ABSTRACT
Myotis atacamensis (Lataste, 1892) was described based on three syntypes from San Pedro de Atacama, Chile. The type series is lost. The original description was based on few external and cranial characters, and the diagnosis became obsolete and useless considering the current diversity of South American Myotis. Based on 12 specimens of M. atacamensis from southern Peru and northern Chile, we provide a morphological comparison with its South American congeners, designate a neotype, and provide a new diagnosis.
KEY WORDS:
Atacama Myotis; desert habitat; Myotinae; neotype; taxonomy
INTRODUCTION
Myotis Kaup, 1829 is the most speciose genus of mammal, with more than 140 species distributed worldwide (Moratelli et al. 2019aMoratelli R, Burgin C, Cláudio VC, Novaes RLM, López-Baucells A, Haslauer R (2019a) Family Vespertilionidae (Vesper Bats). In: Wilson DE, Mittermeier AR (Eds) Handbook of the mammals of the world. Lynx Editions, Barcelona, vol. 9, 716-981., MDD 2021MDD (2021) Mammal Diversity Database (Version 1.4). Zenodo. http://doi.org/10.5281/zenodo.4139818
http://doi.org/10.5281/zenodo.4139818...
). Currently, 22 South American species of Myotis are recognized, and ca. 40% of them were described or recognized in the last decade, demonstrating a considerable recent increase in the taxonomic knowledge of this genus (Moratelli and Wilson 2011Moratelli R, Wilson DE (2011) A new species of Myotis Kaup, 1829 (Chiroptera, Vespertilionidae) from Ecuador. Mammalian Biology 76: 608−614. https://doi.org/10.1016/j.mambio.2010.10.003
https://doi.org/10.1016/j.mambio.2010.10...
, 2014Moratelli R, Wilson DE (2014) A new species of Myotis (Chiroptera, Vespertilionidae) from Bolivia. Journal of Mammalogy 95: 17−25. https://doi.org/10.1644/14-MAMM-149
https://doi.org/10.1644/14-MAMM-149...
, Moratelli et al. 2011Moratelli R, Peracchi AL, Dias D, Oliveira JA (2011) Geographic variation in South American populations of Myotis nigricans (Schinz, 1821) (Chiroptera, Vespertilionidae), with the description of two new species. Mammalin Biology 76: 592−607. https://doi.org/10.1016/j.mambio.2011.01.003
https://doi.org/10.1016/j.mambio.2011.01...
, 2013Moratelli R, Gardner AL, Oliveira JA, Wilson DE (2013) Review of Myotis (Chiroptera, Vespertilionidae) from northern South America, including description of a new species. American Museum Novitates 3780: 1−36. https://doi.org/10.1206/3780.2
https://doi.org/10.1206/3780.2...
, 2016Moratelli R, Wilson DE, Gardner AL, Fisher RD, Gutiérrez EE (2016) A new species of Myotis (Chiroptera, Vespertilionidae) from Suriname. Special Publications of the Museum of Texas Tech University 65: 49-66., 2017Moratelli R, Wilson DE, Novaes RLM, Helgen KM, Gutiérrez EE (2017) Caribbean Myotis (Chiroptera, Vespertilionidae), with description of a new species. Journal of Mammalogy 98: 994-1008. https://doi.org/10.1093/jmammal/gyx062
https://doi.org/10.1093/jmammal/gyx062...
, 2019bMoratelli R, Novaes RLM, Carrión-Bonilla C, Wilson DE (2019b) A new species of Myotis (Chiroptera, Vespertilionidae) from Peru. Special Publications of the Museum of Texas Tech University 71: 239-256., Carrión-Bonilla and Cook 2020Carrión-Bonilla CA, Cook JA (2020) A new bat species of the genus Myotis with comments on the phylogenetic placement of M. keaysi and M. pilosatibialis. Therya 11: 508-532. https://doi.org/10.12933/therya-20-999 ISSN 2007-3364
https://doi.org/10.12933/therya-20-999 I...
, Novaes et al. 2021aNovaes RLM, Cláudio VC, Larsen RJ, Wilson DE, Weksler M, Moratelli R (2021a) The taxonomic status of Myotis nesopolus larensis (Chiroptera, Vespertilionidae) and new insights on the diversity of Caribbean Myotis. ZooKeys 1015: 145-167. https://doi.org/10.3897/zookeys.1015.59248
https://doi.org/10.3897/zookeys.1015.592...
). However, many species described during the 19th and 20th centuries, such as Myotis atacamensis (Lataste, 1892Lataste F (1892) Etudes sur la faune Chilienne. II. Note sur les chauve-souris. Actes de la Société Scientifique du Chile 1: 70−91.), still have their taxonomic limits unresolved.
Myotis atacamensis occurs from southern Peru to central Chile, mostly in xeric habitats from lowlands to the western slope of the Andes (Ossa et al. 2017Ossa G, Vilches K, Faúndez PV (2017) First records of the endangered Atacama Myotis Myotis atacamensis (Chiroptera, Vespertilionidae), at high altitude in the Parinacota Province, northern Chile. Idesia 35: 75-78. https://doi.org/10.4067/S0718-34292017000400075
https://doi.org/10.4067/S0718-3429201700...
, Moratelli et al. 2019aMoratelli R, Burgin C, Cláudio VC, Novaes RLM, López-Baucells A, Haslauer R (2019a) Family Vespertilionidae (Vesper Bats). In: Wilson DE, Mittermeier AR (Eds) Handbook of the mammals of the world. Lynx Editions, Barcelona, vol. 9, 716-981., Rodríguez-San Pedro et al. 2020Rodríguez-San Pedro A, Allendes JL, Beltrán C, Mayorga M, Mella-Ávilla J (2020) Additions to the ecology, distribution, and natural history of the endangered bat Myotis atacamensis (Lataste, 1892) (Chiropter: Vespertilionidae) in Chile. Studies on Neotropical Fauna and Environment 56: 146-152. https://doi.org/10.1080/01650521.2020.1770452
https://doi.org/10.1080/01650521.2020.17...
). Due to a relatively small distribution dependent on specific habitats that are under severe disruption by agricultural activity and expansion of wind farms, this species is classified as endangered on the IUCN Red List (Vargas-Rodríguez et al. 2016Vargas-Rodríguez R, Peñaranda D, Ugarte J, Rodríguez-San Pedro A, Ossa G, Gatica-Castro A (2016) Myotis atacamensis. The IUCN Red List of Threatened Species, e.T14143A22050638. https://doi.org/10.2305/IUCN.UK.2016-1.RLTS.T14143A22050638.en
https://doi.org/10.2305/IUCN.UK.2016-1.R...
).
Myotis atacamensis was originally described as Vespertilio atacamensisLataste, 1892Lataste F (1892) Etudes sur la faune Chilienne. II. Note sur les chauve-souris. Actes de la Société Scientifique du Chile 1: 70−91. and transferred to Myotis by Trouessart (1904Trouessart EL (1904) Catalogus mammalium tam viventium quam fossilium. Quinquennale supplementum anno. R. Friedländer & Sohn, Berlin. https://doi.org/10.5962/bhl.title.61820
https://doi.org/10.5962/bhl.title.61820...
). Lataste’s description was based on three syntypes collected in February 1885 from San Pedro de Atacama, Antofagasta, Chile, and deposited at the Museo Nacional de Historia Natural (Santiago, Chile). The syntypes were a mounted specimen (number 277), a skull (number 1007), and a fluid preserved specimen (number 276). According to LaVal (1973LaVal RK (1973) A revision of the Neotropical bats of the genus Myotis. Natural History Museum of Los Angeles County, Science Bulletin 15: 1−54.), these specimens are lost or, more probable, were destroyed. Since LaVal’s publication, after almost 50 years, efforts to find the type were unsuccessful.
The South American Myotis is a taxonomic puzzle due to morphological similarity among species phylogenetically close. Consequently, there are cryptic species of Myotis and recent systematic reviews have demonstrated the existence of independent evolutionary lineages being treated as a unique species (Larsen et al. 2012Larsen RJ, Knapp MC, Genoways HH, Khan FAA, Larsen PA, Wilson DE, Baker RJ (2012) Genetic diversity of Neotropical Myotis (Chiroptera: Vespertilionidae) with an emphasis on South American species. PLoS One 7: e46578. https://doi.org/10.1371/journal.pone.0046578
https://doi.org/10.1371/journal.pone.004...
, Carrión-Bonilla and Cook 2020Carrión-Bonilla CA, Cook JA (2020) A new bat species of the genus Myotis with comments on the phylogenetic placement of M. keaysi and M. pilosatibialis. Therya 11: 508-532. https://doi.org/10.12933/therya-20-999 ISSN 2007-3364
https://doi.org/10.12933/therya-20-999 I...
