Abstract
A new genus of spittlebug is described to include Gervasiella oakenshieldi sp. nov. (holotype male from Brazil, state of Paraná, municipality of Piraquara, Mananciais da Serra at 25°29'46"S, 48°58'54"W, 1000 m a.s.l., 15.XI.2008, P.C. Grossi leg., deposited in DZUP). In addition, Aeneolamia bucca Paladini & Cavichioli, 2013 is transferred to Gervasiella gen. nov. based on the results of a cladistic analysis. Gervasiella gen. nov. can be distinguished from the other cercopid genera by the following: postclypeus inflated with upper portion black and basal one yellowish; color of tylus distinct from color of head and rostrum, barely reaching mesocoxae. Gervasiella oakenshieldi sp. nov. is diagnosed by having the head black with tylus white, postclypeus in profile inflated and convex with a prominent longitudinal carina; tegmina black with two elongate white maculae near costal margin, one on anterior third and the other on posterior third.
Auchenorrhyncha; Neotropical Region; phylogeny; taxonomy
Insects belonging to Cercopidae are known as spittlebugs due to the bubble nest produced by the nymphs. This family forms a large group of xylem feeding insects with approximately 1500 worldwide species included in 150 genera. Most species are distributed in the tropical and subtropical regions. Adults feed on leaves or stems of a wide variety of plants, nymphs can feed on roots and in some cases they complete their development above the ground (Carvalho & Webb 2005Carvalho GS, Webb MD (2005) Cercopid Spittlebugs of the New World (Hemiptera, Auchenorrhyncha, Cercopidae). Sofia, Pensoft, 271p.).
The Neotropical genera of Cercopidae have been usually defined by characters of the head and pronotum, and by the number of spines on the hind leg. The same set of characters also form the basis of the tribal classification proposed by Fennah (1968Fennah RG (1968) Revisionary notes on the new world genera of cercopid froghoppers (Homoptera, Cercopoidea). Bulletin of Entomological Research 58: 165-190.).
An ongoing study on Neotropical cercopids has revealed a new genus of Ischnorhininae. The new genus and the new species are described and illustrated. Also, we propose a new combination: Aeneolamia bucca Paladini & Cavichioli, 2013 is transferred to Gervasiella gen. nov. The species of Gervasiella gen. nov. are distinguished based on a comparative diagnosis.
MATERIAL AND METHODS
The specimens studied are deposited in the Coleção Entomológica Padre Jesus Santiago Moure, Departamento de Zoologia, Universidade Federal do Paraná, Curitiba, Paraná, Brazil (DZUP). Morphological terminology follows Fennah (1968Fennah RG (1968) Revisionary notes on the new world genera of cercopid froghoppers (Homoptera, Cercopoidea). Bulletin of Entomological Research 58: 165-190.) and Paladini & Cryan (2012Paladini A, Cryan JR (2012) Nine new species of Neotropical spittlebugs. Zootaxa 3519: 53-68.). Techniques for preparation of genital structures follow Oman (1949Oman PW (1949) The Nearctic leafhoppers (Homoptera: Cicadelli dae). A generic classification and check list. Memoirs of the Entomological Society of Washington 3: 1-253.). The dissected parts were stored in micro vials with glycerin. Photographs were obtained with a Leica DFC-550 digital camera attached to the stereomicroscope (Leica MZ16) and captured with the software IM50 (Image Manager; Leica Microsystems Imaging Solutions Ltd, Cambridge, UK), after montage using Auto-Montage Syncroscopy of Taxonline (Rede Paranaense de Coleções). Illus trations were made with the aid of a camera lucida and the final art were finalized using vectors with the software Corel Draw version X5.
Terminal taxa. Besides the Neotropical cercopids present in the matrix analyzed by Paladini et al. (2015Paladini A, Takiya DM, Cavichioli RR, Carvalho GS (2015) Phylogeny and
biogeography of Neotropical spittlebugs (Hemiptera: Cercopidae: Ischnorhininae):
revised tribal classification based on morphological data. Systematic Entomology
40(1): 82-108. doi: 10.1111/syen.12091
https://doi.org/10.1111/syen.12091...
