Abstract
Migration is defined as a seasonal and cyclic population movement observed in all animal classes and studied mainly in vertebrates. A considerable part of the knowledge on migration comes from birds, for which migration is an important aspect of their biology. In the case of bats, females usually migrate larger distances than males in some species. The present study analyzes the seasonal occurrence of Pygoderma bilabiatum (Wagner, 1843) at different elevations, in order to test for a pattern that evidences migration, using data from the states of Espírito Santo, Minas Gerais, Rio de Janeiro, São Paulo, Paraná, Santa Catarina, and Rio Grande do Sul, Brazil. A total of 529 specimens of P. bilabiatum were captured. Pygoderma bilabiatum seems to be more frequent at intermediate and high elevations (over 80% of all captures were made above 250 m a.s.l.) and at latitudes above 22°S, where rainfall is high (over 1,500 mm) and temperatures are mild (16-23°C). Sex ratio varied with elevation; it was skewed towards males at lower elevations (N = 9, r² = 0.60, F = 12.311, p = 0.008, Sex ratio = 0.0004*elevation + 0.976), though females predominated at all altitudinal bands and in all states analyzed.
Atlantic Forest; Brazil; capture efficiency; seasonality; temperature
BIOLOGY
Evidence of vertical migration in the Ipanema bat Pygoderma bilabiatum (Chiroptera: Phyllostomidae: Stenodermatinae)
Carlos E. L. EsbérardI, 1 1 Corresponding author. E-mail: cesberard@superig.com.br ; Isaac P. de LimaI; Pedro H. NobreII; Sérgio L. AlthoffIII; Tássia Jordão-NogueiraIV; Daniela DiasV; Fernando CarvalhoVI; Marta E. FabiánVI; Margareth L. SekiamaVII; Artur Stanke SobrinhoIII
IDepartamento de Biologia Animal, Instituto de Biologia, Universidade Federal Rural do Rio de Janeiro. Antiga estrada Rio-São Paulo, km 47, Caixa Postal 74507, 23890-000 Seropédica, RJ, Brazil
IIDepartamento de Ciências Naturais, Universidade Federal de Juiz de Fora. Rua Visconde Mauá 300, 36015-260 Juiz de Fora, MG, Brazil
IIILaboratório de Biologia Animal, Departamento de Ciências Naturais,Universidade Regional de Blumenau. Rua Antônio da Veiga 140, 89012-900 Blumenau, SC, Brazil
IVLaboratório de Ecologia de Mamíferos, Instituto de Biologia, Universidade do Estado do Rio de Janeiro. Rua São Francisco Xavier 524, 20550-900 Rio de Janeiro, RJ, Brazil
VLaboratório de Biologia e Parasitologia de Mamíferos Silvestres Reservatórios, Fundação Oswaldo Cruz. Avenida Brasil 4365, Pavilhão Arthur Neiva, 21040-900 Rio de Janeiro, RJ, Brazil
VIDepartamento de Zoologia, Universidade Federal do Rio Grande do Sul. Avenida Bento Gonçalves 9500, 91501-970 Porto Alegre, RS, Brazil
VIIDepartamento de Ciências da Natureza, Matemática e Educação, Centro de Ciências Agrárias, Universidade Federal de São Carlos. Rodovia Anhanguera, km 174, 13600-970 Araras, SP, Brazil
ABSTRACT
Migration is defined as a seasonal and cyclic population movement observed in all animal classes and studied mainly in vertebrates. A considerable part of the knowledge on migration comes from birds, for which migration is an important aspect of their biology. In the case of bats, females usually migrate larger distances than males in some species. The present study analyzes the seasonal occurrence of Pygoderma bilabiatum (Wagner, 1843) at different elevations, in order to test for a pattern that evidences migration, using data from the states of Espírito Santo, Minas Gerais, Rio de Janeiro, São Paulo, Paraná, Santa Catarina, and Rio Grande do Sul, Brazil. A total of 529 specimens of P. bilabiatum were captured. Pygoderma bilabiatum seems to be more frequent at intermediate and high elevations (over 80% of all captures were made above 250 m a.s.l.) and at latitudes above 22°S, where rainfall is high (over 1,500 mm) and temperatures are mild (16-23°C). Sex ratio varied with elevation; it was skewed towards males at lower elevations (N = 9, r2 = 0.60, F = 12.311, p = 0.008, Sex ratio = 0.0004*elevation + 0.976), though females predominated at all altitudinal bands and in all states analyzed.
