ABSTRACT
We describe a new genus and a new species in the family Epiphragmophoridae, Minaselates paradoxa sp. n. The new species was found at the National Park Cavernas do Peruaçu, in northern portion of the state of Minas Gerais, Brazil. Minaselates paradoxa sp. n. is classified in Epiphragmophoridae based on the fact that it shares the following diagnostic features of the family: a dart apparatus with a single dart sac, and two unequal mucous glands at the terminal genitalia. Minaselates gen. n. differs from Epiphragmophora Doering, 1874 by having a granulose protoconch, shell spire with blunt apex, complex microsculpture on the teleoconch and closed umbilicus fused with the shell wall. Also, significant differences between the two genera are the presence of a long and thin kidney that extends more than half the length of the pulmonary cavity, the presence of a flagellar caecum, and a smooth jaw in Minaselates gen. n. The finding of this new species and genus is particularly significant to refine the definition of the family, since Epiphragmophoridae has been traditionally diagnosed using the same characters of Epiphragmophora. Dinotropis Pilsbry & Cockerell, 1937, the other valid genus in the family, is monospecific and is only known by the morphology of the shell. In many ways it is similar to Epiphragmophora. A cladistics analysis was made in the present study which supports Minaselates gen. n. as a different entity and as sister group of the Epiphragmophora within Epiphragmophoridae.
KEY WORDS:
Cerrado; Pleurodontidae; Pulmonata; South America; Taxonomy
INTRODUCTION
Epiphragmophoridae is a Pulmonate land snail family exclusively distributed in South America. It is composed of the genera Epiphragmophora Doering, 1874 and Dinotropis Pilsbry & Cockerell, 1937. Epiphragmophora is currently composed of 63 species distributed in Peru, Bolivia, Argentina, Paraguay and southern Brazil with a single extra occurrence in Colombia (Linares and Vega 2011Linares EL, Vega ML (2011) Catalogo preliminar de los Moluscos continentales de Colombia. Bogotá, Biblioteca José Gerónimo Triana 22, Instituto de Ciencias Naturales, Facultad de Ciencias, Universidad Nacional de Colombia.). The species are classified into five subgenera (Epiphragmophora s.s., Angrandiella Ancey, 1886, Doeringiana Ihering, 1929, Karlchmidtia Hass, 1955 and Pilsbrya Ancey, 1887) (Zilch 1959Zilch A (1959-1960) Gastropoda. Euthyneura. In: Handbuch der Paläozoologie 6. Berlin, Borntraeger, 1-825., Richardson 1982Richardson L (1982) Helminthoglyptidae: Catalog of species. Tryonia 6: 1-117., Cuezzo 2006Cuezzo MG (2006) Systematic revision and cladistic analysis of Epiphragmophora Doering from Argentina and Southern Bolivia (Gastropoda: Stylommatophora: Xanthonychidae). Malacologia 49: 121-188. https://doi.org/10.4002/1543-8120-49.1.121
https://doi.org/10.4002/1543-8120-49.1.1...
). Dinotropis is a monotypic genus, known only by D. harringtoni Pilsbry & Cockerel, 1937 from Bolivia.
Epiphragmophoridae is currently diagnosed by the same synapomorphies of Epiphragmophora because Dinotropis is only known by its original description, which is entirely based on shell characters. Based on a cladistic hypothesis (Cuezzo 2006Cuezzo MG (2006) Systematic revision and cladistic analysis of Epiphragmophora Doering from Argentina and Southern Bolivia (Gastropoda: Stylommatophora: Xanthonychidae). Malacologia 49: 121-188. https://doi.org/10.4002/1543-8120-49.1.121
https://doi.org/10.4002/1543-8120-49.1.1...
), Epiphragmophora is characterized by the following synapomorphies i) malleated shell body whorl surface with diagonal ribs, ii) umbilicus overlapping, but not fused to the body whorl, iii) thick, widely reflexed peristome, iv) presence of a dart sac apparatus inserting in the vagina, or directly into the atrium, v) mucous glands unequal in size and shape, vi) insertion of mucous glands ducts in middle portion of dart sac, and vii) penial retractor muscle inserting in epiphallus medial portion. The short duct of the bursa copulatrix, a character that traditionally had been used to define the genus, is characteristic only of a small group of species.
Epiphragmophora was classified by Pilsbry (1894Pilsbry HA (1894) Guide to the study of Helices. In: Manual of Conchology. Philadelphia, Academy of Natural Sciences, vol. 7. 1-366) in the Helicidae, tribe Belogona Euadenia. The members of the latter are distinguished by having mucous glands of typically glandular structure, in contrast to the tube-like glands of the Belogona Siphonadenia. Thiele (1929Thiele J (1929-1935) Handbook of Systematic Malacology 1. Berlin, Jena.) later classified Epiphragmophora in Fruticicolidae: Epiphragmophorinae. Pilsbry (1939Pilsbry HA (1939) Land Mollusca of North America (north of Mexico). Academy of Natural Sciences Monographs 1: 216-227.) assembled all the American dart-bearing helices in Helminthoglyptidae and restricted Epiphragmophora to the South American Epiphragmophorinae Hoffman, 1928 from the same family. Nordsieck (1987Nordsieck H (1987) Revision des Systems der Helicoidea (Gastro poda: Stylommatophora). Archiv für Molluskenkunde 118: 9-50.) maintained Epiphragmophorinae Hoffman, 1928, but moving it into Xanthonychidae, while stating that its reproductive system resembles that of the Cepoliinae: diverticulum usually missing, one dart sac, dart glands unequal, one elongate and the other compact, inserting on the dart sac or on its base. Finally, Schileyko (1991Schileyko AA (1991) Taxonomic status phylogenetic relations and system of the Helicoidea sensu lato. Archiv fur Molluskenkunde 120: 187-236.) elevated Epiphragmophorinae to family within Helicoidea and this classification was followed by Bouchet and Rocroi (2005Bouchet P, Rocroi JP (2005) Classification and Nomenclator of Gastropod Families. Malacologia 47: 1-397.) in the last gastropod family nomenclator. Cuezzo (2006Cuezzo MG (2006) Systematic revision and cladistic analysis of Epiphragmophora Doering from Argentina and Southern Bolivia (Gastropoda: Stylommatophora: Xanthonychidae). Malacologia 49: 121-188. https://doi.org/10.4002/1543-8120-49.1.121
https://doi.org/10.4002/1543-8120-49.1.1...
) studied species of Epiphragmophora from Argentina and part of Bolivia, providing the first phylogenetic analysis of the genus. Species from Peru and Paraguay have been scarcely investigated and are mostly known by their original descriptions. Specimens with their entire body preserved are rare in malacological collections globally. Consequently, comprehensive anatomical or molecular studies are not feasible in most cases. In Brazil there is a single species, Epiphragmophora oresigena bernardius Ihering in Pilsbry, 1900, known from Serra da Bocaina and Campos do Jordão in São Paulo state and from state of Rio Grande do Sul.
