Resumo
From a series of biological samples collected from different freshwater environments in Costa Rica, Central America, the exotic Asian cyclopoid Mesocyclops thermocyclopoides Harada, 1931 was identified. We analyzed the morphology and appendage ornamentation of different Neotropical populations of this species, including specimens from Honduras, southeastern Mexico, and Costa Rica. We also examined Asian specimens from Taiwan, Indonesia, Vietnam, and Thailand, and performed a comparison of the Neotropical and Asian populations including a Principal Component Analysis (PCA). The Neotropical and Asian specimens show subtle morphological variations in the antennules, antennae, mandibles, swimming legs 1-4, and fifth legs. Some characters in the Neotropical group appear to diverge from the Asian pattern and the PCA indicated that intercontinental populations of M. thermocyclopoides are far from being homogeneous. These intra-specific differences are described to expand the known morphological range of this species and to provide the first comparative analysis of an exotic copepod in the Americas. Our analysis suggests that the geographic isolation of the American populations and the subtle morphological divergences with respect to the Asian patterns could be related to speciation processes in the Neotropical region, but also intra-Asian differences are reported. In the Neotropical region this species appears to be restricted to southeastern Mexico, Central America, and one Caribbean island; its potential as biological control of mosquito might enhance its spread in the region.
Crustacean taxonomy; freshwater crustaceans; copepods; exotic species; morphometrics
Crustacean taxonomy; freshwater crustaceans; copepods; exotic species; morphometrics
TAXONOMY AND NOMENCLATURE
Morphological variability and distribution of the exotic Asian Mesocyclops thermocyclopoides (Copepoda: Cyclopoida) in the Neotropical region
Eduardo Suárez-MoralesI, 1 1 Corresponding author. E-mail: esuarez@ecosur.mx ; Nancy F. Mercado-SalasI; Álvaro Morales-RamírezII
IEl Colegio de la Frontera Sur (ECOSUR) Unidad Chetumal, Av. Centenario Km. 5.5. Chetumal, Quintana Roo 77014 Mexico
IICIMAR, Universidad de Costa Rica. San José, Costa Rica CA
ABSTRACT
From a series of biological samples collected from different freshwater environments in Costa Rica, Central America, the exotic Asian cyclopoid Mesocyclops thermocyclopoides Harada, 1931 was identified. We analyzed the morphology and appendage ornamentation of different Neotropical populations of this species, including specimens from Honduras, southeastern Mexico, and Costa Rica. We also examined Asian specimens from Taiwan, Indonesia, Vietnam, and Thailand, and performed a comparison of the Neotropical and Asian populations including a Principal Component Analysis (PCA). The Neotropical and Asian specimens show subtle morphological variations in the antennules, antennae, mandibles, swimming legs 1-4, and fifth legs. Some characters in the Neotropical group appear to diverge from the Asian pattern and the PCA indicated that intercontinental populations of M. thermocyclopoides are far from being homogeneous. These intra-specific differences are described to expand the known morphological range of this species and to provide the first comparative analysis of an exotic copepod in the Americas. Our analysis suggests that the geographic isolation of the American populations and the subtle morphological divergences with respect to the Asian patterns could be related to speciation processes in the Neotropical region, but also intra-Asian differences are reported. In the Neotropical region this species appears to be restricted to southeastern Mexico, Central America, and one Caribbean island; its potential as biological control of mosquito might enhance its spread in the region.
Key words: Crustacean taxonomy; freshwater crustaceans; copepods; exotic species; morphometrics.
The copepod order Cyclopoida includes many species with complex taxonomic problems stemming from their morphological variability (MIRABDULLAYEV & DEFAYE 2004, ALEKSEEV et al. 2006). Several cyclopoid copepod species among the genus Mesocyclops have invaded the Americas from Africa or Asia, each showing distinct distributional patterns (REID & SANDERS 1986, REID & PINTO-COELHO 1994, SUÁREZ-MORALES et al. 1999, HRIBAR & REID 2008). The knowledge of the freshwater copepod fauna, including the occurrence and distribution of exotic species, is still lacking for extensive areas of the Neotropical region, and detailed taxonomical and morphological data are needed to distinguish the nonindigenous species (OKOLODKOV et al. 2007). One of these poorly known areas is Central America, which is known to have a key role in the biogeographical history of the cyclopoid copepod fauna in the Neotropical region (SUÁREZ-MORALES et al. 2004).