Key words: Atlantic Forest; Brazil; capture efficiency; seasonality; temperature.
Migration is defined as a seasonal and cyclic population movement (ALERSTAM & HEDENSTRÖM 1998) observed in all animal classes and studied mainly in vertebrates. A considerable part of the knowledge on migration comes from birds, for which migration is an important aspect of their biology (BISSON et al. 2009). In some bat species, females usually migrate larger distances than males (FLEMING & EBY 2003). Migratory movements may not occur in all populations of a species (MCCRAKEN et al. 1994, RUSSEL et al. 2005).
Migration occurs mainly in species that live in temperate climates, and is more common in winter (SICK 1997). It is, therefore, less frequently observed in the Neotropics (FENTON & KUNZ 1977). Migration may occur over large distances, between localities in different continents or hemispheres. There is also migration that involves only altitudinal, latitudinal or longitudinal movements; these movements, which may be regional or local, are related to resource availability (ALVES 2007).
Even for birds, local and vertical migrations are the least known and the most difficult to record (SICK 1997, MAIA-GOUVEIA et al. 2005). Some authors do not consider smaller spatial movements in bats as migration, because they do not require physiological adaptations (FLEMING & EBY 2003, BISSON et al. 2009).
However, local or regional movements in bats are also expected in the Neotropics (e.g., PASSOS et al. 2003, MELLO et al. 2009), and many authors reported changes in bat assemblages between dry and rainy seasons (e.g., MARINHO-FILHO & SAZIMA 1989, PEDRO & TADDEI 2002, AGUIAR & MARINHO-FILHO 2004, CAMARGO et al. 2009), based mainly on variations in capture rates or species richness. Horizontal, vertical and regional movements may be important to understand temporal variations in the abundance of some species, but they do not necessarily represent migration, because the whole population does not perform them in a cyclic way. Although partial migration can be more frequent than expect by this definition (CHAPMAN et al. 2011).
Little is known about the biology of the Ipanema Broad-Nosed Bat, Pygoderma bilabiatum (Wagner, 1843), a frugivorous species (GARDNER 1977) that feeds on species such as Pouteria caimito (Ruiz & Pav.) Radlk (Sapotaceae), Miconia brasiliensis (Spreng.) (Melostomataceae), Maclura tinctoria (L.) Don ex Steud (Moraceae), Ficus insipida Willd, F. enormis (Mart. ex Miq.) (Moraceae), Solanum sanctae-catharinae, S. granuloso-leprosum Dunal (Solanaceae), and Eugenia (Myrtaceae) (PERACCHI & ALBUQUERQUE 1971, WEBSTER & OWEN 1984, FARIA 1997, PASSOS et al. 2003).
The present study analyzes the seasonal occurrence of P. bilabiatum at different elevations, in order to test for a pattern that evidences migration, using published and unpublished data from southern and southeastern Brazil.
MATERIAL AND METHODS
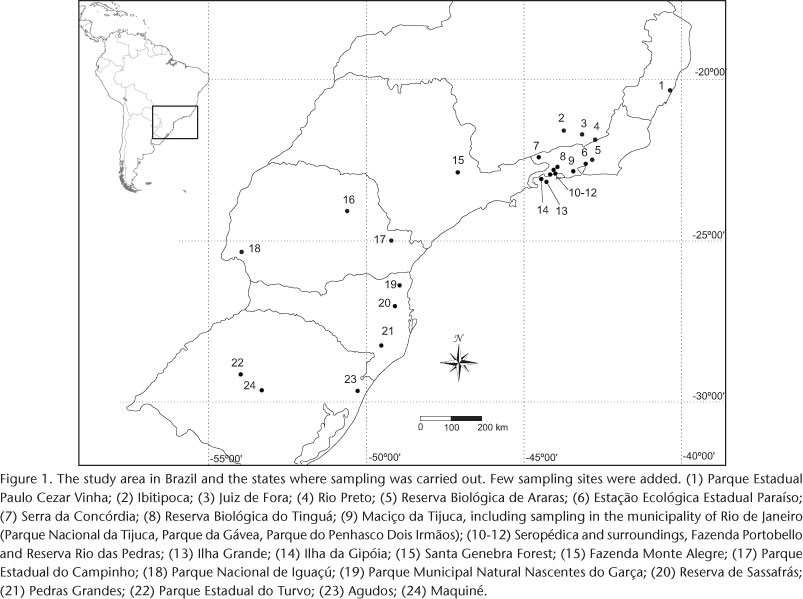
In the present study, 26 samples from 21 localities were analyzed within the sensu strictu Atlantic Forest Biome (URURAHY et al. 1983). Geographic coordinates and elevation of sampling sites are given in Tab. I and Fig. 1. The reproductive condition of females was assessed at the moment of capture and each specimen was assigned to one of the following categories: inactive female, female with palpable fetus and lactating female (e.g., COSTA et al. 2007).
