Abstract
A new subterranean and troglomorphic species of Ituglanis Costa and Bockmann, 1993 is described from the carbonatic karst area of northeastern Goiás state, upper Tocantins River Basin, central Brazil, representing the sixth subterranean species of the genus described from the same region. Ituglanis boticario sp. nov. is diagnosed by a combination of unusual characters for the genus: body pigmentation forming longitudinal stripes, 7-8 pairs of ribs, presence of the anterior segment of the infraorbital laterosensory canal, and usually 8 pectoral-fin rays. Due to the absence of epigean populations and the presence of some degree of morphological specialization to the subterranean environment, it can be classified as a troglobite (i.e., exclusively subterranean). The description of this species increases the importance of the northeastern Goiás region as a biodiversity spot for subterranean ichthyofauna, mainly Ituglanis. The region demands urgent political efforts to ensure the preservation of its speleobiological patrimony, including the Tarimba cave system, one of the largest caves in Brazil and type-locality of I. boticario sp. nov.
Cave fauna; taxonomy; troglobite; upper Tocantins River Basin
TAXONOMY AND NOMENCLATURE
Ituglanis boticario, a new troglomorphic catfish (Teleostei: Siluriformes: Trichomycteridae) from Mambaí karst area, central Brazil
Pedro Pereira RizzatoI,II; Maria Elina BichuetteI
ILaboratório de Estudos Subterrâneos, Departamento de Ecologia e Biologia Evolutiva, Universidade Federal de São Carlos. Rodovia Washington Luís, km 235, Caixa Postal 676, 13565-905 São Carlos, SP, Brazil
IICorresponding author. E-mail: rizzatopp@gmail.com
ABSTRACT
A new subterranean and troglomorphic species of Ituglanis Costa and Bockmann, 1993 is described from the carbonatic karst area of northeastern Goiás state, upper Tocantins River Basin, central Brazil, representing the sixth subterranean species of the genus described from the same region. Ituglanis boticario sp. nov. is diagnosed by a combination of unusual characters for the genus: body pigmentation forming longitudinal stripes, 7-8 pairs of ribs, presence of the anterior segment of the infraorbital laterosensory canal, and usually 8 pectoral-fin rays. Due to the absence of epigean populations and the presence of some degree of morphological specialization to the subterranean environment, it can be classified as a troglobite (i.e., exclusively subterranean). The description of this species increases the importance of the northeastern Goiás region as a biodiversity spot for subterranean ichthyofauna, mainly Ituglanis. The region demands urgent political efforts to ensure the preservation of its speleobiological patrimony, including the Tarimba cave system, one of the largest caves in Brazil and type-locality of I. boticario sp. nov.
Keywords: Cave fauna; taxonomy; troglobite; upper Tocantins River Basin.
Trichomycteridae is a monophyletic assemblage of small catfishes, easily diagnosed by their highly specialized opercular-interopercular apparatus bearing odontodes (de Pinna 1998, de Pinna & Wosiacki 2003, Adriaens et al. 2010, Datovo & Bockmann 2010). The family is one of the most interesting groups of Neotropical catfishes, not only because of the large number of valid species, placing it as the second richest family of Siluriformes (after Loricariidae – Eschmeyer & Fong 2014), but also due to the diversity of life habits and diet types (Adriaens et al. 2010, Datovo & Bockmann 2010). Trichomycterids are also successful colonizers of unusual and extreme habitats, such as torrential, temporary, insular, high elevations, psamophilic, interstitial, infaunal and subterranean environments (Myers & Weitzmann 1966, Nico & de Pinna 1996, de Pinna & Wosiacki 2003, Zuanon & Sazima 2004, Fernández & Schaeffer 2005, Nelson 2006, Zuanon et al. 2006, Castellanos-Morales 2007, Fernández & Vari 2012).
Up to date, Trichomycteridae is the third fish family in richness of known subterranean representatives, with 23 recognized troglobitic species (Proudlove 2010). As expected by their distribution, Brazil holds most of this diversity, including 14 species distributed in the Copionodontinae – with Glaphyropoma de Pinna, 1992 (one species: Glaphyropoma spinosum Bichuette, de Pinna & Trajano, 2008) and Copionodon de Pinna, 1992 (one undescribed species, M.E. Bichuette, pers. comm.) – and Trichomycterinae – with Trichomycterus Valenciennes, 1832 (five species, two of which undescribed – Bichuette & Rizzato 2012, Cordeiro et al. 2013) and Ituglanis Costa & Bockmann, 1993 (seven species including the new described herein, and an undescribed species from the same region – Bichuette & Trajano 2008).
Ituglanis was erected to accommodate nine species previously included in the clearly non-monophyletic Trichomycterus. Ituglanis is a monophyletic group that was proposed based originally on three synapomorphies (Costa & Bockmann 1993), and recently Wosiacki et al. (2012) proposed another four (but see discussion). Currently, 23 valid species are recognized in Ituglanis (Eschmeyer & Fong 2014), and there are several other undescribed (Lima et al. 2013). Herein we describe a new species, collected from subterranean streams in northeastern Goiás State, upper Tocantins River Basin, from where the other five troglobitic species of the same genus were also described: Ituglanis passensis Fernández & Bichuette, 2002, I. bambui Bichuette & Trajano, 2004, I. epikarsticus Bichuette & Trajano, 2004, I. ramiroi Bichuette & Trajano, 2004 and I. mambai Bichuette & Trajano, 2008. We also provide a detailed morphological description of this new species as an aid for future attempts to unravel the phylogenetic affinities within the genus.
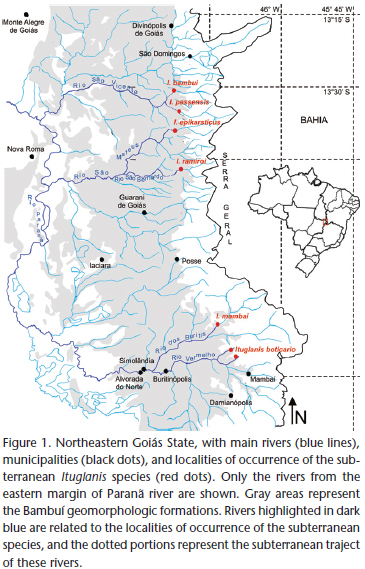
The carbonatic karst areas of northeastern Goiás (Fig. 1) belong to the São Domingos District (sensu Karmann & Sánchez 1979), representing one of the regional expressions of the Bambuí geomorphological unit, the Brazilian largest group of limestone outcrops favorable to occurrence of caves (Karmann & Sánchez 1979).
The region is characterized by extensive limestone outcrops inserted within the Cerrado morphoclimatic domain (the Brazilian savannah-like vegetation – Ab'Saber 1977), with tropical climate, hot and semi-humid, the dry-season occurring between May and September, sometimes extending to October (Nimer 1979). The karst is inserted mostly at the eastern margin of the Paranã River, a tributary of the upper Tocantins River, and is typically crossed by several rivers which originate at "Serra Geral" (a mountain range delimiting the states of Goiás and Bahia). After draining an extensive sandstone landscape, these rivers penetrate the limestone through sinkholes to form predominantly horizontal cave systems. They then reappear at the surface through resurgences and flow to the west, to reach the Paranã River.
Two large karst areas are comparable in northeastern Goiás. To the north, the São Domingos karst area, with large cave systems (some with more than 20 km) inserted within a State Park ("Parque Estadual de Terra Ronca") in the municipality of São Domingos. The subterranean fauna of the São Domingos karst area have been intensively studied (Majer et al. 2003, Rheims & Pellegatti-Franco 2003, Bichuette & Trajano 2003, Simões et al. 2013) and the region is considered an area of high diversity of subterranean ichthyofauna, including four troglobitic Ituglanis (Fig. 1) and many non-troglomorphic cave fishes (Bichuette & Trajano 2003). To the southeast, the Mambaí karst area, which includes the municipalities of Mambaí and Posse, whose cave systems are not as developed as those from São Domingos (the largest cave system in the region and type locality of the new species herein described, the Gruna da Tarimba cave system, is approximately 12 km long – União Paulista de Espeleologia, pers. comm.). The Cerrado in the region is altered, with many plantations and cattle farms in the surroundings of the cave systems. Recent studies are unraveling the speleobiological patrimony of Mambaí karst area, which includes many invertebrates and at least two troglomorphic fishes, the trichomycterid I. mambai and the callichthyid Aspidoras albater Nijssen & Isbrücker, 1976 (Bichuette & Trajano 2008, Secutti et al. 2011). Furthermore, several caves are inhabited by subterranean populations of Ituglanis other than I. mambai. At least one new species, herein described, occurs in the area, in caves of the municipality of Mambaí.
MATERIAL AND METHODS
The specimens were hand-netted, anesthetized in benzocaine or clove-oil solution until death, preserved in formalin and then transferred to 70% alcohol. Some specimens were brought alive to the laboratory and reared in an aquarium for behavioral observations. Measurements were taken point-to-point with a digital caliper under the stereomicroscope, and expressed to the nearest 0.1 mm. All the measurements are described in Table I. One specimen (LIRP-11011) was cleared and double-stained (C&S) for bone and cartilage, following the procedure described by Song & Parenti (1995) until step 9, and two (LIRP-11010 and LESCI-00223) were double-stained for bone and cartilage and dissected (S&D), following the procedure described by Datovo & Bockmann (2010). Specimens not C&S or S&D were radiographed with a Faxitron DX device. Osteological and myological nomenclature follow Winterbottom (1974), Bockmann et al. (2004), Datovo & Bockmann (2010), and Datovo & Vari (2013). In the text and in the figures, names of muscles are graphed in italics. In Fig. 3, laterosensory canals and branches are graphed in bold.
On the branchial skeleton, only the ossified elements are identified as basibranchials, and the remaining cartilaginous elements, anterior to the basibranchial 2 and posterior to the basibranchial 3 are identified as the anterior portion of copula 1 and the copula 2, respectively, according to Srinivasachar (1958). Additionally, the cartilaginous element that connects the ceratobranchial 4 and 5 and the epibranchial 4 is identified as the accessory element of ceratobranchial 4 following Carvalho et al. (2013). Ray counts and symbols follow Bockmann et al. (2004). Vertebrae counts include only free vertebrae (those fused into Weberian complex were not included) plus the compound caudal centrum (PU1 + U1, considered as a single element). Laterosensory-system nomenclature follow Arratia & Huaquin (1995), but the pores of the postotic canal are treated as the preoperculo-mandibular and the pterotic branches of the postotic canal, following Schaeffer & Aquino (2000). These two banches correspond respectively, to the pores po1 and po2 of Bockmann et al. (2004). The comparative material is listed in the Appendix 1.
Abbreviations: (SL) standard length. (HL) head length. (C&S) cleared and stained. (S&D) stained and dissected. (LIRP) Laboratório de Ictiologia de Ribeirão Preto. (LESCI) Coleção de Ictiologia do Laboratório de Estudos Subterrâneos.
TAXONOMY
Ituglanis boticario sp. nov.
