Abstract
The Neotropical lizard Mabuya agmosticha Rodrigues, 2000 is a habitat-specialist of thorny bromeliads in rocky outcrops of northeastern Brazil. Its distribution in the Caatinga Domain is most likely relictual. In recent years, new surveys conducted in northeastern Brazil have revealed new records of the species in the Caatinga and also in the Atlantic Forest Domain. In this study, we add four new records for M. agmosticha, extending its known geographic range in the states of Rio Grande do Norte and Paraíba. In addition, we investigated the potential geographical distribution of the species using ecological niche modeling (ENM), which combines the available occurrence records with environmental variables. Our model revealed a continuous range of areas with suitable climatic conditions for the species, from the state of Rio Grande do Norte to the northeast portion of the state of Bahia, plus some relictual distribution spots, mainly in the states of Bahia, Pernambuco, Ceará and western Rio Grande do Norte. Based on the model, we suggest that the distribution of M. agmosticha is continuous on a large geographic scale. On a smaller spatial scale, however, it is clear that its distribution is clumped, reflecting its specialist habits associated with rupicolous bromeliads.
Biogeography; Caatinga; lizards; skinks; species distribution models
The semiarid Caatinga is a mosaic of thorny shrubs and seasonally dry, xerophytic and
deciduous forests that occupy most of northeastern Brazil. It is bound by the Atlantic
Forest to the east, by the Amazon Forest to the west and by the Cerrado to the south (Leal et al. 2005Leal IR, Silva JMC, Tabarelli M, Lacher Jr TE (2005) Changing the course
of biodiversity conservation in the Caatinga of northeastern Brazil. Conservation
Biology 19: 701-706. doi: 10.1111/j.1523-1739.2005.00703.x
https://doi.org/10.1111/j.1523-1739.2005...
). As the result of centuries of
inappropriate and unsustainable exploitation of its natural resources, the Caatinga is now
seriously threatened (Velloso et al. 2002Velloso AL, Sampaio EVSB, Pareyn FGC (2002) Ecorregiões Propostas para o
Bioma Caatinga. Recife, Associação Plantas do Nordeste, Instituto de Conservação
Ambiental The Nature Conservancy do Brasil, 76p.). The
biodiversity of the Caatinga has remained relatively overlooked despite recent survey
efforts (Albuquerque et al. 2012Albuquerque UP, Araújo EL, El-Deir ACA, Lima ALA, Souto A, Bezerra BM,
Ferraz EMN, Freire EMX, Sampaio EVSB, Las-Casas FMG, Moura GJB, Pereira GA, Melo JG,
Ramos MA, Rodal MJN, Schiel N, Lyra-Neves RM, Alves RRN, Azevedo Júnior SM, Telino
Júnior WR, Severi W (2012) Caatinga revisited: ecology and conservation of an
important seasonal dry forest. The Scientific World Journal 2012: 1-18. doi:
10.1100/2012/205182
https://doi.org/10.1100/2012/205182...
). More specifically,
notwithstanding recent efforts to expand the geographical coverage of the sampled areas
(Freire et al. 2009Freire EMX, Skuk G, Kolodiuk MF, Ribeiro LB, Maggi BS, Rodrigues LS,
Vieira WLS, Falcão ACGP (2009) Répteis Squamata das Caatingas do Seridó do Rio Grande
do Norte e do Cariri da Paraíba: síntese do conhecimento atual e perspectivas, p.
51-84. In: Freire EMX (Ed). Recursos Naturais das Caatingas: Uma Visão
Multidisciplinar. Natal, EDUFRN, 240p., 2012Freire EMX, Jorge JS, Ribeiro LB (2012) First record of Colobosaura
modesta (Reinhardt and Lütken, 1862) (Squamata: Gymno phthal midae) to the Cariri
region, state of Ceará, Brazil, with a map of its geographical distribution. Check
List 8: 970-972. Available online at: http://www.checklist.org.br/getpdf?NGD067-12
[Accessed: 18 February 2015]
http://www.checklist.org.br/getpdf?NGD06...
, 2013Freire EMX, Jorge JS, Sales RFD, Ribeiro MM, Andrade MJM, Sousa PAG
(2013) New record and geographic distribution map of Alexandresaurus camacan
Rodrigues, Pellegrino, Dixo, Verdade, Pavan, Argôlo and Sites Jr, 2007 (Squamata,
Gymnophthalmidae) in northeastern Brazil. Check List 9: 783-784. Available online at:
http://www.checklist.org.br/getpdf?NGD023-13 [Accessed: 18 February
2015]
http://www.checklist.org.br/getpdf?NGD02...