Captures were grouped according to states and altitudinal bands (1-1,600 m a.s.l., in 200 m intervals). Each altitudinal band had at least 24 months of continuous sampling, with a minimum sampling effort of two nights per month, except for the bands over 1,200 m a.s.l., which were only occasionally sampled.
To test for differences in average temperatures between months when the species was present and months when the species was absent in the samples, we used a Student t test (a = 0.05). To test for the effect of rainfall on the local seasonality of P. bilabiatum a linear regression between average rainfall and the number of months when the species was captured was calculated for the 11 localities with higher number of captures.
To test whether males and females prefer different elevations, we calculated a linear regression between sex ratio, expressed as the total number of males divided by the total number of females, and elevation for nine samples with at least ten captures.
RESULTS
For the entire dataset the total sampling effort was 1,141 nights and 9,296 hours in 24 localities (Fig. 1); 529 specimens of P. bilabiatum were captured (0.47 specimens/night and 0.06 specimens/hour), at elevations that varied from 1 to 1,430 m a.s.l.
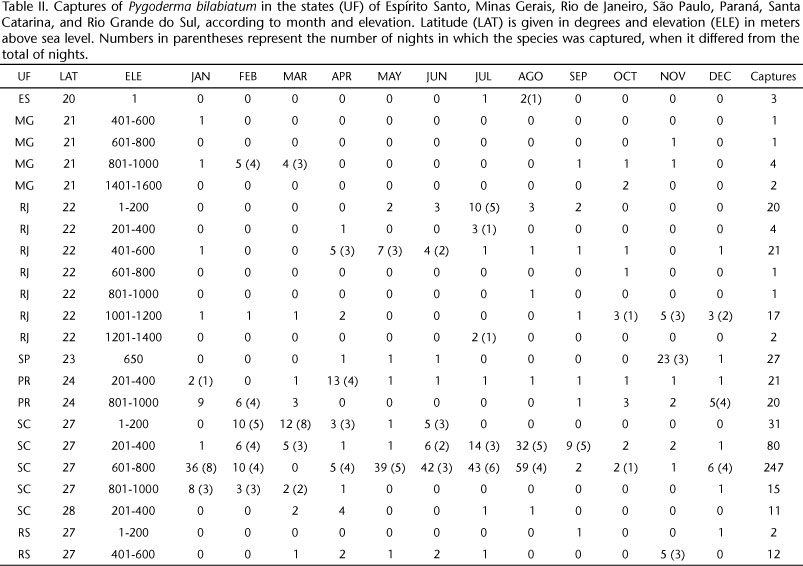
In Espírito Santo, three individuals were captured in July and August 2005: two males and one female (Tab. II). In 16 localities of Rio de Janeiro, P. bilabiatum was observed in all altitudinal bands. Three bands had few captures: 601-800 m a.s.l., 801-1,000 m a.s.l. (one capture each) and 1,200-1,400 m a.s.l. (two captures). At 1,001-1,200 m a.s.l., 17 animals were collected from January to April and from September to December, a rate of 0.03 captures/hour. In the intermediate bands, the presence of P. bilabiatum was confirmed by 21 captures at 401-600 m a.s.l. (0.010 captures/hour) and four captures at 201-400 m a.s.l. (0.01 captures/hour). Bats were captured during all months, except for February, March, June and November. At the lower band, the species was only captured in March and from May to September, with a total of 20 individuals (0.006 captures/hour).
During the dry season, P. bilabiatum was absent from elevation bands at 1,100 m a.s.l. or higher (six months), except for two individuals captured in July at 1,270 m a.s.l. However, it was found during the same period at 1-200 m a.s.l.; at this elevation there are also dry months (five months), but they are warmer. The sex ratio was 1.00 males/females at 1-200 m a.s.l, but at the other bands there was a higher frequency of females: 0.50 males/females at 201-400 m a.s.l., 0.63 males/females at 401-600 m a.s.l. and 0.42 males/females at 1,001-1,200 m a.s.l.
