir (DUARTE & ARAÚJO 2000), an oligotrophic environment constructed between 1905 and 1908, surrounded by well-preserved stretches of Atlantic rainforest (SANTOS et al. 2004). Unlike most reservoirs, the Lajes Reservoir was built by damming only small streams with small fish adapted to high altitudes in the Atlantic Forest. The reservoir has low habitat complexity. The unprotected shoreline is composed mainly of bare soil, lacking consolidated substratum, and harboring only a few shelters due to the lack of vegetation, which was cut prior to the construction of the dams (ARAÚJO & SANTOS 2001). A large amount of the ichythyofauna in the Lajes Reservoir colonized the impounded system or was introduced into it, one of the oldest of this kind in Brazil (SANTOS et al. 2008).
Studies on the reproductive biology of H. affinis in in the Paraíba do Sul River encompass sex ratio (MAZZONI & CARAMASCHI 1995), ovarian de development, fecundity and spawning season (MAZZONI & CARAMASCHi 1997). They concluded that this species adopts an equilibrium (sensu WINEMILLER) reproductive strategy characterized by a suite of attributes associated with parental care, maturation, large eggs and seasonal reproduction. Hypostomus affinis is less abundant than other species in the Lajes Reservoir (ARAÚJO & SANTOS 2001) such as the Siluriformes Loricariichthys castaneus (Castelnau, 1855), Trachelyopterus striatulus (Steindachner, 1877) and Rhamdia quelen (Quoy & Gaimard, 1824), the Characiformes Astyanax parahybae Eigenmann, 1908 and Oligosarcus hepsetus (Cuvier, 1829), and the Perciformes Cichla kelberi Kullander & Ferreira, 2006 and Tilapia rendalli (Boulenger 1897).
The shortage of rocks and other preferable consolidated substrata for H. affinis in the Lajes Reservoir is a major environmental constraint that probably limits fish population densities (DUARTE & ARAÚJO 2000). POWER (1983) reported that loricariid catfish were six to seven times denser in sunny pools (where algae grew about seven times faster) than in shaded pools. LOWE MCCONNELL (1963) identified many species of Hypostomus Lacepède, 1803 as common in small tributary environments with rocky bottoms in the hydrographic basin of the Rupununi River, British Guyana. Several species of Hypostomus known for their association with fast flowing environments are bottom-dwellers, feeding on the attached algae on rocky substratum (BUCK & SAZIMA 1995, GARAVELLO & GARAVELLO 2004); during the day, individuals remain under rocks or submerged logs (WEBER 2003). POWER (1990) and ANTONIASSI et al. (1998) observed the environmental preferences of Hypostomus species for the clean and running waters of the large Brazilian rivers, and reported that this species can survive daming. Furthermore, MAZZONI et al. (2010) reported that species of Hypostomus are normally classified as grazers, frequently inhabiting fast flowing streams in benthic areas close to rock substratum, and/or submerse wood. According to LOWE-MCCONNELL (1963) species of Hypostomus give parental care and have low fecundity, but their reproductive strategy is rather unknown.
In this study we describe features of the reproductive biology of H. affinis and assess the tactics that this species uses to succeed in the Lajes Reservoir, a lentic, low structured impoundment where the preferred habitat of the species (rocks, stones and gravel) are scarce. We hypothesized that some of the reproductive tactics (e.g., sex ratio, size of oocytes, oocytes development) of our population differ from the equilibrium strategies, reported for most loricariid Siluriformes.
MATERIAL AND METHODS
The Lajes Reservoir (22º42'-22º50'S, 43º53'-44º05'W) is a major impoundment in the state of Rio de Janeiro, located at 415 m above mean sea level in the upper slopes of the Cordilheira do Mar, in southeastern Brazil. It was built between 1905 and 1908 to generate energy and has an area of 30 km2 of well preserved oligotrophic system (SOARES et al. 2008). This reservoir has low concentrations of nitrogen (< 10 µg.L-1), phosphate (< 120 µg.L-1) and chlorophyll α (< 2.5 µg.L-1) with a high water residence time (286 days) (SANTOS et al. 2004). The altitude of the reservoir and the tropical humid climate determine two distinct seasons: a wet season, from late spring to early autumn; and a dry season, from late autumn to spring (BARBIERI & KRONEMBERG 1994). Surface water temperature ranges from 15.3 to 30.6ºC, pH between 6 and 8, dissolved oxygen is higher than 4.7 mg.l-1 and Secchi transparency averages 2.30 m ± 0.05 (ARAÚJO & SANTOS 2001). Fluctuation in rainfall combined with a regulated outflow results in peaks in water level in May-June, one or two months after the peak of rainfall (SANTOS et al. 2004). Differences in water level usually range between 3-6 m, but in extremes of flood and drawdown events can reach up to 12 m (SANTOS et al. 2004).
