Abstract
Line-transect surveys are commonly used for sampling large mammals, but estimates of the effort needed to reliably surveying low-diversity assemblages of mammals are scarce. Using data from line-transects and species accumulation curves, I examined whether or not a sampling effort previously suggested to survey mammals elsewhere (ca. 85-100 km) would be satisfactory for surveying a low-diversity assemblage of large mammals in the Rio Negro basin in northern Amazonia. In total, 14 mammals were recorded after an accumulated effort of 690 km walked. The desired threshold of completeness was only achieved in one of six transects after an average effort of 115 km surveyed. Considering the entire landscape (all transects pooled), survey completeness was reached after a much higher effort. Moreover, the theoretical effort required to achieve completeness was estimated to be 150-360 km per transect, and 512 km for the landscape. Further studies are required to fully understand this issue, but meanwhile it is safest to assume that higher sampling efforts should be employed when surveying low-diversity assemblages through diurnal line-transects in northwestern Amazonia to get robust estimates of mammal richness.
Amazonia; species accumulation curves; species richness; survey
SHORT COMMUNICATION
How much effort should be employed for surveying a low-diversity Amazonian mammal assemblage using line-transects?
Ítalo Mourthé
Núcleo de Pesquisas de Roraima, Instituto Nacional de Pesquisas da Amazônia. Rua Coronel Pinto 315, Centro, 69301-150 Boa Vista, Brasil. Email: imourthe@gmail.com
ABSTRACT
Line-transect surveys are commonly used for sampling large mammals, but estimates of the effort needed to reliably surveying low-diversity assemblages of mammals are scarce. Using data from line-transects and species accumulation curves, I examined whether or not a sampling effort previously suggested to survey mammals elsewhere (ca. 85-100 km) would be satisfactory for surveying a low-diversity assemblage of large mammals in the Rio Negro basin in northern Amazonia. In total, 14 mammals were recorded after an accumulated effort of 690 km walked. The desired threshold of completeness was only achieved in one of six transects after an average effort of 115 km surveyed. Considering the entire landscape (all transects pooled), survey completeness was reached after a much higher effort. Moreover, the theoretical effort required to achieve completeness was estimated to be 150-360 km per transect, and 512 km for the landscape. Further studies are required to fully understand this issue, but meanwhile it is safest to assume that higher sampling efforts should be employed when surveying low-diversity assemblages through diurnal line-transects in northwestern Amazonia to get robust estimates of mammal richness.
Key words: Amazonia; species accumulation curves; species richness; survey.
Conducting complete and reliable inventories of mammal assemblages in Neotropical forests is challenging, particularly during surveys limited in time and space. Increasing the sampling effort should improve the chances of recording a representative number of species (i.e., achieve survey completeness), but the necessary effort required to adequately survey sparsely distributed species can be impeditive (MCARDLE 1990). Many methods have been used to survey large mammals (SILVEIRA et al. 2003), line-transect surveys being one of the most frequently used in Neotropical forests. This method allows the coverage of large areas within short time periods at relatively low cost, and provides information on a broad range of species (PERES 1999), being an useful tool to estimate species richness of large vertebrates (DE THOISY et al. 2008). Species richness is a simple but fundamental measurement of community and regional diversity that underlies many ecological models and conservation strategies (GOTELLI & COLWELL 2001). To address some ecological questions or to evaluate rapid-assessment inventories it is important to know to what extent an assemblage was reliably sampled. However, while line-transects are widely used due to its practicality, estimates of the effort needed to adequately survey mammal assemblages using this method are scarce (e.g., DE THOISY et al. 2008), particularly in low-diversity sites.