From a series of biological samples collected from different freshwater environments in the area of El Arenal, of Costa Rica, Central America, copepods were taxonomically examined. The exotic Asian cyclopoid Mesocyclops thermocyclopoides Harada, 1931, was recorded. The specimens examined showed some interesting morphological variations with respect to other known populations of the species, motivating us to perform a deeper morphological analysis of M. thermocyclopoides. These variations are presented here in order to expand the knowledge of the morphological range of the species through a comparative analysis of different populations. We compared the morphology of the Neotropical populations, also including specimens from Honduras and Mexico, with published descriptions and museum specimens from different zones of Asia. Specimens were borrowed from the collection of the National Museum of Natural History, Smithsonian Institution (USNM) and from the Collection of Zooplankton at El Colegio de la Frontera Sur, Chetumal, Mexico (ECO-CHZ). The current distribution of the exotic M. thermocyclopoides in the Neotropical region is also assessed and discussed.
TAXONOMY
Cyclopinae Rafinesque, 1815
Mesocyclops thermocyclopoides Harada, 1931
Material examined. Ten females from Charco Pulsar, Tabasco, Mexico, M.A. Gutiérrez-Aguirre leg., 31-I-1999 (ECOCHZ-1212), female from Laguna Popalillo, Tabasco, Mexico,M.A. Gutiérrez-Aguirre leg., 31-I-1999 (ECO-CHZ-1218), 10 females from Charco Báscula, Tabasco, Mexico, M.A. Gutiérrez-Aguirre leg., 01-II-1999 (ECO-CHZ-1219). Ten females from El Progreso, Honduras, 27-VIII-1991 (ECO-CHZ-1181). Eight adult females from reservoir El Arenal, Costa Rica, collected 2-XI-2007, ethanol-preserved, vial (ECO-CHZ-06595), 2 adult females from same locality and date, semi-permanent slides, sealed with nail varnish (ECO-CHZ-06596). Asian specimens: One female from Bao-Shan Reservoir, Hsin-chu, Taiwan,G. Wyngaard leg., VII-2002 (USNM 1083794); two females from Chachoengsao, Thailand, G. Marten leg., XI-1994 (USNM 271905, USNM 271904); three females from Hai Hung, Phan Boi Village, Vietnam, Mr. Phich leg., 11-II-1994 (USNM 271930); two females from Sword Lake, Hanoi, Vietnam, B.H. Kay leg.,13-VI-1990 (USNM 251632); and two females from undetermined locality in Indonesia, G. Marten leg., I-1994 (USNM 264006).
The specimens from Costa Rica were identified following the keys and illustrations by SUÁREZ-MORALES & GUTIÉRREZ-AGUIRRE (2001), GUTIÉRREZ-AGUIRRE et al. (2003), and HOLYNSKA et al. (2003). The morphological analysis of these specimens revealed some differences with respect to specimens from other Neotropical populations, and from the Asian pattern, as described below.
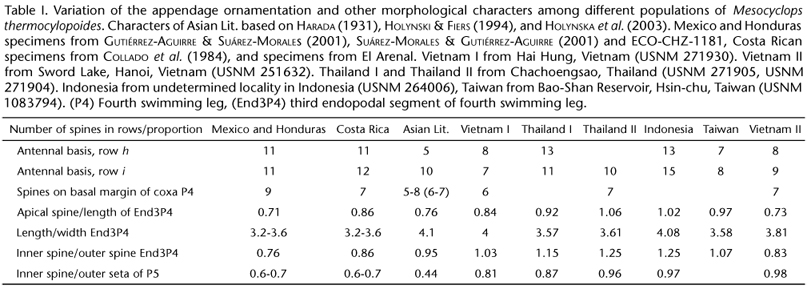
Morphological remarks and comparisons. The main differences between populations from Asia (cf. HOLYNSKA 1994, HOLYNSKA et al. 2003; museum specimens from different geographic areas in Asia), southeastern Mexico (two localities at the state of Tabasco, southeastern Mexico), Honduras (cf. GUTIÉRREZ-AGUIRRE et al. 2003; specimens from El Progreso), and Costa Rica (specimens from El Arenal) are presented in detail in Table I and summarized in Table II. The size of the body of individuals in these populations (excluding caudal rami) differ among the Neotropical populations examined: specimens from Costa Rica are the smallest (0.78-0.80 mm), those from Honduras are the largest (0.97-1.0 mm); specimens from two localities in south-eastern Mexico have intermediate size ranges (Báscula: 0.74-0.89 mm; Pulsar 0.90-1.0 mm). Overall, the Asian specimens tend to be larger than the Neotropical forms; the smallest sizes were found in Vietnam II (0.73-0.88) and the largest were from Thailand (0.95-1.26 mm) and Indonesia (1.01-1.30 mm).
