Abstract
In the present study, we assessed the ecomorphology of two species of Astyanax in streams with different substrates found in the Rio São Francisco Basin. The dominant substrate of each stream was defined as either "fine" (0 to 2 mm), "gravel" (2 to 250 mm), "rock" (> 250 mm), or "leaf bank". We analyzed a total of 22 ecomorphological attributes of Astyanax intermedius Eigenmann, 1908 (127 individuals) and Astyanax rivularis (Lütken, 1875) (238 individuals) adults. We detected significant ecomorphological differences between the populations of A. rivularis and A. intermedius from habitats with different types of substrates. However, the two species did not show the same morphological differences depending on the type of substrate. These results confirmed the hypothesis that individuals from environments with different characteristics may have different ecomorphological patterns. Knowing that morphology is associated with habitat use and available resources, the loss of a resource or a modification in the environment may directly affect the permanence of a species, leading to a loss of morphologic diversity.
Convergence; divergence; ecomorphological attributes; freshwater fish; habitat diversity; intraspecific differences
ECOLOGY
ILaboratório de Ecologia de Peixes, Universidade Federal de Lavras. Campus Universitário, 37200-000 Lavras, MG, Brazil. E-mail: marcelaalvesdesouza@gmail.com
IIPrograma de Pós-Graduação em Ecologia Aplicada, Universidade Federal de Lavras. Campus Universitário, 37200-000 Lavras, MG, Brazil. E-mail: danielafagundes87@gmail.com, c.gontijoleal@gmail.com
IIIDepartamento de Biologia, Universidade Federal de Lavras. Campus Universitário, 37200-000 Lavras, MG, Brazil. E-mail: pompeu@ufla.br
ABSTRACT
In the present study, we assessed the ecomorphology of two species of Astyanax in streams with different substrates found in the Rio São Francisco Basin. The dominant substrate of each stream was defined as either "fine" (0 to 2 mm), "gravel" (2 to 250 mm), "rock" (> 250 mm), or "leaf bank". We analyzed a total of 22 ecomorphological attributes of Astyanax intermedius Eigenmann, 1908 (127 individuals) and Astyanax rivularis (Lütken, 1875) (238 individuals) adults. We detected significant ecomorphological differences between the populations of A. rivularis and A. intermedius from habitats with different types of substrates. However, the two species did not show the same morphological differences depending on the type of substrate. These results confirmed the hypothesis that individuals from environments with different characteristics may have different ecomorphological patterns. Knowing that morphology is associated with habitat use and available resources, the loss of a resource or a modification in the environment may directly affect the permanence of a species, leading to a loss of morphologic diversity.
Keywords: Convergence; divergence; ecomorphological attributes; freshwater fish; habitat diversity; intraspecific differences.
The relationship between the body shape of organisms and the environments they inhabit was suggested by Aristotle in the fourth century BC. However, it was only in 1859 with the publication of The Origin of Species by Charles Darwin that the theoretical base of this relationship was consolidated (FREIRE & AGOSTINHO 2001).
Ecomorphology is an area of evolutionary biology that defines relationships between morphology and ecology by studying the relationship between body shape and organisms' ecological characteristics (GATZ 1979, WATSON & BALON 1984, WIKRAMANAYAKE 1990, WINEMILLER 1991, DOUGLAS & MATTHEWS 1992). The concept is based on the assumption that a phenotype provides useful information about the relationship between form and function, i.e., ecology and morphology are alternative expressions of the same ecological and evolutionary adjustments between phenotype and environmental conditions (WAINWRIGHT 1994). Body shape is the primary phenotypical component of an organism and influences foraging activities, locomotion, reproduction, and predator escape (GUILL et al. 2003). Species specialization is often associated with improved efficiency in the exploitation of the resources of a given habitat (PERES-NETO 1999). Morphological differences between species can result from different selective expressions, and species morphology should, therefore, reflect their habits and adaptations to their environment (GATZ 1979, WATSON & BALON 1984, WIKRAMANAYAKE 1990, WINEMILLER 1991, DOUGLAS & MATTHEWS 1992, BEAUMORD & PETRERE 1994, LANGERHANS et al. 2003, CASATTI & CASTRO 2006).
