Abstract
The aim of the present study was to describe the species composition and reproductive modes of an anuran community from a transition area between the Amazonia and Cerrado biomes. Data were collected in habitats exhibiting different degrees of anthropogenic degradation. The community (35 species) identified during the present study presented a larger number of reproductive modes when compared with those from Cerrado communities, but smaller than those of other sites in the Amazon. While all nine modes were recorded in the gallery forests of local rivers and streams, anthropogenic habitats (rubber tree orchards and soybean fields) were occupied only by species adapted to environments where humidity is low, typical of the Cerrado. Overall, the anuran fauna of the study area was characterized by species that depend on access to water bodies for their reproduction, with only a few specialized species able to reproduce in dry environments.
Amazonia; anuran community; arc of deforestation; ecotone; reproductive diversity
ECOLOGY
Species composition and reproductive modes of anurans from a transitional Amazonian forest, Brazil
Youszef O. C. BitarI,* * Corresponding author: E-mail: youszef@yahoo.com.br ; Leandra P. C. PinheiroI; Pedro S. AbeI; Maria C. Santos-CostaII
IPrograma de Pós-graduação em Zoologia, Universidade Federal do Pará/Museu Paraense Emílio Goeldi, Belém, Pará, Brazil
IIUniversidade Federal do Pará, Instituto de Ciências Biológicas, Lab. Ecologia e Zoologia de Vertebrados, Belém, Pará, Brazil
ABSTRACT
The aim of the present study was to describe the species composition and reproductive modes of an anuran community from a transition area between the Amazonia and Cerrado biomes. Data were collected in habitats exhibiting different degrees of anthropogenic degradation. The community (35 species) identified during the present study presented a larger number of reproductive modes when compared with those from Cerrado communities, but smaller than those of other sites in the Amazon. While all nine modes were recorded in the gallery forests of local rivers and streams, anthropogenic habitats (rubber tree orchards and soybean fields) were occupied only by species adapted to environments where humidity is low, typical of the Cerrado. Overall, the anuran fauna of the study area was characterized by species that depend on access to water bodies for their reproduction, with only a few specialized species able to reproduce in dry environments.
Key words: Amazonia/Cerrado; anuran community; arc of deforestation; ecotone; reproductive diversity.
Amphibians have an ample geographic distribution, and present a variety of physiological, morphological, and behavioral adaptations which allow them to occupy a wide range of habitat types. Given this, they are considered good models for ecological and evolutionary studies (Duellman & Trueb 1986).
One of the principal characteristics of amphibians is their wide array of reproductive modes, which are classified according to a range of variables, such as oviposition site, attributes of the eggs and nests, rate and duration of larval development, and type of parental care (Salthe & Duellman 1973). Due to the extreme vulnerability of early life stages (to desiccation), precipitation is the primary extrinsic factor controlling reproduction in tropical amphibians (Duellman & Trueb 1986). Beyond that, in species that depend on aquatic environments for their reproduction, the availability of water bodies is one of the main factors determining their distribution and abundance (McDiarmid 1994, Zimmerman & Bierregard 1996).
Among vertebrates, anurans display one of the greatest diversity of reproductive modes (Duellman & Trueb 1986), with a total of 39 distinct modes recorded to date, 31 of which can be found in Neotropical species (Haddad & Prado 2005). This diversity, recorded primarily in humid tropical forests, has been attributed to a combination of ecological factors, such as selective pressure from predators, high humidity, the availability of microhabitats, pond desiccation, rainfall unpredictability and the structural complexity of the vegetation (Duellman 1985, Hödl 1990, Magnusson & Hero 1991, Haddad & Prado 2005). Forest-dwelling species, for example, tend to be less dependent on aquatic environments for their reproduction, and in some cases, they may be able to reproduce in the total absence of water in terrestrial environments where humidity is high and constant (Salthe & Duellman 1973, Duellman & Trueb 1986, Duellman 1985, 1989, Hödl 1990).
