Abstract
The reproductive cycle of Squamata reptiles is often associated with environmental conditions, such as rainfall. In this respect, seasonal variations may affect the morphology of the ovarian follicles, which are associated with vitellogenesis. The present study describes histological alterations in the ovarian cycle of two lizard species, Tropidurus hispidus (Spix, 1825) and Tropidurus semitaeniatus (Spix, 1825), which inhabit a caatinga region in the state of Rio Grande do Norte. Our goal was to identify morphological differences in the ovarian follicles at each phase of vitelloge nesis and to ascertain if they are associated with rainfall. Three follicular phases were identified in both species: pre-vitellogenesis, vitellogenesis and follicular atresia. An additional phase, the luteal, was found only in T. hispidus. During the development of these phases, vitellus was deposited inside the oocyte and there were identifiable alterations in the granulosa and thecal layers. Rainfall was found to influence the gonadal cycle.
Ovarian stages; reproductive cycle; semiarid; vitellogenesis
A wide range of reproductive strategies occur among Squamata (Zug et al. 2001Zug GR, Vitt LJ, Caldwell JP (2001) Reproductive ecology and life histories, p. 135-153. In: Zug GR, Vitt LJ, Caldwell JP (Eds) Herpetology: an introductory biology of amphibians and reptiles. San Diego, Academic Press, 2nd ed.), and these strategies vary within and between species. These differences are due to phylogenetic history, the morphological characteristics of the different lineages and their adaptive responses to environmental conditions (Adolph & Porter 1993Adolph SC, Porter WP (1993) Temperature, activity and lizard life histories. The American Naturalist 142: 273-295., Ramírez-Bautista et al. 1998Ramírez-Bautista A, Barba-Torres J, Vitt LJ (1998) Reproductive cycle and brood size of Eumeces lynxe from Pinal de Amoles, Queretero, Mexico. Journal of Herpetology 32: 18-24.). The temporal pattern of reproduction in Squamata is often associated with limiting environmental conditions. In temperate regions, reproductive activity is seasonal and is governed by temperature and day length (Meshaka et al. 2006Meshaka Jr WE, Smith HT, Dean CL (2006) Gonadal cycle and growth of a West Indian Lizard, the Northern Curlytail Lizard (Leiocephalus carinatus armouri), in southern Florida. Herpetological Conservation and Biology 1: 109-115.). In tropical regions, by contrast, where limiting environmental factors are not always easy to identify, Squamata exhibit a wide variety of reproductive patterns, from continuous reproductive cycles to strictly seasonal reproduction (Clerke & Alford 1993Clerke RB, Alford RA (1993) Reproductive biology of four species of tropical Australian lizards and comments on the factors regulating lizard reproductive cycles. Journal of Herpetology 27: 400-406., Vitt 1992Vitt LJ (1992) Diversity of reproductive strategies among Brazilian lizards and snakes: the significance of lineage and adaptation, p. 135-149. In: Hamlett WC (Ed.) Reproductive Biology of South American Vertebrates. New York, Springer-Verlag.). However, in tropical environments that exhibit seasonal variation, the reproductive cycles of lizards are often correlated with rainfall (Ávila et al. 2008Ávila RW, Cunha-Avellar LR, Ferreira VL (2008) Diet and reproduction of the lizard Tropidurus etheridgei in rocky areas of central Brazil. Herpetological Review 39: 430-433., Van Sluys et al. 2002Van Sluys M, Mendes HMA, Assis VB, Kiefer MC (2002) Reproduction of Tropidurus montanus Rodrigues, 1987 (Tropiduridae), a lizard from a seasonal habitat of south-eastern Brazil, and a comparison with other Tropidurus species. Herpetological Journal 12: 89-97.): the reproductive activities of a large number of tropical lizards decrease or come to a halt during the dry season (Fitch 1982Fitch HS (1982) Reproductive cycles in tropical reptiles. Occasional Papers of the Museum of Natural History 96: 1-53.).
Traditionally, two hypotheses have been advanced to explain reproductive seasonality in tropical lizards: 1) microhabitats with adequate humidity for egg development become limited (Wiederhecker et al. 2002Wiederhecker HC, Pinto ACS, Colli GR (2002) Reproductive ecology of Tropidurus torquatus (Squamata: Tropiduridae) in the highly seasonal Cerrado biome of Central Brazil. Journal of Herpetology 36: 82-91.), and 2) food resources for reproduction and/or offspring development become scarce (Vrcibradic & Rocha 1998Vrcibradic D, Rocha, CFD (1998) Reproductive cycle and life-history traits of the viviparous skink Mabuya frenata in southeastern Brazil. Copeia 1998: 612-619.) during certain periods. Furthermore, a series of studies have revealed the influence of temperature, rainfall and day length on lizard reproduction - e.g., Tropidurus torquatus (Wied-Neuwied, 1820) (Wiederhecker et al. 2002Wiederhecker HC, Pinto ACS, Colli GR (2002) Reproductive ecology of Tropidurus torquatus (Squamata: Tropiduridae) in the highly seasonal Cerrado biome of Central Brazil. Journal of Herpetology 36: 82-91.); Eurolophosaurus nanuzae (Rodrigues, 1981) (Galdino et al. 2003Galdino CAB, Assis VB, Kiefer MC, Van Sluys M (2003) Reproduction and fat body cycle of Eurolophosaurus nanuzae (Sauria; Tropiduridae) from a seasonal montane habitat of Southeastern Brazil. Journal of Herpetology 37: 687-694.); Cercosaura schreibersii Wiegmann, 1834; and Contomastix lacertoides (Duméril & Bibron, 1839) (Balestrin et al. 2010Balestrin RL, Cappellari LH, Outeiral AB (2010) Biologia reprodutiva de Cercosaura schreibersii (Squamata, Gymnophthalmidae) e Cnemidophorus lacertoides (Squamata, Teiidae) no Escudo Sul-Riograndense, Brasil. Biota Neotropica 10: 131-139.).