A total of 180 specimens (125 females and 55 males) were examined. Fish were collected monthly from January to December 1994 with gill nets (30 x 4 m, 3-8 cm stretched mesh size). All individuals were sexed and their total length (TL, to the nearest 0.1 mm) and total body mass (BM, to the nearest 0.1 g) were recorded. Gonads were removed and assigned to a given developmental stage, based on shape, size, weight, color and degree of blood irrigation; however, gonads were ultimately classified as either immature (1, juveniles and inactive stages); initial maturation (2a); advanced maturation (2b); partially spawned/spent (4a); and totally spawned/spent (4b); according to BAZZOLI & GODINHO (1991). Mature stages (3) are ephemeral and fish tend to release spermatozoa/oocytes when captured, being therefore hard to find (RIZZO et al. 2002). The diameter of oocytes was measured in a stereomicroscope fitted with an ocular micrometric (precision 0.001 mm). A cross-section from the posterior part of the gonads was fixed in of Bouin solution for 4-8 hours and submitted to ordinary histological techniques. Preserved ovaries were embedded in paraffin, sectioned at 5-7 µm thickness, and stained with hematoxylin and eosin. Histological identification of the various maturity stages were determined according to germinative cellular types for females (i.e. presence/absence of different types of oocytes; whether organized by ovarian lamellae or not) and males (presence/absence of seminiferous tubules, presence/absence of spermatozoa).
The annual gonadal cycle was determined by the variations in the gonadosomatic index (GSI) = 100 (GM × BM-1), where GM is total gonad mass and BM is total body mass. Analysis of variance (ANOVA) was used to compare means of environmental variables and means of GSI among the months. When there were significant differences, an 'a posteriori' SNK test was used to identify which values were significantly different at α = 0.05 level. Raw data was previously Log10 (x+1) transformed to address normality and homocedasticity assumptions of ANOVA. Size structure of males and females was determined by total length (TL) frequency distribution. A chi-square (X2) test was used to assess differences in size structure between sexes across size classes.
RESULTS
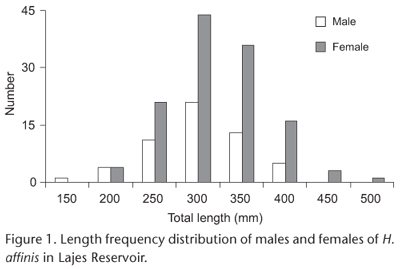
Males ranged from 125 to 425 mm TL and females from 175 to 525 mm TL. Females larger than 275 mm TL (p < 0.05) significantly outnumbered males (Fig. 1). The overall sex ratio was unbalanced (1 male: 2.27 female) with significant predominance of females (p < 0.01, X2 = 32.83) compared with males.
Gonads description
The ovaries are paired structures of different sizes located in the upper part of the coelomatic cavity. The ovaries' ducts lead to a common orifice near the anus. The right ovary is almost two times larger than the left one in advanced stages. The gonads are separated by connective tissue located ventrally to the kidneys and swim bladder.
The pair of testes are elongated, reaching the spermatic duct and do not differ morphologically from each other. The filiform testes are fairly cylindrical, flatted organs, located in the dorso-posterior area attached to the coellomic wall by the mesorc. The proximal part of the testes is thin and tubular, and the distal part changes according to the stage of the reproductive cycle.
At the beginning of the maturation, both testes and ovaries are difficult to visualize and to identify. They are translucid and easily confounded with the grizzly coloration of the peritoneum. The testes change from translucid to milky white as they mature. The ovaries, with the maturation, become laterally compressed, longitudinally voluminous, and asymmetric. A short common duct opens in the uro-genital papillae.
The testes are covered by the conjunctive albuginea tunic, flat muscular cells and blood vessels. They emit septa to the interior of the organ, forming lobules that are filled by seminiferous tubules in the testes. In the testes, the seminiferous tubules form cysts, defined by cytoplasmatic projections of Sertoli cells. In each cyst, the spermatogenic cells have the same phase of development of the cells of spermatogenic lineage. Oocytes in the ovarian lamellae occur in different development phases in the ovaries. They are variable depending on the stage of the reproductive cycle.
Spermatogenesis
Spermatogonias. They are divided in primary and secondary. The primary spermatogonias (Fig. 2) are the largest cells of the spermatogenic lineage cells, having clear cytoplasm, large and prominent nucleus. They occur isolated, not forming cysts. The secondary spermatogonia appear inside the cysts. They are small cells with more condensed chromatin.