During a field trip to the National Park Cavernas do Peruaçu in northern Minas Gerais, Brazil, to collect gastropods, a striking group of land snails was found. Analysis of the specimens collected revealed that they represent a new species of the family Epiphragmophoridae. The objective of the present work is to describe the new species in a new genus of Epiphragmophoridae and to discuss its position among the South American Helicoidea.
MATERIAL AND METHODS
Snails were collected at the National Park Cavernas do Peruaçu (14°56’S, 44°36’W) located in the state of Minas Gerais, Brazil. This conservation unit was created in 1999 with 143,353.84 ha (http://www.icmbio.gov.br), to protect limestone caverns. The calcareous massif is covered by rare and typical deciduous (Caatinga) or semi-deciduous forests called Seasonal Dry Tropical Forest (SDTF) (Pennington et al. 2000Pennington RT, Prado DE, Pendry CA (2000) Neotropical seasonally dry forests and Quaternary vegetation changes. Journal of Biogeography 27: 261-273. https://doi.org/10.1046/j.1365-2699.2000.00397.x
https://doi.org/10.1046/j.1365-2699.2000...
, Prado 2000Prado D (2000) Seasonally dry forests of tropical South Ame rica: from forgotten ecosystems to a new phytogeographic unit. Edinburgh Journal of Botany 57: 437-461. https://doi.org/10.1017/S096042860000041X
https://doi.org/10.1017/S096042860000041...
). Open shrub savannah (Cerrado) also occurs on the top of the mountains, and in poorly drained areas of the Park where the Peruaçu River originates. Shrub density is lower at the watersheds, and there the vegetation is totally herbaceous (Campo Limpo and Veredas), with localized Buriti palm-trees. The Caa tinga, the Cerrado and the Chaco dry areas are extensive, open biomes, and form the dry diagonal in South America, which is a natural phytogeographic unit (Pennington et al. 2000Pennington RT, Prado DE, Pendry CA (2000) Neotropical seasonally dry forests and Quaternary vegetation changes. Journal of Biogeography 27: 261-273. https://doi.org/10.1046/j.1365-2699.2000.00397.x
https://doi.org/10.1046/j.1365-2699.2000...
, Prado 2000Prado D (2000) Seasonally dry forests of tropical South Ame rica: from forgotten ecosystems to a new phytogeographic unit. Edinburgh Journal of Botany 57: 437-461. https://doi.org/10.1017/S096042860000041X
https://doi.org/10.1017/S096042860000041...
). The Cerrado spreads across 2,031,990 km2 of the central Brazilian Plateau and is the second largest of Brazil’s major biomes, after the Amazon. This biodiversity hotspot actually receives abundant rainfall (between 1,100 and 1,600 mm per year), although this rainfall is concentrated in a six to seven month period between October and April. The rest of the year is characterized by a pronounced dry season.
Live specimens and dry shells of Minaselates paradoxa
sp. n. were collected from rocky outcrops in dry deciduous forests of the National Park. Dry shells adhered to rocks or to leaves were abundant but live snails were scarce. The collected specimens were drowned in water for relaxation previous to fixation in ethanol 96%. Their shells were then photographed and measured using the software ImageJ 1.49 (Fig. 1). Anatomical information was obtained by dissecting specimens and studying them under a Leica MZ6 stereoscope, illustrations of the dissected parts where made with the aid of a camera lucida. Photographs of the different organ systems were taken using a Nikon 5000 camera attached to the stereoscope. They were enhanced and finalized using the software Corel Draw version X3. The terminology for the anatomical descriptions follows Tompa (1984Tompa AS (1984) Land Snails (Stylommatophora). In: Tompa AS, Verdonk H H, Van Den Biggelar JA (Eds) The Mollusca. New York, Academic Press, 47-140. https://doi.org/10.1016/B978-0-08-092659-9.50009-0
https://doi.org/10.1016/B978-0-08-092659...
). The terms proximal and distal refer to the position of an organ or part of an organ in relation to the gamete flow from the ovotestis (proximal) to the genital pore (distal), as in previous studies (Cuezzo 1997Cuezzo MG (1997) Comparative anatomy of three species of Epiphragmophora Doering, 1874 (Pulmonata: Xanthonychidae) from Argentina. The Veliger 40: 216-227., Cuezzo 2006Cuezzo MG (2006) Systematic revision and cladistic analysis of Epiphragmophora Doering from Argentina and Southern Bolivia (Gastropoda: Stylommatophora: Xanthonychidae). Malacologia 49: 121-188. https://doi.org/10.4002/1543-8120-49.1.121
https://doi.org/10.4002/1543-8120-49.1.1...
). The limit between the epiphallus and penis is based on the internal sculpture of their inner walls. The radula and the jaw of specimens were observed and photographed with a Jeol Scanning Electron Microscopy 35CF at the Integral Center of Electron Microscopy of the National University of Tucumán, Argentina. Shell microphotographs were obtained with a DSM 950ZEISS SEM at “Centro de Aquisição e Processamento de Imagens” of the Federal University of Minas Gerais, Brazil.
Shell measurements. Abbreviations (AD) dorsal view area, (Aap) apertural area, (Abw) body whorl area, (AL) lateral view area, (Dap) apertural diameter, (DM) major diameter, (Dm) minor diameter, (H) total shell height, (Hap) height of aperture, (Hbw) body whorl height, (Pbw) body whorl perimeter, (PD) dorsal view shell perimeter, (PL) lateral view shell perimeter, (PS) parietal space.
Intitutional abbreviations used in the text: IBN, Instituto de Biodiversidad Neotropical, Tucumán, Argentina; MLP-Ma, Museo de La Plata, Buenos Aires, Argentina; MNRJ, Museu Nacional Rio de Janeiro, Universidade Federal do Rio de Janeiro, Brazil; MCN, Museu Ciências Naturais, Pontifícia Universidade de Minas Gerais, Belo Horizonte, Brazil.
For the cladistic analysis, a matrix of 35 characters from the general anatomy plus shell morphology was generated for 24 terminal taxa (Appendix
1
APPENDIX 1
Appendix 1
Character matrix used in cladistic analysis. Only taxa with described anatomy were included in the matrix. Character codification according to Cuezzo (2003, 2006) with some modifications. “-”are non-applicable characters, “?” are missing characters.