, Moura et al. 2011Moura GJB, Freire EMX, Santos EM, Morais ZMB, Lins EAM, Andrade EVE,
Ferreira JDC (2011) Distribuição geográfica e caracterização ecológica dos répteis do
estado de Pernambuco, p. 229-290. In: Moura GJB, Santos EM, Oliveira MAB, Cabral MCC
(Orgs) Herpetologia no estado de Pernambuco. Brasília, IBAMA, 440p., Garda et al. 2013Garda AA, Costa TB, Faria RG, Mesquita DO, Conceição BM, Silva IRS,
Ferreira AS, Rocha SM, Palmeira CNS, Rodrigues R, Torquato S (2013) Herpetofauna of
protected areas in the Caatinga I: Raso da Catarina Ecological Station. Check List 9:
405-414. Available online at: http://www.checklist.org.br/getpdf?SL115-12 [Accessed:
18 February 2015]
http://www.checklist.org.br/getpdf?SL115...
, Cavalcanti et al.
2014Cavalcanti LBQ, Costa TB, Colli GR, Costa GC, França FGR, Mesquita DO,
Palmeira CNS, Pelegrín N, Soares AHB, Tucker D, Garda AA (2014) Herpetofauna of
protected areas in the Caatinga II: Serra da Capivara National Park. Check List 10:
18-27. doi: 10.15560/10.1.18
https://doi.org/10.15560/10.1.18...
), the herpetofauna of the Caatinga has remained unsatisfactory known (Rodrigues 2003Rodrigues MT (2003) Herpetofauna da Caatinga, p. 489-540. In: Leal IR,
Tabarelli M, Silva JMC (Eds). Ecologia e Conservação da Caatinga. Recife, Ed.
Universitária da UFPE, 822p.).
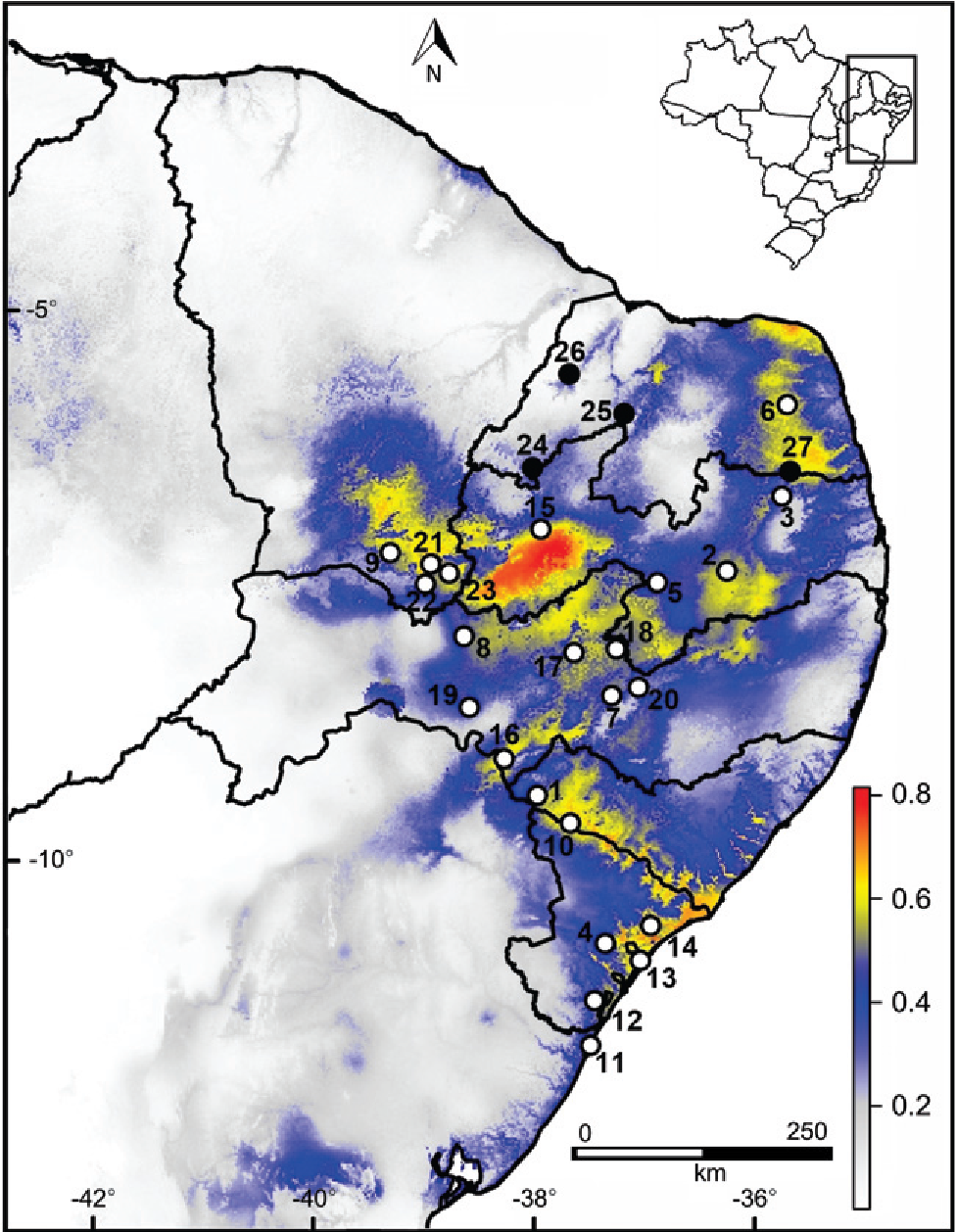
The lizard Mabuya agmosticha Rodrigues, 2000 was described from two sites in the Caatinga (Xingó-AL and Cabaceiras-PB; Fig. 1 - points 1 and 2). It is a habitat-specialist that is intimately associated with clumps of rupicolous bromeliads (Encholirium spectabile Martius ex Schultes f.) popularly known as "macambiras" (Rodrigues 2000Rodrigues MT (2000) A new species of Mabuya (Squamata: Scincidae) from the semiarid Caatingas of northeastern Brazil. Papeis Avulsos de Zoologia 41: 313-328.). For this reason, Rodrigues (2003Rodrigues MT (2003) Herpetofauna da Caatinga, p. 489-540. In: Leal IR, Tabarelli M, Silva JMC (Eds). Ecologia e Conservação da Caatinga. Recife, Ed. Universitária da UFPE, 822p.) suggested that its distribution is likely to remain relictual in the Caatinga even after intensive surveys.
