ABSTRACT
A new species of the leafhopper genus Andanus Linnavuori, 1959 (Hemiptera: Cicadellidae: Deltocephalinae: Athysanini), A. tambopixunensis sp. nov., is described. Materials studied were intercepted using Malaise and light traps in the Tambopata National Reserve (Peru) and the Amazonian Forest (Brazil). The genera Perundanus Zanol, 1993 syn. nov. and Paralaca Lozada, 1998 syn. nov. are recognized as junior synonyms of Andanus based on morphological comparison. The type species of Perundanus and Paralaca, A. raunoi (Zanol, 1993) comb. nov. and A. sordidus (Lozada, 1998) comb. nov., respectively, are transferred to Andanus. A detailed redescription of the genus and illustrations of external and genital morphology are provided as well as a key to the known species. The new species differs from other species in having a pygofer without processes, subgenital plates divergent, and aedeagus with a pair of short dorsoapical and single ventroapical spine. Comparative notes on morphological similarities of Andanus to other Neotropical Athysanini genera are discussed.
KEY WORDS:
Auchenorrhyncha; Membracoidea; Athysanini; Perundanus; Napo; Paralaca
INTRODUCTION
Athysanini Van Duzee, 1892 (Hemiptera: Cicadellidae) with nearly 227 described genera and more than 1170 described species is the largest tribe of Deltocephalinae, comprising 39 tribes, >960 described genera and >7200 described species worldwide, itself the largest subfamily of leafhoppers. The tribe comprises 126 genera distributed in the New World from Canadian boreal forest to the remote southern archipelagos of Chile (Zanol 2008Zanol KMR (2008) Catalogue of the Neotropical Deltocephalinae (Hemiptera: Cicadellidae). Part III-Tribe Athysanini. Acta Biológica Paranaense 37(1-2): 1-104. https://doi.org/10.5380/abpr.v37i0.13191
https://doi.org/10.5380/abpr.v37i0.13191...
). The Neotropical region has many endemic Athysanini that appear to be highly restricted to specific host plants, habitats, or regions (Pinedo-Escatel and Dietrich 2020Pinedo-Escatel JA, Dietrich CH (2020) Review of the enigmatic Neotropical leafhopper genus Brazosa Oman and other potentially related Athysanini genera (Hemiptera: Auchenorrhyncha: Cicadellidae: Deltocephalinae), with descriptions of South American new genera and species. Zootaxa 4830(3): 401-454. https://doi.org/10.11646/zootaxa.4830.3.1
https://doi.org/10.11646/zootaxa.4830.3....
). The tribe has been shown to be a polyphyletic group and mostly comprises genera that do not fit the definitions of other, better-defined deltocephaline tribes (Zahniser and Dietrich 2013Zahniser JN, Dietrich CH (2013) A review of the tribes of Deltocephalinae (Hemiptera: Auchenorrhyncha: Cicadellidae). European Journal of Taxonomy 45: 1-211. https://doi.org/10.5852/ejt.2013.45
https://doi.org/10.5852/ejt.2013.45...
). Relationships among genera of Athysanini remain poorly explored but a recent analysis showed that the endemic New World genera of Athysanini form a distinct lineage that also includes the endemic New World tribes Bahitini, Pendarini and Scaphytopiini (Cao et al. 2022Cao Y, Dietrich CH, Zahniser JN, Dmitriev DA (2022) Dense sampling of taxa and characters improves phylogenetic resolution among deltocephaline leafhoppers (Hemiptera: Cicadellidae: Deltocephalinae). Systematic Entomology: 1-15. https://doi.org/10.1111/syen.12540
https://doi.org/10.1111/syen.12540...
).
Linnavuori (1959Linnavuori R (1959) Revision of the Neotropical Deltocephalinae and some related subfamilies (Homoptera). Annales Botanici Societatis Zoologicae-Botanicae Fennicae “Vanamo” 20(1): 1-370.) erected the endemic South American genus Andanus based on two males and two females of A. bimaculatus Linnavuori, 1959 from Madre de Dios department, southeast Peru. Later, Zanol (1993Zanol KMR (1993) Sobre o material-tipo de Andanus bimaculatus Linnavuori e descrição de um novo gênero e nova espécie (Homoptera: Cicadellidae: Deltocephalinae). Revista Brasileira de Zoologia 10(4): 613-618. https://doi.org/10.1590/S0101-81751993000400006
https://doi.org/10.1590/S0101-8175199300...
) erected another Peruvian leafhopper genus, Perundanus, based on a previously undissected female and male from the type series of A. bimaculatus. Subsequently, Lozada (1998Lozada P (1998) Two new genera of neotropical Deltocephalinae (Insecta: Homoptera: Cicadellidae) related to Alaca Oman. Revista Peruana de Biologia 5(2): 113-117. https://doi.org/10.15381/rpb.v5i2.8328
https://doi.org/10.15381/rpb.v5i2.8328...