Out of 62 individuals analyzed, 10 females were reproductive and among them, four were pregnant: one in September, two in October and one in November. Five lactating females were found: one in August, one in October, two in November and one in December. None of the females captured at 1-200 m a.s.l. were reproductive.
In Minas Gerais, 22 specimens from three localities were analyzed, at elevations that varied from 400 to 1,430 m a.s.l. However, only at 801-1,000 m a.s.l. more than two captures of P. bilabiatum were obtained. There was a predominance of females: 0.47 males/females in the entire sample and 0.42 males/females at 801-1,000 m a.s.l. (N = 17 individuals). Reproductive activity was observed in February, March and April, with pregnant and lactating females captured at 900 m a.s.l. and a lactating female captured at 1,430 m a.s.l. in October.
In Campinas, state of São Paulo, 27 animals were captured; 19 were captured during a single night close to a M. tinctoria in fruit. Captures were carried out from April to June and from November to December. Lactating (N = 16) and pregnant (n = 5) females were observed in November.
In the Parque Nacional do Iguaçú, state of Paraná, 21 specimens were captured every month except for February (12 females and 9 males). Lactating females were observed in January, March and April. In the Fazenda Monte Alegre, a total of 20 specimens were collected at 885 m a.s.l. (nine males and 11 females), with captures concentrated from January to March and from October to December (0.06 captures/hour). Pregnant females were observed in January and lactating females in March. In the Parque Estadual de Campinho, nine individuals were captured in September, January, February and March.
In Santa Catarina, 91 individuals were captured at 201-400 m a.s.l. and 241 animals at 601-800 m a.s.l., resulting in rates of 0.12 and 0.50 captures/hour, respectively. This species was present in all months at these elevation bands. In Joinville and surroundings, 31 animals were captured at 1-200 m a.s.l. from February to June, and in the Reserva Estadual de Sassafrás 14 animals were captured from January to April. Reproductive females were observed in March, April and June at 1-200 m a.s.l.; in January, July and August at 201-400 m a.s.l.; in September, October and December at 601-800 m a.s.l.; and from January to April at 915 m a.s.l. The sex ratio observed was 0.81 males/females at 1-200 m a.s.l., 0.89 males/females at 320 m a.s.l., 0.63 males/females at 640 m a.s.l., and 0.56 males/females at 915 m a.s.l.
In Rio Grande do Sul, 12 specimens were collected at 401-600 m a.s.l. (sex ratio = 0.50 males/females) in March, April, May, June, July and November; a pregnant female was observed in November.
Pygoderma bilabiatum was present in higher sites in months with temperatures over 16°C, as shown in Tab. III for Araras (Rio de Janeiro), Telêmaco Borba (Paraná) and Juiz de Fora (Minas Gerais). At intermediate elevations in Campinas and Rio de Janeiro (401-800 m a.s.l.) there were no differences in average temperature between months when the species was present or absent. In these localities the low density of this species probably results from the lack of specimens captured in other months of the year. At low elevations, such as Rio de Janeiro at 80 m a.s.l. and Espírito Santo at 1 m a.s.l., the species was present in months with temperatures below 23°C. In Santa Catarina, where the species was present throughout the year, it occurred at average temperatures below 21°C.
All localities at intermediate and high elevation (over 600 m a.s.l.) have high rainfall (over 1,500 mm). We observed a positive relationship between the number of months in which the species was captured in each locality and its average rainfall (N = 11 localities, r2 = 0.41, F = 6.361, p = 0.033, occurrence = 0.0928*rainfall - 4.3539).
Sex ratio varied with elevation. It was skewed towards males at lower elevations (N = 9, r2 = 0.60, F = 12.311, p = 0.008, Sex ratio = 0.0004*elevation + 0.976), though females predominated at all elevation bands and in all states analyzed (Fig. 2).
DISCUSSION
Pygoderma bilabiatum seems to be more frequent at intermediate and high elevations (over 80% of all captures were observed above 250 m a.s.l.) and at latitudes above 22°S, where rainfall is high (over 1,500 m) and temperatures are mild (16-23°C). For instance, in the state of Santa Catarina (27°S), where it was the third most frequent frugivorous bat in the Parque Nacional Serra de Itajaí (313 out of 2,252 captures) at 320 and 640 m a.s.l. In Paraguay (24°S), it was also the third most frequent species (522 out of 7,725 captures (GORRESEN & WILLIG 2004, STEVENS et al. 2004), and it was more frequent in sites located between 100 and 300 m a.s.l. (WILLIG et al. 2000). At latitudes below 22°S, this species has been rarely captured (PERACCHI & ALBUQUERQUE 1993, OPREA et al. 2007, FARIA et al. 2006). All these sites have high average temperatures and low rainfall. In Santa Catarina and Rio Grande do Sul, where total rainfall is high and monthly variations are small, this species can be found at all elevations throughout the year.
