ABSTRACT
Megaspiridae include land snails with a tall spire. They occur in Brazil, New Guinea and Australia. Megaspira Lea, 1839 is distributed through the central and southeast regions of Brazil There is controversy regarding the number of species in the genus, and their taxonomic status. The characters used to identify Megaspira include a large cylindrical shell and internal armature in the columella. The goal of the present study is to describe a new species for the genus, based on shell morphology, microsculpture and the inner anatomy. These anatomic characters had not been described before for any of the included species. The material was collected at the Jararaca trail, Ilha Grande (Angra dos Reis, state of Rio de Janeiro). Seven linear measurements were taken from the shells. Megaspira adenticulata sp. nov. differs from the other species of the genus by not having apertural lamella in the shell aperture or in the columella in adult specimens. Also, the shell does not have light brown spots, as observed in other species of Megaspira. The shell has a mean of 16 whorls and height of 27 mm. It is smaller in average size than the shell of other described species. The new species was found under leaf litter, especially near rocks and in shallow soil.
KEY WORDS:
Gastropoda; Jararaca trail; land snail; taxonomy
INTRODUCTION
Megaspiridae Pilsbry, 1904 is a family of land snails characterized by a tall spire. The family includes four recent genera, which occur in Brazil, New Guinea and Australia (Pilsbry 1904Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195.): Callionepion Pilsbry & Vannatta, 1899 in southeast Brazil (Simone 2006Simone LRL (2006) Land and freshwater molluscs of Brazil. EGB, Fapesp, São Paulo, 390 pp., Salvador 2019Salvador RB (2019) Brazilian, Uruguayan and Argentinian terrestrial gastropods in the collection of the Museum of New Zealand Te Papa Tongarewa. Tuhinga 30: 82-98.), MegaspiraLea, 1839Lea I (1839) Description of the new freshwater and land shells. Transactions of the American Philosophical 6: 1-154. in the central and southeastern Brazil (Pilsbry 1904Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195.), Perrieria Tapparone-Canefri, 1878 in western New Guinea and Coelocion Pilsbry, 1903 along the east coast of Queensland, Australia. Besides the genera mentioned above, Pilsbry included, in Megaspiridae, the fossil Eomegaspira Pilsbry, 1903. The fossil has been recorded from the Eocene in Europe (France and England).
The taxonomy of Megaspiridae has changed over time, and the relationships among the included genera and the phylogenetic relationships between Megaspiridae and the other families of Gastropoda have remained unclear. Pilsbry (1904Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195.) proposed the following diagnostic characters for the family: “…an elongated, acute spire, and cylindrical shell, with of a high number of whorls. Aperture of the shell, with a thin outer margin of the peristome, with or without columellar lamellae in the aperture...”.
Although no consensus has been reached about the supra-familiar classification of Megaspiridae, two main classifications have been generally accepted. The first, proposed by Thiele (1931Thiele JH (1931) Handbuch der systematischen weichtierkunde. Verlag Jena, Berlin, vol. 5, 778 pp.), includes Megaspiridae (with Callionepion, Perrieria and Coelocion) within the “lineage” Achatinacea (= Achatinoidea), since it takes the radular characters and the conchilio-morphometric description into account. The second, based on distribution data and shell characters, was advanced by Nordsieck (1986Nordsieck H (1986) The system of the Stylommatophora (Gastropoda), with special regard to the systematic position of the Clausiliidae, importance of the shell and distribution. Archiv für Molluskenkunde 117: 93−116.), who proposed that Megaspiridae is a member of the Orthalicoidea. According to Nordsieck, the presence of inner lamellae, an elongated conical shell, and a thick fold at the base of the columellar side of the aperture are plesiomorphic characters that are also present in some genera of Orthalicoidea and also in Megaspira. Thiele’s proposal was adopted by some (Salgado and Coelho 2003Salgado NC, Coelho ACS (2003) Moluscos terrestres do Brasil (gastrópodes operculados ou não, exclusive Veronicellidae, Milacidade e Limacidae). Revista de Biología Tropical 51: 149-189.), while others (Bouchet et al. 2005Bouchet P, Rocroi JP, Frýda J, Hausdorf B, Ponder W, Valdés Á, Warén A (2005) Classification and nomenclator of gastropod families. Malacologia 47(1-2): 1-397., 2017Bouchet P, Rocroi JP, Hausdorf B, Andrzej K, Yasunori K, Nützel A, Parkhaev P, Schrödl M, Strong E (2017). Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 61(1-2): 1-526., Breure et al. 2010Breure B, Groenenberg DSJ, Schilthuizen M (2010) New insights in the phylogenetic relations within the Orthalicoidea (Gastropoda, Stylommatophora) based on 28S sequence data. Basteria 74: 25-31.) opted for Nordsieck’s classification. Bouchet et al. (2005Bouchet P, Rocroi JP, Frýda J, Hausdorf B, Ponder W, Valdés Á, Warén A (2005) Classification and nomenclator of gastropod families. Malacologia 47(1-2): 1-397.) considered Megaspiridae as part of Orthalicoidea, following Nordsieck (1986Nordsieck H (1986) The system of the Stylommatophora (Gastropoda), with special regard to the systematic position of the Clausiliidae, importance of the shell and distribution. Archiv für Molluskenkunde 117: 93−116.). Both authors, however, questioned their own classification. Using molecular markers (ITS2 and 28S), Breure et al. (2010Breure B, Groenenberg DSJ, Schilthuizen M (2010) New insights in the phylogenetic relations within the Orthalicoidea (Gastropoda, Stylommatophora) based on 28S sequence data. Basteria 74: 25-31.), found compelling evidence that Megaspiridae is a member of Orthalicoidea, with Coelocion grouped with Megalobulimus Miller, 1878 in a clade with low support. Subsequently, Breure and Romero (2012Breure B, Romero P (2012) Support and surprises: molecular phylogeny of the land snail superfamily Orthalicoidea using a three-locus gene analysis with a divergence time analysis and ancestral area reconstruction (Gastropoda: Stylommatophora). Archiv für Molluskenkunde 141: 1-20. https://doi.org/10.1127/arch.moll/1869-0963/141/001-020
https://doi.org/10.1127/arch.moll/1869-0...
