Abstract
The aim of this study was to assess the ecomorphological patterns and diet of four Characiformes fish species in a poorly physically structured tropical reservoir. We tested the hypothesis that body shape and diet are associated, because environmental pressure acts on the phenotype, selecting traits according to the available resources. Ten ecomorphological attributes of 45 individuals of each species - Astyanax cf. bimaculatus (Linnaeus, 1758), Astyanax parahybae Eigenmann, 1908, Oligosarcus hepsetus (Cuvier, 1829), and Metynnis maculatus (Kner, 1858) - , collected between February and November 2003, were analyzed, and the patterns were assessed using Principal Components Analysis (PCA). Diet similarity among fish species was assessed using cluster analysis on feeding index. The first two axes from PCA explained 61.73% of the total variance, with the first axis being positively correlated with the compression index and relative height, whereas the second axis was positively correlated with the pectoral fin aspect. Two well-defined trophic groups, one herbivorous/specialist (M. maculatus) and the other formed by two omnivorous/generalist (A. cf. bimaculatus, A. parahybae) and one insectivorous-piscivorous (O. hepsetus) were revealed by the cluster analysis. Astyanax. cf. bimaculatus and A. parahybae differed. The first has comparatively greater relative height, relative length of the caudal peduncle and lower caudal peduncle compression index. However, we did not detect a close correspondence between diet and body shape in the reservoir, and inferred that the ecomorphological hypothesis of a close relationship between body shape and diet in altered systems could be not effective.
Body shape; diet; freshwater fishes; morphological diversity; niche overlap
ECOLOGY
Ecomorphological relationships among four Characiformes fish species in a tropical reservoir in South-eastern Brazil
Débora de S. Silva-Camacho* * Corresponding author. E-mail: debora_desouza@yahoo.com.br ; Joaquim N. de S. Santos; Rafaela de S. Gomes; Francisco G. Araújo
Laboratório de Ecologia de Peixes, Universidade Federal Rural do Rio de Janeiro. Antiga Rodovia Rio-SP km 47, 23851-970 Seropédica, RJ, Brazil
ABSTRACT
The aim of this study was to assess the ecomorphological patterns and diet of four Characiformes fish species in a poorly physically structured tropical reservoir. We tested the hypothesis that body shape and diet are associated, because environmental pressure acts on the phenotype, selecting traits according to the available resources. Ten ecomorphological attributes of 45 individuals of each species - Astyanax cf. bimaculatus (Linnaeus, 1758), Astyanax parahybae Eigenmann, 1908, Oligosarcus hepsetus (Cuvier, 1829), and Metynnis maculatus (Kner, 1858) - , collected between February and November 2003, were analyzed, and the patterns were assessed using Principal Components Analysis (PCA). Diet similarity among fish species was assessed using cluster analysis on feeding index. The first two axes from PCA explained 61.73% of the total variance, with the first axis being positively correlated with the compression index and relative height, whereas the second axis was positively correlated with the pectoral fin aspect. Two well-defined trophic groups, one herbivorous/specialist (M. maculatus) and the other formed by two omnivorous/generalist (A. cf. bimaculatus, A. parahybae) and one insectivorous-piscivorous (O. hepsetus) were revealed by the cluster analysis. Astyanax. cf. bimaculatus and A. parahybae differed. The first has comparatively greater relative height, relative length of the caudal peduncle and lower caudal peduncle compression index. However, we did not detect a close correspondence between diet and body shape in the reservoir, and inferred that the ecomorphological hypothesis of a close relationship between body shape and diet in altered systems could be not effective.
Keywords: Body shape; diet; freshwater fishes; morphological diversity; niche overlap.
Ecomorphological studies of fishes aim to understand the patterns of association between the morphology of these organisms and their resource use. A major focus of some of these studies is the relationship between morphological variables and feeding behavior, or habitat use (TEIXEIRA & BENNEMANN 2007, OLIVEIRA et al. 2010, FAYE et al. 2012). One of the first studies on the ecomorphological patterns of fishes was published by KEAST & WEEB (1966), who observed that specializations of the oral apparatus determined habitat preferences and contributed to lessening interspecific competition among the fish species of the Opinicon Lake, Canada. Coexistence among several species in fish communities are facilitated because morphological segregation enables spatial and feeding partitioning (WIKRAMANAYAKE 1990).