The reproductive cycle of Tropidurus (Tropiduridae sensu Frost et al. 2001Frost DR, Rodrigues MT, Grant T, Titus TA (2001) Phylogenetics of the lizard genus Tropidurus (Squamata: Tropiduridae: Tropidurinae): direct optimization, descriptive efficiency, and sensitivity analysis of congruence between molecular data and morphology. Molecular Phylogenetics and Evolution 21: 352-371.), a genus that includes oviparous species that are primarily insectivores and heliophiles (Van Sluys 1992Van Sluys M (1992) Aspectos da ecologia do lagarto Tropidurus itambere (Iguanidae) em uma área do sudeste do Brasil. Revista Brasileira de Biologia 52: 181-185., Vitt 1995Vitt LJ (1995). The ecology of tropical lizards in the caatinga of northeast Brazil. Occasional Papers of the Oklahoma Museum of Natural History 1: 1-29.) and which occur in open habitats of South America (Frost et al. 2001Frost DR, Rodrigues MT, Grant T, Titus TA (2001) Phylogenetics of the lizard genus Tropidurus (Squamata: Tropiduridae: Tropidurinae): direct optimization, descriptive efficiency, and sensitivity analysis of congruence between molecular data and morphology. Molecular Phylogenetics and Evolution 21: 352-371., Rodrigues 1987Rodrigues MT (1987) Sistemática, ecologia e zoogeografia dos Tropidurus do grupo torquatus ao Sul do Rio Amazonas (Sauria, Iguanidae). Arquivos de Zoologia do Estado de São Paulo 31: 105-230.), follows a seasonal pattern. The seasonal reproductive cycle of the following species has been documented Tropidurus spinulosus (Cope, 1862) (Cruz et al. 1997Cruz FB, Teisaire E, Nieto L (1997) Reproductive biology of the lizard Tropidurus spinulosus in the chaco of Salta, Argentina. Studies on Neotropical Fauna and Environment 32: 28-32.), Tropidurus itambere Rodrigues, 1987 (Van Sluys 1993Van Sluys M (1993) The reproductive cycle of Tropidurus itambere (Sauria, Tropiduridae) in southeastern Brazil. Journal of Herpetology 27: 28-32.), Tropidurus montanus Rodrigues, 1987 (Van Sluys et al. 2002Van Sluys M, Mendes HMA, Assis VB, Kiefer MC (2002) Reproduction of Tropidurus montanus Rodrigues, 1987 (Tropiduridae), a lizard from a seasonal habitat of south-eastern Brazil, and a comparison with other Tropidurus species. Herpetological Journal 12: 89-97.) and T. torquatus (Wiederhecker et al. 2002Wiederhecker HC, Pinto ACS, Colli GR (2002) Reproductive ecology of Tropidurus torquatus (Squamata: Tropiduridae) in the highly seasonal Cerrado biome of Central Brazil. Journal of Herpetology 36: 82-91.).
Folliculogenesis in both oviparous and viviparous species is characterized by changes in the layers that surround the oocyte (granulosa and thecal layer), as well as in the ooplasma, during vitellus deposition (Manes et al. 2007Manes ME, Noriega T, Casal FC, Apichela S (2007) Ovarian changes during the reproductive cycle of the Tupinambis merianae lizard raised in a temperate environment. Cuadernos de Herpetologia 21: 21-29., Moodley & Van Wyk 2007Moodley GK, Van Wyk JH (2007) Folliculogenesis and ovarian histology of the oviparous gecko, Hemidactylus mabouia (Sauria: Gekkonidae). African Journal of Herpetology 56: 115-135.). To better understand the influence of seasonality on the reproductive activity of lizards, ovarian histological studies seek to characterize the phases of folliculogenesis in different seasons (Goldberg 1970Goldberg SR (1970) Seasonal ovarian histology of the ovoviviparous Iguanid lizard Sceloporus jarrovi Cope. Journal of Morphology 132: 265-276., Manes et al. 2007Manes ME, Noriega T, Casal FC, Apichela S (2007) Ovarian changes during the reproductive cycle of the Tupinambis merianae lizard raised in a temperate environment. Cuadernos de Herpetologia 21: 21-29.). In a previous study we conducted seasonal analyses of the reproductive activity and fat body mass in females and males of T. hispidus and T. semitaeniatus (Ribeiro et al. 2012Ribeiro LB, Silva NB, Freire EMX (2012) Reproductive and fat body cycles of Tropidurus hispidus and Tropidurus semitaeniatus (Squamata, Tropiduridae) in a caatinga area of northeastern Brazil. Revista Chilena de Historia Natural 85: 307-320.). Histological and cytological descriptions of the reproductive activity of Brazilian lizards, particularly those inhabiting the Caatinga, are scarce. In the present study we describe folliculogenesis in T. hispidus and T. semitaeniatus, two lizard species that inhabit the caatinga of northeastern Brazil.
