Abstract
Legitimate flowers visitors pollinate the flower during the visit and thus influence the production of fruits and seeds. We tested whether the visitation rate of potential pollinators is associated with the amount of seeds per fruit produced by the self-compatible bromeliad Tillandsia stricta (Bromeliaceae). We determined whether hummingbirds are legitimate visitors by testing for a correlation between visits and pollination (seed production) at the Guapiaçú Ecological Reserve (Reserva Ecológica de Guapiaçú), state of Rio de Janeiro. We tested 30 flowers, five of which were also monitored to test the possibility of spontaneous self-pollination. The remaining 25 flowers were exposed to floral visitors. Twenty-two flowers formed fruits and seeds, from which three formed seeds without floral visits. The hummingbird Amazilia fimbriata (Gmelin, 1788) was the only legitimate visitor. The average number (± standard deviation) of seeds was 27 units (±15) per fruit. The floral visitation rate by A. fimbriata was 6.6 (±3.4) visits/per flower. The number of floral visits and the amount of seed produced were positively correlated (r² = 0.58, p < 0.01). Thus, A. fimbriata is a legitimate floral visitor of T. stricta, and influences seed production per fruit in this bromeliad.
Atlantic forest; hummingbird; pollination; self-compatibility
Bromeliaceae includes 58 genera, of which Tillandsia Linnaeus is one of
the richest, with about 600 epiphytic or rupicolous species (Luther 2008Luther HE (2008) An alphabetical list of bromeliad binomials. Available
online at: http://www.selby.org/sites/all/files/Bromeliad_Binomial_List_For_Web.pdf
[Accessed: 18/05/2014]
http://www.selby.org/sites/all/files/Bro...
). Species in this family are pollinated mostly by
vertebrates, and hummingbirds are their most frequent visitors (Zanella et al. 2012Zanella CM, Janke A, Palma-Silva C, Kaltchuk-Santos E, Pinheiro FG,
Paggi GM, Soares LES, Goetze M, Büttow MW, Bered F (2012) Genetics, evolution and
conservation of Bromeliaceae. Genetics and Molecular Biology 35(4, Suppl. 1):
1020-1026. doi: 10.1590/S1415-47572012000600017
https://doi.org/10.1590/S1415-4757201200...
). The effects of plant-pollinator interaction on
plant reproduction, however, are still poorly understood (Antonelli & Sanmartín 2011Antonelli A, Sanmartín I (2011) Why are there so many plant species in
the Neotropics? Taxon 60: 403-414., Kamke et al.
2011Kamke R, Schmid S, Zillikens A, Lopes AC, Steiner J (2011) The impor
tance of bees as pollinators in the short corolla bromeliad Aechmea caudata in
southern Brazil. Flora 206: 749-756., Zanella et al. 2012Zanella CM, Janke A, Palma-Silva C, Kaltchuk-Santos E, Pinheiro FG,
Paggi GM, Soares LES, Goetze M, Büttow MW, Bered F (2012) Genetics, evolution and
conservation of Bromeliaceae. Genetics and Molecular Biology 35(4, Suppl. 1):
1020-1026. doi: 10.1590/S1415-47572012000600017
https://doi.org/10.1590/S1415-4757201200...
). Species in
this family have two main breeding systems, and self-compatibility (SC) is more common than
self-incompatibility (SI). SC may be a pre-zygotic reproductive isolation mechanism in
Bromeliaceae populations that are subjected to interspecific pollen flow (Wendt et al. 2008Wendt T, Coser TS, Matallana G, Guilherme FAG (2008) An apparent lack of
prezygotic reproductive isolation among 42 sympatric species of Bromeliaceae in
southeastern Brazil. Plant Systematics and Evolution 275: 31-41. doi:
10.1007/s00606-008-0054-7
https://doi.org/10.1007/s00606-008-0054-...