Spermatocytes. They are divided into primary and secondary (Fig. 3). The nucleus is prominent with filamentous chromatin. The secondary spermatocytes are smaller than the primary ones and the nucleus has chromatin condensed in one of the poles of the cell.
Spermatids. They originate from the secondary spermatocytes and are smaller (Fig. 3).Their cytoplasm has little affinity for dyes. The nucleus is spherical and dense.
Spermatozoa. They are the smallest cells of the spermatogenic lineage (Fig. 4). Their nucleus is round and very dense. They accumulate gradually in the tubular lumen after the breaking of the wall of the cyst. After being released in the reproductive process, there are no spermatozoa in the lumen of the seminiferous tubules and the spermatogonia appear in the wall of the tubules (Fig. 5).
Oogenesis
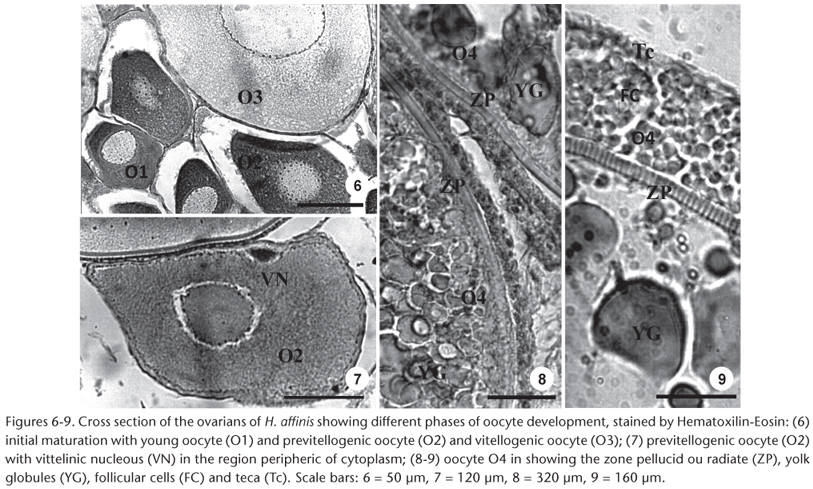
Young Oocyte (O1). The oocyte has small diameter (mean = 150 µm, range = 90-160 µm), vitreous cytoplasm, basophilic, large nucleus, prominent, centralized and with outlying nucleolus (Fig. 6).
Previtellogenic oocyte (O2). Cytoplasm with fine to granulate aspect, with nucleolus attached to the nuclear wrapping. In this phase, in the cytoplasm (mean = 390, range = 350-500 µm) the vitellinic nucleus which is a conspicuous basophilic structure, is observed (Fig. 7). The zone radiata is thin and the follicle cells are paving (Fig. 8).
Oocyte with cortical vesicles (O3). Presence of clear vesicles in the cytoplasm. Nucleus (mean diameter = 300, range = 230-400 µm) in central position but not well delineated and nucleolus distributed randomly (Fig. 6). The cytoplasm loses gradually the basophilic condition. The zone radiata is thin with follicle cells.
Vitellogenic oocytes (O4). Reach the largest size in this stage (mean diameter = 3050, range = 2750-3500 µm), characterized by yolk globules in the cytoplasm. These globules initially appear in the cell periphery as small particles that increase in size as they spread throughout the cell. The striated zone radiata (width ± 40 µm) is thin and the cells' follicles become prismatic (Fig. 9).
Morphologic characteristics of stages of the reproductive cycle
Five stages were described for the males/females according to the distribution of oocytes and spermatogenic lineage cells: resting (1); initial maturation (2a); advanced maturation (2b); partially spent/spawned (4a); totally spent/spawn (4b). The main morphologic characteristics of the testicles and ovaries in the different stages of the reproductive cycle and the gonadosomatic indices by stage of maturation are shown in tables I and II.

Oocytes with diameter between 2 and 3.35 mm were more abundant in the advanced maturation and partially spawn stages, being totally absent in the totally spawned stage, indicating a total release of the vitellogenic oocytes during the reproductive season. Oocytes of the reserve stocks had diameter lesser than 1.0 mm and mode of 0.3 mm (Fig. 10). Thus, the analysis of the frequency distribution of oocytes and the histological observation unveil that spawning in this species is synchronic in two groups, that is, a group of oocytes that underwent the maturation process and another group of oocytes in which the vitellogenesis has not started yet.