) reinforced the inclusion of Megaspiridae in the Orthalicoidea, considering the analyses of the species Megaspira elatior (Spix, 1820) as representative of Megaspira. In their results, M. elatior grouped with Thaumastus largillierti Philippi, 1845 and T. achilles Pfeiffer, 1852. They proposed that Thaumastus and Megaspira are sister-groups within the Bulimulidae. At present, some authors (Salgado and Coelho 2003Salgado NC, Coelho ACS (2003) Moluscos terrestres do Brasil (gastrópodes operculados ou não, exclusive Veronicellidae, Milacidade e Limacidae). Revista de Biología Tropical 51: 149-189., Simone 2006Simone LRL (2006) Land and freshwater molluscs of Brazil. EGB, Fapesp, São Paulo, 390 pp.) consider only Megaspira and Callionepion as members of the Megaspiridae. A recent proposal (Breure and Romero 2012Breure B, Romero P (2012) Support and surprises: molecular phylogeny of the land snail superfamily Orthalicoidea using a three-locus gene analysis with a divergence time analysis and ancestral area reconstruction (Gastropoda: Stylommatophora). Archiv für Molluskenkunde 141: 1-20. https://doi.org/10.1127/arch.moll/1869-0963/141/001-020
https://doi.org/10.1127/arch.moll/1869-0...
), based on molecular data, included other genera, such as ThaumastusAlbers, 1860Albers JC, Martens EC von (1860) Die Heliceen nach naturlicher Verwandtschaft systematisch geordnet von Joh. Christ. Albers Zweite Ausgabe. Engelmann, Leipzig, 359 pp., in Megaspiridae. According to Bouchet et al. 2017Bouchet P, Rocroi JP, Hausdorf B, Andrzej K, Yasunori K, Nützel A, Parkhaev P, Schrödl M, Strong E (2017). Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 61(1-2): 1-526., Perrieria and Coelocion are part of another family (Coelociontidae Iredale, 1937) and not Megaspiridae.
MegaspiraLea, 1839Lea I (1839) Description of the new freshwater and land shells. Transactions of the American Philosophical 6: 1-154. is an interesting genus that includes terrestrial gastropods with elongated and cylindrical shell, large and plicate columella with internal armature (Lea 1839Lea I (1839) Description of the new freshwater and land shells. Transactions of the American Philosophical 6: 1-154., Pilsbry and Vanatta 1899Pilsbry HA, Vanatta EG (1899) Morphological and systematic notes on South America land snails: Achatinidae. Proceedings of the Academy of Natural Sciences of Philadelphia 51: 366-374. https://ur.booksc.eu/book/26042206/9bf827
https://ur.booksc.eu/book/26042206/9bf82...
). According to Lea (1839Lea I (1839) Description of the new freshwater and land shells. Transactions of the American Philosophical 6: 1-154.) the shells of the adult of most species have an inner sculpture, which is usually confined to the last whorls, and consists of several lamellae. There is no consensus on the authorship of the genus, number of species in it, and the taxonomic status of the included species. Thiele 1931Thiele JH (1931) Handbuch der systematischen weichtierkunde. Verlag Jena, Berlin, vol. 5, 778 pp., Simone 2006Simone LRL (2006) Land and freshwater molluscs of Brazil. EGB, Fapesp, São Paulo, 390 pp., Breure and Ablett 2015Breure B, Ablett J (2015) Annotated type catalogue of the Megaspiridae, Orthalicidae, and Simpulopsidae (Mollusca, Gastropoda, Orthalicoidea) in the Natural History Museum, London. Zookeys 470: 17-143. https://doi.org/10.3897/zookeys.470.8548
https://doi.org/10.3897/zookeys.470.8548...
, Breure and Araujo 2017Breure B, Araujo R (2017) The Neotropical land snails (Mollusca, Gastropoda) collected by the Comisión Científica del Pacífico. PeerJ 5: e3065. https://doi.org/10.7717/peerj.3065
https://doi.org/10.7717/peerj.3065...
attributed the authorship of the genus to John C. Jay. Most likely, this attribution owes to the fact that, in the Catalogue on Recent Shells published in 1936, Jay mentioned (sic) “Megaspira ruschenbergiana, Lea. This is a new and interesting land shell from Brazil, for which Mr. Lea considers it necessary to form a new genus. His description of it will appear in the Transactions of American Philosophical Society of Philadelphia.” (Jay 1836Jay JC (1836) A catalogue of recent shells with descriptions of new or rare species in the Collection of John C. Jay. New York, 82 pp. https://www.biodiversitylibrary.org/page/12172590
https://www.biodiversitylibrary.org/page...
). The formal description of Megaspira was made by Lea (1839Lea I (1839) Description of the new freshwater and land shells. Transactions of the American Philosophical 6: 1-154.), when the author designated M. ruschenbergiana Lea, 1839 as the type species as Jay had previously indicated. The distribution of Megaspira is restricted to Brazil, with records mainly from the Atlantic Forest of the southeast region (Pilsbry 1904Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195., Morretes 1949Morretes LF (1949) Ensaio de catálogo dos moluscos do Brasil. Arquivos do Museu Paranaense 7: 1-216. http://doi.org/10.7213/estud.biol.612
http://doi.org/10.7213/estud.biol.612...
, Simone 2006Simone LRL (2006) Land and freshwater molluscs of Brazil. EGB, Fapesp, São Paulo, 390 pp.). According to Pilsbry (1904Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195.), the distribution of Megaspira is Central-Southern Brazil, and information on the habitats of several species is scarce.
Pilsbry (1904Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195.) recognized only two species in Megaspira (M. ruschenbergiana and M. elatior). He established three subspecies (M. elatior gracilis, M. elatior robusta and M. elatior elata). Rehder (1945Rehder HA (1945) A note on Megaspira elata Gould. The Nautilus 59: 67.) increased the number of species to three with the description of Megaspira pilsbryi Rehder, 1945. Brazilian authors such as Salgado and Coelho (2003Salgado NC, Coelho ACS (2003) Moluscos terrestres do Brasil (gastrópodes operculados ou não, exclusive Veronicellidae, Milacidade e Limacidae). Revista de Biología Tropical 51: 149-189.) considered four valid species. They did not mention the status of Pilsbry’s subspecies. Simone (2006Simone LRL (2006) Land and freshwater molluscs of Brazil. EGB, Fapesp, São Paulo, 390 pp.) considered a total of six species, after raising the two subspecies established by Pilsbry (1904Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195.) to species, and synonymizing Megaspira elatior with M. ruschembergiana. The author failed to provide a clear justification for their nomenclatural acts. All species are only known by their shell morphology, with the exception of the radula of Megaspira elatior robusta described by Pilsbry (1904Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195.).