) two species of
Gervasiella
gen. nov. were also included, to test the validity of the new genus
proposed here.
Characters were coded to include most of the morphological variation of the external
morphology of the adult, and male and female genitalia. We included and reanalyzed 108
characters from the cladistics analysis of Paladini et
al. (2015Paladini A, Takiya DM, Cavichioli RR, Carvalho GS (2015) Phylogeny and
biogeography of Neotropical spittlebugs (Hemiptera: Cercopidae: Ischnorhininae):
revised tribal classification based on morphological data. Systematic Entomology
40(1): 82-108. doi: 10.1111/syen.12091
https://doi.org/10.1111/syen.12091...
), and added two characters totaling 110 characters. Each character
was considered a hypothesis of grouping. Primary homologies were proposed by similarity
or topological correspondence (de Pinna 1991de Pinna MCC (1991) Concepts and tests of homology in the cladistics
paradigm. Cladistics 7(4): 367-394.). The
contingent coding was used when novel features appeared and evolved, and this feature
shows variation (Sereno 2007Sereno PC (2007) Logical basis for morphological characters in
phylogenetics. Cladistics 23(6): 565-587.). Multistate
characters were treated as unordered (nonadditive) (Fitch
1971Fitch WN (1971) Toward defining the course of evolution, minimum change
for a specified tree topology. Systematic Zoology 20: 406-416.). Character state polarity was determined by outgroup rooting (Nixon & Carpenter 1993Nixon KC, Carpenter JM (1993) On outgroups. Cladistics 9(4): 413-426.
doi: 10.1111/j.1096-0031.1993.tb00234.x
https://doi.org/10.1111/j.1096-0031.1993...
). Missing data were coded
as '?' and nonapplicable characters were coded as '-'. The data matrix was built using
Winclada v1.00.08 (Nixon 2002Nixon KC (2002) Winclada. New York, Published by the Author, v.
1.00.08.).
Analyses were performed using two character weighting schemes: equal weight and implied
weight. Analyses were conducted using TNT version 1.1 (Goloboff et al. 2008Goloboff PA, Farris JS, Nixon KC (2008) TNT, a free program for
phylogenetic analysis. Cladistics 24(5): 774-786. doi:
10.1111/j.1096-0031.2008.00217.x
https://doi.org/10.1111/j.1096-0031.2008...
) using Traditional Search basing the heuristic search
strategies on RAS + TBR (random addition sequences plus swap by tree bisection and
reconnection), with 1,000 replications with 100 trees saved per replication. The choice
for the best constant of concavity (K) values range for the data followed the
methodology of Paladini et al. (2015Paladini A, Takiya DM, Cavichioli RR, Carvalho GS (2015) Phylogeny and
biogeography of Neotropical spittlebugs (Hemiptera: Cercopidae: Ischnorhininae):
revised tribal classification based on morphological data. Systematic Entomology
40(1): 82-108. doi: 10.1111/syen.12091
https://doi.org/10.1111/syen.12091...
). The best K
range for the data matrix presented here was 8-13. Branch support was calculated using
the relative Bremer support (Goloboff & Farris
2001Goloboff PA, Farris JS (2001) Methods for quick consensus estimation.
Cladistic 17(1): S26-S34. doi: 10.1111/j.1096-0031.2001.tb00102.x
https://doi.org/10.1111/j.1096-0031.2001...
). Nonparametric Bootstrap support values were computed running 1000
bootstrap pseudoreplicates (Felsenstein 1985Felsenstein J (1985) Confidence limits on phylogenies: an approach using
the bootstrap. Evolution 39: 783-791.).
TAXONOMY
Gervasiella gen. nov.
Type species. Gervasiella oakenshieldi sp. nov. by original designation.