DISCUSSION
The sex ratio of H. affinis in the Lajes Reservoir is skewed towards females, which reach larger sizes than males. This pattern is the opposite of that reported by MAZZONI & CARAMASCHI (1995) for this species in the Paraíba do Sul River, where sex ratio is balanced and males are larger than females. VIANA et al. (2008) did not find significant differences in the sex ratio of the congeneric Hypostomus cf. ancistroides in the Ivaí River Basin, state of Paraná. The reasons for such differences in sex ratio between a lentic (colonized) and a lotic (natural) system are rather unknown. In most teleosteans, males are smaller than females, a strategy associated with greater reproductive investment by larger females, which can produce more oocytes. Such strategy is associated with selection toward higher fecundity (LOWE-MCCONNELL 1963, NIKOLSKY 1969, GROSS & SARGENT 1985). By contrast, larger males are associated with territorialism and interference competition by attracting more healthy females (POWER 1984).
The ecological importance of the sex ratio and size structure are still not fully explained. NIKOLSKY (1969) reported that an unbalanced sex ratio can occur between populations of a given species, and at different times in a given population. In the Lajes Reservoir, females probably grow faster and live longer due to physiological factors, or to selection to invest more in the reproductive process, as larger females have a larger peritoneal cavity and thus can lay more eggs. Both fertility and the number of fertilized eggs increase with body size, a general pattern in teleosts (GROSS & SARGENT 1985). Additionally, individuals with a faster growth rate will undergo the most vulnerable phase (smaller size) more quickly, escaping predation in proportionally larger numbers. Smaller males may, in part, be a consequence of selection for early male maturation and reproductive effort, which reduces male growth compared to that of females (ENDLER 1983, ANDERSSON 1994).
The testes of H. affinis are typical of Loricariidae, thin and laterally compressed, with distal part changing according to the stage of the reproductive cycle. Only a few species have filiform or tubular testes, similar to those of Rhinelepis aspera (Agassiz, 1829), which was described by AGOSTINHO et al. (1986, 1990). The size of the testes changes markedly according to the reproductive cycle. The grey color sometimes is confused with the peritoneal layer and/or with the guts. Overall, both testes are similar in size but can also present asymmetry. Ovaries are conspicuous irrespective of their maturation stage, asymmetric, becoming irrigated by the blood during advanced maturation, with yellow color, occupying c.a. 80% of the coelomatic cavity and pressing the liver to a flatted shape. Unlikely L. castaneus in which ovaries are asymmetric only during the partially and totally spawned phases (DUARTE et al. 2007), the ovaries of H. affinis are asymmetric throughout the reproductive cycle.
Several cells in different phases of development occur in the tubular compartment of the testes of H. affinis individuals (cell of spermatogenic lineage):spermatogonia, spermatocytes, spermatids and spermatozoa. Similarly to R. aspera (AGOSTINHO et al. 1987), changes in testicular morphology occur during cell maturation. All cell of germinative lineage are contained in cysts, except spermatogonia and spermatozoa. The seminiferous tubules appear in the partially spent stage with the walls of the tubules containing spermatogonia and cysts with cells in different phases of the spermatogenesis. AGOSTINHO et al (1987) reported that the germinative cells are annually replaced from a permanent stock of spermatogonia. According to AGOSTINHO et al. (1987), spermatozoa that occur in the spermatic ducts, similarly to those from the seminiferous tubules, are immersed in fluids produced by the cell walls. Such fluid can be associated with spermatozoa nutrition. The presence of spermatozoa throughout the year in H. affinis may serve as a reserve for the next reproductive cycle. HENDERSON (1962) reported that phagocytes invade the lobules and spermatic ducts to engulf residual spermatozoa. Furthermore, LOPES et al. (2004) reported that the spermatid may participate in the transport of spermatozoa, in the secretion of substances that form the seminal fluid, and in the re-absorption of residual spermatozoa. Resting adult males had testis with seminiferous tubules organized, with numerous spermatogonia.
According to the classification by WALLACE & SELMAN (1981), H. affinis have synchronic oocyte development with two distinct oocyte populations occurring at the same time in the ovaries: one in the reserve stock and the other which will mature synchronically and be released during the spawning season. By contrast, MAZZONI & CARAMASCHI (1997) reported an asynchronic oocyte development for H. affinis in the Paraíba do Sul River, an indication of the reproductive plasticity of this species. It is expected that the events of the reproductive cycle are locked to, and synchronized with, seasonal environmental changes. AGOSTINHO et al. (1991), describing the reproductive cycle of Hypostomus commersonii (Valenciennes, 1840), found that spawning in different periods according to size stratum is an adaptation that reduces intraspecific competition per spawning site. Limitation in spawning sites for H. affinis is very likely to occur in the Lajes Reservoir, where crevices in the rocky substratum are not common. We believe that such reservoir characteristics could be a constraint for the spawning acivity of H. affinis and it could trigger plasticity in reproductive tactics.