) proposed two monotypic Peruvian genera taken from Pakitza National Park, Paralaca with six specimens of both sexes of P. sordidus Lozada, 1998 and Adlaca based on five males of Adlaca dubiosa Lozada, 1998. Zanol (2005Zanol KMR (2005) Nova sinonímia em Deltocephalinae (Hemiptera, Cicadellidae) e primeira ocorrência de Andanus Linnavuori no Brasil. Acta Biológica Paranaense 34(1-4): 89-90. https://doi.org/10.5380/abpr.v34i0.955
https://doi.org/10.5380/abpr.v34i0.955...
) considered A. dubiosa to be a junior synonym of Andanus bimaculatus and recorded this genus in Brazil for the first time. Herein, we revised the mentioned genera and recognized only Andanus as valid. Two species, Paralaca sordidus and Perundanus raunoi, are transferred to the valid genus and a fourth species from Peru is newly described.
MATERIAL AND METHODS
The identification of specimens was based mainly on morphological comparison of male genitalia. Terminology for head and thorax follows Dietrich (2005Dietrich CH (2005) Keys to the families of Cicadomorpha and subfamilies and tribes of Cicadellidae (Hemiptera: Auchenorrhyncha). Florida Entomologist 88(4): 502-517. https://doi.org/10.1653/0015-4040(2005)88[502:KTTFOC]2.0.CO;2
https://doi.org/10.1653/0015-4040(2005)8...
), wing venation is based on Anufriev and Emeljanov (1988Anufriev GA, Emeljanov AF (1988) Suborder Cicadinea (Auchenorrhyncha). In: Lehr PA (Ed.) Keys to the Insects of the Far East of the USSR. Homoptera and Hemiptera. Nauka Publishing House, Leningrad, 1-496.), and Rakitov (1998Rakitov RA (1998) On differentiation of cicadellid leg chaetotaxy (Homoptera: Auchenorrhyncha: Membracoidea). Russian Entomological Journal 6(3-4): 7-27.) was used for leg chaetotaxy. Male abdomens were removed and cleared following Oman’s (1949Oman PW (1949) The Nearctic leafhoppers (Homoptera: Cicadellidae). A generic classification and check list. Memoirs of the Entomological Society of Washington 3: 1-253.) protocol but increasing heating time to 20 minutes and neutralizing remaining KOH solution with acetic acid after washing with water. Abdomens and dissected genitalia are stored in glycerin in microvials pinned beneath the specimen. Labels are quoted exactly as written. Specimen labels are quoted verbatim. The total length was measured using an ocular micrometer mounted to the stereomicroscope. The distributional map was elaborated based on material studied below and data included in Zanol (1993Zanol KMR (1993) Sobre o material-tipo de Andanus bimaculatus Linnavuori e descrição de um novo gênero e nova espécie (Homoptera: Cicadellidae: Deltocephalinae). Revista Brasileira de Zoologia 10(4): 613-618. https://doi.org/10.1590/S0101-81751993000400006
https://doi.org/10.1590/S0101-8175199300...
, 2005Zanol KMR (2005) Nova sinonímia em Deltocephalinae (Hemiptera, Cicadellidae) e primeira ocorrência de Andanus Linnavuori no Brasil. Acta Biológica Paranaense 34(1-4): 89-90. https://doi.org/10.5380/abpr.v34i0.955
https://doi.org/10.5380/abpr.v34i0.955...
), Lozada (1998Lozada P (1998) Two new genera of neotropical Deltocephalinae (Insecta: Homoptera: Cicadellidae) related to Alaca Oman. Revista Peruana de Biologia 5(2): 113-117. https://doi.org/10.15381/rpb.v5i2.8328
https://doi.org/10.15381/rpb.v5i2.8328...
), and Pinedo-Escatel and Dietrich (2020Pinedo-Escatel JA, Dietrich CH (2020) Review of the enigmatic Neotropical leafhopper genus Brazosa Oman and other potentially related Athysanini genera (Hemiptera: Auchenorrhyncha: Cicadellidae: Deltocephalinae), with descriptions of South American new genera and species. Zootaxa 4830(3): 401-454. https://doi.org/10.11646/zootaxa.4830.3.1
https://doi.org/10.11646/zootaxa.4830.3....
).
The studied materials are deposited in the following collections: USML - Universidad Nacional Mayor de San Marcos, Lima, Peru; INPA - Instituto Nacional de Pesquisas da Amazônia, Coleção Sistemática da Entomologia, Manaus, Amazonas, Brazil; DZRJ - Coleção Entomológica Prof. José Alfredo Pinheiro Dutra, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; INHS - Illinois Natural History Survey, University of Illinois at Urbana-Champaign, Champaign, USA; USNM - United States National Museum of Natural History, Smithsonian Institution, Washington, DC, USA.