Amazonian anurans tend to use terrestrial habitats more than non-forest communities, and their reproductive modes are strongly associated with this type of environment, which makes them less dependent on aquatic habitats for their reproduction than species inhabiting open areas, were eggs are more susceptible to desiccation (Hödl 1990, Haddad & Prado 2005). However, in areas of more open vegetation, anurans tend to reproduce in aquatic environments, with embryonic development occurring in the water, or in foam nests (Hödl 1990). Species that inhabit open areas with few water bodies normally have reproductive modes that are relatively resistant to dehydration, such as modes 11 (Foam nest floating on pond), 13 (Foam nest floating on water accumulated in constructed basins) and 30 (Foam nest with eggs and early larval stages in subterranean constructed nests and subsequent to flooding). Those modes are found in species of Physalaemus and Leptodactylus (Hödl 1990, Haddad & Prado 2005, Vieira et al. 2009).
The reproductive strategies of anurans living in environments characterized by open vegetation and a pronounced dry period, such as the Amazonian savannas and transition areas within the Cerrado biome, have been poorly studied and are consequently not well understood (Neckel-Oliveira et al. 2000, Barbosa et al. 2005). Differences between environments may result in communities with different compositions and distinct reproductive adaptations (Duellman & Trueb 1986, Hödl 1990, Haddad & Prado 2005). Changes in the landscape, such as deforestation and habitat fragmentation, may also lead to changes in the reproductive modes found in a particular region, local species extinction, and possibly more specialized reproductive modes (e.g. Bernarde & Macedo 2008). However, it is also possible to observe reproductive plasticity in amphibians that do not succumb in the face of environmental changes, for instance Dendropsophus ebraccatus (Cope, 1874). This species can lay eggs both in water bodies and on terrestrial habitats, depending on environmental conditions, and select oviposition sites in response to factors that can promote egg desiccation (Touchon & Warkentin 2008).
Given these considerations, the present study focused on the anuran communities in an ecotonal region between the Amazonian and Cerrado biomes in the Brazilian state of Mato Grosso. Species composition and the diversity of reproductive modes were compared among the different environments found within the transitional forest in order to describe the relationship between reproductive modes and habitat.
MATERIAL AND METHODS
The study was carried out at Tanguro farm (12°54'S, 52°22'W), located in the municipality of Querência, state of Mato Grosso, Brazil (Fig. 1), in an area of transition between the Amazon and Cerrado biomes. This site is located within the "Arc of Deforestation", at the southern rim of the Amazon basin, which suffers constant pressure from ranchers and soybean growers. The property has a total area of 82,000 ha, of which approximately 44,000 ha are covered with primary forest or regenerating gallery forest along the headwaters of the Tanguro and Darro Rivers, the region's main rivers. They are part of the upper Xingu River Basin, with the Tanguro flowing directly into the Xingu, and the Darro into the Suiá-miçu River (ANA 2002).
The region's climate can be classified as tropical with a well-defined dry season, i.e., precipitation of less than 100 mm in the driest month, and mean temperature above 18°C in the coldest month (Peel et al. 2007). The mean annual precipitation is approximately 1,900 mm, and there are two well-defined seasons, with the peak of the dry season occurring between June and September, and intense rains between December and March. The other months are considered transitional periods (IPAM 2007).
As a result of intense agricultural activities, the Tanguro farm encompasses a mosaic of various environments with ecologically distinct characteristics. Forest areas are characterized by relatively low vegetation diversity and reduced canopy height in comparison with the typical Amazonian forest (Ivanauskas et al. 2004). Within the study area, the forest is mostly located within permanent reserves along watercourses, which vary in width from 30 m to more than 1 km. These reserves are located along small streams and springs, which overflow to form small marginal pools in the rainy season (Fig. 2), and along the Tanguro River itself (Fig. 3), which has steep banks where small lentic water bodies may form in the rainy season, as a result of the emersion of the water table.
Rubber tree orchards (Hevea brasiliensis) for the extraction of latex are also found within the study area (Fig. 4). These orchards are characterized by the lack of undergrowth, and leaf litter composed only of the detritus of H. brasiliensis. The property also has a large area of approximately 38,000 ha dedicated primarily to the cultivation of soybean (Fig. 5), with a number of buildings, including housing, grain silos, and storage facilities, as well as unpaved roads. The soil of the soybean fields is constantly subjected to modifications from planting to harvesting, including plowing and the application of pesticides and herbicides. All water bodies in the rubber tree orchard and soy fields are temporary. They are formed by the accumulation of rainwater, and have no marginal vegetation (Figs 4 and 5).