Morphological divergences among species can be assessed by certain indices that can be interpreted as indicators of life strategies for habitat colonization (FREITAS et al. 2005) and use of food resources (WAINGHWRITH & RICHARD 1995). Correlations between the diversity of morphological patterns and resource partitioning have been used to test ecomorphological hypotheses in fish communities (WIKRAMANAYAKE 1990, HUGENY & POUILLY 1999). However, in the current literature there is no consensus about the direct relationship between ecology and morphology. Some studies maintain that there is a strong relationship between the morphology of organisms and their use of resources (MOYLE & SENANAYAKE 1984, WIKRAMANAYAKE 1990), whereas others have not found support for this relationship (GROSSMAN 1986, MOTTA et al. 1995), or have found only a weak correlation (CLIFTON & MOTTA 1998).
The use of ecomorphological approaches in the Neotropical region may be particularly relevant to address questions on niches and shared resources, since the region is characterized by a high diversity of fish (WINEMILLER 1991). This approach has been taken to study fish species inhabiting reservoirs, and which possess morphological and reproductive plasticity to adapt to modified environmental conditions (DUARTE et al. 2011). Moreover, impoundments can cause changes in the feeding strategies of species (HAHN & FUGI 2007), through selection: traits that are more suited for the colonization and use of available resources in the altered environment will be selected (CUNICO & AGOSTINHO 2006). This study describes the ecomorphological patterns of four Characiformes fish species in the Lajes Reservoir, and evaluates the relationship between their diet and ecomorphological variables associated with their feeding behavior, locomotion, and their use of the water column. We tested the hypothesis that the shape of the body and diet of fishes are closely associated, since the environmental pressure acts on the phenotype, selecting traits according to the available resources.
MATERIAL AND METHODS
Fishes were collected using gillnets between February and November 2003, from the Lajes Reservoir (22°42'-22°50'S, 43°53'-44°05'W) and were fixed in 10% formalin during 48 hours and transferred to 70% ethanol. Four abundant Characiformes species were analyzed and forty-five individuals of each species were selected: Astyanax cf. bimaculatus (Linnaeus, 1758), Standard Length average (SL) = 96.65 ± 9.69 mm standard deviation, Astyanax parahybae Eigenmann, 1908, SL = 104.37 ± 7.27 mm, Oligosarcus hepsetus (Cuvier, 1829), SL = 144.28 ± 23.5 mm, and Metynnis maculatus (Kner, 1858) SL = 110.11 ± 6,81 mm. Voucher specimens were deposited in the fish collection of the Laboratory of Fish Ecology, Universidade Federal Rural do Rio de Janeiro, Brazil under numbers 1636 to 1641.
The Lajes Reservoir is a major impoundment in the State of Rio de Janeiro and was built between 1905 and 1908 by damming small streams. Initially built to generate hydroelectric power, the reservoir today is a strategic water supply for the municipality of Rio de Janeiro because it provides good quality water. This reservoir, situated at 415 m a.s.l., has a poorly structured physical habitat, lacking routes for fish migration because the tributaries that it receives in the slopes of the Serra do Mar are small (ARAÚJO & SANTOS 2001). Water levels range between 5-9 m during the year (ARAÚJO & SANTOS 2001, SANTOS et al. 2004).
Thirteen morphometric measurements were taken for each individual: standard length, body height, body width, head height, head length, pectoral fin length, pectoral fin width, caudal peduncle length, caudal peduncle height, caudal peduncle width, eye height, mouth height and mouth width. Absolute measurements were taken on the left side of each specimen with a caliper accurate to 0.01 mm. Only adults above L50 (average length of first gonadal maturation, according to data from VAZZOLER 1996) were used in order to avoid eventual allometric effects.Ecomorphological attributes of compression index (CI), relative height (RH), relative length of the caudal peduncle (RLP), caudal peduncle compression index (CPC), pectoral fin aspect (PFA), eye position (EP), relative length of the head (RLH), relative width of the mouth (RWM), relative height of the mouth (RHM) and mouth aspect (MA) were calculated based on morphometric measurements interpreted as indicators of lifestyle or adjustments to the different habitats and diets, as described in several studies (GATZ 1979a, b, MAHON 1984, WATSON & BALON 1984, BALON et al. 1986, BARRELLA et al. 1994, FREIRE & AGOSTINHO 2001).