, Novaes et al. 2021aNovaes RLM, Cláudio VC, Larsen RJ, Wilson DE, Weksler M, Moratelli R (2021a) The taxonomic status of Myotis nesopolus larensis (Chiroptera, Vespertilionidae) and new insights on the diversity of Caribbean Myotis. ZooKeys 1015: 145-167. https://doi.org/10.3897/zookeys.1015.59248
https://doi.org/10.3897/zookeys.1015.592...
, 2021bNovaes RLM, Wilson DE, Moratelli R (2021b) A new species of Myotis (Chiroptera, Vespertilionidae) from Uruguay. Vertebrate Zoology 71: 711-722. https://doi.org/10.3897/vz.71.e73146
https://doi.org/10.3897/vz.71.e73146...
, 2021cNovaes RLM, Cláudio VC, Carrión-Bonilla C, Abreu EF, Wilson DE, Maldonado JE, Weksler M (2021c) Variation in the Myotis keaysi complex (Chiroptera, Vespertilionidae), with description of a new species from Ecuador. Journal of Mammalogy: gyab139. https://doi.org/10.1093/jmammal/gyab139
https://doi.org/10.1093/jmammal/gyab139...
). Myotis atacamensis is at the center of this debate, where populations from northern and central western Peru misidentified as M. atacamensis were recently described as a new species (Moratelli et al. 2019bMoratelli R, Novaes RLM, Carrión-Bonilla C, Wilson DE (2019b) A new species of Myotis (Chiroptera, Vespertilionidae) from Peru. Special Publications of the Museum of Texas Tech University 71: 239-256.). Furthermore, there is evidence of the existence of unidentified lineages in northern and central Chile, probably occurring in sympatry with M. atacamensis (Novaes et al. 2018Novaes RLM, Wilson DE, Ruedi M, Moratelli R (2018) The taxonomic status of Myotis aelleni Baud, 1979 (Chiroptera, Vespertilionidae). Zootaxa 4446: 257-264. https://doi.org/10.11646/zootaxa.4446.2.5
https://doi.org/10.11646/zootaxa.4446.2....
). This scenario demonstrates the importance of name-bearing type to define the nominal taxon objectively, considering the nomenclatural dynamism that the Myotis is experiencing in the Neotropics.
In accordance with Article 75 of the International Code of Zoological Nomenclature (ICZN 1999ICZN (1999) International Code of Zoological Nomenclature. International Union of Biological Sciences, London, 4th ed., 306 p.), we designate a neotype for M. atacamensis and present a redescription of the species, including a morphological diagnosis based on current knowledge about the taxonomy of neotropical species of Myotis.
MATERIAL AND METHODS
The redescription is based on 12 specimens of M. atacamensis from southern Peru and northern Chile deposited in the following zoological collections: Field Museum of Natural History (FMNH, Chicago, USA), Museum of Vertebrate Zoology (MVZ, Berkeley, USA), Smithsonian National Museum of Natural History (USNM, Washington, D.C., USA), and Muséum d’Histoire Naturelle (MHNG, Geneva, Switzerland). The identification of specimens was made from morphological characters available in the literature (e.g., Lataste 1892Lataste F (1892) Etudes sur la faune Chilienne. II. Note sur les chauve-souris. Actes de la Société Scientifique du Chile 1: 70−91., Miller and Allen 1928Miller GS, Allen GM (1928) The American bats of the genera Myotis and Pizonyx. Bulletin of the United States National Museum 144: 1-218. https://doi.org/10.5479/si.03629236.144.i
https://doi.org/10.5479/si.03629236.144....
, LaVal 1973LaVal RK (1973) A revision of the Neotropical bats of the genus Myotis. Natural History Museum of Los Angeles County, Science Bulletin 15: 1−54., Moratelli et al. 2019bMoratelli R, Novaes RLM, Carrión-Bonilla C, Wilson DE (2019b) A new species of Myotis (Chiroptera, Vespertilionidae) from Peru. Special Publications of the Museum of Texas Tech University 71: 239-256.).
Comparative analyses were based on 368 specimens from seven South American Myotis species deposited in 12 zoological collections from Argentina, Switzerland, and United States (Appendix 1 APPENDIX Appendix 1. Specimens examined and localities of occurrence for Myotis species deposited in the following biological collections: Museo Argentino de Ciencias Naturales Bernardino Rivadavia (MACN, Buenos Aires, Argentina), Museo Miguel Lillo De Ciencias Naturales (CML, San Miguel de Tucumán, Argentina), Muséum d’Histoire Naturelle (MHNG, Geneva, Switzerland), American Museum of Natural History (AMNH, New York, USA), Carnegie Museum of Natural History (CM, Pittsburgh, USA), Field Museum of Natural History (FMNH, Chicago, USA), Museum of Natural History of the Kansas University (KU, Lawrence, USA), Museum of Natural Science, Louisiana State University (LSUMZ, Baton Rouge, USA), Museum of Vertebrate Zoology (MVZ, Berkeley, USA), Sam Noble Oklahoma Museum of Natural (OMNH, Norman, USA), Museum of Texas Tech University (TTU, Lubbock, TTU), and Smithsonian National Museum of Natural History (USNM, Washington D.C., USA). Myotis albescens (N = 109): Argentina: Tucumán, La Cocha, Dique San Ignacio (OMNH 18877); Tucumán, Leales, Dique San Ignacio (OMNH 18878); Santiago del Estero, Pellegrini, Santo Domingo (OMNH 23772, 23773, 23774). Peru: Ayacucho, Río Apurimac, Hacienda Luisiana (LSUMZ 16621, 16622); Ayacucho, San José, Río Santa Rosa (LSUMZ 16623-16625); Cusco, Quispicanchi (FMNH 68471, 68473-68478); Huánuco, Leonicio Prado, 1 km S of Tingo Maria (CM 98854); Huánuco, Río Huallaga, ca. 4 km NE of Tingo Maria (LSUMZ 14265); Loreto, Río Curaray (AMNH 71643); Loreto, Maynas, Puerto Indiana, Amazon River (AMNH 73235, 73237, 73239, 73242); Loreto, Maynas, Orosa, Amazon River (AMNH 74018, 74019, 74021); Loreto, San Jacinto (KU 158160); Madre de Dios, Manú, Maskoitania, 13.4 km NW Atalaya, left bank of Rio Alto Madre de Dios (FMNH 174919, 174921); Madre de Dios, Manú, Quebrada Aguas Calientes, left bank, Rio Alto Madre de Dios, 2.75 km E of Shintuya (FMNH 170275); Madre de Dios, Mouth of Rio La Torre, south bank of Rio Tambopata (LSUMZ 24562); Madre de Dios, Pakitza (USNM 564391, 564392, 566560-566563); Pasco, Oxapampa (AMNH 230746-230748, 230750-230757); Pasco, San Juan (USNM 364442-364480); Pasco, unknown localities (AMNH 213428, 213430); Ucayali, Balta, Río Curanja (LSUMZ 12272, 12274-12279); Ucayali, Coronel Portillo, Yarinacocha (LSUMZ 12253, FMNH 62178-62188). Myotis atacamensis (N = 12). Chile: Tarapacá, Minimini (USNM 391786 [neotype]; 19°10’S, 69°41’W); Tarapacá, Iquique, Los Canchones (FMNH 23618; MHNG 1748-43, 1748-44, 1748-45, 1748-46; 20°27’00”S, 69°37’00”W). Peru: Arequipa, Chucarapi, Tambo Valley (MVZ 116638; FMNH 50783, 51063; 17°04’S, 71°43’W); Arequipa, Arequipa, Patasagua, 3 km W of Tiabaya (FMNH 49790, 49791, 49792; 16°27’S, 71°35’W). Myotis bakeri (N = 4): Peru: Lambayeque, 12 km N of Olmos (LSUMZ 21306, 21307); Lima, 7 km SE of Chilca (MVZ 137906, 137907-holotype). Myotis