Abstract
The immature stages (eggs, larvae and pupae), oviposition and larval behavior of Altinote ozomene (Godart, 1819) are described here for the first time. Larvae were reared from egg clutches collected from the host plants Erato vulcanica (Klatt) H.Rob and Munnozia senecionidis Benth (Asteraceae). Eggs were laid in groups on the undersides of leaves. The number of instars varied from five to eight within the same egg clutch, and the corresponding development time from larva to adult varied from 91 to 115 days. Most (72%) larvae pupated during the sixth instar. The first four instars fed only on the leaf cuticle, whereas later instars consumed the whole leaf. Larvae were gregarious during all instars but rested together only during the day in later instars, either hidden inside dry leaves, on the stem at the base of the host plants, or in the leaf litter. Larvae showed similar morphology and behavior to those previously described for species of Actinote Hübner, 1819 from southeastern Brazil and the Andes.
Host plant; immature behavior; Lepidoptera; life cycle; neotropical
BIOLOGY
Immature stages and natural history of the Andean butterfly Altinote ozomene (Nymphalidae: Heliconiinae: Acraeini)
Patricia Duque Velez1, 11 Corresponding Author. ; Hugo Hernando Vargas MontoyaII; Marta WolffII
IMuseo Entomológico Piedras Blancas, Comfenalco Antioquia. Medellín-Colombia. E-mail: paduve12@gmail.com
IIGrupo de Entomología Universidad de Antioquia GEUA, Universidad de Antioquia. Calle 67, no. 53-108, Medellín-Colombia, AA 1226. E-mail: mwolff@matematicas.udea.edu.co
ABSTRACT
The immature stages (eggs, larvae and pupae), oviposition and larval behavior of Altinote ozomene (Godart, 1819) are described here for the first time. Larvae were reared from egg clutches collected from the host plants Erato vulcanica (Klatt) H.Rob and Munnozia senecionidis Benth (Asteraceae). Eggs were laid in groups on the undersides of leaves. The number of instars varied from five to eight within the same egg clutch, and the corresponding development time from larva to adult varied from 91 to 115 days. Most (72%) larvae pupated during the sixth instar. The first four instars fed only on the leaf cuticle, whereas later instars consumed the whole leaf. Larvae were gregarious during all instars but rested together only during the day in later instars, either hidden inside dry leaves, on the stem at the base of the host plants, or in the leaf litter. Larvae showed similar morphology and behavior to those previously described for species of Actinote Hübner, 1819 from southeastern Brazil and the Andes.
Key words: Host plant; immature behavior; Lepidoptera; life cycle; neotropical.
The nymphalid Acraeini (Lepidoptera) comprises between one and seven genera, depending on the taxonomy adopted, and occurs both in the Neotropics and the Old World, with the greatest diversity in Africa (SILVA-BRANDÃO et al. 2008). Three genera are found in the Neotropics: Actinote Hübner, 1819, Abananote Potts, 1943 and Altinote Potts, 1943 (LAMAS 2004). According to the most recent phylogeny of Acraeini, which was proposed by SILVA-BRANDÃO et al. (2008), Altinote and Abananote are embedded within Actinote. Several species, however, were not included in that study, including Altinote ozomene(Godart, 1819). Given the current disarray in the systematics of Acraeinae at the generic level, knowledge of the morphology and biology of the immature stages of the species in this subfamily could be of particular value in developing a stable classification (HARVEY 1991, PENZ 1999, PENZ & PEGGIE 2003, FREITAS & BROWN 2004, SILVA-BRANDÃO et al. 2008).
Most of the existing descriptions of the immature stages of Neotropical Acraeini involve members of Actinote (PALUCH et al. 1999, 2001, FREITAS et al. 2009a, b, 2010). Currently, no detailed descriptions exist for species of Altinote, except for brief notes on Altinote ozomene nox (H.W. Bates, 1864) (sensu LAMAS 2004, HARVEY 1983, DEVRIES 1987). The present paper thus provides the most complete description to date of the biology and morphology of the immature stages of an Altinote species, focusing on Al. ozomene.