TAXONOMY
Cicadellidae Latreille, 1825
Deltocephalinae Dallas, 1870
Athysanini Van Duzee, 1892
Andanus Linnavuori, 1959
AndanusLinnavuori, 1959Linnavuori R (1959) Revision of the Neotropical Deltocephalinae and some related subfamilies (Homoptera). Annales Botanici Societatis Zoologicae-Botanicae Fennicae “Vanamo” 20(1): 1-370.: 237.
AdlacaLozada, 1998Lozada P (1998) Two new genera of neotropical Deltocephalinae (Insecta: Homoptera: Cicadellidae) related to Alaca Oman. Revista Peruana de Biologia 5(2): 113-117. https://doi.org/10.15381/rpb.v5i2.8328
https://doi.org/10.15381/rpb.v5i2.8328...
: 113.
PerundanusZanol, 1993Zanol KMR (1993) Sobre o material-tipo de Andanus bimaculatus Linnavuori e descrição de um novo gênero e nova espécie (Homoptera: Cicadellidae: Deltocephalinae). Revista Brasileira de Zoologia 10(4): 613-618. https://doi.org/10.1590/S0101-81751993000400006
https://doi.org/10.1590/S0101-8175199300...
: 616, syn. nov.
ParalacaLozada, 1998Lozada P (1998) Two new genera of neotropical Deltocephalinae (Insecta: Homoptera: Cicadellidae) related to Alaca Oman. Revista Peruana de Biologia 5(2): 113-117. https://doi.org/10.15381/rpb.v5i2.8328
https://doi.org/10.15381/rpb.v5i2.8328...
: 113, syn. nov.
Type species. Andanus bimaculatusLinnavuori, 1959Linnavuori R (1959) Revision of the Neotropical Deltocephalinae and some related subfamilies (Homoptera). Annales Botanici Societatis Zoologicae-Botanicae Fennicae “Vanamo” 20(1): 1-370..
Diagnosis. Medium-sized and moderately robust leafhoppers with overall body coloration yellowish with brown or orange marks. Crown distinctly short, anterior and posterior margins parallel, surface longitudinally striate, with pair of large-round black spots on anterior margin adjacent to eyes. Pronotum narrower than head, slightly declivous with weak lateral carina. Forewing macropterous, without extra crossveins; anal veins without crossvein. Male pygofer pointed distally, without processes; subgenital plates divergent in ventral view; connective with anterior arms fused anteriorly; aedeagus with gonoduct sclerotized basad of atrium; segment X long, sclerotized laterally and/or dorsally.
Redescription. External morphology. Medium-sized leafhoppers (4.52-5.15 mm) moderately robust. Body coloration yellowish and usually with orange markings on crown; face entirely yellowish or with orange and black marking on gena and lorum; pronotum mostly yellowish or with a brownish band near mid-length; scutellum yellowish with whitish and brownish marks; outline of veins on clavus yellowish or brown; a well-defined round pair of black spots above ocelli; face yellowish with or without brownish pigment on lora and anteclypeus (Figs 1-3, 9-11, 17-19).
Holotype of Andanus tambopixunensis sp. nov.: (1) Habitus, dorsal view; (2) habitus, lateral view; (3) Face, anterior view; (4) pygofer, lateral view; (5) subgenital plate and style, dorsal view; (6) style, ventral view; (7) aedeagus and connective, lateral view; (8) aedeagus and connective, ventral view.
Male of Andanus bimaculatus: (9) habitus, dorsal view; (10) habitus, lateral view; (11) face, anterior view; (13) pygofer, lateral view; (13) apodemes of sternite I, ventral view; (14) subgenital plates, valve, and styles, ventral view; (15) aedeagus and connective, lateral view; (16) apex of aedeagus, caudal view.
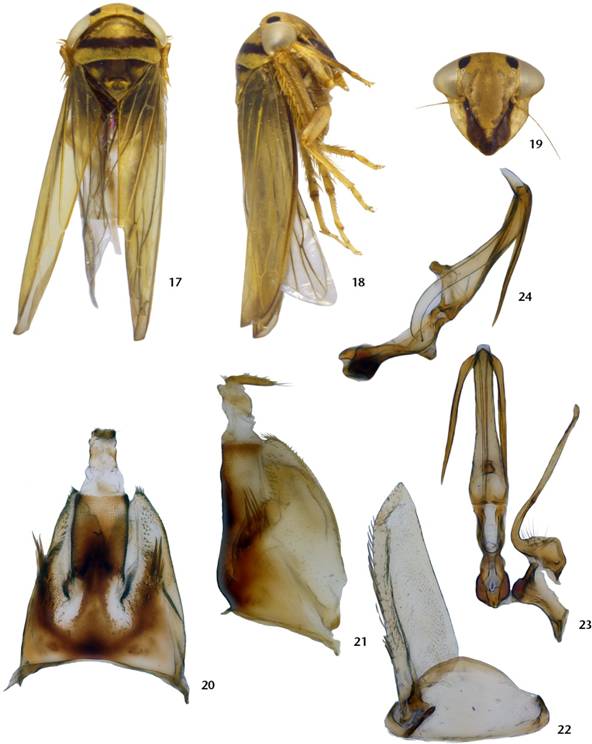
Male of Andanus raunoi comb. nov.: (17) habitus, dorsal view; (18) habitus, lateral view; (19) face, anterior view; (20) pygofer and segment X, dorsal view; (21) pygofer, lateral view; (22) subgenital plate and valve, ventral view; (23) aedeagus, connective, and style, ventral view; (24) aedeagus and connective, lateral view.