Vertical migration can only be proved through recaptures of previously marked animals. However, inferring from these data, P. bilabiatum probably moves from higher regions during colder months in the states of Rio de Janeiro and Paraná; it is found at intermediate elevations throughout the year and also at sea level in the months when it is absent from the highlands. These movements of P. bilabiatum may not cover large distances, since from the base to the top of Serra do Mar in Rio de Janeiro the linear distance is shorter than 30 km. Similarly, smaller densities of Sturnira lillium (E. Geoffroy, 1810) were observed in a high locality in southeastern Brazil during the colder months, though food-plant availability was not reduced when temperatures were lower (MELLO et al. 2008).
Although the inferred partial migration (CHAPMAN et al. 2001) of P. bilabiatum populations of southeastern Brazil and Paraná from highlands to lowlands occurs during the driest four months of the year, when less than 25% of the annual rainfall occurs, rainfall seems to be less important than temperature at this latitude (22 to 24°S). Phenology of food-plants and foraging strategies of bats seem to be more important than rainfall (MELLO 2009).
If P. bilabiatum moves to intermediate or low elevations, it finds higher average temperatures of up to 9.0°C in southeastern Brazil (RAMOS et al. 2009). Pygoderma bilabiatum seems to prefer elevations with average temperatures equal or above 16-17°C and to avoid temperatures above 22-23°C in southeastern Brazil and Paraná. In Santa Catarina, the t test did not show significant differences; minimum temperatures were around 16°C both in months with and without captures. Migration to lowlands in this state would lead the population to areas with temperatures only 2°C higher. Temperature has been pointed out as a critical factor for frugivorous (SPEAKMAN & THOMAS 2003) and non-frugivorous phyllostomids (MCNAB 1973). Temperature shortens the reproductive season at higher elevations (MELLO et al. 2009). Capture rates are directly related to local density of bats (e.g., FLEMING 1988), and lower rates are observed in the coldest months, in winter (e.g., MELLO et al. 2009).
There is probably latitudinal variation in migration; it may not occur in localities with high average temperatures or it may last longer in colder climates, such as in high elevations. Further data are needed to test for latitudinal variation. There are differences between data collected in Santa Catarina and in southeastern Brazil and Paraná, as P. bilabiatum shows no seasonality at lower elevations, whereas captures in lowlands of Paraná and Rio de Janeiro were restricted to the period between March and September.
Our evidence on migration in P. bilabiatum is only circumstantial. However, the low capture frequency of P. bilabiatum observed above 800 m a.s.l. in Rio de Janeiro during the coldest months together with its high capture frequency observed at 1-200 m a.s.l. during the same period may be considered as indirect evidence of migration. The absence of P. bilabiatum above 800 m a.s.l. in winter should not be attributed to insufficient sampling effort, since even in the least sampled altitudinal band in Rio de Janeiro (1,000-1,200 m a.s.l.) sampling comprised more than 42 nights and 550 hours, and at 1-200 m a.s.l. it comprised more than 2,500 hours.
Similar migration patterns are known for birds, such as Turdus albicollis Vieillot, 1818, Platycichla flavipes (Vieillot, 1818) and Aburria jacutinga (Spix, 1825), which exchange highlands in summer for lowlands in winter (SICK 1997). The pattern described here fits the model described by COHEN (1967), in which migration is motivated by less favorable climate and by lower local food availability.
The lower the elevation the more the sex ratio is skewed towards males. This is evidence that males and females use different strategies, be it vertical migration in different seasons or preference for different elevations. Other bat species exhibit similar patterns, with samples skewed towards one sex in different seasons and in different localities, as was observed in Lasiurus cinereus (Beauvois, 1796) and Lasionycteris noctivagans (La Conte, 1831) (e.g., CRYAN 2003, KURTA 2010). Among bats there are species in which females move more than males; one explanation is that females have higher nutritional requirements and need environments with proper conditions to breed (CRYAN 2003). Elevation segregation has been already described in some insectivorous bat species (BARCLAY 1991, CRYAN et al. 2000). In the Rocky Mountains, male Myotis lucifugus (Le Conte, 1831) are more frequent in summer at proportions that reach 93% of the population, whereas females predominate in the lowlands (BARCLAY 1991). In P. bilabiatum, males seem to be more abundant at low elevations and females at intermediate and high elevations.