Despite the high diversity of most Amazonian localities, some areas are known to harbor relatively few species and low abundance, as those found in the Rio Negro basin in northwestern Amazonia (MENDES PONTES et al. 2012). This paucity is probably due to the low soil fertility and low productivity (EMMONS 1984). Surveying low-diversity assemblages (i.e., those with relatively low species richness and low numbers of individuals), should be particularly hard because many species usually have low densities, increasing the difficulty for recording new individuals and, consequently, new species (EMMONS 1984, GOTELLI & COLWELL 2001). Therefore, surveys on these low-diversity assemblages might require higher sampling efforts, but there are no estimates of the amount of effort required to achieve mammal survey completeness in those sites where such assemblages occur. I tested whether the minimum sampling effort suggested to achieve survey completeness elsewhere in Amazonia (e.g., 85-100 km; DE THOISY et al. 2008) is enough to survey a low-diversity mammal assemblage (MENDES PONTES 2004) in one locality in the Rio Negro basin, northwestern Amazonia. This basin extends over a large area with little human disturbance, thus showing high conservation potential (MENDES PONTES et al. 2012).
The study of the diurnal, mid- to large-sized mammal assemblage was carried out at Maracá Ecological Station (MES; 3°21'44"N, 61°26'01"W), a large riverine tropical forest island (ca. 1013 km2) located in the state of Roraima, Brazil (MENDES PONTES 2004). Mean annual rainfall is ~2100 mm, with a pronounced dry season (Oct-Mar). The predominant vegetation found in the eastern part of the island where this study was carried out is the upland terra-firme forest, interspersed with small portions of savanna and swamps dominated by Mauritia flexuosa palms (MILLIKEN & RATTER 1998, MENDES PONTES 2004). This undisturbed forest has a canopy height of ca. 25-35 m, and can reach up to 54 m2/ha basal area of trees $10 cm diameter at breast height (dbh). Tree species diversity is not high by Amazonian standards, and only 80 spp. $10 cm dbh were recorded in a 1.5 ha plot (MILLIKEN & RATTER 1998). The mammal assemblage at MES is relatively species-poor and many species have low densities (MENDES PONTES 2004), compared to other mammal assemblages elsewhere (JANSON & EMMONS 1990, MALCOLM 1990, HAUGAASEN & PERES 2005, DE THOISY et al. 2008).
I conducted diurnal line-transect surveys from April 2009 to April 2010 on six 5 km-long, east-west transects, arranged in a 5 × 5 km grid, positioned at the eastern part of the island; transects were surveyed twice every month, totaling 690 km. Adjacent transects were located 1 km apart and ran parallel to each other. The suggested minimum sampling effort to estimate the species richness of large mammals using line-transects (ca. 85 km; DE THOISY et al. 2008) was exceeded in all transects (mean = 115 km; Table I). Mammal surveys were conducted following a standardized protocol (PERES 1999). In brief, two consecutive one-way transects were surveyed at ~1.5 km/h on a daily basis from 06:30-10:30 h and 13:00-17:00 h. Transects were surveyed in sequence and consecutive surveys along the same transects were made in alternating directions at 3-6 d intervals. This rotation schedule was used to minimize any temporal bias and maximize the independence of records.
Initially, I generated extrapolated species richness (ESRi) for each 5-km survey using the specaccum function (random method, 1000 permutations) in the vegan package in R 2.14. This randomization eliminates the influence of the order in which each transect was added, thus producing smoothed species accumulation curves. Then, to evaluate survey completeness relative to sampling effort invested, and to project species accumulation curves, ESRi were fitted to the asymptotic Clench function S(x)= ax/(1+bx) (MORENO & HALFFTER 2000), through non-linear regression; where S(x) is the number of species at a given effort, x is the effort employed (distance sampled), a represents the rate of increase at the beginning of the sampling, and b is a parameter related to the shape of species accumulation of new species during the sampling (a and b are regression-derived coefficients). This model is appropriate because it assumes the probability of adding new species increases with sampling effort until an upper limit is reached. The maximum number of species predicted (i.e., asymptote) was calculated as a/b (SOBERÓN & LLORENTE 1993). Survey completeness was assessed by calculating the proportion of the asymptote recorded at the end of sampling. Following MORENO & HALFFTER (2000), I considered that a survey has achieved completeness when ESRi were $90% of the asymptote. Finally, to estimate the theoretical effort required to reach this level of completeness (tq), the following equation tq = q/[b(1-q)] (SOBERÓN & LLORENTE 1993) was applied, where q is the desired proportion of the total fauna (in this case, 0.9) for which the required effort is estimated, and b is the species accumulation as aforementioned.