dinellii (N = 73): Argentina: Buenos Aires, General Guido, Canal 2 y Ruta 2 (MACN 15740); Catamarca (CM 42934-42937); Catamarca, Trancas, 50 km NO Catamarca Ciudad, Las Juntas, Estancia de los Figueroa (OMNH 18977-18979); Catamarca, Capayán, 6 km NW Chumbicha, Balneario El Caolin (OMNH 19366, 19367); Catamarca, Capayán, 1 km NW of Balneario by road, Chumbicha (OMNH 23777-23782); Catamarca, Ambato, Estancia Narvaez, 5.5 km N Las Chacritas on Ruta Provincial No. 1 (OMNH 27931); Catamarca, Ambato, 21.4 km S Humaya (OMNH 36211); Catamarca, Tinogasta, 36.7 km W Fiambalá by road (OMNH 34545); Catamarca, Tinogasta, 57 km W Fiambalá by road (OMNH 34547); Catamarca, El Alto, Bella Vista (OMNH 36208); Catamarca, Capayán, 5.2 km NW Chumbicha (OMNH 36209, 36210); Catamarca, Andalgalá, Pucará (OMNH 36212, 36213); Córdoba (USNM 142560-142562); Córdoba, San Javier (TTU 32524, 32525, 32528, 32529); Córdoba, Calamuchita (TTU 64334); Córdoba, Cruz del Eje (TTU 64335, 64336); Córdoba, Río Cuarto (TTU 64337-64345, 66489, 66490, 66491); Córdoba, Pocho (TTU 66483-6488); Mendoza (MVZ 150861); La Pampa, Pampa Central, Caleu (MACN 49.163, 49.165); La Rioja, San Blás de Los Sauces, 4 km SE de San Blas (CML 5439, 5444, 5448, 5449); Mendoza, La Valle, Reserva Telteca (OMNH 23783); Salta, Guachipas, 25 km SE La Viña (OMNH 36214, 36217); Salta, San Carlos, Los Sauces (OMNH 36215); San Juan, Sarmiento, Pedernal (OMNH 23786); San Luis, Ayacucho, Quebrada de López, San Francisco del Monte de Oro (OMNH 23787); Tucumán (CM 42938-42942); Tucumán, La Cocha, Dique San Inacio (AMNH 256987); Tucumán, Tafí Viejo, 5 km SW Siambón (OMNH 36216). Myotis chiloensis (N = 48): Argentina: Chubut (MVZ 150842, 150847-150851, 150853-150858); Neuquen (MVZ 150862-150869, 150883, 150884, 150892, 150894-150898, 162181-162183); Rio Negro (MVZ 150902-150907, 150917, 150918, 150927, 150929, 152150, 152153, 154461). Chile: Valparaíso Region, Zapallar (USNM 391785); Valdivia, Rinihue (FMNH 23613, 23614); Chiloe Island, Chiloe (FMNH 24029 [neotype]). Myotis keaysi (N = 43): Argentina: Tucumán, Burruyacú (CML 6177, 7600, 8938, 9839, MACN 16795, 16855, 16857, OMNH 23499, 36207, TTU 32588). Peru: Amazonas, ca. 20 km of La Peca by trail (LSUMZ 21488); Ayacucho, Puncu, ca. 30 km NE of Tambo (15688); Cusco, Cordillera Vilcabamba (AMNH 214371); Cusco, Cordillera Vilcabamba, west side (AMNH 233850, 233851, 233853, 233854, 233857, 236134); Cusco, Hacienda Cadena (FMNH 78686); Huánuco, Bosque Cutirragra, S of Huaylaspampa (LSUMZ 17898); Huánuco, Trail to Hacienda Paty below Carpish Pass (LSUMZ 18434); Huánuco, 7 km NW of Carpish Pass by road (AMNH 216117); Junín, Chanchamayo (FMNH 65751); Lambayeque, 16 km N and 25 km E of Olmos (MVZ 135620, 135621); Pasco, Santa Cruz, ca. 9 km SE of Oxapampa (LSUMZ 25907); Piura, Batan, on Zapalache-Carmen trail (LSUMZ 26898); Piura, 15 km E of Canchaque by road (LSUMZ 19213); Puno, Inca Mines (AMNH 15814); Puno, Inca Mines (AMNH 15814 [holotype]), Puno, Oconeque, 10 miles N of Limbani (MVZ 116050). Myotis oxyotus (N = 19): Argentina: Tucumán, Tafí Viejo, 5 km SW Siambón (OMNH 36218). Peru: Ancash, 31 km E of Pariacoto by road (LSUMZ 22131); Cajamarca, Celendin, Hacienda Limón (FMNH 19969); Cusco, Iquente (USNM 195146); Cusco, Marcapata (FMNH 66375, 66376); Cusco, Santa Ana (USNM 194452, 194453, 195141, 195147, 195149); Cusco, 6 miles N of Paucartambo (MVZ 116008); Huancavelica, Rumicruz (AMNH 60598); Huánuco, Ambo (FMNH 24864-24866); Junín, Rio Palca, 15 Km W of San Ramon (USNM 507204); Lima, Bujama Baja, 95 km south of Lima by road (AMNH 216118); Lima, Huaros, Bosque de Zarate, San Bartolomé (FMNH 129208). Myotis riparius (N = 41): Argentina: Corrientes, Capital, Laguna Paivas, Barrio Los Lomas (CML 2994); Formosa, Rio Porteno, km 64, a 5 km al sur de Estancia Sta. Catalina (OMHN 18889); Formosa, Río Bermejo, 10 millas al S de Cólonia km 503 (CML 5412); Formosa, Parque Nacional Pilcomayo (MACN 20881, 20895); Formosa, Jecc. Cassinera, R. Teuco (MACN 20938); Jujuy, Santa Bárbara, Laguna “La Brea”, 25 km antes de Palma Sola, sobre Ruta 1 (OMNH 18890, 18891); Salta, San Martín, 6 km al W de Piquirenda Viejo (CML 3157); Tucumán, Burruyacú, Balneário Piedra Tendida, 6 km al O del El Cajón (CML 3155); Tucumán, Reserva Provincial Santa Ana (El Salton), Rio Chico (CML 3158). Peru: Ayacucho, San José, Río Santa Rosa (LSUMZ 16631); Cusco, Camisea, Armihuari (USNM 582875, 582876); Cusco, Camisea, San Martín-3 (USNM 582877, 582878); Cusco, Cordillera Vilcabamba (AMNH 233859, 233860); Cusco, Marcapata (FMNH 68479-68482); Cusco, Paucartambo, Consuelo (FMNH 174941); Cusco, Paucartambo, San Pedro (FMNH 172162); Cusco, Quispicanchi, Hacienda Cadena (FMNH 75150); Cusco, Ridge Camp (USNM 588040); Huánuco, Agua Caliente, Río Pachitea (FMNH 55400); Huánuco, Leoncio Prado (TTU 46348); Loreto, Río Curaray (AMNH 71645); Loreto, San Jacinto (KU 158162); Madre de Dios, Lago Sandoval, Río Madre de Dios (MVZ 157782); Madre de Dios, Manú, Maskoitania (FMNH 174933); Madre de Dios, Mouth of Rio La Torre, south bank of Rio Tambopata (LSUMZ 24561); Madre de Dios, Rio Tambopata, 30 km up from Mouth (USNM 530919); Pasco, Oxapampa, San Pablo (AMNH 230775-230777); Ucayali, Balta, Río Curanja (LSUMZ 12268-12271). ). Species selected for comparisons include those that occur in the western Andes and Southern Cone: M. albescens (É. Geoffroy, 1806), M. bakeri Moratelli, Novaes, Carrión-Bonilla & Wilson, 2019, M. chiloensis (Waterhouse, 1840), M. dinellii Thomas, 1902, M. keaysi J.A. Allen, 1914, M. oxyotus (Peters, 1866), and M. riparius Handley, 1960.
Qualitative morphological comparisons follow the external and cranial characters described by Moratelli et al. (2011Moratelli R, Peracchi AL, Dias D, Oliveira JA (2011) Geographic variation in South American populations of Myotis nigricans (Schinz, 1821) (Chiroptera, Vespertilionidae), with the description of two new species. Mammalin Biology 76: 592−607. https://doi.org/10.1016/j.mambio.2011.01.003
https://doi.org/10.1016/j.mambio.2011.01...
, 2013Moratelli R, Gardner AL, Oliveira JA, Wilson DE (2013) Review of Myotis (Chiroptera, Vespertilionidae) from northern South America, including description of a new species. American Museum Novitates 3780: 1−36. https://doi.org/10.1206/3780.2
https://doi.org/10.1206/3780.2...
) and Novaes et al. (2021aNovaes RLM, Cláudio VC, Larsen RJ, Wilson DE, Weksler M, Moratelli R (2021a) The taxonomic status of Myotis nesopolus larensis (Chiroptera, Vespertilionidae) and new insights on the diversity of Caribbean Myotis. ZooKeys 1015: 145-167. https://doi.org/10.3897/zookeys.1015.59248
https://doi.org/10.3897/zookeys.1015.592...