Diagnosis. Ituglanis boticario sp. nov. is diagnosed by a combination of four unusual but not exclusive characters in the genus: body pigmentation forming longitudinal stripes, vs. pigmentation uniform, forming regular or irregular spots of different sizes, or reduced to absent – except for I. parahybae (Eigenmann, 1918), I. cahyensis Sarmento-Soares, Martins-Pinheiro, Aranda & Chamon, 2006, I. australis Datovo & de Pinna, 2014 and I. apteryx Datovo, 2014 –; large number of ribs, 7-8, vs. 6 or fewer – except for the subterranean species I. passensis, I. bambui, I. ramiroi, and I. mambai; not seen for I. guayaberensis (Dahl, 1960), I. herberti (Miranda-Ribeiro, 1940) and I. metae (Eigenmann, 1917) –; presence of the anterior segment of the infraorbital laterosensory canal – except for the epigean I. proops (Miranda-Ribeiro, 1908), I. paraguassuensis Campos-Paiva & Costa, 2007, I. agreste Lima, Neves & Campos-Paiva, and I. australis –; and large number of pectoral-fin rays, usually 8, vs. 7 or fewer (except for the subterranean I. passensis, I. bambui, I. epikarsticus, I. ramiroi, and I. mambai). The new species can be diagnosed from its geographically closest congener, I. mambai, by the body pigmentation forming longitudinal stripes (vs. body pigmentation homogeneous light brown with irregular small spots distributed mainly on dorsum and flanks in I. mambai), smaller eyes and longer barbels, especially the maxillary and the nasal, and by the presence of the anterior segment of the infraorbital laterosensory canal in most specimens (vs. absent) (see "Intrageneric comparisons").
Description. Morphometric data of holotype and paratypes are given in Table II.
External morphology: Take as reference Figs 2-6. Body bulky, elongate, semi-cylindrical, becoming uniformly compressed towards caudal fin (Figs 2-4). Dorsal profile of body straight in lateral view, with slight and straight slope from tip of snout to posterior portion of head (Fig. 2). Ventral profile of body straight. Anterior portion of body slightly deeper than caudal peduncle. Dorsal and ventral profile of caudal peduncle straight, dorsal and ventral margins of caudal fin straight or slightly divergent, similar to a brush. Urogenital and anal openings on vertical through anterior end of dorsal-fin base, covered by distal portions of pelvic fins.
Head relatively wide and depressed, trapezoidal in dorsal view (Fig. 5). Eyes small (on average 8.9% of HL), visible as small black dots on anterior half of head, close to posterior nostrils. Anterior nostril transversally ovoid and slightly smaller than posterior, surrounded laterally by a tubular flap of skin continuous with nasal barbels. Posterior nostril rounded, surrounded anteriorly by a flap of integument. Mouth slightly subterminal, straight to slightly convex in dorsal view, rictus laterally directed and partially continuous with rictal barbel (Fig. 6). Barbels long, especially the maxillary (on average 126% of HL) and the nasal (113% of HL). Nasal barbel originating at posterolateral portion of integumentary flap around anterior nostril. Maxillary and rictal barbel originating at lateral portion of rictus. When adpressed to body, maxillary barbel extending at least to origin of pectoral fin; nasal barbel extending at least to opercular patch of odontodes; and submaxilary barbel extending at least to posterior portion of interopercular patch of odontodes. Opercular patch of odontodes small, circular or slightly elliptical longitudinally, in some individuals pointing upward. Interopercular odontodes forming a slightly convex patch throughout ventral margin of interopercle. Posterior margin of interopercular patch of odontodes not reaching the opercular one.
Dorsal-fin origin at posterior half of body, with first pterygiophore posterior to neural spine of 21th (9 specimens) or 22th (3) vertebra. Dorsal fin semicircular or approximately retangular in lateral view, distal margin rounded, with variable ray count: 9 (12), rarely 8 (2) rays, the two first almost always unbranched (except for one specimen, LESCI-00223B) and the last occasionally unbranched; in addition, one or two micro rays anterior to first ray. Number of rays or branching of last ray not related to size of specimen. Eight narrow pterygiophores at dorsal fin, basal radials with espatulate distal region curved backwards bearing one ray each, except the last one, which bears the last two rays splitting from a posterior laminar expansion.
Anal-fin origin at posterior half of body, slightly posterior to dorsal-fin origin, with first pterygiophore posterior to neural spine of 22th (6) or 23th (6) vertebrae. Anal fin rectangular or approximately semicircular in lateral view, distal margin rounded. Anal fin with 7 (12), rarely 8 (2, LIRP-11010B and LIRP-11012) rays, the two first almost always unbranched (except for one specimen, LIRP-11010C) and the last branched or unbranched; in addition, one to three micro rays anterior to first ray. Number of rays or branching of the last ray not related to size of specimen. Six narrow pterygiophores at anal fin, basal radials with espatulate distal region curved backwards bearing one ray each, except the last one, which bears the last two rays splitting from a posterior laminar expansion.
Pectoral fin triangular in dorsal view, lateral and distal margins rounded, with wide base and variable ray count: usually 8 (I7, rarely I6I) or less commonly 9 (I8 or I7I). First ray always unbranched, and prolonged as a short filament in some specimens. Last ray unbranched in half of specimens. Pectoral-fin ray count asymmetrical in about one-third of specimens. Number of pectoral-fin rays not related to size of specimen.
Pelvic fin rectangular in ventral view, lateral margins straight and distal margin rounded, usually with five rays (I3I or I4). First ray always unbranched. Last ray unbranched in most specimens. Pelvic-fin ray count asymmetrical for about one-third of specimens. Pelvic-fin origin at posterior half of body, anterior to dorsal-fin origin. Pelvic-girdle base at level of 19th vertebra. Distal margin of pelvic fins covering urogenital and anal openings. Pelvic-fin bases adjacent to each other.
Margin of caudal fin straight or slightly rounded, with 13 principal rays, six in upper lobe attached to the hypural plate 3+4+5, (first principal ray unbranched), seven in lower lobe attached to parhypural + hypural plate 1+2 (first principal ray unbranched); additionally, 5 to 15 dorsal and ventral procurrent rays.
General morphology of cranium: Anterior cranial fontanel absent (Fig. 7). Posterior cranial (= parieto-supraoccipital) fontanel very small, reduced to a round orifice at the posterior half of parieto-supraoccipital, between the insertions for contralateral supracarinalis anterioris. Conspicuous depressions at dorsoposterior portion of skull forming a ridge at dorsal region of parieto-supraoccipital, for insertion of supracarinalis anterioris. Foramens of lateral accessory nerves relatively large (larger than parieto-supraoccipital fontanel), elliptical, slightly posterior to parieto-supraoccipital fontanel and adjacent to posterior semicircular canal of inner ear, covered by the supracarinalis anterioris. Parieto-supraoccipital approximately rectangular, connected to frontals by a straight or convex suture and to complex vertebra of weberian capsule by a slightly convex suture. Frontals wide and long, tightly joined to each other medially except at the anteriormost portion, where they are separated by posterior portion of mesethmoid. Lateral external processes of frontals at mid-length of the bone, forming the dorsanterior portion of postorbital process of skull.
Mesethmoid long and narrow, T-shaped, with anterior margin straight or slightly concave. Posterior half of mesethmoid sharpening posteriorly between anterior portions of frontals and covering the lateral ethmoids dorsally. Anterior half of mesethmoid, posterior to cornua, wider than posterior, with lateral flaps of bone surrounding dorsally the distal end of vomer to form the internal borders of nasal chambers. Pre-maxilla rectangular, slightly curved backwards, with internal half wider than external half, sometimes almost triangular, with two almost regular rows of conical, sharp teeth curved backwards. Posterodorsal margins of pre-maxilla and of lateral processes of mesethmoid covered by a flap of elastic cartilage extending to internal lateral margin of antorbital and continuous with nasal barbels, forming the anterior margin of nasal chamber. Maxilla boomerang-shaped, without teeth, covering dorsally the rictal barbel nerve. Posterior margin of external half of maxilla covered by an elastic cartilage continuous with maxillary barbel. Ventral anterior margin of internal half of maxilla covered by a flap of elastic cartilage continuous to rictal barbel. Internal tip of maxilla adjacent and anterior to palatine, adjacent and lateral to external margin of pre-maxilla, and connected with the antorbital by a narrow ligament. Antorbitals small, drop-shaped, rounded anteriorly and sharp posteriorly, connected to internal tip of maxilla and to sesamoid supraorbital by ligaments. Sesamoid supraorbital very long and narrow, cylindrical, slightly curved outwards, joined to frontal by a narrow ligament, with small process on the external margin of its anterior half, pointing backwards and supporting the anterior portion of the ocular connective tissue. External margin of sesamoid supraorbital adjacent and dorsal to trigemino-facial nerves, and continuous posteriorly with the ocular connective tissue. Internal margin of sesamoid supraorbital covered by a connective tissue continuous with anterior margin of frontals and posterior half of mesethmoid, covering dorsally the lateral ethmoids and forming the posterior margin of nasal chamber.
Epioccipitals pentagonal, on posterior diagonals of parieto-supraoccipital between pterotic and weberian capsule, covering dorsally part of the posterior semicircular canal of inner ear (Figs 7 and 8). Pterotic wide, connected dorsally to epioccipitals and parieto-supraoccipital internally, to posttemporo-suprachleitrum posteriorly, and to sphenotic-prootic-pterosphenoid anteriorly; and ventrally to sphenotic-prootic-pterosphenoid anteriorly and to epioccipital posteriorly. Posterior margin of pterotic covered by the epaxialis. External margin of pterotic with a concavity to which the levator operculi is attached. Sphenotic-prootic-pterosphenoid long and wide, adjacent dorsally to parieto-supraoccipital and to frontals, with anterior portion directed anteriorly forming with the lateral process of frontals the postorbital process of skull from which emerges the infraorbital canal of laterosensory cephalic system and to which attaches, ventrally, the levator arcus palatini. Lateral external margin of sphenotic-prootic-pterosphenoid covered partially by the secondary section of dilatator operculi. Sphenotic-prootic-pterosphenoids connected ventrally to pterotic and basiexoccipital posteriorly, to orbitosphenoid anteriorly and to each other medially, covered by the posterior process of basisphenoid. Sphenotic-prootic-pterosphenoid and pterotic with a ventrolateral, continuous depression to which articulates the hyomandibula. Sphenotic-prootic-pterosphenoid recovering dorsally and ventrally the anterior semicircular canal of inner ear. Three rounded foramens at ventral portion of sphenotic-prootic-pterosphenoid. Orbitosphenoid wide, connected dorsolaterally to frontals, anteriorly to lateral ethmoids, posteriorly to sphenotic-pootic-pterosphenoid and medially to posterior portion of vomer at the anterior half and to anterior process of basisphenoid at the posterior half. Optic foramen small, at the lateral portion of orbitosphenoid. Trigemino-facial nerve foramen wide, at the lateral contact of sphenotic-prootic-pterosphenoid and orbitosphenoid. Lateral ethmoid wide, with a wide dorsal concavity covered by connective tissue, from which emerges the olfactory tract. Lateral ethmoids separated from each other dorsally by mesethmoid and ventrally by vomer, connected posteriorly to frontals dorsally and to orbitosphenoids ventrally. Lateral ethmoid with an anteroventral cartilaginous articulation with palatine and anterodorsally connected to mesethmoid and vomer by a block of cartilage.
Vomer arrow-shaped, extending from ventral surface of mesethmoid to mid-length of orbitosphenoids. Margins of "setae" bearing cartilaginous articulations with palatine. Basisphenoid long, almost circular at medial portion, with two long, narrow and sharpening anterior processes surrounding laterally the posterior portion of vomer, and with a sharpening, wider and shorter posterior process covering the contact of contralateral sphenotic-prootic-pterosphenoids and anterior half of basioccipital. Basiexoccipital wide, connected to sphenotic-prootic- pterosphenoids and pterotics anteriorly, and posteriorly to complex vertebrae of weberian capsule. Complex vertebrae of weberian capsule wide and triangular dorsally, adjacent to large, wide, spherical capsules that enclose the gas bladder. Weberian capsules with lateral external, tubular processes connected to posttemporo-supracleithrum, forming a round orifice from which emerges the trunk canal of laterosensory system. Posttemporo-supracleithrum wide, connecting the lateral tubular process of weberian capsule to the posterior portion of pterotic, covered dorsomedially by levator internus 4 (Fig. 8), and with a small, narrow posterior internal process surrounding laterally the posterodorsal process of cleithrum.
