ABSTRACT
Two new species of Drosophilidae from Uruguay are described and illustrated: Drosophila montevidensis sp. nov. (Holotype male in MZSP: Facultad de Agronomía, Universidad de la República, Montevideo city, Department of Montevideo), and Scaptomyza pipinna sp. nov. (Holotype male in MZSP: Sarandí del Consejo, near north shore of Laguna de Castillos, Department of Rocha). The former species belongs to the D. tripunctata group and is sibling to Drosophila nappae Vilela, Valente & Basso-da-Silva, 2004, differing mainly in characters of the aedeagus. The latter is closely related to Scaptomyza striaticeps Wheeler & Takada, 1966, from which it can be distinguished by color and terminalia characters. The new Drosophila species was successfully cultured in a modified banana-agar medium which is provided. Photomicrographs of mitotic and meiotic chromosomes of D. montevidensis sp. nov. are also included.
KEY WORDS:
Argentina; Drosophila tripunctata group; karyotypes; Mesoscaptomyza; taxonomy
For decades, Drosophila nappaeVilela, Valente & Basso-da-Silva, 2004Vilela CR, Valente VLS, Basso-da-Silva L (2004) Drosophila angustibucca Duda sensu Frota-Pessoa is an undescribed species (Diptera, Drosophilidae). Revista Brasileira de Entomologia 48: 233-238. doi: 10.1590/S0085-56262004000200012
https://doi.org/10.1590/S0085-5626200400...
, a South American species belonging to the D. tripunctata group, was misidentified under the nominal species Drosophila angustibucca Duda, 1925 described from Central America. Drosophila angustibucca is apparently endemic to Costa Rica and adjacent countries, whereas D. nappae occurs in the Atlantic forest of southeastern and southern Brazil and most probably also in Paraguay (Vilela et al. 2004Vilela CR, Valente VLS, Basso-da-Silva L (2004) Drosophila angustibucca Duda sensu Frota-Pessoa is an undescribed species (Diptera, Drosophilidae). Revista Brasileira de Entomologia 48: 233-238. doi: 10.1590/S0085-56262004000200012
https://doi.org/10.1590/S0085-5626200400...
).
During a preliminary survey of the drosophilid fauna of Uruguay, conducted during the last two decades, the first author collected some specimens of an unknown Drosophila Fallén, 1823 species which had been cited by Goñi et al. (1997Goñi B, Martínez ME, Daguer P (1997) Studies of two Drosophila (Diptera, Drosophilidae) communities from urban Montevideo, Uruguay. Revista Brasileira de Entomologia 41: 89-93., 1998Goñi B, Martinez ME, Valente VLS, Vilela CR (1998) Preliminary data on the Drosophila species (Diptera, Drosophilidae) from Uruguay. Revista Brasileira de Entomologia 42: 131-140.) as D. gr. tripunctata and as an unidentified species of the D. tripunctata group, respectively. At that time, Goñi and colleagues believed that their unknown specimens of Drosophila belonged to an undescribed species, which would be later described as D. nappae (Vilela et al. 2004Vilela CR, Valente VLS, Basso-da-Silva L (2004) Drosophila angustibucca Duda sensu Frota-Pessoa is an undescribed species (Diptera, Drosophilidae). Revista Brasileira de Entomologia 48: 233-238. doi: 10.1590/S0085-56262004000200012
https://doi.org/10.1590/S0085-5626200400...
) from flies collected at Porto Alegre, state of Rio Grande do Sul, southern Brazil. More recently, we became convinced that the unidentified specimens from Uruguay cited by Goñi et al. (1997Goñi B, Martínez ME, Daguer P (1997) Studies of two Drosophila (Diptera, Drosophilidae) communities from urban Montevideo, Uruguay. Revista Brasileira de Entomologia 41: 89-93., 1998Goñi B, Martinez ME, Valente VLS, Vilela CR (1998) Preliminary data on the Drosophila species (Diptera, Drosophilidae) from Uruguay. Revista Brasileira de Entomologia 42: 131-140.) do not belong either to D. angustibucca or to Drosophila nappae , but are members of a sibling and still undescribed species referred to as D. aff. nappae in Goñi et al. (2012Goñi B, Remedios M, González-Vainer P, Martínez ME, Vilela CR (2012). Species of Drosophila (Diptera: Drosophilidae) attracted to dung and carrion baited pitfall traps in the Uruguayan Eastern Serranías. Zoologia 29: 308-317. doi: 10.1590/S1984-46702012000400004
https://doi.org/10.1590/S1984-4670201200...
). The specimens unidentified to species rank were collected in southern Uruguay by net sweeping over fallen, decaying fleshy seeds of maidenhair tree (Ginkgo biloba L.) and decaying fruits of both native and exotic plants or emerging from such fruits (Goñi et al. 1997Goñi B, Martínez ME, Daguer P (1997) Studies of two Drosophila (Diptera, Drosophilidae) communities from urban Montevideo, Uruguay. Revista Brasileira de Entomologia 41: 89-93., 1998Goñi B, Martinez ME, Valente VLS, Vilela CR (1998) Preliminary data on the Drosophila species (Diptera, Drosophilidae) from Uruguay. Revista Brasileira de Entomologia 42: 131-140.), or attracted to dung and carrion baited pitfall traps (Goñi et al. 2012Goñi B, Remedios M, González-Vainer P, Martínez ME, Vilela CR (2012). Species of Drosophila (Diptera: Drosophilidae) attracted to dung and carrion baited pitfall traps in the Uruguayan Eastern Serranías. Zoologia 29: 308-317. doi: 10.1590/S1984-46702012000400004
https://doi.org/10.1590/S1984-4670201200...
). Only by checking the male terminalia, mainly the shape of their aedeagi, of this pair of sibling species it is possible to tell them apart. Additionally, a single male of an undescribed species of Scaptomyza Hardy, 1849, closely related to the Colombian Scaptomyza striaticepsWheeler & Takada, 1966Wheeler MR, Takada H (1966) The Nearctic and Neotropical Species of Scaptomyza Hardy (Diptera: Drosophilidae). University of Texas Publications 6615: 37-78., was collected by net sweeping over grass by our colleague Maria E. Martínez at the eastern wetlands (bañados in Spanish) of the Uruguayan Department of Rocha.
The purpose of the present paper is to erect names to these two species new to science, and to present their formal descriptions, including the karyotype description of the new Drosophila species.
MATERIAL AND METHODS
Specimens examined are deposited in the Entomological Collection (Diptera), Facultad de Ciencias, Universidad de la República, Montevideo, Uruguay (FCE-D) and the Museu de Zoologia, Universidade de São Paulo, São Paulo, Brazil (MZSP).