). External measurements, including the head-body length (HB), tail length (TL), hind foot length (HF), ear length (EL), and body mass (BM), were recorded from skin labels, and reported to the nearest millimeter and to the nearest gram. Cranial and other external dimensions were taken using digital calipers accurate to 0.01 mm, including: forearm length (FA), third metacarpal length (3MC), length of dorsal hair (LDF), length of ventral hair (LVF), greatest length of skull (GLS), condylocanine length (CCL), condylobasal length (CBL), condylo-incisive length (CIL), basal length (BAL), zygomatic breadth (ZB), mastoid breadth (MAB), braincase breadth (BCB), interorbital breadth (IOB), postorbital breadth (POB), breadth across canines (BAC), breadth across molars (BAM), maxillary toothrow length (MTL), length of the upper molars (M1-3), mandibular length (MAL), and mandibular toothrow length (MAN). These measurements are described in detail by Novaes et al. (2021bNovaes RLM, Wilson DE, Moratelli R (2021b) A new species of Myotis (Chiroptera, Vespertilionidae) from Uruguay. Vertebrate Zoology 71: 711-722. https://doi.org/10.3897/vz.71.e73146
https://doi.org/10.3897/vz.71.e73146...
). Capitalized color nomenclature follows Ridgway (1912Ridgway R (1912) Color standards and color nomenclature. Privately published, Washington, DC, 110 p. https://doi.org/10.5962/bhl.title.144788
https://doi.org/10.5962/bhl.title.144788...
).
The designation of the neotype was made considering specimen preservation, biogeographic context, and the access to museum in a global context, following all directives from Article 75 of ICZN (1999).
TAXONONY
Myotis atacamensis ( Lataste, 1892Lataste F (1892) Etudes sur la faune Chilienne. II. Note sur les chauve-souris. Actes de la Société Scientifique du Chile 1: 70−91. )
Atacama Myotis - vernacular name.
Vespertilio atacamensisLataste, 1892Lataste F (1892) Etudes sur la faune Chilienne. II. Note sur les chauve-souris. Actes de la Société Scientifique du Chile 1: 70−91.: 79.
Myotis chiloensis atacamensis: Miller and Allen, 1928Miller GS, Allen GM (1928) The American bats of the genera Myotis and Pizonyx. Bulletin of the United States National Museum 144: 1-218. https://doi.org/10.5479/si.03629236.144.i
https://doi.org/10.5479/si.03629236.144....
: 192.
Myotis nigricans nicholsoniSanborn, 1941Sanborn CC (1941) Descriptions and records of Neotropical bats. Field Museum of Natural History, Zoology Series 27: 371-387.: 382.
Neotype. Adult female (USNM 391786; Figs 1-5) collected by W. Mann and S. Mann on January 1944. The skin and skeleton are complete, and skull is partially damaged but in good condition. The neotype is deposited in the National Museum of Natural History, Washington, D.C., USA.
New type-locality. Near Minimini, Tarapacá, Chile (19°10’S, 69°41’W; elevation 1,800 m), inside Atacama desert.
Diagnosis. Small size (FA 30.6-34.1 mm; GLS 12.6-13.6); dorsal fur long (7-9 mm), silky, and tricolored, with dark-brown bases (near Bone Brown), pale yellowish middle portions (near Pale Olive-Buff), and yellowish-brown tips (near Light Ochraceous Buff); dorsal surface of the uropatagium covered by dense fur extending just beyond the knees; presence of a fringe of sparse hairs on the distal border of the uropatagium; plagiopatagium connected to the feet by a broad band of membrane. Sagittal crest usually absent; elongated and narrow skull; braincase remarkably inflated and high in profile; braincase roof formed by the parietal bone is straight; forehead steeply sloping in lateral view; narrow and short rostrum; posterior region of the braincase rounded and quite projected beyond the limit of the occipital condyles; mastoid processes narrow.
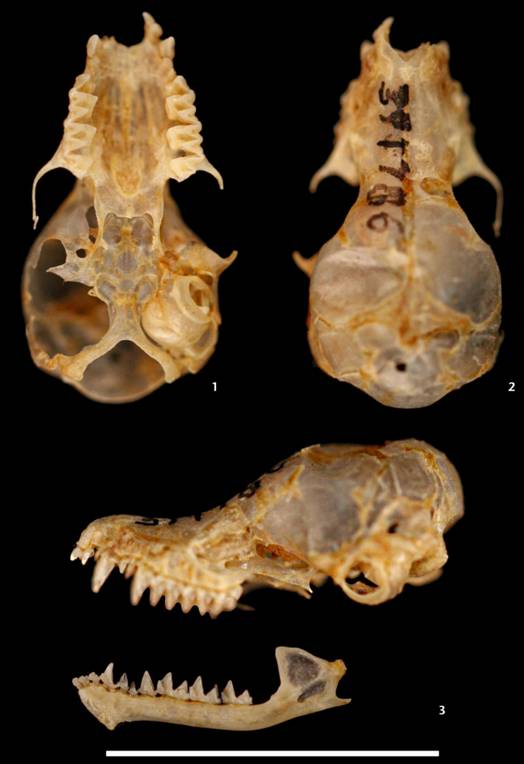
Description and comparisons. Dental formula is I 2/3, C 1/1, PM 3/3, M 3/3 (2x) = 38, and the teeth are small. Skull small (measurements in Table 1); forehead steeply sloping with inflated braincase; braincase roof is straight; mastoid processes narrow and poorly developed; rostrum narrow and comparatively short; the sagittal crest and lambdoidal crests are usually absent; and the occipital region is rounded and conspicuously projected beyond the posterior surfaces of the occipital condyles (Figs 1-3). The second upper premolar (P3) is a little smaller than the first upper premolar (P2), aligned in the toothrow and visible labially in all individuals (Figs 1-3).
Ventral (1), dorsal (2), and lateral (3) views of the skull, and lateral view of the mandible of the neotype of Myotis atacamensis (USNM 391786) from Atacama Desert, Tarapacá, Chile. Scale bar = 10 mm. Photos by Melissa Hawkins.5
Ears are moderate in size, but not reaching the nostrils when extended forward. The tragus is elongated, with a broad base and a narrower terminal half; the front edge is almost straight and the tip rounded. Membranes and ears are medium-brown. Plagiopatagium is connected to the feet at the level of the toes by a broad band of membrane; a fringe of scattered hairs on the distal border of the uropatagium is present and visible only under magnification (although less evident than in M. albescens). Dorsal surface of the uropatagium barely furred, with hairs not extending beyond to the knees. Silky and long fur; dorsal hairs tricolored, with dark-brown bases (2/5 of total hair length), pale yellowish middle portions (2/5 of total hair length), and yellowish-brown tips (1/5 of total hair length), however, the contrast between tip and middle portion of the hair is quite subtle (Figs 6, 7). Ventral fur strongly bicolored, with Bone Brown bases (2/3 of total hair length) and Pallid Brownish Drab tips (1/3 of total hair length). The whitish-gray venter contrasts with the yellowish-brown dorsum (Figs 6, 7).
Dorsal (4) and ventral (5) views of the skin of the neotype of Myotis atacamensis (USNM 391786) from Atacama Desert, Tarapacá, Chile. Photos by Melissa Hawkins.
Details of tricolored dorsal fur (6) and bicolored ventral fur (7) of Myotis atacamensis (MVZ 116638).
Myotis atacamensis differs from all South American congeners by the tricolored dorsal fur. In addition, considering either the assemblage of Myotis that occurs on the west side of the Andes (M. albescens, M. bakeri, M. chiloensis, M. diminutus, M. oxyotus, and M. riparius) or the taxa that occur in mountainous or open environments of the Southern Cone (M. albescens, M. dinellii, M. keaysi, M. oxyotus, and M. riparius), it can also be distinguished from all by the set of diagnostic traits reported above. Among them, M. atacamensis is externally closer to M. bakeri, from which it can be distinguished by slightly lighter dorsal fur (near Light Ochraceous Buff on the tips in M. atacamensis and near Buckthorn Brown on the tips in M. bakeri); presence of a fringe of hairs along the trailing edge of uropatagium (absent in M. bakeri); and narrower skull (e.g., POB < 3.5 in M. atacamensis, POB ≥ 3.5 in M. bakeri; see Moratelli et al. 2019bMoratelli R, Novaes RLM, Carrión-Bonilla C, Wilson DE (2019b) A new species of Myotis (Chiroptera, Vespertilionidae) from Peru. Special Publications of the Museum of Texas Tech University 71: 239-256.).
From M. albescens, M. atacamensis can be distinguished by the paler dorsal fur color (dorsal fur medium to dark brown on the bases [4/5 of the total fur length] and yellowish on the tips [1/5] in M. albescens, conveying a brownish general appearance to the dorsum; in contrast with bases and tips strongly contrasting and general yellowish appearance in M. atacamensis). Additionally, M. albescens have the throat yellowish, grading to whitish towards the abdomen and sides of the body, and a more globular braincase (see Moratelli and Oliveira 2011Moratelli R, Oliveira JA (2011) Morphometric and morphological variation in South American populations of Myotis albescens (Chiroptera: Vespertilionidae). Zoologia (Curitiba) 28: 789-802. https://doi.org/10.1590/S1984-46702011000600013
https://doi.org/10.1590/S1984-4670201100...