The goal of the present study is to describe a new species of Megaspira based on shell morphology and microsculpture, and to describe the inner anatomy of main anatomy systems.
MATERIAL AND METHODS
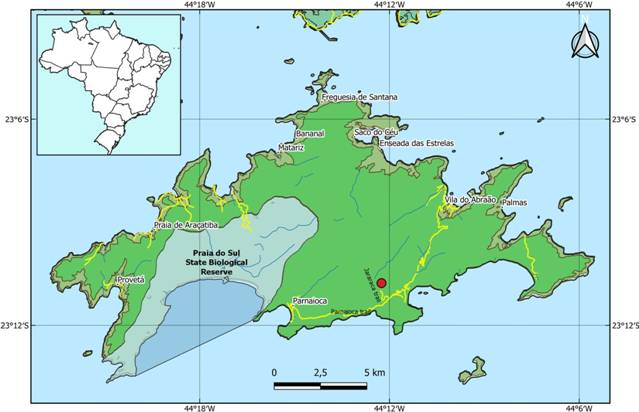
The material was collected from the Jararaca trail, Ilha Grande State Park (PEIG), Angra dos Reis, in southwestern Rio de Janeiro state (Fig. 1). This protected area covers 62.5% of the island and is also part of the Tamoios Environmental Protection Area (Bastos et al. 2009Bastos M, Prado RM, Santiago AMA, Birman P, Catão H, Mendonça TM, Bakker A, Ferrarez A, Gilayn H, Mendonça M, Wiedemann M, Zanatta R, Pereira V, Cruz A, Roseiro T, Araújo A, Attianezi M (2009) Estrutura econômica e organização sociocultural e política. In: Bastos M, Callado C (Eds) O Ambiente da Ilha Grande. Universidade do Estado do Rio de Janeiro, Centro de Estudos e Desenvolvimento Sustentável, Rio de Janeiro, vol. 1, 215-250.).
Map of the Ilha Grande showing the site (the Jararaca trail in red circle) where the material of Megaspira adenticulata sp. nov. was collected.
The specimens were collected in 1995, using the hand collection method, where specimens are captured directly from the substrate. The material was deposited in the Malacological Collection of the Laboratório de Malacologia Límnica e Terrestre, Universidade do Estado do Rio de Janeiro. In 2015, the same methodology was used to collect additional material to obtain live specimens for studies of the inner anatomy. The collecting locality, however, was not in the exact same spot as in 1995.
The shells were measured using calipers (0.01 mm precision) and seven linear measurements were taken: major diameter (D), minor diameter (d), diameter of body whorl (dw), total shell height (h), teleoconch height (th), apertural height (ah) and apertural length (al) (Fig. 2). Due to the size of the shells, a 0.5 × reduction lens was used and the scales of all drawings were standardized. The number of whorls (NW) was obtained following the method of Diver (1931Diver CA (1931) Method of determining the number of the whorls of a shell and its application to Cepaea hortensis and C. nemoralis. Proceedings of the Malacological Society of London 19(5): 234-239.) (Fig. 3). Also, the shells were drawn with the help of a camera lucida (Nikon SMZ 800) in order to take the angular measurements according the methodology explained in Parodiz (1951Parodiz JJ (1951) Métodos de conquiliometria. Physis 38: 241-248.): spiral angle (SpA), suture angle (SA), columellar angle (CA), growth angle (GA) and maximum angle (MA) (Fig. 4).
Shell measurements of Megaspira adenticulata sp. nov.: (2) linear measurements taken from specimens; (3) apical view showing the partial angle of the first two whorls that part of the protoconch; (4) angular measurements taken from specimens: maximum angle in red, spiral angle in blue, growth angle in green, columellar angle in pink, suture angle in orange. (h) Height, (sh) spire height, (ah) aperture height, (al) aperture width, (D) major diameter.
Five adult specimens were dissected and used to describe the pallial, digestive (jaw and radula) and reproductive systems. Due to the high number of whorls in the shell and the thick columellar muscle, it was necessary to remove the entire animal by dissolving the shell. To do this, the specimens were transferred from 70º alcohol to Raillet Henry solution (93% distilled water, 2% glacial acetic acid, 5% formaldehyde, and 6 g sodium chloride per liter). Five specimens remained in Raillet Henry solution for 24 hours to allow the shell to decalcify. After that, the soft parts were washed in distilled water to remove any remaining Raillet Henry and returned to 70º alcohol for dissections.
Dissections were carried out under a Leica MZ6 stereoscopic microscope, the pallial and reproductive systems were dissected following the methodology of Cuezzo (1997Cuezzo MG (1997) Comparative anatomy of three species of Epiphragmophora Doering, 1874 (Pulmonata: Xanthonychidae) from Argentina. The Veliger 40: 216-227.) and anatomical systems were drawn with the help of a camera lucida. The material was compared with type material from the Malacology Collection of the Academy of Natural Sciences of Philadelphia (ANSP) (Table 1): Megaspira elatior gracilis (lectotype: ANSP 25039), Megaspira pilsbryi (lectotype: ANSP 25041) (Figs 5, 6), Megaspira iheringi (lectotype: ANSP 100532) (Figs 7-9) and Megaspira elatior robusta (lectotype: ANSP 71925 and paralectotype ANSP 25040) (Figs 10-12).
Radulas, jaws (three) and a portion of the columellar axis were cleaned and placed on stubs to be analyzed in JSM-6510LV scanning microscopy following the methodology of Ploeger and Breure (1977Ploeger S, Breure ASH (1977) A rapid procedure for preparation of radulae for routine research with the Scanning Electron Microscope. Basteria 4: 47-52.) in the Scanning Electronic Microscopy Laboratory in the Department of Chemistry of UERJ (PPGQ).