Additional differences among the Neotropical populations include the ornamentation of the antennular segments 4 and 7, as follows: the specimens from Costa Rica and Asia bear four rows of spinules on the fourth antennular segment vs. two in both the Honduran and Mexican populations (Fig. 1). On the seventh antennular segment, the Asian specimen has six groups of tiny spinules, vs. five such groups in both Mexico and Honduras and nine in the Costa Rican specimens.
The ornamentation of the antennal basis includes several rows of spines which have been marked and denominated by their position following GUTIÉRREZ-AGUIRRE et al. (2003). The range of the number of spines per row is different in all groups of specimens examined (Tabs I and II). None of the Neotropical populations have less than seven and more than 11 spines in row h. Only the Asian populations examined have less than six spines in row i. We did not consider the number of spinules in row c because it often continues on the anterior surface, making counting difficult. We observed, however, that the specimens from Costa Rica (Fig. 2) have clearly larger and stronger spinules in this row when compared with the Asian, Mexican, and Honduran populations (GUTIÉRREZ-AGUIRRE et al. 2003, HOLYNSKA et al. 2003). The length ratio of the medial basipodal setae (arrowed in Fig. 2) is alike in the Neotropical populations (Mexico, Honduras, Costa Rica) (1:1) and differs from the Asian figure: 1.2-1.47:1 (n = 12).
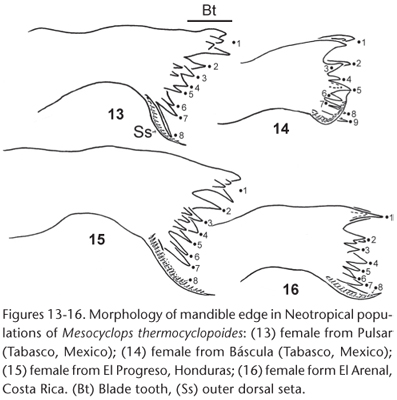
The mandible gnathobase and the teeth number show clear interspecific variation in Mesocyclops (SUÁREZ-MORALES et al. 2003). In line with that prediction, we found some differences among the Neotropical populations investigated (Figs 13-16). Specimens from Báscula (Tabasco, Mexico) have nine teeth vs. eight in the Costar Rican, Honduran and Pulsar (Tabasco, Mexico) populations (Figs 13-16); among the latter populations, only the Costar Rican specimens do not bear accessory spines. The mandibular size relative to the body size (MSI index, SUÁREZ-MORALES et al. 2003) was measured; the Costar Rican (3.17) and Báscula (3.11) populations showed the highest MSI values. Lower values were found in the Honduran specimens (2.79) and in the population from Pulsar (Tabasco) (2.67). These mandibular characters were not evaluated in the Asian specimens.
The spine-like seta on the frontal surface of the maxillules of both the Mexican and the Honduran specimens reaches half the length of the chitinized spine, whereas in the Asian and the Costar Rican populations this seta almost reaches the distal end of the spine (arrow in Fig. 5). Also, the inner and outer endopodal setae of the maxillular palp are subequal in the Asian, Mexican, and Honduran populations, but the medial seta is clearly shorter than the other setae in specimens from Costa Rica (arrowed in Fig. 4). Ornamentation and elements of the maxilla are practically the same in all specimens (Fig. 6).