Refer to Bächli et al. (2004Bächli G, Vilela CR, Escher SA, Saura A (2004) The Drosophilidae (Diptera) of Fennoscandia and Denmark. Leiden, Brill, Fauna Entomologica Scandinavica, vol. 39, 362p.) for the terminology used in the descriptions, to Vilela (1983Vilela CR (1983) A revision of the Drosophila repleta species group (Diptera, Drosophilidae). Revista Brasileira de Entomologia 27: 1-114.) and Vilela & Bächli (1990Vilela CR, Bächli G (1990) Taxonomic studies on Neotropical species of seven genera of Drosophilidae (Diptera). Mitteilungen der schweizerischen Entomologischen Gesellschaft 63 (Suppl.): 1-332.) for measurements and indices, to Kaneshiro (1969Kaneshiro KY (1969) A study of the relationships of Hawaiian Drosophila species based on external male genitalia. University of Texas Publications 6918: 55-70.) and Wheeler & Kambysellis (1966Wheeler MR, Kambysellis MP (1966) Notes on the Drosophilidae (Diptera) of Samoa. University of Texas Publications 6615: 533-565.) for details on the methods of preparing the terminalia. Type labels include clarifying notes in square brackets; a slash indicates a label change. In the descriptions, average measurements are followed by range in parentheses. All types were dissected and their terminalia photomicrographed. The disarticulated terminalia are kept in a microvial filled with glycerin and attached by the stopper to the pinned specimen. Photomicrographs of imagoes were taken with an Olympus camera (PM2) loaded with an analog 35 mm Fujichrome Professional 64T film and attached to an Olympus stereomicroscope (SZ11) with a ring illuminator. Photomicrographs of the aedeagi+aedeagal apodeme, inner spermathecal capsules, and oviscapt valves were taken using a black & white APX25 Agfa Pan analog film in Zeiss photomicroscope. The originally analog films (slides and negatives) were later converted to a digital format using an Epson scanner (Perfection 4180 Photo). Line drawings of the terminalia were made using a Zeiss microscope, under an objective 40x, attached to a camera lucida (1.8x). Whenever in the same plate, all line drawings were drawn to the same scale and all photomicrographs were taken and enlarged to the same magnification.
Four Drosophila isofemale lines (coded Q23F2, Q37F53, Q37F56, and Q49F1, see material examined) were used as non-type material to determine the species karyotypes and analyze the male meiotic chromosomes. The latter strain was also used for the analyses of the general body and eye colors, eggs and puparia. Additionally, adult males and females from the line Q37F55 were double mounted and photomicrographed. For comparison purposes, male and female specimens of D. nappae sampled from two isofemale lines (I42F56 and I73F254), derived from wild females collected at the Forest Reserve of IB-USP (São Paulo city, state of São Paulo, Brazil), 26-28.III.1996 and 26-28.VIII.1997 respectively, V. Ratcov and C.R. Vilela leg. (MZSP), plus one male of the same species collected at Morro Santana, Porto Alegre (state of Rio Grande do Sul), III.1995, L. Basso da Silva leg. (MZSP) were dissected and their terminalia photomicrographed. The cited isofemale lines were cultured in a cheap, long-lasting, and suitably modified banana-agar culture medium (detailed below) at constant temperature (18 ± 1 °C) and photoperiod (١٣h light: ١٠h dark). Because this modified medium is also used to culture many other species of this genus, including Drosophila melanogaster Meigen, 1830, and its mutants, it is worthwhile mentioning here its recipe, as detailed below.
Ingredients. Tap water (1000 ml), agar (10 g), five medium-sized, peeled, and overripe bananas (preferentially Nanica cultivar) blended in advance with 125 ml of tap water (to get a banana purée, which may also be stored at -20 °C for up to one year), aqueous solution of brewer yeast (15 g of powder in 125 ml of tap water), 10% Nipagin(r) solution in ethanol (18 ml), both latter solutions prepared in advance.
Cooking instructions. Prepare a wet mixture of tap water and agar, bring it to a boil in a pot, stirring once in a while to prevent clumping, add banana purée and brewer yeast solution and boil it again, stir the mixture, turn off fire and let it cool to about 60 °C before adding Nipagin. Stir well, pour it into cylindrical glass vials (20 x 100 mm) and plug them. After cooling and drying at room temperature the vials medium may be preserved for up to three weeks in the refrigerator (ca. 8 °C). After transferring the imagines to a new vial, a tiny ball (about the size of a homeopathic pill) of live baker's yeast should be added.
The following tips may be useful to maintain the strains at a temperature of 18 °C, ideal for keeping specimens of most of the species of the D. tripunctata group in laboratory. The emerged adults are to be transferred to new vials once a week. Turning and keeping the vials upside down until the adult flies reach sexual maturity (ca. two weeks) and also during the subsequent week, reduces the adult mortality caused mainly by the cataleptic behaviour observed in many of these flies whenever they are disturbed (Frota-Pessoa 1954Frota-Pessoa O (1954) Revision of the tripunctata group of Drosophila with description of fifteen new species (Drosophilidae, Diptera). Arquivos do Museu Paranaense 10: 253-304.). The vials from the first two weeks are usually devoid of eggs and larvae and must be discarded. The sexually mature flies (ca. 14-day old) are transferred into the new vial kept upside down to oviposit for one week. Later on, flies can be either discarded or transferred into new vial, if needed. Alternatively, sexually mature flies could be transferred to vials with powdered milk-agar medium for egg laying and larval development (Bächli et al. 2000Bächli G, Vilela CR, Ratcov V (2000) Morphological differences among Drosophila paraguayensis Duda, 1927 and its close relatives (Diptera, Drosophilidae). Mitteilungen der schweizerischen Entomologischen Gesellschaft 73: 67-92.). No additional yeast needs to be added at this point. One week later, the medium becomes double-layered due to the presence of large amount of growing larvae. At this stage ca. four V-shaped filter paper strips (ca. 1,8 x 10 cm) are inserted into the medium to reduce the excess of humidity.
Mitotic and meiotic chromosomes of the new Drosophila species were obtained by applying the technique of Imai et al. (1977Imai HT, Crozier RH, Taylor RW (1977) Karyotype evolution in Australian ants. Chromosoma 59: 341-393. doi: 10.1007/BF00327974
https://doi.org/10.1007/BF00327974...
, 1988Imai HT, Taylor RW, Crossland MW, Crozier RH (1988) Modes of spontaneous chromosomal mutation and karyotype evolution in ants with reference to the minimum interaction hypothesis. Japanese Journal of Genetics 63: 159-185. doi: 10.1266/jjg.63.159
https://doi.org/10.1266/jjg.63.159...
) and Matsuda et al. (1983Matsuda M, Imai HT, Tobari YN (1983) Cytogenetic analysis of recombination in males of Drosophila ananassae . Chromosoma 88: 286-292. doi: 10.1007/BF00292905
https://doi.org/10.1007/BF00292905...
). Cytology procedure follows Vilela & Goñi (2015Vilela CR, Goñi B (2015) Is Drosophila nasuta Lamb, 1914 (Diptera, Drosophilidae) currently reaching the status of a cosmopolitan species? Revista Brasileira de Entomologia 59: 346-350. doi: 10.1016/j.rbe.2015.09.007
https://doi.org/10.1016/j.rbe.2015.09.00...
). Cytological preparations were made from single individuals by using the isofemale lines coded Q23F2 and Q37F53 (see material examined, and observed in an Olympus BX60(r) microscope equipped with an Olympus U-MAD-3(r) camera, under an objective 100x and 1.6x optovar magnification changer. Selected mitotic and meiotic cells were photomicrographed using the Image-Pro Plus(r) version 5.1 image analysis software and further edited in GIMP 2.8.14 (GNU Image Manipulation Program).