Adaptation is the most important concept for understanding the relationship between morphology and ecology, and it is described as structures that increase fitness (ELDREDGE 1989). This understanding states that adaptations can be detected by relating physiology to environmental variables (PERES-NETO 1999). A key feature is that the variation among individuals or species may be related to differences in performance and, ultimately, a change in resource use and fitness (WAINWRIGHT 1994). Ecomorphology is thus an alternative method of studying adaptation (MOTTA et al. 1995).
Intra and interspecific ecomorphological differences may be related to different environmental and/or biological pressures as well as phylogenetic factors (LANGERHANS et al. 2003, CASSATTI & CASTRO 2006, LEAL et al. 2011, 2013). Elements of the structural complexity of habitats, including substrates of different types and sizes and the presence of sites with different water velocities and depths, such as pools and rapids, are important in this regard (LEAL et al. 2011, 2013). Other factors may also influence habitat complexity and species distribution, including physical and chemical water parameters, as well as species and individuals interactions (JACKSON et al. 2001). Different habitats lead to divergent intraspecific diversification, but the degree of divergence is constrained by the mixture of individuals from alternative environments (LANGERHANS et al. 2003).
Conversely, significant relationships between morphology and ecology can occur due to phylogenetic factors. The integration of phylogenetic information and ecomorphological analyses is the only way to objectively identify cases of morphological and adaptive convergence and divergence (CASATTI & CASTRO 2006). Importantly, this fusion allows the comprehension of larger evolutionary patterns within the communities (PERES-NETO 1999).
Astyanax encompasses morphologically diverse species that are highly abundant and widely distributed in the São Francisco River Basin. For this reason, these species can be good models for ecomorphological studies. Comparing the ecomorphology of populations of two species of Astyanax inhabiting streams characterized by different substrate types, this study addressed the following questions: I) are there intraspecific, differences in morphology related to the different stream substrate types?; and II) do different species show the same substrate related morphological differences?
MATERIAL AND METHODS
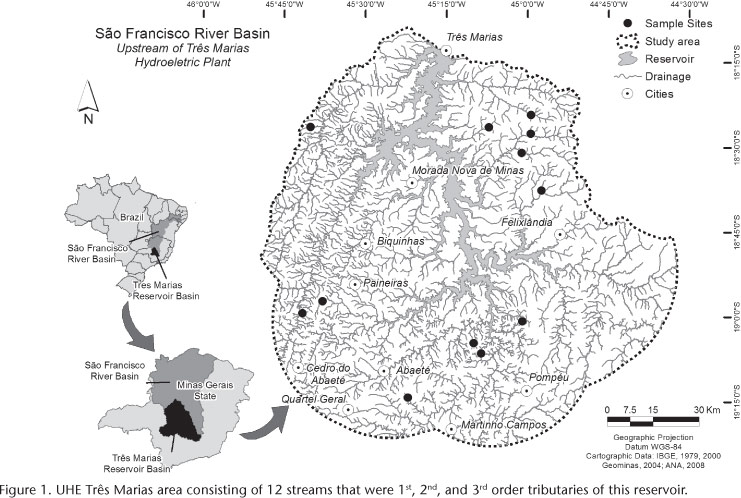
We conducted the present study in 12 1st-3rd order tributaries (Fig. 1) of the reservoir of the Três Marias hydroelectric dam (UHE Três Marias) located in the Upper Rio São Francisco Basin, in the Brazilian cerrado (Table I). The study area is characterized mainly by pasture and small family farms as well as small cities and towns (LIGEIRO et al. 2013).
We collected the data during September 2010, and each stream was sampled once. We collected fish using seines and kick nets, labeled them and fixed them in a 10% formaldehyde solution.
The substrate characterization of each stream was conducted through a visual estimation along a stretch of 40 times the stream width, with the total length divided into 10 sections. Within each section, 15 equidistant measurements of the following substrate classes was made: "fine" (silt, clay, and/or sand, from 0 to 2 mm), "gravel" (fine gravel, coarse gravel, and/or block, from 2 to 250 mm), "rock" (boulders, wide boulders, and smooth and/or rough rocks, > 250 mm), and "leaf bank". The dominant substrate of each stream was defined as the one that occurred in 50% or more of the positions within that stream.