MATERIAL AND METHODS
The study was conducted between January 2006 and December 2009 in the Parque Ecológico Piedras Blancas nature reserve in the northeastern region of Medellín, Colombia (6º17'40.68"N, 75º30'4.32"W). The altitude at this site is 2350 m, and the average temperature is 15ºC. The area consists primarily of large pine plantations interspersed with small fragments of native forest dominated by Quercus humboldtii Bonpl (Fagaceae). Observations of the behavior of the immature stages were conducted in the field. Host plants were identified, collected and deposited in the Herbarium of the Universidad de Antioquia (HUA).
Eggs were collected in the field and taken to the laboratory where they were reared in plastic containers with host plant leaves. To determine the time spent in the embryonic phase (from oviposition to eclosion of the eggs), five egg clusters were observed daily after oviposition. To determine the time spent in each larval instar, 4680 eggs from 39 egg clusters collected in the field were monitored daily from the hatching of larvae to the emergence of adults.
The samples of first instar larvae were placed in acetic acid for 48 hours, and the samples of other instars were fixed in Kahle solution (BORROR & DELONG 1971) and then transferred to 80% ethyl alcohol. Adults were mounted on entomological pins and deposited in the Museo Entomológico de Piedras Blancas (MEPB) and in the Laboratorio de Colecciones Entomológicas, Universidad de Antioquia (CEUA). In the following descriptions, T1-T3 refer to thorax segments and A1A10 to abdominal segments. Chaetotaxy of the first instar is described using the terminology of STEHR (1987). Body length was measured at each stage using a millimeter rule under a stereomicroscope.
RESULTS
Host plants and oviposition
Eggs of Al. ozomene were collected from leaves of the Asteraceae plants Erato vulcanica (Klatt) H. Rob (Fig. 1) and Munnozia senecionidis Benth (Fig. 2; VELEZ et al. 2008). The former is a shrub, 4 m in height, with a 5-cm diameter stem; the latter has a prostrate habit of growth. Both species are abundant in the study area, especially in open areas, scrub and forest edges and along stream margins, paths and tracks (TORO 2000).
Eggs were laid in clusters on the undersides of leaves. Females typically alighted on the underside of the leaf around noon and remained there for over 2.5 hours, laying eggs (Fig. 3). Eggs and/or larvae were often found concentrated on plants within close proximity. Eggs were sometimes laid on plants where eggs or larvae were already present (up to two egg clutches were found on the same plant), generally on different leaves on large plants, and rarely on the same leaf. The number of eggs in each clutch varied from 100 to 380 (n = 39 clutches). Several clutches included four or five infertile eggs (Fig. 6). Larvae from clutches that contained fewer than 30 viable eggs (either because oviposition was interrupted or because most of the eggs died from fungal attack) died within the first three instars.
Development cycle
Eggs from a single clutch hatched within 24 hours of one another. In 92% (36) of the clutches, the number of instars varied within the same clutch from five to eight (Tab. I). In each of the other three clutches, all larvae showed the same number of instars: five in one clutch and six in the other two clutches. Most of the larvae that reached adulthood (72%, n = 349) pupated during the sixth instar, and 17% pupated during the seventh instar (Tab. I).
The total developmental time was directly related to the number of molts: the time from egg to adult was 91, 97, 110 and 115 days for larvae that passed through five to eight instars, respectively (Tab. I). This is related to the fact that all instars are of similar duration (8-10 days), except for the final instar (whether it is the fifth or the eighth), which typically lasts 13-14 days (Tab. I). However, a few larvae that passed through more than five instars pupated after only two or three days in their last instar. No relationship was observed between either the number of instars or the total developmental time and the sex of the adult butterfly. After emerging from pupae, the adults spend two or three days hanging, eliminating meconium, before they fly.