Characters
1
2
3
4
5
6
7
8
9
10
1
2
3
4
5
6
7
8
9
20
1
2
3
4
5
6
7
8
9
30
1
2
3
4
Pleurodonte
4
0
0
1
0
0
0
1
[01]
1
0
0
-
-
-
-
-
-
-
0
1
2
2
1
0
2
0
0
[01]
-
0
0
0
0
0
E. argentina
2
[03]
0
1
0
1
0
1
0
0
1
1
1
0
1
0
1
0
0
1
0
1
2
1
1
0
1
0
1
1
0
0
0
0
0
E. cryptomphala
2
[01]
[12]
1
0
1
0
1
0
0
1
1
0
1
1
1
1
1
1
0
1
1
1
0
1
1
0
0
0
0
1
0
0
0
0
E. escoipensis
0
1
1
0
0
[01]
0
1
0
2
1
1
0
1
1
1
0
1
1
0
0
1
1
0
0
1
0
0
1
0
0
0
0
0
0
E. hemiclausa
2
1
0
1
0
1
0
1
0
2
1
1
1
0
1
0
0
0
0
0
1
1
2
1
0
1
1
0
1
1
0
0
0
0
0
E. guevarai
0
2
0
0
0
1
0
1
0
2
1
1
0
1
1
1
0
0
1
2
1
1
0
0
0
1
0
0
0
0
1
0
0
0
0
E. hieronymi
0
2
0
0
0
[01]
0
0
0
0
1
1
0
1
1
1
0
1
1
1
1
2
0
0
1
1
1
0
0
0
0
1
0
0
0
E. jujuyensis
2
1
1
1
1
1
0
1
0
0
1
1
0
1
1
0
1
0
0
2
1
1
0
0
1
1
0
0
1
1
1
1
0
0
0
E. parodizi
3
1
0
1
0
1
0
1
0
0
1
1
0
1
1
1
0
1
1
2
0
0
1
0
0
1
0
0
1
0
1
0
0
0
0
E. puella
4
2
0
2
0
0
1
0
0
0
1
1
0
?
?
?
0
1
?
?
?
?
0
?
?
1
?
?
0
0
1
?
0
0
0
E. puntana
3
1
1
0
0
1
0
1
0
0
1
1
0
1
1
1
1
1
1
2
1
1
1
0
0
1
0
0
0
0
0
0
0
0
0
E. quirogai
0
2
0
0
0
[01]
0
1
0
2
1
1
0
1
1
1
0
1
1
0
1
2
0
0
1
1
0
0
0
1
1
1
0
0
0
E. rhathymos
2
3
0
1
0
1
0
1
0
0
1
1
0
1
1
1
0
1
1
1
0
1
1
0
0
1
0
0
0
0
1
0
0
0
0
E. saltana
3
1
1
1
0
1
0
1
0
0
1
1
0
1
1
1
0
1
0
1
0
1
1
0
0
1
0
1
1
0
0
0
0
0
0
E. tomsici
2
1
1
1
0
0
0
1
0
0
1
1
0
1
1
1
0
1
0
1
0
0
1
0
0
0
0
1
0
0
1
0
0
0
0
E. trenquelleonis
0
1
0
0
0
1
0
1
0
0
1
1
0
1
1
1
0
1
1
2
1
1
0
2
1
1
0
0
[01]
0
1
0
0
0
0
E. trifasciata
1
2
0
0
0
1
0
1
0
0
1
1
0
1
1
1
0
0
1
2
1
1
0
0
1
1
0
0
0
0
1
0
0
0
0
), following characters and codifications of Cuezzo (2003Cuezzo MG (2003) Phylogenetic analysis of the Camaenidae with special emphasis on The American taxa. Zoological Journal of the Linnean Society 138: 449-476. https://doi.org/10.1046/j.1096-3642.2003.00061.x
https://doi.org/10.1046/j.1096-3642.2003...
) for Pleurodonte Fischer, 1807 and Labyrinthus Beck, 1837 and Cuezzo (2006Cuezzo MG (2006) Systematic revision and cladistic analysis of Epiphragmophora Doering from Argentina and Southern Bolivia (Gastropoda: Stylommatophora: Xanthonychidae). Malacologia 49: 121-188. https://doi.org/10.4002/1543-8120-49.1.121
https://doi.org/10.4002/1543-8120-49.1.1...
) for species of Epiphragmophora, with modifications. The characters used in the analysis are listed in Appendix 2
APPENDIX 2
Appendix 2. Character list used in the cladistics analysis of species of Epiphragmophora with known anatomy plus the new genus and species, Minaselates paradoxa sp. n.
0. Body whorl surface: with thin growth lines = 0; with thick growth ridges = 1; malleated with diagonal ribs = 2; with axial ribs regularly distributed = 3; pustules/granules to wrinkles = 4; triangular lamella in axial rows = 5. [additive].
1. Umbilicus: Fused with basal lip of peristome = 0; overlapped but not fused to body whorl = 1; perspective wide not overlapped by peristomal lip = 2; perspective narrow slightly overlapped = 3; wide partly overlapped = 4.
2. Shape of the aperture: sub circular = 0; oval horizontal = 1; sub quadrangular = 2.
3. Peristome: Thin expanded slightly reflexed = 0; thick wide reflexed = 1; thin highly expanded = 2.
4. Basal callus in peristome: absent = 0; present = 1.
5. Peripheral bands: absent = 0; present = 1.
6. Body whorl periphery: convex = 0; equatorially subcarinated = 1; supraequatorially subcarinated = 2; carinated = 3. [additive].
7. Aperture respect to body whorl: not descending = 0; descending = 1.
8. Protoconch sculpture: smooth = 0; granulose = 1.
9. Spire apex: pointed = 0; dull = 1; not evident = 2.
10. Mucous glands in terminal genitalia: absent = 0; present = 1.
11. Dart apparatus: absent = 0; present = 1.
12. Shape of dart sac: long finger-like usually with constriction = 0; short, cylindrical, no constriction = 1.
13. Dart sac insertion: in vagina = 0; in atrium = 1.
14. Dart sac papillae: absent = 0; present = 1.
15. Relation between ducts of both mucous glands: separated = 0; distally fused or contiguous = 1.
16. Position of left mucous gland duct: distal respect to the body of the gland = 0; equatorial respect to the body of the gland = 1.
17. Shape of right mucous gland: not sac-like = 0; sac-Like = 1.
18. Right mucous gland: not fused with atrium wall = 0; distally fused with atrium wall = 1.
19. Penis length respect to epiphallus: half epiphallus length = 0; as long as epiphallus = 1; longer than epiphallus length = 2.
20. Penial papillae (= verge): absent = 0; present = 1.
21. Penial retractor muscle: inserts in distal epiphallus = 0; inserts in medial zone of epiphallus = 1; inserts in proximal epiphallus = 2; inserts in proximal penis = 3.
22. Duct of bursa copulatrix: extremely short not longer than sac = 0; medium = 1; long = 2. [additive].
23. Vagina: short = 0; medium to long = 1; extremely long = 2. [additive].
24. Atrium:short = 0; medium to long = 1.
25. Flagellum: thin, long = 0; finger-like, short to medium = 1; Pleurodonte-like = 2; Labyrinthus-like = 3. [additive].
26. Penial muscular band: absent = 0; present = 1.
27. Penial sheath (penial tunica): simple = 0; double or multilayer = 1.
28. Microhabitat associated to: rocks = 0; tree trunks = 1.
29. Vas deferens: surrounding dart sac = 0; not surrounding dart sac = 1.
30. Epiphallus proximal portion: Not widen at point entrance vas deferens = 0; Widen at point of entrance of vas deferens = 1.