Morphometric analyses were obtained from 131 adult individuals of Astyanax intermedius Eigenmann, 1908 and 240 of Astyanax rivularis (Lütken, 1875). Specimens of both species are deposited in the Coleção Ictiológica da UFLA at the Universidade Federal de Lavras (CIUFLA 307 and 308). We obtained a total of 21 linear and six area measurements for each specimen and selected these measurements based on WINEMILLER (1991), CASATTI & CASTRO (2006), OLIVEIRA et al. (2010), and LEAL et al. (2011). Linear measurements were made using a digital caliper (0.01 mm precision), and area measurements were obtained from drawings of the contours of the body and fins of the individuals. The drawings were then scanned and their areas calculated using the program ImageJ. From these measurements, 22 ecomorphological attributes were calculated: 1) Compression index (CI): the maximum body depth divided by the maximum body width (GATZ 1979). High CI values indicate laterally compressed fishes inhabiting environments with slow-flowing waters (WATSON & BALON 1984). 2) Relative depth (RD): the maximum body depth divided by the standard body length (GATZ 1979). RD is inversely related to water velocity and directly related to the fish's ability to perform vertical movements (GATZ 1979). 3) Index of ventral flattening (IVF): the average body height (vertical distance from the midline to the ventrum, with the midline defined as an imaginary line crossing the eye pupil towards the center of the ultimate vertebra) divided by the maximum body depth (GATZ 1979). Low IVF values indicate fishes living in environments with fast-flowing waters; these characteristics enable the fish to stay in position with no swimming (HORA 1930). 4) Mouth orientation (MO): the angle formed between the tangential plane to both lips and the perpendicular plane to the longitudinal axis of the body when the mouth is open (GATZ 1979). MO is characterized as follows: superior = between 10° and 80°; terminal = 90°; inferior = between 100°; and 170° and ventral = 180° (FREIRE & AGOSTINHO 2001). Degree values were converted to a decimal scale where 1° = 60 (CASSATI & CASTRO 2006). 5) Relative eye position (REP): the depth of the eye at the midline divided by the head depth (WATSON & BALON 1984). REP is assumed to be related to vertical habitat preference (GATZ 1979), and high values correspond to benthic fishes with dorsally located eyes (WATSON & BALON 1984). 6) Relative eye area (REA): this index is related to food detection and provides information on the use of vision in predation activities (PANKHURST 1989, POUILLY et al. 2003). 7) Relative height of head (RHH): the head depth divided by the body depth (OLIVEIRA et al. 2010). Larger relative values of head height are found in fishes that feed on larger prey. Larger values for this index are expected for piscivores (WINEMILLER 1991, WILLIS et al. 2005). 8) Relative width of head (RWH): the head width divided by the body width (OLIVEIRA et al. 2010). Larger relative values of head width are found in fishes that feed on larger prey. Larger values for this index are expected for piscivores (WINEMILLER 1991, WILLIS et al. 2005). 9) Relative head length (RHL): the head length divided by the standard length (GATZ 1979). Larger relative values of head length are found in fishes that feed on larger prey. This index should be larger for piscivores (WATSON & BALON 1984, WINEMILLER 1991, POUILLY et al. 2003, WILLIS et al. 2005). 10) Relative caudal peduncle length (RCPL): the caudal peduncle length (distance from a vertical line at the level of the posterior margin of the base of the most posterior median fin to the terminus of the vertebral column) divided by the standard length (GATZ 1979). Fishes with long caudal peduncles are goods swimmers. However, fishes adapted to rapid water flow, but not necessarily nektonic as armored catfishes, also presented long caudal peduncules for propulsion over short distances (HORA 1930, WATSON & BALON 1984, WINEMILLER 1991). 11) Caudal peduncle compression index (CPCI): the depth of the caudal peduncle divided by the width of the caudal peduncle taken at the narrowest section (GATZ 1979). High values indicate compressed peduncles, and this feature is assumed to be typical of less active swimmers (GATZ 1979). 12) Relative height of caudal peduncle (RHCP): the peduncle height divided by the body depth (OLIVEIRA et al. 2010). Lower values indicate greater maneuverability potential (WINEMILLER 1991). 13) Relative width of caudal peduncle (RWCP): the peduncle width divided by the body width (OLIVEIRA et al. 2010). Higher relative values indicate better continuous swimmers (WINEMILLER 1991). 