Head wider than pronotum. Crown slightly convex, relatively short, surface weakly striate longitudinally, anterior margin broadly rounded and parallel to posterior margin, width between eyes 1.3x wider than eye width, transition of crown to face rounded and shagreened. Ocellocular area widened toward antennal pit, lateral frontal sutures extended to ocelli. Ocelli on anterior margin of head, close to eyes and separated from latter by less than one ocellar diameter. Frontoclypeus wide, not tumid, parallel-sided through most of length with short erect fine seta beside frontal suture. Antennal ledge weakly developed, not carinate. Anteclypeus widened apically, not tumid, apex slightly surpassing natural curve of gena. Lorum width subequal to anteclypeus near base, extended to lower margin of face. Gena incised below eye.
Thorax. Pronotum with anterior margin slightly produced and posterior margin slightly concave; lateral margins weakly carinate, shorter than half eye width; convex in lateral view. Scutellum not protuberant, same length as pronotum. Forewing macropterous, transparent, appendix restricted to anal margin, apex rounded, without extra crossveins, apical cells subequal in width, base of outer apical cell large and delimited by crossvein, three anteapical cells present, inner cell open (without m-cu2 crossvein). Clavus with crossvein connecting Pcu to claval suture, Pcu and A1 free throughout length, extra crossveins absent. Hind wing venation usual for Athysanini and complete with RP-MA and MP-CuA each separated by crossvein. Front femur with distinct long AM1 near ventral margin, IC setae long and thin (15-17), row AV with 23-27 short stout setae, AV1 differentiated from IC row. Front tibia usually with dorsal macrosetal formula 1+4 (AD+PD) (females rarely 1+5), PV without setae. Middle trochanter without setae. Middle femur row AV with long stout setae. Hind femur macrosetal formula 2+2+1, without extra setae basad of usual set. Hind tarsomere I as long as II and III combined, apex not expanded, plantar setae simple, pecten with 3 platellae, inner apical seta platelliform.
Abdomen. Apodemes of sternite I present, distinct, meeting or nearly meeting medially, relatively long, narrow, reaching to mid-length or nearly to posterior margin of sternite III. Apodemes of sternite II broader, shorter than those of sternite I, lying laterad of apodemes of sternite I (Fig. 13).
Male genitalia. Pygofer longer than tall, sclerotized dorsally at base, incised dorsally beyond mid-length, posterior margin narrowly rounded in lateral view, macrosetae long and abundant or not and distributed on distal 1/3 of pygofer but absent on posterior margin (Fig. 12), short stout setae on posteroventral margin, basolateral cleft reaching half pygofer length with adjacent small fine setae, without long processes (Fig. 4) or with ventral margin serrate (Fig. 32). Segment X longer than wide, about half-length of pygofer, narrow, well sclerotized laterally and/or dorsally with limited mobility or fused to pygofer (Figs 12, 28). Valve and subgenital plates free, articulated to pygofer. Valve triangular, wider than long, anterior margin straight and posterior pointed or broadly rounded to nearly straight. Subgenital plate shape variable, shorter than pygofer, longer than wide, inner margin straight or strongly incised (sinuous) at apical third, apex pointed or expanded, with few fine or stout setae arranged apically or not, lateral margin usually without macrosetae or (A. raunoi) with one marginal row of macrosetae. Style bilobed basad, lateral and medial anterior lobes subequal in size or not, lateral preapical lobe variably developed, apophysis elongate or not (A. sordidus) and slender, surface smooth, without setae, apex rounded, truncate or pointed. Connective shorter than aedeagus, anterior arms curved mesad and fused anteriorly, stem usually longer than arms and partially fused with aedeagus. Aedeagus with preatrium moderately developed, dorsal apodeme short, shaft tubular, curved dorsad, moderately slender, without or with pair of long processes directed anterad along shaft, without or with pair of sublateroapical flanges, with or without one or two pairs of apical or dorsopreapical spines directed anterad or caudad, without or with single minute spine ventro-apically or -medially; gonoduct well sclerotized basad of atrium, recurved toward or beyond connective; gonopore preapical on dorsal surface, as wide as shaft.