Shell of Megaspira species: (5, 6) lectotype of Megaspira pilsbryi (ANSP 25041) apertural and dorsal views of the shell: (7-9) lectotype of Megaspira iheringi (ANSP 100532) apertural, dorsal views of the shell and detail of inner collumelar lamellae; (10-12) lectotype of Megaspira elatior robusta (ANSP 71925) apertural, dorsal views of the shell and detail of inner collumelar lamellae. Scale bars: 5 mm.
Comparisons among Megaspira adenticulata sp. nov. from the Jararaca trail and other species/subspecies of Megaspira.
TAXONOMY
Gastropoda Cuvier, 1795
Eupulmonata Haszprunar & Huber, 1990
Orthalicoidea Martens, 1860 in Albers & Martens, 1860Albers JC, Martens EC von (1860) Die Heliceen nach naturlicher Verwandtschaft systematisch geordnet von Joh. Christ. Albers Zweite Ausgabe. Engelmann, Leipzig, 359 pp.
Megaspiridae Pilsbry, 1904Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195.
Megaspira I. Lea in Jay, 1836Jay JC (1836) A catalogue of recent shells with descriptions of new or rare species in the Collection of John C. Jay. New York, 82 pp. https://www.biodiversitylibrary.org/page/12172590
https://www.biodiversitylibrary.org/page...
Type species Megaspira ruschenbergiana I. Lea in Jay, 1836Jay JC (1836) A catalogue of recent shells with descriptions of new or rare species in the Collection of John C. Jay. New York, 82 pp. https://www.biodiversitylibrary.org/page/12172590
https://www.biodiversitylibrary.org/page...
type by monotype.
Megaspira adenticulata sp. nov.
http://zoobank.org/08B4C9CF-8909-474B-9C3E-798A197DA331
Diagnosis. Shell with 16 whorls and 27 mm in height, on average. Aperture with no lamella folded on inner lip of smooth aperture. Columella sculpture with a rough texture over entire surface.
Description. Cephalo-pedal mass brownish in all its extension, especially close to cephalic portion, and presence of several ridges along body (Figs 13, 14). Thin, cylindrical tentacles, with central axis of dark gray. Foot with gradual tapering in its posterior portion, no external gland in the posterior terminal part of the foot. Sole almost entire, groove not deep in its median portion.
Living specimen of Megaspira adenticulata sp. nov. and shell morphology in different views: (13, 14) specimen showing the pigmentation of the cephalopedal mass; (15-18) apertural, dorsal and lateral views of the shell. Scale bars: 1 mm. Photos: Regiana Salgado de Mello.
Shell. Oblong (in juvenile specimens) or conical-elongated (in adults) (Figs 15-18), shell height from 18.2 to 32.8 mm with 16 convex whorls on average (Table 2). Spire high with moderate growth, spiral angle with 15.8° on average (Table 2). Aperture oval, with apertural height and length almost similar on average (Table 2; Figs 15, 16), with lower lip slightly enlarged; lamella folded on inner lip of aperture, absence of parietal teeth; peristome with reflected edges, mainly on the columellar lip side (Fig. 16). Umbilicus closed covered by peristome reflection. Protoconch smooth, without sculpture, with 2.5 to 2.75 whorls (Figs 19, 20). Third whorl, with faint incomplete radial lines (Fig. 20). Teleoconch sculpture with numerous oblique ribs, well marked, arranged close to each other (Fig. 21). Columellar axis microsculpture with a rough texture along its entire length surface, without internal columellar lamellae (Figs 22-24).
Megaspira adenticulata sp. nov.: (19-21) shell of young specimen in SEM showing the absence of apertural lamellae and detail of the microsculpture of the teleoconch: (22-24) detail of microsculptura of columela in SEM showing small papillae on the entire surface; (25) jaw; (26-28) detail of radula showing the rachidian, lateral and marginal tooth. Scale bars: 19, 20, 22 = 1 mm; 21, 24 = 200 µm; 24, 26, 28 = 20 µm; 25 = 100 µm; 27 = 10 µm.
Minimum, maximum, mean and standard deviation of linear (in mm) and angular (degree) measurements for 30 adult specimens of Megaspira adenticulata sp. nov. from the Jararaca trail. (SD) Standard deviation, (D) major diameter, (d) minor diameter, (dw) diameter of body whorl, (h) total shell height, (th) teleoconch height, (ah) apertural height, (al) apertural length, (NW) number of whorls, (SpA) spiral angle, (SA) suture angle, (CA) columellar angle, (GA) growth angle, (MA) maximum angle.
Jaw. Shaped like horseshoe, with rectangular curved central plate; lateral plates of smaller size, overlapped each other on both sides of jaw (Fig. 25). Lateral plate surfaces with fine, parallel lines, evident on the outer margin without forming true sculpture (Fig. 25).
Radula formula: 20 + 18 + 1 + 18 + 20 and 18 + 16 + 1 + 16 + 18 (M + L + R + L + M), being: M: Marginal, L: Lateral, and R: Rachidian. Rachidian tooth symmetric, tricuspid with mesocone well developed, triangular blunt end, trapezoidal basal plate; ecto- and endocone smaller than mesocone, without accessory cusps (Figs 26, 27). Lateral teeth, asymmetric, bicuspid, with larger and conical mesocone; ectocone smaller than mesocone, triangular, rounded end; basal plate rectangular (Figs 25, 26). Marginal teeth bicuspid, with oblique insertion, basal plate reduced to small square-shaped plate, mesocone elongated, ectocone triangular, smaller than mesocone (Fig. 28).
Pallial system. Kidney elongated, triangular, thin, about ¼ of pallial cavity in length, between pericardial cavity and rectum. Pericardial cavity located on left side of kidney (Figs 29-31); pulmonary vein visible in median and distal region of mantle roof (Figs 30, 31). Slender vessels on both sides of main pulmonary vein (close to distal portion of pallial system).
Megaspira adenticulata sp. nov.: detail of proximal, median and distal portions of pallial system. (A) Anus, (MB) Mantle border, (Ki) kidney, (MV) Mantle vessels, (Pe) Pericardium, (PV) Pulmonar vein, (Pn) Pneumostome, (R) Rectum, (Ur) Ureter). Scale bars: 20-24 = 1 mm.