31. Penial retractor: not = 0; forming a loop around vas deferens before insertion in epiphallus = 1.
32. Flagellar caecum: absent = 0; present = 1.
33. Kidney length respect to pulmonary roof: not exceeding half of pulmonary roof length = 0; more than half the pulmonary roof = 1.
34. Jaw: ribbed = 0; smooth = 1.
. In the text, characters and state characters numbers are located between parenthesis. Only species for which the anatomy had been described were included in the character matrix. The data matrix was built in Winclada, v. 1.00.08 (Nixon 2002Nixon KC (2002) Winclada Computer program. Ithaca, Published by the author, ver. 1.00.08.). Non-applicable data were coded as “-” and multistate characters were treated as additive. Cladistic analyses were performed with TNT, version 1.5 (Goloboff et al. 2008Goloboff P, Farris JS, Nixon KC (2008) TNT, a free program for phylogenetic analysis. Cladistics 24: 774-786. https://doi.org/10.1111/j.1096-0031.2008.00217.x
https://doi.org/10.1111/j.1096-0031.2008...
) with Character Weighting and Traditional Search basing the strategy on RAS + TBR (random addition sequences plus swap by tree bisection and reconnection), with 1,000 replications and 100 trees saved per replication. The default concavity (K) value was used in all analyses (K = 3000). In the analysis, trees were rooted in Pleurodonte based on Wade et al. (2007Wade CM, Hudelot C, Davison A, Mordan PB (2007) Molecular phylogeny of the helicoid land snails (Pulmonata: Stylommatophora: Helicoidea), with special emphasis on the Camaenidae. Journal of Molluscan Studies 73: 411-415. https://doi.org/10.1093/mollus/eym030
https://doi.org/10.1093/mollus/eym030...
) hypothesis on the phylogenetic relationships of the Helicoidea. Clade support was estimated using symmetric resampling (Goloboff et al. 2003Goloboff P, Farris J, Kallersjo M, Oxelmann B, Ramirez M, Szumik C (2003) Improvements to resampling measures of group support. Cladistics 19: 324-332. https://doi.org/10.1111/j.1096-0031.2003.tb00376.x
https://doi.org/10.1111/j.1096-0031.2003...
), because the resulting values obtained under this procedure are not distorted by character weighting. Additionally, support for the obtained clades was calculated using the Jackknife resampling method.
TAXONOMY
Supra superfamily classification follows Bouchet and Rocroi (2005Bouchet P, Rocroi JP (2005) Classification and Nomenclator of Gastropod Families. Malacologia 47: 1-397.) and Ponder and Lindberg (1997Ponder WF, Lindberg DR (1997) Towards a phylogeny of gastropod molluscs: an analysis using morphological characters. Zoological Journal of the Linnean Society 119: 83-265. https://doi.org/10.1111/j.1096-3642.1997.tb00137
https://doi.org/10.1111/j.1096-3642.1997...
). This classification tries to integrate the results of recent cladistics work by using the unranked “clade” above the rank of superfamily while still using the traditional Linnaean ranks for superfamilies and all taxa below the rank of superfamily.
Class Gasteropoda Cuvier, 1795
Clade Heterobranchia Burmeister, 1837
Clade Stylommatophora Schmidt, 1855
Superfamily Helicoidea Rafinesque, 1815
Epiphragmophoridae Hoffmann, 1928
Minaselates gen. n.
http://zoobank.org/48537C28-29CA-4488-97FF-1C570990D9F9
Diagnosis. Minaselates gen. n. is distinguished by the following characters: 1) shell globose with blunt apex; 2) protoconch sculptured with granules; 3) teleoconch sculptured with complex microstructures; 4) umbilicus imperforate, parietal wall fused with columellar zone of peristome; 5) wavy spiral lines below the periphery and over ventral teleoconch surface; 6) genitalia with a dart apparatus composed by a single dart sac and two unequal mucous glands, one globose and the other oval; 7) presence of a flagellar caecum; 8) bursa copulatrix duct short, no longer than the sac.
Type species. Minaselates paradoxa sp. n. by original designation.
Description. Shell globose, with 4 to 5 convex whorls. Spire conic with blunt apex. Protoconch granulose. Teleoconch sculptured. Wavy spiral grooves at the ventral teleoconch surface. Aperture subcircular with thin peristome. Umbilicus closed. Presence of spiral brownish bands more pronounced in the body whorl. Kidney long and thin, more than half the lung roof length. Genitalia with a dart apparatus and two unequal mucous glands.
Etymology. Minaselates is a compound name formed by Minas in honor to the Brazilian state where the species was found, and selates, a noun in the genitive singular, that derives from the Greek meaning “snail” (Brown 1979Brown RW (1979) Composition of scientific words. A manual of methods and a lexicon of materials for the practice of logotechnics. Washington, DC, Smithsonian Institution Press.).
Remarks. Minaselates gen. n. is classified in Epiphragmophoridae because it has a dart apparatus and two unequal mucous glands at the terminal genitalia. These structures are diagnostic of Epiphragmophoridae (Helicoidea) and their morphology serve to differentiate this family from the remaining helicoidean groups. Dinotropis differs from Minaselates in its depressed shell with an acute peripheral keel and open umbilicus. Minaselates differs from Epiphragmophora in its general shell shape with blunt apex, granulose protoconch and complex sculpture of the teleoconch surface. The wavy spiral grooves at the ventral teleoconch surface in Minaselates are lacking in both, Epiphragmophora and Dinotropis. The presence of a long and thin kidney in Minaselates is very different to the kidney shape in Epiphragmophora, which is triangular and shorter.
Minaselates paradoxa sp. n.
http://zoobank.org/9AACED00-8AFB-4736-85DC-14A3399A04CB
Figs 1 - 26
Diagnosis. Shell globular, with three spiral continuous pigmented bands, the middle, equatorial band thinner. Protoconch granulose. Dorsal side of teleoconch with axial lines bearing triangular lamellae, ventral teleoconch with wavy, concentric, spiral grooves. Imperforate umbilicus. Jaw smooth. Kidney triangular, long and thin, of about 60 to 70% the length of the lung roof. Vas deferens insertion in lower portion of flagellar caecum. Strong, short muscular penial retractor inserting at proximal epiphallus.
Etymology. The species name derives from the Greek paradoxos meaning “strange, contrary to expectation” (Brown 1979Brown RW (1979) Composition of scientific words. A manual of methods and a lexicon of materials for the practice of logotechnics. Washington, DC, Smithsonian Institution Press.) as this is a species of Epiphragmophoridae that was not expected to occur in the state of Minas Gerais, Brazil.