Diagnosis. Gervasiella gen. nov. can be distinguished from all other cercopids genera by the following combination of characters: 1) postclypeus inflated with upper portion black and basal one yellowish; 2) tylus with a distinct color from the head; 3) rostrum barely reaching mesocoxae; 4) subgenital plates shorter than pygofer, in ventral view quadrangular with apex truncate; 5) paramere long and slender, apex rounded, a unique subapical spine quadrangular; 6) paramere's spines located upon a lateral concavity similar to a hole; 7) aedeagus slender with quandrangular and wide base, one pair of dorsal processes long and slender turned upward.
Description. Head triangular with two deep impressions on the vertex near the median line; tylus quadrangular; ocelli near to each other than the compound eyes. Antennae with pedicel visible in dorsal view, flagellum normal in length, with ovoid basal body and an arista almost as long as pedicel; postclypeus inflated, convex in profile with a well-marked longitudinal carina; rostrum barely reaching mesocoxae. Pronotum hexagonal, surface smooth; anterior and lateral anterior margins straight; posterior and lateroposterior margins slightly sinuated; tegmina long and slender. Hindwings with Cu1 not thickened at base. Hind tibiae with two lateral spines and a row of apical spines; hind basitarsus with apical spines distributed in two irregular rows. Pygofer with one process between anal tube and subgenital plate in lateral view; subgenital plate quandrangular in ventral view. Aedeagus long, slender with one pair of dorsal processes; parameres slender, dorsal margin with two processes, one subapical spine quadrangular and sclerotized. First valvulae of ovipositor with two processes near the base; second valvulae with dorsal margin smooth.
Etymology. The genus is named in honor of Prof. Dr. Gervásio Silva Carvalho, a specialist of Neotropical cercopids, in recognition of his expertise and several contributions to the taxonomy of the group.
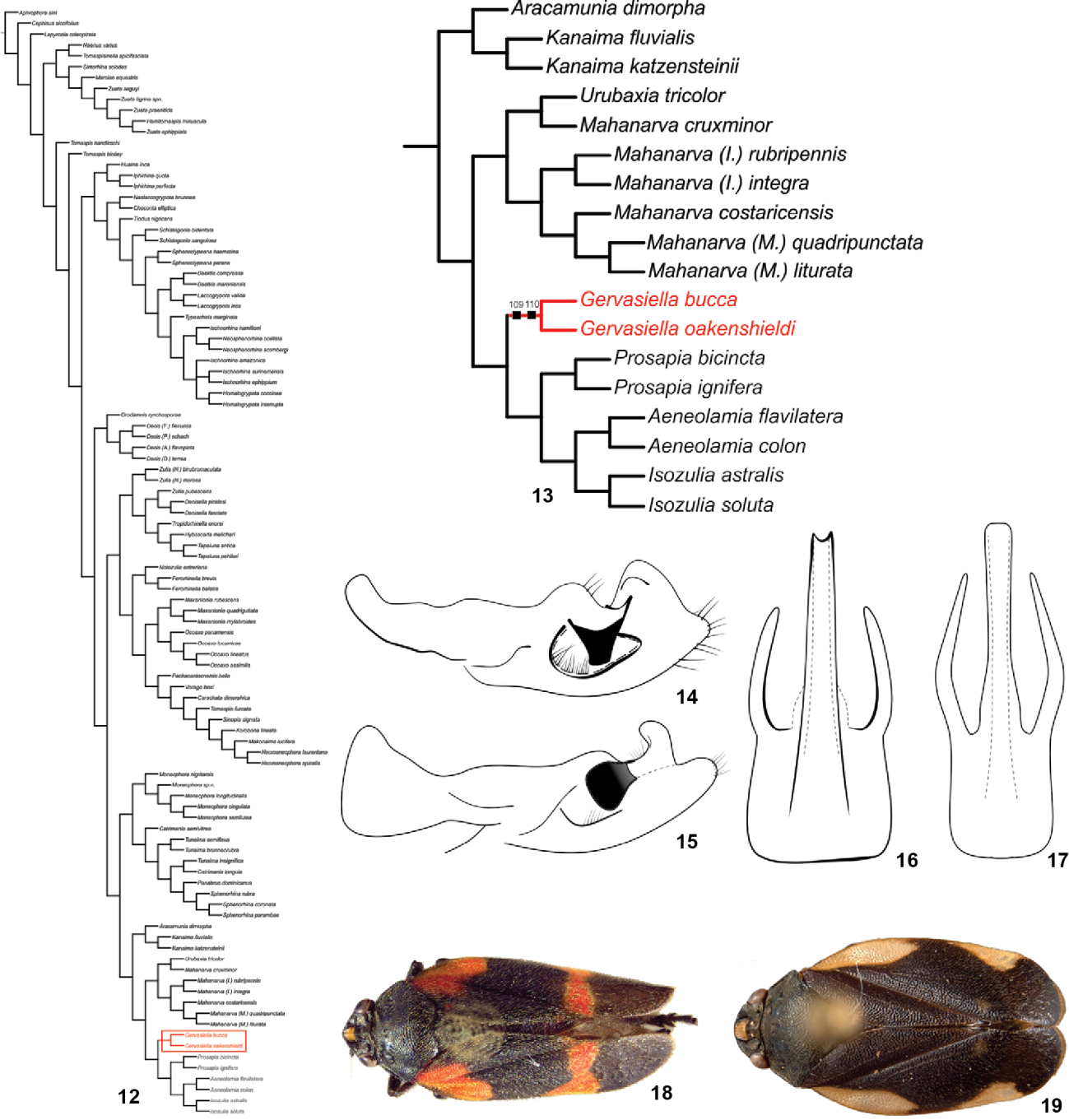
Remarks. Based on the tree resulting from the cladistics analysis (Figs. 12 and 13), Gervasiella gen. nov. is sister group of the clade including Prosapia Fennah, 1949, Aeneolamia Fennah, 1949 and Isozulia Fennah, 1953. In these genera the aedeagus presents a long and slender dorsal process inserted medially. Gervasiella gen. nov. is supported by two synapomorphies: paramere with a concavity under the spine (Figs. 14 and 15) and aedeagus base quadrangular (Figs. 16 and 17).
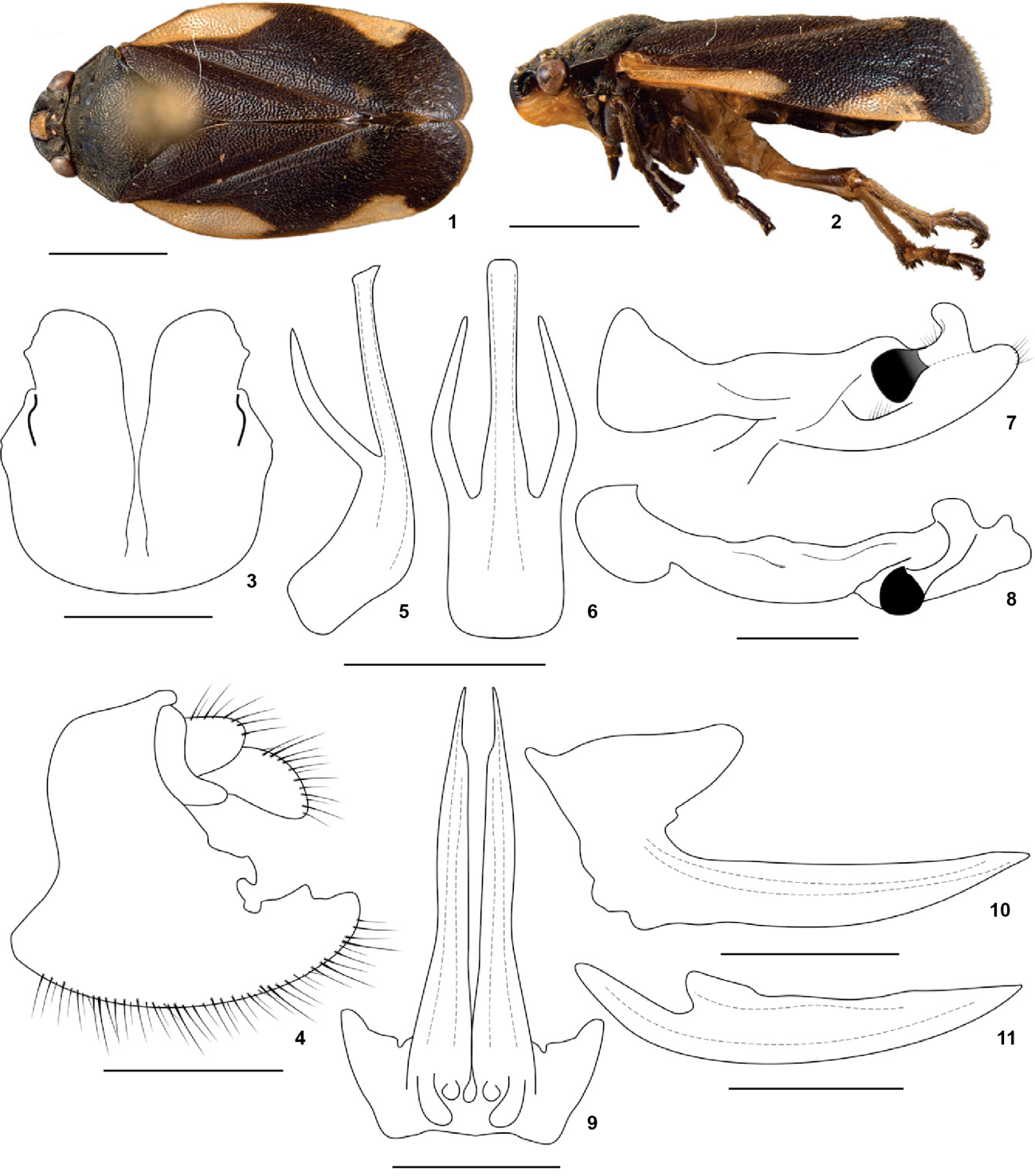