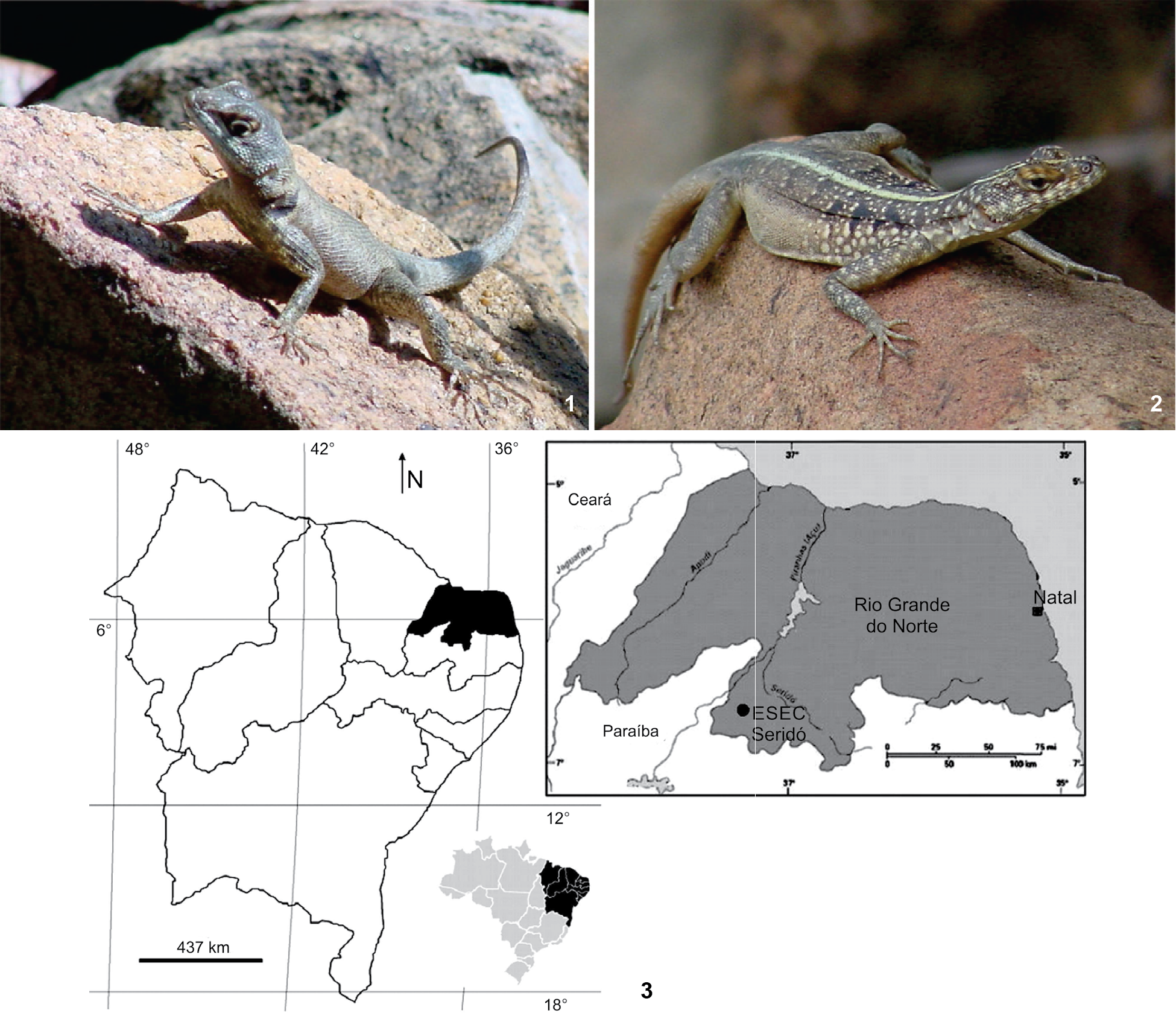
The study of the ovarian follicles of T. hispidus and T. semitaeniatus (Figs. 1 and 2) was conducted at the Cell Biology/Cytology and Histology Laboratory of the Agrarian Sciences Campus of the Universidade Federal do Vale do São Francisco, Petrolina, state of Pernambuco, between August 2013 and February 2014. A total of 60 lizards, 34 T. hispidus (dry, n = 22; wet, n = 12) and 26 T. semitaeniatus (dry, n = 12; wet, n = 14), were collected from the Ecological Station of Seridó (ESEC Seridó), Serra Negra do Norte, state of Rio Grande do Norte (Fig. 3), between October 2006 and May 2008. The climate there is semiarid (Ab'Sáber 1974Ab'sáber AN (1974) O domínio morfoclimático semiárido das Caatingas brasileiras. Geomorfologia 43: 1-139.), with a short wet season between March and May and average annual rainfall ranging between 500 and 700 mm.
(1-2) Specimens of Tropidurus hispidus (1) and Tropidurus semitaeniatus (2) at the Ecological Station of Seridó, Brazil. (3) Location of the Ecological Station of Seridó (black circle = ESEC Seridó) in the southwest portion of the state of Rio Grande do Norte, Brazil.