Female. Based on A. raunoi. Female sternite VII protuberant and 1.8x longer than wide, narrowing towards posterior margin, apex rounded. Pygofer relatively short, with ~20 macrosetae toward apical 1/4. Base of first valvula expanded laterally, with two lobes; surface covered by numerous short fine hairs. First valvula curved, lanceolate; dorsal sculpturing strigate to reticulate; dorsal margin with unsculptured band; with indistinctly delimited ventroapical sculptured area. Second valvula relatively short and broad, shorter than first valvula; distinctly broadened medially; with numerous serrated teeth along dorsal margin on apical 2/3. Gonoplac short, broad; without macrosetae; with a ventral row of very short, fine setae.
Distribution. Peru and Brazil (Fig. 37).
Female of Andanus raunoi comb. nov.: (25) sternite VII, ventral view; (26) first valvula, lateral view; (27) sculpturing of first valvula, lateral view; (28) second valvula, lateral view.
Male of Andanus sordidus comb. nov.: (29) habitus, dorsal view; (30) habitus, lateral view; (31) Face, anterior view; (32) pygofer and segment X, dorsal view; (33) pygofer, lateral view; (34) subgenital plates and valve, ventral view; (35) aedeagus, connective, and styles, ventral view; (36) aedeagus and connective, lateral view.
Species of Andanus (males)
Andanus bimaculatusLinnavuori, 1959Linnavuori R (1959) Revision of the Neotropical Deltocephalinae and some related subfamilies (Homoptera). Annales Botanici Societatis Zoologicae-Botanicae Fennicae “Vanamo” 20(1): 1-370. - Peru (Madre de Dios) and Brazil (Ipixuna).
Andanus raunoi (Zanol, 1993Zanol KMR (1993) Sobre o material-tipo de Andanus bimaculatus Linnavuori e descrição de um novo gênero e nova espécie (Homoptera: Cicadellidae: Deltocephalinae). Revista Brasileira de Zoologia 10(4): 613-618. https://doi.org/10.1590/S0101-81751993000400006
https://doi.org/10.1590/S0101-8175199300...
) comb. nov. - Peru (Madre de Dios).
Andanus tambopixunensis sp. nov. - Peru (Tambopata) and Brazil (Ipixuna).
Andanus sordidus (Lozada, 1998Lozada P (1998) Two new genera of neotropical Deltocephalinae (Insecta: Homoptera: Cicadellidae) related to Alaca Oman. Revista Peruana de Biologia 5(2): 113-117. https://doi.org/10.15381/rpb.v5i2.8328
https://doi.org/10.15381/rpb.v5i2.8328...
) comb. nov. - Peru (Madre de Dios).
Key to species of Andanus
1. Pygofer with ventral margin serrated (Figs 32, 33); aedeagus with pair of sublateroapical flanges with single subdorsoapical spine and single ventromedial spine (Fig. 36); subgenital plates very long, constricted medially, expanded and clublike toward apices (Fig. 34) .................... A. sordidus
1’. Pygofer without processes (Figs 12, 20, 21); aedeagus with pair of apical or dorsopreapical spines and with or without single ventroapical spine; subgenital plates not as above (Figs 5, 14, 22) .................... 2
2. Pygofer with numerous long macrosetae covering entire dorsoapical half (Fig. 12); aedeagus with two pairs of apical spines directed caudad (Figs 15, 16), without single spine on ventral surface or long spines along shaft .................... A. bimaculatus
2’. Pygofer with macrosetae limited to smaller patch not extended to apex (Figs 4, 21); aedeagus with one pair of long spines along shaft (Figs 7, 24), with or without preapical spines directed anterad and single spine on ventral surface .................... 3
3. Style with lateral lobe broadly rounded near base, apophysis without triangular lateral projection near mid-length (Fig. 23); aedeagus without short distal spines (Fig. 24) .................... A. raunoi
3’. Style with lateral lobe near base acute, apophysis with triangular lateral projection (Fig. 6); aedeagus with pair of short dorsoapical and single ventroapical spine (Fig. 7) .................... A. tambopixunensis sp. nov.
Andanus tambopixunensis sp. nov.
http://zoobank.org/91F3EAEA-59B6-4014-800B-CC2B3CC7BE55
Diagnosis. Andanus tambopixunensis is easily separated from other species of the genus by lack of a transverse medial band on the pronotum and orange marks on crown, subgenital plate mesal margin sinuate and excavated toward apex, style anterolateral lobe rounded, style apophysis without lateral projection, and aedeagus with pair of long processes arising near apex and paralleling shaft.
Description. External morphology. Body coloration yellowish. Face entirely yellowish or with few orange specks; anteclypeus and gena yellowish. Pronotum yellowish, wider than long and slightly protuberant in lateral view. Forewing with membrane hyaline, anal and Pcu veins bright yellow, remainder of veins dull yellow. Dorsum of abdomen diffuse brown medially, venter yellowish, terminalia light brown with black or pale macrosetae (Figs 1-3).