TAXONOMY
Drosophilidae Rondani, 1856
Drosophila (Drosophila )montevidensis sp. nov.
Figs. 1-8, 17-49, 54-55, 58-63
urn:lsid:zoobank.org:act:DDAFE6AD-5838-4141-84F9-2DB89A1DE856
D. gr. tripunctata (subgroup I) Goñi et al., 1997Goñi B, Martínez ME, Daguer P (1997) Studies of two Drosophila (Diptera, Drosophilidae) communities from urban Montevideo, Uruguay. Revista Brasileira de Entomologia 41: 89-93.: 90 (table 1, collected in banana-baited traps).
Unidentified species of the tripunctata group of DrosophilaGoñi et al., 1998Goñi B, Martinez ME, Valente VLS, Vilela CR (1998) Preliminary data on the Drosophila species (Diptera, Drosophilidae) from Uruguay. Revista Brasileira de Entomologia 42: 131-140.: 134 (table 2, geographic distribution), 137 (table 3, breeding site), 139 [affiliation = not D. angustibucca Duda sensu Frota-Pessoa (1954Frota-Pessoa O (1954) Revision of the tripunctata group of Drosophila with description of fifteen new species (Drosophilidae, Diptera). Arquivos do Museu Paranaense 10: 253-304.)].
Drosophila aff. nappaeGoñi et al., 2012Goñi B, Remedios M, González-Vainer P, Martínez ME, Vilela CR (2012). Species of Drosophila (Diptera: Drosophilidae) attracted to dung and carrion baited pitfall traps in the Uruguayan Eastern Serranías. Zoologia 29: 308-317. doi: 10.1590/S1984-46702012000400004
https://doi.org/10.1590/S1984-4670201200...
: 308 (abstract), 312 (table 2, feeding site), 313 (distribution, level of association), 314 (abundance), 316 (abundance and richness).
Types. Holotype male, labeled "Uruguay - Montevideo, Montevideo city, Facultad de Agronomía, (34°50'69"S, 56°13'44"W), Beatriz Goñi coll. /net swept over fallen fleshy seeds of Ginkgo biloba 17.IV.2005/from isofemale line F6/Drosophila montevidensis ♂ Goñi and Vilela/HOLOTIPO [red label]" (MZSP). Paratypes: five males and one female same data as holotype, except isofemale line label (F1-F5), plus two males collected on the same place (MZSP). The latter two specimens were collected as follows: one male net swept over fallen, decaying fruits of Syagrus romanzoffiana (Cham.) Glassman (Arecaceae), and the other male over fallen, decaying fruits of Psidium cattleianum Afzel. ex. Sabine (Myrtaceae) but on 01.IV.2005.
Diagnosis. Scutum subshining tan, darkening gradually from anterior to posterior region, anterior half with two narrow, diffuse and slightly darker stripes between and just adjacent to the dorsocentral rows; scutellum darker, dull; facial carina large and broad, not sulcate; wing brownish, crossveins strongly clouded, C index = 3.6-4.2; three strong black setae in a line at base of metatarsomere I; abdomen shining yellow, tergites 2-6 with posterior dark brown bands medially slightly interrupted, not reaching lateral margins, and a variable (diffuse to well delimited) median, light brownish to dark brown longitudinal stripe; epandrium devoid of upper and lower setae (3-6 upper setae present in Drosophila nappae ), gonopod subdistally microtrichose (without macrotrichia in D. nappae ), aedeagus anterodorsally bearing a conspicuous pair of slightly membranous, dorsally somewhat sclerotized, finger-shaped processes, 2/3 the length of aedeagus (1/3 the length of aedeagus in D. nappae ), backwards directed and covered with tiny spines, aedeagus distal end medially bearing a V-shaped (U-shaped in D. nappae ) membranous area in dorsal view, ventral rod as long as aedeagus (shorter than aedeagus in D. nappae ), ventral margin of aedeagus and posterior margin of ventral rod abruptly converging, as seen in lateral view (mildly converging in D. nappae ); spermathecal capsule spherical (larger and somewhat elliptical in D. nappae ), devoid of basal furrows (present in D. nappae ).
Description. Male (n = 8). Head. Frons mostly yellowish-brown, dull; frontal length 0.32 (0.29-0.37) mm, frontal index = 0.78 (0.71-0.86), top to bottom width ratio = 1.70 (1.56-1.93). Frontal triangle light brown, not well-defined, about 67-86% of frontal length; ocellar triangle dark brown, about 33-42% of frontal length, ocelli surrounded by conspicuous black crescents along inward-directed margins. Orbital plates light brown, subshining, about 80-108% of frontal length. Orbital setae black, or2 just outside of or1, shorter and about one-half diameter of larger setae of pedicel, distance of or3 to or1 = 50-80% of or3 to vtm, or1/or3 ratio = 0.73 (0.67-0.82), or 2/or 1 ratio = 0.40 (0.33-0.50), postocellar setae 64-83%, ocellar setae 86-108% of frontal length, vt index 1.17. Postocellar setae cruciate at tip. Face light brown, dull; facial carina slightly darker, broad, divergent downwards, not sulcate; vibrissal index = 0.45 (0.30-0.63). Cheek index = 8.10 (5.20-11.0). Eye red, dorsally remarkable darker. Eye index = 1.28 (1.19-1.39). Antenna brown to light brown; pedicel, dorsally darker, with two larger setae of about same size. First flagellomere short-haired; length to width ratio = 1.69 (1.50-2.00). Arista with 6 upper and 3-4 lower branches, plus terminal fork; 7-10 inner branches. Proboscis brown, palpus light brown with a row of about 5 long setae, decreasing in length from tip to middle area, plus several fine setulae.
Thorax. Length = 1.21 (1.15-1.32) mm. Scutum tan, posteriorly darker, subshining, 5-7 irregular rows of acrostichals. h index = 0.82 (0.67-0.91). Transverse distance of dorsocentral setae 180-243% of longitudinal distance; dc index = 0.82 (0.79-0.85). Prescutellar setae absent. Scutellum dark brown, dull, distance between apical scutellar setae about 73-90% of that between apical and basal one, basal setae divergent, apical setae cruciate at median region; scut index = 1.05 (1.00-1.10). Pleura light brown at anterior lower half, brownish dorsally and posteriorly, shining, sterno index = 0.52 (0.50-0.57); median katepisternal seta 67-100% of anterior one and noticeably thinner than other two, posterior one thicker. Proepisternal seta absent. Halter stalk pale yellow, halter knob proximally brown, yellowish at distal region. Legs uniformly light brown. Three strong black setae in line at base of inner surface of metatarsomere I, which is slightly wider than metatarsomere II, but not twice as wide, as it conspicuously occurs in Drosophila platitarsusFrota-Pessoa, 1954Frota-Pessoa O (1954) Revision of the tripunctata group of Drosophila with description of fifteen new species (Drosophilidae, Diptera). Arquivos do Museu Paranaense 10: 253-304.. One small thick black seta at base of inner surface of mesotarsomere I. Apical setae on protibia and mesotibia, the latter spur-shaped; preapicals on all three.