Known records (circles) and distribution model (colored area) of Mabuya agmosticha in northeastern Brazil. White circles represent literature records, and black circles are the new records. Colors indicate the environmental suitability predicted by the model, which ranges from 0 (white) to 1 (red). See Table I for locality names and geographic coordinates.
Localities and coordinates (in decimals) used in the distribution model for Mabuya agmosticha, including the new records. States: (AL) Alagoas, (BA) Bahia, (CE) Ceará, (PB) Paraíba, (PE) Pernambuco, (RN) Rio Grande do Norte, (SE) Sergipe. Biomes: (CA) Caatinga, (AF) Atlantic Forest.
As predicted by Rodrigues (2003Rodrigues MT (2003) Herpetofauna da Caatinga, p. 489-540. In: Leal IR,
Tabarelli M, Silva JMC (Eds). Ecologia e Conservação da Caatinga. Recife, Ed.
Universitária da UFPE, 822p.), M.
agmosticha has been subsequently found in other Caatinga areas (Fig. 1) in the states of Paraíba (Arzabe et al. 2005Arzabe C, Skuk G, Santana GG, Delfim FR, Lima YCC, Abrantes SHF (2005)
Herpetofauna da área do Curimataú, Paraíba, p. 264-280. In: Araújo FS, Rodal MJN,
Barbosa MRV (Eds). Análise das Variações da Biodiversidade do Bioma Caatinga: Suporte
a Estratégias Regionais de Conservação. Brasília, Ministério do Meio Ambiente,
446p., Freire et al.
2009Freire EMX, Skuk G, Kolodiuk MF, Ribeiro LB, Maggi BS, Rodrigues LS,
Vieira WLS, Falcão ACGP (2009) Répteis Squamata das Caatingas do Seridó do Rio Grande
do Norte e do Cariri da Paraíba: síntese do conhecimento atual e perspectivas, p.
51-84. In: Freire EMX (Ed). Recursos Naturais das Caatingas: Uma Visão
Multidisciplinar. Natal, EDUFRN, 240p., Magalhães Júnior et al. 2014Magalhães Júnior AJC, Pereira LCM, Nicola PA, Ribeiro LB, Azevedo Júnior
SM (2014) Distribuição geográfica de Psychosaura agmosticha (Rodrigues, 2000)
(Squamata, Mabuyidae). Biotemas 27: 217-222. doi:
10.5007/2175-7925.2014v27n2p217
https://doi.org/10.5007/2175-7925.2014v2...
),
Pernambuco (Moura et al. 2011Moura GJB, Freire EMX, Santos EM, Morais ZMB, Lins EAM, Andrade EVE,
Ferreira JDC (2011) Distribuição geográfica e caracterização ecológica dos répteis do
estado de Pernambuco, p. 229-290. In: Moura GJB, Santos EM, Oliveira MAB, Cabral MCC
(Orgs) Herpetologia no estado de Pernambuco. Brasília, IBAMA, 440p., Magalhães Júnior et al. 2014Magalhães Júnior AJC, Pereira LCM, Nicola PA, Ribeiro LB, Azevedo Júnior
SM (2014) Distribuição geográfica de Psychosaura agmosticha (Rodrigues, 2000)
(Squamata, Mabuyidae). Biotemas 27: 217-222. doi:
10.5007/2175-7925.2014v27n2p217
https://doi.org/10.5007/2175-7925.2014v2...