Data were collected during seven excursions between March 2006, and February 2009. The sites were selected according to the presence of water bodies, and represented all different environments within the study area (gallery forests of springs and streams, margins of the Tanguro River, rubber tree orchards, and open areas).
The first five excursions lasted on 30 days each, on average, and were conducted between March 2006, and July 2008. They took place in four preserved areas, which consisted of permanent forest reserves centered on perennial watercourses. Specimens were captured by active searchand pitfall traps. The latter consisted of five 60-liter buckets set in lines of 40 m. Three traps were set in each forest reserve. During the two final excursions (between December 2008, and February 2009), which lasted about 45 days each, active searches were based on both visual and auditory cues (during nocturnal sampling), and were conducted in 54 half-hectare plots distributed among the four principal environments identified within the study area (gallery forest, rubber orchard, margin of the Tanguro river, and areas cleared for soybean cultivation). The species inventory was supplemented by specimens encountered in other parts of the study area, such as the base camp, roadsides, and dams.
The reproductive mode of each species was classified according to the scheme proposed by Haddad & Prado (2005), and complemented by observations in the field. Additional information on the reproductive modes of the species was obtained from Heyer (1969, 1974), Duellman (1985), Hödl (1990), Hero (1990), Zimmerman & Simberloff (1996), Eterovick & Sazima (2000), Prado et al. (2002, 2005a, b), Kokubum & Giaretta (2005), Pombal & Haddad (2005, 2007), and Silva & Giaretta (2008, 2009). Some of the species identified in this study, in particular those with an ample geographic distribution _ e.g. Leptodactylus andreae Müller, 1923, Pristimantis aff. fenestratus and Rhaebo guttatus (Schneider, 1799) _, may represent members of more than one specie, masked on a single taxon. Given this, the identification of the reproductive mode of these species was based on observations of their field behavior and, when the mode had not been previously recorded, it was classified according to the mode displayed by similar species in the same taxonomic group.
RESULTS
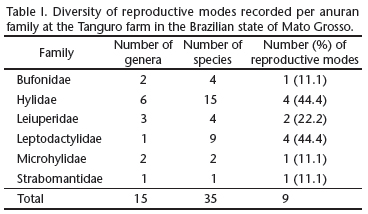
A total of 35 anuran species were recorded in the study area, with nine different reproductive modes (modes 1, 2, 4, 11, 13, 23, 24, 30, and 32). Hylidae and Leptodactylidae were the best represented families in terms of the number of species (15 and 9, respectively), and also in the number of reproductive modes, each with four (Tab. I). All other families, except the Leiuperidae (n = 2), had only a single reproductive mode.
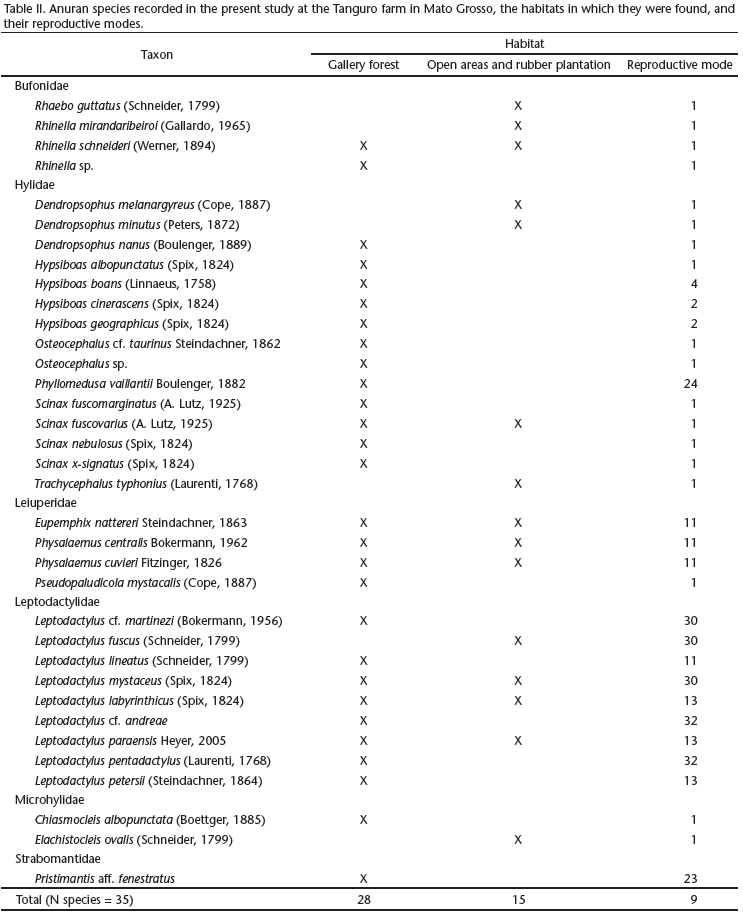