Gervasiella oakenshieldi sp. nov.: (1-2, 9-11) female paratype, (3-8) male holotype: (1) habitus, dorsal view; (2) habitus, lateral view; (3) subgenital plates and pygofer, ventral view; (4) pygofer and subgenital plates, lateral view; (5) aedeagus, lateral view; (6) aedeagus, dorsal view; (7) paramere, lateral view; (8) paramere, dorsal view; (9) first valvulae of ovipositor, ventral view; (10) first valvulae of ovipositor, lateral view; (11) second valvulae of ovipositor, lateral view. Scale bars: 1-2 = 2 mm, 3, 4, 9-11 = 0.5 mm, 5-8 = 0.25 mm.
Phylogenetic relationships of Ischnorhininae and diagnostic characters of Gervasiella gen. nov.: (12) unique most parsimonious tree resulting from the analysis of morphological data with the implied weighting scheme using the optimal constant of concavity interval of K8-12, highlighting the position of Gervasiella gen. nov.; (13) clade including Gervasiella gen. nov.; (14) Gervasiella bucca comb. nov. paramere in lateral view; (15) Gervasiella oakenshieldi sp. nov. parameres in lateral view; (16) Gervasiella bucca comb nov. aedeagus in dorsal view; (17) Gervasiella oakenshieldi sp. nov. aedeagus in dorsal view; (18) Gervasiella bucca dorsal habitus; (19) Gervasiella oakenshieldi sp. nov. dorsal habitus.
Gervasiella oakenshieldi sp. nov. Figs. 1-11, 19
Measurements. Length, male 6.8 mm; females 6.7-7.8 mm.