The reproductive cycle of H. affinis can be classified in the following stages of gonadal development according to macro and microscopy observations: 1) resting/immature, 2a) initial maturation, 2b) advanced maturation, 4a) partially spawned/spent, 4b) totally spawned/spent. The mature stage corresponds to the climax of the gonadal maturation, the exact time of ovulation or spermiation. As this event is very brief (SELMAN & WALLACE 1989) it is hardly found and identified. Another concern is the distinction between immature and resting individuals, which are differentiated histologically by presence of the ovuligers lamellae in the later, and absent in immature females. In males, the resting testes have numerous spermatogonia and scarce interstitial stroma; by contrast, the immature stages have abundant interstitial stroma and spermatogonia randomly distributed as reported by SOARES et al. (1995). Hypostomus affinis individuals produce large oocytes (2.00-3.35 mm) and Hypostomus species produce the largest eggs among all teleostans of the Paraíba do Sul basins, as reported by MENEZES & CARAMASCHI (1994), MAZZONI & CARAMASCHI (1997). SUZUKI et al. (2000) reported that Hypostomus ternetzi (Boulenger, 1895) individuals produce the largest mature eggs (mean oocyte diameter = 4.36 mm) and the smallest clutches relative to adult mass in the Paraná River. MAZONI & CARRAMASCHI (1997) found larger mature eggs of H. affinis in the Lajes Reservoir (ranging from 339 mm) than the eggs we found. Presumably, H. affinis individuals in the Lajes Reservoir allocate their energy such as to produce the same number of eggs (average fecundity = 2374, oocyte diameter 2-3.35 mm in diameter) as populations of the Paraiba do Sul River (average fecundity = 2350, oocyte diameter 3-3.8 mm), but smaller MAZONI & CARRAMASCHI (1997) found mature eggs for H. affinis ranging from 3-39 mm, which are larger than our findings for this species in the Lajes Reservoir. Presumably, the energy in H. affinis in the Lajes Reservoir is allocated to produce the same number of eggs (average fecundity = 2374; oocyte diameter 2-3.35 mm in diameter) but more little, compared with populations of the Paraíba do Sul River (average fecundity = 2350, oocyte diameter 3-3.8 mm). The smaller size of the oocytes of H. affinis individuals become more obvious when we compare the maximum size reached by individuals of this species in the Paraíba do Sul River (Lmax = 425 mm males; Lmax = 525 females) with that of other species of Hypostomus elsewhere, such as H. ternetzi, in the Paraná River (Lmax = 357 mm males; Lmax = 324 mm females, SUZUKI et al. 2000), which are comparatively smaller than H. affinis. The reasons for differences in the size of the oocytes of H. affinis individuals between the river and the reservoir could be asso-ciated with different types of habitats and their available resources. The Lajes Reservoir is an oligotrophic system compared with the eutrophic Paraíba do Sul River (SANTOS et al. 2004) and limitation in resources could shift reproductive tactics to a better use of the available energy. Furthermore, environmental stress in reservoirs, such as oxygen deficits (POTTS 1984), creates harsher living conditions. The production of comparatively smaller eggs in fishes from the Lajes Reservoir could be a mechanism to take advantage of more suitable environmental conditions (e.g., peaks of flooding with more food and shelters availability) to spawn a comparatively large number smaller eggs, thus increasing offspring survival, configuring an opportunist reproductive strategy sensu WINEMILLER (1989).
The absolute fecundity of H. affinis in the Lajes Reservoir was 2,374 oocytes (range = 1235-4304 oocytes) as reported by DUARTE & ARAÚJO (2002), and these numbers are greater than those recorded by MAZZONI & CARAMASCHI (1997) (1,784 oocytes; range = 1235-4304 oocytes). Other species of Hypostomus such as P. ancistroides (Ihering, 1911) 970 oocytes (NOMURA et al. 1975), Hypostomus luetkeni Lacepede, 1803 446-936 oocytes, and Plecostomus commersoni 500 oocytes (AGOSTINHO et al. 1991) had fecundity comparatively lower than H. affinis.
According to BAGENAL (1978), changes in fecundity act as a mechanism to regulate populations, depending on their densities. In spite of having high fecundity, H. affinis has relatively low densities in the Lajes Reservoir (SANTOS et al. 2008), probably associated to the lack of a rocky substratum, which provides feeding and shelter. According to POWER (1990), most of the species that belong to Hypostomus are limited to the bottom and forage on algae. The feeding behavior of species of Hypostomus is favored by a very specialized digestive tract, with a ventral mouth adapted to suck (OLIVEIRA E SILVA 1965, NOMURA & MUELLER 1980, FUGI & HAHN 1991) which prevents Hypostomus individuals to see their food while they are ingesting it (POWER 1983). Species of Hypostomus are detritivores/iliophagues (FUGI & HAHN 1991) and benefit from the excess of food in epiphytic communities on submerged rocks of dammed rivers.