The use of lentic water bodies (mode 1) for oviposition and tadpole development was recorded in 51.4% of the species (Tab. II), all of which belonged to Hylidae, Bufonidae, Microhylidae or Leiuperidae. Mode 11 _ oviposition in aquatic foam nests _ was the second most common (11.4%), and was found in Leptodactylus lineatus (Schneider, 1799), and all the members of the Leiuperidae, with the exception of Pseudopaludicola mystacalis (Cope, 1887), which presented mode 1. Three species of leptodactylids (8.6%) presented mode 30, with foam nests placed in subterranean nests and aquatic tadpoles, while three others deposited foam nests in aquatic depressions (mode 13). The remaining modes were represented by only one (modes 4, 23, and 24) or two species (modes 2 and 32).
A total of 28 species with nine reproductive modes were recorded in gallery forest, where species of the families Hylidae and Leptodactylidae presented the greatest reproductive diversity, each with four modes (44.4%). Most of the species observed in gallery forest (71.4%, n = 20) had more specific reproductive habitat preferences, and were restricted to these environments (Tab. II). These species included P. aff. fenestratus (mode 23), Leptodactylus pentadactylus (Laurenti, 1768) (mode 32), L. cf. andreae (mode 32), Phyllomedusa vaillantii Boulenger, 1882 (mode 24), and Hypsiboas boans (Linnaeus, 1758) (mode 4).
In the rubber orchard and open areas, 15 species were recorded, seven of which were exclusive to these habitats (Tab. II). Hylidae and Leptodactylidae were each represented by four species, the Bufonidae and Leiuperidae by three, and the Microhylidae by only one. Reproductive mode 1 was the most common, being recorded in eight species (53%). This mode, and the others recorded in these environments (11, 13, and 30) require water bodies for eggs or larvae to develop.
DISCUSSION
The anuran fauna of the Tanguro farm was characterized by reproductive modes typical of forest habitats (modes 23, 24, and 32), as well as those characteristic of open areas, with adaptations for low precipitation and humidity, high temperatures, and limited access to water, such as modes 1, 11, and 30. The presence of reproductive modes with distinct characteristics in different habitats may be the result of the coexistence, within the study area, of species from the two biomes. By contrast, P. vaillantii, Pristimantis aff. fenestratus, Leptodactylus paraensis Heyer, 2005 and H. boans, are typically distributed in the Amazon forest, H. albopunctatus, Physalaemus cuvieri Fitzinger, 1826, P. centralis, Eupemphix nattereri Steindachner, 1863, and Scinax fuscovarius (A. Lutz, 1925) are typical of the Cerrado. This finding also reinforces the classification of the area as part of the transitional forest formation between these biomes (IBGE 2005). The observed syntopy among these species may be further reinforced by the diversity of habitats found within the study area, which would favor species with different ecological requirements.