Hyomandibular wide (Figs 7 and 9 ), with membranous bone outgrow directed anterodorsally below trigeminal and facial nerves, reaching the membranous bone outgrow of quadrate and the metapterygoid. Hiomandibular articulating with sphenotic-prootic-pterosphenoid and pterotic dorsally by convex cartilaginous margin, with processes at anterior and posterior angles connected to sphenotic-prootic-pterosphenoid and pterotic, respectively, by a short ligament. Hiomandibular with a deep medial depression on which the levator arcus palatine is inserted. Hiomandibular tightly attached to quadrate by a block of cartilage. Quadrate rod-like, robust, articulating with anguloarticular by a hinge joint, and with membranous bone outrgrow anterodorsally directed below trigeminal and facial nerves, bordered by the hyomandibular membranous bone outrgrow posteriorly and by the metapterygoid anteriorly. Metapterygoid small, trapezoidal, joined to quadrate by a small block of cartilage, with its posterior margin reaching hyomandibular membranous bone outgrowth, and partially covered by the lateroposterior process of autopalatine. Autopalatine extending from lateral-ethmoid to pre-maxilla, articulating anteriorly with maxilla and pre-maxilla by a convex cartilaginous margin, and posteriorly with vomer internally and with lateral ethmoid externally. Medial concavity of autopalatine conspicuous to poorly developed. Lateroposterior process of autopalatine covering dorsally the metapterygoid, and covered by extensor tentaculi. Anguloarticular robust, articulating with quadrate posteriorly and diverging anteriorly to form a convex margin connected to Meckel's cartilage. Anguloarticular with a well developed posterior process ventral to the joint articulation with quadrate, connected to dorsanterior process of interopercle externally and to external tip of posterior ceratohial internally by long ligaments. Anguloarticular with a well developed dorsanterior process bordering Merckel's cartilage dorsally and reaching the coronoid process of dentary. Dentary robust, triangular externally, with two almost regular row of conical, sharp teeth curved backwards. Coronoid process of dentary well developed, wide, to which attaches adductor mandibulae, and connected to maxillary barbel elastic cartilage by the coronomeckelian fibrocartilage. Ventral internal margin of dentary with a depression to which attaches intermandibularis.
Preopercle long and narrow, curved inwards, tightly attached to hyomandibular and quadrate, forming the lateroventral border of hyomandibular arch, covered by adductor mandibulae anteriorly and from which originates protractor operculi posteriorly. Interopercle long and narrow, convex ventrally, with three to four almost regular rows of long odontodes, the posterior ones gradually longer than the anterior. Dorsanterior process of interopercle T-shaped, connected anteriorly to posterior process of anguloarticular, medially to posterior ceratohyal and posteriorly to the ventral process of opercle by ligaments. Another ligament also connects the ventral tip of opercle to the dorsal, internal margin of interopercle. Opercle long, reaching ventrally interopercle and dorsally the posterior process of hyomandibula, articulating with hyomandibula at medial anterior margin. Posterior process of opercle robust, bearing a patch of odontodes on posterior margin. Ventral tip of opercle medial to interopercle and connected to its internal margin by a robust ligament. Ventral process of opercle connected to dorsanterior process of interopercle posteriorly. Dorsal process of opercle with insertion for adductor operculi medially and for levator operculi externally.
Branchial skeleton and associated structures: Urohyal robust, with long, very narrow posterior process, very long lateral sharpening processes forming a broad convex posterior margin for insertion of sternohyoideus, and anterior T-shaped process with condyles articulating ventrally with ventral hypohyals (Fig. 10). Urohyal foramen ovoid. Ventral hypohyal and anterior and posterior ceratohyals robust, tightly connected to each other by cartilages. Ventral hypohyal with deep articular fossa for the condyles of urohyal. Anterior ceratohyal with narrowing medial portion. External tip of posterior ceratohyal inserted between anterior portion of dorsanterior process of interopercle and anterior margin of interopercle. Two ligaments on posterior ceratohyal, one inserted on dorsal portion of posterior ceratohyal and connected to posterior portion of angulo-articular, and the other inserted on posterior tip of posterior ceratohyal, and connected dorsally to hyomandibula. Contralateral hyohyoideus inferioris inserted on anteroventral margins of anterior and posterior ceratohyals, and connected to each other by a median raphe. Contralateral interhyoideus inserted at ventral portion of the cartilage between anterior and posterior ceratohyals, passing ventrally to hyohyoideus inferioris and connected to each other by a median raphe. Seven or eight branchiostegal-rays, connected to each other by hyohyoideous superioris. Rays 1, 2, and 3 shorter and narrower, cylindrical, ray 1 sometimes reduced or absent. Rays 4, 5, and 6 with enlarged distal extremity supporting the elastic cartilage of opercle membrane, and attached proximally to the cartilage at the ventral contact of anterior and posterior ceratohyals. Ray 7 covered by interopercle and reaching the ventral margin of opercular patch of odontodes.
Basibranchials 2, hypobranchials 3, ceratobranchials 5, epibranchials 4, pharyngobranchials 2 (Fig. 10). Basibranchial 1 and 4 absent as ossified elements. Anterior portion of copula 1 cartilaginous, T-shaped, fused ventrally to dorsal margin of anterior process of urohyal and with small lateral processes connected to posterior, internal margins of ventral hypohyals. Basibranchial 2 and 3 ossified, connected to each other by their cartilaginous tips, forming a long rod. Basibranchial 3 with concave lateral margins for insertion of obliquus ventralis 2. Copula 2 cartilaginous, approximately hexagonal shaped. Anterior portion of copula 2 and posterior tip of basibranchial 3 covered dorsally by cartilaginous posterior portion of hypobranchials 3. Ventral margin of copula 2 with insertion for obliquus ventralis 3. Anterior margins of copula 2 bordered by cartilaginous posterior portion of hypobranchials 3, lateral and posterior margins bordered by cartilaginous anterior tips of ceratobranchial 4 and ceratobranchial 5, respectively.
Hypobranchial 1 wide with cartilaginous tips. Hypobranchial 2 boomerang-shaped, posterior half cartilaginous, anterior half ossified, forming a long anterior process that almost reaches the external posterior margin of hipobranchial 1 and from which originates obliquus ventralis 1. Hypobranchial 3 almost completely cartilaginous, with only the anterior tip ossified in a triangular shape, closely joined to anterior cartilaginous tip of ceratobranchial 3 and slightly displaced ventrally, with insertion for three muscles: at its ventral margin, for rectus comunis, which originates at the posterior margin of ventral hypohyal and inserts on the ventral margin of ceratobranchial 5; at its anterior tip, for rectus ventralis 2, which originates at the internal posterior margin of anterior ceratohyal; and at its external margin, for rectus ventralis 4, which originates at the internal margin of ceratobranchial 4.
Ceratobranchials slightly curved, with cartilaginous tips. Ceratobranchial 1 with internal tip wider than external, with a notch at the posterior internal margin for insertion of obliquus ventralis 1. Ceratobranchial 2 with a shallow concavity at its posterior margin, for insertion of obliquus ventralis 2. Ceratobranchial 3 with a pronounced concavity at its posterior margin limited posteriorly by a small process, for insertion of obliquus ventralis 3. Ceratobranchial 4 without notches or processes, with a ventral depression anteriorly to which attaches laterally the transversus ventralis 4, connecting the contralateral ceratobranchials 4. Internal margin of ceratobranchial 4 with insertion for adductor 4, which originates at the internal distal margin of epibranchial 4. Ceratobranchial 5 enlarged, bearing a patch of small, narrow conical teeth pointed dorsally at its medial portion, and connected to the accessory element of ceratobranchial 4 only by the external half of the posterior margin. Internal posterior margin of ceratobranchial 5 with insertion for adductor 5, which originates at the internal proximal margin of epibranchial 4. External margin of ceratobranchial 5 with insertion for transversus ventralis 5. Obliquus posterioris connecting the external posterior margin of ceratobranchial 5 to the cartilaginous connection of ceratobranchial 4 and epibranchial 4, covered externally by the accessory element of ceratobranchial 4.
Epibranchials 1, 2 and 3 narrow, rod-like, with cartilaginous tips. Epibranchial 1 with long, narrow and sharp anterior process, pointed outwards in acute angle, forming the margin of the first gill slit. External margin of epibranchial 1 with insertion for levator externus 1. Epibranchial 2 with small, acute process anteriorly forming the margin of the second gill slit, and with small, poorly developed process forming the margin of the third gill slit. External margin of epibranchial 2 with insertion for levator externus 2. Internal, cartilaginous tips of Epibranchial 1 and 2 placed anteriorly to pharyngobranchial 3. Levator internus 3 posteriorly directed, passing internally to levator internus 3 and 4, and inserted on the cartilaginous connection of epibranchial 2 and pharyngobranchial 3. Epibranchial 3 connected internally to external margin of pharyngobranchial 4, with conspicuous, posteriorly directed, uncinate process for attachment of obliquus dorsalis 3, which originates at the lateral external margin of pharyngobranchial 3. Internal dorsal margin of epibranchial 3 with insertion for levator externus 3, anteriorly directed, passing ventrally to obliquus dorsalis 3. Epibranchial 4 large, curved, with a wide dorsal margin slightly convex, covered with cartilage, joined to posterior half of tooth plate. Dorsal posterior margin of epibranchial 4 with insertion for obliquus dorsalis 4, which originates at the lateral external margin of pharyngobranchial 4. Internal dorsal margin of epibranchial 4 with insertion for levator externus 4, anteriorly directed, passing ventrally to obliquus dorsalis 4 and dorsally to obliquus dorsalis 3.
Pharyngobranchials 1 and 2 absent. Pharyngobranchial 3 elongate, narrow, rod-like, slightly depressed, with cartilaginous tips. Pharyngobranchial 4 ossified, curved, with ventral margin cartilaginous, joined tightly to dorsanterior half of tooth plate. Tooth plate well developed, curved, with one row of long, conic, internally curved teeth. Contralateral pharyngobranchials 3 and tooth plates connected to each other by transversi dorsalis 3 and 4, respectively.
Postcranial skeleton. Total vertebrae 39 (11), one specimen with 38 (LESCI-00258C). First complete hemal canal at 8th to 9th vertebrae. Usually 7 pairs of ribs, sometimes 8. Rib count asymmetrical in 4 specimens (one third of total counted). First ribs thicker, posteriormost gradually thinner.
Pectoral girdle with wide, curved cleithrum, becoming narrower at anterior half, joined together by very narrow anterolateral margin (Figs 11 and 12). Posterodorsal process of cleithrum reaching ventral surface of skull, inserted between the posterior internal process of postteporo-supracleithrum and the weberian capsule. External anterior margin of cleithrum with insertion for levator pectoralis, that attaches to ventral external border of posttemporo-supracleithrum. Scapulocoracoid narrow, with anterior process joined tightly to ventral surface of cleihtrum, and lateral processes forming two arches, one with the external margin (sometimes incomplete) and the other with the posterior margin of cleithrum (to which attaches proximal radial 2). External arch of scapulocoracoid separating arrector dorsalis and ventralis. Posterior portion of scapulocoracoid cartilaginous, articulating with the cartilaginous proximal radial 1. Proximal radial 1 cartilaginous, surrounded by the proximal portion of first pectoral fin. Proximal radial 2 ossified at its medial portion, with a narrow cartilaginous margin anteriorly articulating with internal depression of scapulocoracoid at the posterior arch of cleithrum, and a convex cartilaginous margin posteriorly for attachment of pectoral-fin rays. Distal radial 1 present as a very small, rounded cartilage posterior to the proximal radial 1 cartilage. First pectoral-fin ray robust, with relatively wide proximal process to which attaches, on an external concavity, the arrector dorsalis.