Diet analysis was based on stomach contents examined under a stereoscopic microscope and identified to the lowest taxonomic level. Food items were identified according to BRUSCA & BRUSCA (2007) and MUGNAI et al. (2010). Empty stomachs were excluded from the analyses. The feeding index (IAi according to KAWAKAMI & VAZZOLER 1980) was calculated to obtain the relative importance of each food item - , using the wet weight of each item.
Principal Components Analysis (PCA) was performed using a correlation matrix of the 10 ecomorphological attributes of the four species to characterize them according to their morphological characteristics. The broken-stick criterion (JACKSON 1993) was used to select the most significant axes in the PCA. This criterion selects axes with eigenvalues higher than those expected by chance (KING & JACKSON 1999). We performed a Discriminant Function Analysis (DFA) to identify the ecomorphological attributes that best discriminated morphological characteristics among A. cf. bimaculatus and A. parahybae. This procedure was performed using Statistica 7.0 software.
Fish diets were analyzed by cluster analysis on the feeding index based on wet weight of food items, using the UPGMA linkage method and the Euclidian Distance matrix. To perform this analysis we used the software Primer 6.0. The amplitude of the trophic niche of each species was estimated using the Shannon's index of niche breadth (H' log2). This index varies from 0 (only one kind food item is present in the species' diet) to 1 (similar quantities of many food items are present in the species' diet). Feeding overlap among species was assessed using PIANKA's (1973) index, with the help of the software EcoSim700. This index varies from zero (signifying no overlap) to one (complete overlap).
The relationships between diet and ecomorphological attributes among the fishes were explored using Canonical Correspondence Analysis (CCA), a direct gradient analysis technique that ordinates a set of observations (in this case species) by directly relating them to two series of associated variables (ecomorphological attributes and diet) (TER BRAAK 1986). CCA is a modification of Correspondence Analysis that adds a multiple regression step and simultaneously relates the primary set of variables (in this case diet) with the secondary variables (ecomorphological attributes), constraining the ordination in such a manner that scores represent the maximum correlation between diet and morphology. A permutation test was used to assess the statistical significance of the relationship. The analysis was performed on log10-transformed ecomorphological attributes and dietary data with the CANOCO software, version 4.5.
RESULTS
Principal Component Analysis of the ecomorphological attributes produced two axes with eigenvalues higher than those expected by chance, explaining 61.73% of the total variance (Table I). The first component (PC1) explained 42.37% of the variability and was positively correlated with the compression index and relative height, and negatively correlated with the relative length of the caudal peduncle, relative height of the mouth and aspect of the mouth. Metynnis maculatus was positively correlated with axis 1 and was characterized by a laterally compressed and high body, whereas O. hepsetus was characterized by large mouth opening and elongated caudal peduncle, being negatively correlated with axis 1. The species A. cf. bimaculatus and A. parahybae positioned close to the origin of the ordination diagram and were characterized by a fusiform body. Despite this similarity, A. cf. bimaculatus had a comparatively wider mouth and more compressed caudal peduncle, whereas A. parahybae had a longer caudal peduncle, higher head and higher and narrow mouth. The second component (PC2) explained 19.36% of the variability and was positively correlated with the aspect of the pectoral fin. This axis was negatively correlated with A. cf. bimaculatus, which had a shorter and wider pectoral fin and was positively correlated with M. maculatus, O. hepsetus and A. cf. bimaculatus, which had a longer and narrower pectoral fin (Table I, Fig. 1).
According to the Discriminant Function Analyses, there are significant morphological differences between A. parahybae and A. cf. bimaculatus (Wilks' l = 0.147, p < 0.0001). The most important attributes for discriminating among the studied species were relative height, relative length of the caudal peduncle, and caudal peduncle compression index.