Different strategies adopted by each sex can be of advantage if mating occurs at lower elevations, where males are more abundant, as observed in the present study. Migration can only be considered an evolutionary advantage for the species if the benefit obtained is higher than the costs (e.g., costs of the movement itself, search for new roosts, time spent to adapt to a new foraging site) (RICHTER & GUMMING 2006).
ACKNOWLEDGEMENTS
We acknowledge the support of: the Centro de Primatologia da Fundação Estadual de Engenharia do Meio Ambiente, the Parque Nacional da Tijuca, the Prefeitura da cidade do Rio de Janeiro, the Reserva Biológica de Araras, the Reserva Biológica do Tinguá, the Centro Estadual de Desenvolvimento Sustentável da Universidade do Estado do Rio de Janeiro, the Fazenda da Gipóia - SOGIM, the Club Méd, the Santuário Serra da Concórdia, Klabin S/A (Fazenda Monte Alegre), the Parque Estadual de Ibitipoca, the Parque Nacional Serra de Itajaí, the Parque Nacional do Iguaçu and the Reserva Biológica Estadual de Sassafrás. The Fundação de Amparo à Pesquisa no Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) funded the field work (Processes E-16/170.449/07 and 471983/2007-1) of C.E.L. Esbérard. The CNPq granted C.E.L. Esbérard a research productivity fellowship (Process 301061/2007-6); FAPERJ granted C.E.L. Esbérard a 'Jovem Cientista do Nosso Estado' fellowship; FAPERJ granted I.P. de Lima a PhD and a Postdoctoral fellowship (Processes E-26/152.621/2005 and E-26/100.021/2009). The CNPq granted D. Dias a PhD scholarship (141602/2003-1); The Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) granted T. Jordão-Nogueira and F. Carvalho Master's scholarships; FURB granted S. L. Althoff a permission for completing his PhD; CNPq/PIBIC/FURB granted A. S. Sobrinho an undergrad scholarship; Fazenda Monte Alegre and Empresa de Pesquisa Agropecuária e Extensão Rural de Santa Catarina provided us with meteorological data; Deborah Faria (UESC), Monik Oprea (UFG), Dayana Bolzan (UFRRJ), Adriano L. Peracchi (UFRRJ), Ricardo Moratelli (FIOCRUZ), Júlia L. Luz (UFRRJ) and Luciana M. Costa (UFRRJ) shared unpublished data with us; L.M. Costa revised early versions of this paper. Field assistants participated in data collection for the projects of T. Jordão-Nogueira (UFRJ), Fernando Carvalho (UFRGS) and Margareth L. Sekiama (UEL), I.P. Lima (UFRRJ), P.H. Nobre (UFJF), S.L. Althoff (UFRS), D. Dias (UFRRJ) and C.E.L. Esbérard (UERJ). The study in the state of Rio de Janeiro was authorized under a special collection license from Instituto Brasileiro de Meio Ambiente (processes 1755/89-SUPES/RJ/IBAMA and 4156/95-46 AC-SUPES/DF/IBAMA), and a permanent collection license from SISBIO (10356-1) was granted to C.E.L. Esbérard. A special collection license for Reserva Biológica do Tinguá (027/2000, 042/2004 and 239/2005) was granted to D. Dias. In Minas Gerais, collection was carried out under the special licenses (19510-2, 14882-2, 14882-3, 22213-1, and 10767-2) granted to P.H. Nobre. The fieldwork in Santa Catarina was authorized under the special collection license (24580-1) for S.L. Althoff.
LITERATURE CITED
Submitted: 10.VII.2011; Accepted: 10.X.2011.
Editorial responsibility: Diego Astúa de Moraes
- AGUIAR, L.M.S. & J. MARINHO-FILHO. 2004. Activity patterns of nine phyllostomid bat species in a fragment of the Atlantic Forest in southeastern Brazil. Revista Brasileira Zoologia 21 (2): 385-390.
- ALERSTAM, T. & A. HEDENSTRÖM. 1998. The development of bird migration theory. Journal of Avian Biology 29: 343-369.