Wing. Brownish, slightly pointed at tip of R4+5, crossveins clouded, tips of longitudinal veins slightly darkened; length 2.74 (2.46-2.93) mm, length to width ratio = 2.27 (2.20-2.35). Indices: C = 3.86 (3.63-4.16), ac = 1.74 (1.54-2.11), hb = 0.47 (0.45-0.52), 4C = 0.72 (0.66-0.76), 4v = 1.59 (1.52-1.75), 5x = 0.88 (0.79-1.00), M = 0.40 (0.38-0.43), prox. x = 0.76 (0.69-0.83).
Abdomen. Shining brownish-yellow, tergites 2-6 with a posterior, medially slightly interrupted dark brown band, not reaching lateral margins, and a variable (diffuse to well delimited) median, light brownish to dark brown longitudinal stripe, which may be completely absent in some specimens.
Terminalia (Figs. 17-24, 26-49). Epandrium almost bare, slightly microtrichose on posterior dorsal area; upper and lower setae absent; ventral lobe roundish, slightly covering surstylus. Cercus slightly microtrichose on dorsal area, linked to epandrium by membranous tissue. Surstylus not microtrichose, with about 7 cone-shaped prensisetae, about 4 long, strong outer setae and about 11 long, thin, mostly inner setae. Decasternum as in Fig. 18. Hypandrium (Fig. 19) as long as epandrium, anterior margin convex; posterior hypandrial process absent; dorsal arch present, strongly sclerotized; gonopod subdistally microtrichose, fused to paraphysis, bearing one long seta on median inner margin. Aedeagus (Figs. 20-24, 26-49) lateroventrally strongly sclerotized, anterodorsally bearing a distinctive pair of membranous, dorsally slightly sclerotized, finger-shaped, backwards directed processes, which are shorter than aedeagus (ca. 2/3 its length) and lateroventrally covered with tiny spines. Aedeagus in dorsal view (Fig. 20) bearing a small, slightly sclerotized, crescent-shaped plate at subdistal area which embraces the gonopore of endophallus; distal end medially bearing a V-shaped membranous area, distal margin rounded in dorsal as well as in ventral view (Figs. 31-35, 41-45, 48, 49) (remarkably angled in Drosophila nappae , Figs. 52-53). Aedeagal apodeme rod-shaped, laterally flattened, slightly shorter than aedeagus; anteriorly expanded dorsoventrally in aged males (Figs. 28, 38). Ventral rod completely fused to aedeagal apodeme, relatively long, remarkably right-angled in relation to ventral margin of the aedeagus as seen in lateral view (in D. nappae it is much shorter than aedeagus and obtuse-angled in relation to ventral margin of the aedeagus).
Male and female adults of two sibling species of the Drosophila tripunctata group: (1, 5, 9, 13) left lateral view; (2, 6, 10, 14) laterodorsal view; (3-4, 7-8, 11-12, 15-16) dorsal (thorax and abdomen) views. (1-8) Drosophila montevidensis sp. nov.: (1-4) male (isofemale line Q37F55, Montevideo, Uruguay); (5-8) female (isofemale line Q37F51, idem). (9-16) Drosophila nappae (isofemale line I73F254, São Paulo, Brazil): (9-12) male; (13-16) female. Scale bar: 1 mm.
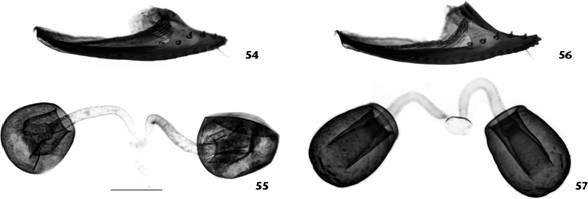
Drosophila montevidensis sp. nov. male holotype, terminalia: (17) epandrium, cerci, surstyli and decasternum, oblique posterior view; (18) surstyli and decasternum, posterior view; (19) hypandrium and gonopods+paraphyses [fused], posterior view; (20-24) aedeagus+aedeagal apodeme in several views from dorsal through ventral. Scale bar: 0.1 mm.
Left oviscapt valve of the female paratype of Drosophila montevidensis sp. nov.: outer lateral view. Scale bar: 0.1 mm.
Aedeagi + aedeagal apodeme of two sibling species of the Drosophila tripunctata group: (26-30, 36-40, 46-47, 50-51), left lateral view; (31-35, 41-45, 48, 49, 52, 53), dorsal view. (26-49) Drosophila montevidensis sp. nov.: (36, 41) holotype; (26-35, 37, 38, 42, 43) paratypes; (39, 44) Montevideo (Cerro Montevideo, Parque Vaz-Ferreira); (40, 45, 46-49) Rocha (Laguna Negra, Don Bosco camping), specimen E; (46, 48) idem, specimen U; (47, 49) idem, specimen W; (50-53) Drosophila nappae: (50, 52) isofemale line I42F56, São Paulo (Forest Reserve of IB-USP); (51, 53) wild-caught, Porto Alegre (RS). Scale bar: 0.1 mm.
Female (n = 1). Main differences from male: usually larger; median anteroposterior stripe on tergite 6 seems to be thinner and paler than that of male.
Measurements. Frontal length 0.37 mm; frontal index = 0.79, top to bottom width ratio 1.58. Ocellar triangle 33% of frontal length. Orbital plates 93% of frontal length. Distance of or3 to orb1 = 67% of or3 to vtm, or1/or3 ratio not determined (or3 missing), or2/orb1 ratio = 0.33, postocellar setae = 60%, ocellar setae = 100% of frontal length, vt index = 1.25, vibrissal index = 0.36. Cheek index 7. Eye index 1.4. First flagellomere short-haired; length to width ratio 2.00. Arista with 7 upper and 3 lower branches, plus terminal fork; 8 inner branches. Thorax length 1.34 mm. h index = 0.75. Transverse distance of dorsocentral setae 250% of longitudinal distance; dc index not determined (bristles missing). Distance between apical scutellar setae about 82% of that between apical and basal one; scut index not determined (bristles missing), sterno index = 0.50, median katepisternal seta about 82% of anterior one. Wing length 2.98 mm, length to width ratio not determined (wings posteriorly broken). Indices: C = 3.96, ac = 1.69, hb = 0.41, 4C = 0.67, 4v = 1.52, 5x and M not determined (wings posteriorly broken), prox. x = 0.64.
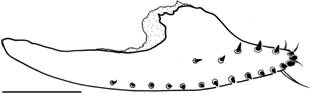
Female terminalia (Figs. 25, 54, 55). Valves of oviscapt (Figs. 25, 54) pointed at tip, ventrally convex, dorsally rounded subdistally (angled in D. nappae , Fig. 56), with ca. 15 marginal and 5 discal peg-like ovisensilla; inner trichoid-like ovisensilla: 3 thin, distally positioned and 1 long, curved, subterminal. Spermathecal capsule (Fig. 55) spherical (larger and somewhat elliptical in D. nappae , Fig. 57), devoid of basal furrows (present in D. nappae ); spermathecal duct distally dilated, becoming gradually wider apically, sclerotized.