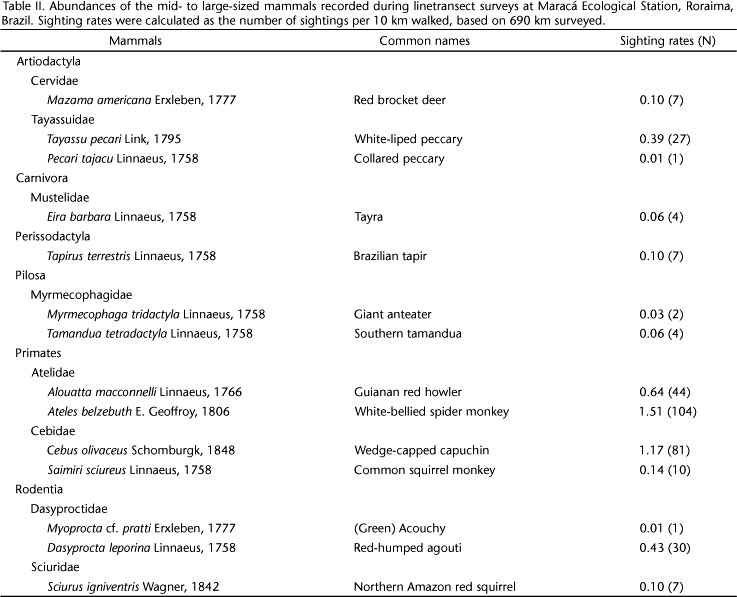
In all, 14 mammals were recorded after 690 km walked, considering the entire landscape (all transects pooled) in the eastern part of MES (regional diversity) (Table II). On average, 9.3 (± 0.9 SE; range 7-13) mammals were recorded per transect (local diversity) (Table I, Fig. 1). Although some species were ubiquitous across the study area, the distribution of the sightings of many species, mainly those with low abundance, varied considerably among transects (Fig. 1). Around 64% of the mammal assemblage at MES was composed by species of low abundance (#0.14 sightings/10 km walked; Table II, Fig. 1). The cumulative number of species increased with the number of surveys in all transects sampled (Fig. 2). Although extrapolated curves tended to stabilize around 100 km, only one of six transects sampled have effectively achieved completeness within the effort employed (Table I). Considering the entire landscape, survey completeness was reached within the total effort of 690 km (Table I). The minimum theoretical effort estimated to obtain the desired level of completeness varied from 150-360 km per transect, and 512 km for the landscape (Table I, Figs 2 and 3).
Line-transect surveys worked reasonably well when surveying the large mammal assemblage at MES, but at least eight species known to occur in the area were not found during this study. At least 22 diurnal mammals were previously found at MES during a higher survey effort (~1100 km; MENDES PONTES 2004). Moreover, most transects sampled in the present study did not achieved the desired level of completeness, either individually or pooled, within the suggested effort of 85-100 km walked (DE THOISY et al. 2008) and theoretical estimations of the effort required to achieve survey completeness showed higher values (Table I). Taken together, these results show that a reliable survey in this low-diversity assemblage requires a higher effort.
The effort needed to record new species using asymptotic species accumulation models increases substantially as the proportion of species recorded approaches the number of species present (i.e., when the probability of observing new species approaches zero; MORENO & HALFFTER 2000). Further effort increases the number of species recorded, but at the expense of an extensive field work (MCARDLE 1990). Indeed, an almost two-fold higher effort resulted in 36% more mammals recorded at MES (MENDES PONTES 2004). Low soil fertility and forest productivity, and strong seasonality (EMMONS 1984, TOGNELLI & KELT 2004, HANYA et al. 2011), can affect mammal richness and abundances negatively. For instance, EMMONS (1984) suggested that mammalian diversity is expected to be low along large areas of Amazonia, covered by remarkably poor soils. Although some mammals attain high abundances at MES, many other found during this study (Table II, Fig. 1) or missed, have low abundance (MENDES PONTES 2004). Indeed, rare and cryptic species are usually the last to be found in line-transect surveys (DE THOISY et al. 2008). In Amazonia, species-poor assemblages of mammals tended to show low evenness in number of individuals from species to species (EMMONS 1984). Therefore, recording each additional species, and consequently achieving survey completeness, might demand a high effort in low-diversity assemblages (GOTELLI & COLWELL 2001), as found here.