); whereas in M. atacamensis the entire venter, from the throat to the abdomen, is whitish-gray and the skull is narrower and the braincase is less globular.
Myotis atacamensis can be distinguished from M. diminutus and M. oxyotus by the dorsal fur paler and with strong contrast between bases and tips, and the ventral fur whitish-gray; whereas the dorsal fur is darker and slightly bicolored, and the ventral fur is yellowish in M. diminutus and M. oxyotus. Myotis atacamensis is smaller than M. oxyotus in virtually all external and cranial measurements (e.g., GLS 12.6-13.6 mm in M. atacamensis, 14.1-15.0 mm in M. oxyotus; POB 3.0-3.2 mm in M. atacamensis, 3.3-3.9 mm in M. oxyotus; MAL 8.6-9.3 mm in M. atacamensis, 9.9-11.2 mm in M. oxyotus), and tends to be smaller than M. diminutus in forearm length (FA 30.6-34.1 mm in M. atacamensis; 33.3-33.4 mm in M. diminutus), although there is an overlap with larger individuals of M. atacamensis, both in external and in cranial measurements (see Moratelli and Wilson 2011Moratelli R, Wilson DE (2011) A new species of Myotis Kaup, 1829 (Chiroptera, Vespertilionidae) from Ecuador. Mammalian Biology 76: 608−614. https://doi.org/10.1016/j.mambio.2010.10.003
https://doi.org/10.1016/j.mambio.2010.10...
, 2015Moratelli R, Wilson DE (2015) A second record of Myotis diminutus (Chiroptera: Vespertilionidae): its bearing on the taxonomy of the species and discrimination from M. nigricans. Proceedings of the Biological Society of Washington 127: 533−542. https://doi.org/10.2988/0006-324X-127.4.533
https://doi.org/10.2988/0006-324X-127.4....
, Moratelli et al. 2013Moratelli R, Gardner AL, Oliveira JA, Wilson DE (2013) Review of Myotis (Chiroptera, Vespertilionidae) from northern South America, including description of a new species. American Museum Novitates 3780: 1−36. https://doi.org/10.1206/3780.2
https://doi.org/10.1206/3780.2...
).
From M. chiloensis, it can be distinguished by the silky, brighter, and longer fur. Myotis atacamensis has dorsal fur with strong contrast between bases (blackish) and tips (yellowish), and whitish-gray venter; whereas in M. chiloensis, the fur is woolly and shorter; dorsal pelage with weak contrast between bases (dark brown) and tips (reddish-brown) and yellowish-brown venter (see Novaes et al. 2018Novaes RLM, Wilson DE, Ruedi M, Moratelli R (2018) The taxonomic status of Myotis aelleni Baud, 1979 (Chiroptera, Vespertilionidae). Zootaxa 4446: 257-264. https://doi.org/10.11646/zootaxa.4446.2.5
https://doi.org/10.11646/zootaxa.4446.2....
). Beyond the very distinct dorsal and ventral fur colors, M. atacamensis can be distinguished from M. chiloensis by generally smaller size (FA 30.6-34.1 mm in M. atacamensis, 35.5-41.2 mm in M. chiloensis; GLS 12.6-13.6 mm in M. atacamensis, 13.8-15.3 mm in M. chiloensis).
In relation to M. dinellii, it can be distinguished by lighter dorsal fur (near Light Ochraceous Buff on the tips in M. atacamensis and from Buckthorn Brown to Dresden Brown on the tips in M. dinellii) and membranes (almost blackish in M. dinellii); shorter ears; general smaller size (FA 30.6-34.1 mm in M. atacamensis, 34.4-40.6 mm in M. dinellii; GLS 12.6-13.6 mm in M. atacamensis, 13.9-15.2 in M. dinellii); skull higher in lateral view, braincase more globose, and frontals conspicuously steeply sloping.
Myotis atacamensis can be distinguished from M. keaysi and M. riparius by its general smaller size (FA 30.6-34.1 mm in M. atacamensis, 38.5-43.4 mm in M. keaysi, 36.0-38.4 mm in M. riparius; GLS 12.6-13.6 mm in M. atacamensis, 13.9-14.7 mm in M. keaysi, 13.3-14.4 mm in M. riparius), paler, longer, and silky fur (shorter, woolly, and either brownish or reddish-brown in M. keaysi and M. riparius). In relation to skull morphology, it differs from M. keaysi and M. riparius by sagittal crest absent, mastoid process narrower and poorly developed, and shorter rostrum.
In addition to these characters aforementioned, M. atacamensis differs from all its South American congeners, excepts M. albescens, M. levis (I. Geoffroy, 1824), and M. dinellii, by the presence of a fringe of scattered hair on the distal edge of the uropatagium.
Distribution. It is known from western Peru to central Chile (Fig. 8). Myotis atacamensis is associated with arid and semiarid formations in elevations from sea level to 3,475 m, where it inhabits xeric vegetational formations, as absolute deserts, desert scrublands, and sclerophyllous forest (Rodríguez-San Pedro et al. 2014Rodríguez-San Pedro A, Allendes JL, Castillo MLC, Peñaranda DA, Peña-Gómez FT (2014) Distribution externsion and new record of Myotis atacamensis (Lataste, 1892) (Chiroptera: Vespertilionidae) in Chile. Check List 10: 1164-1166. http://doi.org/10.15560/10.5.1164
http://doi.org/10.15560/10.5.1164...
, 2015Rodríguez-San Pedro A, Peñaranda DA, Allendes JL, Castillo MLC (2015) Update distribution of Myotis atacamensis (Chiroptera: Vespertilionidae): southernmost record and description of its echolocation calls. Chiroptera Neotropical 21: 1342-1346., 2020Rodríguez-San Pedro A, Allendes JL, Beltrán C, Mayorga M, Mella-Ávilla J (2020) Additions to the ecology, distribution, and natural history of the endangered bat Myotis atacamensis (Lataste, 1892) (Chiropter: Vespertilionidae) in Chile. Studies on Neotropical Fauna and Environment 56: 146-152. https://doi.org/10.1080/01650521.2020.1770452
https://doi.org/10.1080/01650521.2020.17...
, Moratelli et al. 2019aMoratelli R, Burgin C, Cláudio VC, Novaes RLM, López-Baucells A, Haslauer R (2019a) Family Vespertilionidae (Vesper Bats). In: Wilson DE, Mittermeier AR (Eds) Handbook of the mammals of the world. Lynx Editions, Barcelona, vol. 9, 716-981., 2019bMoratelli R, Novaes RLM, Carrión-Bonilla C, Wilson DE (2019b) A new species of Myotis (Chiroptera, Vespertilionidae) from Peru. Special Publications of the Museum of Texas Tech University 71: 239-256.).
Occurrence of Myotis atacamensis, including the new type-locality (red star); records by museum specimens (red circles); and records obtained from the literature (yellow circles; Rodríguez-San Pedro et al. 2014Rodríguez-San Pedro A, Allendes JL, Castillo MLC, Peñaranda DA, Peña-Gómez FT (2014) Distribution externsion and new record of Myotis atacamensis (Lataste, 1892) (Chiroptera: Vespertilionidae) in Chile. Check List 10: 1164-1166. http://doi.org/10.15560/10.5.1164
http://doi.org/10.15560/10.5.1164... , 2015Rodríguez-San Pedro A, Peñaranda DA, Allendes JL, Castillo MLC (2015) Update distribution of Myotis atacamensis (Chiroptera: Vespertilionidae): southernmost record and description of its echolocation calls. Chiroptera Neotropical 21: 1342-1346., 2020Rodríguez-San Pedro A, Allendes JL, Beltrán C, Mayorga M, Mella-Ávilla J (2020) Additions to the ecology, distribution, and natural history of the endangered bat Myotis atacamensis (Lataste, 1892) (Chiropter: Vespertilionidae) in Chile. Studies on Neotropical Fauna and Environment 56: 146-152. https://doi.org/10.1080/01650521.2020.1770452
https://doi.org/10.1080/01650521.2020.17... ).
DISCUSSION
Myotis atacamensis was described based on few external and cranial characters (Lataste 1892Lataste F (1892) Etudes sur la faune Chilienne. II. Note sur les chauve-souris. Actes de la Société Scientifique du Chile 1: 70−91.) and its original diagnosis became obsolete and useless considering the current diversity of South American Myotis. However, the information available in the original description, as well as the posterior refinements in the morphological description (e.g., Miller and Allen 1928Miller GS, Allen GM (1928) The American bats of the genera Myotis and Pizonyx. Bulletin of the United States National Museum 144: 1-218. https://doi.org/10.5479/si.03629236.144.i
https://doi.org/10.5479/si.03629236.144....