Egg (n = 6). Length ca. 0.50 mm; four slender, divergent filaments; anterior pair slightly shorter than posterior pair.
Puparium (n = 3). Reddish-brown; horn index ca. 3.9; each anterior spiracle with about 14 branches; anterior stalk as long as branches length; tip of stalk of anterior spiracle blackish brown; posterior spiracles relatively long, only slightly shorter than anterior spiracles stalks.
Chromosomes (Figs. 58-63). Basic diploid chromosome number of 2n = 12, XX in females and XY in males (Figs. 58-61), with the haploid karyotype formula: 5R, 1D. The 4 pairs of autosomes range from medium to small-sized acrocentrics but smaller than any of the sex chromosomes. In early C-banded prometaphase cells, the autosomes show a short heterochromatic arm (Fig. 59). The X chromosome, the biggest of the complement, has a large block of pericentromeric heterochromatin that covers almost half of its length and a tiny heterochromatic short arm, while the Y is heterochromatic and shorter than the X (Fig. 60, 61). Dot chromosomes are quite small. Prophase I cells of male meiosis show four bivalents representing the pairs 2, 3, 4, 5, the small dot chromosomes, and the heterologous XY association (Figs. 62, 63). The autosome bivalents pair along their length excepting the centric and paracentromeric regions. The sex chromosomes pair at a specific site, located at the distal heterochromatic region in the X and the proximal region in the Y chromosome (Figs. 62, 63).
Left oviscapt valve and pair of inner spermathecal capsules of two sibling species of the Drosophila tripunctata group, outer lateral and lateral views respectively: (54, 55) Drosophila montevidensis sp. nov., female paratype; (56, 57) Drosophila nappae , isofemale line I42F56, São Paulo (Forest Reserve of IB-USP). Scale bar: 0.1 mm.
Mitotic and meiotic chromosomes of Drosophila montevidensis sp. nov. (58-59) female and (60-61) male metaphase plates from larval neuroblasts; (59) female prometaphase cell showing C-banded proximal heterochromatin in all chromosomes; (62-63) male meiotic prometaphase I chromosomes showing four bivalents representing the autosomes 2, 3, 4, 5 and the small dot chromosome, and the heterologous XY association. Sex chromosomes are indicated in all cells, and arrows indicate the dot chromosomes. Scale bar: 10 μm.
Distribution. Neotropical: Argentina (Buenos Aires) and Uruguay (Lavalleja, Montevideo, Rocha).
Remarks. This species belongs to the subgroup I (cf. Vilela 1992Vilela CR (1992) On the Drosophila tripunctata species group (Diptera, Drosophilidae). Revista Brasileira de Entomologia 36: 197-221.: 198) of the Drosophila tripunctata species group of the subgenus Drosophila . It shares with D. angustibucca , D. mediocris , D. medioobscurata , D. nappae , D. neoguaramunu , D. platitarsus, D. rostrata , and D. setula , the following remarkable features: aedeagus dorsally with a small, sclerotized, crescent-shaped plate at subdistal area, and anterodorsally bearing a pair of finger-shaped (sometimes diffuse) and backwards directed processes. The processes are small, diffuse and mostly membranous in all species except in D. nappae , where they are only slightly membranous and proportionally longer; and they are even longer in D. montevidensis sp. nov. Additionally, all of them but the latter two, have in common a sclerotized, inverted T-shaped area partially surrounded by the pair of processes in the anterodorsal, mostly membranous surface of aedeagus.
The karyotype of four out of the eight species included in the subgroup I of the Drosophila tripunctata group, namely D. nappae (originally referred to as D. angustibucca ) (see Franck et al. 1984Franck G, Valente VLS, Morales NB (1984) Chromosome characteristics of Drosophila angustibucca Duda. Revista Brasileira de Genética 7: 241-253. and Pires 2000Pires AO (2000). Karyotypes of species of the Drosophila tripunctata species group in a secondary semideciduous forest (Diptera, Drosophilidae). Thesis Abstracts. Genetics and Molecular Biology 23: 249. [as Drosophila sp. U3]), D. neoguaramunu (see Frydenberg 1956Frydenberg O (1956) Two new species of Drosophila from Peru (Drosophilidae, Diptera). Revista Brasileira de Entomologia 6: 57-64.), D. platitarsus (see Pires 2000Pires AO (2000). Karyotypes of species of the Drosophila tripunctata species group in a secondary semideciduous forest (Diptera, Drosophilidae). Thesis Abstracts. Genetics and Molecular Biology 23: 249.) and D. setula (see Clayton & Wasserman 1957Clayton FE, Wasserman M (1957) Chromosomal Studies of Several Species of Drosophila . University of Texas Publications 5721: 125-131.), were reported. Apparently, the observed haploid karyotype of D. montevidensis sp. nov. (n = 6) is indistinguishable from D. nappae , with 5R, 1D (Y chromosome is a rod shorter than X); however, it differs remarkably from the haploid karyotype formula of D. neoguaramunu (n = 3), with 3V (X chromosome considerably shorter than rod-shaped Y chromosome), in samples collected from Peru (Frydenberg 1956Frydenberg O (1956) Two new species of Drosophila from Peru (Drosophilidae, Diptera). Revista Brasileira de Entomologia 6: 57-64.). The karyotype of these species differ from that of D. platitarsus (n = 6), being 4R, 1V, 1D (V-shaped X and J-shaped Y), with heterochromatic pericentromeric regions in the rod-shaped pairs, the dots, but differ in the shape of the Y, and the location of the heterochromatin in the X chromosome (interstitially located in one arm, and proximally, at the pericentromeric region, in the other arm), in samples of an isofemale line derived from an individual collected at the Forest Reserve of the IB-USP, located in the Cidade Universitária "Armando de Salles Oliveira", São Paulo city, state of São Paulo, Brazil (Pires 2000Pires AO (2000). Karyotypes of species of the Drosophila tripunctata species group in a secondary semideciduous forest (Diptera, Drosophilidae). Thesis Abstracts. Genetics and Molecular Biology 23: 249.). They also differ from that of D. setula (n = 6) with 4R, 1V, 1D in a strain from Santa Martha, Colombia, and (n = 5[?]) with 3R, 1V, 1D (?) in a strain from Barro Colorado Is., Canal Zone, Panama (Clayton & Wasserman 1957Clayton FE, Wasserman M (1957) Chromosomal Studies of Several Species of Drosophila . University of Texas Publications 5721: 125-131.). There is no description of the sex chromosomes for D. setula in the latter reference. These data reveal that, excepting for D. montevidensis sp. nov. and D. nappae, a high interspecific karyotype variation is found among three species of the subgroup I of D. tripunctata group. The polytene chromosomes map of D. nappae (misidentified as D. angustibucca ) described by Franck et al. (1984Franck G, Valente VLS, Morales NB (1984) Chromosome characteristics of Drosophila angustibucca Duda. Revista Brasileira de Genética 7: 241-253.) allows the opportunity to investigate the genetic divergence between this pair of sibling species by using polytene banding pattern as primary chromosomes markers. The feasibility to maintain laboratory strains of both sibling species let to investigate other biological aspects, such as life cycle, the existence or not of reproductive isolation mechanisms. So far, D. montevidensis sp. nov. has been collected in natural areas of southern Uruguay as well as in urban areas along both margins of Rio de la Plata (Buenos Aires and Montevideo cities), as well as other localities of the province of Buenos Aires and at southern and southeastern Departments of Uruguay (as detailed in material examined). Up to date, it has been absent in drosophilid samples emerged from decaying fruits collected in northern Uruguay, Department of Salto (B. Goñi, unpublished data). However, the apparently allopatric distribution of D. montevidensis sp. nov. and its sibling species, D. nappae , remains to be confirmed.