Behavioural plasticity has been recurrently mentioned for Hypostomus species (e.g., BUCK & SAZIMA 1995, CASATTI et al. 2005). VIANA et al. (2008) found that H. ancistroides seemed to be associated with the physiographical features of the Bonito River. The fast and slow flowing stretches, the rocky substratum, with little sediment deposition, and the depths not exceeding 1.5 m throughout most of the year, constituted a favorable environment for the macro algae to settle and develop. In conclusion, H. affinis change some reproductive tactics in the Lajes Reservoir by decreasing oocyte size and increasing fecundity. Moreover, females reach larger sizes than males and have synchronic oocytes development. Such changes in tactics configure a trend from the equilibrium to the opportunistic strategy, and it is reasonable to suppose that it is a probable mechanism to withstand environmental constraints and to get success in this oligotrophic and poorly structured reservoir.
ACKNOWLEDGMENTS
We thank all the technicians and undergraduate students at Fish Ecology Laboratory, Universidade Federal Rural do Rio de Janeiro, for helping in field and laboratory. We also tank Mônica Cândida Pereira Ricardo, Laboratory of Ictio-histology, Universidade Federal de Minas Gerais for helping in histological techniques. We thank specially Luis A.B. Grande, Ricardo Bichara and Rinaldo Rocha from Light Energia S.A. for their encouragement and support. The project was partially financed by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Light Energia S.A. provides financial throughout its Research and Development Program (P & D).
LITERATURE CITED
Submitted: 03.XII.2010; Accepted: 14.VII.2011.
Editorial responsibility: Cassiano Monteiro Neto
1 Corresponding author. E-mail: gerson@ufrrj.br
- AGOSTINHO, A.A.; G. BARBIERI; J.R. VERANI & C.S. AGOSTINHO. 1986. Ciclo reprodutivo e primeira maturação de Rhinelepis aspera (Agassiz, 1829), (Teleostei Loricariidae) no rio Paranapanema. Revista Unimar 8 (1): 17-27.
- AGOSTINHO, A.A.; M.C. BARBIERI; C. AGOSTINHO & G. BARBIERI. 1987. Biologia reprodutiva de Rhinelepis aspera (Agassiz, 1829) (Teleostei, Loricariidae) no rio Paranapanema. I. Estrutura dos testículos e escala de maturidade. Revista Brasileira de Biologia 47 (3): 309-317.
- AGOSTINHO, A.A; G. BARBIERI; J.R. VERANI & N.S. HAHN. 1990. Variação do fator de condição e do índice hepatossomático e suas relações com o ciclo reprodutivo em Rhinelepis aspera (Agassiz, 1829) (Osteichthyes, Loricariidae) no rio Paranapanema. Ciência e Cultura 42 (9): 711-714.
- AGOSTINHO, A.A; N.S. HAHN & C.S. AGOSTINHO. 1991. Ciclo reprodutivo e primeira maturação de fêmeas de Hypostomus commersonii (Valenciennes. 1840) (Siluriformes, Loricariidae) no reservatório Capivari-Cachoeira, PR. Revista Brasileira de Biologia 51 (1): 31-37.
- ANDERSSON, M. 1994. Sexual selection, p. 595-624. In: J. KREBS & T.T. CLUTTON-BROCK (Eds). Monographs in behavior and ecology. Princeton, NJ, Princeton University Press, 599p.
- ANTONIASSI, L.E.; A.A. AGOSTINHO; L.C. GOMES & N.S. HAHN. 1998. Ecologia trófica de peixes em dois riachos da bacia do Rio Paraná. Revista Brasileira de Biologia 58 (2): 273-285.
- ARAÚJO, F.G. & L.N. SANTOS. 2001. Composition and structure of the fish community in the Lajes Reservoir, RJ. Revista Brasileira de Biologia 61 (4): 1-6
- BAGENAL, T.B. 1978. Aspects of fish fecundity, p. 75-101. In: S.D. GERKING (Ed.). Ecology of freshwater fish production. Oxford, Blackwell Scientific Publications, 348p.
- BARBIERI, E.B. & D.M.P. KRONEMBERGER. 1994. Climatologia do Litoral Sul Sudeste do Estado do Rio de Janeiro. Caderno de Geografia 12: 57-73
- BAZZOLI, N. & H.P. GODINHO. 1991. Reproductive biology of the Acestrorhynchus lacustriis (Reinhand, 1874) (Pisces, Characidae) from Três Marias Reservoir Brazil. Zoologisher Anzeiger 226 (5/6): 285-299.