), Sergipe (S.M. Rocha unpubl. data), Rio
Grande do Norte (Jorge & Freire 2010Jorge JS, Freire EMX (2010) Geographic Distribution. Mabuya agmosticha
(NCN). Herpetological Review 41: 512-513.) and Ceará
(Ribeiro et al. 2012Ribeiro SC, Roberto IJ, Sales DL, Ávila RW, Almeida WO (2012) Amphibians
and reptiles from the Araripe bioregion, northeastern Brazil. Salamandra 48:
133-146., Magalhães Júnior et al. 2014Magalhães Júnior AJC, Pereira LCM, Nicola PA, Ribeiro LB, Azevedo Júnior
SM (2014) Distribuição geográfica de Psychosaura agmosticha (Rodrigues, 2000)
(Squamata, Mabuyidae). Biotemas 27: 217-222. doi:
10.5007/2175-7925.2014v27n2p217
https://doi.org/10.5007/2175-7925.2014v2...
). In addition, more recent records have
extended the distribution of this species to the Atlantic Forest Domain: Dias & Rocha (2013Dias EJR, Rocha CFD (2013) Ecpleopus gaudichaudi Duméril and Bibron,
1839 (Squamata: Gymnophthalmidae) and Psychosaura agmosticha (Rodrigues, 2000)
(Squamata: Scincidae): Distribution extension and new records from Atlantic Forest in
Bahia state, Brazil. Check List 9: 607-609. Available online at:
http://www.checklist.org.br/getpdf?NGD173-11 [Accessed: 18 February
2015]
http://www.checklist.org.br/getpdf?NGD17...
) recorded it in restinga in the
state of Bahia, and (F.R. Delfim unpubl. data), after examination of specimens deposited in
seven herpetological collections in Brazil, provided new records from various localities in
the state of Sergipe.
In this study, we add four new locations for M. agmosticha in the
Caatinga, three in the state of Rio Grande do Norte and one in the state of Paraíba. We
identified the specimens by comparing them with the diagnostic characters provided by Rodrigues (2000Rodrigues MT (2000) A new species of Mabuya (Squamata: Scincidae) from
the semiarid Caatingas of northeastern Brazil. Papeis Avulsos de Zoologia 41:
313-328.). All specimens are deposited in the
Herpetological Collection of the Federal University of Rio Grande do Norte (CHBEZ).
Additionally, we investigated the potential geographical distribution of M.
agmosticha using ecological niche modeling (ENM), which combines the available
occurrence records with environmental variables. The potential application of ENM
techniques in biogeography is promising, especially with respect to species that are
scarce, have cryptic habits, are restricted in distribution or have been poorly sampled
(Pearson et al. 2007Pearson RG, Raxworthy CJ, Nakamura M, Peterson AT (2007) Predicting
species distributions from small numbers of occurrence records: a test case using
cryptic geckos in Madagascar. Journal of Biogeography 34: 102-117. doi:
10.1111/j.1365-2699.2006.01594.x
https://doi.org/10.1111/j.1365-2699.2006...
). Through modeling, and
considering the hypothesis of Rodrigues (2003Rodrigues MT (2003) Herpetofauna da Caatinga, p. 489-540. In: Leal IR,
Tabarelli M, Silva JMC (Eds). Ecologia e Conservação da Caatinga. Recife, Ed.
Universitária da UFPE, 822p.), we
investigated the influence of abiotic factors on the distribution pattern of M.
agmosticha. We expected the model to predict a relictual pattern of areas that
offer suitable environmental conditions for the species.
The four new records (Table I, Fig. 1) were obtained from 2012 to 2014 in the states of Rio Grande do Norte (RN) and Paraíba (PB). The first record, on December 16, 2012,was obtained at Serra de João do Vale, municipality of Jucurutu-RN, during a short-term survey in the area; three of us (RFDS, MFK and MMR) collected one specimen of M. agmosticha (CHBEZ 3981) within a clump of E. spectabile on a rocky outcrop. The second record is from April 26, 2013 at Serra da Barriguda, municipality of Alexandria-RN; MJMA collected one adult specimen of M. agmosticha (CHBEZ 3975) within a clump of E. spectabile on a rocky outcrop. The third record was obtained during a field expedition of the Research Program on Biodiversity (PPBio Semiárido/CNPq) to the Chapada do Apodi, municipality of Felipe Guerra-RN; we collected one specimen of M. agmosticha (CHBEZ 4085) within a clump of E. spectabile on an extensive limestone rocky outcrop. The fourth record is from Parque Estadual da Pedra da Boca, municipality of Araruna-PB. On a visit to this protected area, JSJ observed and photographed several individuals of M. agmosticha in clumps of E. spectabile (Fig. 2).
Live specimen of Mabuya agmosticha on a leaf of "macambira" (Encholirium spectabile) in Parque Estadual da Pedra da Boca, Paraíba state, Brazil. In the lower right corner, an individual of E. spectabile (diameter: ~60 cm).
The distribution model was generated using Maxent 3.3.3k (Phillips et al. 2006Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of
species geographic distributions. Ecological Modelling 190: 231-259. doi:
10.1016/j.ecolmodel.2005.03.026
https://doi.org/10.1016/j.ecolmodel.2005...