- ALVES, M.A.S. 2007. Sistemas de migrações de aves em ambientes terrestres no Brasil: exemplos, lacunas e propostas para o avanço do conhecimento. Revista Brasileira de Ornitologia 15 (2): 231-238.
- BARCLAY, R.M.R. 1991. Population structure of temperate zone bats in relation to foraging behaviour and energy demand. Journal of Animal Ecology 60 (1):165-178.
- BISSON, I-A; K. SAFI & R.A. HOLLAND. 2009. Evidence for Repeated Independent Evolution of Migration in the Largest Family of Bats. PLoS ONE 4 (10): e7504.
- CAMARGO, G.; E. FISCHER; F. GONÇALVES; G. FERNANDES & S. FERREIRA. 2009. Morcegos do Parque Nacional da Serra da Bodoquena, Mato Grosso do Sul, Brasil. Chiroptera Neotropical 15 (1): 417-424.
- CHAPMAN, B.B.; C. BRÖNMARK; J. NILSON & L. HANSSON. 2011. The ecology of partial migration. Oikos 120: 1764-1775.
- COHEN, D. 1967. Optimization of seasonal migratory behavior. American Naturalist 101: 5-17.
- COSTA L.M.; J.C. ALMEIDA & C.E.L. ESBÉRARD. 2007. Dados de reprodução de Platyrrhinus lineatus em estudo de longo prazo no estado do Rio de Janeiro. Iheringia, Série Zoologia 97 (2): 175-176.
- CRYAN, P.M. 2003. Seasonal distribution of migratory tree bats (Lasiurus and Lasionycteris) in North America. Journal of Mammalogy 84 (2): 579-593.
- CRYAN, P.M.; M.A. BOGAN & J.S. ALTENBACH. 2000. Effect of elevation on distribution of female bats in the Black Hills, South Dakota. Journal of Mammalogy 81 (3): 719-725.
- FARIA, D. 1997. Reports on the diet and reproduction of the Ipanema fruit bat (Pygoderma bilabiatum) in a Brazilian forest fragment. Chiroptera Neotropical 3 (2): 65-66.
- FARIA, D.; B.S. SOARES-SANTOS & E. SAMPAIO. 2006. Bats from the Atlantic rainforest of Southern Bahia. Biota Neotropica 6 (2): 1-13.
- FENTON, M.B. & T.H. KUNZ. 1977. Movements and behavior, p. 351-364. In: R.J. BAKER; J.K. JONES & D.C. CARTER (Eds). Biology of bats of the New World Family Phyllostomatidae. Part II. Lubbock, Texas Tech University Museum, Special Publications, vol. 13, 364p.
- FLEMING, T.H. 1988. The short-tailed fruit bat: a study in plant-animal interactions. Chicago, Chicago University Press, 365p.
- FLEMING, T.H. & P. EBY. 2003. Ecology of bat migration, p. 156-208. In: T.H. KUNZ, & M.B. FENTON, (Eds). Bat ecology. Chicago, University of Chicago Press, 779p.
- GARDNER, A.L. 1977. Feeding habits. In Biology of bats of the New World family Phyllostomidae, p. 293-350. In: Biology of bats of the New World Family Phyllostomatidae. Part II. Lubbock, Texas Tech University Museum, Special Publications, vol. 13, 364p.
- GORRESEN, P.M. & M.R. WILLIG. 2004. Landscape responses of bats to habitat fragmentation in Atlantic forest of Paraguay. Journal of Mammalogy 85 (3): 688-697.
- KURTA, A. 2010. Reproductive timing, distribution, and sex ratios of tree bats in Lower Michigan. Journal of Mammalogy 91 (3): 586-592.
- MAIA-GOUVÊA, E.R.; E. GOUVÊA & A. PIRATELLI. 2005. Comunidade de aves de sub-bosque em uma área de entorno do Parque Nacional do Itatiaia, Rio de Janeiro, Brasil. Revista Brasileira de Zooogia 22 (4): 859-866.
- MARINHO-FILHO, J.S. & I. SAZIMA. 1989. Activity patterns of six Phyllostomidae bat species in Southeastern Brazil. Revista Brasileira de Biologia 49 (3): 777-782.
- MCNAB, B.K. 1973. Energetics and distribution of vampire bats. Journal of Mammalogy 54 (1): 131-144.
- MELLO, M.A.R. 2009. Temporal variation in the organization of a Neotropical assemblage of leaf-nosed bats (Mammalia: Chiroptera). Acta Oecologica 35 (2): 280-286.