Basipterygium of pelvic girdle with external anterior process narrower and slightly shorter than internal anterior process (Fig. 13). Internal processes directed medially, sometimes joined together by a small cartilage. A short medial process may occur. Posterior margin of basipterygium with convex border to which attaches the pelvic-fin rays. Pelvic splint present.
Hemal and neural spine of the penultimate preural centrum (pu2) single, not divided, the hemal spine generally strongly compressed and slightly enlarged (Fig. 14). Epural absent. Neural spine of compound centrum reduced. Compound centrum with conspicuous ventral triangular process directed to but not reaching the hemal spine of penultimate preural centrum. Neural arch of compound centrum sometimes incomplete. Uroneural long, adjacent dorsally but not fused to upper hypural plate and reaching its distal margin. Parhypural fused to hypural 1+2, forming a trapezoidal lower hypural plate fused proximally to compound centrum. Hypural 3, 4, and 5 fused to each other, forming an autogenous, triangular upper hypural plate.
Laterosensory system. Laterosensory system reduced, with non-dendritic tubulae and simple pores (Fig. 7). Mandibular, preopercular and supratemporal canals, and parietal branch, absent. Supraorbital (divided in anterior and posterior segments), infraorbital (divided in anterior and posterior segments), otic, postotic and trunk canals present, with variable number of pores.
Supraorbital divided in: anterior segment with 2 pores, s1 and s2, and posterior segment with 2 pores, s3 and s6, in almost all specimens. Anterior and posterior segments continuous with three pores, s1, s2+s3 and s6, on left side of one specimen (LIRP-11010A) and on right side of four specimens. An additional pore between s3 and s6 (possibly s4) is present in one specimen on left side (LIRP-11010E) and in one specimen on right side (LESCI-00223B). Another additional pore medially to s6 is present in two specimens on left side (LIRP-11010A and LESCI-00223B). Anterior segment short and superficial, above the skin, medially to the contralateral nares, in the space between mesethmoid and the nasal cavity. Posterior segment free at its anterior portion, and entering the neurocranium at the anterior portion of frontals, running longitudinally throughout this bone until the anterior process of sphenotic-prootic-pterosphenoid, where it connects with the infraorbital and otic canals.
Infraorbital divided in: anterior segment, with 2 pores, i1 and i3, in about two thirds of specimens; and posterior segment, with 2 pores, i10 and i11, in all specimens (except on one specimen, LIRP-11010C, on left side). An additional pore is present on the medial posterior diagonal of the right i11 pore, in two specimens. Another additional pore is present on the medial posterior diagonal of the additional pore above cited, in one specimen (LIRP-11010B). Anterior segment short and superficial, above skin, lateral to nares. Posterior segment as a short, tubular canal entering the neurocranium by the anterior process of sphenotic-prootic-pterosphenoid, where it connects with the posterior segment of the supraorbital and the otic canals.
Otic without pores in all specimens. Canal running longitudinally throughout sphenotic-prootic-pterosphenoid, between the connection with the supraorbital and infraorbital canals anteriorly, at the postorbital process of skull, and the preoperculo-mandibular branch of postotic canal posteriorly.
Postotic with two pores, po1 (preoperculo-mandibular branch) and po2 (pterotic branch). One additional pore is present between po1 and po2 in one specimen on left side (LIRP-11010E). Canal running longitudinally throughout the pterotic and posttemporo-supracleithrum, with two dorsolateral external branches, the preoperculo-mandibular branch anteriorly (which opens as the po1) in the contact between pterotic and sphenotic-prootic-pterosphenoid, and the pterotic branch posteriorly (which opens externally as the po2) in the contact between pterotic and posttemporo-supracleithrum. The canal extends posteriorly from the neurocranium by the shared aperture of posttemporo-supracleithrum and weberian capsule, forming a tubule continuous with the ll1 pore.
Trunk canal with two pores, ll1 and ll2 in all specimens (except one specimen, LIRP-11010A, on right side). Trunk canal as a short, superficial tubule above the skin connecting the pores ll1 and ll2, in some specimens surrounded by a tubular ossification.
Coloration. Body background mostly yellowish to brownish on dorsal and lateral regions, gradually brighter ventrally. Brown to grey melanophores distributed on dorsal and lateral profiles, but absent on ventral profile. Melanophores aggregated on spots, that can vary in size from small dots (smaller than eye diameter) to big blotches (bigger than eye diameter), scattered throughout the body, aligned longitudinally and coalescing in longitudinal lateral stripes in all specimens. Dorsal central region of head darkened by concentration of melanophores, both superficial (above skin) and profound(above skull, below skin, seen by transparence), forming a conspicuous dark blotch, more visible in some specimens. Two small, dark blotches laterally external to the nares, sometimes barely visible. Fins translucent, with scattered melanophores on the base of fin-rays, except for the pelvic fin, on which melanophores are absent. Barbels usually with scattered melanophores, mostly at the base. In alcohol, coloration similar to life but becoming gradually paler (Figs 2-6 and 15 -16 ).
Type material. Holotype. Brazil, Goiás: Mambaí (Gruna da Tarimba cave system, main entrance, river conduit, 14°24'42.9"S, 46° 10'29.7"W, elevation 742 m), upper Tocantins River Basin, 69.7 mm SL, 29 Apr 2013, Bichuette, M.E., Gallão, J.E., Von Schimonsky, D.M., Rizzato, P.P., Borghezan, R. leg., LIRP-11009). Paratypes. Brazil, Goiás: Mambaí (Gruna da Tarimba cave system, main entrance, river conduit, 14°24'42.9"S, 46°10' 29.7"W, elevation 742 m), upper Tocantins River Basin: 4 specimens (3 alc, 1 S&D) collected with the holotype, 47.1-68.9 mm SL, LIRP-11010; 3 specimens (2 alc., 1 S&D), 55.2-73.5 mm SL, 27 Oct 2012, Bichuette, M.E., Simões, L.B., Sorbo-Fernandes, C. leg, LESCI-00223; 1 specimen, 71.1 mm SL, 27 Oct. 2012 (death in 10 Feb 2013), Bichuette, M.E., Simões, L.B., Sorbo-Fernandes, C. leg, LESCI-00228; 1 specimen (C&S), 76.5 mm SL, 27 Oct. 2012 (death in 13 Nov 2013), Bichuette, M.E., Simões, L.B., Sorbo-Fernandes, C. leg, LIRP-11011. Mambaí (Gruna da Tarimba, passage 2, 14°24'42.9"S, 46°10'29.7"W, elevation 742 m), upper Tocantins River Basin, 3 specimens, 29,1-62,8 mm SL, 30 Apr. 2013 (death in 10 Feb 2013 Bichuette, M.E., Gallão, J.E., Von Schimonsky, D.M., Rizzato, P.P., Borghezan, R. leg., LIRP-11012. Mambaí (Nova Esperança cave system, 14°25'54.1"S, 46°09' 16.4"W, elevation 743 m), upper Tocantins River Basin: 1 specimen, 53.9 mm SL, 31 Oct 2004, LESCI-00041; 1 specimen, 46.1 mm SL, 31 Oct 2004, LIRP-11013.
Distribution. Ituglanis boticario sp. nov. is known only from subterranean streams on the Mambaí karst area, belonging to the Rio Vermelho sub-basin, a tributary of Rio Paranã, upper Tocantins River Basin, on the northeastern region of Goiás state. Its presence was reported for at least two cave systems on the Mambaí municipality region (from north to south): the Tarimba cave system and the Nova Esperança cave system.
Etymology. The specific epithet – boticario – is in honor of Fundação O Boticário de Proteção à Natureza (FBPN) which financially supported a project for effective protection of Tarimba cave system, including the proposition of a Conservation Unit at Goiás State. A noun in apposition.
DISCUSSION
Taxonomic assignment. The genus Ituglanis was proposed by Costa & Bockmann (1993) based on the presence of three autapomorphies: the parieto-supraoccipital fontanel present as a small orifice at the posterior portion of the parieto-supraoccipital bone, sometimes absent (in I. apteryx, in the miniaturized I. macunaima Datovo & Landim, 2005, and in some specimens of the subterranean I. epikarsticus and I. mambai, at least – Datovo & Landim 2005, Bichuette & Trajano 2008, Datovo 2014); the presence of a concavity at the median portion of the autopalatine, sometimes shallow (in the subterranean I. mambai, at least – Bichuette & Trajano 2008); and the anterior extremity of sphenotic-prootic-pterosphenoid anteriorly directed. All three synapomorphies are present in the new species, justifying its inclusion in the genus.
Wosiacki et al. (2012), based on a sample of Ituglanis, proposed four additional characters as synapomorphies for the species included in the genus. Two were confirmed in the new species: five or more abdominal vertebrae and two or fewer vertebrae between the first pterygiophores of the dorsal and anal fins. The first may be in fact a synapomorphy for Ituglanis (Datovo 2014), but the latter occurs also in other members of Trichomycterinae and in some members of Glanapteryginae and Vandelinae (Datovo & de Pinna 2014).
The third character, the parapophysis of the first four free vertebrae directed medially, is dubious since only some, but not all, of those parapophysis in I. boticario are likewise directed. The same was observed by Datovo & de Pinna (2014) for the species of Ituglanis examined by them.
The fourth character, presence of 23 or more free vertebrae anterior to the first pterygiophore of the dorsal fin, is also dubious. As pointed out by Datovo & de Pinna (2014), besides occurring in the more derived glanapterygines, stegophilines and vandelines, and at least in the trichomycterine Trichomycterus paolence (Eigenmann, 1917), this character is not present in many Ituglanis species: at leastin I. nebulosus de Pinna & Keith, 2003, I. paraguassuensis, I. parahybae, and some individuals of I. australis, I. macunaima, and I. proops. The character was absent in the new species herein described, and also in the following species analyzed by us: I. passensis, I. bambui, I. ramiroi, and I. mambai.
Other characters proposed by Costa & Bokmann (1993) as useful to identify species of Ituglanis, although not exclusive, are also present in the new species: mouth subterminal, barbels well developed, eyes of moderate size, parasphenoid with a long posterior process (absent on the species of the TSVSG clade) and vomer with a long posterior process. Datovo & Bockmann (2010) proposed as synapomorphy for Trichomycterinae strictu sensu (excluding the "T." hasemani group and including Ituglanis) the levator internus 4 originating from the dorsal face of the posttemporo-supracleithrum. This character state is present in the new species, and also in the following species analyzed by us: Ituglanis passensis, I. bambui, I. epikarsticus, I. ramiroi, I. mambai, Ituglanis sp. 1 and Ituglanis sp 2, in addition to other species of Ituglanis and Trichomycterus cited in Datovo et al. (2012), Datovo (2014), and Datovo & de Pinna (2014).