The diversity of reproductive modes recorded at the study site (nine modes) also reflects a pattern that is intermediate between those of the Amazon forest and the Cerrado. Amazonian rainforest sites tend to be relatively diverse with regards to reproductive modes. For instance, twelve modes were recorded at both the Adolpho Ducke Forest Reserve in the Brazilian state of Amazonas (Lima et al. 2006) and Serra do Divisor, in Acre (Souza 2009). In the Cerrado, by contrast, only four modes were recorded at Rio Claro, São Paulo (Toledo et al. 2003), six at Serra do Cipó, in Minas Gerais (Eterovick & Sazima 2004), and seven in Silvânia, Goiás (Bastos et al. 2003). The diversity of reproductive modes in different areas may be related to environmental factors, rather than just the similarity in species composition. This is because any resemblance in species composition does not necessarily reflect similarities in a region's reproductive diversity. For example, in the Caatinga, Vieira et al. (2009) recorded only a few of the species observed in the present study, but identified seven of the reproductive modes encountered here. By contrast, the species composition recorded by studies in Cerrado-dominated vegetation in Rondônia is more similar to that of the present study (14 shared species), but once again, with seven reproductive modes in common (Bernarde & Macedo 2008, Turci & Bernarde 2008). In other words, even though distinct sets of species may be present at different sites, the similarities in their reproductive modes may be determined by the ecological characteristics of the site.
In the present study, the greatest diversity of reproductive modes was recorded in the families Hylidae and Leptodactylidae, a pattern also observed at a number of other Neotropical sites (Zimmerman & Simberloff 1996, Haddad & Prado 2005, Vieira et al. 2009). The relative diversity of reproductive modes in these two families results from a combination factors such as the high diversity of species and the ecological adaptations these species display to a variety of habitats, most of which have to do with the colonization of terrestrial environments (Duellman 1985). Morphological and reproductive adaptations allow these species to occupy habitats successfully (Haddad & Prado 2005, Pombal & Haddad 2007), as observed in the Brazilian Atlantic Forest, where the hylids presented four arboreal reproductive modes and three reproductive modes associated with the forest floor, whereas the leptodactylids had only three modes associated with the vegetation, and 10 modes adapted to terrestrial environments (Haddad & Sawaya 2000, Haddad & Prado 2005).
The large number of species that depend on water for all reproductive phases (25 species with modes 1, 2, 4 or 11) or for the larval development (three species with mode 30) indicates that the local fauna is primarily adapted to habitats with sparse vegetation, unpredictable precipitation patterns, and low atmospheric humidity (Duellman 1989, Hödl 1990, Magnusson & Hero 1991, Haddad & Prado 2005, Vieira et al. 2009). In the present study, some reproductive modes _ the most specialized and dependent on specific habitat types _ were recorded only in gallery forest with perennial water bodies, in forest-dwelling species typical of the Amazon basin (Tab. II). The conditions found in the gallery forest, such as the larger number of microhabitats available for oviposition and the higher humidity, likely account for the occurrence of more specialized reproductive modes in these areas, especially those that encompass non-aquatic oviposition (modes 24 and 32), or direct development of terrestrial eggs, i.e., mode 23 (Hödl 1990, Zimmerman & Simberloff 1996, Pearman 1997). This is because the diversity of specialized reproductive modes, and of modes that do not depend on the water tend to be greater in environments that have more humidity and a greater diversity of microhabitats (Duellman 1989, Hödl 1990, Haddad & Prado 2005, Pombal & Haddad 2005).
Haddad & Prado (2005) have found that anuran species typical of the Cerrado will commonly colonize areas of the Atlantic Forest following deforestation, because these species are well adapted to more open conditions. Invasive species are more resistant to dehydration than forest-dwelling species, and tend to have either more generalist reproductive modes (1 and 2), or strategies that are better adapted to insolation (modes 11, 13, 30, 31, and 32). A situation of this type was recorded in the present study: species with reproductive modes adapted for open areas, such as Physalaemus centralis (mode 11), P. cuvieri (mode 11), E. nattereri (mode 11), S. fuscovarius (mode 1), Elachistocleis ovalis (Schneider, 1799) (mode 1), Rhinella mirandaribeiroi (Gallardo, 1965) (mode 1), and R. schneideri (mode 1), were found in the rubber orchard and soybean fields. Mode 1 was the most common in these anthropogenic habitats, and occurred with specific adaptations to unpredictable precipitation, such as pigmented eggs, which absorb more heat and energy, and thus accelerate the development of the embryo (Crump 1974, León 1975).