Since funds for wildlife surveying are limited in the tropics, sampling efforts may depend on the primary objective of the survey. For instance, during a rapid-assessment survey, an effort of at least 150 km per transect could be enough to estimate mammal richness, but when high-quality estimates are mandatory, 360 km per transect should be the minimum effort required for surveying low-diversity assemblages. The theoretical effort needed to reach completeness in individual transects was lower than for the entire landscape (Table I). Surveying wide areas (i.e., landscape level) may demand a higher effort (512 km) than small habitat patches due to habitat heterogeneity and seasonal variation in the use of habitat by mammals (MORENO & HALFFTER 2000, DE THOISY et al. 2008). Since species detectability may be influenced by the period of day and season (DE THOISY et al. 2008), future studies should evaluate possible temporal effects on the relationship between survey completeness and sampling effort aiming to find most appropriate survey periods.
In conclusion, an effort of 100-km was not enough to produce robust estimates of species richness for large mammals in the low-diversity assemblage at MES. Since this data came from a single site, extrapolations should be undertaken with caution, as the robustness of the estimators may be affected by assemblage characteristics, environmental heterogeneity, and other limitations of line-transect method, discussed at length by DE THOISY et al. (2008). In addition, adjacent transects were relatively close to each other in the present study considering the dispersal capabilities of some large mammals (FRAGOSO 1997, 1998). This implies potential drawbacks due to the risk of multiple sightings of the same individuals in different transects (DE THOISY et al. 2008). However, while a few species has large home ranges and could move long distances in relatively short periods, most of those mammals recorded here have limited ranges, thus diminishing the chances that multiple sightings of the same individuals in different transects have occurred during this study. Therefore, I acknowledge that my sampling design is not optimal, but I believe that the extrapolated species richness estimated in this study were not severely affected by distance among transects. To eliminate or at least minimize this problem, it is recommended that future studies use transects more distant from one another. Until further work is undertaken, however, it would be safest to assume that higher sampling efforts are needed, when surveying low-diversity sites, such as those located in the wide area of the Rio Negro basin (MENDES PONTES et al. 2012) in the northwestern Amazonia. At least 150-360 km per transect, or 512 km for the entire landscape are suggested as an adequate effort to get robust estimates of mammal richness through diurnal line-transect surveys conducted over a yearly schedule in these areas.
ACKNOWLEDGEMENTS
This manuscript derived from my doctoral dissertation for the Graduate Program in Ecology at Instituto Nacional de Pesquisas da Amazônia (INPA). I am grateful to ICMBio and INPA-RR staffs for providing permits and logistic support. I also wish to thank to Adriano Chiarello, Daniel Munari, Fabiana Couto, Luciano Naka, Maíra Benchimol, Renato Cintra, Rossano Mendes Pontes, and the anonymous reviewers which provided insightful criticisms and comments on earlier drafts. The Mohamed bin Zayed Species Conservation Fund, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Fundação Estadual do Meio Ambiente e Recursos Hídricos de Roraima, and Idea Wild funded this study.