Intrageneric comparisons. The new species herein described exhibits a set of character states that are unusual in the genus, including some of the highest counts for some characters, for example, the number of ribs, 7 or 8, and the number of pectoral-fin rays, 8 or 9. The presence of the anterior segment of the infraorbital laterosensory canal is another very unusual condition in the genus, shared only by four other species: I. proops, I. paraguassuensis, I. agreste and I. australis. Finally, the pigmentation forming longitudinal stripes is shared only by I. parahybae, I. cahyensis, I. australis, and I. apteryx. Taken in combination, these characters can be used to differentiate the new species from all congeners.
The pigmentation forming longitudinal stripes differs the new species from all congeners except for I. parahybae, I. cahyensis, I. australis and I. apteryx. From I. parahybae, I. cahyensis and I. apteryx, the new species differs by the absence of the anterior cranial fontanel (vs. presence), the presence of the anterior segment of the infraorbital laterosensory canal (vs. absence) and by the pectoral fin mostly with 8, sometimes 9 rays (vs. 5 or fewer rays). From I. parahybae, it is further distinguished by the presence of the supraorbital laterosensory canal (vs. absence). From I. cahyensis, it is further distinguished by the presence of the anterior segment of the supraorbital laterosensory canal (vs. absence), the number of vertebrae, 38-39 (vs. 40) and the number of ribs, 7-8 pairs (vs. 4 pairs). From I. apteryx, it is further distinguished by the presence of the pelvic girdle and fin (vs. absence), the number of vertebrae, 38-39 (vs. 43-45) and the interruption of the supraorbital laterosensory canal in two segments, anterior and posterior, with four pores (vs. supraorbital laterosensory canal complete, with three pores). From I. australis, it differs in the greater number of pectoral-fin rays, 8-9 (vs. 6), the number of ribs, 7-8 (vs. 4-6 pairs) and the presence of a single upper hypural plate (vs. two upper hypural plates)
The number of ribs, 7-8 pairs, differs from the condition found in all congeners (6 or fewer; not seen for I. guayaberensis, I. herberti and I. metae) except for the subterranean species I. passensis, I. bambui, I. ramiroi and I. mambai. From the subterranean I. passensis, I. bambui, and I. ramiroi, it differs by the presence of well developed pigmentation (vs. absence), the presence of the anterior segment of the infraorbital laterosensory canal (vs. absence) and the larger eye, more than 7.0%, on average 8.9% of HL (vs. smaller than 8.0% of HL). From I. passensis and I. bambui, it is further distinguished by the presence of the supraorbital laterosensory canal (vs. absence). From I. ramiroi, it is further distinguished by the number of vertebrae, 38-39 (vs. 36‑37).
The presence of the anterior segment of the infraorbital laterosensory canal differs from the condition found in all other congeners except for the epigean I. proops, I paraguassuensis, I. agreste and I. australis. From these species, it can be distinguished by the large number of ribs, 7-8 pairs (vs. 6 or fewer), the number of vertebrae, 38-39 (vs. 39-41 for I. proops and I. australis, 36 or fewer in I. paraguassuensis and I. agreste) and the pectoral-fin ray count, usually 8, less commonly 9 (vs. 7 or fewer). From I. proops, I. paraguassuensis and I. agreste, it can be further distinguished by the pigmentation forming longitudinal stripes and the absence of the anterior cranial fontanel (vs. presence; not seen for I. australis).
The number of pectoral-fin rays, 8, less commonly 9, differs from the condition found in all other congeners (7 or fewer) except for the subterranean I. passensis, I. bambui, I. epikarsticus, I. ramiroi, and I. mambai. From I. epikarsticus, it differs by the presence of well developed pigmentation (vs. absence), the presence the supraorbital and infraorbital laterosensory canal (vs. absence), the larger eye, more than 7.0%, on average 8.9% of HL (vs. smaller than 3.0% of HL), the number of ribs, 7-8 pairs (vs. 5), the number of vertebrae, 39 (vs. 36) and the larger body, reaching more than 70 mm SL (vs. maximum 45.7 mm SL).
The geographically closest congener of Ituglanis boticario is the subterranean I. mambai. Both species occur in the tributaries of the Rio Corrente, an affluent of Rio Paranã, in the neighborhood of Mambaí municipality. However, the caves in which these species occur are located in different sub-basins: the Lapa do Sumidouro cave-system, type-locality of I. mambai, in the Rio dos Buritis sub-basin, and the Gruna da Tarimba and Nova Esperança cave-systems, type-localities of I. boticario, in the Rio Vermelho sub-basin (Fig. 1). Although adjacent, these two river basins are parallel to each other and separated by areas of higher elevations, precluding transversal migrations between them. Additionally, morphological differences between I. boticario and I. mambai enable the distinction of the former as a new species.
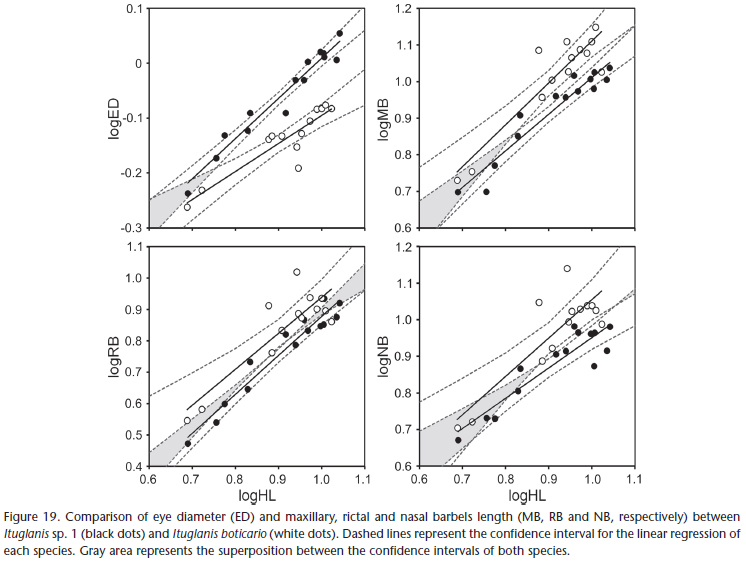
The main differences between I. boticario and I. mambai are the pigmentation pattern, and eye and barbel size. In I. boticario, all specimens analyzed exhibit longitudinal stripes on the body, which are absent from all specimens of I. mambai analyzed (compare Figs 15 -16 with Figs 17 -18 ). In both species, the pigmentation pattern (longitudinal stripes in I. boticario; homogeneous light brown with irregular small spots distributed mainly on dorsum and flanks of I. mambai) is ontogenetically stable (juvenile specimens of I. mambai may only be less pigmented than adults; Bichuette & Trajano 2008). The eyes of I. boticario are also conspicuously smaller when compared to I. mambai, whereas the barbels are longer (Fig. 19).
A fourth character could be used to differentiate I. mambai and I. boticario, the presence of the anterior segment of the infraorbital laterosensory canal. In I. boticario, this segment is present in most specimens (about two thirds of the specimens analyzed), whereas in I. mambai it is absent, except for the left side in three specimens. This variability in the presence of the anterior segment of the infraorbital laterosensory canal was also reported for I. australis (Datovo & de Pinna 2014) and may occur in other trichomycterines. In I. proops, however, it was present in all specimens analyzed by us. In other species of Ituglanis, the anterior segment of the infraorbital is present only in I. paraguassuensis and I. agreste, but no information about variability is presented by the authors. Although the presence/absence of laterosensory canal segments is useful to differentiate and diagnose species within Ituglanis (Datovo & Landim 2005, Sarmento-Soares et al. 2006, Campos-Paiva & Costa 2007, Wosiacki et al. 2012, Lima et al. 2013, Datovo 2014, Datovo & de Pinna 2014), informations about variability should be provided in order to reinforce the utility of these characters.
Comments on the phylogeny of Ituglanis
Up to date, the relationships between the species of Ituglanis are still obscure. The only attempt to identify monophyletic groups within the genus was made by de Pinna & Keith (2003), who suggested a separation in two distinct clades, one encompassing species from northern South America (from Amazon to Guyana) which share a small number of ribs, three pairs or fewer, and the other encompassing species from southern South America (Paraná/Paraguay, Ribeira do Iguape, Paraíba do Sul and Southeastern drainages in Brazil and Uruguay) which share a greater rib count, more than five pairs. The species described subsequently (Bichuette & Trajano 2004, Datovo & Landim 2005, Sarmento-Soares et al. 2006, Campos-Paiva & Costa 2007, Bichuette & Trajano 2008, Wosiacki et al. 2012, Lima et al. 2013, Datovo 2014, Datovo & de Pinna 2014) conformed to that distribution, giving partial support for the hypothesis of de Pinna & Keith (2003).
The two described epigean species of Ituglanis from the Rio Tocantins Basin, I. macunaima (Araguaia River Basin, a tributary of Tocantins river) and I. ina Wosiacki, Mendonça & Dutra, 2012 (lower Tocantins River Basin) have fewer ribs, being more closely related to the northern South American species of Ituglanis. However, all species from the upper Tocantins River Basin have a greater number of ribs, including the new species herein described. Therefore, all species from the upper Rio Tocantins basin are probably more closely related to the southern South American species.
The reduction in the number of ribs was interpreted by de Pinna & Keith (2003) as an apomorphic condition of Ituglanis, since basal trichomycterids have nine or more pairs of ribs. The extreme reduction to three or fewer, seen in the northern South American clade, should be interpreted as a derived character within the genus, and therefore the large number of ribs of the southern South American species must be considered a plesiomorphic condition for Ituglanis. Other possibly plesiomorphic conditions are also seen in some species from the southern South America (see below), suggesting that they represent the most basal members of the genus.
The anterior segment of the infraorbital laterosensory canal is present only in five species of Ituglanis, all of which are from the southern South American group: I. proops, I. paraguassuensis, I. agreste, I. australis, and I. boticario. Since the anterior segment of the infraorbital laterosensory canal is present in the most basal subfamilies of Trichomycteridae, Copionodontinae (de Pinna 1992, Campanario & de Pinna 2000, Bichuette et al. 2008) and Trichogeninae (de Pinna et al. 2010), and also in many trichomycterines (Arratia & Huaquin 1995), it is more parsimonious to interpret its absence as a derived condition shared by most Ituglanis species, and its presence as a plesiomorphic condition shared by the six species cited above. Accordingly, those species (except I. australis) also exhibit other putatively plesiomorphic character states, for example, greater number of pectoral-fin rays, lower number of vertebrae and insertion of the first pterygiophore of the dorsal-fin before the 23th vertebrae, besides the large number of ribs. Therefore, it is possible that those five species are some of the most basal members of the genus, and a more detailed analysis of the characters exhibited by them would be useful to investigate the evolutionary relationships between the species of Ituglanis.
Troglobitic status
Since the discovery of the first cave animals, many authors have been trying to apply a useful classification of the subterranean fauna that reflects their evolutionary and ecological relationships to their environment (for a critical review – see Trajano 2012). Currently, the most used and useful classification is the so-called Schiner-Racovitza, which after important contributions from Barr (1967) and Trajano (2012), can be presented as follows: animals with source populations in epigean environments using the hypogean environment as part of their habitat, but which must leave it in order to complete their life cycles, are called "trogloxenes"; animals with source populations both in hypogean and epigean habitats, and whose individuals commute from these habitats, thus maintaining the gene flow between the populations, are called "troglophiles"; and animals with source populations exclusively in hypogean environments, even when having sink populations in epigean habitats, are called "troglobites". This classification provides a useful scheme to analyze the ecological relationship of the populations/species to the subterranean environment even in the absence of accentuated troglomorphisms, typical (but not exclusive) of troglobites and generally used to identify them in the absence of other lines of evidence. Following this scheme, the new species herein described can be classified as a troglobite, since it is found only in subterranean streams of two contiguous cave systems of the Mambaí municipality, neither of which connected directly with epigean streams (through sinkholes or resurgences, for instance).