Laboratory Cultures. It is cultivated at 18 °C with a modified banana-agar culture medium (recipe above).
Etymology. The specific name is an adjective in allusion to the type locality (Montevideo city).
Material examined. Type series (8 males, 1 female, as detailed above) plus 318 non-type specimens (142 males, 103 females, and 73 adults not sexed) which were analyzed for distributional and ecological purposes only, as detailed below. Argentina. Buenos Aires: Azul city (36°46'39"S, 59°51'48"W), 1 female, net swept over fallen, decaying infructescence of Ficus cairica L. (Moraceae), 06.IV.2006, B. Goñi leg. (MZSP); Buenos Aires city, Universidad de Buenos Aires, Facultad de Agronomía, Jardín Botánico "Lucien Hauman (34°35'36"S, 58°29'03"W), 2 males (MZSP) and 5 females (FCE-D), net swept over fallen, decaying fruits of Butia capitata (Mart.) Becc. (Arecaceae), 2 males, 3 females (MZSP), plus 10 males, 2 females (offspring of isofemale line Q23F2, MZSP), net swept over fallen, decaying fruits of Ocotea acutifolia , Crataegus sp. and Butia yatay (Mart.) Becc. 1916 (Arecaceae) (locally known as butiá), 11, 18, 27.IV.2006, B. Goñi leg. Uruguay. Lavalleja: Sierra de Minas (woodland sierra, riparian forest and pine plantation habitats, between 34°30'59"S, 55°20'07"W and 34°30'54"S, 55°19'53"W), 10 males, 4 females (dung traps), 4 males, 3 females (carrion traps), V.2002-IV.2003, P. González-Vainer leg. (FCE-D) (Goñi et al. 2012Goñi B, Remedios M, González-Vainer P, Martínez ME, Vilela CR (2012). Species of Drosophila (Diptera: Drosophilidae) attracted to dung and carrion baited pitfall traps in the Uruguayan Eastern Serranías. Zoologia 29: 308-317. doi: 10.1590/S1984-46702012000400004
https://doi.org/10.1590/S1984-4670201200...
). Montevideo: Montevideo city, Cerro Montevideo, Parque Vaz Ferreira (near waterpond, 34°53'49"S, 56°15'41"W), 3 males, 1 female in banana-baited traps, I, VI.1994, B. Goñi & M.E. Martinez leg. (FCE-D) (Goñi et al. 1997); Universidad de la República, Facultad de Agronomía (backyard garden, 34°50'69"S, 56°13'44"W) as follows: 1 female, emerged from fallen, decaying fruit of Butia yatay (Mart.) Becc. 1916 (Arecaceae) (locally known as yatay), 1 female in banana-baited trap, IV, V, XII.2000, B. Goñi, P. Fresia, M. Calviño & M.J. Ferreiro leg. (FCE-D); 5 males, 2 females, emerged from fallen, decaying fleshy seeds of Ginkgo biloba (locally known as ginko), V.2005, B. Goñi & M.E. Martinez leg. (FCE-D); 10 males (MZSP), plus 10 males, 3 females (isofemale line Q37F51), 10 males, 5 females (Q37F53), 11 males, 3 females (Q37F54), 9 males, 5 females (Q37F55), 7 males, 2 females (Q37F56), 5 males, 2 females (Q37F57) and 4 males (Q37F58), net swept over fallen, decaying fleshy seeds of Ginkgo biloba , 6, 10, 16.V.2006, Goñi & M.E. Martinez leg. (MZSP); the following 127 specimens were net swept over fallen, decaying fleshy seeds of Ginkgo biloba , detailed as follows: 6 males, 5 females, III-V.2005, B. Goñi & M.E. Martinez leg. (FCE-D); 73 adults not sexed, 6, 10, 16.V.2006, idem leg. (FCE-D), and 14 males and 29 females offspring from eight isofemales (Q49F1 to F3, and Q49F5 to F9), 19.IV-15.V.2007, B. Goñi leg. (FCE-D). Rocha: Boca del Sarandí, on the western shore of the Laguna Negra (34°00'36"S, 53°45'26"W), 1 female, emerged from fallen, decaying cladode of Opuntia arechavaletai Speg. 1905 (Cactaceae) (locally known as opuntia), 4.VII.1995, M.E. Martinez leg. (FCE-D) (Goñi et al. 1998Goñi B, Martinez ME, Valente VLS, Vilela CR (1998) Preliminary data on the Drosophila species (Diptera, Drosophilidae) from Uruguay. Revista Brasileira de Entomologia 42: 131-140.); Laguna Negra, Don Bosco camp gallery forest, (53°45'18"W; 34°05'24"S), 20 males, 25 females, net swept over fallen, decaying fruits of Schinus longifolius (Lindl.) Speg. (Anacardiaceae) (locally known as molle), 17-25.V.2003, B. Goñi, M.E. Martinez, I. Machado, M. Gandelman, I. Corvo & M.J. Cabrera leg. (FCE-D).
Scaptomyza (Mesoscaptomyza )pipinna sp. nov.
Figs. 64-78
urn:lsid:zoobank.org:act:0BCA88CF-93ED-4A78-85A6-89D36ED04183
Types. Holotype male (dissected), labelled: Uruguay - Departament of Rocha, Sarandí del Consejo [creek, near north shore of Laguna (lagoon) de Castillos], 34°16'86"S, 53°59'08"W, M.E. Martinez coll. /segada [net swept over grass], 15.X.1994/pradera [prairie]/Scaptomyza pipinna ♂ Goñi & Vilela/HOLOTIPO [red label]" (MZSP).
Diagnosis. Frons yellowish-brown, dull, lacking defined stripe (present in Scaptomyza striaticeps ), orbits and anterior region lighter, ocellar triangle dark brown, antennae brown, medial vertical convergent and conspicuously long (longer than frontal length), vt index 1.63; scutum light brown, with three prominent brown longitudinal stripes, the central one extending to the end of scutellum; one pair of small presutural dorsocentrals; a single dorsal pleural stripe extending to postscutellum; wing clear; epandrium devoid of upper and lower setae (lower setae present in S. striaticeps ), cercus slightly fused to epandrium on anteroventral corner (not fused in S. striaticeps ), aedeagus straight (bent upwards in S. striaticeps ) in lateral view.