14) Caudal fin aspect ratio (CFAR): the square caudal fin depth divided by the caudal fin area (GATZ 1979). This index is directly proportional to the amount of swimming by a fish (GATZ 1979). 15) Pectoral fin aspect ratio (PcFAR): the pectoral fin length divided by its width (GATZ 1979). High values suggest long fins typical of fishes with remarkable swimming ability (WATSON & BALON 1984). 16) Pelvic fin aspect ratio (PlFAR): the pelvic fin length divided by its width (GATZ 1979). Low values are observed in fishes that use their pelvic fins for braking and swimming forward, while high values are observed in fishes that use their pelvic fins to swim backwards and to maintain their position in the water column (GATZ 1979). 17) Relative pectoral fin length (RPcFL): the pectoral fin length divided by the SL (GATZ 1979). High values correspond to fishes that are able to perform many slow maneuvers and inhabit slow-flowing waters (GATZ 1979). 18) Relative pelvic fin length (RPlFL): the pelvic fin length divided by the SL (GATZ 1979). This index relates to the choice of habitat and is longer in rocky habitat-dwelling species and shorter in nektonic species (GATZ 1979). 19) Relative caudal fin area (RCFA): the caudal fin area divided by the total body area (WATSON & BALON 1984). High values indicate fins capable of quick propelling movements typical of benthic fishes (WATSON & BALON 1984). 20) Relative pectoral fin area (RPcFA): the pectoral fin area divided by the total body area (GATZ 1979). High values indicate slow swimmers using their fins to maneuver, although high values may also indicate fishes inhabiting fast-flowing waters that use their fins as water deflection surfaces to help maintain their body close to the substrate (WATSON & BALON 1984). 21) Relative pelvic fin area (RPlFA): the pelvic fin area divided by the total body area (GATZ 1979). Benthic fishes have relatively large RPlFAs (GATZ 1979). 22) Relative dorsal fin area (RDFA): the dorsal fin area divided by the total body area (CASATTI & CASTRO 2006). The dorsal fin provides fishes with stability, and small areas are assumed to be more efficient in fast-flowing waters (GOSLINE 1971).
We performed a principal components analysis (PCA) based on the morphological attributes to observe specimen distribution in morphological space. This ordination technique enables the simultaneous evaluation of several variables. The first two axes, representing the higher values of explained variance, were retained for biological interpretation.
We performed discriminant function analyses (DFA) for each species separately using the default method of Statistica 7.0 (STATSOFT 2004). We used this approach to search for ecomorphological differences between individuals from different substrates. In DFA, the squared Mahalanobis distances are used to measure the distances between the centroids of each group. The farther one group is from another group, the greater confidence is that they are different (STATSOFT 2004). Afterwards, we tested the attributes selected by the DFA for differences by analysis of variance (ANOVA) or by Kruskal-Waliis when assumptions of normality and homogeneity of variance were not met. The Tukey test or Fisher LDS (Post-Hoc) were subsequently applied to identify groups of streams responsible for the differences. The analyses were performed using Statistica 7.0 (STATSOFT 2004).
RESULTS
The first two PCA axes for A. intermedius accounted for 29.0% of the variance; the first axis represented 18.1%, and the second axis 10.9% (Fig. 2). The contributions of the variables to the PCA axes (Table II) showed that the pelvic fin aspect ratio and the pectoral fin aspect ratio negatively influenced the first axis. Moreover, the relative pelvic fin area, the relative pectoral fin area, and the relative dorsal fin area positively influenced the first axis. The second axis had a positive contribution from the relative width of the caudal peduncle and a negative contribution from the caudal peduncle compression index.
The ecomorphological gradient indicates that on the first axis there was high segregation among individuals present in the streams with a fine, rocky substrate, while they overlap on the second axis. The ecomorphological attributes that most contributed to the observed differences were as follows: the compression index (CI), the relative width of the caudal peduncle (RWCP), the relative width of the head (RWH), and the relative dorsal fin area (RDFA) (Table III). The percentage of individuals within each group was 88.3% for the fine substrate and 80% for the rocky substrate.