), a well-established algorithm (Elith et al. 2006Elith J, Graham CH, Anderson RP, Dudý'k M, Ferrier S, Guisan A, Hijmans
RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G,
Moritz C, Nakamura M, Nakazawa Y, Overton JM, Peterson AT, Phillips SJ, Richardson
KS, Scachetti-Pereira R, Schapire RE, Sobero'n J, Williams S, Wisz MS, Zimmermann NE
(2006) Novel methods improve prediction of species' distributions from occurrence
data. Ecography 29: 129-151. doi: 10.1111/j.2006.0906-7590.04596.x
https://doi.org/10.1111/j.2006.0906-7590...
) that works well for small samples (Pearson et al. 2007Pearson RG, Raxworthy CJ, Nakamura M, Peterson AT (2007) Predicting
species distributions from small numbers of occurrence records: a test case using
cryptic geckos in Madagascar. Journal of Biogeography 34: 102-117. doi:
10.1111/j.1365-2699.2006.01594.x
https://doi.org/10.1111/j.1365-2699.2006...
). Maxent uses a probability
distribution of maximum entropy to predict approximate species distributions from presence
data. Environmental variables were obtained from the WorldClim database (www.worldclim.org,
Hijmans et al. 2005Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high
resolution interpolated climate surfaces for global land areas. International Journal
of Climatology 25: 1965-1978. doi: 10.1002/joc.1276
https://doi.org/10.1002/joc.1276...
) as generic grids with 30
arc-seconds resolution (~1 km). We used Bioclim climate variables for current conditions
(~1950-2000). The bioclimatic layers were cropped to span from latitudes from 0°30' to
17°00'S and longitudes from 44°30' to 34°30'W; this includes the Caatinga Domain and the
northern part of the Atlantic Forest. Eleven of the 19 original climate variables were
removed due to high correlations (r > 0.8), to minimize multicollinearity among the
layers. The variables used were Bio1 (annual mean temperature), Bio3 (isothermality), Bio4
(temperature seasonality), Bio7 (temperature annual range), Bio12 (annual precipitation),
Bio17 (precipitation of driest quarter), Bio18 (precipitation of warmest quarter), Bio19
(precipitation of coolest quarter), and altitude. We performed 20 bootstrap replications,
with 25% of random test points to evaluate the model. The model was generated with the
dismo package under the R software (Hijmans et al.
2013Hijmans RJ, Phillips S, Leathwick J, Elith J (2013) Dismo: Species
distribution modeling. R package version 0.9-3. Available online at:
http://CRAN.R-project.org/package=dismo [Accessed: 17 October 2013]
http://CRAN.R-project.org/package=dismo...
).
The distribution model for M. agmosticha is shown in Fig. 1. The area under the curve (Test AUC) was 0.884 ± 0.041, indicating that the model performed well. The environmental suitability was explained primarily by precipitation of coolest quarter (26.0%), temperature seasonality (22.3%), and precipitation of driest quarter (12.2%). The optimal niche occupied by the species (environmental suitability area more than 0.5) is defined by precipitation of coolest quarter between 45 and 745 mm, temperature seasonality above 11.4°C, and precipitation of driest quarter between 4 and 177 mm.
Our results extend the known geographical distribution of M. agmosticha 220 km to the west and 160 km to the north of the nearest known localities (Santa Maria-RN and Coremas-PB, respectively). Mabuya agmosticha is difficult to collect in the habitat where it occurs (rupicolous thorny bromeliads). Pitfall traps (one of the main methods used in herpetofauna surveys) are ineffective for sampling in rocky outcrops, the only habitat where the species is regularly found. Moreover, it is difficult to find and to collect M. agmosticha using active visual search because individuals often hide within bromeliad clumps (pers. obs.). This is perhaps one of the main reasons for the low representation of M. agmosticha in herpetological collections, together with insufficient sampling efforts. The distribution model (Fig. 1), however, suggests that the potential distribution of the species is much broader than it is currently documented. It encompasses most of the states of Paraíba, Alagoas and Sergipe, as well as considerable portions of the states of Rio Grande do Norte, Pernambuco and Ceará. It is also possible that the species occurs in inland portions of the state of Bahia.
Besides the Caatinga, the model predicts the occurrence of M. agmosticha
in the Atlantic Forest in the states of Rio Grande do Norte, Paraíba, Pernambuco, Alagoas,
Sergipe and northeastern portion of Bahia. However, the occurrence of the species in the
Atlantic Forest may be limited by biotic factors, such as the availability of suitable
microhabitats (bromeliads). The geographic distribution of E. spectabile,
the main bromeliad used by M. agmosticha (Magalhães Junior et al. 2014Magalhães Júnior AJC, Pereira LCM, Nicola PA, Ribeiro LB, Azevedo Júnior
SM (2014) Distribuição geográfica de Psychosaura agmosticha (Rodrigues, 2000)
(Squamata, Mabuyidae). Biotemas 27: 217-222. doi:
10.5007/2175-7925.2014v27n2p217
https://doi.org/10.5007/2175-7925.2014v2...