Other adaptations can be observed in the families Leptodactylidae and Leiuperidae, whose members deposit their eggs in foam nests. Regardless of the type of larval development, this characteristic can be considered a first step towards a more terrestrial mode of reproduction. The foam has a number of functions that are essential in this type of habitat, such as protection against predators (Downie 1990, Menin & Giaretta 2003), protection from dehydration (Heyer 1969, Magnusson & Hero 1991), maintenance of the oxygen level appropriate for the eggs (Seymour & Loveridge 1994), and temperature control (Dobkin & Gettinger 1985), as well as providing a source of food for the tadpoles of some species (Tanaka & Nishihara 1987). Furthermore, species in the Leptodactylus pentadactylus group, such as L. labyrinthicus, exhibit extra strategies that guarantee larval survival in unpredictable habitats, such as trophic eggs to feed the larvae (e.g. Prado et al. 2005a, Silva & Giaretta 2008).
Overall, while the study area has been included in the Amazon biome (IBGE 2005), the Cerrado has a clear influence on the local landscapes. The local anuran fauna is characterized by species typical of both biomes. However, while species with reproductive modes that do not depend on the water are found mainly in gallery forests, a clear difference was found between habitat types: typical Cerrado species predominated in the disturbed habitats, as they are able to better tolerate the conditions imposed by more open environments (Vieira et al. 2009), and the Amazonian species are more common in forested areas. Given this, it is clear that the preservation of areas of forest with access to water bodies and humid microhabitats, such as the gallery forests of the Tanguro, is essential for the maintenance of anuran diversity in these transitional forests of the Amazon-Cerrado ecotone.
ACKNOWLEDGEMENTS
The authors are grateful to O. Portela and A. Portela, A. Pedroso (Bibal), J. Serrão (Donga), Darlison and Sandro for their invaluable assistance in the field, to O. Carvalho Jr and W. Silva of IPAM (Institute for Amazonian Environmental Research) for infrastructure and logistic support during fieldwork. We thank M. Kokubum and U. Galatti for their help with the identification of the reproductive mode of Leptodactylus martinezi (Bokermann, 1956) and Leptodactylus gr. pentadactylus, M. Hoogmoed for the identification and confirmation of some of the species, and L.P.P. Albarelli de Castro for help with the map. We also thank two anonymous reviewers that provided valuable comments and helped us improve the manuscript. We are grateful to CAPES and CNPq for the grants provided, and to PPG7 (MCT/CNPq/PPG7 48/2005) for financing part of the fieldwork.
LITERATURE CITED
Submitted: 11.VII.2011;
Accepted: 22.XII.2011.
Editorial responsibility: Mauricio O. Moura
- ANA. 2002. Bacia Amazônica. Agência Nacional de Águas. Available online at: http//www.ana.gov.br [Accessed: 02/I/2009]
- Barbosa, R.I.; H.A.M. Xaud; & J.M.C. Souza. 2005. Savanas de Roraima: Etnoecologia, biodiversidade e potencialidades agrosilvopastoris. Boa Vista, FEMACT, 202p.
- Bastos, R.P.; J.A.O. Motta; L.P. Lima & L.D. Guimarães. 2003. Anfíbios da Floresta Nacional de Silvânia, Estado de Goiás. Goiânia, Stylo, 82p.
- Bernarde, P.S. & L.C. Macedo. 2008. Impacto do desmatamento e formação de pastagens sobre a anurofauna de serapilheira em Rondônia. Iheringia, Série Zoológica 98 (4): 454-459.
- Crump, M.L. 1974. Reproductive strategies in a tropical anuran community. Miscellaneous Publication of Museum Natural History University of Kansas 61: 1-68.
- Dobkin, D.S. & R.D. Gettinger. 1985. Thermal aspects of anuran foam nests. Journal of Herpetology 19: 271-275.
- Downie, J.R. 1990. Functions of the foam in foam-nesting leptodactylids: Anti-predator effects of Physalaemus pustulosus foam. Herpetological Journal 1: 501-503.
- Duellman, W.E. 1985. Reproductive modes in anuran amphibians: phylogenetic significance of adaptive strategies. South African Journal of Science 81: 174-178.
- Duellman, W.E. 1989. Alternative life-history styles in anuran amphibians: Evolutionary and ecological implications; p. 101-126. In: M.N. Bruton (Ed.). Alternative Life-History Styles of Animals. Dordrecht, Kluwer Academic Publishers.