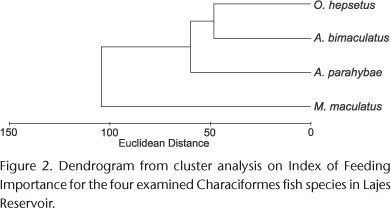
Astyanax parahybae (H' = 0.25), A. cf. bimaculatus (H' = 0.57) and O. hepsetus (H' = 0.31) clustered in a single group in the results of the cluster analysis of the diet (Index of Feeding Importance), whereas M. maculatus (H' = 0.03) formed a separated branch (Fig. 2, Table II). The diets of the former three species consist mainly of insects and the items in the diets of A. cf. bimaculatus and O. hepsetus, which have a wider trophic niche, overlap by 78% (Table III). Moreover, seeds are an important food item in the diet of A. parahybae, which have a comparatively narrower trophic niche than A. cf. bimaculatus. On the other hand, the diet of M. maculatus consists mainly on vegetal fragments (algae), and the trophic niche of this species is narrower.
The Canonical Correspondence Analysis did not show significant correlation between diet and morphology (p = 0.567, test with 1000 permutations). The sum of the eigenvalues for all axes defined by CCA was only 0.011, suggesting that differences in dietary composition were poorly explained by variations in morphological variables.
DISCUSSION
The four studied species in the Lajes Reservoir presented clear differentiated ecomorphological traits, suggesting that these species potentially use different resources. We found a lack of correspondence between diet and morphology, although the patterns of habitat use (locomotion and position in the water column) were consistent with the ecomorphological hypothesis. This finding suggests that the fish species were more influenced by spatial than by trophic structure.
Metynnis maculatus presented higher CI and RH than the other species examined. These attributes are associated with the use of structured habitats that have a variety of shelters and different types of substrate, where fish with high and laterally compressed bodies can perform maneuvers such as pitch or yaw (ALEXANDER 1967). Structured habitats are limited in the Lajes Reservoir, especially during the dry season, when most of the shoreline is exposed. Then, structured areas, which are favorable to the herbivorous feeding habits of M. maculatus, are restricted to some shallow bays, which house shrubs and grasses. Conversely, during the wet season, the submerged vegetation in the shoreline is well structured and should allow for the maneuvers of M. maculatus. The herbivorous habit of M. maculatus inhabiting reservoirs of the middle and lower Tiete River was described by SMITH et al. (2003). It is made possible by the small terminal mouth of this species, which enables the capture of plant fragments in the water column. Moreover, herbivorous feeding habits suit slow swimmers that have the ability to perform vertical movements in habitats where dynamism is low (BALON et al. 1986), such as reservoirs. Their results were corroborated by our cluster analysis, in which M. maculatus formed an isolated branch in the dendrogram based on its herbivorous feeding habits (mostly algae and seeds), thus characterizing this species as the most specialist among the species studied.
In contrast to M. maculatus, O. hepsetus had lower CI and RH, and higher RHM, MA and RLP, indicating a narrow mouth with large gap and a longer head and caudal peduncle. This set of attributes are typical of fishes that feed on large prey items that have good swimming capacity, for instance piscivorous predators that inhabit benthic habits, dwelling in more dynamic environments (GATZ 1979a, b, WATSON & BALON 1984, BALON et al. 1986, FREIRE & AGOSTINHO 2001). Although fishes were the main prey of this species, insects and fish eggs were also consumed, suggesting that the trophic plasticity of O. hepsetus is high. The insectivorous-piscivorous habits of O. hepsetus at the Lajes Reservoir were described by ARAÚJO et al. (2005).