Description. Shell (Figs 2-13) dextral, helicoidal with 4¾ convex, solid whorls. Coloration pale brown with three spiral darker brown bands more separated from each other in the body whorl. Medial pigmented band thinner than the other bands (Figs 2-7). Suture impressed. Protoconch of 1½ whorls, covered by oval, tightly arranged pustules (Figs 8, 9). Dorsal side of teleoconch with slightly curved axial growth lines that, at higher magnifications, appear as axial thin lines bearing broad-base triangular lamellae. These axial lines are not continuous and are separated by narrow spaces with wrinkles. Each lamellae has an axis from the base to the upper extreme of the triangle (Figs 10-13). The long axes of the lamellae are perpendicular to the shell sutures. This sculpture looks like granules at naked eye. Wavy spiral grooves below the periphery, and in the basal surface area. Peristome thin, reflexed, some specimens showing a basal thickening and or an incipient palatal tooth. Aperture roundish, without angulation, and rounded in the palatal zone, well reflected in the columellar zone covering the umbilicus. Shell imperforate. Shell measurements. Table 1, Fig. 1. Digestive system. Radula (Figs 14-17) Central tooth long, unicuspid. Lateral teeth long, with incipient lateral cusps, of about 54-56 µm (n = 10). Marginal teeth bicuspid, similar to laterals in shape and size. Jaw (Figs 18, 19) Horseshoe slightly arched, translucent, with no division. Surface almost smooth with thin, transverse grooves visible with high magnification. Pallial system (Figs 20, 21) Kidney triangular, long and thin, of about 60 to 70% the length of the lung roof. Main pulmonary vein thick, splitting into two secondary branches before reaching mantle collar; pulmonary roof dark grey in color, furrowed by well marked, but lesser minor transverse veins. Secondary ureter runs parallel to rectum, completely closed until reaching mantle collar. Ureteric interramus triangular in shape, deeply excavated. Genital System (Figs 22-26) Terminal genitalia with dart apparatus and two mucous glands (Figs 22-24). Right ommatophore retractor crosses the distal genital system between penis and vagina. Vagina long. Penis and vagina entering side by side in atrium. Bursa copulatrix with a globular sac and short, thick duct. Bursa copulatrix slightly longer than free oviduct. Single dart sac muscular, with medial constriction, ending in the atrium. Upper dart sac inverted pear shaped, lower portion of dart sac bellow constriction cylindrical, thicker (Fig. 24). Two unequal mucous glands with their respective thin efferent ducts inserting independently above the dart sac constriction (Fig. 25). One of the glands bean shaped with medial duct and the other oval with one end bulkier than the other. Vas deferens is a long, narrow duct that passes between one of the mucous glands and the dart sac, over the penial sheath, going down it adheres to penis-vagina at angle with connective tissue and then rises parallel to the penis complex to insert bellow the flagellar caecum. Vas deferens insertion marks the limit between epiphallus and flagellum. Penial complex tubular without external differentiation between penis and epiphallus. Short distal muscular penis sheath. Penial retractor muscle thick inserting in proximal epiphallus. Penial cavity occupied by several thin wavy pilasters and an oval verge sculptured with overlapping lamellae (Fig. 26). Penis longer than epiphallus, limits differentiated through their particular inner sculpture. Penial sheath short of about 1/5 of penial length. Epiphallus inner cavity with three thick pilasters. Proximal epiphallus with a rounded caecum where the vas deferens inserts. Flagellum tubular, longer than epiphallus. Spermoviduct long with uterus zone plicated transversal to the longitudinal axis. Albumen gland bean shaped with a prominent fertilization pouch-spermathecal complex (FPSC). Atrium short.
Shell morphology: (2) Dorsal view of Holotype, MNRJ 34.580; (3) lateral position of holotype shell and soft body; (4) dorsal, (5) ventral, and (6) lateral view of paratype, IBN 861; (7) live snail. Scale bar: 3-5 = 10 mm.
Shell dimensions in mm or mm2 (n = 14). DM major diameter, Dm minor diameter, AD dorsal area, PD dorsal perimenter, H total height, HBw body whorl height, AL lateral area, PL lateral perimeter, ABw body whorl area, PBw body whorl perimeter, Dap diameter of the aperture, Hap height of the aperture, EP length parietal space, AAp apertural area, Pap apertural perimeter, DP penultimate whorl diameter, DPr protoconch diameter (see Fig. 1).
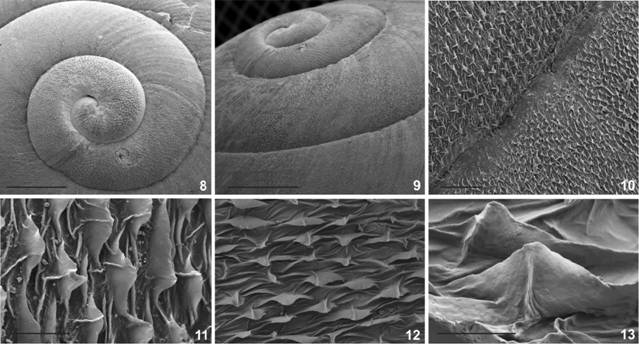
Shell morphology: (8) Protoconch in dorsal view, scale bar = 4mm; (9) lateral view of the protoconch and first whorls, scale bar = 4mm; (10) general view of the teleoconch microsculpture, scale bar = 100μ; (11) body whorl microsculpture consisting on axial rows of triangular lamella separated by wrinkles; (12) perpendicular view of the lamella, scale bar = 5μ; (13) detail of a triangular lamellae showing its central axis, scale bar = 2 μm.
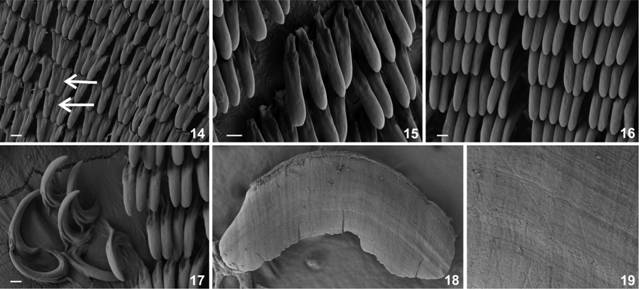
Digestive system: (14) Dorso-lateral view of the radula, arrows points to central tooth in two transverse rows of teeth; (15) detail on a dorso-lateral view of the lateral teeth;(16) detail of lateral teeth close to margin in dorsal view (17) margin of the radula showing curve shaped marginal teeth; (18) dorsal view of the smooth, fragile jaw; (19) detail of the dorsal surface of the jaw showing transversal shallow grooves. Scale bars = 10 μm.
Pallial system: (20) photograph of the ventral zone of the pulmonary roof, note the long kidney, with respect to the total length of the lung; (21) line drawing of the same region detailing the limits between kidney and ureter and the shallow veins crossing the pulmonary roof. Scale bar: 21 = 5mm. Abbreviations (k) kidney, (mc) mantle collar, (pv) pericardic vein, (r) rectum, (su) secondary ureter.