The coxal ornamentation of P1 is similar in all groups examined. The populations from Honduras, Mexico, and Asia all have a semi-circular row of spines on the anterior surface of the distal margin of the basipodite, near the insertion of the exopodite (arrowed in Fig. 7). Furthermore, specimens from Mexico and Honduras also have a row of tiny spinules near the insertion of the endopodite. In the Costa Rican specimens (Fig. 7), the inner coxal seta reaches the distal margin of the first endopodal segment, whereas this seta is distinctly longer in the Asian, Mexican, and Honduran populations, reaching the distal margin of the second endopodal segment (see HOLYNSKA et al. 2003). The length ratio of the apical endopodal seta/segment length is 0.71 in Asian specimens vs. 1.04 in Costa Rican specimens (Fig. 7). The inner apical seta of this segment also differs between populations; in Asian specimens this seta is clearly longer than the inner spine, being relatively shorter in the Costa Rican specimens. The seta on the lateral margin of the same segment is longer than the apical spine in Asian specimens, whereas this spine barely exceeds half of the length of the spine in specimens from Costa Rica (arrowed in Fig. 7).
The ornamentation of both the coxopodite and basipodite of the second swimming leg differs among the examined populations. Specimens from Costa Rica bear only a single row of hair-like setae on the outer coxal margin (Fig. 8), whereas specimens from Mexico and Honduras have three additional transverse rows of setal elements on the frontal surface (GUTIÉRREZ-AGUIRRE et al. 2003). The ornamentation of the third swimming leg also differs among the three Neotropical groups of specimens; all populations bear a row of hair-like setae on the outer coxal margin, but the Mexican and Honduran specimens have a second row of shorter hair-like setae on the proximal surface of same inner margin (see GUTIÉRREZ-AGUIRRE et al. 2003: fig. 3b). Another difference is the position and size of a transverse row of hair-like setae along the distal margin of the coxopodite. In both the Mexican and Honduran specimens this row is located medially near the insertion of the coxopodite, whereas in the Costa Rican specimens the position of this row is on the middle coxal surface and closer to the outer margin (arrowed in Fig. 9).
The coxal ornamentation of the fourth swimming leg is almost the same in all populations examined (Fig. 10). The four groups of specimens have a group of hair-like setae on the inner margin of the basipodite, including a transverse row of hair-like setae close to the insertion of the inner coxal seta. Differences in P4 ornamentation and proportions include the ranges of length ratio of the apical spine/third endopodal segment, length/width ratio of the same segment, and inner spine/ outer spine ratio (Tabs I and II). Only in the Asian populations less than six spines on the basal margin of coxopodite have been observed. Additionally, specimens from Mexico and Honduras have a unique pattern of ornamentation of tiny spinules on the anterior surface of the three endopodal segments. This pattern was not observed in the Costa Rican or in the Asian populations.
The length ratio of the inner spine/outer seta of the fifth leg was also evaluated; all populations examined have variable ratios (Fig. 11, Tabs I and II). The proportion and relative lengths of the setal elements and the ornamentations of the caudal rami of specimens from Costa Rica, Mexico, Honduras and specimens from Asia are within the range known from the type Asian population (HOLYNSKA 1994, HOLYNSKA et al. 2003).
In order to define the extent and relevance of the differences found among the different populations of M. thermocyclopoides examined, we performed a Principal Component Analysis (PCA), which is a statistical tool commonly used to evaluate the morphometric and meristic characters among populations of invertebrates (COSTA-PAIVA & PAIVA 2007). We used the PRIMER 6 software with log10-transformed meristic and morphometrical data. The resulting plot (Fig. 17) included all individuals of the different populations examined. The first principal component (PC1) accounted for 42.2% of the variation, the second (PC2) for 20% among the samples (Tab. III). The plot of the component variants PC1 and PC2 (Fig. 17) shows a discrimination of population scores of different groups along PC1, including the Asian populations from Indonesia (marked as "In" in Fig. 17) with highest scores and also the two Thailand populations (TA and TB) with lower ones. Also, the Mexican populations are aligned along the PC2, thus forming a uniform group. The Honduran specimens clearly diverge in character 2, number of spines on row "i" of the antennal basis. The other Central America specimens, from Costa Rica, appear to be more closely related to the Mexican pattern that to the Honduran or Asian ones. Among the Asian populations, there is a divergence between the Indonesia and Thailand forms and the Vietnam (Vi1, Vi2) and Taiwan (Tw) populations (Fig. 17). Examination of the distance of variables from the origin revealed that the main observed differences (characters 1 and 2) were related to the number of spines on the rows of the antennal basis. Characters 6 and 7, the ratios of length/width of the third endopodal segment of the fourth leg and the inner/outer spines of the same segment, respectively, were also important. Characters 8 and 9 (inner spine of the fifth leg and ratio of the outer apical spines of the third endopodal segment of the fourth leg) have no detectable variations.