), encompasses the entire distribution range of
M. agmosticha predicted by the model, except for coastal areas of the
Atlantic Forest Domain, where this bromeliad does not occur (Forzza 2005Forzza RC (2005) Revisão taxonômica de Encholirium Mart. ex Schult.
& Schult. f. (Pitcairnioideae - Bromeliaceae). Boletim de Botânica da
Universidade de São Paulo 23: 1-49. doi:
10.11606/issn.2316-9052.v23i1p1-49
https://doi.org/10.11606/issn.2316-9052....
). Another limiting biotic factor is the presence of competitors,
such as Mabuya macrorhyncha Hoge, 1946, sister taxon of M.
agmosticha (Miralles & Carranza
2010Miralles A, Carranza S (2010) Systematics and biogeography of the
Neotropical genus Mabuya, with special emphasis on the Amazonian skink Mabuya
nigropunctata (Reptilia, Scincidae). Molecular Phylogenetics and Evolution 54:
857-869. doi: 10.1016/j.ympev.2009.10.016
https://doi.org/10.1016/j.ympev.2009.10....
), which has similar ecological attributes, including bromelicolous habit (Freire 1996Freire EMX (1996) Estudo ecológico e zoogeográfico sobre a fauna de
lagartos (Sauria) das dunas de Natal, Rio Grande do Norte, e da restinga de Ponta de
Campina, Cabedelo, Paraíba, Brasil. Revista Brasileira de Zoologia 13: 903-921. doi:
10.1590/S0101-81751996000400012
https://doi.org/10.1590/S0101-8175199600...
). Recent research (Dias & Rocha 2013Dias EJR, Rocha CFD (2013) Ecpleopus gaudichaudi Duméril and Bibron,
1839 (Squamata: Gymnophthalmidae) and Psychosaura agmosticha (Rodrigues, 2000)
(Squamata: Scincidae): Distribution extension and new records from Atlantic Forest in
Bahia state, Brazil. Check List 9: 607-609. Available online at:
http://www.checklist.org.br/getpdf?NGD173-11 [Accessed: 18 February
2015]
http://www.checklist.org.br/getpdf?NGD17...
), however, reported the presence of M.
agmosticha in an Atlantic Forest restinga in Bahia, associated with tank
bromeliads (Aechmea sp.) in sympatry and sintopy with M.
macrorhyncha, a record that requires further investigation. Furthermore,
according to Dias & Rocha (2013Dias EJR, Rocha CFD (2013) Ecpleopus gaudichaudi Duméril and Bibron,
1839 (Squamata: Gymnophthalmidae) and Psychosaura agmosticha (Rodrigues, 2000)
(Squamata: Scincidae): Distribution extension and new records from Atlantic Forest in
Bahia state, Brazil. Check List 9: 607-609. Available online at:
http://www.checklist.org.br/getpdf?NGD173-11 [Accessed: 18 February
2015]
http://www.checklist.org.br/getpdf?NGD17...
), M.
agmosticha inhabits terrestrial tank-bromeliads besides rupicolous bromeliads
("macambiras").
The distribution model indicates a continuous range of areas that have favorable climatic conditions for t M. agmosticha (environmental suitability > 0.4), extending from the state of Rio Grande do Norte to the northeast portion of the state of Bahia (Fig. 1). The model also predicts some relictual spots of distribution, mainly in the states of Bahia, Pernambuco, Ceará and western Rio Grande do Norte (Fig. 1). The occurrence of the species in Chapada do Apodi - RN (Fig. 1, point 26), for instance, is in a relictual spot according to the model.
In conclusion, based on the model, we suggest that the distribution of M.
agmosticha is continuous on a large geographic scale. On a smaller spatial
scale, however, it is clear that its distribution is clumped and is strictly associated
with its microhabitat (rupicolous bromeliads). The absence of M. agmosticha
from some occurrence areas as predicted by the model may be the result of biotic
(competitors, availability of microhabitats) and historic factors (low dispersal capacity
of the species, which is a habitat-specialist). For instance, the suitability of the
northeastern coast of the state of Bahia is intermediate (blue color in Fig. 1), but the Restinga de Costa Azul (Fig. 1, point 11) seems to be the southern limit of the
species' range, at least on the coast, since it was not found in the southernmost restingas
of Bahia surveyed by Dias & Rocha (2014Dias EJR, Rocha CFD (2014) Habitat Structural Effect on Squamata Fauna
of the Restinga Ecosystem in Northeastern Brazil. Anais da Academia Brasileira de
Ciências 86: 359-371. doi: 10.1590/0001-3765201420130006
https://doi.org/10.1590/0001-37652014201...
).
Certainly, a greater number of herpetological surveys will help to better understand the
pattern of distribution of this species, and also of other lizard species of the
Caatinga.