, LaVal 1973LaVal RK (1973) A revision of the Neotropical bats of the genus Myotis. Natural History Museum of Los Angeles County, Science Bulletin 15: 1−54., Moratelli et al. 2019bMoratelli R, Novaes RLM, Carrión-Bonilla C, Wilson DE (2019b) A new species of Myotis (Chiroptera, Vespertilionidae) from Peru. Special Publications of the Museum of Texas Tech University 71: 239-256.), are sufficient for the identification of the specimens. Based on few specimens, Miller and Allen (1928Miller GS, Allen GM (1928) The American bats of the genera Myotis and Pizonyx. Bulletin of the United States National Museum 144: 1-218. https://doi.org/10.5479/si.03629236.144.i
https://doi.org/10.5479/si.03629236.144....
) considered M. atacamensis as a subspecies of M. chiloensis. This arrangement was followed by Osgood (1943Osgood WH (1943) The mammals of Chile. Field Museum of Natural History, Zoological Series 30: 1-268. https://doi.org/10.5962/bhl.title.3842
https://doi.org/10.5962/bhl.title.3842...
) when analyzing pale specimens from Coquimbo and Tarapaca, Chile, considering a wide overlap in size between these two taxa. However, according to LaVal (1973LaVal RK (1973) A revision of the Neotropical bats of the genus Myotis. Natural History Museum of Los Angeles County, Science Bulletin 15: 1−54.), the specimen from Coquimbo analyzed by Osgood is not M. atacamensis, but its identity has not yet been determined. Based on morphological comparisons from a large number of specimens examined, LaVal (1973LaVal RK (1973) A revision of the Neotropical bats of the genus Myotis. Natural History Museum of Los Angeles County, Science Bulletin 15: 1−54.) recognized M. atacamensis as a species distinct from M. chiloensis and provided a more detailed morphological description for the species.
LaVal (1973LaVal RK (1973) A revision of the Neotropical bats of the genus Myotis. Natural History Museum of Los Angeles County, Science Bulletin 15: 1−54.) indicated that the distribution of M. atacamensis extends from central Peru (near Lima) to northern Chile, and identified variation in size and shape of skull among M. atacamensis from central and southern Peru. Moratelli et al. (2019bMoratelli R, Novaes RLM, Carrión-Bonilla C, Wilson DE (2019b) A new species of Myotis (Chiroptera, Vespertilionidae) from Peru. Special Publications of the Museum of Texas Tech University 71: 239-256.) also found morphological differences between the northernmost forms from Peru and those in southern Peru and Chile, and recognized the northern population as a distinct species, described as M. bakeri.
It is possible that there is still a hidden diversity of Myotis in the lowland areas of central Chile. Novaes et al. (2018Novaes RLM, Wilson DE, Ruedi M, Moratelli R (2018) The taxonomic status of Myotis aelleni Baud, 1979 (Chiroptera, Vespertilionidae). Zootaxa 4446: 257-264. https://doi.org/10.11646/zootaxa.4446.2.5
https://doi.org/10.11646/zootaxa.4446.2....
) demonstrated that individuals originally identified as M. chiloensis from Coquimbo and Limache do not fit morphometrically within populations of M. chiloensis from Argentina and southern Chile. These specimens were examined by us and, although they have the pale venter, cannot be identified as M. atacamensis due to the larger external and cranial size and distinct skull shape. Therefore, a taxonomic review of the Myotis from Chile is still needed.
The tricolored dorsal pelage of M. atacamensis is unique among its South American congeners, but may be less evident in specimens preserved in alcohol. It is possible that this may have driven the perception that the fur of this species is bicolored, as appears in studies by LaVal (1973LaVal RK (1973) A revision of the Neotropical bats of the genus Myotis. Natural History Museum of Los Angeles County, Science Bulletin 15: 1−54.) and Moratelli et al. (2019aMoratelli R, Burgin C, Cláudio VC, Novaes RLM, López-Baucells A, Haslauer R (2019a) Family Vespertilionidae (Vesper Bats). In: Wilson DE, Mittermeier AR (Eds) Handbook of the mammals of the world. Lynx Editions, Barcelona, vol. 9, 716-981., 2019bMoratelli R, Novaes RLM, Carrión-Bonilla C, Wilson DE (2019b) A new species of Myotis (Chiroptera, Vespertilionidae) from Peru. Special Publications of the Museum of Texas Tech University 71: 239-256.). However, the tricolored dorsal coloration pattern is an important diagnostic character for M. atacamensis and should be considered when examining specimens.
ACKNOWLEDGMENTS
We are grateful to the curators of the collections referred in the text for providing access to the specimens; to Melissa T. Hawkins (Smithsonian Institution) by photographs of the specimen used in the designation of the neotype; and to three anonymous referees for the valuable suggestions and corrections that considerably improved the original version of the manuscript. RLMN receives a financial support from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ, process E-26/204.243/2021). RM receives financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, process 313963/2018-5) and FAPERJ (process E-26/200.967/2021).
LITERATURE CITED
- Carrión-Bonilla CA, Cook JA (2020) A new bat species of the genus Myotis with comments on the phylogenetic placement of M. keaysi and M. pilosatibialis Therya 11: 508-532. https://doi.org/10.12933/therya-20-999 ISSN 2007-3364
» https://doi.org/10.12933/therya-20-999 ISSN 2007-3364 - ICZN (1999) International Code of Zoological Nomenclature. International Union of Biological Sciences, London, 4th ed., 306 p.
- Larsen RJ, Knapp MC, Genoways HH, Khan FAA, Larsen PA, Wilson DE, Baker RJ (2012) Genetic diversity of Neotropical Myotis (Chiroptera: Vespertilionidae) with an emphasis on South American species. PLoS One 7: e46578. https://doi.org/10.1371/journal.pone.0046578
» https://doi.org/10.1371/journal.pone.0046578 - Lataste F (1892) Etudes sur la faune Chilienne. II. Note sur les chauve-souris. Actes de la Société Scientifique du Chile 1: 70−91.
- LaVal RK (1973) A revision of the Neotropical bats of the genus Myotis Natural History Museum of Los Angeles County, Science Bulletin 15: 1−54.
- MDD (2021) Mammal Diversity Database (Version 1.4). Zenodo. http://doi.org/10.5281/zenodo.4139818
» http://doi.org/10.5281/zenodo.4139818 - Miller GS, Allen GM (1928) The American bats of the genera Myotis and Pizonyx Bulletin of the United States National Museum 144: 1-218. https://doi.org/10.5479/si.03629236.144.i
» https://doi.org/10.5479/si.03629236.144.i - Moratelli R, Oliveira JA (2011) Morphometric and morphological variation in South American populations of Myotis albescens (Chiroptera: Vespertilionidae). Zoologia (Curitiba) 28: 789-802. https://doi.org/10.1590/S1984-46702011000600013
» https://doi.org/10.1590/S1984-46702011000600013 - Moratelli R, Wilson DE (2011) A new species of Myotis Kaup, 1829 (Chiroptera, Vespertilionidae) from Ecuador. Mammalian Biology 76: 608−614. https://doi.org/10.1016/j.mambio.2010.10.003
» https://doi.org/10.1016/j.mambio.2010.10.003 - Moratelli R, Wilson DE (2014) A new species of Myotis (Chiroptera, Vespertilionidae) from Bolivia. Journal of Mammalogy 95: 17−25. https://doi.org/10.1644/14-MAMM-149
» https://doi.org/10.1644/14-MAMM-149 - Moratelli R, Wilson DE (2015) A second record of Myotis diminutus (Chiroptera: Vespertilionidae): its bearing on the taxonomy of the species and discrimination from M. nigricans Proceedings of the Biological Society of Washington 127: 533−542. https://doi.org/10.2988/0006-324X-127.4.533
» https://doi.org/10.2988/0006-324X-127.4.533 - Moratelli R, Peracchi AL, Dias D, Oliveira JA (2011) Geographic variation in South American populations of Myotis nigricans (Schinz, 1821) (Chiroptera, Vespertilionidae), with the description of two new species. Mammalin Biology 76: 592−607. https://doi.org/10.1016/j.mambio.2011.01.003
» https://doi.org/10.1016/j.mambio.2011.01.003 - Moratelli R, Gardner AL, Oliveira JA, Wilson DE (2013) Review of Myotis (Chiroptera, Vespertilionidae) from northern South America, including description of a new species. American Museum Novitates 3780: 1−36. https://doi.org/10.1206/3780.2
» https://doi.org/10.1206/3780.2 - Moratelli R, Wilson DE, Gardner AL, Fisher RD, Gutiérrez EE (2016) A new species of Myotis (Chiroptera, Vespertilionidae) from Suriname. Special Publications of the Museum of Texas Tech University 65: 49-66.