We chose the first two PCA axes for the interpretation of the A. rivularis data. The first axis explained 14.6% of the variance, and the second axis accounted for 14.0% (Fig. 3). The contributions of the variables to the PCA showed that the first axis was negatively influenced by the relative height of the caudal peduncle, the relative pelvic fin area, and the relative pectoral fin area, and it was positively influenced by the pectoral fin aspect ratio (Table II). In the second axis, relative head length had a positive contribution, and relative caudal peduncle length and relative depth made negative contributions. It can be observed that for A. rivularis, the population from streams with abundant leaf banks appeared to be more segregated from the others in relation to the second axis of the PCA gradient, but the discriminant analysis still showed that all substrates were different.
The discriminant function analysis showed significant differences among the ecomorphological attributes of populations of A. rivularis present in different substrate types (Wilk's l = 0.182, F66,633 = 7.39, p < 0.01). The attributes that contributed most to this result were the compression index, the relative head length, the relative width of head, the relative eye position, the caudal fin aspect ratio, the pectoral fin aspect ratio, the relative caudal fin area, the relative pectoral fin area, and the mouth orientation (Table III). All Mahalanobis distances were significantly different (p < 0.05) for the substrate pairs. The percentage of individuals within each group was 95.7% for the fine substrate, 51.6% for the rocky substrate, 83% for the gravel, and 76.9% for the leaf bank. When separately evaluating the ecomorphological attributes of A. intermedius by discriminant analysis, streams with rocks showed lower values for the compression index (F (1, 125) = 12.6, p < 0.01) and the relative dorsal fin area (F (1,125) = 53.5, p < 0.01). However, no differences were found among the substrates for the values of the relative width of the caudal peduncle (F (1,125) = 0.08, p = 0.76) or for the values of the relative head width (F (1, 125) = 1.71, p = 0.19) (Fig. 4).
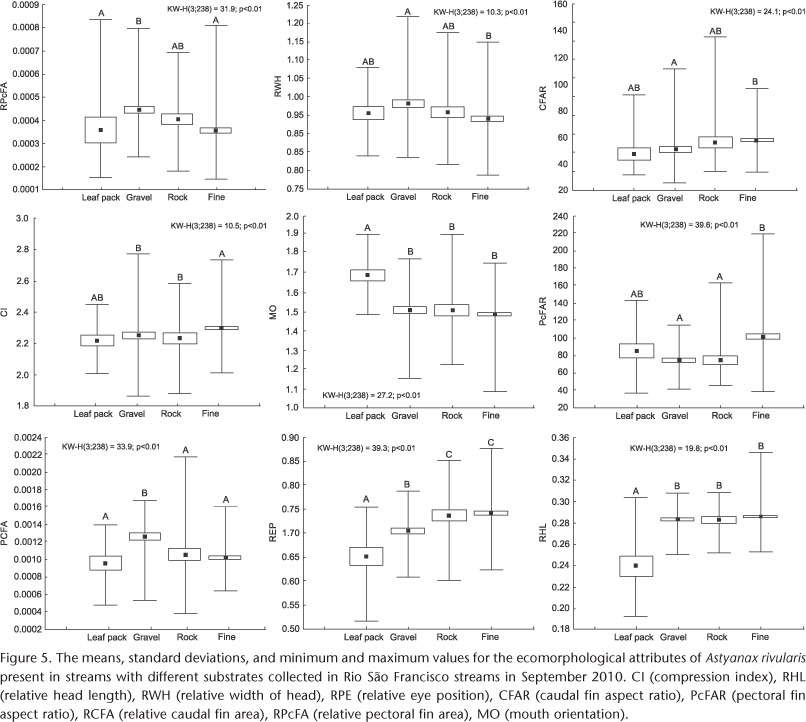
For A. rivularis, the ecomorphological attributes indicated by the discriminant analysis differed primarily for the leaf bank substrate when compared with the other substrates. In streams with a leaf bank substrate, we observed lower values for the relative head length (RHL) and the relative eye position (REP) as well as greater values for the mouth orientation (MO). Populations from streams with a predominantly gravel substrate showed increased values for the relative caudal fin area (RCFA). For the other attributes, differences occurred between pairs of substrates, but there were no clear patterns (Fig. 5).