ACKNOWLEDGEMENTS
This study was supported by Biodiversity Research Program - PPBio Semiarid/CNPq (Process 558317/2009-0) and by research grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to EMXF (Process 309424/2011-9), JSJ (Process 107757/2010-9) and MMR (Process 504028/2010-3), and from the Coordernação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) to MJMA and RFDS. G. Costa and two anonymous reviewers provided useful comments on the manuscript.
- Arzabe C, Skuk G, Santana GG, Delfim FR, Lima YCC, Abrantes SHF (2005) Herpetofauna da área do Curimataú, Paraíba, p. 264-280. In: Araújo FS, Rodal MJN, Barbosa MRV (Eds). Análise das Variações da Biodiversidade do Bioma Caatinga: Suporte a Estratégias Regionais de Conservação. Brasília, Ministério do Meio Ambiente, 446p.
- Albuquerque UP, Araújo EL, El-Deir ACA, Lima ALA, Souto A, Bezerra BM, Ferraz EMN, Freire EMX, Sampaio EVSB, Las-Casas FMG, Moura GJB, Pereira GA, Melo JG, Ramos MA, Rodal MJN, Schiel N, Lyra-Neves RM, Alves RRN, Azevedo Júnior SM, Telino Júnior WR, Severi W (2012) Caatinga revisited: ecology and conservation of an important seasonal dry forest. The Scientific World Journal 2012: 1-18. doi: 10.1100/2012/205182
» https://doi.org/10.1100/2012/205182 - Carvalho CM, Vilar JC, Oliveira FF (2005) Répteis e Anfíbios, p. 39-61. In: Carvalho CM, Vilar JC (Eds). Parque Nacional Serra de Itabaiana - Levantamento da Biota. Aracajú, IBAMA, Biologia Geral e Experimental, Universidade Federal de Sergipe, 131p.
- Cavalcanti LBQ, Costa TB, Colli GR, Costa GC, França FGR, Mesquita DO, Palmeira CNS, Pelegrín N, Soares AHB, Tucker D, Garda AA (2014) Herpetofauna of protected areas in the Caatinga II: Serra da Capivara National Park. Check List 10: 18-27. doi: 10.15560/10.1.18
» https://doi.org/10.15560/10.1.18 - Dias EJR, Rocha CFD (2013) Ecpleopus gaudichaudi Duméril and Bibron, 1839 (Squamata: Gymnophthalmidae) and Psychosaura agmosticha (Rodrigues, 2000) (Squamata: Scincidae): Distribution extension and new records from Atlantic Forest in Bahia state, Brazil. Check List 9: 607-609. Available online at: http://www.checklist.org.br/getpdf?NGD173-11 [Accessed: 18 February 2015]
» http://www.checklist.org.br/getpdf?NGD173-11 - Dias EJR, Rocha CFD (2014) Habitat Structural Effect on Squamata Fauna of the Restinga Ecosystem in Northeastern Brazil. Anais da Academia Brasileira de Ciências 86: 359-371. doi: 10.1590/0001-3765201420130006
» https://doi.org/10.1590/0001-3765201420130006 - Elith J, Graham CH, Anderson RP, Dudý'k M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, Overton JM, Peterson AT, Phillips SJ, Richardson KS, Scachetti-Pereira R, Schapire RE, Sobero'n J, Williams S, Wisz MS, Zimmermann NE (2006) Novel methods improve prediction of species' distributions from occurrence data. Ecography 29: 129-151. doi: 10.1111/j.2006.0906-7590.04596.x
» https://doi.org/10.1111/j.2006.0906-7590.04596.x - Forzza RC (2005) Revisão taxonômica de Encholirium Mart. ex Schult. & Schult. f. (Pitcairnioideae - Bromeliaceae). Boletim de Botânica da Universidade de São Paulo 23: 1-49. doi: 10.11606/issn.2316-9052.v23i1p1-49
» https://doi.org/10.11606/issn.2316-9052.v23i1p1-49 - Freire EMX (1996) Estudo ecológico e zoogeográfico sobre a fauna de lagartos (Sauria) das dunas de Natal, Rio Grande do Norte, e da restinga de Ponta de Campina, Cabedelo, Paraíba, Brasil. Revista Brasileira de Zoologia 13: 903-921. doi: 10.1590/S0101-81751996000400012
» https://doi.org/10.1590/S0101-81751996000400012 - Freire EMX, Skuk G, Kolodiuk MF, Ribeiro LB, Maggi BS, Rodrigues LS, Vieira WLS, Falcão ACGP (2009) Répteis Squamata das Caatingas do Seridó do Rio Grande do Norte e do Cariri da Paraíba: síntese do conhecimento atual e perspectivas, p. 51-84. In: Freire EMX (Ed). Recursos Naturais das Caatingas: Uma Visão Multidisciplinar. Natal, EDUFRN, 240p.