Diagnosis. Head black with tylus white, postclypeus in profile, inflated and convex with a prominent longitudinal carina; tegmina black with two elongate white maculae near the costal margin, one on the anterior third and the other on the posterior third.
Description. Head triangular black; rostrum yellowish with the third segment black; compound eyes black, rounded, arranged transversely; vertex smooth, rectangular, with a prominent median carina; ocelli reddish near to each other than to compound eyes; tylus white, smooth, quadrangular, lacking a median carina; antennae black, pedicel scarcely setose, basal body of flagellum ovoid with an arista almost as long as the pedicel; postclypeus inflated, convex in profile with a wide longitudinal carina, lateral grooves slightly marked, apical portion black and two basal thirds yellowish. Thorax black; pronotum black, flattened, hexagonal, lacking median carina, anterior margin straight, lateral-anterior margins straight, lateral-posterior margin slightly sinuous, posterior margin with a light groove; scutellum black, with a slight central concavity and transversal grooves. Tegmina black with two white maculae: the first one elongated, located near the costal margin, extending from its base until the median third of the tegmina; the second one rounded located between the median and apical third; apical plexus of vein poorly developed; hindwings hyaline with brown venation; vein Cu1 not thickened at base; legs brownish; metathoracic tibia with two lateral spines (basal spine equal in size to spines in apical crown; apical spine larger than spines in apical crown); apical crown of spines on tibia consisting of two rows; basitarsus with one row of spines covered by sparse setae; subungueal process absent.
Male genitalia. Pygofer with one quadrangular process between the anal tube and subgenital plates; subgenital plates short, quadrangular with a rounded apex, dorsal margin produced in a rectangular process (Fig. 3); parameres long and slender with a quandrangular sclerotized spine turned backwards located over a concavity on the external side, dorsal margin with a finger like process turned to the inner side (Figs. 7 and 8); aedeagus cylindrical with a pair of dorsal processes long and slender turned upward, aedeagus base quadrangular and wide, apex quadrangular (Figs. 5 and 6).
Female. First valvulae of ovipositor long and slender with acute apex and two basal process poorly developed, rounded, directed ventrally (Figs. 9 and 10); second valvulae long and slender, dorsal margin smooth (Fig. 11), third valvulae short and wide, with long setae ventrally.
Etymology. Noun in genitive singular after to a fictional character surname of the novel The Hobbit, Thorin Oakenshield; in honor to J.R.R Tolkien an English writer known as the author of classic fantasy books.
Remarks. Gervasiella oakenshieldi sp. nov. (Figs. 12 and 19) superficially resembles Gervasiella bucca comb. nov. (Fig. 18) in having the same color pattern but the paramere is slender, with the concavity under the spine less pronounced (Fig. 15).
Examined material. Holotype male from Brazil, Paraná: Piraquara (Mananciais da Serra 25°29'46"S, 48°58'54"W, 1000 m a.s.l., 15.XI.2008), P.C. Grossi leg. Paratypes: 1 female same data as holotype; 1 female, same locality as holotype but 25.III.2012, light trap; 1 female same locality as holotype but flight interception [trap], XI.2007, P.C. Grossi & D. Parizotto leg. All deposited in DZUP.
Gervasiella bucca (Paladini & Cavichioli, 2013) comb. nov. Figs. 14, 16, 18
Aeneolamia bucca Paladini & Cavichioli, 2013Paladini A, Cavichioli RR (2013) A new species of Aeneolamia (Hemiptera:
Cercopidae: Tomaspidinae) from the Neotropi cal Region. Zoologia 30(3): 353-355. doi:
10.1590/S1984-46702013000300016
https://doi.org/10.1590/S1984-4670201300...
: 353.
Diagnosis. General coloration black; tegmina with basal red macula and one apical red stripe; Pygofer short with finger-like process between anal tube and subgenital plates; aedeagus with one pair of dorsal, slender processes directed upward.