- Duellman, W.E. & L. Trueb. 1986. Biology of Amphibians. New York, McGraw-Hill, 670p.
- Eterovick, P.C. & I. Sazima. 2000. Description of the tadpole of Leptodactylus syphax, with a comparison of morphological and ecological characters of tadpoles and adults of the species in the Leptodactylus pentadactylus group (Anura, Leptodactylidae). Amphibia- Reptilia 21: 341-350.
- Eterovick, P.C. & I. Sazima. 2004. Amphibians from the Serra do Cipó, Minas Gerais, Brazil. Belo Horizonte, PUC Minas.
- Haddad, F.B. & R.J. Sawaya. 2000. Reproductive modes of Atlantic Forest hylid frogs: a general overview and description of a new mode. Biotropica 32 (4): 862-871.
- Haddad, C.F.B. & C.P.A. Prado. 2005. Reproductive modes in frogs and their unexpected diversity in the Atlantic Forest of Brazil. Bioscience 55 (3): 207-217.
- Hero, J.M. 1990. An illustrated key to tadpoles occurring in the Central Amazon rainforest, Manaus, Amazonas, Brazil. Amazoniana 11: 201-262.
- Heyer, W.R. 1969. The adaptive ecology of the species groups of the genus Leptodactylus (Amphibia, Leptodactylidae). Evolution 23: 421-428.
- Heyer, W.R. 1974. Relationships of the marmoratus species group (Amphibia, Leptodactylidae) within the subfamily Leptodactylinae. Contributions in Science of the Natural History Museum of the Los Angeles County 253: 1-46.
- Hödl, W. 1990. Reproductive diversity in Amazonian lowland frogs. Fortschr Zool 38: 41-60.
- IBGE. 2005. Mapa de biomas e mapa de vegetação do Brasil Instituto Brasileiro de Geografia e Estatística. Available online at: http//www.ibge.gov.br [
- IPAM. 2007 Instituto de Pesquisa Ambiental da Amazônia. Available online at: http//www.ipam.org.br [
- Ivanauskas, N.M.; R. Monteiro & R.R. Rodrigues. 2004. Estrutura de um trecho de floresta Amazônica na bacia do alto rio Xingu. Acta Amazonica 34 (2): 281-305.
- Kokubum, M.N.C. & A.A. Giaretta. 2005. Reproductive ecology and behaviour of a species of Adenomera (Anura, Leptodactylidae) with endotrophic tadpoles: systematic implications. Journal of Natural History 39: 1745-1758.
- León, J.R. 1975. Desarrollo temprano y notas sobre la historia natural de la larva de Hyla x-signata (Amphybia: Hylidae). Caribbean Journal of Science 15 (1-2): 57-65.
- Lima, A.P.; W.E. Magnusson; M. Menin; L.K. Erdtmann; D.J. Rodrigues; C. Keller & W. Hödl. 2006. Guia de sapos da reserva Adolpho Ducke. Manaus, Áttema Design Editorial, 168p.
- Magnusson, W.E. & J.M. Hero. 1991. Predation and evolution of complex oviposition behaviour in Amazon rainforest frogs. Oecologia 86: 310-318.
- McDiarmid, R.W. 1994. Amphibian diversity and natural history: An overview, p. 5-15. In: W.R. Heyer; M.A. Donnelly; R.W. McDiarmid; L.A.C. Hayek & M.S. Foster (Eds). Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians. Washington, D.C., Smithsonian Institution Press.
- Menin, M. & A.A. Giaretta. 2003. Predation on foam nests of leptodactyline frogs (Anura: Leptodactylidae) by larvae of Beckeriella niger (Diptera: Ephydridae). Journal of Zoology 26: 1-5.
- Neckel-Oliveira, S.; W.E. Magnusson; A.P. Lima & L.K. Albernaz. 2000. Diversity and distribution of frogs in an Amazonian savanna in Brazil. Amphibia-Reptilia 21: 317-326.
- Pearman, P.B. 1997. Correlates of amphibians diversity in an altered landscape of Amazon Acuador. Conservation Biology 11: 1211-1225.
- Peel, M.C.; B.L. Finlayson & T.A. Mcmahon. 2007. Updated world map of the Köppen-Geiger climate classification. Hydrology Earth System Science 11: 1633-1644.