Male genitalia. Pygofer elongate and rounded posteriorly without process, macrosetae on distal third of disk separated from posterior margin (Fig. 4). Valve acutely angulate posteriorly. Subgenital plate mesal margin deeply incised on distal third (Fig. 5). Connective stem 1.7x longer than arms. Style with medial cleft separating broad, bilobed base from medial lobe and apophysis; medial lobe digitiform; apophysis with triangular projection near mid-length, long with apex curved and blunt (Fig. 6). Aedeagus curved dorsad with long lateral processes closely appressed to shaft, with additional pair of short dorsopreapical spines directed anterad and single minute spine ventroapically (Figs 7-8).
Measurements (mm). Body: total length male 4.52-4.95; width 1.51-1.59. Head: width 1.37-1.41; mid-length 0.19-0.25; eye width 0.48-0.52; eye length 0.14-0.18; crown mid width before eyes 0.72-0.76; crown posterior width between eyes 0.70-0.75; distance between ocelli 0.59-0.63; frontoclypeus width 0.57-0.62; frontoclypeus length 0.79-0.85; anteclypeus width 0.20-0.24; anteclypeus length 0.26-0.27; lorum width 0.11-0.14; lorum length 0.28-0.31; gena width 0.52-0.55; gena length 0.32-0.35. Pronotum: width 1.24-1.27; length 0.61-0.65. Scutellum: width 0.94-0.97; length 0.64-0.68. Forewing length 3.52-3.59. Male capsule: pygofer height 0.49-0.57; pygofer length 1.51-1.57; valve width 0.56-0.60; valve length 0.38-0.51; subgenital plate: apex width 0.08-0.12; mid width 0.25-0.30, base width 0.36-0.40; plate length 0.80-0.83; style length 0.50-0.56; aedeagus length 0.81-0.87.
Type-material. Holotype, PERU male: Madre de Dios, Tambopata Research Center, Rio Tambopata, 662ft., 13°08.305’S, 69°36.502’W, C.R. Bartlett Coll. (USML). Paratypes, BRASIL 1 male: AM [Amazonas], Ipixuna, Rio Liberdade, Com. [Comunidade] São Vicente no Estirão da Preta, ٠٧°21’47”S, 071°52’07”W, 175m, 11-14.v.2011, light trap, Cavichioli, Gonçalves and Takiya Colls. [voucher GoLife Delt 35-41] (INPA); BRASIL 1 male: AM, Ipixuna, Rio Liberdade, Com. São Vicente no Estirão da Preta, 07°21’47”S, 071°52’07”W, 175m, 11-14.v.2011, light trap, Cavichioli, Gonçalves and Takiya Colls. [voucher GoLife Delt 35-41] (DZRJ); PERU 2 male: same data for holotype (USML); PERU 1 male: Madre de Dios, Tambopata Research Center, 13°08.305’S, 69°36.502’W, 190 m, 3-7 May 2004, C.R. Bartlett Coll. PE04-1 [DNA voucher GoLife Delt 45-53.1] (INHS).
Etymology. The species epithet was formed by combining parts of the names of both localities where the species was collected, the Tambopata National Reserve, Peru, and the Ipixuna municipality, Amazonas, Brazil.
Andanus bimaculatus Linnavuori, 1959
Andanus bimaculatusLinnavuori, 1959Linnavuori R (1959) Revision of the Neotropical Deltocephalinae and some related subfamilies (Homoptera). Annales Botanici Societatis Zoologicae-Botanicae Fennicae “Vanamo” 20(1): 1-370.: 238.
Adlaca dubiosaLozada, 1998Lozada P (1998) Two new genera of neotropical Deltocephalinae (Insecta: Homoptera: Cicadellidae) related to Alaca Oman. Revista Peruana de Biologia 5(2): 113-117. https://doi.org/10.15381/rpb.v5i2.8328
https://doi.org/10.15381/rpb.v5i2.8328...
: 114.
Material examined. PERU 1 male: Madre de Dios, Rio Tambopata, Posada Amazonas, 662ft., S12°48’08.4”W, 69°17’59.4”, Sept 2004, J.R. Cryan, J.M. Urban [voucher GoLife Delt 42-18] (INHS). PERU 1 male: Rio Tambopata, Explorer’s Inn - Rio Tower, S12 50.208’ W069 17.603’, 10-XII-2003, G. Svenson [voucher GoLife Delt 40-56] (INHS). 2 males: Madre de Dios, Manu, Pakitza, 11°56’S, 71°18’W, 12-18.IX.1989, kitchen stream, malaise trap, night, N. Adams et al. (USNM). 1 male: Madre de Dios, Manu, Pakitza, 12°07’S, 70°58’W, 250 m, 14-23 Sept 1988, O. Flint, N. Adams, Trail 2, 1st stream, malaise trap, day and night (USNM).