, Matallana et al. 2010Matallana G, Godinho MAS, Guilherme FAG, Belisario M, Coser TS, Wendt T
(2010) Breeding systems of Bromeliaceae species: Evolution of selfing in the context
of sympatric occurrence. Plant Systematics and Evolution 289: 57-65.).
In SI species, an increase in the visitation rate of pollinators is expected to be
positively correlated with fecundity, since pollinators increase the number of pollen
grains transported (Longo & Fischer 2006Longo JM, Fischer E (2006) Efeito da taxa de secreção de néctar sobre a
polinização e a produção de sementes em flores de Passiflora speciosa Gardn.
(Passifloraceae) no Pantanal. Revista Brasileira de Botânica 29(3):
481-488.) only
if pollen is limited (Hargreaves et al. 2009Hargreaves AL, Lawrence DH, Johnson SD (2009) Consumptive emasculation:
the ecological and evolutionary consequences of pollen theft. Biological Reviews 84:
259-276. doi: 10.1111/j.1469-185X.2008.00074.x
https://doi.org/10.1111/j.1469-185X.2008...
). This
relationship occurs because cross-pollination is necessary for fertilization (Takayama & Isogai 2005Takayama S, Isogai A (2005) Self-incompatibility in plants. Annual
Review of Plant Biology 56: 467-489. doi:
10.1146/annurev.arplant.56.032604.144249
https://doi.org/10.1146/annurev.arplant....
). However, even in SC
bromeliad species, floral visits may increase pollen transfer between floral structures and
positively influence fecundity (Siqueira Filho &
Machado 2001Siqueira-Filho JA, Machado ICS (2001) Biologia reprodutiva de Canistrum
aurantiacum E. Morren (Bromeliaceae) em remanescente da floresta atlântica, nordeste
do Brasil. Acta Botanica Brasilica 15(3): 427-443.).
Tillandsia stricta Sol is an epiphytic, ornithophilous species with a SC
reproductive system (Matallana et al 2010Matallana G, Godinho MAS, Guilherme FAG, Belisario M, Coser TS, Wendt T
(2010) Breeding systems of Bromeliaceae species: Evolution of selfing in the context
of sympatric occurrence. Plant Systematics and Evolution 289: 57-65.). It is
widely distributed in Brazil (Pontes & Agra
2006Pontes RAS, Agra MF (2006) Flora da Paraíba, Brasil: Tillandsia L.
(Bromeliaceae). Rodriguésia 57(1): 47-61., Cogliatti-Carvalho et al. 2008Cogliatti-Carvalho L, Rocha-Pessôa TC, Nunes-Freitas AF, Rocha CFD
(2008) Bromeliaceae species from coastal restinga habitats, Brazilian states of Rio
de Janeiro, Espírito Santo, and Bahia. Check List 4(3):234-239.),
occurring in the Atlantic forest and its associated ecossistems (Cogliatti-Carvalho et al. 2001Cogliatti-Carvalho L, Nunes-Freitas AF, Rocha CFD, Van Sluys M (2001)
Variação na estrutura e composição de Bromeliaceae em cinco zonas de vegetação no
Parque Nacional da restinga de Jurubatiba, Macaé, RJ. Revista Brasileira de Botânica
24(1): 1-9., Bonnet
& Queiroz 2006Bonnet A, Queiroz MH (2006) Estratificação vertical de bromélias
epifíticas em diferentes estádios sucessionais da Floresta Ombrófila Densa, Ilha de
Santa Catarina, Santa Catarina, Brasil. Revista Brasileira de Botânica 29:
217-228., Machado & Semir
2006Machado CG, Semir J (2006) Fenologia da floração e biologia floral de
bromeliáceas ornitófilas de uma área da Mata Atlântica do Sudeste brasileiro. Revista
Brasileira de Botânica 29(1): 163-174.). This species receives floral visits from different species of hummingbirds
(Apodiformes: Trochilidae) and from the bird Bananaquit, Coereba flaveola
(Linnaeus, 1758) (Passeriformes: Thraupidae), in addition to different insect species
(Sazima et al. 1996Sazima I, Buzato S, Sazima M (1996) An Assemblage of
Hummingbird-pollinated Flowers in a Montane Forest in Southeastern Brazil. Botanica
Acta 109: 149-160. doi: 10.1111/j.1438-8677.1996.tb00555.x
https://doi.org/10.1111/j.1438-8677.1996...