Reproductive system. Ovotestis with five or six branches of enlarged digitiform acini, each branch with 12 to 15 acini simple or bifurcated (Fig. 32). Hermaphrodite duct tubular, contorted, with same diameter along its extension (Fig. 33). Albumen gland elongated, irregularly shaped, translucent, formed by small irregular acini (Figs 32, 34). Oviduct cylindrical, elongated, accompanying shape of spire (Figs 33, 34). Penial complex tubular, long and slender, with three regions differentiated (proximal phallus, median epiphallus and distal flagellum), proportions of those regions 4: 1: 1 length respectively, each delimited by well-defined narrowing (Figs 32, 34, 35). Prostate with numerous digitiform acini over oviduct surface. Genital diverticle in club shaped, between crossing penial complex and female system (Fig. 35). Genital atrium short, cylindrical, with insertion of bursa copulatrix (also called the copulation pouch), six times length of genital flagellum, bursa sac slightly elongated in its terminal portion (Fig. 35).
General view of reproductive system dissected out of Megaspira adenticulata sp. nov.: (32, 34) dorsal and ventral views of reproductive; (33) detail of ovotestis; (35) detail of distal portion of reproductive system showing the genital atrium and genital diverticle. (Ov) Ovoteste, (HD) Hermaphrodite Duct), (AG) Albumen gland, (BC) Bursa copulatrix, (BD) Bursa duct, (DV) Deferens vas, (Ep) epiphallus, (Ph) proximal phallum, (GA) Genital atrium, (Fl) Flagellum, (Ov) Ovotestis, (Pr) Prostate, (U: uterus), (BD) Bursa duct, (GD) Genital diverticle, (Ph) Proximal phallum, (GA) Genital atrium, (U) Ureter). Scale bars: 32-35 = 1 mm.
Type locality. Brazil: state of Rio de Janeiro, municipality of Angra dos Reis, Ilha Grande, Jararaca trail, 23.17950° S, 44.20421°W.
Type material. Holotype. Shell and soft parts preserved in alcohol. Original label; Daniel VR, Ovando XMC, Santos SB, Souza ST, Lacerda LEM leg. “Col. Mol. UERJ 11395-A”. Paratypes: Eleven. Collected with Holotype. Col. Mol. UERJ 11395-B; One. Collected with Holotype. MNRJ 23.760.
Additional material examined. BRAZIL, Ilha Grande, 1 shell, Jararaca trail; 23.17950° S, 44.20421° W; 22 Mar. 1997; Santos SB et al. leg.; Col. Mol. UERJ 695 • 1 shell Same locality; 20 Sep. 1995; Queiroz V leg.; Col. Mol. UERJ 697 • 2 shells, Same locality; 15 Aug. 1996; Queiroz V leg.; Col. Mol. UERJ 701 • 1 (1 shell), Same locality; 15 Aug. 1996; Queiroz V leg.; Col. Mol. UERJ 1076 • 4 (2 shells, 2 specimens preserved in ethanol), Same locality; 29 Aug. 2002, Santos SB et al. leg.; Col. Mol. UERJ 1742 • 2 (1 shell, 1 specimen preserved in ethanol), Same locality; 09 Jan. 2002, Santos SB et al. leg.; Col. Mol. UERJ 1749 • 4 shells, Same locality; 09 Jan. 2002, Santos SB et al. leg.; Col. Mol. UERJ 1771 • 7 shells, Same locality; 13 Apr. 2002; Santos SB et al. leg.; Col. Mol. UERJ 1794 • 6 (3 shells, 3 specimens preserved in ethanol), Same locality; 20 Oct. 2000; Santos SB et al. leg.; Col. Mol. UERJ 2076 • 4 shells, Same locality; 07 Jan. 2001; Santos SB Ribeiro F leg.; Col. Mol. UERJ 2135 • 10 (6 shells, 4 specimens preserved in ethanol), Same locality; 21 Apr. 2001; Santos SB et al. leg.; Col. Mol. UERJ 2167 • 4 shells, Same locality; 27 Oct. 2001, Santos SB et al. leg.; Col. Mol. UERJ 2228 • 2 (1 shell, 1 specimen preserved in ethanol), Same locality; 31 Jul. 2001; Monteiro SP leg; Col. Mol. UERJ 2239 • 2 shells, Same locality; 13 Apr. 2003; Santos SB leg.; Col. Mol. UERJ 2957 • 3 (2 shells, 1 specimen preserved in ethanol) Same locality; 25 Jan. 2005; Miyahira IC leg.; Col. Mol. UERJ 6677 • 13 (5 shells, 8 specimens preserved in ethanol), Same locality; 25 Oct. 2015; Daniel VR, Ovando XMC, Santos SB, Souza ST, Lacerda LE leg.; Col. Mol. UERJ 11395.
Etymology. The epithet “adenticulata”, meaning without teeth, refers to the absence of parietal teeth in the shell aperture. The presence of a parietal tooth is considered a diagnostic character for other species of Megaspira.
Habitat and distribution. The species was found under leaf litter, especially near rocks and in shallow soil. Only known from the type locality.
Comparison with other species. Megaspira adenticulata sp. nov. have an aperture without lamella parietal. This structure is present in the other species of Megaspira. Also, the columellar sculpture in M. adenticulata sp. nov. shows a rough texture and not inner lamellas, characteristic of Megaspira iheringiPilsbry, 1925Pilsbry HA (1925) South American Land and Fresh Water Mollusks: Notes and Descriptions: V. Proceedings of the Academy of Natural Sciences 77: 311-315. https://www.jstor.org/stable/4063977
https://www.jstor.org/stable/4063977...
, M. pilsbryi and M. elatior robusta. In M. iheringi and M. elatior robusta have three small lamellae that rise 3 ½ whorls inward (Pilsbry 1904, 1925). The shell in M. adenticulata sp. nov. has 16 whorls on average and 27 mm in height whereas M. iheringi have 14 whorls and 28 mm. In Megaspira elatior robusta the shell is 16.7 mm in height and has 39 whorls (Table 1). The shell does not have light brown spots, as observed by Pilsbry (1925) in Megaspira iheringi and M. elatior.