Remarks. Gervasiella bucca was originally described in Aeneolamia due to a superficial resemblance in the morphology of the male genitalia, although other features indicated that those species were not congeneric. Subsequently, Gervasiella gen. nov. was erected to accommodate G. bucca and the newly described G. oakenshieldi sp. nov., based on the examination of additional specimens from Southern Brazil (from the municipality of Piraquara, Paraná State). Diagnostic traits of this genus were previously mentioned in the generic diagnosis. These features were later recovered as synapomorphies validating the monophyly of Gervasiella gen. nov., as inferred in our morphology-based phylogenetic analysis of Ischnorhininae that included both G. bucca and G. oakenshieldi sp. nov.
CLADISTIC ANALYSIS
List of new characters. The complete list of characters can be found in Paladini et al. (2015Paladini A, Takiya DM, Cavichioli RR, Carvalho GS (2015) Phylogeny and
biogeography of Neotropical spittlebugs (Hemiptera: Cercopidae: Ischnorhininae):
revised tribal classification based on morphological data. Systematic Entomology
40(1): 82-108. doi: 10.1111/syen.12091
https://doi.org/10.1111/syen.12091...
) and the full data matrix is
available in Appendix S1
Appendix S1
Appendix S1. Full data matrix.
MS Excel file:
1
1
Available as Online Supplementary Material accessed with the online version of the
manuscript at
. The two new characters included in the analysis
are: Male genitalia: (109) Paramere, lateral view, concavity under the main spine: (0)
absent; (1) present; and (110) Aedeagus, shape of base in dorsal view: (0) rectangular;
(1) quadrangular. The data matrix included 102 taxa and 110 characters (Appendix
Appendix S1
Appendix S1. Full data matrix.
MS Excel file:
S11
1
Available as Online Supplementary Material accessed with the online version of the
manuscript at
).
The equal weights analysis produced 30 equally parsimonious trees (length = 1061 steps,
CI = 14, RI = 61). The strict consensus cladogram had 59 collapsed nodes. The
relationships among genera were poorly resolved. In the analysis with implied weighting
scheme the best K range for the data matrix presented here was 8-12; this range was
chosen based on Paladini et al. (2015Paladini A, Takiya DM, Cavichioli RR, Carvalho GS (2015) Phylogeny and
biogeography of Neotropical spittlebugs (Hemiptera: Cercopidae: Ischnorhininae):
revised tribal classification based on morphological data. Systematic Entomology
40(1): 82-108. doi: 10.1111/syen.12091
https://doi.org/10.1111/syen.12091...
). The five
trees obtained with the best K range were had the same topology,(Fig. 12) which will be used as the hypothesis to infer the
phylogenetic relationship and monophyly of Gervasiella
gen. nov. The topology obtained in the present analysis is similar to that
of Paladini et al. (2015Paladini A, Takiya DM, Cavichioli RR, Carvalho GS (2015) Phylogeny and
biogeography of Neotropical spittlebugs (Hemiptera: Cercopidae: Ischnorhininae):
revised tribal classification based on morphological data. Systematic Entomology
40(1): 82-108. doi: 10.1111/syen.12091
https://doi.org/10.1111/syen.12091...
) except for the
inclusion of the new genus. Only unambiguous characters were optimized in the resultant
cladogram.
The main goal of this cladistics analysis was to evaluate and to support the description of a new genus. The clade Gervasiella gen. nov. (Fig. 13) has a relative Bremer support of 71 and a Bootstrap support of 99. The genus is supported by two synapomorphies: paramere with a concavity located under the main spine (1091) and aedeagus with a quadrangular base (1101). and 10 a homoplasious character-state transformations: vertex shape narrow (30); antennae with basal body of flagellum ovoid (61); posterior margin of pronotum slightly grooved (310); tegmina venation almost indistinct (372); tegmina with apical plexus of veins reduced (401); basitarsus of the posterior leg with two rows of spines (471); subgenital plates short compared to pygofer (530); apex of subgenital plates truncated (502); spine of paramere oriented vertically (691); ovipositor with two basal processes (1031).