, Alves et al. 2000Alves MAS, Rocha CFD, Van Sluys M, Bergallo HG (2000) Guildas de
beija-flores polinizadores de quatro espécies de Bromeliaceae de Mata Atlântica da
Ilha Grande, RJ, Brasil: composição e taxas de visitação, p. 171-185. In: Alves MAS,
Silva JMC, Van Sluys M, Bergallo HG, Rocha CFD (Eds.). A Ornitologia n Brasil:
pesquisa atual e perspectivas. Rio de Janeiro, EdUERJ, 352p., Machado & Semir
2006Machado CG, Semir J (2006) Fenologia da floração e biologia floral de
bromeliáceas ornitófilas de uma área da Mata Atlântica do Sudeste brasileiro. Revista
Brasileira de Botânica 29(1): 163-174.). In the present study we tested the hypothesis that flower visits by
potential pollinators of T. stricta are positively correlated with the
number of seeds produced per fruit.
The study took place in the Atlantic Rainforest within the Guapiaçú Ecological Reserve (22°24'S, 42°44'W, 7,000 ha) near the city of Cachoeiras de Macacu, state of Rio de Janeiro. We set up the experiment and observed flower visitors during six days in January 2013, and returned to collect fruits in March 2013.
We monitored 30 flowers from six individuals of T. stricta, including five flowers used to test for spontaneous self-pollination. The remaining 25 flowers were bagged with tulle fabric to prevent access of flower visitors.
It was not possible to limit our survey to one flower per individual because the plants were difficult to assess. We used one to three flowers per individual per day to make the observations on flower visitors. As we needed up to three days to conclude de observations of each flower, several flowers per individual were necessary to achieve a minimum sample size (about 12 flowers observed per day). The distance between the individuals sampled was about 20 m, and these individuals were in similar environmental conditions: two meters up from ground level in riparian vegetation. Each inflorescence had one to five flowers in anthesis per day.
During anthesis, we uncovered the flowers and exposed them to flower visitors between 6:00 a.m. and 6:00 p.m. After that we bagged them again. Observations were made for 12 consecutive hours on each flower sampled. We applied a thin layer of the non-toxic resin Tanglefoot(r) at the base of each flower stalk to block access by non-winged insects (Del-Claro et al. 1996Del-Claro K, Berto V, Reu W (1996) Effect of herbivore deterrence by ants on the fruit set of an extra floral nectary plant, Qualea multi flora (Vochysiaceae). Journal of Tropical Ecology 12: 887-892.). This process was repeated until the flower entered into senescence (three days). Stigma viability was determined using hydrogen peroxide (Kearns & Inouye 1993Kearns CA, Inouye D (1993) Techniques for pollinations biologists. Niwot, University of Colorado Press, 579p.) on unmonitored flowers of the same individuals. During focal observations we recorded floral visitor species and visit legitimacy (Inouye 1980Inouye DW (1980) The terminology of floral larceny. Ecology 61: 1251-1253.). After approximately 60 days, we collected fruits from the monitored flowers and determined the amount of seeds in each fruit. The flower stalks and resin were removed after the experiment. We tested the influence of the frequency of hummingbird floral visits on the number of seeds using a simple linear regression.