External morphology of the immature stages
Eggs (Figs 4-5) of Al. ozomene are 0.6-0.8 mm in diameter, 0.9-1.1 in height (n = 14) and barrel shaped, with a flat base adhered to the leaf and a flat apical region. The micropyle region resembles a rosette, with tiny ridges surrounded by a ring of polygonal cells, and a similar ring is present in the preapical area. Slightly elevated longitudinal ridges run from the apex to the base, varying in number from 17 to 22 (n = 16) and separated by slight grooves. Poorly defined transverse ridges are also observed. Eggs are ivory-colored from oviposition until the day preceding eclosion, when they become translucent, with the dark-colored head of the larva visible in the apical region. In many cases, the apical region of the egg was observed to be covered with a drop of viscous, transparent liquid throughout the embryonic period. Eggs without this liquid were observed to develop a fungus.
First instar larvae (Figs 6-12) have a round, smooth head without scoli and with golden setae. The body is cylindrical, without scoli and with simple, dark brown setae arising from sclerotized brown pinacula standing out slightly from the skin. The head is black, and the body is ivory-colored immediately after hatching, becoming greenish-white the next day, once food is observable through the translucent skin. The prothoracic shield and anal shield are dark brown. The thorax becomes brown or chestnut and is darkest in T1 and fades through T3. A1 is brown or dark yellowish ochre, and A2 is similar but has diffuse brown dorsolateral marks. The rest of abdomen is pale, clear yellow or greenish-yellow due to food showing through the skin.
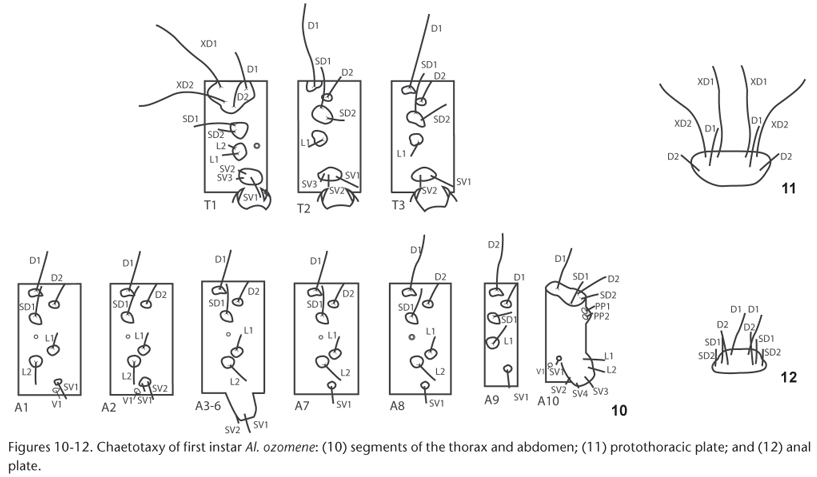
Chaetotaxy is illustrated in figures 10-12. D1 and D2 are present in all body segments. D1 is posterior and dorsal to D2 in T1 and anterior and dorsal to D2 in the remaining segments. D1 is shorter in A1-A7 than in A8-A9. SD2 is absent in A1-A9. L2 is absent in T2, T3 and A9, and L3 is absent in all segments. SV1 is present in all segments. SV2 is absent in A1, A7, A8 and A9. SV1 and SV2 originate on the same sclerotized plate in A2 and are located on the lateral shield, outside of the prolegs, in A3-A6, with SV2 being anterior and shorter and SV1 lateral and longer. SV3 is found on T1 and A10 and sometimes on T2 and/or T3. V1 is present on A1, A2 and A10. The coxae of the thoracic legs are surrounded by a laterally open, chitinous horseshoe-shaped plate with five setae: one anterior, one posterior and three setae on the internal mesal margin. EXV1 is located very close to the midline in A10, and the setae on both sides of the body originate on the same plate. The spiracles are circular and are larger on T1 and A8, with protruding edges.