Andanus raunoi (Zanol, 1993), comb. nov.
Perundanus raunoiZanol, 1993Zanol KMR (1993) Sobre o material-tipo de Andanus bimaculatus Linnavuori e descrição de um novo gênero e nova espécie (Homoptera: Cicadellidae: Deltocephalinae). Revista Brasileira de Zoologia 10(4): 613-618. https://doi.org/10.1590/S0101-81751993000400006
https://doi.org/10.1590/S0101-8175199300...
: 616.
Material examined. PERU 1 male, 1 female: Cusco, Estacion Biologica Villa Carmen, trail 8 mark 8-1924, 721 m, 12°54’08”S, 71°24’38”W, malaise trap, 1-7 Jan 2013, T. Förster (USNM).
Andanus sordidus (Lozada, 1998), comb. nov.
Paralaca sordidaLozada, 1998Lozada P (1998) Two new genera of neotropical Deltocephalinae (Insecta: Homoptera: Cicadellidae) related to Alaca Oman. Revista Peruana de Biologia 5(2): 113-117. https://doi.org/10.15381/rpb.v5i2.8328
https://doi.org/10.15381/rpb.v5i2.8328...
: 114.
Material examined. PERU 1 male: Madre de Dios, Manu, Pakitza, 11°56’S, 71°18’W, 12-18.IX.1989, kitchen stream, malaise trap, night, N. Adams et al. (USNM).
DISCUSSION
The three genera here considered synonyms were originally described based on single species. Our examination of available specimens indicates that these three species, in addition to the new species described here, are very similar in external morphology, including body size, structure, coloration, leg chaetotaxy and wing venation. They also share several unusual characteristics of the male genitalia including an elongate pygofer and segment X, subgenital plate with macrosetae reduced or absent, connective with anterior arms fused, gonoduct well sclerotized anterad of aedeagal atrium, and aedeagus with paired distal processes. Compared to these shared traits, the characters separating the species, including the shape and ornamentation of the male pygofer and plates, shape of the style, and length and orientation of aedeagal processes are similar to the kinds of variation occurring among species of many other genera of Cicadellidae. Therefore, although these species differ substantially in details of the male genital structures, we think it is preferable to recognize their close relationship by including them all in a single genus.
Peru is home to 16 genera of Athysanini (Andanus; Atanus Oman, 1938; Bolotheta Kramer, 1963; Brasilanus Linnavuori, 1959; Brazosa Oman, 1938; Caranavia Linnavuori, 1959; Chimaerotettix Dietrich & Rakitov, 2002; Huancabamba Linnavuori, 1959; Napo Linnavuori & DeLong, 1976; Pachytettix Linnavuori, 1959; Paratanus Young, 1957; Pseudonapo Pinedo-Escatel, 2020; Sincholata DeLong, 1982; Spaltumtettix Pinedo-Escatel & Dietrich, 2020; Tingolix Linnavuori & DeLong, 1978; Yungasia Linnavuori, 1959), of which most have been described from eastern forests including Ecuadorian, Bolivian, and Brazilian Amazonian forests as well as Andean cloud forests. This fauna includes multiple Athysanini genera manifesting particular morphological characteristics not shared among other athysanine faunas in northern or southern latitudes (Pinedo-Escatel and Dietrich 2020Pinedo-Escatel JA, Dietrich CH (2020) Review of the enigmatic Neotropical leafhopper genus Brazosa Oman and other potentially related Athysanini genera (Hemiptera: Auchenorrhyncha: Cicadellidae: Deltocephalinae), with descriptions of South American new genera and species. Zootaxa 4830(3): 401-454. https://doi.org/10.11646/zootaxa.4830.3.1
https://doi.org/10.11646/zootaxa.4830.3....
).
The species treated here share some unusual features with other Neotropical Athysanini genera, including Napo and Pseudonapo. These include the structure and coloration of the crown of the head, which is relatively short, smooth, and wide between the eyes, and rounded to the face with symmetrical black marks on the anterior margin. In addition, the pronotum is distinctly convex, which is an unusual feature. Some features of the male terminalia of these genera are also unusual among Athysanini. These include the relatively long, dorsally sclerotized segment X, the reduction or loss of macrosetae on the subgenital plate, and the connective with the arms often curved mesad apically and partially fused to aedeagus. More detailed taxonomic studies in combination with phylogenetic research are needed to understand the limits of some of these genera and clarify relationships in the tribe.
ACKNOWLEDGEMENTS
We thank Charles Bartlett (University of Delaware) and Daniela Takiya (Federal University of Rio de Janeiro) who kindly provided recently collected specimens for study. To Edith Blanco-Rodriguez who kindly imaged specimens studied. This research was supported in part by U.S. National Science Foundation grant DEB1639601. The opinions expressed by individuals in this report do not necessarily represent the policies of the U.S. Department of Agriculture.