Gonads previously embedded in paraffin blocks were sectioned at 5 µm thick. Next, slides were prepared using the hematoxylin-eosin (HE) staining technique. Slides were analyzed under an optical microscope for morphological characterization of the ovarian follicles. Structures such as the granulosa and thecal layer cells, the vitelline membrane and the vitelline reservoir in the ooplasma were observed. The arrangement of these structures allowed us to determine the phases of folliculogenesis in these lizards. Images of these follicular phases were photographed with a digital camera coupled to a microscope and were labeled with the season in which the animals were collected.
Clutch size was estimated consistent with a previous study (see Ribeiro et al. 2012Ribeiro LB, Silva NB, Freire EMX (2012) Reproductive and fat body cycles of Tropidurus hispidus and Tropidurus semitaeniatus (Squamata, Tropiduridae) in a caatinga area of northeastern Brazil. Revista Chilena de Historia Natural 85: 307-320.) by counting the number of vitellogenic follicles or eggs in the oviducts. In the case of females with simultaneous occurrence of vitellogenic follicles and eggs in the oviducts or corpora lutea, clutch size was estimated only by the number of oviductal eggs. However, given that the folliculogenesis of tropical lizards, particularly in phylogenetically related species of Tropidurus, is poorly known, we had to compare our results with data on evolutionarily distant species, such as teiids, gekkonids and mabuyids. The rainfall data were obtained from the Laboratório de Recursos Hídricos e Saneamento Ambiental (LARHISA), the Federal University of Rio Grande do Norte, Natal, Rio Grande do Norte. The relationship between number of reproductive lizards and monthly rainfall was tested using linear regression analysis (Zar 1999Zar JH (1999) Biostatistical analysis. Upper Sandle River, Prentice-Hall Inc., 4th ed., 663p.). Statistical analysis was conducted using SPSS 13.0 and the significance level adopted was 0.05.
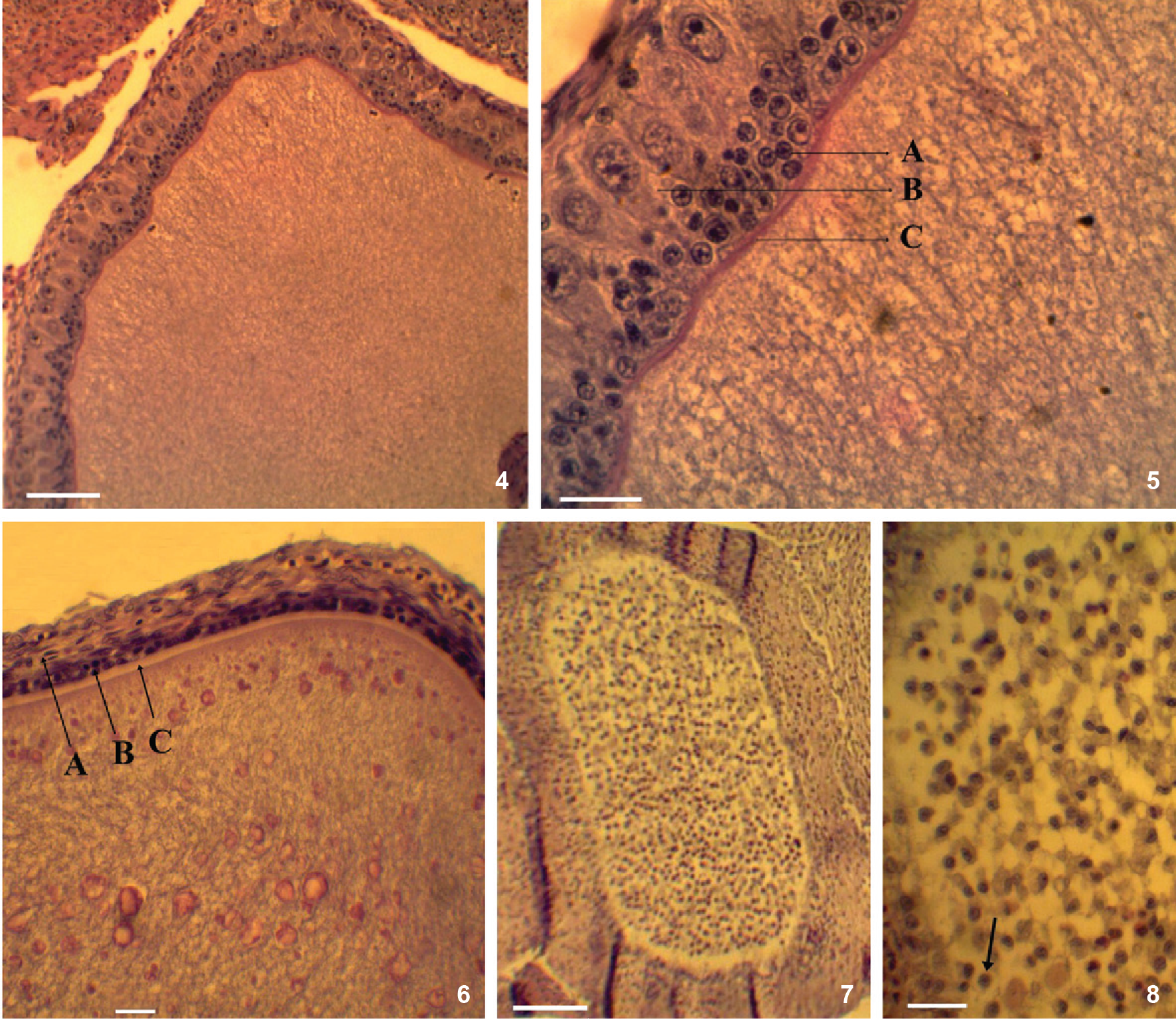
The histology of the ovary of T. hispidus and T. semitaeniatus revealed three gonad phases that are common to both species: pre-vitellogenic (Figs. 4 and 5), vitellogenic (Fig. 6) and follicular atresia (Figs. 7 and 8). An additional phase, corresponding to the post-ovulatory or luteal phase, was observed only in T. hispidus (Figs. 9 and 10). In T. semitaeniatus, luteal cell mass was not visualized in any section of the gonad. The snout-vent length (SVL) of females and sample size for each reproductive condition, and a description of the folliculogenesis, are in Table I.