DISCUSSION
We present new information on the anatomy, microsculpture, radula and morphometry of the shell of a Megaspira species. Megaspira adenticulata sp. nov. is characterized based on its morphology (shell and inner anatomy) and compared with the shells of the other species of Megaspira. Lea (1839Lea I (1839) Description of the new freshwater and land shells. Transactions of the American Philosophical 6: 1-154.) considered the presence of inner lamellae as diagnostic of Megaspira (Figs 7-9). Instead of a complex with inner lamellae or inner folds, characteristic of several other species such as Megaspira elatior, M. robusta, M. pilsbryi and M. iheringi, the entire surface of the columella of Megaspira adenticulata sp. nov. has a rough texture. However, the inner columellar lamellae are also present in other genera of terrestrial gastropods such as Holospira Martens, 1860. Therefore, it is not an exclusive character of Megaspira. The adult shell of species of Holospira has an internal barrier formed by inner lamellae usually in the last 1-4 whorls. Basically, the barrier consisting of four lamellae: the columellar, parietal, the basal and the palatal (Thompson and Mahalcik 2005Thompson FG, Mahalcik EL (2005) Urocoptid landsnails of the genus Holospira from southern Mexico. Bulletin Florida Museum Natural History 43: 63-124.) are similar to the condition observed in some species of Megaspira (ST Vieira, unpublished data). On the other hand, Pilsbry (1904Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195.) also mentioned the presence of a lamella located on the inner lip of the aperture and another on the parietal surface as a diagnostic character of Megaspiridae. Megaspira adenticulata sp. nov. lacks those apertural lamella mentioned by Pilsbry (1904Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195.) and the columellar axis has only two small folds in its basal portion (Fig. 15).
Even though the height of the shell of M. adenticulata sp. nov. is almost one-third the height of the shell of M. elatior, the general shell morphology and teleoconch sculpture of M. adenticulata sp. nov. matches the generic diagnosis proposed by Lea (1839Lea I (1839) Description of the new freshwater and land shells. Transactions of the American Philosophical 6: 1-154.) and Pilsbry (1904Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195.).
Similar to the observations of Pilsbry (1904Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195.) on Megaspira ruschenbergiana, the jaw of M. adenticulata sp. nov. also has the central plate larger than the lateral plate. (Fig. 25). Unfortunately, we have no information on the morphology of the other species, but we suspect that this character is diagnostic for the genus.
Callionepion is another genus that is accepted as a member of the Megaspiridae (Salgado and Coelho 2003Salgado NC, Coelho ACS (2003) Moluscos terrestres do Brasil (gastrópodes operculados ou não, exclusive Veronicellidae, Milacidade e Limacidae). Revista de Biología Tropical 51: 149-189.) and has also been described from Brazil. Megaspira adenticulata sp. nov. shows differences when compared to the type species Callionepion iheringiPilsbry & Vanatta, 1899Pilsbry HA, Vanatta EG (1899) Morphological and systematic notes on South America land snails: Achatinidae. Proceedings of the Academy of Natural Sciences of Philadelphia 51: 366-374. https://ur.booksc.eu/book/26042206/9bf827
https://ur.booksc.eu/book/26042206/9bf82...
, mainly in the shell sculpture and reproductive system (Figs 32-35). Callionepion iheringi has a protoconch sculpture consisting of round nodules and simple columellar lamella (Pilsbry and Vanatta 1899Pilsbry HA, Vanatta EG (1899) Morphological and systematic notes on South America land snails: Achatinidae. Proceedings of the Academy of Natural Sciences of Philadelphia 51: 366-374. https://ur.booksc.eu/book/26042206/9bf827
https://ur.booksc.eu/book/26042206/9bf82...
, Salvador 2019Salvador RB (2019) Brazilian, Uruguayan and Argentinian terrestrial gastropods in the collection of the Museum of New Zealand Te Papa Tongarewa. Tuhinga 30: 82-98.), while the protoconch in M. adenticulata sp. nov. is smooth. Pilsbry and Vanatta (1899Pilsbry HA, Vanatta EG (1899) Morphological and systematic notes on South America land snails: Achatinidae. Proceedings of the Academy of Natural Sciences of Philadelphia 51: 366-374. https://ur.booksc.eu/book/26042206/9bf827
https://ur.booksc.eu/book/26042206/9bf82...
) pointed out that the reproductive system of C. iheringi does not have accessory organs, while in M. adenticulata sp. nov., a genital diverticle is present between the bursa copulatrix and the vagina.
There are no similar characters in the reproductive system, radula and jaw among some species of Orthalicoidea and M. adenticulata sp. nov. In M. adenticulata sp. nov. the central plate of the jaw is larger than the plates that extend laterally (Fig. 25). This differs from other genera of Orthalicoidea, for instance Orthalicus Beck, 1837; Bostryx Troschel, 1847 and Thaumastus, which have a central plate that is smaller than the marginal plates (Pena et al. 2011Pena MS, Salgado NC, Coelho AS (2011) Two new species of Thaumastus (Gastropoda: Pulmonata: Orthalicidae: Bulimulinae) from the state of Minas Gerais, Brazil. Zoologia (Curitiba) 28(4): 531-537. http://doi.org/10.1590/s1984-46702011000400016
http://doi.org/10.1590/s1984-46702011000...
, Miranda and Cuezzo 2014Miranda M, Cuezzo MG (2014) Taxonomic revision of the Bostryx stelzneri species complex, with description of a new species (Gastropoda: Orthalicoidea: Bulimulidae). American Malacological Bulletin 32: 74-93. http://doi.org/10.4003/006.032.0107
http://doi.org/10.4003/006.032.0107...
). When Lea (1839Lea I (1839) Description of the new freshwater and land shells. Transactions of the American Philosophical 6: 1-154.) described the jaw of Megaspira ruschenbergiana, he highlighted the presence of a central plate that is larger than the lateral plate. The radula of M. adenticulata sp. nov. has a rachidian tooth with a well-developed mesocone, and both the ecto and endocone (Figs 26-28) are smaller than the mesocone, which differ from some Thaumastus (Pena et al. 2011Pena MS, Salgado NC, Coelho AS (2011) Two new species of Thaumastus (Gastropoda: Pulmonata: Orthalicidae: Bulimulinae) from the state of Minas Gerais, Brazil. Zoologia (Curitiba) 28(4): 531-537. http://doi.org/10.1590/s1984-46702011000400016
http://doi.org/10.1590/s1984-46702011000...