When compared with other troglobitic fish species from all over the world (Romero & Paulson 2001, Proudlove 2006), even from other Brazilian regions (Trajano & Bichuette 2010), the troglobite fishes from northeastern Goiás are much less specialized, exhibiting low degree of troglomorphism (Bichuette & Trajano 2003, 2008, Trajano et al. 2004, Trajano & Bichuette 2010). It has been argued that there is a positive correlation between the time of isolation in the subterranean environment and the degree of specialization (troglomorphisms) of a given species, in such a way that species isolated for longer periods of time have more developed and less variable troglomorphic characters (Wilkens 1982, Trajano 1995, Bichuette & Trajano 2008 – but see also Trajano 2007). From this, it can be concluded that the subterranean ichthyofauna of northeastern Goiás has been isolated for a shorter time when compared with other Brazilian regions (Trajano et al. 2004).
Considering all subterranean species of Ituglanis collectively, there is a wide range of interspecific variation in their degree troglomorphism: in I. mambai the eyes are comparable to those of epigean species but the pigmentation is slightly reduced; in I. boticario, the pigmentation is comparable to epigean species but the eyes are slightly reduced; in I. bambui, the pigmentation and eyes are reduced, but are still intermediary between the epigen Ituglanis and the more troglomorphic I. passensis, I. epikasticus and I. ramiroi, which have very reduced pigmentation and eyes. This particular situation, in which several related troglomorphic species exhibit a range of troglomorphism from less to more specialized, is very rare, and is known to occur only in the North American amblyopsids (Poulson 1963, Niemiller & Poulson 2010). Still, the case of the subterranean Ituglanis is remarkable, since its range is very restricted, and there is geological and morphological evidence (e.g., the mosaic of characters exhibited by the species – see Table III) that the colonization of the subterranean environment occurred independently in each species (Bichuette & Trajano 2008). This particularity makes Ituglanis an excellent model to test several hypotheses on the origin and evolution of subterranean animals.
Conservation remarks
Bichuette & Trajano (2008) expressed concern about the conservation of the Mambaí karst area, where I. boticario occurs. The authors described, for the same region, the subterranean I. mambai, which occur in a relatively large population at the Lapa do Sumidouro cave. In contrast, I. boticario occurs in small population densities, about 0.05-0.3 inds.m-2, and at the Tarimba cave system its distribution is restricted to the upstream part of the cave system. The fish inhabit base level streams, with small depths (less than 0.80 cm) and no more than 1m width, with moderate water current (Figs 20-27). The bottom of the river is formed mainly by pebbles and silt. The microhabitats specificity exhibited by I. boticario makes it vulnerable to continuous cave usage (e.g. touristic visitation) or alterations or interferences of the epigean areas nearby the subterranean system (e.g., deforestation, pollution). Furthermore, the cave system recharges are apparently far from the surroundings of the cave system, which implies that the cave is very fragile, since there are many deforested areas in the region.
The Tarimba cave system is one of the most important caves in Goías and it is the largest in the Mambaí region, extending for about 12 km (União Paulista de Espeleologia, pers. comm.). However, Mambaí is a poorly developed region, with native vegetation altered by deforestation, and there are no short- to medium-term conservation projects for the area. The only conservation unit in the region is the "Área de Proteção Ambiental (APA) das Nascentes do Rio Vermelho", created in 2001, but which has not been effective to ensure the preservation of the subterranean systems and the epigean areas nearby.
The occurrence of a troglobitic species is considered sufficient to elevate the cave system to the higher relevance category predicted by the current Brazilian legislation. Besides the troglobitic I. boticario, the cave system is also inhabited by at least one species of characin (Astyanax aff. fasciatus), two species of bats and a diversity of troglobitic and non-troglobitic invertebrates (M.E. Bichuette, unpubl. data), representing an area of high diversity for subterranean fauna in Brazil. Based on this, we strongly recommend the creation of a conservation unit of higher category including at least all the Tarimba cave system and its areas of influence, in order to ensure the integral protection of the subterranean system and its biospeleological patrimony.
ACKNOWLEDGEMENTS
We thank the financial support from Fundação Grupo Boticário de Proteção à Natureza for the project "Diagnóstico Ambiental da Área de Influência e Ambientes Subterrâneos do Sistema Cárstico da Gruna da Tarimba (Mambaí-GO): Proposta Para a Delimitação de Unidade de Conservação de Proteção Integral" (#0941-20121), which provided the opportunity to study the Tarimba cave system and other localities of the Mambaí karstic area, in order to generate scientific arguments for the proposition of a Conservation Unit in the area, and also the União Paulista de Espeleologia (UPE), for information on the development of the Tarimba cave system. We also thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), for the continuous financial support of our research with cave fishes (MEB: 2010/08459-4; PPR: 2009/15030-7, 2011/06736-3 and 2011/15429-7). R. S. Martinelli provided some photos and authorization for their reproduction. We are greatly indebted to the colleagues who helped us in the field, Jonas E. Gallão, Diego M. Von Schimonsky, and Emílio Calvo. Ricardo Castro and Flávio Bockmann provided the authorization for the use of the radiographic device housed in the Laboratório de Ictiologia de Ribeirão Preto. Instituto Chico Mendes de Conservação da Biodiversidade (ICMBIO) and Sistema de Autorização e Informação em Biodiversidade (SISBio) provided the licenses for the collection of specimens. Two anonymous reviewers gave valuable contributions to the final version of this manuscript.
LITERATURE CITED
Ab'Saber, A.N. 1977. Os domínios morfoclimáticos na América do Sul. Geomorfologia 52: 1-21.
Adriaens, D.; J.N. Baskin & H. Coppens. 2010. Evolutionary morphology of trichomycterid catfishes: about hanging on and digging in, p. 337-362. In: J.S. Nelson; H.P. Schultze & M.V.H. Wilson (Eds). Origin and phylogenetic interrelationships of Teleosts. Verlag, Dr. Friedrich Pfeil, 480p.
Arratia, G. & L. Huaquin. 1995. Morphology of the lateral line system and of the skin of diplomystid and certain primitive loricarioid catfishes and systematic and ecological considerations. Bonner Zoologische Monographien 36: 1‑110.
Barr, T.C. 1967. Observations on the ecology of caves. The American Naturalist 101 (922): 475-491
Bichuette, M.E. & P.P. Rizzato. 2012. A new species of cave catfish from Brazil, Trichomycterus rubbioli sp. nov., from Serra do Ramalho karstic area, São Francisco River basin, Bahia State (Siluriformes: Trichomycteridae). Zootaxa 3480: 48-66.
Bichuette, M.E. & E. Trajano. 2003. Epigean and subterranean ichthyofauna from the São Domingos karst area, Upper Tocantins River basin, Central Brazil. Journal of Fish Biology 63: 1100-1121.
Bichuette, M.E. & E. Trajano. 2004. Three new subterranean species of Ituglanis from Central Brazil (Siluriformes: Trichomycteridae). Ichthyological Exploration of Freshwaters 15 (3): 243-256.
Bichuette, M.E. & E. Trajano. 2008. Ituglanis mambai, a new subterranean catfish from a karst area of Central Brazil, rio Tocantins basin (Siluriformes: Trichomycteridae). Neotropical Ichthyology 6 (1): 9-15.
Bichuette, M.E.; M.C.C. de Pinna & E. Trajano. 2008. A new species of Glaphyropoma: the first subterranean copionodontine catfish and the first occurrence of opercular odontodes in the subfamily (Siluriformes: Trichomycteridae). Neotropical Ichthyology 6 (3): 301-306. doi: 10.1590/S1679-62252008000300002.
Bockmann, F.A.; L. Casatti & M.C.C. de Pinna. 2004. A new species of trichomycterid catfish from the Rio Paranapanema basin, southeastern Brazil (Teleostei: Siluriformes), with comments on the phylogeny of the family. Ichthyological Exploration of Freshwaters 15 (3): 225-242.
Campanario, C.M. & M.C.C. de Pinna. 2000. A new species of the primitive trichomycterid subfamily Copionodontinae from norheastern Brazil (Teleostei: Trichomycteridae). Ichthyological Explorations of Freshwaters 11: 369-375.
Campos-Paiva, R.M. & W.J.E.M. Costa. 2007. Ituglanis paraguassuensis sp. nov. (Teleostei: Siluriformes: Trichomycteridae): a new catfish from the rio Paraguaçu, northeastern Brazil. Zootaxa 1471: 53-59.
Carvalho, M.; F.A. Bockmann & M.R. de Carvalho. 2013. Homology of the fifth epibranchial and accessory elements of the ceratobranchials among Gnathostomes: insights from the development of Ostaiophysans. PLOS ONE 8 (4): 1-21. doi:10.1371/journal.pone.0062389
Castellanos-Morales, C.A. 2007. Trichomycterus santanderensis: A new species of troglomorphic catfish (Siluriformes: Trichomycteridae) from Colombia. Zootaxa 1541: 49-55
Cordeiro, L.M.; R. Borghezan & E. Trajano. 2013. Distribuição, riqueza e conservação dos peixes troglóbios da Serra da Bodoquena, MS (Teleostei: Siluriformes). Revista da Biologia 10 (2): 21-27.
Costa, W.J.E.M. & F.A. Bockmann. 1993. Un nouveau genre néotropical de la famille des Trichomycteridae (Siluriformes: Loricarioidei). Revue Française d'Aquariologie et Herpetologie 20 (2): 43-46.
Datovo, A. 2014. A new species of Ituglanis from the Rio Xingu basin, Brazil, and the evolution of pelvic fin loss in trichomycterid catfishes (Teleostei: Siluriformes: Trichomycteridae). Zootaxa 3790 (3): 466-476. doi: 10.11646/zootaxa.3790.3.5
Datovo, A. & F.A. Bockmann. 2010. Dorsolateral head muscles of the catfish families Nematogenyidae and Trichomycteridae (Siluriformes: Loricarioidei): comparative anatomy and phylogenetic analysis. Neotropical Ichthyology 8 (2):193-246. doi: 10.1590/S1679-62252010000200001
Datovo, A. & M.C.C. de Pinna. 2014. A new species of Ituglanis representing the southernmost record of the genus, with comments on phylogenetic relationships (Teleostei: Siluriformes: Trichomycteridae). Journal of Fish Biology 84 (2): 314-327. doi: 10.1111/jfb.12285
Datovo, A. & M.I. Landim. 2005. Ituglanis macunaima, a new catfish from the rio Araguaia basin, Brazil (Siluriformes: Trichomycteridae). Neotropical Ichthyology 3 (4): 455-464. doi: 10.1590/S1679-62252005000400002
Datovo, A. & R.P. Vari. 2013. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLOS ONE 8: e60846. doi: 10.1371/journal.pone.0060846
Datovo, A.; M. Carvalho & J. Ferrer. 2012. A new species of the catfish genus Trichomycterus from the La Plata River basin, southern Brazil, with comments on its putative phylogenetic position (Siluriformes: Trichomycteridae). Zootaxa 3327: 33-44.
de Pinna, M.C.C. 1992. A new subfamily of Trichomycteridae (Teleostei, Siluriformes), lower loricarioid relationships and a discussion on the impact of additional taxa for phylogenetic analysis. Zoological Journal of the Linnean Society 106: 175-229. doi: 10.1111/j.1096-3642.1992.tb01247.x.
de Pinna, M.C.C. 1998. Phylogenetic relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses, p. 279-330. In: L.R. Malabarba; R.E. Reis; R.P. Vari; Z.M.S. Lucena & C.A.S. Lucena (Eds). Phylogeny and Classification of Neotropical Fishes. Porto Alegre, Edipucrs, 603p.
de Pinna, M.C.C. & P. Keith. 2003. A new species of the catfish genus Ituglanis from French Guyana (Osteichthyes: Siluriformes: Trichomycteridae). Proceedings of the Biological Society of Washington 116 (4): 873-882.