DISCUSSION
Because of the rarity of cosmopolitan forms in Mesocyclops, earlier Neotropical records of M. thermocyclopoides were suspected to represent undescribed taxa, but GUTIÉRREZ-AGUIRRE et al. (2003) confirmed the presence of the strict form of this species in the Neotropics. Our survey provides detailed morphological information about subtle differences among Neotropical and Asian populations of this species. Intraspecific morphological variations among some cyclopoids have been related to seasonal changes, but such variations in presumably cosmopolitan species are, in many species, related to the formation of species complexes (LEE 2000, MIRABDULLAYEV & DEFAYE 2003).
Overall, the morphological differences described herein are not consistently related to either the Neotropical or the Asian populations groups of M. thermocyclopoides. Our analysis showed that the characters evaluated, which are usually those used to distinguish species within the genus, show subtle but detectable intra-specific differences among the populations examined; it is clear that these meristic and morphometric characters are not uniform throughout the species range. There is some indication that the American populations are diverging from the Asian ones and that even among the Asian forms there is some degree of differentiation, particularly between the Indonesia-Thailand and Taiwan-Vietnam groups. Among the Neotropical groups, the Honduran diverges at least in one of the most variable characters, and the Mexican and Costa Rican specimens are more closely related to each other than to most of the other groups examined. Overall, the geographic isolation and the presumed reproductive isolation of the immigrant Neotropical populations, and also the subtle but consistent morphological divergence with respect to the Asian populations, suggest that a process of speciation could be occurring among the American populations of M. thermocyclopoides, particularly in Central America and southern Mexico. This isolation is expressed in subtle differences not only between the continents but also within them.
Originally described from Taiwan, the known distributional range of M. thermocyclopoides in Asia includes Japan, southern China, Burma, Vietnam, Thailand, Indochina, Malaysia, Indonesia, and Java (HOLYNSKA 1994, GUO 2000, HOLYNSKA et al. 2003). American records from Central America and the Caribbean most probably represent introduced populations (GUTIÉRREZ-AGUIRRE et al. 2003, HOLYNSKA et al. 2003). In Central America M. thermocyclopoides was first recorded from Costa Rica by COLLADO et al. (1984), from 14 localities, and later on by HERNÁNDEZ-CHAVARRÍA & SCHAPER (2000). It has also been recorded from Puerto Rico (MARTEN, 1994), Honduras (MARTEN et al. 1994) and southeastern Mexico (GUTIÉRREZ-AGUIRRE & SUÁREZ-MORALES 2001, SUÁREZ-MORALES & GUTIÉRREZ-AGUIRRE 2001, GUTIÉRREZ-AGUIRRE et al. 2006), together with other exotic species (SUÁREZ-MORALES et al. 2011), thus confirming the growing occurrence of populations of exotic copepods in the neotropics (Fig. 18).
Other exotic Asian or Afro-Asian Mesocyclops known in the Neotropical region are M. aspericornis (SUÁREZ-MORALES et al. 2011) and M. pehpeiensis (SUÁREZ-MORALES et al. 2005, MENÉNDEZ-DÍAZ et al. 2006). These exotic species have been successfully tested as biological control agents of mosquito larvae. Mesocyclops thermocyclopoides has been deemed an efficient biological control agent of mosquito larvae in Asia (KUMAR & RAO 2003) and also in the Americas (SOTO et al. 1999); its life cycle has important advantages over that of congeners in terms of maturation (SUÁREZ-MORALES et al. 2007). Other native Neotropical Mesocyclops have ranked low as potential mosquito control (TRANCHIDA et al. 2009) agents. Despite the risks involved in the introduction and spread of biological controls (SIMBERLOFF & STILLING 1996), it is expected that the success and potential use of M. thermocyclopoides for the biological control of mosquitoes could favor its spread into other tropical regions of the Americas.
ACKNOWLEDGEMENTS
We thank Chad Walter for processing the loan of specimens of the NMNH. The comments of two reviewers were useful to improve this contribution.