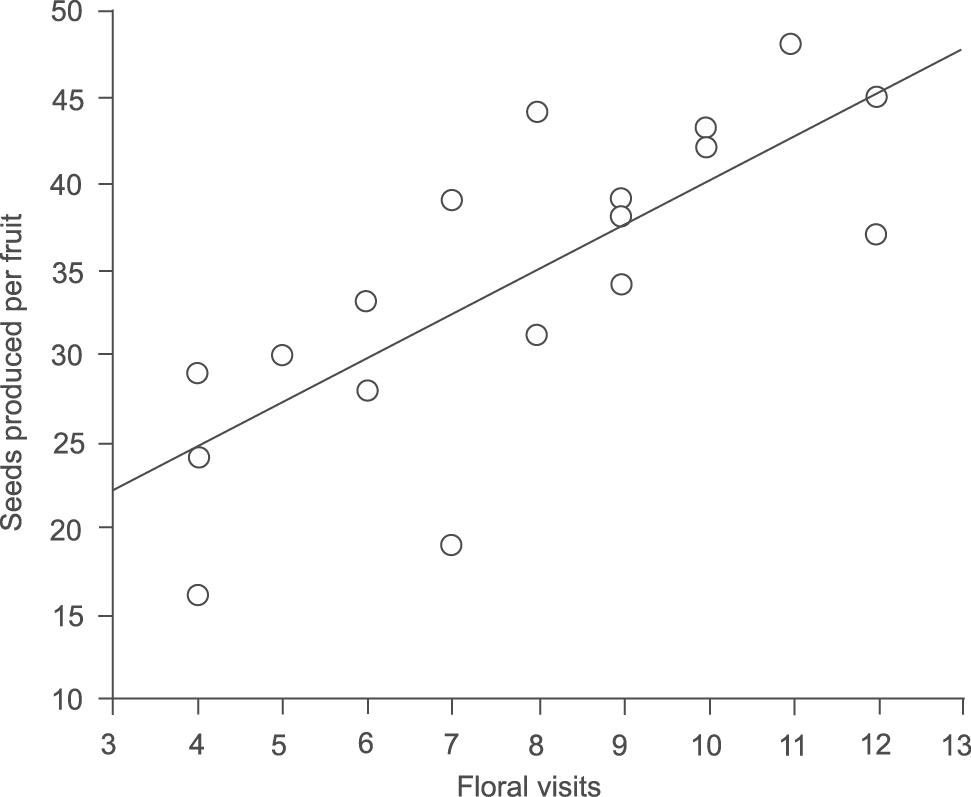
During the six-days of observation, flowers were visited only by the hummingbird Amazilia fimbriata (Gmelin, 1788). The flowers opened at dawn and closed at dusk. Each flower lasted for two to three days before entering into senescence. Among the five flowers monitored for spontaneous self-pollination, two formed fruits without the help of floral visitors. Of the other 25 monitored flowers, 22 formed fruits during the period considered (60 days), including three flowers that formed fruits without receiving floral visits. The remaining 19 flowers received an average (± standard deviation) of 6.6 (±3.4) visits throughout their duration. Each fruit had an average of 27 (±15) seeds. Fruits formed by spontaneous self-pollination produced less seeds with an average of 9.3 (±2.3) seeds per fruit. The amount of floral visits and the number of seeds produced per fruit were positively correlated (r² = 0.58, F = 24, df = 1.17, p < 0.01) (Fig. 1).
Linear regression between the rate of visitation of Amazilia fimbriata and the prodution of Tillandsia stricta seeds.
Flowers of T. stricta are typically receptive for three days, and may only be receptive the day after anthesis, even though pollen can be released soon after anthesis (Machado & Semir 2006Machado CG, Semir J (2006) Fenologia da floração e biologia floral de bromeliáceas ornitófilas de uma área da Mata Atlântica do Sudeste brasileiro. Revista Brasileira de Botânica 29(1): 163-174.). The anthers of the T. stricta flowers monitored by us released pollen and their stigmas were receptive shortly after anthesis. Increased seed production as a consequence of an increase in the number of visits by A. fimbriata shows that this bird is a legitimate pollinator. Similarly, hummingbird visitation resulted in greater seed production by Canistrum aurantiacum E. Morren (Bromeliaceae) than spontaneous self-pollination or manual pollination (Siqueira Filho & Machado 2001Siqueira-Filho JA, Machado ICS (2001) Biologia reprodutiva de Canistrum aurantiacum E. Morren (Bromeliaceae) em remanescente da floresta atlântica, nordeste do Brasil. Acta Botanica Brasilica 15(3): 427-443.).