- Moratelli R, Wilson DE, Novaes RLM, Helgen KM, Gutiérrez EE (2017) Caribbean Myotis (Chiroptera, Vespertilionidae), with description of a new species. Journal of Mammalogy 98: 994-1008. https://doi.org/10.1093/jmammal/gyx062
» https://doi.org/10.1093/jmammal/gyx062 - Moratelli R, Burgin C, Cláudio VC, Novaes RLM, López-Baucells A, Haslauer R (2019a) Family Vespertilionidae (Vesper Bats). In: Wilson DE, Mittermeier AR (Eds) Handbook of the mammals of the world. Lynx Editions, Barcelona, vol. 9, 716-981.
- Moratelli R, Novaes RLM, Carrión-Bonilla C, Wilson DE (2019b) A new species of Myotis (Chiroptera, Vespertilionidae) from Peru. Special Publications of the Museum of Texas Tech University 71: 239-256.
- Novaes RLM, Wilson DE, Ruedi M, Moratelli R (2018) The taxonomic status of Myotis aelleni Baud, 1979 (Chiroptera, Vespertilionidae). Zootaxa 4446: 257-264. https://doi.org/10.11646/zootaxa.4446.2.5
» https://doi.org/10.11646/zootaxa.4446.2.5 - Novaes RLM, Cláudio VC, Larsen RJ, Wilson DE, Weksler M, Moratelli R (2021a) The taxonomic status of Myotis nesopolus larensis (Chiroptera, Vespertilionidae) and new insights on the diversity of Caribbean Myotis ZooKeys 1015: 145-167. https://doi.org/10.3897/zookeys.1015.59248
» https://doi.org/10.3897/zookeys.1015.59248 - Novaes RLM, Wilson DE, Moratelli R (2021b) A new species of Myotis (Chiroptera, Vespertilionidae) from Uruguay. Vertebrate Zoology 71: 711-722. https://doi.org/10.3897/vz.71.e73146
» https://doi.org/10.3897/vz.71.e73146 - Novaes RLM, Cláudio VC, Carrión-Bonilla C, Abreu EF, Wilson DE, Maldonado JE, Weksler M (2021c) Variation in the Myotis keaysi complex (Chiroptera, Vespertilionidae), with description of a new species from Ecuador. Journal of Mammalogy: gyab139. https://doi.org/10.1093/jmammal/gyab139
» https://doi.org/10.1093/jmammal/gyab139 - Osgood WH (1943) The mammals of Chile. Field Museum of Natural History, Zoological Series 30: 1-268. https://doi.org/10.5962/bhl.title.3842
» https://doi.org/10.5962/bhl.title.3842 - Ossa G, Vilches K, Faúndez PV (2017) First records of the endangered Atacama Myotis Myotis atacamensis (Chiroptera, Vespertilionidae), at high altitude in the Parinacota Province, northern Chile. Idesia 35: 75-78. https://doi.org/10.4067/S0718-34292017000400075
» https://doi.org/10.4067/S0718-34292017000400075 - Ridgway R (1912) Color standards and color nomenclature. Privately published, Washington, DC, 110 p. https://doi.org/10.5962/bhl.title.144788
» https://doi.org/10.5962/bhl.title.144788 - Rodríguez-San Pedro A, Allendes JL, Castillo MLC, Peñaranda DA, Peña-Gómez FT (2014) Distribution externsion and new record of Myotis atacamensis (Lataste, 1892) (Chiroptera: Vespertilionidae) in Chile. Check List 10: 1164-1166. http://doi.org/10.15560/10.5.1164
» http://doi.org/10.15560/10.5.1164 - Rodríguez-San Pedro A, Peñaranda DA, Allendes JL, Castillo MLC (2015) Update distribution of Myotis atacamensis (Chiroptera: Vespertilionidae): southernmost record and description of its echolocation calls. Chiroptera Neotropical 21: 1342-1346.
- Rodríguez-San Pedro A, Allendes JL, Beltrán C, Mayorga M, Mella-Ávilla J (2020) Additions to the ecology, distribution, and natural history of the endangered bat Myotis atacamensis (Lataste, 1892) (Chiropter: Vespertilionidae) in Chile. Studies on Neotropical Fauna and Environment 56: 146-152. https://doi.org/10.1080/01650521.2020.1770452
» https://doi.org/10.1080/01650521.2020.1770452 - Sanborn CC (1941) Descriptions and records of Neotropical bats. Field Museum of Natural History, Zoology Series 27: 371-387.
- Trouessart EL (1904) Catalogus mammalium tam viventium quam fossilium. Quinquennale supplementum anno. R. Friedländer & Sohn, Berlin. https://doi.org/10.5962/bhl.title.61820
» https://doi.org/10.5962/bhl.title.61820 - Vargas-Rodríguez R, Peñaranda D, Ugarte J, Rodríguez-San Pedro A, Ossa G, Gatica-Castro A (2016) Myotis atacamensis The IUCN Red List of Threatened Species, e.T14143A22050638. https://doi.org/10.2305/IUCN.UK.2016-1.RLTS.T14143A22050638.en
» https://doi.org/10.2305/IUCN.UK.2016-1.RLTS.T14143A22050638.en
ADDITIONAL NOTES
-
Zoobank register
http://zoobank.org/F224E29C-E6C2-485E-A075-0A4D033B1ADB -
How to cite this article
Novaes RLM, LaVal RK, Wilson DE, Moratelli R (2022) Redescription of Myotis atacamensis (Chiroptera, Vespertilionidae) with neotype designation. Zoologia (Curitiba) 39: e21026. https://doi.org/10.1590/S1984-4689.v39.e21026 -
Published by
Sociedade Brasileira de Zoologia at Scientific Electronic Library Online (https://www.scielo.br/zool)
APPENDIX
Appendix 1. Specimens examined and localities of occurrence for Myotis species deposited in the following biological collections: Museo Argentino de Ciencias Naturales Bernardino Rivadavia (MACN, Buenos Aires, Argentina), Museo Miguel Lillo De Ciencias Naturales (CML, San Miguel de Tucumán, Argentina), Muséum d’Histoire Naturelle (MHNG, Geneva, Switzerland), American Museum of Natural History (AMNH, New York, USA), Carnegie Museum of Natural History (CM, Pittsburgh, USA), Field Museum of Natural History (FMNH, Chicago, USA), Museum of Natural History of the Kansas University (KU, Lawrence, USA), Museum of Natural Science, Louisiana State University (LSUMZ, Baton Rouge, USA), Museum of Vertebrate Zoology (MVZ, Berkeley, USA), Sam Noble Oklahoma Museum of Natural (OMNH, Norman, USA), Museum of Texas Tech University (TTU, Lubbock, TTU), and Smithsonian National Museum of Natural History (USNM, Washington D.C., USA).
Myotis albescens (N = 109): Argentina: Tucumán, La Cocha, Dique San Ignacio (OMNH 18877); Tucumán, Leales, Dique San Ignacio (OMNH 18878); Santiago del Estero, Pellegrini, Santo Domingo (OMNH 23772, 23773, 23774). Peru: Ayacucho, Río Apurimac, Hacienda Luisiana (LSUMZ 16621, 16622); Ayacucho, San José, Río Santa Rosa (LSUMZ 16623-16625); Cusco, Quispicanchi (FMNH 68471, 68473-68478); Huánuco, Leonicio Prado, 1 km S of Tingo Maria (CM 98854); Huánuco, Río Huallaga, ca. 4 km NE of Tingo Maria (LSUMZ 14265); Loreto, Río Curaray (AMNH 71643); Loreto, Maynas, Puerto Indiana, Amazon River (AMNH 73235, 73237, 73239, 73242); Loreto, Maynas, Orosa, Amazon River (AMNH 74018, 74019, 74021); Loreto, San Jacinto (KU 158160); Madre de Dios, Manú, Maskoitania, 13.4 km NW Atalaya, left bank of Rio Alto Madre de Dios (FMNH 174919, 174921); Madre de Dios, Manú, Quebrada Aguas Calientes, left bank, Rio Alto Madre de Dios, 2.75 km E of Shintuya (FMNH 170275); Madre de Dios, Mouth of Rio La Torre, south bank of Rio Tambopata (LSUMZ 24562); Madre de Dios, Pakitza (USNM 564391, 564392, 566560-566563); Pasco, Oxapampa (AMNH 230746-230748, 230750-230757); Pasco, San Juan (USNM 364442-364480); Pasco, unknown localities (AMNH 213428, 213430); Ucayali, Balta, Río Curanja (LSUMZ 12272, 12274-12279); Ucayali, Coronel Portillo, Yarinacocha (LSUMZ 12253, FMNH 62178-62188).