DISCUSSION
Ecomorphology studies the relationship between morphology and ecology across individuals, populations, and communities, and it examines the evolutionary consequences of these relationships, which are primarily adaptive convergence and divergence (GATZ 1979, WINEMILLER 1991). Comparisons between related organisms occupying different habitats have been used as evidence of the role the environment plays in evolution and of the number of optimal adaptive solutions to a given habitat (CODY & MONEY 1978). Adaptive characteristics are those for which adaptive convergence occurs between different groups or for which adaptive divergence occurs within a group. Thus, these adaptations occur as evolutionary novelties for individuals and require minimal knowledge of the phylogenetic relationships between groups. The integration of phylogenetic information and ecomorphological analysis is the only way to identify cases of morphological and adaptive convergence and divergence, allowing the understanding of evolutionary patterns within communities (PERES-NETO 1999).
In the present study, we detected significant morphological differences among populations of A. rivularis and A. intermedius present in streams with different types of substrates. This finding confirmed the hypothesis that individuals from environments with different characteristics (i.e., velocity, depth, and substrate type) may have different ecomorphological patterns (LANGERHANS et al. 2003, NEVES & MONTEIRO 2003, LEAL et al. 2011, 2013). Our findings also support the idea that the morphology of individuals is shaped by different environments with different selection pressures, resulting in adaptations in body shape (LANGERHANS et al. 2003, JACKSON et al. 2001). However, in this study, the two species did not exhibit the same differences in morphological attributes across substrates.
Our study did detect ecomorphological differences between fish from rocky streams and fish from other substrates, with lower values observed for the compression index and the relative dorsal fin area for A. intermedius in rocky streams. These differences may be related to the faster water velocity in these streams. According to WATSON & BALON (1984), greater compression index values indicate laterally compressed fishes, which tend to reside in locations with a slow water velocity. The fin is responsible for the stability of the fish, and it is thought that dorsal fins with small areas are more effective in faster waters (GOSLINE 1971).
For A. rivularis, the attributes differed primarily between fish from the leaf bank substrate and fish from the other substrate types. Fish from the leaf bank substrate exhibited reduced values for the relative head length and the relative eye position as well as greater values for mouth orientation. The relative head length value is directly related to the size of the prey; therefore, larger values suggest predatory species of relatively large prey (GATZ 1979). Mouth orientation can reflect a species' foraging behavior, and the greater values here likely related to increased resource supply within the leaf bank, as many invertebrates are associated with this type of substrate. The importance of the substrate type for the composition of the stream benthic macroinvertebrate community is widely recognized in the literature and results from the differential availability of food resources and refuge from predation or flow disturbances (LIGEIRO et al. 2010). The importance of riparian vegetation inputs for stream substrates has also been emphasized by ANGERMEIER & KARR (1984) and JACKSON et al. (2001). These substrates locally modify the water flow and attract fish to these lower energy areas, in addition to being suitable areas for foraging, providing protection from predators, and serving as a substrate for aquatic invertebrates. Through these studies, accompanied by the significant results observed for the leaf bank substrate in our study, it is possible to highlight the ecological importance of the organic matter input in streams.
CASSATTI & CASTRO (2006) documented both a case of ecomorphological divergence between phylogenetically close Loricariidae species, indicating that the divergence occurred before their differentiation, and a case of ecomorphological convergence among Characidiinae and Parodontidae, which are phylogenetically distant groups. In our study, the two phylogenetically close species showed different ecomorphological attributes and thus represent adaptive divergence.
Considering that species morphology is associated with habitat use and resource availability (GATZ 1979), the loss and modification of habitats could directly affect the permanence of a species (CUNICO & AGOSTINHO 2006). Therefore, several aspects of physical habitat (i.e., stream size, channel gradient, habitat complexity and cover, channel morphology, substrate, and channel-riparian interactions) should be emphasized, as they might be impacted by human activities in different ways, resulting in habitat loss or homogenization (KAUFMANN et al. 1999). The loss of natural flow regimes have different impacts on the distribution of biodiversity in space and time, as well as on the functions and services provided by aquatic ecosystems (NILSSON et al. 2005).