The morphological attributes related to body height and apparatuses to capture food of Astyanax cf. bimaculatus and A. parahybae were intermediate between M. maculatus and O. hepsetus. The scores obtained for individuals of Astyanax, close to the origin in the ordination diagram, indicate a more fusiform body, a characteristic that is associated with generalist feeding habits (OLIVEIRA et al. 2010). According to UIEDA (1984) and CASATTI et al. (2001), species of Astyanax have a continuous swimming behavior across different parts of the water column, which is favored by their laterally positioned eyes and small and relatively elongate pectoral fins. Such characteristics suggest a lack of specialization to explore a particular feeding resource. These species, which are similar in phenotypic traits, present similar adaptations to use resources and thus have a strong potential to compete with one another (WOOTTON 1990). However, in the tropics, competition seems to be reduced, owing to the feeding plasticity of most species, resource partitioning (ARAÚJO-LIMA et al. 1995) and resource availability (WINEMILLER & JEPSEN 1998), as well as the phenotypic characteristics of each species (WAINWRIGHT & RICHARD 1995, LABROPOULOU & ELEFTHERIOU 1997, BELLWOOD & WAINWRIGHT 2001). Contrarily to the classical results of the traditional niche theory, species that use similar resources may coexist if they are sufficiently similar in their skills to compete for the limiting resources (FAGERSTRÖM 1988). This pattern was confirmed for the Lajes Reservoir because of the high trophic niche overlap of Astyanax species, although their ecomorphological differences allow the exploration of differentiated resources that probably decrease competition.
In spite of exhibiting a more generalist body shape, the two Astyanax species have morphological differences that enable them to use resources differently. This pattern was also found by SANTOS et al. (2011) for the same species in the Funil Reservoir. Astyanax parahybae fish have phenotypic characteristics that enable them to capture larger prey items, or prey items that have faster movements, than the items eaten by A. cf. bimaculatus. This is possible because A. parahybae has narrower mouth, greater gap and longer caudal peduncle, whereas A. cf. bimaculatus has a higher RH, CI and CPC, and a wider mouth. Astyanax cf. bimaculatus is more frequent and abundant than A. parahybae in the Lajes Reservoir (ARAÚJO & SANTOS 2001), presenting wide niche breadth. We speculate that the generalist feeding habits and the morphological characteristics of A. cf. bimaculatus are more suitable for standing waters and enable them to be more successful exploring a wide range of trophic resources compared with A. parahybae.
According to the cluster analysis, A. cf. bimaculatus presented a diet more similar to O. hepsetus than to A. parahybae because they have a comparatively wider niche breadth and prey mainly on insects, whereas A. parahybae eats a great amount of seeds. Although the two species of Astyanax had high niche overlap, they seem to concentrate on different food items. While A. cf. bimaculatus use a variety of food items, A. parahybae concentrates on a few food items such as insects and seeds. These findings show the trophic plasticity of A. parahybae, since they use other kinds of food items in other systems (HIRT et al. 2011). Moreover, A. cf. bimaculatus seems to be better adapted to lentic environments (e.g. height body, compressed peduncle), which help this species to better explore different food items, while A. parahybae seems to be less efficient in obtaining access to a wide range of food resources, thus restricting its diet to a fewer number of items.
No significant association was found between the eco-morphological attributes and diet of these four Characiformes species in the reservoir. The lack of correspondence between morphology and diet can be related to the trophic plasticity of species such as O. hepsetus, A. cf. bimaculatus and A.parahybae. As these species present great flexibility in their feeding behavior, it is unlikely that we will observe a strong link between diet and morphology, because they belong to the same broad trophic category, as indicated by cluster analysis. Moreover, HUGUENY & POUILLY (1999) suggested that food availability may weaken the relationship between diet and morphology because stomach contents probably reflect food availability more than morphological adaptation.
Trophic plasticity can also be associated with morphological divergence of fish species in reservoirs. SANTOS et al. (2011) suggested that there is morphological divergence between A. cf. bimaculaus and A. parahybae in the Funil Reservoir, Southeastern Brazil, as consequence of impoundment. Similarly, FRANSSEN (2011), studying fish species of Central Plains of the USA, found that, although the components of body shape are plastic, anthropogenic habitat modifications may drive trait divergence in native populations in reservoir-altered habitats. According to LANGERHANS (2008), phenotypic variation can be adaptive in conditions of low flow and in habitats with high densities of predators, and these two factors could be driving the observed morphological shifts. Morphological divergence between phylogenetically related species has been reported to occur when morphological variations are found as responses to selective pressures of the environment (CASATTI & CASTRO 2006, OLIVEIRA et al. 2010). Thus, the mechanism involved in competition in altered environments may suggest an intensification of generalist habits, rather than the specialization predicted by the classical competition theory (GABLER & AMUNDSEN 2009). Therefore, altered systems like the Lajes Reservoir, which have a simple habitat structure, may favor trophic generalist fish species and contribute to the lack of correlation between morphology and diet.