Second instar larvae (Fig. 13) exhibit a shiny black head with thin pale setae, a translucent white clypeus, a brown labrum and a brown mandible. The thorax and A1 are generally brown, and the other abdominal segments are creamy yellow, with or without diffuse brown marks. On a few individuals, the abdomen is dark brown. From the second instar on, the larvae have ivory-colored infraspiracular bands that generally extend from T1 to A10, and the subventral and ventral regions of the abdomen are creamy yellow. Scoli are generally brown on the thorax and on A1, and the abdominal infraspiracular scoli are creamy yellow. The other scoli vary from brown to creamy yellow. The scoli setae range from chestnut and creamy yellow to brown.
The head is smooth, without scoli, spines or chalazae. A pair of dorsal scoli is arranged linearly on each segment except for A10, where it is absent. The T1 scoli are slightly longer than the remaining thoracic scoli, and the abdominal scoli are uniform in size and slightly smaller than those of T3. From A1 to A8, a second line of supraspiracular scoli appears below the dorsal scoli, each slightly anterior to a spiracle. One scolus is present adjacent to the anal shield on A10, in line with the other supraspiracular scoli on the abdomen. In the lateral region of the thorax, one scolus appears between T1 and T2 and another between T2 and T3; both of these are in line with the abdominal spiracles. A third line of infraspiracular scoli that are posterior to the spiracles also appears on A1 to A8. These scoli are the same size as the supraspiracular scoli and slightly shorter than the dorsal scoli on the abdomen.
The chitinous prothoracic shield is shiny black and almost oval in shape, with two setae that are longer than the dorsal scoli of the same segment. The anal shield is black, round and convex. Chitinous plate on T1-T3 and A9, located below dorsal scolus: on T1 above spiracle, almost triangular in shape, with several setae, on T2, T3 and A9 rounded in shape; very small plate under these: on T1 anterior and opposite spiracle, almost square in shape, with several setae, on T2 and T3, posterior to lateral scoli, rounded in shape. Only the plates on T1 protrude from the skin. Subventral, elliptic verruca with numerous setae are present above each thoracic leg, and these are arched in T2 and T3. Several ventral setae are clustered together on A1, A2, A7 and A8, with one seta in each cluster that is longer than the others. The circular spiracles are the same size on T1 and A8 and larger on the remaining segments. The spiracle on T1 is slightly more ventrally located.
The chitinous plate surrounding the coxa of each thoracic leg is horseshoe-shaped, outwardly open, and of the same dark brown or gray color as the legs. Prolegs exhibit dark grey plates on their lateral margins, with uniserial, biordinal crochets arranged in a mesal penellipse. Two small grey verrucae are present in the region posterior to A10 under the anus, one of which is inferior and smaller.
The arrangement of scoli, plates, and verrucae in the third through eighth instars is the same as described for the second instar.
Third instar larvae (Figs 14-15) are slightly variable in coloration, but the thorax is generally brown, the abdomen is brown or dark chestnut, and the thorax and A1 are darker than the abdomen. The abdomen is creamy yellow below the infraspiracular band. The scoli on the thorax and the back of the abdomen are dark brown, the abdominal supraspiracular and infraspiracular scoli are creamy yellow, and the supraspiracular scoli on A1 are typically brown. The spiracles on T1 and A8 are elliptical, equal in size and slightly larger than those on the rest of the abdomen, which are circular.
Fourth instar larvae (Figs 16-18) exhibit a shiny black head and a dark brown, almost black body. The scoli are generally brown, although the infraspiracular scoli tend to be creamy yellow but are also occasionally brown. All of the body plates, including the prothoracic and anal shields, are shiny and black.