We detected significant ecomorphological differences among the populations of A. rivularis and A. intermedius from habitats with different types of substrates. However, the two species did not show the same morphological differences depending on the type of substrate. These results confirmed the hypothesis that individuals from environments with different characteristics may have different ecomorphological patterns. Knowing that species morphology is associated with habitat use and available resources, the loss and modification of these factors may directly affect the permanence of a species and can cause a loss of morphological diversity.
ACKNOWLEDGMENTS
We thank our colleagues from Laboratório de Ecologia de Peixes (UFLA), NuVelhas (UFMG) and CEFET-MG for their help during fieldwork, Miriam A. de Castro for lab work and species identification, Diego Macedo for the map, Daniel Tregidgo, Hannah Griffiths, and Natalie Swan for English editing, IBAMA for collecting permits, and landowners for allowing access to their properties. Anonymous reviewers gave us important suggestions to improve the manuscript. This study was made possible by funding from Cemig, Fapemig, Capes, and CNPq. MAS and DCF received undergraduate scholarships, CGL received a doctoral fellowship, and PSP received a research fellowship from Fapemig and CNPq.
LITERATURE CITED
Submitted: 17.VII.2013
Accepted: 20.XII.2013
Editorial responsibility: Vinícius Abilhoa
- ANGERMEIER, P. L. & J.R. KARR. 1984. Relationships between woody debris and fish habitat in a small warmwater stream. Transactions of the American Fisheries Society 113: 716-726.
- BEAUMORD, A.C.; M. PETRERE. 1994. Fish communities of Manso River, Chapada dos Guimarães, MT, Brazil. Acta Biologica Venezuelica152: 21-35.
- CASATTI, L.& R.M.C. CASTRO. 2006. Testing the ecomorphological hypothesis in a headwater riffles fish assemblage of the rio São Francisco, southeastern Brazil. Neotropical Ichthyology 4 (2): 203-214.
- CODY, M.L. & H.A. MOONEY. 1978. Convergence versus nonconvergence in Mediterranean-climate ecosystems. Annual Review of Ecology and Systematics 9: 265-321.
- CUNICO, A.M. & A.A. AGOSTINHO. 2006. Morphological patterns of fish and their relationships with reservoirs hydrodynamics. Brazilian Archives of Biology and Technology 49 (1): 125-134.
- DOUGLAS, M.E.; W.J. MATTHEWS. 1992. Does morphology predict ecology? Hypothesis testing within a freshwater fish assemblage. Oikos 65: 213-224.
- ELDREDGE, N. 1989. Macroevolutionary Dynamics. New York, McGraw- Hill, 226p.
- FREIRE, A.G. & A.A. AGOSTINHO. 2001. Ecomorfologia de oito espécies dominantes da ictiofauna do reservatório de Itaipu (Paraná/Brasil). Acta Limnologica Brasiliensia 13 (1): 1-9.
- GATZ JÚNIOR, A.J. 1979. Ecological morphology of freshwater stream fishes. Tulane Studies in Zoology and Botany 21: 91-124.
- GOSLINE, W.A. 1971. Functional morphology and classification of teleostean fishes. Honolulu, University of Hawaii, 208p.
- GUILL, J.M.; C.S. HOOD & D.C. HEINS. 2003. Body shape variation within and among three species of darters (Perciformes: Percidae). Ecology of Freshwater Fish 12: 134-140.
- HORA, S.L.1930. Ecology, bionomics and evolution of the torrential fauna, with special reference to the organs of attachment. Philosophical Transactions ofthe Royal Society of London 28: 171-282.
- JACKSON, D.A.; P.R. PERES-NETO & J.D. OLDEN. 2001. What controls who is where in freshwater fish communities - the roles of biotic, abiotic, and spatial factors. Canadian Journal of Fisheries and Aquatic Sciences 58: 157-170.
- KAUFMANN, P.R; P. LEVINE; E.G. ROBISON; C. SEELIGER & D.V. PECK. 1999. Quantifying Physical Habitat in Wadeable Streams. Washington, D.C., U.S. Environmental Protection Agency, 149p.
- LANGERHANS, R.B.; C.A. LAYMAN; A.K. LANGERHANS & T.J. DEWITT. 2003. Habitat associated morphological divergence in two neotropical fish species. Biological Journal of the Linnean Society 80: 689-698.