), Bulimulus Leach, 1814 and Bostryx species (Miranda and Cuezzo 2014Miranda M, Cuezzo MG (2014) Taxonomic revision of the Bostryx stelzneri species complex, with description of a new species (Gastropoda: Orthalicoidea: Bulimulidae). American Malacological Bulletin 32: 74-93. http://doi.org/10.4003/006.032.0107
http://doi.org/10.4003/006.032.0107...
). In those, the rachidian tooth does not differ in morphology from the lateral teeth.
Megaspira adenticulata sp. nov. was found in only one location of Ilha Grande (Fig. 1). Despite exhaustive field survey carried out in different trails of the island, we were unable to find any live specimens or dry shells of either M. adenticulata sp. nov. or any other species of Megaspira. Ilha Grande is inserted within the Atlantic Forest Biome, which is one of the world’s biodiversity “hotspots” (Mittermeier et al. 2011Mittermeier RA, Turner WR, Larsen FW, Brooks TM Gascon C (2011) Global biodiversity conservation: the critical role of hotspots. In: Zachos FE, Habel JC (Eds) Biodiversity Hotspots. Springer Publishers, London, 3-22.). Ilha Grande is considered an area of endemism for certain vertebrate species (Rocha et al. 2018Rocha CFD, Telles BFS, Vrcibradic D, Nogueira-Costa P (2018). The Herpetofauna from Ilha Grande (Angra dos Reis, Rio de Janeiro, Brazil): updating species composition, richness, distribution and endemisms. Papeis Avulsos de Zoologia 58: e20185825. https://doi.org/10.11606/1807-0205/2018.58.25
https://doi.org/10.11606/1807-0205/2018....
) and is one of the largest continuous remnants of Atlantic Forest in the state of Rio de Janeiro state. Megaspira iheringi and M. elatior have been recorded from the state of Rio de Janeiro (Macaé as type locality and Rio de Janeiro municipality respectively) (Pilsbry 1925Pilsbry HA (1925) South American Land and Fresh Water Mollusks: Notes and Descriptions: V. Proceedings of the Academy of Natural Sciences 77: 311-315. https://www.jstor.org/stable/4063977
https://www.jstor.org/stable/4063977...
, Rangel et al. 2021Rangel FCS, Gomes SR, Canuto T, Rodrigues PS, Thiengo SC (2021) Diversity of non-marine gastropods of the Fiocruz Atlantic Forest Biological Station and adjacents urban áreas. Anais da Academia Brasileira de Ciências 93(2): e20190691. https://doi.org/10.1590/0001-3765202120190691
https://doi.org/10.1590/0001-37652021201...
). The most recent separation of Ilha Grande from the continent is estimated at seven thousand years and the island is located 110 km off the coast of Rio de Janeiro. It is possible that M. adenticulata sp. nov. has been present at the island since the separation. It is also possible that it was transported there by oceanic birds. We think that transport by birds is unlikely because the shells of these snails are large.
In the near future we expect to carry out a taxonomic review of the genus Megaspira in order to better resolve the status of the nominal species, and generate a phylogeny based on molecular data, to better understand the relationship between the species and to clarify the position of the genus within Orthalicoidea.
ACKNOWLEDGMENTS
Many thanks to Jessica Machado, for taking the photographs on the SEM of the material of M. adenticulata sp. nov. Also thanks to Thiago Vieira, for providing information on some species of Megaspira from Brazil. Thanks also to Joel C. Creed from improving the English and other comments. We appreciate and are thankful to the reviewers and editors for their suggestions and constructive comments, which have improved the quality of this manuscript. XCO is a visiting scholar in the Universidade Federal de Juiz de Fora. This research has been financially supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ APQ 1 E-26/110.362/2012, E-26/111.573/2013, E-26/010.0011639/2014) awarded to SBS, by Conselho Nacional de Desenvolvimento Científica e Tecnológico (CNPq/PIBIC scholarship from 2014-2019) awarded to VRD, and PR2/UERJ (Prociência Grant) awarded to SBS. We also thank CEADS/PR2/UERJ for facilities. The field work was carried out under licenses IBAMA/SISBIO 10812-1 and INEA/RJ 18/2007 to SBS.
- Albers JC, Martens EC von (1860) Die Heliceen nach naturlicher Verwandtschaft systematisch geordnet von Joh. Christ. Albers Zweite Ausgabe. Engelmann, Leipzig, 359 pp.
- Bastos M, Prado RM, Santiago AMA, Birman P, Catão H, Mendonça TM, Bakker A, Ferrarez A, Gilayn H, Mendonça M, Wiedemann M, Zanatta R, Pereira V, Cruz A, Roseiro T, Araújo A, Attianezi M (2009) Estrutura econômica e organização sociocultural e política. In: Bastos M, Callado C (Eds) O Ambiente da Ilha Grande. Universidade do Estado do Rio de Janeiro, Centro de Estudos e Desenvolvimento Sustentável, Rio de Janeiro, vol. 1, 215-250.
- Bouchet P, Rocroi JP, Frýda J, Hausdorf B, Ponder W, Valdés Á, Warén A (2005) Classification and nomenclator of gastropod families. Malacologia 47(1-2): 1-397.
- Bouchet P, Rocroi JP, Hausdorf B, Andrzej K, Yasunori K, Nützel A, Parkhaev P, Schrödl M, Strong E (2017). Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 61(1-2): 1-526.
- Breure B, Ablett J (2015) Annotated type catalogue of the Megaspiridae, Orthalicidae, and Simpulopsidae (Mollusca, Gastropoda, Orthalicoidea) in the Natural History Museum, London. Zookeys 470: 17-143. https://doi.org/10.3897/zookeys.470.8548
» https://doi.org/10.3897/zookeys.470.8548 - Breure B, Araujo R (2017) The Neotropical land snails (Mollusca, Gastropoda) collected by the Comisión Científica del Pacífico. PeerJ 5: e3065. https://doi.org/10.7717/peerj.3065
» https://doi.org/10.7717/peerj.3065 - Breure B, Groenenberg DSJ, Schilthuizen M (2010) New insights in the phylogenetic relations within the Orthalicoidea (Gastropoda, Stylommatophora) based on 28S sequence data. Basteria 74: 25-31.