Genital system: (22, 23) line drawing and photograph of the complete dissected out reproductive system (ag) albumen gland, (bc) bursa copulatrix, (ds) dart sac, (ec) flagellar caecum, (fl) flagellum, (go) genital opening, (hd) hermaphroditic duct, (mg1) mucous gland 1, (mg2) mucous gland 2, (p) penis, (pr) penial retractor muscle, (s) spermoviduct, (vd) vas deferens; (24) detail of the terminal genitalia (pl) penial plates, (ps) penial sheath, (v) verge; (25) vas deferens and insertion of mucous gland ducts into dart sac (mgd) mucous gland duct; (26) inner sculpture of the penial complex showing the verge inside the penis. Scale bars: 22 and 25 = 5 mm.
Type locality. Brazil, Minas Gerais: Itacarambi, National Park Cavernas do Peruaçu, Vale dos Sonhos (523m, X = 0599645, Y = 8343426), M.S. Pena, A. Suhett, D.C. Souza leg., December 2010, (MNRJ 34.580), Holotype (ethanol preserved specimen).
Other material examined. Brazil, Minas Gerais: Itacarambi, National Park Cavernas do Peruaçu, Nossa Senhora Aparecida Farm (532 m, X = 0589284, Y = 8328970), M.S. Pena, A. Suhett, D.C. Souza leg., December 2010, (IBN 21-S, MLP-Ma 14216), dry shells, (IBN 861), ethanol preserved specimens. Paratypes. Brazil, Minas Gerais: Itacarambi, National Park Cavernas do Peruaçu, Brejal, Peruaçu River side (663 m, X = 0579404, Y = 8332170), (MCN 192), dry shells. Brazil, Minas Gerais: Itacarambi, Natiomal Park Cavernas do Peruaçu, Janelão Cave (714 m, X = 0581514, Y = 8329046) M.S. Pena, A. Suhett, D.C. Souza leg., December 2010, (MCN 208).
Distribution. Thus far known only from National Park Cavernas do Peruaçu, northern region of Minas Gerais, Brazil.
Remarks. Minaselates differs from all known species of Epiphragmophora by having a granulose protoconch, the shell spire with a blunt apex, and by the wavy, spiral grooves in the ventral region of the shell. The fused, imperforate umbilicus on the shells of this new species is not typical of Epiphragmophora. Most of species of the genus present an open, perspective umbilicus. The exceptions are E. argentina (Holmberg, 1909) and some specimens of E. variegata Hylton Scott, 1962. The presence of wavy spiral grooves at the base of the shell of M. paradoxa sp. n. is noteworthy, sharply contrasting with the condition found in all other species of Epiphragmophora, where it is absent, except for E.(Pylsbrya) farrisi (Pfeiffer, 1859), which has shallow spiral lines. Minaselates paradoxa sp. n. also differs from the species classified in Epiphragmophora in the shape and length of the kidney, and the smooth jaw. In the species of Epiphragmophora for which the anatomy has been studied, the kidney is no more than half the length of the pulmonary roof, while the jaw is ribbed. A noteworthy character present in Minaselates paradoxa sp. n. is the presence of a flagellar caecum, a structure not found in Epiphragmophora. In this new species, the vas deferens inserts in the lower portion of the caecum. The penial retractor muscle in Epiphragmophora is mostly long and thin, inserting in the epiphallus, while in Minaselates paradoxa sp. n. this muscle is stronger, inserting in the caecum. M. paradoxa sp. n. is isolated from the area of distribution of Epiphragmophora, whose area of highest species richness is in the western portion of South America.
Minaselates paradoxa sp. n. resembles some species of Pleurodontidae by the presence of complex structures in the terminal genitalia of the male, such as the flagellar caecum. It is also similar to some Pleurodontidae in its long and thin kidney, the crowded granules in the surface of the shell protoconch, the globular general shape of the shell, the complex microsculpture on the teleoconch and in its smooth jaw. The presence of a flagellar caecum is noteworthy in Minaselates, this structure being absent in Epiphragmophoridae, while it is characteristic of some Pleurodontidae such as Polydontes Montfort, 1810 and Pleurodonte incerta (Férussac, 1823) (Cuezzo 2003Cuezzo MG (2003) Phylogenetic analysis of the Camaenidae with special emphasis on The American taxa. Zoological Journal of the Linnean Society 138: 449-476. https://doi.org/10.1046/j.1096-3642.2003.00061.x
https://doi.org/10.1046/j.1096-3642.2003...
).
In Minaselates, however, the vas deferens inserts in the flagellar pouch while in Polydontes the vas deferens inserts above this caecum. At first glance, the shell of this new species is very similar to some species of Pleurodonte (Pleurodontidae), except for the presence of concentric sculpture below the periphery, and in the basal area. It also differs in the morphology of the terminal genitalia that has a dart complex and a different flagellum shape. In Pleurodonte, the vas deferens is twisted around the epiphallus, descending to the peni-oviducal angle, while in M. paradoxa sp. n. the vas deferens runs straight, parallel to the penis complex, without looping around the epiphallus. Most of the Helicoidean families have a ribbed jaw or ‘odontognath jaw’, except for the Sagdidae with a ‘stegognath jaw’ and the Sphicterochilidae and Cepoliinae with a smooth jaw (‘oxygnath jaw’) (Cuezzo 2003Cuezzo MG (2003) Phylogenetic analysis of the Camaenidae with special emphasis on The American taxa. Zoological Journal of the Linnean Society 138: 449-476. https://doi.org/10.1046/j.1096-3642.2003.00061.x
https://doi.org/10.1046/j.1096-3642.2003...
). In M. paradoxa sp. n. the jaw was found to be smooth, only having fine transverse lines visible with electron microscopy, similar to jaws in Caracolus Montfort, 1810 and Labyrinthus.
Minaselates paradoxa sp. n. is distributed within a National Park area where Cerrado and Caatinga are the dominant biomes. These are considered high diversity hotspot areas. Within these hotspots areas specimens were collected in typical deciduous or semi-deciduous forests called Seasonally Dry Tropical Forests (SDTF) (Pennington et al. 2000Pennington RT, Prado DE, Pendry CA (2000) Neotropical seasonally dry forests and Quaternary vegetation changes. Journal of Biogeography 27: 261-273. https://doi.org/10.1046/j.1365-2699.2000.00397.x
https://doi.org/10.1046/j.1365-2699.2000...
, Prado 2000Prado D (2000) Seasonally dry forests of tropical South Ame rica: from forgotten ecosystems to a new phytogeographic unit. Edinburgh Journal of Botany 57: 437-461. https://doi.org/10.1017/S096042860000041X
https://doi.org/10.1017/S096042860000041...