- Freire EMX, Jorge JS, Ribeiro LB (2012) First record of Colobosaura modesta (Reinhardt and Lütken, 1862) (Squamata: Gymno phthal midae) to the Cariri region, state of Ceará, Brazil, with a map of its geographical distribution. Check List 8: 970-972. Available online at: http://www.checklist.org.br/getpdf?NGD067-12 [Accessed: 18 February 2015]
» http://www.checklist.org.br/getpdf?NGD067-12 - Freire EMX, Jorge JS, Sales RFD, Ribeiro MM, Andrade MJM, Sousa PAG (2013) New record and geographic distribution map of Alexandresaurus camacan Rodrigues, Pellegrino, Dixo, Verdade, Pavan, Argôlo and Sites Jr, 2007 (Squamata, Gymnophthalmidae) in northeastern Brazil. Check List 9: 783-784. Available online at: http://www.checklist.org.br/getpdf?NGD023-13 [Accessed: 18 February 2015]
» http://www.checklist.org.br/getpdf?NGD023-13 - Garda AA, Costa TB, Faria RG, Mesquita DO, Conceição BM, Silva IRS, Ferreira AS, Rocha SM, Palmeira CNS, Rodrigues R, Torquato S (2013) Herpetofauna of protected areas in the Caatinga I: Raso da Catarina Ecological Station. Check List 9: 405-414. Available online at: http://www.checklist.org.br/getpdf?SL115-12 [Accessed: 18 February 2015]
» http://www.checklist.org.br/getpdf?SL115-12 - Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965-1978. doi: 10.1002/joc.1276
» https://doi.org/10.1002/joc.1276 - Hijmans RJ, Phillips S, Leathwick J, Elith J (2013) Dismo: Species distribution modeling. R package version 0.9-3. Available online at: http://CRAN.R-project.org/package=dismo [Accessed: 17 October 2013]
» http://CRAN.R-project.org/package=dismo - Jorge JS, Freire EMX (2010) Geographic Distribution. Mabuya agmosticha (NCN). Herpetological Review 41: 512-513.
- Leal IR, Silva JMC, Tabarelli M, Lacher Jr TE (2005) Changing the course of biodiversity conservation in the Caatinga of northeastern Brazil. Conservation Biology 19: 701-706. doi: 10.1111/j.1523-1739.2005.00703.x
» https://doi.org/10.1111/j.1523-1739.2005.00703.x - Magalhães Júnior AJC, Pereira LCM, Nicola PA, Ribeiro LB, Azevedo Júnior SM (2014) Distribuição geográfica de Psychosaura agmosticha (Rodrigues, 2000) (Squamata, Mabuyidae). Biotemas 27: 217-222. doi: 10.5007/2175-7925.2014v27n2p217
» https://doi.org/10.5007/2175-7925.2014v27n2p217 - Miralles A, Carranza S (2010) Systematics and biogeography of the Neotropical genus Mabuya, with special emphasis on the Amazonian skink Mabuya nigropunctata (Reptilia, Scincidae). Molecular Phylogenetics and Evolution 54: 857-869. doi: 10.1016/j.ympev.2009.10.016
» https://doi.org/10.1016/j.ympev.2009.10.016 - Moura GJB, Freire EMX, Santos EM, Morais ZMB, Lins EAM, Andrade EVE, Ferreira JDC (2011) Distribuição geográfica e caracterização ecológica dos répteis do estado de Pernambuco, p. 229-290. In: Moura GJB, Santos EM, Oliveira MAB, Cabral MCC (Orgs) Herpetologia no estado de Pernambuco. Brasília, IBAMA, 440p.
- Pearson RG, Raxworthy CJ, Nakamura M, Peterson AT (2007) Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. Journal of Biogeography 34: 102-117. doi: 10.1111/j.1365-2699.2006.01594.x
» https://doi.org/10.1111/j.1365-2699.2006.01594.x - Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecological Modelling 190: 231-259. doi: 10.1016/j.ecolmodel.2005.03.026
» https://doi.org/10.1016/j.ecolmodel.2005.03.026 - Ribeiro SC, Roberto IJ, Sales DL, Ávila RW, Almeida WO (2012) Amphibians and reptiles from the Araripe bioregion, northeastern Brazil. Salamandra 48: 133-146.
- Rodrigues MT (2000) A new species of Mabuya (Squamata: Scincidae) from the semiarid Caatingas of northeastern Brazil. Papeis Avulsos de Zoologia 41: 313-328.
- Rodrigues MT (2003) Herpetofauna da Caatinga, p. 489-540. In: Leal IR, Tabarelli M, Silva JMC (Eds). Ecologia e Conservação da Caatinga. Recife, Ed. Universitária da UFPE, 822p.
- Velloso AL, Sampaio EVSB, Pareyn FGC (2002) Ecorregiões Propostas para o Bioma Caatinga. Recife, Associação Plantas do Nordeste, Instituto de Conservação Ambiental The Nature Conservancy do Brasil, 76p.
Publication Dates
-
Publication in this collection
Jan-Feb 2015
History
-
Received
08 Apr 2014 -
Reviewed
29 Sept 2014 -
Accepted
06 Dec 2014