- Breure B, Romero P (2012) Support and surprises: molecular phylogeny of the land snail superfamily Orthalicoidea using a three-locus gene analysis with a divergence time analysis and ancestral area reconstruction (Gastropoda: Stylommatophora). Archiv für Molluskenkunde 141: 1-20. https://doi.org/10.1127/arch.moll/1869-0963/141/001-020
» https://doi.org/10.1127/arch.moll/1869-0963/141/001-020 - Cuezzo MG (1997) Comparative anatomy of three species of Epiphragmophora Doering, 1874 (Pulmonata: Xanthonychidae) from Argentina. The Veliger 40: 216-227.
- Diver CA (1931) Method of determining the number of the whorls of a shell and its application to Cepaea hortensis and C. nemoralis Proceedings of the Malacological Society of London 19(5): 234-239.
- Jay JC (1836) A catalogue of recent shells with descriptions of new or rare species in the Collection of John C. Jay. New York, 82 pp. https://www.biodiversitylibrary.org/page/12172590
» https://www.biodiversitylibrary.org/page/12172590 - Lea I (1839) Description of the new freshwater and land shells. Transactions of the American Philosophical 6: 1-154.
- Miranda M, Cuezzo MG (2014) Taxonomic revision of the Bostryx stelzneri species complex, with description of a new species (Gastropoda: Orthalicoidea: Bulimulidae). American Malacological Bulletin 32: 74-93. http://doi.org/10.4003/006.032.0107
» http://doi.org/10.4003/006.032.0107 - Mittermeier RA, Turner WR, Larsen FW, Brooks TM Gascon C (2011) Global biodiversity conservation: the critical role of hotspots. In: Zachos FE, Habel JC (Eds) Biodiversity Hotspots. Springer Publishers, London, 3-22.
- Morretes LF (1949) Ensaio de catálogo dos moluscos do Brasil. Arquivos do Museu Paranaense 7: 1-216. http://doi.org/10.7213/estud.biol.612
» http://doi.org/10.7213/estud.biol.612 - Nordsieck H (1986) The system of the Stylommatophora (Gastropoda), with special regard to the systematic position of the Clausiliidae, importance of the shell and distribution. Archiv für Molluskenkunde 117: 93−116.
- Parodiz JJ (1951) Métodos de conquiliometria. Physis 38: 241-248.
- Pena MS, Salgado NC, Coelho AS (2011) Two new species of Thaumastus (Gastropoda: Pulmonata: Orthalicidae: Bulimulinae) from the state of Minas Gerais, Brazil. Zoologia (Curitiba) 28(4): 531-537. http://doi.org/10.1590/s1984-46702011000400016
» http://doi.org/10.1590/s1984-46702011000400016 - Pilsbry HA (1904) Family Megaspiridae. In: Tryon GW, Pislbry HA (Eds) Manual of Conchology. Academy of Natural Sciences, Philadelphia, vol. 16, 175-195.
- Pilsbry HA (1925) South American Land and Fresh Water Mollusks: Notes and Descriptions: V. Proceedings of the Academy of Natural Sciences 77: 311-315. https://www.jstor.org/stable/4063977
» https://www.jstor.org/stable/4063977 - Pilsbry HA, Vanatta EG (1899) Morphological and systematic notes on South America land snails: Achatinidae. Proceedings of the Academy of Natural Sciences of Philadelphia 51: 366-374. https://ur.booksc.eu/book/26042206/9bf827
» https://ur.booksc.eu/book/26042206/9bf827 - Ploeger S, Breure ASH (1977) A rapid procedure for preparation of radulae for routine research with the Scanning Electron Microscope. Basteria 4: 47-52.
- Rangel FCS, Gomes SR, Canuto T, Rodrigues PS, Thiengo SC (2021) Diversity of non-marine gastropods of the Fiocruz Atlantic Forest Biological Station and adjacents urban áreas. Anais da Academia Brasileira de Ciências 93(2): e20190691. https://doi.org/10.1590/0001-3765202120190691
» https://doi.org/10.1590/0001-3765202120190691 - Rehder HA (1945) A note on Megaspira elata Gould. The Nautilus 59: 67.
- Rocha CFD, Telles BFS, Vrcibradic D, Nogueira-Costa P (2018). The Herpetofauna from Ilha Grande (Angra dos Reis, Rio de Janeiro, Brazil): updating species composition, richness, distribution and endemisms. Papeis Avulsos de Zoologia 58: e20185825. https://doi.org/10.11606/1807-0205/2018.58.25
» https://doi.org/10.11606/1807-0205/2018.58.25 - Salgado NC, Coelho ACS (2003) Moluscos terrestres do Brasil (gastrópodes operculados ou não, exclusive Veronicellidae, Milacidade e Limacidae). Revista de Biología Tropical 51: 149-189.
- Salvador RB (2019) Brazilian, Uruguayan and Argentinian terrestrial gastropods in the collection of the Museum of New Zealand Te Papa Tongarewa. Tuhinga 30: 82-98.
- Simone LRL (2006) Land and freshwater molluscs of Brazil. EGB, Fapesp, São Paulo, 390 pp.
- Thiele JH (1931) Handbuch der systematischen weichtierkunde. Verlag Jena, Berlin, vol. 5, 778 pp.
- Thompson FG, Mahalcik EL (2005) Urocoptid landsnails of the genus Holospira from southern Mexico. Bulletin Florida Museum Natural History 43: 63-124.
ADDITIONAL NOTES
-
Zoobank register
http://zoobank.org/BA8B3A25-8620-46B1-BE21-6F9FC6764B37 -
How to cite this article
Daniel VR, Ovando XMC, Santos SB (2022) A new species of Megaspira (Stylommatophora: Megaspiridae) from Ilha Grande, Southeast Brazil. Zoologia (Curitiba) 39: e21022. https://doi.org/10.1590/S1984-4689.v39.e21022 -
Published by
Sociedade Brasileira de Zoologia at Scientific Electronic Library Online (https://www.scielo.br/zool)
Edited by
Editorial responsibility
Publication Dates
-
Publication in this collection
11 Apr 2022 -
Date of issue
2022
History
-
Received
30 Nov 2021 -
Accepted
18 Jan 2022