). These types of forest are drought-adapted, tree-dominated ecosystems with a more or less continuous canopy. Grasses are not a significant element in these forests, which are currently scattered in eastern South America. A hypothesis has been advanced predicting that SDTFs were more widespread during drier glacial climates, and that their fragmentation during the current wet interglacial period has contributed to speciation and the distribution patters of species observed today (Pennington et al. 2000Pennington RT, Prado DE, Pendry CA (2000) Neotropical seasonally dry forests and Quaternary vegetation changes. Journal of Biogeography 27: 261-273. https://doi.org/10.1046/j.1365-2699.2000.00397.x
https://doi.org/10.1046/j.1365-2699.2000...
, Prado 2000Prado D (2000) Seasonally dry forests of tropical South Ame rica: from forgotten ecosystems to a new phytogeographic unit. Edinburgh Journal of Botany 57: 437-461. https://doi.org/10.1017/S096042860000041X
https://doi.org/10.1017/S096042860000041...
). This hypothesis is for the most part based on the assessment of floristic links among species assemblages in the SDTF areas (Sarkinen et al. 2011Sarkinen T, Iganci JR, Linares-Palomino R, Simon M, Prado D (2011) Forgotten forests - issues and prospects in biome mapping using Seasonally Dry Tropical Forests as a case study. BMC Ecology 11: 1-16. https://doi.org/10.1186/1472-6785-11-27
https://doi.org/10.1186/1472-6785-11-27...
). Land snails inhabiting this type of dry forest, from the Cerrado and Caatinga hotspots areas, have been scarcely studied and consequently their conservation status is unknown. Most vertebrates and plants have been more extensively documented in the Cerrado and Caatinga hotspots areas (Overbeck et al. 2015Overbeck GE, Velez-Martin E, Scarano FR, Lewinsohn TM, Fonseca CR, Meyer ST, Müller SC, Ceotto P, Dadalt L, Durigan G, Ganade G, Gossner MM, Guadagnin DL, Lorenzen K, Jacobi CM, Weisser WW, Pillar VD (2015) Conservation in Brazil needs to include non-forest ecosystems. Diversity and Distributions 21: 1455-1460. https://doi.org/10.1111/ddi.12380
https://doi.org/10.1111/ddi.12380...
), but this is not the case with many invertebrate groups.
CLADISTIC ANALYSIS
The main goal of the analysis performed was to evaluate and support the description of a new genus. For this, only the species of Epiphragmophora with known complete anatomical and shell morphology information were used. The morphological data set analyzed under the implied weights approach resulted in two most parsimonious trees. The resulting strict consensus tree is illustrated (Fig. 27). Synapomorphies that support Minaselates gen. n. as a new genus, different from Epiphragmophora are: body whorl surface covered with triangular lamellae (0: 5); duct of bursa copulatrix extremely short, not longer than sac (22:0); presence of a flagellar caecum (32:1).
Consensus tree obtained from two trees under implied weight approach highlighting the position of Minaselates gen. n. Only taxa with described anatomy were included in the matrix. Numbers below branches are node numbers. Symmetric resampling values (left number) and jackknife values (right number) are located above branches. Only values above 50% are illustrated.
Epiphragmophora (node 29) was also recovered as a monophyletic genus in all optimal trees, supported by the following synapomorphies: Body whorl surface with axial ribs regularly distributed (0:3); umbilicus overlapped but not solded to body whorl (1: 1); protoconch smooth (8:0), and Penial retractor muscle inserting in medial zone of epiphallus (21:1). Within Epiphragmophora, the clade [E. variegata [E. hemiclausa [E. tucumanensis+E. argentina]]] (node 27) has the highest SR and jackknife support and resulted monophyletic in all trees obtained.
Minaselates gen. n. is the sister group of Epiphragmophora in both optimal trees. This relationship is supported by the following synapomorphies: Presence of mucous glands in terminal genitalia (10: 1); presence of a dart apparatus (11:1); medium to short length of the bursa copulatrix duct (22:01) and a finger-like short to medium flagellum (25:1).
ACKNOWLEDGEMENTS
Thanks to the Instituto Chico Mendes de Conservação da Biodiversidade for the working permits and support (SISBIO-ICMBIO 19133-1 de 08/04/2009) and Fundo de Incentivo à Pesquisa (FIP), Pontifícia Universidade Católica de Minas Gerais for financial support to MSP. MGC is a researcher of the Argentine National Council for Scientific Research (CONICET). Financial support to MGC has been received through PIP 0055 (CONICET). We also thank the anonymous reviewers and the editor for their valuable comments.
LITERATURE CITED
- Bouchet P, Rocroi JP (2005) Classification and Nomenclator of Gastropod Families. Malacologia 47: 1-397.
- Brown RW (1979) Composition of scientific words. A manual of methods and a lexicon of materials for the practice of logotechnics. Washington, DC, Smithsonian Institution Press.
- Cuezzo MG (1997) Comparative anatomy of three species of Epiphragmophora Doering, 1874 (Pulmonata: Xanthonychidae) from Argentina. The Veliger 40: 216-227.
- Cuezzo MG (2003) Phylogenetic analysis of the Camaenidae with special emphasis on The American taxa. Zoological Journal of the Linnean Society 138: 449-476. https://doi.org/10.1046/j.1096-3642.2003.00061.x
» https://doi.org/10.1046/j.1096-3642.2003.00061.x - Cuezzo MG (2006) Systematic revision and cladistic analysis of Epiphragmophora Doering from Argentina and Southern Bolivia (Gastropoda: Stylommatophora: Xanthonychidae). Malacologia 49: 121-188. https://doi.org/10.4002/1543-8120-49.1.121
» https://doi.org/10.4002/1543-8120-49.1.121 - Goloboff P, Farris J, Kallersjo M, Oxelmann B, Ramirez M, Szumik C (2003) Improvements to resampling measures of group support. Cladistics 19: 324-332. https://doi.org/10.1111/j.1096-0031.2003.tb00376.x
» https://doi.org/10.1111/j.1096-0031.2003.tb00376.x - Goloboff P, Farris JS, Nixon KC (2008) TNT, a free program for phylogenetic analysis. Cladistics 24: 774-786. https://doi.org/10.1111/j.1096-0031.2008.00217.x
» https://doi.org/10.1111/j.1096-0031.2008.00217.x - Linares EL, Vega ML (2011) Catalogo preliminar de los Moluscos continentales de Colombia. Bogotá, Biblioteca José Gerónimo Triana 22, Instituto de Ciencias Naturales, Facultad de Ciencias, Universidad Nacional de Colombia.
- Nixon KC (2002) Winclada Computer program. Ithaca, Published by the author, ver. 1.00.08.
- Nordsieck H (1987) Revision des Systems der Helicoidea (Gastro poda: Stylommatophora). Archiv für Molluskenkunde 118: 9-50.