The fact that the only floral visitor of T. stricta in our study was a hummingbird contrasts with the results of two studies on species of the genus: Alves et al. (2000Alves MAS, Rocha CFD, Van Sluys M, Bergallo HG (2000) Guildas de beija-flores polinizadores de quatro espécies de Bromeliaceae de Mata Atlântica da Ilha Grande, RJ, Brasil: composição e taxas de visitação, p. 171-185. In: Alves MAS, Silva JMC, Van Sluys M, Bergallo HG, Rocha CFD (Eds.). A Ornitologia n Brasil: pesquisa atual e perspectivas. Rio de Janeiro, EdUERJ, 352p.) found that insects were the most frequent visitors of this and other Tillandsia species (Varassim & Sazima 2000), and Machado & Semir (2006Machado CG, Semir J (2006) Fenologia da floração e biologia floral de bromeliáceas ornitófilas de uma área da Mata Atlântica do Sudeste brasileiro. Revista Brasileira de Botânica 29(1): 163-174.) found more than one hummingbird species visiting Tillandsia flowers. Our results indicate that increased foraging activity by one hummingbird species can stimulate seed production in T. stricta. We believe that an increased number of pollinator species, leading to higher visitation rate, could help to increase the fertility of this bromeliad, but this hypothesis needs to be tested.
ACKNOWLEDGMENTS
We thank the owners and employees of Guapiaçú Ecological Reserve for the support offered during this this study; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro for the scholarship and support given to Maria Alice S. Alves (processes 305.798/2014-6 and E-26/102.837/2012, respectively). CNPq also granted the first author a master's scholarship (process 133520/2012-9) in the Programa de Pós-Graduação em Ecologia, Universidade Federal do Rio de Janeiro.
- Alves MAS, Rocha CFD, Van Sluys M, Bergallo HG (2000) Guildas de beija-flores polinizadores de quatro espécies de Bromeliaceae de Mata Atlântica da Ilha Grande, RJ, Brasil: composição e taxas de visitação, p. 171-185. In: Alves MAS, Silva JMC, Van Sluys M, Bergallo HG, Rocha CFD (Eds.). A Ornitologia n Brasil: pesquisa atual e perspectivas. Rio de Janeiro, EdUERJ, 352p.
- Antonelli A, Sanmartín I (2011) Why are there so many plant species in the Neotropics? Taxon 60: 403-414.
- Bonnet A, Queiroz MH (2006) Estratificação vertical de bromélias epifíticas em diferentes estádios sucessionais da Floresta Ombrófila Densa, Ilha de Santa Catarina, Santa Catarina, Brasil. Revista Brasileira de Botânica 29: 217-228.
- Cogliatti-Carvalho L, Nunes-Freitas AF, Rocha CFD, Van Sluys M (2001) Variação na estrutura e composição de Bromeliaceae em cinco zonas de vegetação no Parque Nacional da restinga de Jurubatiba, Macaé, RJ. Revista Brasileira de Botânica 24(1): 1-9.
- Cogliatti-Carvalho L, Rocha-Pessôa TC, Nunes-Freitas AF, Rocha CFD (2008) Bromeliaceae species from coastal restinga habitats, Brazilian states of Rio de Janeiro, Espírito Santo, and Bahia. Check List 4(3):234-239.
- Del-Claro K, Berto V, Reu W (1996) Effect of herbivore deterrence by ants on the fruit set of an extra floral nectary plant, Qualea multi flora (Vochysiaceae). Journal of Tropical Ecology 12: 887-892.