Follicular phase. (4-6) Tropidurus semitaeniatus: (4-5) pre-vitellogenic: small cells (A), granulosa layer with the presence of pyriform cells (B), vitelline membrane (C); (6) vitellogenic: thecal layer (A), note the thickness of the granulosa layer with a single strand of cuboid cells (B), pellucid zone around the radiate zone (C); (7-8) Tropidurus hispidus, follicular atresia, showing hypertrophy in the granulosa layer. Presence of amoeboid macrophages (arrow) and significant vacuolization. Scale bars: 4, 6, 7 = 100 µm, 5, 8 = 30 µm.
Folliculogenesis in Tropidurus hispidus and Tropidurus semitaeniatus, indicating snout-vent length (SVL) of the females and sample size for each follicular phase with the basic description of the histological changes.
The pre-vitellogenic phase preceded the reproductive activity, with little deposition of vitellus granules in the cytoplasm of the oocyte (Fig. 4). The granulosa layer was formed by cuboid follicular cells that stratified and became polymorphic with their development, increasing the thickness of the granulosa. Pyriform cells were the largest granulosa cells, exhibiting a voluminous nucleus and thin, long apex facing the vitelline membrane of the oocyte. In this phase, small cells and the vitelline membrane can be observed (Fig. 5). While the exact function of the granulosa layer, in relation to pyriform cells, is uncertain, some authors consider that these cells play a role in vitellus production (Betz 1963Betz TW (1963) The ovarian histology of the diamond-backed water snake, Natrix rhombifera during the reproductive cycle. Journal of Morphology 113: 245-260., Goldberg 1970Goldberg SR (1970) Seasonal ovarian histology of the ovoviviparous Iguanid lizard Sceloporus jarrovi Cope. Journal of Morphology 132: 265-276., Saha et al. 1984Saha B, Manna D, Sircar A (1984) Ovarian histology of the water snake Xenochrophis piscator. Annals of Zoology 21: 47-58.).
The vitellogenic phase (Fig. 6) was characterized by intense vitellogenesis with a large number of vitellus granules filling the oocyte cytoplasm. The granulosa layer became thinner, with a single layer of cuboid cells, contrasting with the thecal layer, which became hypertrophied. Vitellus granules accumulated in the vitellogenesis phase will feed the future developing embryo (Varma 1970Varma SK (1970) Morphology of ovarian changes in the garden lizard, Calotes versicolor. Journal of Morphology 131: 95-210., Uribe et al. 1995Uribe MCA, Omana MEM, Quintero JEG, Guillette LJ (1995) Seasonal variation in ovarian histology of the viviparous lizard Sceloporus torquatus torquatus. Journal of Morphology 226: 103-119.). Vitellogenic ovarian hypertrophy marked the onset of the ovarian cycle of both species of Tropidurus. This observation is consistent with previous studies on Hemidactylus mabouia (Moreau de Jonnès, 1818) (Moodley & Van Wyk 2007Moodley GK, Van Wyk JH (2007) Folliculogenesis and ovarian histology of the oviparous gecko, Hemidactylus mabouia (Sauria: Gekkonidae). African Journal of Herpetology 56: 115-135.).
The origin of the cells of the granulosa layer during folliculogenesis has been widely questioned. According to Betz (1963Betz TW (1963) The ovarian histology of the diamond-backed water snake, Natrix rhombifera during the reproductive cycle. Journal of Morphology 113: 245-260.) and Boyd (1940Boyd MMM (1940) The structure of the ovary and the formation of the corpus luteum in Hoplodactylus maculatus. Quarterly Journal of Microscopical Science 82: 337-376.), intermediate and pyriform cells emerge from the differentiation of small cells, each from a different lineage. On the other hand, Neaves (1971Neaves WB (1971). Intercellular bridges between follicle cells and oocyte in the lizard. Anolis carolinensis. The Anatomical Record 170: 285-301.) and Van Wyk (1984Van Wyk JH (1984) Ovarian morphological changes during the annual breeding cycle of the rock lizard Agama atra (Sauria: Agamidae). Navorsinge van die Nasionale Museum Bloemfontein 4: 237-275.) suggested that pyriform cells are derived from intermediate cells, which in turn, derive from small cells. Given that the cytological features of all three types of cells are similar, they may represent a sequence of development (Goldberg 1970Goldberg SR (1970) Seasonal ovarian histology of the ovoviviparous Iguanid lizard Sceloporus jarrovi Cope. Journal of Morphology 132: 265-276.). In the progression from the pre-vitellogenic to the vitellogenic phase there is a decrease in the size of the granulosa layer. In H. mabouia, the granulosa becomes drastically reduced prior to the onset of the vitellogenesis (Moodley & Van Wyk 2007Moodley GK, Van Wyk JH (2007) Folliculogenesis and ovarian histology of the oviparous gecko, Hemidactylus mabouia (Sauria: Gekkonidae). African Journal of Herpetology 56: 115-135.). It has been suggested that this reduction in size is associated with programmed cell death (apoptosis) (Motta et al. 1996Motta C, Filosa S, Andreuccetti P (1996) Regression of the epithelium in the late previtellogenic follicles of Podarcis sicula: a case of apoptosis. Journal of Experimental Zoology 266: 233-241.).