De Pinna, M.C.C. & W.B. Wosiacki. 2003. Family Trichomycteridae (Pencil or parasitic catfishes), p. 270-290. In: R.E. Reis; S.O. Kullander & C.J. Ferraris. Check list of the freshwater fishes of South and Central America. Porto Alegre, Edipucrs, 729p.
De Pinna, M.C.C.; J.L. Helmer; H.A. Britski & L.R. Nunes. 2010. A new species of Trichogenes from the rio Itapemirim drainage, southeastern Brazil, with comments on the monophyly of the genus (Siluriformes: Trichomycteridae). Neotropical Ichthyology 8 (4): 707-717. doi: 10.1590/S1679-62252010000400002
Eschmeyer, W.N. & J.D. Fong. 2014. Species of Fishes by family/subfamily. Available online at: http://researcharchive. calacademy.org/research/Ichthyology/catalog/fishcatmain.asp [Accessed March, 2014] .
Fernández, L. & S.A. Schaeffer. 2005. New Trichomycterus (Siluriformes: Trichomycteridae) from an offshore island of Colombia. Copeia 2005 (1): 68-76. doi: 10.1643/CI-04-177R1
Fernández, L. & R. Vari. 2012. New species of Trichomycterus (Teleostei: Siluriformes) from the Andean Cordillera of Argentina and the second record of the genus in thermal waters. Copeia 2012 (4): 631-636. doi: 10.1643/CI-12-035
Karmann, I. & L.E. Sánchez. 1979. Distribuição das rochas carbonáticas e províncias espeleológicas do Brasil. Espeleo-Tema 13: 105-167.
Lima, S.M.Q.; C.P. Neves; R.M. Campos-Paiva. 2013. Ituglanis agreste, a new catfish from the rio de Contas basin, northeastern Brazil (Siluriformes: Trichomycteridae). Neotropical Ichthyology 11 (3): 523-524. doi: 10.1590/S1679-62252013000300005
Majer, A.P.; F.P. Santos; P.A. Basile & E. Trajano. 2003. Invertebrados aquáticos de cavernas da Área Cárstica de São Domingos, Nordeste de Goiás. O Carste 15: 126-131.
Myers, G.S. & S.H. Weitzman. 1966. Two remarkable new trichomycterid catfishes from the Amazon basin in Brazil and Colombia. Journal of Zoology 149 (3): 277-287. doi: 10.1111/j.1469-7998.1966.tb04049.x
Nelson, J.S. 2006. Fishes of the world. New York, John Wiley and Sons, 4th ed., 624p.
Nico, L.G. & M.C.C. de Pinna. 1996. Confirmation of Glanapteryx anguilla (Siluriformes, Trichomycteridae) in the Orinoco River Basin, with notes on the distribution and habitats of the Glanapteryginae. Ichthyological Exploration of Freshwaters 7 (10): 27-32.
Niemiller, M.L. & T.L. Poulson. 2010. Subterranean fishes of North America: Amblyopsidae, p. 169-280. In: E. Trajano; M.E. Bichuette & B.G. Kapoor (Eds). Biology of Subterranean Fishes. Enfield, Science Publishers, 480p.
Nimer, E. 1979. Climatologia do Brasil. Rio de Janeiro, SUPREN, 421p.
Poulson, T.L. 1963. Cave adaptation in amblyopsid fishes. American Midland Naturalist 70: 257-290. doi: 10.2307/2423056
Proudlove, G.S. 2006. Subterranean fishes of the world: an account of the subterranean (hypogean) fishes described up to 2003 with a bibliography 1541-2004. Moulis, International Society for Subterranean Biology, 300p.
Proudlove, G.S. 2010. Biodiversity and distribution of the subterranean fishes of the world, p. 41-63. In: E. Trajano; M.E. Bichuette & B.G. Kapoor (Eds). Biology of Subterranean Fishes. Enfield, Science Publishers, 480p.
Rheims, C.A. & F. Pellegatti-Franco. 2003. Invertebrados terrestres de cavernas da área cárstica de São Domingos, Nordeste de Goiás. O Carste 15 (4): 132-127.
Romero, A. & K.M. Paulson. 2001. It's a wonderful hypogean life: a guide to the troglomorphic fishes of the world. Environmental Biology of Fishes 62: 13-41.
Sarmento-Soares, L.M.; R.F. Martins-Pinheiro; A.T. Aranda & C.C. Chamon. 2006. Ituglanis cahyensis, a new catfish from Bahia, Brazil (Siluriformes: Trichomycteridae). Neotropical Ichthyology 4 (3): 309-318. doi: 10.1590/S1679-62252006000300002
Schaefer, S.A. & A.E. Aquino. 2000. Postotic laterosensory canal and pterotic branch homology in catfishes. Journal of Morphology 246: 212-227. doi: 10.1002/1097-4687(200012) 246:3%3C212::AID-JMOR5%3E3.0.CO;2-S
Secutti, S.; R.E. Reis & E. Trajano. 2011. Differentiating cave Aspidoras catfish from a karst area of Central Brazil, upper rio Tocantins basin (Siluriformes: Callichthyidae). Neotropical Ichthyology 9 (4): 689-695. doi: 10.1590/S1679-62252011005000045
Song, J. & L.R. Parenti. 1995. Clearing and staining whole fish specimens for simultaneous demonstrations of bone, cartilage and nerves. Copeia 1995 (1): 114-118. doi: 10.2307/1446805
Simões, L.B.; T.S. Ferreira & M.E. Bichuette. 2013. Aquatic biota of different karst habitats in epigean and subterranean systems of Central Brazil – visibility versus relevance of taxa. Subterranean Biology 11: 55-74.
Srinivasachar, H.R. 1958. Development of the skull in catfishes. Part V. Development of the skull in Heteropneustes fossilis (Bloch). Proceeding of the National Institute of Sciences of India Part B 1958: 165-190.
Trajano, E. 1995. Evolution of tropical troglobites: Applicability of the model of Quaternary climatic fluctuations. Serie documents – Laboratoire souterrain du C.N.R.S, Moulis 22: 203-209.
Trajano, E. 2007. The challenge of estimating the age of subterranean lineages: examples from Brazil. Time in Karst: 191-198
Trajano, E. 2012. Ecological classification of subterranean organisms, p. 275-277. In: W.B. White & D.C. Culver (Eds). Encyclopedia of caves. Amsterdam, Academic Press, 2nd ed., 945p.
Trajano, E. & M.E. Bichuette. 2010. Subterranean Fishes of Brazil, p. 331-355. In: E. Trajano; M.E. Bichuette & B.G. Kapoor (Eds). Biology of Subterranean Fishes. Enfield, Science Publishers, 480p.
Trajano, E.; R.E. Reis & M.E. Bichuette. 2004. Pimelodella spelaea: a new cave catfish from Central Brazil, with data on ecology and evolutionary considerations (Siluriformes: Heptapteridae). Copeia 2004 (2): 315-325. doi: 10.1643/CI-03-144R1.
Wilkens, H. 1982. Regressive evolution and phylogenetic age: the history of colonization of freshwaters of Yucatan by fish and crustacea. Texas Memorial Museum Bulletin 28 (1982): 237-243.
Winterbottom, R. 1974. A descriptive synonymy of the striated muscles of the Teleostei. Proceedings of the Academy of Natural Sciences of Philadelphia 215 (12): 225-317.
Wosiacki, W.B.; G.M. Dutra & M.B. Mendonça. 2012. Description of a new species of Ituglanis (Siluriformes: Trichomycteridae) from Serra dos Carajás, rio Tocantins basin. Neotropical Ichthyology 10 (3): 547-554.
Zuanon, J. & I. Sazima. 2004. Natural history of Stauroglanis gouldingi (Siluriformes: Trichomycteridae), a miniature sand-dwelling candiru from central Amazonian streamlets. Ichthyological Exploration of Freshwaters 15: 201-208.
Zuanon, J.; F.A. Bockmann & I. Sazima. 2006. A remarkable sand-dwelling fish assemblage from central Amazonia, with comments on the evolution of psamophily in South American freshwater fishes. Neotropical Ichthyology 4 (1) 107-118. doi: 10.1590/S1679-62252006000100012
Submitted: 05.V.2014
Accepted: 21.X.2014
- Ab'Saber, A.N. 1977. Os domínios morfoclimáticos na América do Sul. Geomorfologia 52: 1-21.
- Adriaens, D.; J.N. Baskin & H. Coppens. 2010. Evolutionary morphology of trichomycterid catfishes: about hanging on and digging in, p. 337-362. In: J.S. Nelson; H.P. Schultze & M.V.H. Wilson (Eds). Origin and phylogenetic interrelationships of Teleosts. Verlag, Dr. Friedrich Pfeil, 480p.
- Arratia, G. & L. Huaquin. 1995. Morphology of the lateral line system and of the skin of diplomystid and certain primitive loricarioid catfishes and systematic and ecological considerations. Bonner Zoologische Monographien 36: 1‑110.
- Barr, T.C. 1967. Observations on the ecology of caves. The American Naturalist 101 (922): 475-491
- Bichuette, M.E. & P.P. Rizzato. 2012. A new species of cave catfish from Brazil, Trichomycterus rubbioli sp. nov., from Serra do Ramalho karstic area, São Francisco River basin, Bahia State (Siluriformes: Trichomycteridae). Zootaxa 3480: 48-66.
- Bichuette, M.E. & E. Trajano. 2003. Epigean and subterranean ichthyofauna from the São Domingos karst area, Upper Tocantins River basin, Central Brazil. Journal of Fish Biology 63: 1100-1121.
- Bichuette, M.E. & E. Trajano. 2004. Three new subterranean species of Ituglanis from Central Brazil (Siluriformes: Trichomycteridae). Ichthyological Exploration of Freshwaters 15 (3): 243-256.
- Bichuette, M.E. & E. Trajano. 2008. Ituglanis mambai, a new subterranean catfish from a karst area of Central Brazil, rio Tocantins basin (Siluriformes: Trichomycteridae). Neotropical Ichthyology 6 (1): 9-15.
- Bichuette, M.E.; M.C.C. de Pinna & E. Trajano. 2008. A new species of Glaphyropoma: the first subterranean copionodontine catfish and the first occurrence of opercular odontodes in the subfamily (Siluriformes: Trichomycteridae). Neotropical Ichthyology 6 (3): 301-306. doi: 10.1590/S1679-62252008000300002.
- Bockmann, F.A.; L. Casatti & M.C.C. de Pinna. 2004. A new species of trichomycterid catfish from the Rio Paranapanema basin, southeastern Brazil (Teleostei: Siluriformes), with comments on the phylogeny of the family. Ichthyological Exploration of Freshwaters 15 (3): 225-242.
- Campanario, C.M. & M.C.C. de Pinna. 2000. A new species of the primitive trichomycterid subfamily Copionodontinae from norheastern Brazil (Teleostei: Trichomycteridae). Ichthyological Explorations of Freshwaters 11: 369-375.
- Campos-Paiva, R.M. & W.J.E.M. Costa. 2007. Ituglanis paraguassuensis sp. nov. (Teleostei: Siluriformes: Trichomycteridae): a new catfish from the rio Paraguaçu, northeastern Brazil. Zootaxa 1471: 53-59.