LITERATURE CITED
Submitted: 08.IX.2010; Accepted: 24.IX.2011.
Editorial responsibility: Marcos D.S. Tavares
- ALEKSEEV,V.; H.J. DUMONT; J. PENSAERT; D. BARIBWEGURE & J.R. VANFLETEREN. 2006. A redescription of Eucyclops serrulatus (Fischer, 1851) (Crustacea: Copepoda: Cyclopoida) and some related taxa, with a phylogeny of the E. serrulatus-group. Zoologica Scripta 35: 123-147.
- COLLADO, C.; D. DEFAYE; B.H. DUSSART & C.H. FERNANDO. 1984. The freshwater Copepoda of Costa Rica with notes on some species. Hydrobiologia 119: 89-99.
- COSTA-PAIVA, E.M. & P.C. PAIVA. 2007. A morphometric analysis of Eunice (Annelida, Polychaeta). Revista Brasileira de Zoologia 24: 353-358.
- GUO, X. 2000. Two new species of Mesocyclops from southern China and notes on the genus Mesocyclops in China. Hydrobiologia 429: 115-131.
- GUTIÉRREZ-AGUIRRE, M.A. & E. SUÁREZ-MORALES. 2001. Diversity and distribution of freshwater copepods (Crustacea) in southeastern Mexico. Biodiversity and Conservation 10: 659-672.
- GUTIÉRREZ-AGUIRRE, M.A.; J.W. REID & E. SUÁREZ-MORALES. 2003. An Afro-Asian species of Mesocyclops (Copepoda:Cyclopoida) in Central America and Mexico. Journal of Crustacean Biology 23 (2): 352-363.
- GUTIÉRREZ-AGUIRRE, M.A.; E. SUÁREZ-MORALES & A. CERVANTES. 2006. Distribución de las especies de Mesocyclops (Copepoda: Cyclopoida) en el sureste mexicano y región norte de Guatemala. Hidrobiológica 16: 259-265.
- HERNÁNDEZ-CHAVARRÍA, F. & S. SCHAPER. 2000. Mesocylops thermocyclopoides (Copepoda: Cyclopoidea): a scanning electron microscopy study. Revista Latinoamericana de Microbiología 42: 53-56.
- HOLYNSKA, M. 1994. A redescription of Mesocyclops thermocyclopoides Harada, 1931 (Copepoda, Cyclopidae). Bulletin de l'Institut Royal des Sciences Naturelles de Belgique 64: 99-110.
- HOLYNSKI, M. & F. FIERS. 1994. Mesocyclops thermocyclopoides species-group: redefinition and content. Hydrobiologia 292/293: 41-51.
- HOLYNSKA, M.; J.W. REID & H. UEDA. 2003. Genus Mesocyclops Sars, 1914, p. 12-213 In: H. UEDA & JW REID (Eds). Copepoda: Cyclopoida. Genera Mesocyclops and Thermocyclops. Guides to the Identification of the Microinvertebrates of the Contiental Waters of the world 20. Leiden, Backhuys Publishers.
- HRIBAR, L.J. & J.W. REID. 2008. New records of copepods (Crustacea) from the Florida Keys. Southeastern Naturalist 7 (2): 219-228.
- LEE, C.E. 2000. Global phylogeography of a cryptic copepod species complex and reproductive isolation between genetically proximate "populations". Evolution 54: 2014-2027.
- KUMAR, R. & R. RAO. 2003. Predation on Mosquito larvae by Mesocyclops thermocyclopoides (Copepoda: Cyclopoida) in the presence of alternate prey. International Review of Hydrobiology 88: 570-581.
- MARTEN, G.G.; E.S. BORDES & M. NGUYEN. 1994. Use of cyclopoid copepods for mosquito control. Hydrobiologia 292/293: 491-496.
- MENÉNDEZ-DÍAZ, Z.; J.W. REID; I. CASTILLO & I. VALDÉS-RAMOS. 2006. A new record of Mesocyclops pehpeiensis Hu, 1943 (Copepoda: Cyclopoida) for Cuba. Journal of Vector Ecology 31: 193-195.
- MIRABDULLAYEV, I. & D. DEFAYE. 2003. On the taxonomy of the Acanthocyclops robustus species-complex (Copepoda, Cyclopidae): Acanthocyclops brevispinosus and A. einslei sp. nov. Vestnik Zoologii 38 (5): 27-37.