- Hargreaves AL, Lawrence DH, Johnson SD (2009) Consumptive emasculation: the ecological and evolutionary consequences of pollen theft. Biological Reviews 84: 259-276. doi: 10.1111/j.1469-185X.2008.00074.x
» https://doi.org/10.1111/j.1469-185X.2008.00074.x - Inouye DW (1980) The terminology of floral larceny. Ecology 61: 1251-1253.
- Kamke R, Schmid S, Zillikens A, Lopes AC, Steiner J (2011) The impor tance of bees as pollinators in the short corolla bromeliad Aechmea caudata in southern Brazil. Flora 206: 749-756.
- Kearns CA, Inouye D (1993) Techniques for pollinations biologists. Niwot, University of Colorado Press, 579p.
- Longo JM, Fischer E (2006) Efeito da taxa de secreção de néctar sobre a polinização e a produção de sementes em flores de Passiflora speciosa Gardn. (Passifloraceae) no Pantanal. Revista Brasileira de Botânica 29(3): 481-488.
- Luther HE (2008) An alphabetical list of bromeliad binomials. Available online at: http://www.selby.org/sites/all/files/Bromeliad_Binomial_List_For_Web.pdf [Accessed: 18/05/2014]
» http://www.selby.org/sites/all/files/Bromeliad_Binomial_List_For_Web.pdf - Machado CG, Semir J (2006) Fenologia da floração e biologia floral de bromeliáceas ornitófilas de uma área da Mata Atlântica do Sudeste brasileiro. Revista Brasileira de Botânica 29(1): 163-174.
- Matallana G, Godinho MAS, Guilherme FAG, Belisario M, Coser TS, Wendt T (2010) Breeding systems of Bromeliaceae species: Evolution of selfing in the context of sympatric occurrence. Plant Systematics and Evolution 289: 57-65.
- Pontes RAS, Agra MF (2006) Flora da Paraíba, Brasil: Tillandsia L. (Bromeliaceae). Rodriguésia 57(1): 47-61.
- Sazima I, Buzato S, Sazima M (1996) An Assemblage of Hummingbird-pollinated Flowers in a Montane Forest in Southeastern Brazil. Botanica Acta 109: 149-160. doi: 10.1111/j.1438-8677.1996.tb00555.x
» https://doi.org/10.1111/j.1438-8677.1996.tb00555.x - Siqueira-Filho JA, Machado ICS (2001) Biologia reprodutiva de Canistrum aurantiacum E. Morren (Bromeliaceae) em remanescente da floresta atlântica, nordeste do Brasil. Acta Botanica Brasilica 15(3): 427-443.
- Takayama S, Isogai A (2005) Self-incompatibility in plants. Annual Review of Plant Biology 56: 467-489. doi: 10.1146/annurev.arplant.56.032604.144249
» https://doi.org/10.1146/annurev.arplant.56.032604.144249 - Varassin IG, Sazima M (2000) Recursos de Bromeliaceae utilizados por beija-flores e borboletas em Mata Atlântica no Sudeste do Brasil. Boletim do Museu de Biologia Mello Leitão 11(12): 57-70.
- Wendt T, Coser TS, Matallana G, Guilherme FAG (2008) An apparent lack of prezygotic reproductive isolation among 42 sympatric species of Bromeliaceae in southeastern Brazil. Plant Systematics and Evolution 275: 31-41. doi: 10.1007/s00606-008-0054-7
» https://doi.org/10.1007/s00606-008-0054-7 - Zanella CM, Janke A, Palma-Silva C, Kaltchuk-Santos E, Pinheiro FG, Paggi GM, Soares LES, Goetze M, Büttow MW, Bered F (2012) Genetics, evolution and conservation of Bromeliaceae. Genetics and Molecular Biology 35(4, Suppl. 1): 1020-1026. doi: 10.1590/S1415-47572012000600017
» https://doi.org/10.1590/S1415-47572012000600017
Publication Dates
-
Publication in this collection
May-Jun 2015
History
-
Received
14 July 2014 -
Reviewed
14 Dec 2014 -
Accepted
22 Apr 2015