- Carvalho, M.; F.A. Bockmann & M.R. de Carvalho. 2013. Homology of the fifth epibranchial and accessory elements of the ceratobranchials among Gnathostomes: insights from the development of Ostaiophysans. PLOS ONE 8 (4): 1-21. doi:10.1371/journal.pone.0062389
- Castellanos-Morales, C.A. 2007. Trichomycterus santanderensis: A new species of troglomorphic catfish (Siluriformes: Trichomycteridae) from Colombia. Zootaxa 1541: 49-55
- Cordeiro, L.M.; R. Borghezan & E. Trajano. 2013. Distribuição, riqueza e conservação dos peixes troglóbios da Serra da Bodoquena, MS (Teleostei: Siluriformes). Revista da Biologia 10 (2): 21-27.
- Costa, W.J.E.M. & F.A. Bockmann. 1993. Un nouveau genre néotropical de la famille des Trichomycteridae (Siluriformes: Loricarioidei). Revue Française d'Aquariologie et Herpetologie 20 (2): 43-46.
- Datovo, A. 2014. A new species of Ituglanis from the Rio Xingu basin, Brazil, and the evolution of pelvic fin loss in trichomycterid catfishes (Teleostei: Siluriformes: Trichomycteridae). Zootaxa 3790 (3): 466-476. doi: 10.11646/zootaxa.3790.3.5
- Datovo, A. & F.A. Bockmann. 2010. Dorsolateral head muscles of the catfish families Nematogenyidae and Trichomycteridae (Siluriformes: Loricarioidei): comparative anatomy and phylogenetic analysis. Neotropical Ichthyology 8 (2):193-246. doi: 10.1590/S1679-62252010000200001
- Datovo, A. & M.C.C. de Pinna. 2014. A new species of Ituglanis representing the southernmost record of the genus, with comments on phylogenetic relationships (Teleostei: Siluriformes: Trichomycteridae). Journal of Fish Biology 84 (2): 314-327. doi: 10.1111/jfb.12285
- Datovo, A. & M.I. Landim. 2005. Ituglanis macunaima, a new catfish from the rio Araguaia basin, Brazil (Siluriformes: Trichomycteridae). Neotropical Ichthyology 3 (4): 455-464. doi: 10.1590/S1679-62252005000400002
- Datovo, A. & R.P. Vari. 2013. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLOS ONE 8: e60846. doi: 10.1371/journal.pone.0060846
- Datovo, A.; M. Carvalho & J. Ferrer. 2012. A new species of the catfish genus Trichomycterus from the La Plata River basin, southern Brazil, with comments on its putative phylogenetic position (Siluriformes: Trichomycteridae). Zootaxa 3327: 33-44.
- de Pinna, M.C.C. 1992. A new subfamily of Trichomycteridae (Teleostei, Siluriformes), lower loricarioid relationships and a discussion on the impact of additional taxa for phylogenetic analysis. Zoological Journal of the Linnean Society 106: 175-229. doi: 10.1111/j.1096-3642.1992.tb01247.x.
- de Pinna, M.C.C. 1998. Phylogenetic relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses, p. 279-330. In: L.R. Malabarba; R.E. Reis; R.P. Vari; Z.M.S. Lucena & C.A.S. Lucena (Eds). Phylogeny and Classification of Neotropical Fishes. Porto Alegre, Edipucrs, 603p.
- de Pinna, M.C.C. & P. Keith. 2003. A new species of the catfish genus Ituglanis from French Guyana (Osteichthyes: Siluriformes: Trichomycteridae). Proceedings of the Biological Society of Washington 116 (4): 873-882.
- De Pinna, M.C.C. & W.B. Wosiacki. 2003. Family Trichomycteridae (Pencil or parasitic catfishes), p. 270-290. In: R.E. Reis; S.O. Kullander & C.J. Ferraris. Check list of the freshwater fishes of South and Central America. Porto Alegre, Edipucrs, 729p.
- De Pinna, M.C.C.; J.L. Helmer; H.A. Britski & L.R. Nunes. 2010. A new species of Trichogenes from the rio Itapemirim drainage, southeastern Brazil, with comments on the monophyly of the genus (Siluriformes: Trichomycteridae). Neotropical Ichthyology 8 (4): 707-717. doi: 10.1590/S1679-62252010000400002
- Eschmeyer, W.N. & J.D. Fong. 2014. Species of Fishes by family/subfamily. Available online at: http://researcharchive. calacademy.org/research/Ichthyology/catalog/fishcatmain.asp [Accessed March, 2014]
- Fernández, L. & S.A. Schaeffer. 2005. New Trichomycterus (Siluriformes: Trichomycteridae) from an offshore island of Colombia. Copeia 2005 (1): 68-76. doi: 10.1643/CI-04-177R1
- Fernández, L. & R. Vari. 2012. New species of Trichomycterus (Teleostei: Siluriformes) from the Andean Cordillera of Argentina and the second record of the genus in thermal waters. Copeia 2012 (4): 631-636. doi: 10.1643/CI-12-035
- Karmann, I. & L.E. Sánchez. 1979. Distribuição das rochas carbonáticas e províncias espeleológicas do Brasil. Espeleo-Tema 13: 105-167.
- Lima, S.M.Q.; C.P. Neves; R.M. Campos-Paiva. 2013. Ituglanis agreste, a new catfish from the rio de Contas basin, northeastern Brazil (Siluriformes: Trichomycteridae). Neotropical Ichthyology 11 (3): 523-524. doi: 10.1590/S1679-62252013000300005
- Majer, A.P.; F.P. Santos; P.A. Basile & E. Trajano. 2003. Invertebrados aquáticos de cavernas da Área Cárstica de São Domingos, Nordeste de Goiás. O Carste 15: 126-131.
- Myers, G.S. & S.H. Weitzman. 1966. Two remarkable new trichomycterid catfishes from the Amazon basin in Brazil and Colombia. Journal of Zoology 149 (3): 277-287. doi: 10.1111/j.1469-7998.1966.tb04049.x
- Nelson, J.S. 2006. Fishes of the world. New York, John Wiley and Sons, 4th ed., 624p.
- Nico, L.G. & M.C.C. de Pinna. 1996. Confirmation of Glanapteryx anguilla (Siluriformes, Trichomycteridae) in the Orinoco River Basin, with notes on the distribution and habitats of the Glanapteryginae. Ichthyological Exploration of Freshwaters 7 (10): 27-32.
- Niemiller, M.L. & T.L. Poulson. 2010. Subterranean fishes of North America: Amblyopsidae, p. 169-280. In: E. Trajano; M.E. Bichuette & B.G. Kapoor (Eds). Biology of Subterranean Fishes. Enfield, Science Publishers, 480p.
- Nimer, E. 1979. Climatologia do Brasil. Rio de Janeiro, SUPREN, 421p.
- Poulson, T.L. 1963. Cave adaptation in amblyopsid fishes. American Midland Naturalist 70: 257-290. doi: 10.2307/2423056
- Proudlove, G.S. 2006. Subterranean fishes of the world: an account of the subterranean (hypogean) fishes described up to 2003 with a bibliography 1541-2004. Moulis, International Society for Subterranean Biology, 300p.
- Proudlove, G.S. 2010. Biodiversity and distribution of the subterranean fishes of the world, p. 41-63. In: E. Trajano; M.E. Bichuette & B.G. Kapoor (Eds). Biology of Subterranean Fishes. Enfield, Science Publishers, 480p.
- Rheims, C.A. & F. Pellegatti-Franco. 2003. Invertebrados terrestres de cavernas da área cárstica de São Domingos, Nordeste de Goiás. O Carste 15 (4): 132-127.
- Romero, A. & K.M. Paulson. 2001. It's a wonderful hypogean life: a guide to the troglomorphic fishes of the world. Environmental Biology of Fishes 62: 13-41.
- Sarmento-Soares, L.M.; R.F. Martins-Pinheiro; A.T. Aranda & C.C. Chamon. 2006. Ituglanis cahyensis, a new catfish from Bahia, Brazil (Siluriformes: Trichomycteridae). Neotropical Ichthyology 4 (3): 309-318. doi: 10.1590/S1679-62252006000300002
- Schaefer, S.A. & A.E. Aquino. 2000. Postotic laterosensory canal and pterotic branch homology in catfishes. Journal of Morphology 246: 212-227. doi: 10.1002/1097-4687(200012) 246:3%3C212::AID-JMOR5%3E3.0.CO;2-S
- Secutti, S.; R.E. Reis & E. Trajano. 2011. Differentiating cave Aspidoras catfish from a karst area of Central Brazil, upper rio Tocantins basin (Siluriformes: Callichthyidae). Neotropical Ichthyology 9 (4): 689-695. doi: 10.1590/S1679-62252011005000045
- Song, J. & L.R. Parenti. 1995. Clearing and staining whole fish specimens for simultaneous demonstrations of bone, cartilage and nerves. Copeia 1995 (1): 114-118. doi: 10.2307/1446805
- Srinivasachar, H.R. 1958. Development of the skull in catfishes. Part V. Development of the skull in Heteropneustes fossilis (Bloch). Proceeding of the National Institute of Sciences of India Part B 1958: 165-190.
- Trajano, E. 2007. The challenge of estimating the age of subterranean lineages: examples from Brazil. Time in Karst: 191-198
- Trajano, E. 2012. Ecological classification of subterranean organisms, p. 275-277. In: W.B. White & D.C. Culver (Eds). Encyclopedia of caves. Amsterdam, Academic Press, 2nd ed., 945p.
- Trajano, E. & M.E. Bichuette. 2010. Subterranean Fishes of Brazil, p. 331-355. In: E. Trajano; M.E. Bichuette & B.G. Kapoor (Eds). Biology of Subterranean Fishes. Enfield, Science Publishers, 480p.
- Trajano, E.; R.E. Reis & M.E. Bichuette. 2004. Pimelodella spelaea: a new cave catfish from Central Brazil, with data on ecology and evolutionary considerations (Siluriformes: Heptapteridae). Copeia 2004 (2): 315-325. doi: 10.1643/CI-03-144R1.
- Wilkens, H. 1982. Regressive evolution and phylogenetic age: the history of colonization of freshwaters of Yucatan by fish and crustacea. Texas Memorial Museum Bulletin 28 (1982): 237-243.
- Winterbottom, R. 1974. A descriptive synonymy of the striated muscles of the Teleostei. Proceedings of the Academy of Natural Sciences of Philadelphia 215 (12): 225-317.
- Wosiacki, W.B.; G.M. Dutra & M.B. Mendonça. 2012. Description of a new species of Ituglanis (Siluriformes: Trichomycteridae) from Serra dos Carajás, rio Tocantins basin. Neotropical Ichthyology 10 (3): 547-554.
- Zuanon, J. & I. Sazima. 2004. Natural history of Stauroglanis gouldingi (Siluriformes: Trichomycteridae), a miniature sand-dwelling candiru from central Amazonian streamlets. Ichthyological Exploration of Freshwaters 15: 201-208.
- Zuanon, J.; F.A. Bockmann & I. Sazima. 2006. A remarkable sand-dwelling fish assemblage from central Amazonia, with comments on the evolution of psamophily in South American freshwater fishes. Neotropical Ichthyology 4 (1) 107-118. doi: 10.1590/S1679-62252006000100012
Publication Dates
-
Publication in this collection
22 Jan 2015 -
Date of issue
Dec 2014
History
-
Received
05 May 2014 -
Accepted
21 Oct 2014