Myotis atacamensis (N = 12). Chile: Tarapacá, Minimini (USNM 391786 [neotype]; 19°10’S, 69°41’W); Tarapacá, Iquique, Los Canchones (FMNH 23618; MHNG 1748-43, 1748-44, 1748-45, 1748-46; 20°27’00”S, 69°37’00”W). Peru: Arequipa, Chucarapi, Tambo Valley (MVZ 116638; FMNH 50783, 51063; 17°04’S, 71°43’W); Arequipa, Arequipa, Patasagua, 3 km W of Tiabaya (FMNH 49790, 49791, 49792; 16°27’S, 71°35’W).
Myotis bakeri (N = 4): Peru: Lambayeque, 12 km N of Olmos (LSUMZ 21306, 21307); Lima, 7 km SE of Chilca (MVZ 137906, 137907-holotype).
Myotis dinellii (N = 73): Argentina: Buenos Aires, General Guido, Canal 2 y Ruta 2 (MACN 15740); Catamarca (CM 42934-42937); Catamarca, Trancas, 50 km NO Catamarca Ciudad, Las Juntas, Estancia de los Figueroa (OMNH 18977-18979); Catamarca, Capayán, 6 km NW Chumbicha, Balneario El Caolin (OMNH 19366, 19367); Catamarca, Capayán, 1 km NW of Balneario by road, Chumbicha (OMNH 23777-23782); Catamarca, Ambato, Estancia Narvaez, 5.5 km N Las Chacritas on Ruta Provincial No. 1 (OMNH 27931); Catamarca, Ambato, 21.4 km S Humaya (OMNH 36211); Catamarca, Tinogasta, 36.7 km W Fiambalá by road (OMNH 34545); Catamarca, Tinogasta, 57 km W Fiambalá by road (OMNH 34547); Catamarca, El Alto, Bella Vista (OMNH 36208); Catamarca, Capayán, 5.2 km NW Chumbicha (OMNH 36209, 36210); Catamarca, Andalgalá, Pucará (OMNH 36212, 36213); Córdoba (USNM 142560-142562); Córdoba, San Javier (TTU 32524, 32525, 32528, 32529); Córdoba, Calamuchita (TTU 64334); Córdoba, Cruz del Eje (TTU 64335, 64336); Córdoba, Río Cuarto (TTU 64337-64345, 66489, 66490, 66491); Córdoba, Pocho (TTU 66483-6488); Mendoza (MVZ 150861); La Pampa, Pampa Central, Caleu (MACN 49.163, 49.165); La Rioja, San Blás de Los Sauces, 4 km SE de San Blas (CML 5439, 5444, 5448, 5449); Mendoza, La Valle, Reserva Telteca (OMNH 23783); Salta, Guachipas, 25 km SE La Viña (OMNH 36214, 36217); Salta, San Carlos, Los Sauces (OMNH 36215); San Juan, Sarmiento, Pedernal (OMNH 23786); San Luis, Ayacucho, Quebrada de López, San Francisco del Monte de Oro (OMNH 23787); Tucumán (CM 42938-42942); Tucumán, La Cocha, Dique San Inacio (AMNH 256987); Tucumán, Tafí Viejo, 5 km SW Siambón (OMNH 36216).
Myotis chiloensis (N = 48): Argentina: Chubut (MVZ 150842, 150847-150851, 150853-150858); Neuquen (MVZ 150862-150869, 150883, 150884, 150892, 150894-150898, 162181-162183); Rio Negro (MVZ 150902-150907, 150917, 150918, 150927, 150929, 152150, 152153, 154461). Chile: Valparaíso Region, Zapallar (USNM 391785); Valdivia, Rinihue (FMNH 23613, 23614); Chiloe Island, Chiloe (FMNH 24029 [neotype]).
Myotis keaysi (N = 43): Argentina: Tucumán, Burruyacú (CML 6177, 7600, 8938, 9839, MACN 16795, 16855, 16857, OMNH 23499, 36207, TTU 32588). Peru: Amazonas, ca. 20 km of La Peca by trail (LSUMZ 21488); Ayacucho, Puncu, ca. 30 km NE of Tambo (15688); Cusco, Cordillera Vilcabamba (AMNH 214371); Cusco, Cordillera Vilcabamba, west side (AMNH 233850, 233851, 233853, 233854, 233857, 236134); Cusco, Hacienda Cadena (FMNH 78686); Huánuco, Bosque Cutirragra, S of Huaylaspampa (LSUMZ 17898); Huánuco, Trail to Hacienda Paty below Carpish Pass (LSUMZ 18434); Huánuco, 7 km NW of Carpish Pass by road (AMNH 216117); Junín, Chanchamayo (FMNH 65751); Lambayeque, 16 km N and 25 km E of Olmos (MVZ 135620, 135621); Pasco, Santa Cruz, ca. 9 km SE of Oxapampa (LSUMZ 25907); Piura, Batan, on Zapalache-Carmen trail (LSUMZ 26898); Piura, 15 km E of Canchaque by road (LSUMZ 19213); Puno, Inca Mines (AMNH 15814); Puno, Inca Mines (AMNH 15814 [holotype]), Puno, Oconeque, 10 miles N of Limbani (MVZ 116050).
Myotis oxyotus (N = 19): Argentina: Tucumán, Tafí Viejo, 5 km SW Siambón (OMNH 36218). Peru: Ancash, 31 km E of Pariacoto by road (LSUMZ 22131); Cajamarca, Celendin, Hacienda Limón (FMNH 19969); Cusco, Iquente (USNM 195146); Cusco, Marcapata (FMNH 66375, 66376); Cusco, Santa Ana (USNM 194452, 194453, 195141, 195147, 195149); Cusco, 6 miles N of Paucartambo (MVZ 116008); Huancavelica, Rumicruz (AMNH 60598); Huánuco, Ambo (FMNH 24864-24866); Junín, Rio Palca, 15 Km W of San Ramon (USNM 507204); Lima, Bujama Baja, 95 km south of Lima by road (AMNH 216118); Lima, Huaros, Bosque de Zarate, San Bartolomé (FMNH 129208).
Myotis riparius (N = 41): Argentina: Corrientes, Capital, Laguna Paivas, Barrio Los Lomas (CML 2994); Formosa, Rio Porteno, km 64, a 5 km al sur de Estancia Sta. Catalina (OMHN 18889); Formosa, Río Bermejo, 10 millas al S de Cólonia km 503 (CML 5412); Formosa, Parque Nacional Pilcomayo (MACN 20881, 20895); Formosa, Jecc. Cassinera, R. Teuco (MACN 20938); Jujuy, Santa Bárbara, Laguna “La Brea”, 25 km antes de Palma Sola, sobre Ruta 1 (OMNH 18890, 18891); Salta, San Martín, 6 km al W de Piquirenda Viejo (CML 3157); Tucumán, Burruyacú, Balneário Piedra Tendida, 6 km al O del El Cajón (CML 3155); Tucumán, Reserva Provincial Santa Ana (El Salton), Rio Chico (CML 3158). Peru: Ayacucho, San José, Río Santa Rosa (LSUMZ 16631); Cusco, Camisea, Armihuari (USNM 582875, 582876); Cusco, Camisea, San Martín-3 (USNM 582877, 582878); Cusco, Cordillera Vilcabamba (AMNH 233859, 233860); Cusco, Marcapata (FMNH 68479-68482); Cusco, Paucartambo, Consuelo (FMNH 174941); Cusco, Paucartambo, San Pedro (FMNH 172162); Cusco, Quispicanchi, Hacienda Cadena (FMNH 75150); Cusco, Ridge Camp (USNM 588040); Huánuco, Agua Caliente, Río Pachitea (FMNH 55400); Huánuco, Leoncio Prado (TTU 46348); Loreto, Río Curaray (AMNH 71645); Loreto, San Jacinto (KU 158162); Madre de Dios, Lago Sandoval, Río Madre de Dios (MVZ 157782); Madre de Dios, Manú, Maskoitania (FMNH 174933); Madre de Dios, Mouth of Rio La Torre, south bank of Rio Tambopata (LSUMZ 24561); Madre de Dios, Rio Tambopata, 30 km up from Mouth (USNM 530919); Pasco, Oxapampa, San Pablo (AMNH 230775-230777); Ucayali, Balta, Río Curanja (LSUMZ 12268-12271).
Edited by
Editorial responsibility
Data availability
Data citations
MDD (2021) Mammal Diversity Database (Version 1.4). Zenodo. http://doi.org/10.5281/zenodo.4139818
Publication Dates
-
Publication in this collection
20 May 2022 -
Date of issue
2022
History
-
Received
28 Sept 2021 -
Accepted
07 Feb 2022