We conclude that the ecomorphological hypothesis of a close relationship between fish body shape and diet was not corroborated in the present study for the altered system studied. However, the patterns of habitat use of the four species studied corresponded to the expectation of the ecomorphological approach. In altered systems, the patterns of resource use can be broad enough to allow fish species to change their choices and to respond to local biotic and/or abiotic conditions, contradicting the traditional ecological theories. Further studies are necessary to give a more detailed picture on the relationship between body shape and diet, especially in dammed rivers, because the species that persist in this new system had to adapt to new environmental constraints.
ACKNOWLEDGMENTS
Specimens of fish used in this study were obtained from the Project "Fish ecology in Lajes Reservoir", which is financially support by a Research & Development Program by ANNEEL/LIGHT/UFRRJ consortium. FAPERJ - Rio de Janeiro Carlos Chagas Filho Research Support Agency also supplied financial support for this project.
LITERATURE CITED
Submitted: 12.IV.2013
Accepted: 23.VI.2013
Editorial responsibility: Adriano S. Melo
- ALEXANDER, R.M. N. 1967. Functional design in fishes. London, Hutchinson University Library, 160p.
- ARAÚJO, F.G. & L.N. SANTOS. 2001. Distribution and composition of fish assemblages in Lajes Reservoir, Rio de Janeiro, Brazil. Revista Brasileira de Biologia 61: 563-576.
- ARAÚJO, F.G.; C.C. ANDRADE; R.N. SANTOS; A.F.G.N. SANTOS & L.N. SANTOS. 2005. Spatial and seasonal changes in the diet of Oligosarcus hepsetus (Characiformes: Characidae) in a Brazilian reservoir. Revista Brasileira de Biologia 65: 1-8.
- ARAÚJO-LIMA, C.A.R.M.; A.A. AGOSTINHO & N.N. FABRÉ. 1995. Trophic aspects of fish communities in Brazilian rivers and reservoirs, p. 105-136. In: J.G. TUNDISI; C.E.M. BICUDO & T. MATSUMURA-TUNDISI (Eds). Limnology in Brazil. Rio de Janeiro, ABC/SBL, 376p.
- BALON, E.K.; S.S. CRAWFORD & A. LELEK. 1986. Fish communities of the upper Danube River (Germany, Austria) prior to the new Rhein-Main-Donau connection. Environmental Biology of Fishes 15: 243-271.
- BARRELLA, W.; A.C. BEAUMORD & M. PETRERE-JR. 1994. Comparison between the fish communities of Manso river (MT) and Jacare Pepira river (SP), Brazil. Acta BiologicaVenezuelica 15: 11-20.
- BELLWOOD, D.R. & P.C. WAINWRIGHT. 2001. Locomotion in labrid fishes: implications for habitat use and cross-shelf biogeography on the Great Barrier Reef. Coral Reefs 20 (2): 139-150.
- BRUSCA, R.C. & G.J. BRUSCA. 2007. Invertebrados 2nd ed. Rio de Janeiro, Guanabara Koogan, XXII+968p.
- CASATTI, L. & R.M.C. CASTRO. 2006. Testing the ecomorphological hypothesis in a headwater riffles fish assemblage of the rio São Francisco, southeastern Brazil. Neotropical Ichthyology 4 (2): 203-2014.
- CASATTI, L.; F. LANGEANI & R.M.C. CASTRO. 2001. Peixes de riacho do parque estadual morro do Diabo, bacia do alto rio Paraná, SP. Biota Neotropica 1 (1): 1-15.
- CLIFTON K.B. & P.J. MOTTA. 1998. Feeding morphology, diet and ecomorphological relationships among five caribbean labrids (Teleostei, Labridae). Copeia (4): 953-966.