- Overbeck GE, Velez-Martin E, Scarano FR, Lewinsohn TM, Fonseca CR, Meyer ST, Müller SC, Ceotto P, Dadalt L, Durigan G, Ganade G, Gossner MM, Guadagnin DL, Lorenzen K, Jacobi CM, Weisser WW, Pillar VD (2015) Conservation in Brazil needs to include non-forest ecosystems. Diversity and Distributions 21: 1455-1460. https://doi.org/10.1111/ddi.12380
» https://doi.org/10.1111/ddi.12380 - Pennington RT, Prado DE, Pendry CA (2000) Neotropical seasonally dry forests and Quaternary vegetation changes. Journal of Biogeography 27: 261-273. https://doi.org/10.1046/j.1365-2699.2000.00397.x
» https://doi.org/10.1046/j.1365-2699.2000.00397.x - Pilsbry HA (1894) Guide to the study of Helices. In: Manual of Conchology. Philadelphia, Academy of Natural Sciences, vol. 7. 1-366
- Pilsbry HA (1939) Land Mollusca of North America (north of Mexico). Academy of Natural Sciences Monographs 1: 216-227.
- Ponder WF, Lindberg DR (1997) Towards a phylogeny of gastropod molluscs: an analysis using morphological characters. Zoological Journal of the Linnean Society 119: 83-265. https://doi.org/10.1111/j.1096-3642.1997.tb00137
» https://doi.org/10.1111/j.1096-3642.1997.tb00137 - Prado D (2000) Seasonally dry forests of tropical South Ame rica: from forgotten ecosystems to a new phytogeographic unit. Edinburgh Journal of Botany 57: 437-461. https://doi.org/10.1017/S096042860000041X
» https://doi.org/10.1017/S096042860000041X - Richardson L (1982) Helminthoglyptidae: Catalog of species. Tryonia 6: 1-117.
- Sarkinen T, Iganci JR, Linares-Palomino R, Simon M, Prado D (2011) Forgotten forests - issues and prospects in biome mapping using Seasonally Dry Tropical Forests as a case study. BMC Ecology 11: 1-16. https://doi.org/10.1186/1472-6785-11-27
» https://doi.org/10.1186/1472-6785-11-27 - Schileyko AA (1991) Taxonomic status phylogenetic relations and system of the Helicoidea sensu lato. Archiv fur Molluskenkunde 120: 187-236.
- Thiele J (1929-1935) Handbook of Systematic Malacology 1. Berlin, Jena.
- Tompa AS (1984) Land Snails (Stylommatophora). In: Tompa AS, Verdonk H H, Van Den Biggelar JA (Eds) The Mollusca. New York, Academic Press, 47-140. https://doi.org/10.1016/B978-0-08-092659-9.50009-0
» https://doi.org/10.1016/B978-0-08-092659-9.50009-0 - Wade CM, Hudelot C, Davison A, Mordan PB (2007) Molecular phylogeny of the helicoid land snails (Pulmonata: Stylommatophora: Helicoidea), with special emphasis on the Camaenidae. Journal of Molluscan Studies 73: 411-415. https://doi.org/10.1093/mollus/eym030
» https://doi.org/10.1093/mollus/eym030 - Zilch A (1959-1960) Gastropoda. Euthyneura. In: Handbuch der Paläozoologie 6. Berlin, Borntraeger, 1-825.
-
Editorial responsibility:
Rosana M. da Rocha
-
Zoobank:
http://zoobank.org/1B7B6395-EE91-46AA-9774-89832FE0F47A
APPENDIX 1
APPENDIX 2
Appendix 2. Character list used in the cladistics analysis of species of Epiphragmophora with known anatomy plus the new genus and species, Minaselates paradoxa sp. n.
0. Body whorl surface: with thin growth lines = 0; with thick growth ridges = 1; malleated with diagonal ribs = 2; with axial ribs regularly distributed = 3; pustules/granules to wrinkles = 4; triangular lamella in axial rows = 5. [additive].
1. Umbilicus: Fused with basal lip of peristome = 0; overlapped but not fused to body whorl = 1; perspective wide not overlapped by peristomal lip = 2; perspective narrow slightly overlapped = 3; wide partly overlapped = 4.
2. Shape of the aperture: sub circular = 0; oval horizontal = 1; sub quadrangular = 2.
3. Peristome: Thin expanded slightly reflexed = 0; thick wide reflexed = 1; thin highly expanded = 2.
4. Basal callus in peristome: absent = 0; present = 1.
5. Peripheral bands: absent = 0; present = 1.
6. Body whorl periphery: convex = 0; equatorially subcarinated = 1; supraequatorially subcarinated = 2; carinated = 3. [additive].
7. Aperture respect to body whorl: not descending = 0; descending = 1.
8. Protoconch sculpture: smooth = 0; granulose = 1.
9. Spire apex: pointed = 0; dull = 1; not evident = 2.
10. Mucous glands in terminal genitalia: absent = 0; present = 1.
11. Dart apparatus: absent = 0; present = 1.
12. Shape of dart sac: long finger-like usually with constriction = 0; short, cylindrical, no constriction = 1.
13. Dart sac insertion: in vagina = 0; in atrium = 1.
14. Dart sac papillae: absent = 0; present = 1.
15. Relation between ducts of both mucous glands: separated = 0; distally fused or contiguous = 1.
16. Position of left mucous gland duct: distal respect to the body of the gland = 0; equatorial respect to the body of the gland = 1.
17. Shape of right mucous gland: not sac-like = 0; sac-Like = 1.
18. Right mucous gland: not fused with atrium wall = 0; distally fused with atrium wall = 1.
19. Penis length respect to epiphallus: half epiphallus length = 0; as long as epiphallus = 1; longer than epiphallus length = 2.
20. Penial papillae (= verge): absent = 0; present = 1.
21. Penial retractor muscle: inserts in distal epiphallus = 0; inserts in medial zone of epiphallus = 1; inserts in proximal epiphallus = 2; inserts in proximal penis = 3.
22. Duct of bursa copulatrix: extremely short not longer than sac = 0; medium = 1; long = 2. [additive].
23. Vagina: short = 0; medium to long = 1; extremely long = 2. [additive].
24. Atrium:short = 0; medium to long = 1.
25. Flagellum: thin, long = 0; finger-like, short to medium = 1; Pleurodonte-like = 2; Labyrinthus-like = 3. [additive].
26. Penial muscular band: absent = 0; present = 1.
27. Penial sheath (penial tunica): simple = 0; double or multilayer = 1.
28. Microhabitat associated to: rocks = 0; tree trunks = 1.
29. Vas deferens: surrounding dart sac = 0; not surrounding dart sac = 1.
30. Epiphallus proximal portion: Not widen at point entrance vas deferens = 0; Widen at point of entrance of vas deferens = 1.
31. Penial retractor: not = 0; forming a loop around vas deferens before insertion in epiphallus = 1.
32. Flagellar caecum: absent = 0; present = 1.
33. Kidney length respect to pulmonary roof: not exceeding half of pulmonary roof length = 0; more than half the pulmonary roof = 1.
34. Jaw: ribbed = 0; smooth = 1.
Publication Dates
-
Publication in this collection
2017
History
-
Received
27 May 2016 -
Reviewed
11 Nov 2016 -
Accepted
29 Nov 2016