Fifth to eighth instar larvae (Figs 19-21) are very similar to the fourth instar and to each other in color and morphology (except in the shape of the spiracle; see below), whether in their last instar or not. For example, all sixth instar larvae are similar to each other regardless of whether they then molt to a seventh instar larvae or whether they pupate. After the fifth instar, all body spiracles are elliptical in shape.
Body length varies considerably within and between the fifth to eighth instars (Tab. II). For example, in some cases, fifth instar larvae have the same body length as those of the sixth, seventh or eighth instars; yet at the same time, sibling larvae at the same instar can exhibit differences in length of up to 10 mm. No relationship was found between the size of the larvae and the number of molts. The larvae pupated independently of their final size (25-40 mm). Although it was not determined whether adult size is related to the size of the last instar larvae, it seems likely that the smaller pupating larvae become male butterflies, which are smaller than females.
Pupae (Figs 23-24) are white, with thin, discontinuous black lines on the dorsal and lateral surfaces of the abdomen and a thick discontinuous band on both sides of the ventral midline that forms a barrel-like figure on each segment. A black outline is present on the proboscis, legs, antennae, eyes, labrum and wings. The clypeus and labrum are black. Barely noticeable tiny black setae are distributed over the entire body, except the wing pads, antennae and legs. The cephalic region contains a pair of small protuberances, and the pronotum and mesonotum each possess a pair of aligned dorsal protuberances; these proturbances decrease in size on the metanotum, where they appear only as small buttons. T2 exhibits a lateral protuberance at the base of the wings, crossed by a thick black line. Lateral projections are present at the base of the wing pads. The spiracles on T1 are crescent-shaped and partially visible. The spiracles on A1 are hidden, those on A2 are hidden at the posterior end, and all others are elliptical and black. Five pairs of identically shaped black dorsal spines without ramifications or setae appear on A2 to A6, along the same line as the thoracic protuberances. A V-shaped marking that opens toward the anterior end appears at the base of each spine on A3-A7. Posterior to this V-shaped marking, there is a transverse line and several supraspiracular lines. A cremaster formed by the ventral region of A9 and the dorsal and ventral regions of A10 is completely modified into a corrugated black structure.
Feeding and gregarious behavior
Larvae emerge at the preapical region of the egg (Fig. 5) and immediately feed on the chorion and on infertile eggs, if present. All activities including feeding, molting, resting and displacing are gregarious, from hatching to adulthood.
Within the first several hours after hatching, larvae form a close line such that each individual is in physical contact with its neighbors and begin to feed simultaneously, starting at the edge of the leaf (Fig. 9). The first to fourth instars eat only the leaf cuticle (Figs. 9 and 16) and frequently consume less than half of the leaf before passing on to the next leaf. Third and fourth instars rapidly devour the leaf cuticle; the leaf then dries and folds and the two ends are joined with silk (Fig. 18). The first instars weave silk threads on the leaf surface, forming a dense web, under which they move and deposit their feces (Figs. 9 and 18) or spend periods of rest or premolt.
Beginning at the fifth or sixth instar, larvae rest during the day and become active in the early evening. Beginning at 06:00 to 07:00 h, they climb down the stem, one after the other, and hide either among dry leaves attached to branches close to the ground, at the base of the stem, among cracks in the stem or under dry leaves on the ground near the plant (Fig. 22). Between 18:30 and 19:30 h, the larvae emerge from their hiding place and climb, single file, back up the stem of the same plant until they reach the leaves, where they form small groups to feed. In the later instars, the larvae feed simultaneously on the leaves from the edges (Fig. 19) and either eat the whole leaf or leave only the veins uneaten.
During the first four instars, larvae from different egg clutches do not mix; each group remains on its own leaf. However, groups of older larvae may hide in the same places close to or on the ground, and these groups may mix when they climb back up the plant to feed. One or two later-instar larvae have been observed together with early-instar larvae on the same leaf. When larvae begin to pupate, they individually move three or four meters away from the host plant and attach themselves to the undersides of leaves on other plants at a minimum height of 60 cm above the ground.