- CUNICO, A.M. & A.A. AGOSTINHO. 2006. Morphological patterns of fish and their relationships with reservoirs hydrodynamics. Brazilian Archives of Biology and Technology 49 (1): 125-134.
- DUARTE, S.; F.G. ARAÚJO; N. BAZZOLI. 2011. Reproductive plasticity of Hypostomus affinis (Siluriformes: Loricariidae) as a mechanism to adapt to a reservoir with poor habitat complexity. Zoologia 28 (5): 577-586.
- FAGERSTRÖM, T. 1988. Lotteries in communities of sessile organisms. Trends in Ecology and Evolution 3: 303-306.
- FAYE, D.; F. LE LOC'H; O.T. THIAW & L.T. MORAIS. 2012. Mechanisms of food partitioning and ecomorphological correlates in ten fish species from a tropical estuarine marine protected area (Bamboung, Senegal, West Africa). African Journal of Agricultural Research 7 (3): 443-455.
- FRANSSEN, N.R. 2011. Anthropogenic habitat alteration induces rapid morphological divergence in a native stream fish. Evolutionary Applications 4 (6): 791-804.
- FREIRE, A.G. & A.A. AGOSTINHO. 2001. Ecomorfologia de oito espécies dominantes da ictiofuna do reservatório de Itaipu (Paraná/Brasil). Acta Limnologica Brasiliensia 13 (1): 1-9.
- FREITAS, C.E.C.; E.L. COSTA & M.G.M. SOARES. 2005. Ecomorphological correlates of thirteen dominant fish species of Amazonian floodplain lakes. Acta Limnologica Brasiliensia 17 (3): 339-347.
- GATZ JR, A.J. 1979a. Ecological morphology of freshwater stream fishes. Tulane Studies of Zoology and Botany 21: 91-124.
- GATZ JR, A.J. 1979b. Community organization in fishes as indicated by morphological features. Ecology 60: 711-718.
- GABLER, H.M. & P.A. AMUNDSEN. 2009. Feeding strategies, resource utilisation and potential mechanisms for competitive coexistence of Atlantic salmon and alpine bullhead in a sub-Arctic river. Aquatic ecology 44 (2): 325-336.
- GROSSMAN, G.D. 1986. Food resource partitioning in a rocky intertidal fish assemblage. Journal of Zoology 1: 317-355.
- HAHN, N.S. & R. FUGI. 2007. Alimentação de peixes em reservatórios brasileiros: alterações e conseqüências nos estágios iniciais do represamento. Oecologia Brasiliensis 11 (4): 469-480.
- HIRT, L.M.; P.R. ARAYALA & S.A. FLORES. 2011. Population structure, reproductive biology and feeding of Astyanax fasciatus (Cuvier, 1819) in an Upper Paraná River Tributary, Misiones, Argentina. Acta Limnologica Brasiliensia 23 (1): 1-12.
- HUGUENY, B. & M. POUILLY. 1999. Morphological correlates of diet in an assemblage of West African freshwater fishes. Journal of Fish Biology 54 (6):1310-1325.
- JACKSON, D.A. 1993. Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74: 2201-2214.
- KAWAKAMI, E. & G. VAZZOLER. 1980. Método gráfico e estimativa de índice alimentar aplicado no estudo de alimentação de peixes. Boletim do Instituto Oceanográfico 29 (2): 205-207.
- KEAST, A. & D. WEBB. 1966. Mouth and body form relative to feeding ecology in the fish fauna of a small lake, Lake Opinicon, Ontario. Journal of the Fisheries Research Board of Canada 23: 1846-1874.
- KING, J.R. & D.A. JACKSON. 1999. Variable selection in large environmental data sets using principal components analysis. Environmetrics 10: 67-77.
- LABROPOULOU, M. & A. ELEFTHERIOU. 1997. The foraging ecology of two pairs of congeneric demersal fish species: importance of morphological characteristics in prey selection. Journal of Fish Biology 50: 324-340.
- LANGERHANS, R. B. 2008. Predictability of phenotypic differentiation across flow regimes in fishes. Integrative and Comparative Biology 48: 750-768.