LITERATURE CITED
Submitted: 25.VII.2012
Accepted: 11.XI.2012
Editorial responsibility: Mauricio O. Moura
- DE THOISY, B.; S. BROSSE & M.A. DUBOIS. 2008. Assessment of large-vertebrate species richness and relative abundance in Neotropical forest using line-transect censuses: what is the minimal effort required? Biodiversity and Conservation 17: 2627-2644. doi: 10.1007/s10531-008-9337-0
- EMMONS, L.H. 1984. Geographic variation in densities and diversities on non flying mammals in Amazonia. Biotropica 16: 210-222. URL: http://www.jstor.org/stable/2388054
- FRAGOSO, J.M.V. 1997. Tapir-generated seed shadows: scale-dependent patchiness in the Amazon rain forest. Journal of Ecology 85: 519-529. URL: http://www.jstor.org/stable/2960574
- FRAGOSO, J.M.V. 1998. Home range and movement patterns of white-lipped peccary (Tayassu pecari) herds in the northern Brazilian Amazon. Biotropica 30: 458-469. doi: 10.1111/j.1744-7429.1998.tb00080.x
- GOTELLI, N.J. & R.K. COLWELL. 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379-391. doi: 10.1046/j.1461-0248.2001.00230.x
- HANYA, G.; P. STEVENSON; M. VAN. NOORDWIJK; S.T. WONG; T. KANAMORI; N. KUZE; S.-I. AIBA; C.A. CHAPMAN & C. VAN SCHAIK. 2011. Seasonality in fruit availability affects frugivorous primate biomass and species richness. Ecography 34: 1009-1017. doi: 10.1111/j.1600-0587.2010.06775.x
- HAUGAASEN, T. & C.A. PERES. 2005. Mammal assemblage structure in Amazonian flooded and unflooded forests. Journal of Tropical Ecology 21: 133-145. doi: 10.1017/S026646740400207x
- JANSON, C.H. & L.H. EMMONS. 1990. Ecological structure of the nonflying mammal community at Cocha Cashu Biological Station, Manu National Park, Peru, p. 314-338. In A.H. GENTRY (Ed.). Four Neotropical Rainforests. New Haven, Yale University Press.
- MALCOLM, J.R. 1990. Estimation of mammalian densities in continuous forest north of Manaus, p. 339-357. In: A.H. GENTRY (Ed.). Four Neotropical Rainforests. New Haven Yale University Press.
- MCARDLE, B.H. 1990. When are rare species not there? Oikos 57: 276-277.
- MENDES PONTES, A.R. 2004. Ecology of a community of mammals in a seasonally dry forest of Roraima, Brazilian Amazon. Mammalian Biology 69: 319-336. doi: 10.1078/1616-5047-00151
- MENDES PONTES, A.R.; M.D. PAULA & W.E. MAGNUSSON. 2012. Low primate diversity and abundance in Northern Amazonia and its implications for conservation. Biotropica 44 (6): 834-839. doi: 10.1111/j.1744-7429.2012.00873.x
- MILLIKEN, W. & J.A. RATTER. 1998. The vegetation of the Ilha de Maracá, p. 71-112. In: W. MILLIKEN & J.A. RATTER (Eds). Maracá - The Biodiversity and Environment of an Amazonian Rainforest. Chichester, John Wiley & Sons.
- MORENO, C.E. & G. HALFFTER. 2000. Assessing the completeness of bat biodiversity inventories using species accumulation curves. Journal of Applied Ecology 37: 149-158. doi: 10.1046/j.1365-2664.2000.00483.x
- PERES, C.A. 1999. General guidelines for standardizing line-transect surveys of tropical forest primates. Neotropical Primates 7: 11-16.
- SILVEIRA, L.; A.T.A. JÁCOMO & J.A.F. DINIZ-FILHO. 2003. Camera trap, line transect census and track surveys: a comparative evaluation. Biological Conservation 114: 351-355. doi: 10.1016/S0006-3207(03)00063-6
- SOBERÓN, J. & J. LLORENTE. 1993. The use of species accumulation functions for the prediction of species richness. Conservation Biology 7: 480-488. doi: 10.1046/j.1523-1739.1993.07030480.x
- TOGNELLI, M.F. & D.A. KELT. 2004. Analysis of determinants of mammalian species richness in South America using spatial autoregressive models. Ecography 27: 427-436. doi: 10.1111/j.0906-7590.2004.03732.x
Publication Dates
-
Publication in this collection
06 Mar 2013 -
Date of issue
Feb 2013
History
-
Received
25 July 2012 -
Accepted
11 Sept 2012