Gervasiellagen. nov. is included in the clade Tomaspidini and is sister group to Prosapia, Aeneolamia, and Isozulia
ACKNOWLEDGEMENTS
We thank Paschoal C. Grossi (UFRPE) and Daniele Parizzoto for collecting and generously providing specimens; Olivia Envangelista (MZUSP) for her valuable suggestions; the anonymous reviewers and associate editor for their constructive comments and significant improvement on an earlier version of this manuscript. This work was supported by a CNPq postdoctoral grant (process 150163/2013-4) to the senior author. This research is also partially funded by the advisor's grant (RRC) from PROTAX/CNPq (processes 561298/2010-6 and 303127/2010-4). This paper is the contribution number 1917 of the Departamento de Zoologia, Universidade Federal do Paraná.
- Carvalho GS, Webb MD (2005) Cercopid Spittlebugs of the New World (Hemiptera, Auchenorrhyncha, Cercopidae). Sofia, Pensoft, 271p.
- de Pinna MCC (1991) Concepts and tests of homology in the cladistics paradigm. Cladistics 7(4): 367-394.
- Fennah RG (1968) Revisionary notes on the new world genera of cercopid froghoppers (Homoptera, Cercopoidea). Bulletin of Entomological Research 58: 165-190.
- Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783-791.
- Fitch WN (1971) Toward defining the course of evolution, minimum change for a specified tree topology. Systematic Zoology 20: 406-416.
- Goloboff PA, Farris JS (2001) Methods for quick consensus estimation. Cladistic 17(1): S26-S34. doi: 10.1111/j.1096-0031.2001.tb00102.x
» https://doi.org/10.1111/j.1096-0031.2001.tb00102.x - Goloboff PA, Farris JS, Nixon KC (2008) TNT, a free program for phylogenetic analysis. Cladistics 24(5): 774-786. doi: 10.1111/j.1096-0031.2008.00217.x
» https://doi.org/10.1111/j.1096-0031.2008.00217.x - Nixon KC (2002) Winclada. New York, Published by the Author, v. 1.00.08.
- Nixon KC, Carpenter JM (1993) On outgroups. Cladistics 9(4): 413-426. doi: 10.1111/j.1096-0031.1993.tb00234.x
» https://doi.org/10.1111/j.1096-0031.1993.tb00234.x - Oman PW (1949) The Nearctic leafhoppers (Homoptera: Cicadelli dae). A generic classification and check list. Memoirs of the Entomological Society of Washington 3: 1-253.
- Paladini A, Cavichioli RR (2013) A new species of Aeneolamia (Hemiptera: Cercopidae: Tomaspidinae) from the Neotropi cal Region. Zoologia 30(3): 353-355. doi: 10.1590/S1984-46702013000300016
» https://doi.org/10.1590/S1984-46702013000300016 - Paladini A, Cryan JR (2012) Nine new species of Neotropical spittlebugs. Zootaxa 3519: 53-68.
- Paladini A, Takiya DM, Cavichioli RR, Carvalho GS (2015) Phylogeny and biogeography of Neotropical spittlebugs (Hemiptera: Cercopidae: Ischnorhininae): revised tribal classification based on morphological data. Systematic Entomology 40(1): 82-108. doi: 10.1111/syen.12091
» https://doi.org/10.1111/syen.12091 - Sereno PC (2007) Logical basis for morphological characters in phylogenetics. Cladistics 23(6): 565-587.
-
1
Available as Online Supplementary Material accessed with the online version of the manuscript at
Appendix S1
Appendix S1. Full data matrix.
MS Excel file:
https://minio.scielo.br/documentstore/1984-4670/DLZKSWpMqst7HYjXcJywnHw/a03bbab6a36315dc21627fb849651265851b387c.xlsPublication Dates
-
Publication in this collection
Jan-Feb 2015
History
-
Received
29 Sept 2014 -
Reviewed
21 Jan 2015 -
Accepted
05 Feb 2015