- MAHON, R. 1984. Divergent structure in fish taxocenes of north temperate stream. Canadian Journal of Fisheries and Aquatic Sciences 41: 330-350.
- MOTTA, J.P.; K.B. CLIFTON; P. HERNANDEZ & B.T. EGGOLD. 1995. Ecomorphological correlates in ten species of subtropical seagrass fishes: diet and microhabitat utilization. Environmental Biology of Fishes 44: 37-60.
- MOYLE, P.B. & F.R. SENANAYAKE. 1984. Resource partitioning among the fishes of rainforest streams in Sri Lanka. Journal of Zoology 202: 195-223.
- MUGNAI, R.; J.L. NESSIMIAN & D.F. BAPTISTA. 2010. Manual de identificação de macroinvertebrados aquáticos do Estado do Rio de Janeiro. Rio de Janeiro, Technical Books, 174p.
- OLIVEIRA, E.F.; E. GOULART; L. BREDA; C.V. MINTE-VERA, L.R.S. PAIVA & M.R. VISMARA. 2010. Ecomorphological patterns of the fish assemblage in a tropical floodplain: effects of trophic, spatial and phylogenetic structures. Neotropical Ichthyology 8 (3): 569-586.
- PIANKA, E.R. 1973. The structure of lizard communities. Annual Review of Ecology and Systematics 4: 53-74.
- SANTOS, A.B.I; F.L. CAMILO; R.J. ALBIERI & F.G. ARAÚJO. 2011. Morphological patterns of five fish species (four characiforms, one perciform) in relation to feeding habits in a tropical reservoir in south-eastern Brazil. Journal of Applied Ichthyology 27: 1360-1364.
- SANTOS, A.F.G.N.; L.N. SANTOS & F.G. ARAÚJO. 2004. Water level influences on body condition of Geophagus brasiliensis (Perciformes, Cichlidae) in a Brazilian oligotrophic reservoir. Neotropical Ichthyology 2: 151-156.
- SMITH, W.S.; C.C.G. F. PEREIRA; E.L.G. ESPÍNDOLA & O. ROCHA. 2003. A importância da zona litoral para a disponibilidade de recursos alimentares à comunidade de peixes em reservatórios, p. 233-248. In: R. HENRY (Ed.). Nas interfaces dos ecossistemas aquáticos. São Carlos, Rima, 350p.
- TEIXEIRA, I. & S.T. BENNEMANN. 2007. Ecomorfologia refletindo a dieta dos peixes em um reservatório no sul do Brasil. Biota Neotropica 7 (2): 67-76.
- TER BRAAK, C.J.F. 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate gradient analysis. Ecology 67: 1167-1179.
- UIEDA, V.S. 1984. Ocorrência e distribuição dos peixes em um riacho de água doce. Revista Brasileira de Biologia 44: 203-213.
- VAZZOLER, A.M.A. DE M. 1996. Biologia da reprodução de peixes teleósteos: teoria e prática. Maringá, EDUEM-SBI, 169p.
- WAINWRIGHT, P.C. & B.A. RICHARD. 1995. Predicting patterns of prey use from morphology of fishes. Environmental Biology of Fishes 44: 97-113.
- WATSON, D.J. & E.K. BALON. 1984. Ecomorphological analysis of taxocenes in rainforest streams of northern Borneo. Journal of Fish Biology 25: 371-384.
- WIKRAMANAYAKE, E.D. 1990. Ecomorphology and biogeography of a tropical stream fish assemblage: evolution of assemblage structure. Ecology 71: 1756-1764.
- WINEMILLER, K.O. 1991. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecological Monographs 61: 343-365.
- WINEMILLER, K. & D.B. JEPSEN. 1998. Effects of seasonality and fish movement on tropical river food webs. Journal of Fish Biology 53 (Suppl. A): 267-296.
- WOOTTON, R.J. 1990. Ecology of teleost fishes. London, Chapman and Hall, 404p.
Publication Dates
-
Publication in this collection
21 Mar 2014 -
Date of issue
Feb 2014
History
-
Received
12 Apr 2013 -
Accepted
23 June 2013