- OKOLODKOV, Y.B.; R. BASTIDA-ZAVALA; A.L. IBÁÑEZ; J.W. CHAPMAN; E. SUÁREZ-MORALES; F. PEDROCHE & F.J. GUTIÉRREZ-MENDIETA. 2007. Especies acuáticas no indígenas en México. Ciencia y Mar 9: 29-67.
- REID, J.W. & R.M. PINTO-COELHO. 1994. An Afro-Asian continental copepod, Mesocyclops ogunnus, found in Brazil; with a new key to the species of Mesocyclops in South America and a review of intercontinental introductions of copepods. Limnologica 24: 359-368.
- REID, J.W. & J.F. SAUNDERS. 1986. The distribution of Mesocyclops aspericornis (von DADAY) in South America. Journal of Crustacean Biology 6: 820-824.
- SIMBERLOFF, D. & P. STILLING. 1996. Risks of species introduced for biological control. Biological Conservation 78: 185-192.
- SOTO, L.; S. SHAPER; L. ANGULO & F. HERNÁNDEZ. 1999. Mesocyclops thermocyclopoides y el control biológico de Aedes: ejemplo de un plan de acción comunitaria en Chacarita, Puntarenas. Revista Costarricense de Ciencias Médicas 20: 45-50.
- SUÁREZ-MORALES, E.; M.A. GUTIÉRREZ-AGUIRRE. 2001. Morfología y taxonomía de los Mesocyclops (Crustacea: Copepoda: Cyclopoida) de México. México, CONACYT/ECOSUR.
- SUÁREZ-MORALES, E.; J.A. MCLELLAND & J.W. REID. 1999. The planktonic copepods of coastal saline ponds of the Cayman Islands with special reference to the occurrence of Mesocyclops ogunnus Onabamiro, an apparently introduced Afro-Asian cyclopoid. Gulf Research Reports 11: 51-56.
- SUÁREZ-MORALES, E.; M.A. GUTIÉRREZ-AGUIRRE & M. ELÍAS-GUTIÉRREZ. 2003. Observations on the structure of the mandible edge in some American Mesocyclops (Copepoda: Cyclopidae). Proceedings of the Biological Society of Washington 116 (3): 742-753.
- SUÁREZ-MORALES, E.; M.A. GUTIÉRREZ-AGUIRRE & F. MENDOZA. 2011. The Afro-Asian cyclopoid Mesocyclops aspericornis (Crustacea: Copepoda) in eastern Mexico with comments on the distribution of exotic copepods. Revista Mexicana de Biodiversidad 81: 109-115.
- SUÁREZ-MORALES, E.; J.W. REID; F. FIERS & T.M. ILIFFE. 2004. Historical biogeography and distribution of the freshwater cyclopine copepods (Copepoda, Cyclopoida, Cyclopinae) of the Yucatan Peninsula, Mexico. Journal of Biogeography 31: 1051-1063.
- SUÁREZ-MORALES, E.; M. A. GUTIÉRREZ-AGUIRRE; J.L. TORRES & F. HERNÁNDEZ. 2005. The Asian Mesocyclops pehpeiensis Hu, 1943 (Copepoda, Cyclopidae) in Southeast Mexico with comments on the distribution of the species. Zoosystema 27: 245-256.
- SUÁREZ-MORALES, E.; G.A. WYNGAARD; M.A. GUTIÉRREZ-AGUIRRE & J. COSTANZO. 2007. Life history traits of Mesocyclops thermocyclopoides Harada, 1931 (Copepoda, Cyclopoida) with observations on naupliar morphology. Crustaceana 80: 1205-1222.
- TRANCHIDA, M.C.; M.V. MICIELI; A. MACIÁ & J.J. GARCÍA. 2009. Native Argentinean cyclopoids (Crustacea: Copepoda) as predators of Aedes aegypti and Culex pipiens (Diptera: Culicidae) mosquitoes. Revista de Biología Tropical 57: 1057-1068.
Datas de Publicação
-
Publicação nesta coleção
11 Nov 2011 -
Data do Fascículo
Out 2011
Histórico
-
Recebido
08 Set 2010 -
Aceito
24 Set 2011