- Pombal Jr, J.P. & C.F.B. Haddad. 2005. Estratégias e modos reprodutivos de anuros (Amphibia) em uma poça permanente na Serra de Paranapiacaba, sudeste do Brasil. Papéis Avulsos de Zoologia 45 (15): 201-213.
- Pombal Jr, J.P. & C.F.B. Haddad. 2007. Estratégias e Modos reprodutivos em Anuros, p. 101-116. In: L.B. Nascimento & M.E. Oliveira (Eds). Herpetologia no Brasil II. Belo Horizonte, Sociedade Brasileira de Herpetologia.
- Prado, C.P.A.; M. Uetanabaro & C.F.B. Haddad. 2002. Description of a new reproductive mode in Leptodactylus (Anura, Leptodactylidae), with a review of the reproductive specialization toward terrestriality in the genus. Copeia 4: 1128-1133.
- Prado, C.P.A.; L.F. Toledo; J. Zina; & C.F.B. Haddad. 2005a. Trophic eggs in the foam nests of Leptodactylus labyrinthicus (Anura, Leptodactylidae): an experimental approach. Herpetological Journal 15: 279-284.
- Prado, C.P.A.; M. Uetanabaro & C.F.B. Haddad. 2005b. Breeding activity patterns, reproductive modes, and habitat use by anurans (Amphibia) in a seasonal environment in the Pantanal, Brazil. Amphibia-Reptilia 26 (2): 211-221.
- Salthe, S.N. & W.E. Duellman. 1973. Quantitative constraints with reproductive mode in anurans, p. 229-249. In: J.L. Vial (Ed.). Evolutionary biology of the anurans. Columbia, University of Missouri Press.
- Seymour, R.S. & J.P. Loveridge. 1994. Embryonic and larval respiration in the arboreal foam wests of the African frog Chiromantis xerampetina Journal of Experimental Biology 197: 31-46.
- Silva, W.R. & A.A. Giaretta. 2008. Further notes on the natural history of the South American pepper frog, Leptodactylus labyrinthicus (Anura, Leptodactylidae). Brazilian Journal of Biology 68: 403-407.
- Silva, W.R. & A.A. Giaretta. 2009. On the natural history of Leptodactylus syphax with comments on the evolution of reproductive features in the L. pentadactylus species group (Anura, Leptodactylidae). Journal of Natural History 43: 191-203.
- Souza, M.B. 2009.Anfíbios: reserva extrativista do Alto Juruá e Parque Nacional da Serra do Divisor, Acre. Campinas, IFCH, 77p.
- Tanaka, S. & M. Nishihara. 1987. Foam nest as a potential food source for anuran larvae: A preliminary experiment. Journal of Ethology 5: 86-88.
- Toledo, L.F.; J. Zina & C.F.B. Haddad. 2003. Distribuição especial e temporal de uma comunidade de anfíbios anuros do município de Rio Claro, São Paulo, Brasil. Holos Environment 3 (2): 136-149.
- Touchon, J.C. & K.M. Warkentin. 2008. Reproductive mode plasticity: aquatic and terrestrial oviposition in a treefrog. Proceedings of the National Academy of Sciences 105: 7495-7499.
- Turci, L.C.B. & O.S. Bernarde. 2008. Levantamento herpetofaunístico em uma localidade no município de Coacal, Rondônia, Brasil. Bioikos 22 (2): 101-108.
- Vieira, W.L.S.; G.M. Santana & C. Arzabe. 2009. Diversity of reproductive modes in anurans communities in the Caatinga (dryland) of northeastern Brasil. Biodiversity and Conservation 18: 55-66.
- Zimmerman, B.L. & R.O. Bierregaard. 1986. Relevance of the equilibrium theory of island biogeography and species _ area relations to conservation with a case from Amazonia. Journal of Biogeography 13: 133-143.
- Zimmerman, B.L. & D. Simberloff. 1996. An historical interpretation of habitat use by frogs in a Central Amazonian forest. Journal of Biogeography 23: 27-46.
Publication Dates
-
Publication in this collection
15 Mar 2012 -
Date of issue
Feb 2012
History
-
Received
11 July 2011 -
Accepted
22 Dec 2011