Description. Male (n = 1). Head. Frons mostly yellowish brown, dull, anterior 1/3 light yellow and medially brownish, devoid of a broad blackish stripe from ocelli to anterior margin; frontal length 0.24 mm, frontal index = 0.83, top to bottom width ratio = 1.42. Frontal triangle indistinct; ocellar triangle prominent dark brown, about 40% of frontal length. Orbital plates light brown, subshining, apically divergent from eye margin, about 80% of frontal length. Orbital setae black, or2 just outside of or1, shorter and about one-half diameter of proclinate setae of pedicel, distance of or3 to or1 = 40% of or3 to vtm, or1/or3 ratio = 0.86, or2/or1 ratio = 0.50, postocellar setae 80%, ocellar setae 80% of frontal length. Medial vertical convergent and remarkably long (longer than frontal length) (Fig. 64), vt index 1.62. Postocellar setae cruciate at tip. Face yellowish, dull; facial carina rudimentary, not sulcate; vibrissal index = 0.57. Cheek index = 7.50. Eye index = 1.25. Antenna brown, globose, pedicel with two larger setae, anterior ones proclinate and larger, reaching tip of flagellomere I, posterior ones directed outwards. First flagellomere with median-sized hairs; length to width ratio = 1.00. Arista with 3 upper and 2 lower branches, plus one long terminal fork; 6 inner branches. Proboscis light brown, elongated, palpus dark brown with 1 terminal seta as long as vibrissa, but thinner, 1 smaller subterminal seta half the length of terminal, plus several fine setulae.
Thorax. Length = 0.78 mm. Scutum light brown, subshining, bearing three conspicuous, brown, longitudinal stripes, the central one extending to the tip of scutellum, the two lateral ones lying outside the dorsocentrals, extending onto the sides of scutellum (Fig. 66); one dorsal, brown, conspicuous, pleural stripe, narrow at proepisternum, widening at katepisternum, extending to postscutellum and including meso and metathoracic spiracles. Apparently 2 irregular rows of acrostichals (most of them are broken off) at presutural area. Apparently only one postpronotal. Transverse distance of dorsocentral setae 143% of longitudinal distance; dc index undetermined. A pair of additional small dorsocentrals, twice as long as adjacent acrostichal setulae, just anterior to transverse suture. Prescutellar setae absent. Scutellum with a large, diffuse, brown stripe at center, light brown laterally, subshining; distance between apical scutellar setae about 67% of that between apical and basal one, basal setae parallel, apical setae cruciate at median region; scut index undetermined. Pleura yellow at lower half, with a brownish stripe at upper half, subshining, sterno index undetermined; median katepisternal seta 50% of anterior one and noticeably thinner. Proepisternal seta absent. Halter long, pale yellow, contrasting with brownish background of pleural longitudinal stripe. Legs uniformly yellow, except metatarsomere V, brownish. One black seta at base of inner surface of mesotarsomere I, apical setae on protibia and mesotibia, the latter spur-shaped; preapicals on all three.
Wing. Hyaline, length 2.00 mm, length to width ratio = 2.42. Indices: C = 3.31, ac = 2.00, hb = 0.35, 4C = 0.67, 4v = 1.46, 5x = 1.57, M = 0.46, prox. x = 0.42.
Abdomen shining dark brown with a dorsal pattern of pale yellow areas except on the last tergite.
Terminalia (Figs. 67-78). Epandrium microtrichose on posterior area; upper and lower setae absent; ventral lobe pointed at tip, bearing 1 robust terminal seta and 2 thin, subterminal setae (Fig. 67) arranged in tandem, not covering surstylus. Cercus mostly microtrichose on anterior area, slightly fused to epandrium on anteroventral corner; ventral surface without any sclerotized spine, but medially bearing four setae emerging from an irregular, sclerotized spot, preceded by a membranous, turned frontwards, rectangular area; ventral cercal lobe absent. Surstylus not microtrichose extensively fused to epandrium, with about 4 long, marginal setae, and three smaller, thinner setae on outer surface (Fig. 68). Decasternum with straight anterior and posterior margins, the former slightly incised at middle (not shown in Fig. 67 because anterior margin is curved frontwards), the latter medially protruding sharply backwards, as in Fig. 67. Hypandrium short, about half the length of epandrium, anteriorly narrow, anterior margin convex; posterior hypandrial process and dorsal arch absent (Fig. 69); gonopod posteriorly more sclerotized, laterally double-walled, linked to paraphysis by membranous tissue, bearing one tiny setula on middle inner margin (Figs. 70, 71). Aedeagus tiny, straight in lateral view, tube-shaped; distal tip membranous (Figs. 70-76). Paraphysis triangle-shaped (Fig. 74), ca. 2/3 aedeagus length, double-walled (Fig. 76), blunt at tip, bearing two tiny terminal setulae (Figs. 73, 74). Aedeagal apodeme rod-shaped, longer than aedeagus, posteriorly fused to it (Figs. 75, 76). Ventral rod absent.
Scaptomyza pipinna sp. nov. male holotype, adult: (64) left lateral view; (65) laterodorsal view, (66) dorsal view. Scale bar: 1 mm.
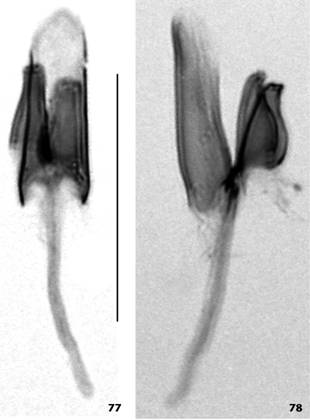
Scaptomyza pipinna sp. nov. male holotype, terminalia: (67) epandrium, cerci, surstyli and decasternum, posterior view; (68) idem, oblique posterior view; (69) left side of hypandrium and left gonopod (right side of hypandrium accidentally broken and right gonopod accidentally detached), posterior view; (70) left gonopod, posterior view; (71) right gonopod, posterior view; (72-76) aedeagus+aedeagal apodeme, and paraphyses, several views from dorsal through ventral. Scale bar: 0.1 mm.
Scaptomyza pipinna sp. nov. male holotype, aedeagi+aedeagal apodeme and paraphyses: (77) dorsal view; (78) left lateral view. Scale bar: 0.1 mm.
Female. Unknown.
Distribution. So far only known from its type locality.
Remarks. Scaptomyza pipinna sp. nov. belongs to the subgenus Mesoscaptomyza . The male terminalia shows similarities with those of two species depicted by Hackman (1959Hackman W (1959) On the genus Scaptomyza Hardy (Dipt., Drosophilidae) with descriptions of new species from various parts of the world. Acta Zoologica Fennica 97: 1-73.) and Wheeler & Takada (1966Wheeler MR, Takada H (1966) The Nearctic and Neotropical Species of Scaptomyza Hardy (Diptera: Drosophilidae). University of Texas Publications 6615: 37-78.: figs. 15.10 and 18.10).The straight aedeagus, although cylinder-shaped, reminds to those of S. paravittata Wheeler, 1952, from California, and S. setosa Wheeler & Takada, 1966, from Ecuador, which are somewhat cone-shaped; its decasternum and epandrium are similar to those of the Colombian S. striaticeps , from which it differs mainly by the absence of the paralobe sensu Hackman (1959Hackman W (1959) On the genus Scaptomyza Hardy (Dipt., Drosophilidae) with descriptions of new species from various parts of the world. Acta Zoologica Fennica 97: 1-73.).