- BUCK, S. & I. SAZIMA. 1995. An assemblage of mailed catfishes (Loricariidae) in southeastern Brazil: distribution, activity, and feeding. Ichthyology Exploration of Freshwaters 6 (4): 325-332.
- CASATTI, L.; F.C. ROCHA & D.C. PEREIRA. 2005. Habitat use by two species of Hypostomus (Pisces, Loricariidae) in Southeastern Brazilian streams. Biota Neotropica 5 (2): 1-9.
- DUARTE, S. & F.G. ARAÚJO. 2000. Distribuição espacial e temporal de Hypostomus affinis na Represa de Ribeirão das Lajes. Acta Biologica Leopoldensia 22: 261-276.
- DUARTE, S. & F.G. ARAÚJO. 2002. Fecundity of the Hypostomus affinis (Siluriformes, Loricariidae) in the Lajes Reservoir, Rio de Janeiro, Brazil. Revista de Biologia Tropical 50 (1): 193-197.
- DUARTE, S.; F.G. ARAÚJO; A. SALES & N. BAZZOLI. 2007. Morphology of gonads, scale of maturity and spawing season of Loricariichthys spixii (Siluriformes, Loricariidae), in a subtropical reservoir, Rio de Janeiro, Brazil. Brazilian Archives of Biology and Technology 50 (6): 1019-1032
- ENDLER, J.A. 1983. Natural and sexual selection on color patterns in Poeciliid fishes. Environmental Biology of Fishes 9: 173-190.
- FUGI, R. & N.S. HAHN. 1991. Espectro alimentar e relações morfológicas com o aparelho digestivo de três espécies de peixes comedores de fundo do rio Paraná, Brasil. Revista Brasileira de Biologia 51 (4): 873-879.
- GARAVELLO, J.C. & J.P. GARAVELLO. 2004. Spatial distribution and interaction of four species of the catfish genus Hypostomus Lacepède with bottom of Rio São Francisco, Canindé do São Francisco, Sergipe, Brazil (Pisces, Loricariidae, Hypostominae). Brazilian Journal of Biology 64 (3): 591-598.
- GROSS, M.R. & R.C. SARGENT. 1985. The evolution of male and female parental care in fishes. American Zoologist 25: 807-822
- HARDIE S.A.; R.W.G WHITE & L.A. BARMUTA. 2007. Reproductive biology of the threatened golden galaxies Galaxias auratus Johnston and the influence of lake hydrology. Journal of Fish Biology 71: 1820-1840.
- HENDERSON, N.E. 1962. The annual cycle in the testis of the eastern brook trout, Salvelinus fotinalis (Mitchill). Canadian Journal of Zoology 40: 631-641.
- LASSALA, M.D.P. & E. RENESTO. 2007. Reproductive strategies and genetic variability in tropical freshwater fish. Genetics and Molecular Biology 30: 690-697.
- LOPES, D.C.J.R.; N. BAZZOLI; M.F.G. BRITO & T.A. MAIA. 2004. Male reproductive system in the South American catfish Conorhynchus conirostris Journal of Fish Biology 64: 1419-1424.
- LOWE MCCONNELL, R.H. 1963. The fishes of the Rupununi savanna district of British Guiana, South America. Part 1. Ecological groupings of fish species and effects of the seasonal cycle of the fish. Journal of the Linnean Society, Series Zoology, 45 (304): 103-144.
- MAZZONI, R. & E.P. CARAMASCHI. 1995. Size, structure, sex ratio and onset of sexual maturity of two species of Hypostomus Journal of Fish Biology 47: 841-849.
- MAZZONI, R. & E.P. CARAMASCHI. 1997. Spawing season, ovarian development and fecundity of Hypostomus affinis (Osteichthyes, Loricariidae). Revista Brasileira de Biologia 57 (3): 455-462.
- MAZZONI, R.; C.F. REZENDE & L.R. MANNA. 2010. Feeding ecology of Hypostomus punctatus Valenciennes, 1840 (Osteichthyes, Loricariidae) in a costal stream from Southeast Brazil. Brazilian Journal of Biology 70: 569-574.
- MENEZES, M.S. & E.P. CARAMASCHI. 1994. Características reprodutivas de Hypostomus grupo H. punctatus no rio Ubatiba. Marica, RJ (Osteichthyes, Siluriformes). Revista Brasileira de Zoologia 54 (3): 503-513.
- NIKOLSKY, G.V. 1969. Theory of fish population dynamics. Edimburgh, Oliver & Bloyd, V+323p.
- NOMURA, H. & I.M.M. MUELLER. 1980. Biologia do cascudo, Plecostomus hermanni Ihering, 1905. do rio Mogi guaçu. São Paulo (Osteichthyes, Loricariidae). Revista Brasileira de Biologia 40 (2): 267-275.