Follicular atresia was characterized by a polymorphic granulosa layer, which consisted of large, irregular cells with circular to oval nuclei. With the progression of atresia, the granulosa hypertrophied and began to invade the oocyte. Although this phase can occur in both follicles during pre-vitellogenesis and vitellogenesis (Moodley & Van Wyk 2007Moodley GK, Van Wyk JH (2007) Folliculogenesis and ovarian histology of the oviparous gecko, Hemidactylus mabouia (Sauria: Gekkonidae). African Journal of Herpetology 56: 115-135.), it is more common during pre-vitellogenesis (Gomez & Ramirez-Pinilla 2004Gomez D, Ramirez-Pinilla MP (2004) Ovarian histology of the Placentrophic Mabuya mabouya (Squamata, Scincidae). Journal of Morphology 259: 90-105.). In our results, atresia was present only in the pre-vitellogenic phase of both species. Within the atresic folli cle there are numerous amoeboid macrophages, indicating phagocytary activity, thereby increasing vacuolization (Figs. 7 and 8). The hypertrophied theca and thin strands of thecal conjunctive tissue penetrated the granulosa tissue. Follicular atresia was completed with a mixture of granulosa layer cells, connective tissue of the thecal layer, fibroblasts and phagocytary cells distributed throughout the oocyte (see Goldberg 1970Goldberg SR (1970) Seasonal ovarian histology of the ovoviviparous Iguanid lizard Sceloporus jarrovi Cope. Journal of Morphology 132: 265-276.). The role of atresia can include limiting the number of eggs during a particular reproductive cycle through digestion and/or removal of ooplasma content (Hernandez-Franyutti et al. 2005Hernandez-Franyutti A, Uribe M, Guillette L (2005) Oogenesis in the viviparous matrophic lizard Mabuya brachypoda. Journal of Morphology 265: 152-164.). At ESEC Seridó, the average clutch size of T. hispidus was 8.1 ± 2.0 eggs, whereas that of T. semitaeniatus was 2.1 ± 0.6 eggs (Ribeiro et al. 2012Ribeiro LB, Silva NB, Freire EMX (2012) Reproductive and fat body cycles of Tropidurus hispidus and Tropidurus semitaeniatus (Squamata, Tropiduridae) in a caatinga area of northeastern Brazil. Revista Chilena de Historia Natural 85: 307-320.). Tropidurus semitaeniatus individuals are dorsoventrally flattened and are morphologically and behaviorally adapted to utilize rock crevices to escape predation. According to Vitt (1981Vitt LJ (1981) Lizard reproduction: habitat specificity and constraints on relative clutch mass. The American Naturalist 117: 506-514.), if a species is relatively more adapted to crevices than a closely related species, as T. hispidus, its relative clutch mass should be correspondingly lower.
The post-ovulatory or luteal phase identified in T. hispidus was characterized by granulosa cells filling the follicular cavity forming a central mass of heterogeneous or luteal cells. This is consistent with observations made on Salvator merianae (Duméril & Bibron, 1839) (Manes et al. 2007Manes ME, Noriega T, Casal FC, Apichela S (2007) Ovarian changes during the reproductive cycle of the Tupinambis merianae lizard raised in a temperate environment. Cuadernos de Herpetologia 21: 21-29.). These cells exhibit basophil nuclei and their morphology varies from circular to oval. The difference between the inner and outer theca was not evident and collagen fibers and fibroblasts started to invade the center of the luteal cell mass (Figs. 9 and 10). Studies with reptiles have suggested that the role of the corpus luteum is to maintain embryonic development through the secretion of progesterone (Gomez & Ramírez-Pinilla 2004Gomez D, Ramirez-Pinilla MP (2004) Ovarian histology of the Placentrophic Mabuya mabouya (Squamata, Scincidae). Journal of Morphology 259: 90-105., Xavier 1987Xavier F (1987) Functional morphology and regulation of the corpus luteum, p. 241-282. In: Norris DO, Jones RE (Eds) Hormones and Reproduction in fishes, amphibians and reptiles. New York, Plenum Press.). Most reproductive activity of T. hispidus and T. semitaeniatus at ESEC Seridó occurred after the middle of the dry season and during the rainy season. This was confirmed by histological analyses showing the greater occurrence of ovarian follicles in the vitellogenesis phase (Figs. 11 and 12), corresponding to cyclical reproduction. The regression analysis indicated a relationship between the number of reproductive individuals and rainfall regimen at ESEC Seridó for T. hispidus (R = 0.561, df = 1, p = 0.01) and T. semitaeniatus (R = 0.637, df = 1, p = 0.003). Seasonal changes in ovarian folliculogenesis are commonly reported in studies of temporal changes in the reproductive cycles of both oviparous (H. mabouia, S. merianae) and viviparous (Mabuya brachypoda) lizards (Moodley & Van Wyk 2007Moodley GK, Van Wyk JH (2007) Folliculogenesis and ovarian histology of the oviparous gecko, Hemidactylus mabouia (Sauria: Gekkonidae). African Journal of Herpetology 56: 115-135., Manes et al. 2007Manes ME, Noriega T, Casal FC, Apichela S (2007) Ovarian changes during the reproductive cycle of the Tupinambis merianae lizard raised in a temperate environment. Cuadernos de Herpetologia 21: 21-29., Hernandez-Franyutti et al. 2005Hernandez-Franyutti A, Uribe M, Guillette L (2005) Oogenesis in the viviparous matrophic lizard Mabuya brachypoda. Journal of Morphology 265: 152-164.). Inasmuch as descriptions of cytological changes during the ovarian cycle have received little attention, the present study provides an unprecedented contribution to the histological characterization of the ovarian follicles of two species of li zards that are widely distributed in the semiarid region of Brazil. Furthermore, it revealed the existence of seasonal variation in the ovarian folliculogenesis of T. hispidus and T. semitaeniatus, in agreement with the results of other studies on Squamata reptiles. Modifications in the granulosa and thecal layers allowed a better understanding of the different phases of folliculogenesis. This folliculogenesis of the ovaries of both species of Tropidurus correspond to the general pattern described for oviparous lizards.