Etymology. The specific name pipinna is a noun in apposition, in allusion to the tiny size (~ 81 µm long) of the aedeagus.
Note. While transferring the terminalia sclerites from the microscope slides to the glass microvial, the epandrium and hypandrium have been accidentally lost and only the aedeagus+aedeagal apodeme and paraphyses, together with the remains of abdominal tergites and sternites, are preserved in the microvial attached to the double-mounted holotype.
ACKNOWLEDGMENTS
The authors are grateful to Francisco Flauzino and Lyria Mori, for technical assistance during fly culture and chromosome preparation, respectively, to Denise S. Sheepmaker for providing the photo stereomicroscope system, to Luciano Basso da Silva and Maria E. Martinez for collecting and donating the male specimen of D. nappae from Porto Alegre, and the male specimen of S. pipinna sp. nov., respectively. We thank the following persons who participated in the collections of adult flies, fruits and fleshy seeds: María Jimena Cabrera, Martin Calviño, Ileana Corvo, Pablo Fresia, Mandi Gandelman, Maria José Ferreiro, Irene Machado, Maria E. Martinez, Clemente Olivera and Vilma Ratcov. We also thank Fernando Luces for English revision. This study was partly financed by the PDT-DINACYT Bilateral Joint Project Uruguay-Argentina 2005, FPR/F/BI/42/10 in collaboration with Alicia Basso (Universidad de Buenos Aires), to whom we thank specially. Beatriz Goñi was supported by the Comisión Sectorial de Investigación (CSIC), Universidad de la República, and Programa de Desarrollo de las Ciencias Básicas (PEDECIBA), Universidad de la República - Ministerio de Educación y Cultura, Uruguay. Thanks are due to two reviewers and the associate editor for their valuable comments.
LITERATURE CITED
- Bächli G, Vilela CR, Ratcov V (2000) Morphological differences among Drosophila paraguayensis Duda, 1927 and its close relatives (Diptera, Drosophilidae). Mitteilungen der schweizerischen Entomologischen Gesellschaft 73: 67-92.
- Bächli G, Vilela CR, Escher SA, Saura A (2004) The Drosophilidae (Diptera) of Fennoscandia and Denmark. Leiden, Brill, Fauna Entomologica Scandinavica, vol. 39, 362p.
- Clayton FE, Wasserman M (1957) Chromosomal Studies of Several Species of Drosophila . University of Texas Publications 5721: 125-131.
- Franck G, Valente VLS, Morales NB (1984) Chromosome characteristics of Drosophila angustibucca Duda. Revista Brasileira de Genética 7: 241-253.
- Frota-Pessoa O (1954) Revision of the tripunctata group of Drosophila with description of fifteen new species (Drosophilidae, Diptera). Arquivos do Museu Paranaense 10: 253-304.
- Frydenberg O (1956) Two new species of Drosophila from Peru (Drosophilidae, Diptera). Revista Brasileira de Entomologia 6: 57-64.
- Goñi B, Martínez ME, Daguer P (1997) Studies of two Drosophila (Diptera, Drosophilidae) communities from urban Montevideo, Uruguay. Revista Brasileira de Entomologia 41: 89-93.
- Goñi B, Martinez ME, Valente VLS, Vilela CR (1998) Preliminary data on the Drosophila species (Diptera, Drosophilidae) from Uruguay. Revista Brasileira de Entomologia 42: 131-140.
- Goñi B, Remedios M, González-Vainer P, Martínez ME, Vilela CR (2012). Species of Drosophila (Diptera: Drosophilidae) attracted to dung and carrion baited pitfall traps in the Uruguayan Eastern Serranías. Zoologia 29: 308-317. doi: 10.1590/S1984-46702012000400004
» https://doi.org/10.1590/S1984-46702012000400004 - Hackman W (1959) On the genus Scaptomyza Hardy (Dipt., Drosophilidae) with descriptions of new species from various parts of the world. Acta Zoologica Fennica 97: 1-73.
- Imai HT, Crozier RH, Taylor RW (1977) Karyotype evolution in Australian ants. Chromosoma 59: 341-393. doi: 10.1007/BF00327974
» https://doi.org/10.1007/BF00327974 - Imai HT, Taylor RW, Crossland MW, Crozier RH (1988) Modes of spontaneous chromosomal mutation and karyotype evolution in ants with reference to the minimum interaction hypothesis. Japanese Journal of Genetics 63: 159-185. doi: 10.1266/jjg.63.159
» https://doi.org/10.1266/jjg.63.159 - Kaneshiro KY (1969) A study of the relationships of Hawaiian Drosophila species based on external male genitalia. University of Texas Publications 6918: 55-70.
- Matsuda M, Imai HT, Tobari YN (1983) Cytogenetic analysis of recombination in males of Drosophila ananassae . Chromosoma 88: 286-292. doi: 10.1007/BF00292905
» https://doi.org/10.1007/BF00292905 - Pires AO (2000). Karyotypes of species of the Drosophila tripunctata species group in a secondary semideciduous forest (Diptera, Drosophilidae). Thesis Abstracts. Genetics and Molecular Biology 23: 249.
- Vilela CR (1983) A revision of the Drosophila repleta species group (Diptera, Drosophilidae). Revista Brasileira de Entomologia 27: 1-114.
- Vilela CR (1992) On the Drosophila tripunctata species group (Diptera, Drosophilidae). Revista Brasileira de Entomologia 36: 197-221.
- Vilela CR, Bächli G (1990) Taxonomic studies on Neotropical species of seven genera of Drosophilidae (Diptera). Mitteilungen der schweizerischen Entomologischen Gesellschaft 63 (Suppl.): 1-332.
- Vilela CR, Goñi B (2015) Is Drosophila nasuta Lamb, 1914 (Diptera, Drosophilidae) currently reaching the status of a cosmopolitan species? Revista Brasileira de Entomologia 59: 346-350. doi: 10.1016/j.rbe.2015.09.007
» https://doi.org/10.1016/j.rbe.2015.09.007 - Vilela CR, Valente VLS, Basso-da-Silva L (2004) Drosophila angustibucca Duda sensu Frota-Pessoa is an undescribed species (Diptera, Drosophilidae). Revista Brasileira de Entomologia 48: 233-238. doi: 10.1590/S0085-56262004000200012
» https://doi.org/10.1590/S0085-56262004000200012 - Wheeler MR, Kambysellis MP (1966) Notes on the Drosophilidae (Diptera) of Samoa. University of Texas Publications 6615: 533-565.
- Wheeler MR, Takada H (1966) The Nearctic and Neotropical Species of Scaptomyza Hardy (Diptera: Drosophilidae). University of Texas Publications 6615: 37-78.
-
Editorial responsibility:
Ângelo Parise Pinto -
ZooBank:
urn:lsid:zoobank.org:pub:52E45989-B00A-403F-9DA9-6D9553C313BB
Publication Dates
-
Publication in this collection
2016
History
-
Received
06 Aug 2016 -
Reviewed
13 Nov 2016 -
Accepted
26 Nov 2016