Natural enemies and defense
Larvae of the syrphid fly Xanthandrus bucephalus (Wiedemann, 1830) (Diptera: Syrphidae) were observed preying on first- to third-instar Al. ozomene larvae (Fig. 15). This solitary predator apparently goes unnoticed inside the silk mass of larvae and feces. Occasionally, spiders were also seen preying on larvae. Larvae and pupae collected in the field were often found to be parasitized by different species of Tachinidae (Diptera) and Braconidae (Hymenoptera). Predation on adults was not observed.
During the first three or four instars, when one individual in a group is threatened, the whole group reacts simultaneously by raising their bodies, standing on the last pair of prolegs (Fig. 17) and/or moving the anterior half of the body rhythmically from side to side. Older larvae entwine themselves together and fall freely from the leaf, suspended by a silk thread. When the pupae are disturbed, they move the first abdominal segments from one side to the other, forming an angle of up to 120º.
Adult behavior
Altinote ozomene adults were normally observed in open areas and at forest edges. Males (Fig. 25) fly low, frequently alighting on the ground, and can be caught by hand. Adults form small groups of four or five individuals while feeding on the ground or on avian (duck) excrement on the banks of a reservoir. On several occasions, adults were observed feeding on the ground together with males of Gnathotriche mundina steinii (Dewitz, 1877) (Lepidoptera: Nymphalidae), an unrelated species with a very similar wing pattern. Females (Fig. 26) were observed less often than males.
DISCUSSION
Altinote ozomene feed on Asteraceae, as has also been observed of Al. ozomene nox (sensu LAMAS 2004; HARVEY 1983, DEVRIES 1987) and species of Actinote (DEVRIES 1987, PALUCH et al. 1999, 2001, 2005, FRANCINI et al. 2004, 2005, FRANCINI & FREITAS 2010, FREITAS et al. 2009a, b, 2010). VALENCIA et al. (2005) recorded Clibadium sp. (Asteraceae) as a host plant for Al. ozomene in the Western Cordillera of the Colombian Andes.
As in all known species of Actinote, Al. ozomene lays egg clutches under leaves (DEVRIES 1987, PALUCH et al. 1999, 2001, 2005, FRANCINI et al. 2004, 2005, FRANCINI & FREITAS 2010, FREITAS et al. 2009a, b, 2010). The maximum number of eggs per oviposition in Al. ozomene is notably less than that in Actinote pellenea pellenea Hübner, 1821 (FRANCINI & FREITAS 2010) and slightly less than that in Actinote surima (Schaus, 1902) (PALUCH et al. 1999) and Ac. carycina Jordan (PALUCH et al. 2001; Table III). The number of eggs per oviposition in Al. ozomene is likely a limiting factor for larval survival because first-instar larvae in groups of fewer than 20 died and larvae of the first four instars generally only survived if they were in large groups, which was probably due to a better response to leaf structural defenses. When the number of larvae is small, the silk web on which the larvae move is equally small (pers. obs.), leaving the leaf trichomes exposed, which may impede larval displacement and anchorage on the leaf and therefore affect feeding, as suggested by FORDYCE & AGRAWAL (2001). In addition, oviposition in Al. ozomene tends to occur in a grouped fashion, on closely spaced plants or on leaves of the same plants, as has been described for Ac. pellenea pellenea (FRANCINI & FREITAS 2010). Other factors, including habitat and plant characteristics, may also affect female preference during oviposition. Although the general behavior of Al. ozomene is similar to that described for species of Actinote (Tab. IV), nocturnal gregarious feeding has only been recorded in Al. ozomene, and hiding in dry leaves during later instars has only recently been described for Ac. conspicua (FREITAS et al. 2010). However, in contrast to Al. ozomene, larvae of Ac. conspicua hide individually or in pairs and only during the last instar.