- NOMURA, H.; M.J. OLIVEIRA; A.M.P. LELLIS & B.E. CALDO. 1975. Caracteres merísticos e biologia do cascudo bugio Plecostomus ancistroides Ihering, 1911 (Pisces, Loricariidae). Científica 3 (2): 231-245.
- OLIVEIRA E SILVA, S.L. 1965. Aspectos histológicos do tubo digestive de Plecostumus sp (Pisces, Actinopterigii, Loricariidae) Rio de Janeiro. Acta da Sociedade de Biologia 4: 63-68.
- POWER, M.E. 1983. Grazing responses of tropical freswaters fishes to different scales of variation in their food. Environmental Biology of Fishes 9 (2): 103-115.
- POWER, M.E. 1984. The importance of sediment in the grazing ecology and size class interactions of an armored catfish, Ancistrus spinosus Environmental Biology of Fishes 10: 173-181.
- POWER, M.E. 1990. Resource enhacement by indirect effects of grazers: Armored Catfish, Algae, and Sediment. Ecology 71, 897-904.
- POTTS, G.W. 1984. Parental behavior in temperate marine teleosts with special reference to the development of nest structures, p. 223-244. In: G.W. POTTS & R.J. WOOTON (Eds). Fish reproduction: strategies and tactics. San Diego, Academic Press, 424p.
- RIZZO, E.; Y. SATO; B.P. BARRETO & H.P. GODINHO. 2002. Adhesividade and supface pattern of eggs in neotropical freshwater teleosts. Journal of Fish Biology 61: 615-632.
- SANTOS, A.F.G.N.; L.N. SANTOS & F.G. ARAÚJO. 2004. Water level influences on body condition of Geophagus brasiliensis (Perciformes, Cichlidae) in a Brazilian oligotrophic reservoir. Neotropical Ichthyology 2: 151-156.
- SANTOS, L.N.; F.G. ARAÚJO & D.S. BROTO. 2008. Artificial structures as tools for fish habitat rehabilitation in a neotropical reservoir. Aquatic Conservation 18: 896-908,
- SELMAN, K. & R.A. WALLACE. 1989. Review cellular aspects of oocyte growth in teleosts. Zoological Scientist 6: 211-231.
- SOARES, M.G.; A.C. DABES; Y. SATO & N. BAZZOLI. 1995. Tamanho da primeira maturação sexual do Schizodon knerii e do Leporinus piau (Teleostei, Anastomidae) na represa de Três Marias, MG. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 48: 47-54.
- SOARES, M.C.S.; M.M. MARINHO; V.L.M. HUSZAR; C.W. BRANCO & S.M.F.O. AZEVEDO. 2008. The effects of water retention time and watershed features on the limnology of two tropical reservoirs in Brazil. Lakes Reservoir Research Management 13: 257-269.
- SUZUKI H.I.; A.A. AGOSTINHO & K.O.WINEMILLER. 2000. Relationship between oocyte morphology and reproductive strategy in Loricariid catfishes of the Parana River, Brazil. Journal of Fish Biology 57: 791-807.
- TAMADA, K. 2009. Variations in clutch and egg sizes in the amphidromous goby Rhinogobius sp. CB along a river course and within a spawning season. Ichthyology 56: 69-75.
- VIANA, D.; L.L. WOLLF; T. ZALESKI; S. ROMÃO; G. BERTOLDI & L. DONATTI. 2008. Population structure and somatic indeces of Hypostomus cf. ancistroides (Siluriformes, Loricariidae) collected from the Bonito River, Ivaí River Basin, Turvo, Paraná. Brazilian Archives of Biology and Technology 51 (3): 493-502.
- WALLACE, R.A. & K. SELMAN. 1981. Cellular and dynamic aspects of oocyte growth in Teleost. American Zoologist 21: 325-343.
- WEBER, C. 2003. Subfamily Hypostominae, p. 351-372. In: R.E. REIS; S.O. KULLANDER & C.J. FERRARIS-JR. (Orgs). Check list of the freshwater fishes of South and Central America. Porto Alegre, Editora da Pontifícia Universidade Católica do Rio Grande do Sul, 729p.
- WINEMILLER, K.O. 1989. Patterns of variation in life history among South American fishes in seasonal environments. Oecologia 81: 225-241.
- WOOTTON, R.J. 1989. Introduction: strategies and tactics in fish reproduction, p. 1-12. In: G.W. POTTS & M.N. WOOTTON (Eds). Fish reproduction: strategies and tactics. San Diego, Academic Press, 424p.
Publication Dates
-
Publication in this collection
11 Nov 2011 -
Date of issue
Oct 2011
History
-
Accepted
14 July 2011 -
Received
03 Dec 2010