Follicular phase of Tropidurus hispidus, corpus luteum showing a mass filled with luteal cells. Cells with basophil nuclei and shapes varying from circular to oval. Presence of collagen fibers from the thecal layer (arrow). Scale bars: 9 = 100 µm, 10 = 30 µm.
Reproductive condition of Tropidurus hispidus (11) and Tropidurus semitaeniatus (12), showing follicular phases (PV: pre-vitellogenic, V: vitellogenic, PV/A: pre-vitellogenic in atresia, L: luteal) in association with rainfall between October 2006 and May 2008 at the Ecological Station of Seridó, Brazil. Numbers above bars indicate sample size.
ACKNOWLEDGEMENTS
We thank the Programa PELD/CNPq - Caatinga: Estrutura e Funcionamento for logistic support, and three anonymous reviewers for their critical and constructive reviews of an earlier version of this manuscript. H.S. Santos received a research grant from CNPq (Process 139179/2012-7) and FACEPE (Process BIC-0113-2.04/13). Research involving animals was in accord with Ethics Comittee in Animal Use of Universidade Federal do Vale do São Francisco, and under permission of IBAMA (Permit 206/2006 and Process 02001.004294/03-15).
- Ab'sáber AN (1974) O domínio morfoclimático semiárido das Caatingas brasileiras. Geomorfologia 43: 1-139.
- Adolph SC, Porter WP (1993) Temperature, activity and lizard life histories. The American Naturalist 142: 273-295.
- Ávila RW, Cunha-Avellar LR, Ferreira VL (2008) Diet and reproduction of the lizard Tropidurus etheridgei in rocky areas of central Brazil. Herpetological Review 39: 430-433.
- Balestrin RL, Cappellari LH, Outeiral AB (2010) Biologia reprodutiva de Cercosaura schreibersii (Squamata, Gymnophthalmidae) e Cnemidophorus lacertoides (Squamata, Teiidae) no Escudo Sul-Riograndense, Brasil. Biota Neotropica 10: 131-139.
- Betz TW (1963) The ovarian histology of the diamond-backed water snake, Natrix rhombifera during the reproductive cycle. Journal of Morphology 113: 245-260.
- Boyd MMM (1940) The structure of the ovary and the formation of the corpus luteum in Hoplodactylus maculatus. Quarterly Journal of Microscopical Science 82: 337-376.
- Clerke RB, Alford RA (1993) Reproductive biology of four species of tropical Australian lizards and comments on the factors regulating lizard reproductive cycles. Journal of Herpetology 27: 400-406.
- Cruz FB, Teisaire E, Nieto L (1997) Reproductive biology of the lizard Tropidurus spinulosus in the chaco of Salta, Argentina. Studies on Neotropical Fauna and Environment 32: 28-32.
- Fitch HS (1982) Reproductive cycles in tropical reptiles. Occasional Papers of the Museum of Natural History 96: 1-53.
- Frost DR, Rodrigues MT, Grant T, Titus TA (2001) Phylogenetics of the lizard genus Tropidurus (Squamata: Tropiduridae: Tropidurinae): direct optimization, descriptive efficiency, and sensitivity analysis of congruence between molecular data and morphology. Molecular Phylogenetics and Evolution 21: 352-371.
- Galdino CAB, Assis VB, Kiefer MC, Van Sluys M (2003) Reproduction and fat body cycle of Eurolophosaurus nanuzae (Sauria; Tropiduridae) from a seasonal montane habitat of Southeastern Brazil. Journal of Herpetology 37: 687-694.
- Goldberg SR (1970) Seasonal ovarian histology of the ovoviviparous Iguanid lizard Sceloporus jarrovi Cope. Journal of Morphology 132: 265-276.
- Gomez D, Ramirez-Pinilla MP (2004) Ovarian histology of the Placentrophic Mabuya mabouya (Squamata, Scincidae). Journal of Morphology 259: 90-105.