Most species of Actinote have univoltine or bivoltine life cycles (PALUCH et al. 1999, 2001, 2005, FRANCINI et al. 2005, FREITAS et al. 2009a, b, 2010). A multivoltine cycle is presently known for only Ac. pellenea pellenea (FRANCINI & FREITAS 2010). Since 2004, we have found Al. ozomene frequently throughout the year, at low densities, except during the second half of 2005, when no adults or immature stages were found.
A variable number of instars, as observed here in Al. ozomene, has never been recorded in Actinote (PALUCH et al. 1999, 2001, PALUCH et al. 2005, FRANCINI et al. 2005, FREITAS et al. 2009a, b, 2010). However, our observations suggest that such variation may occur in other species of Altinote (unpubl. data), and further investigation of this phenomenon might reveal both why it occurs and whether it is restricted to this genus.
The immature stages of Al. ozomene are similar to those of some species of Actinote from Brazil and the Andes in several morphological features. These include the initial color, shape and size of the eggs, the body color and arrangement of the setae in the first instar, the absence of spines on the head, the arrangement of scoli in other instars, the shape of the pupa and the number and arrangement of its abdominal spines (PALUCH et al. 1999, 2001, FRANCINI et al. 2005, FREITAS et al. 2009a, b, 2010). Al. ozomene differs from the species of Actinote in egg color during the days following oviposition (the eggs become reddish in Actinote) and in the color pattern of the larvae after the first instar. Additional studies are clearly needed to identify additional characters that vary within the tribe and thus might provide useful information for phylogenetic studies.
ACKNOWLEDGEMENTS
We thank Eliana Flórez, Juan D. Marín, Diana Grisales, Andrés Vélez, and Marisol Ortega for help with rearing the larvae in the laboratory and Alvaro Idarraga for the identification of the host plants. This study was funded by Comfenalco-Antioquia.
LITERATURE CITED
Submitted: 28.I.2011; Accepted: 16.IX.2011.
Editorial responsibility: Pedro Gnaspini
- BORROR, D.J. & D.M. DELONG. 1971. An Introduction to the Study of Insects. New York, Holt, Rinehart and Winston. 812p.
- BROWER, A.V.Z. 2010. Neotropical Actinote clade. Version 13 February 2010, The Tree of Life Web Project, available online at: http://tolweb.org/neotropical_Actinote_clade/118461/2010.02.13 [Accessed: 04/III/2011]
- DEVRIES, P.J. 1987. The butterflies of Costa Rica and their natural history. Princeton, Princeton Academic Press, 327p.
- FORDYCE, J.A. & A.A. AGRAWAL. 2001. The role of plant trichomes and larvae group size on growth and defence of the pipevine swallowtail Battus phileno. Journal of Animal Ecology 70: 997-1005
- FRANCINI, R.B.; A.V.L. FREITAS & C.M. PENZ. 2004. Two new species of Actinote (Lepidoptera, Nymphalidae) from Southeastern Brasil. Zootaxa 719: 1-10.
- FRANCINI, R.B.; A.V.L. FREITAS & K.S. BROWN JR. 2005. Rediscovery of Actinote zikani (D'Almeida) (Nymphalidae, Heliconiinae, Acraeini): Natural history, population biology and conservation of an endangered butterfly in SE Brazil. Journal of the Lepidopterists' Society 59 (3): 134-142.
- FRANCINI, R.B. & A.V.L. FREITAS. 2010. Aggregated oviposition in Actinote pellenea pellenea Hübner (Lepidoptera: Nymphalidae). The Journal of Research on the Lepidoptera 42: 74-78.
- FREITAS, A.V.L. & K.S. BROWN JR. 2004. Phylogeny of the Nymphalidae (Lepidoptera). Systematic Biology 53 (3): 363-383.
- FREITAS, A.V.L.; L.A. KAMINSKI; R.G. MATOS & K.L. SILVA-BRANDÃO. 2009a. Immature stages of the Andean butterfly Actinote rufina (Nymphalidae: Heliconiinae: Acraeini). Tropical Lepidoptera Research 19 (1): 18-21.