- LEAL, C.G.; N.T. JUNQUEIRA & P.S. POMPEU 2011. Morphology and habitat use by fishes of the Rio das Velhas basin in south-eastern Brazil. Environmental Biology of Fishes 90: 143-157.
- LEAL, C.G.; N.T. JUNQUEIRA; H.A. SANTOS & P.S. POMPEU. 2013. Variações ecomorfológicas e de uso de habitat em Piabina argentea (Characiformes, Characidae) da bacia do rio das Velhas, Minas Gerais, Brasil. Iheringia, Série Zoologia, 103 (3): 222-231.
- LIGEIRO, R.; A.S. MELO & M. CALLISTO. 2010. Spatial scale and the diversity of macroinvertebrates in a Neotropical catchment. Freshwater Biology 55 (2): 424-435.
- LIGEIRO, R.; R.M. HUGHES; P.R. KAUFMANN; D.R. MACEDO; K.R. FIRMIANO; W.R. FERREIRA; D. OLIVEIRA; A.S. MELO & M. CALLISTO. 2013. Defining quantitative stream disturbance gradients and the additive role of habitat variation to explain macroinvertebrate taxa richness. Ecological Indicators 25: 45-57.
- MOTTA, P.J.; S.F. NORTON & J.J. LUCZKOVICH. 1995. Perspectives on the ecomorphology of bony fishes. Environmental Biology of Fishes 44 (1-3): 11-20.
- NEVES, F.M. & L.R. MONTEIRO. 2003. Body shape and size divergence among populations of Poecilia vivipara in coastal lagoons of south-eastern Brazil. Journal of Fish Biology 63: 928-941.
- NILSSON, C.; C.A. REIDY; M. DYNESIUS & C. REVENGA. 2005. Fragmentation and flow regulation of the world's large river systems. Science 308 (5720): 405-408.
- OLIVEIRA, E.F.; E. GOULART; L. BREDA;C.V. MINTE VERA; L.R.S. PAIVA & M.R. VISMARA. 2010. Ecomorphological patterns of the fish assemblage in a tropical floodplain: effects of trophic, spatial and phylogenetic structures. Neotropical Ichthyology 8 (3): 569-586.
- PANKHURST, N.W.1989. The relationship of ocular morphology to feeding modes and activity periods in shallow marine teleosts from New Zealand. Environmental Biology of Fishes 26: 201-211.
- PERES-NETO, P.R. 1999. Alguns métodos e estudos em Ecomorfologia de peixes de riacho, p. 209-236. In: E.P. CARAMASCHI; R. MAZZONI & P.R. PERES-NETO. Ecologia de Peixes de Riachos. Rio de Janeiro, PPGE/UFRJ, vol. 4.
- POUILLY, M.; F. LINO; J.G. BRETENOUX & C. ROSALES. 2003. Dietary morphological relationships in a fish assemblage of the Bolivian Amazonian floodplain. Journal of Fish Biology 62: 1137-1158.
- STATSOFT INC. 2004. STATISTICA: data analysis software system, version 10. Available online at: www.statsoft.com [Accessed: 17.VI.2013]
- WAINWRIGHT, P.C. 1994. Functional morphology as a tool in ecological research, p. 42-59. In: P.C. WAINWRIGHT & S.M. REILLY (Eds). Ecological morphology: integrative organismal biology. Chicago, University of Chicago Press.
- WATSON, D.J. & E. BALON. 1984. Ecomorphological analysis of taxocenes in rainforest streams of northern Borneo. Journal of Fish Biology 25: 371-384.
- WILLIS, S.C.; K.O. WINEMILLER & H. LOPEZ-FERNANDES. 2005. Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecologia 142: 284-295.
- WIKRAMANAYAKE, E.D. 1990. Ecomorphologyand biogeography of a tropical stream fish assemblage: evolution of assemblage structure. Ecology 71: 1756-1764.
- WINEMILLER, K.O. 1991. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecological Monographs 61 (4): 343-365.
Ecomorphology of Astyanax species in streams with different substrates
Publication Dates
-
Publication in this collection
21 Mar 2014 -
Date of issue
Feb 2014
History
-
Accepted
20 Dec 2013 -
Received
17 July 2013