- MELLO, M.A.R.; E.K.V. KALKO & W.R. SILVA. 2009. Ambient temperature is more important than food availability in explaining reproductive timing of the bat Sturnira lillium (Mammalia: Chiroptera) in a montane Atlantic Forest. Canadian Journal of Zoology 87: 239-245.
- MELLO, M.A.R.; W.R. SILVA & E.K.V. KALKO. 2008. Diet and abundance of the bat Sturnira lilium (Chiroptera: Phyllostomidae) in a Brazilian Montane Atlantic Forest. Journal of Mammalogy 89 (2): 485-492.
- MCCRACKEN, G.F.; M. K. MCCRACKEN & M.A.T. VAWTER. 1994. Genetic Structure in Migratory Populations of the Bat Tadarida brasiliensis mexicana Journal of Mammalogy 75 (2): 500-514.
- OPREA, M.; M. POLIANA; T.B. VIEIRA; V.T. PIMENTA, D. BRITO & A.D. DITCHFIELD. 2007. Mammalia, Chiroptera, Phyllostomidae, Phyllostomus hastatus and Pygoderma bilabiatum: First occurrence in the Brazilian coastal shrubland ecosystem. Check List 3 (3): 175-179.
- PASSOS, F.C.; W.R. SILVA; W.A. PEDRO & M.R. BONIN. 2003. Frugivoria em morcegos (Mammalia, Chiroptera) no Parque Estadual Intervales, sudeste do Brasil. Revista Brasileira de Zoologia 20 (3): 511-517.
- PEDRO, W. & V.A. TADDEI. 2002. Temporal distribution of five bat species (Chiroptera, Phyllostomidae) from Panga Reserve, South-eastern Brazil. Revista Brasileira de Zoologia 19 (3): 951-954.
- PERACCHI, A.L. & S.T. ALBUQUERQUE. 1971. Lista provisória dos quirópteros dos Estados do Rio de Janeiro e Guanabara, Brasil (Mammalia: Chiroptera). Revista Brasileira de Biologia 31 (3): 405-413.
- PERACCHI, A.L. & S.T. ALBUQUERQUE. 1993. Quirópteros do município de Linhares, Estado do Espírito Santo, Brasil (Mammalia, Chiroptera). Revista Brasileira de Biologia 53 (4): 575-581.
- RAMOS, A.M.; L.A.R. SANTOS & L.T.G. FORTES. 2009. Normais Climatólogicas do Brasil 1961-1990. Brasilia, Instituto Nacional de Meteorologia, 465p.
- RICHTER, H.V. & G.S. CUMMING. 2006. Food availability and annual migration of the straw-colored fruit bat (Eidolon helvum). Journal of Zoology 268: 35-44.
- RUSSEL, A.L.; R.A. MEDELLÍN & G.F. MCCRAKEN. 2005. Genetic variation and migration in the Mexican free-tailed bat (Tadarida brasiliensis mexicana). Molecular Ecology 14 (7): 2207-2222.
- SICK, H. 1997. Ornitologia Brasileira. Rio de Janeiro, Nova Fronteira, 827p.
- SPEAKMAN, J. & D. THOMAS. 2003. Physiological ecology and energetics of bats, p. 430-492. In: T.H. KUNZ & M.B. FENTON (Eds). Bat ecology. Chicago, University of Chicago Press, 779p.
- STEVENS, R.D.; M.R. WILLIG & I.G. DE FOX. 2004. Comparative community ecology of bats in Eastern Paraguay: taxonomic, ecological, and biogeographic perspectives. Journal of Mammalogy 85 (4): 698-707.
- URURAHY, J.C.C.; J.E.R. COLLARES; M.M. SANTOS & R.A.A. BARRETOS. 1983. As regiões fitoecológicas, sua natureza e seus recursos econômicos. Estudo fitogeográfico. Rio de Janeiro, Projeto RadamBrasil, vol. 4, 780p.
- WEBSTER, D. & R.D. OWEN. 1984. Pygoderma bilabiatum Mammalian Species 220: 1-3.
- WILLIG, M.R.; S.J. PRESLEY; P.D. OWEN & C. LOPEZ-GONZALEZ. 2000. Composition and structure of bat assemblages in Paraguay: A subtropical-temperate interface. Journal of Mammalogy 81 (2): 386-401.
Publication Dates
-
Publication in this collection
17 Jan 2012 -
Date of issue
Dec 2011
History
-
Received
10 July 2011 -
Accepted
10 Oct 2011