LITERATURE CITED
- Anufriev GA, Emeljanov AF (1988) Suborder Cicadinea (Auchenorrhyncha). In: Lehr PA (Ed.) Keys to the Insects of the Far East of the USSR. Homoptera and Hemiptera. Nauka Publishing House, Leningrad, 1-496.
- Cao Y, Dietrich CH, Zahniser JN, Dmitriev DA (2022) Dense sampling of taxa and characters improves phylogenetic resolution among deltocephaline leafhoppers (Hemiptera: Cicadellidae: Deltocephalinae). Systematic Entomology: 1-15. https://doi.org/10.1111/syen.12540
» https://doi.org/10.1111/syen.12540 - Dietrich CH (2005) Keys to the families of Cicadomorpha and subfamilies and tribes of Cicadellidae (Hemiptera: Auchenorrhyncha). Florida Entomologist 88(4): 502-517. https://doi.org/10.1653/0015-4040(2005)88[502:KTTFOC]2.0.CO;2
» https://doi.org/10.1653/0015-4040(2005)88[502:KTTFOC]2.0.CO;2 - Linnavuori R (1959) Revision of the Neotropical Deltocephalinae and some related subfamilies (Homoptera). Annales Botanici Societatis Zoologicae-Botanicae Fennicae “Vanamo” 20(1): 1-370.
- Lozada P (1998) Two new genera of neotropical Deltocephalinae (Insecta: Homoptera: Cicadellidae) related to Alaca Oman. Revista Peruana de Biologia 5(2): 113-117. https://doi.org/10.15381/rpb.v5i2.8328
» https://doi.org/10.15381/rpb.v5i2.8328 - Oman PW (1949) The Nearctic leafhoppers (Homoptera: Cicadellidae). A generic classification and check list. Memoirs of the Entomological Society of Washington 3: 1-253.
- Pinedo-Escatel JA, Dietrich CH (2020) Review of the enigmatic Neotropical leafhopper genus Brazosa Oman and other potentially related Athysanini genera (Hemiptera: Auchenorrhyncha: Cicadellidae: Deltocephalinae), with descriptions of South American new genera and species. Zootaxa 4830(3): 401-454. https://doi.org/10.11646/zootaxa.4830.3.1
» https://doi.org/10.11646/zootaxa.4830.3.1 - Rakitov RA (1998) On differentiation of cicadellid leg chaetotaxy (Homoptera: Auchenorrhyncha: Membracoidea). Russian Entomological Journal 6(3-4): 7-27.
- Zahniser JN, Dietrich CH (2013) A review of the tribes of Deltocephalinae (Hemiptera: Auchenorrhyncha: Cicadellidae). European Journal of Taxonomy 45: 1-211. https://doi.org/10.5852/ejt.2013.45
» https://doi.org/10.5852/ejt.2013.45 - Zanol KMR (1993) Sobre o material-tipo de Andanus bimaculatus Linnavuori e descrição de um novo gênero e nova espécie (Homoptera: Cicadellidae: Deltocephalinae). Revista Brasileira de Zoologia 10(4): 613-618. https://doi.org/10.1590/S0101-81751993000400006
» https://doi.org/10.1590/S0101-81751993000400006 - Zanol KMR (2005) Nova sinonímia em Deltocephalinae (Hemiptera, Cicadellidae) e primeira ocorrência de Andanus Linnavuori no Brasil. Acta Biológica Paranaense 34(1-4): 89-90. https://doi.org/10.5380/abpr.v34i0.955
» https://doi.org/10.5380/abpr.v34i0.955 - Zanol KMR (2008) Catalogue of the Neotropical Deltocephalinae (Hemiptera: Cicadellidae). Part III-Tribe Athysanini. Acta Biológica Paranaense 37(1-2): 1-104. https://doi.org/10.5380/abpr.v37i0.13191
» https://doi.org/10.5380/abpr.v37i0.13191
ADDITIONAL NOTES
-
Zoobank register
http://zoobank.org/6D8A329C-51BB-4C36-89F0-13454C313165 -
How to cite this article
Pinedo-Escatel JA, Dietrich CH, Zahniser JN (2020) Andanus tambopixunensis sp. nov., a new species of the remarkable leafhopper genus Andanus (Hemiptera: Cicadellidae: Deltocephalinae), generic redescription and two new synonyms with new placements. Zoologia (Curitiba) 39: e21037. https://doi.org/10.1590/S1984-4689.v39.e21037 -
Published by
Sociedade Brasileira de Zoologia at Scientific Electronic Library Online (https://www.scielo.br/zool)
Edited by
Editorial responsibility
Publication Dates
-
Publication in this collection
20 May 2022 -
Date of issue
2022
History
-
Received
24 Nov 2021 -
Accepted
15 Feb 2022