- FREITAS, A.V.L.; R.B. FRANCINI & T.S. SOUZA. 2009b. Immature stages and natural history of the threatened butterfly Actinote quadra (Nymphalidae: Heliconiinae: Acraeini). Tropical Lepidoptera Research 19 (2): 82-88.
- HARVEY, D.J. 1983. Actinote leucomelas, p. 679-680. In: D.H. JANZEN (Ed.). Costa Rican Natural History. Chicago, University of Chicago Press, 816p.
- HARVEY, D.J. 1991. Higher classification of the Nymphalidae, p. 225-273. In: H.F. NIJHOUT (Ed.). The development and evolution of butterfly wing patterns. Washington, D.C, Smithsonian Series in Comparative Evolutionary Biology, 297p.
- LAMAS, G. 2004. Heliconiinae, p. 262-274. In: J.B. HEPPNER (Ed.). Atlas of Neotropical Lepidoptera. Gainesville, Association for Tropical Lepidoptera, Scientific Publishers, vol. 5A, 439p.
- PALUCH, M.; M.M. CASAGRANDE & O.H.H. MIELKE. 1999. Estágios imaturos de Actinote surima (Schaus) (Lepidoptera, Nymphalidae, Acraeinae). Revista Brasileira de Zoologia, 16 (Supl. 2): 129-140.
- PALUCH, M.; M.M. CASAGRANDE & O.H.H. MIELKE. 2001. Estágios imaturos de Actinote carycina Jordan (Lepidoptera, Nymphalidae, Acraeinae). Revista Brasileira de Zoologia 18 (3): 883-896.
- PALUCH, M.; M.M. CASAGRANDE & O.H.H. MIELKE. 2005. Comportamento de agregação noturna dos machos de Actinote surima surima (SCHAUS) (Lepidoptera, Heliconiinae, Acraeini). Revista Brasileira de Zoologia 22: 410-418.
- PENZ, C.M. 1999. Higher level phylogeny for the passion-vine butterflies (Nymphalidae, Heliconiinae) based on early stage and adult morphology. Zoological Journal of the Linnean Society 127: 277-344.
- PENZ, C.M. & P. DJUNIJANTI. 2003. Phylogenetic relationships among Heliconiinae genera based on morphology (Lepidoptera: Nymphalidae). Systematic Entomology 28: 451-479.
- SILVA-BRANDÃO, K.L.; N. WAHLBERG; R.B. FRANCINI; A.M.L. AZEREDO ESPIN; K.S. BROWN JR; M. PALUCH; D.C. LEES & A.V.L. FREITAS. 2008. Phylogenetic relationship of butterflies of the tribe Acraeini (Lepidoptera, Nymphalidae, Heliconiinae) and the evolution of host plant use. Molecular Phylogenetics and Evolution 46: 515-531.
- STEHR, F.W. 1987. Order Lepidoptera, p. 288-596. In: F.W. STEHR (Ed.) Immature Insects. Dubuque, Kendal/Hunt, 750p.
- TORO, J.L. 2000. Árboles y arbustos del Parque Regional Arví. Medellín, Corantioquia, 281p.
- VÉLEZ, A.; P. DUQUE & M. WOLFF. 2008. Mariposas del parque ecológico Piedras Blancas Guía de campo. Medellín, Fondo Editorial Comfenalco Antioquia, 204p.
- VALENCIA, C.A.; Z.N. GIL & L.M. CONSTANTINO. 2005. Mariposas diurnas de la zona central cafetera Colombiana. Guía de campo. Chinchiná, Cenicafé, 244p.
Publication Dates
-
Publication in this collection
11 Nov 2011 -
Date of issue
Oct 2011
History
-
Accepted
16 Sept 2011 -
Received
28 Jan 2011