- Hernandez-Franyutti A, Uribe M, Guillette L (2005) Oogenesis in the viviparous matrophic lizard Mabuya brachypoda. Journal of Morphology 265: 152-164.
- Manes ME, Noriega T, Casal FC, Apichela S (2007) Ovarian changes during the reproductive cycle of the Tupinambis merianae lizard raised in a temperate environment. Cuadernos de Herpetologia 21: 21-29.
- Meshaka Jr WE, Smith HT, Dean CL (2006) Gonadal cycle and growth of a West Indian Lizard, the Northern Curlytail Lizard (Leiocephalus carinatus armouri), in southern Florida. Herpetological Conservation and Biology 1: 109-115.
- Moodley GK, Van Wyk JH (2007) Folliculogenesis and ovarian histology of the oviparous gecko, Hemidactylus mabouia (Sauria: Gekkonidae). African Journal of Herpetology 56: 115-135.
- Motta C, Filosa S, Andreuccetti P (1996) Regression of the epithelium in the late previtellogenic follicles of Podarcis sicula: a case of apoptosis. Journal of Experimental Zoology 266: 233-241.
- Neaves WB (1971). Intercellular bridges between follicle cells and oocyte in the lizard. Anolis carolinensis. The Anatomical Record 170: 285-301.
- Ramírez-Bautista A, Barba-Torres J, Vitt LJ (1998) Reproductive cycle and brood size of Eumeces lynxe from Pinal de Amoles, Queretero, Mexico. Journal of Herpetology 32: 18-24.
- Ribeiro LB, Silva NB, Freire EMX (2012) Reproductive and fat body cycles of Tropidurus hispidus and Tropidurus semitaeniatus (Squamata, Tropiduridae) in a caatinga area of northeastern Brazil. Revista Chilena de Historia Natural 85: 307-320.
- Rodrigues MT (1987) Sistemática, ecologia e zoogeografia dos Tropidurus do grupo torquatus ao Sul do Rio Amazonas (Sauria, Iguanidae). Arquivos de Zoologia do Estado de São Paulo 31: 105-230.
- Saha B, Manna D, Sircar A (1984) Ovarian histology of the water snake Xenochrophis piscator. Annals of Zoology 21: 47-58.
- Uribe MCA, Omana MEM, Quintero JEG, Guillette LJ (1995) Seasonal variation in ovarian histology of the viviparous lizard Sceloporus torquatus torquatus. Journal of Morphology 226: 103-119.
- Van Sluys M (1992) Aspectos da ecologia do lagarto Tropidurus itambere (Iguanidae) em uma área do sudeste do Brasil. Revista Brasileira de Biologia 52: 181-185.
- Van Sluys M (1993) The reproductive cycle of Tropidurus itambere (Sauria, Tropiduridae) in southeastern Brazil. Journal of Herpetology 27: 28-32.
- Van Sluys M, Mendes HMA, Assis VB, Kiefer MC (2002) Reproduction of Tropidurus montanus Rodrigues, 1987 (Tropiduridae), a lizard from a seasonal habitat of south-eastern Brazil, and a comparison with other Tropidurus species. Herpetological Journal 12: 89-97.
- Van Wyk JH (1984) Ovarian morphological changes during the annual breeding cycle of the rock lizard Agama atra (Sauria: Agamidae). Navorsinge van die Nasionale Museum Bloemfontein 4: 237-275.
- Varma SK (1970) Morphology of ovarian changes in the garden lizard, Calotes versicolor. Journal of Morphology 131: 95-210.
- Vitt LJ (1992) Diversity of reproductive strategies among Brazilian lizards and snakes: the significance of lineage and adaptation, p. 135-149. In: Hamlett WC (Ed.) Reproductive Biology of South American Vertebrates. New York, Springer-Verlag.
- Vitt LJ (1995). The ecology of tropical lizards in the caatinga of northeast Brazil. Occasional Papers of the Oklahoma Museum of Natural History 1: 1-29.
- Vitt LJ (1981) Lizard reproduction: habitat specificity and constraints on relative clutch mass. The American Naturalist 117: 506-514.
- Vrcibradic D, Rocha, CFD (1998) Reproductive cycle and life-history traits of the viviparous skink Mabuya frenata in southeastern Brazil. Copeia 1998: 612-619.
- Wiederhecker HC, Pinto ACS, Colli GR (2002) Reproductive ecology of Tropidurus torquatus (Squamata: Tropiduridae) in the highly seasonal Cerrado biome of Central Brazil. Journal of Herpetology 36: 82-91.
- Xavier F (1987) Functional morphology and regulation of the corpus luteum, p. 241-282. In: Norris DO, Jones RE (Eds) Hormones and Reproduction in fishes, amphibians and reptiles. New York, Plenum Press.
- Zar JH (1999) Biostatistical analysis. Upper Sandle River, Prentice-Hall Inc., 4th ed., 663p.
- Zug GR, Vitt LJ, Caldwell JP (2001) Reproductive ecology and life histories, p. 135-153. In: Zug GR, Vitt LJ, Caldwell JP (Eds) Herpetology: an introductory biology of amphibians and reptiles. San Diego, Academic Press, 2nd ed.
Publication Dates
-
Publication in this collection
Jan-Feb 2015
History
-
Received
12 July 2014 -
Reviewed
08 Nov 2014 -
Accepted
23 Jan 2015