Abstract
This study evaluated the mode of reproduction, the meiotic behavior and the pollen viability of three tetraploid plants (2n=4x=40) originated from somatic chromosome duplication of Paspalum notatum plants. The plant WKS 3 changed the mode of reproduction after duplication and became apomictic. The plants WKS 63 and WKS 92 confirmed sexual mode of reproduction identical to that of the original genotype. The analyzed plants presented meiotic abnormalities related to tetraploidy, and the chromosome pairing were variable, but it did not hinder the meiotic products, which were characterized by regular tetrads and satisfactory pollen fertility, ranging from 88.7 to 95.7%. Results show that all plants are meiotically stable and that they can be used in intraspecific crosses in the breeding program of Paspalum notatum.
Key words:
Chromosome duplication; cytogenetic analysis; genetic breeding; intraspecific crosses
INTRODUCTION
The accelerated degradation of natural pastures of the state of Rio Grande do Sul has led to the loss of genetic diversity of forage species and of quality forage supply to cattle in the state (Macedo 2009Macedo MCM (2009) Integração lavoura e pecuária: o estado da arte e inovações tecnológicas. Revista Brasileira de Zootecnia 38: 133-146.). Among the several forage species that form native pastures, Paspalum notatum Flugge stands out for having excellent forage value and for being present in all natural pastures of the state (Nabinger and Dall'Agnol 2008Nabinger C and Dall'Agnol M (2008) Principais gramíneas nativas do RS: características gerais, distribuição e potencial forrageiro. In Dall'Agnol M, Nabinger C and Santos RJ (eds) Anais do 3( simpósio de forrageiras e produção animal. UFRGS, Porto Alegre, p.7-54.). The breeding of this species is an alternative to the use of old and/or exotic varieties, with the search of selected materials; also, they are eligible for registration at the Ministry of Agriculture, Livestock and Supply (MAPA) and subsequent seed commercialization.
In the Paspalum genus, there is close correlation between ploidy level and mode of reproduction; diploidy is correlated with sexual reproduction, and allogamy and tetraploidy are correlated with apomixis (Quarin 1992Quarin CL (1992) The nature of apomixis and its origin in Panicoid grasses. Apomixis Newslett 5: 7-15.). Apomictic genotypes preserve the genetic diversity, which is made available, and enables crosses with sexual genotypes. The new gene combinations allow selecting individuals that solve problems related to these species, since they are more adapted to different environments, consequently mitigating risk where biotic agents, especially pests and diseases threaten the development and the production. The possibility of artificial chromosome duplication of sexual diploid plants from natural populations of variety Pensacola of P. notatum and their use in intraspecific crosses schemes in breeding programs (Burton and Forbes 1961Burton GW and Forbes I (1961) Cytology of diploids, natural and induced tetraploids, and intraspecies hybrids of bahiagrass,Paspalum notatum Flugge. Crop Science 1: 402-406.) makes it possible to generate superior genotypes of apomictic reproduction. Therefore, it is possible to protect the developed cultivar.
Weiler et al. (2015Weiler RL, Krycki KC, Guerra D, Simioni C and Dall'Agnol M (2015) Chromosome doubling in Paspalum notatum var. saure (cultivar Pensacola). Crop Breeding and Applied Biotechnology 15: 106-111.) artificially duplicated chromosomes of three individuals of P. notatum (Pensacola bahiagrass) by immersing flower buds and seeds for different exposure time in various concentrations of colchicine, which is an antimitotic agent responsible for chromosome duplication. The three duplicated plants, WKS 3, WKS 63, and WKS 92, confirmed the tetraploid level of ploidy and the complete euploidy by the root tip analysis and meiotic cells diakinesis. The objective of this study was to evaluate these duplicated plants regarding the mode of reproduction, the meiotic behavior, and the pollen viability in order to use them as parents in intraspecific hybridization schemes in the breeding program.
MATERIAL AND METHODS
This work was carried out in the Cytogenetics Laboratory of the Department of Forage Plants and Agrometeorology of the Department of Agronomy of the Federal University of Rio Grande do Sul. The three plants tetraploidized by Weiler et al. (2015Weiler RL, Krycki KC, Guerra D, Simioni C and Dall'Agnol M (2015) Chromosome doubling in Paspalum notatum var. saure (cultivar Pensacola). Crop Breeding and Applied Biotechnology 15: 106-111.) were evaluated, nominated WKS 3, WKS 63, and WKS 92, regarding the cytoembriyological, cytogenetic and pollen viability analysis.
Cytoembryological analysis
Analyses of the mode of reproduction of the plants were carried out with inflorescences in anthesis. Flowers were dissected and fixed in FAA (95% ethanol: 40 mL; distilled water: 14mL, 40% formalin: 3 mL; and glacial acetic acid: 3 mL) for 24 hours. After that, they were stored in 70% alcohol under refrigeration until ovaries extraction, which went through clearing process by means of a series of alcohol dehydration with methyl salicylate, following the protocol of Young et al. (1979Young BA, Sherwood RT and Bashaw EC (1979) Cleared-pistil and thick-sectioning techniques for detecting aposporus apomixis in grasses. Canadian Journal of Botany 57: 1668-1672.), modified by Acuña et al. (2007Acuña CA, Blount AR, Quesenberry KH, Hanna WW and Kenworthy KE (2007) Reproductive characterization of bahiagrass germplasm. Crop Science 47: 1711-1717.), and were stored in methyl salicylate solution (100%) until the analysis in interference contrast optical microscope. At least 30 ovaries per plant were analyzed to determine the mode of reproduction.
Cytogenetic analysis
For the analysis of meiotic behavior, inflorescences of plants were collected at several development stages, and were fixed in an absolute ethanol solution: glacial acetic acid (3: 1) for 24 hours, transferred to 70% ethanol, and stored under refrigeration (Araújo et al. 2005Araújo ACG, Nóbrega JM, Pozzobon MT and Carneiro VTC (2005) Evidence of sexuality in induced tetraploids of Brachiaria brizantha (Poaceae). Euphytica 144: 39-40., Dahmer et al. 2008Dahmer N, Schifino-Wittmann MT, Dall'Agnol M and Castro B (2008) Cytogenetic data for Paspalum notatum Flugge accessions. Scientia Agricola 65: 381-388. ). For the preparation of the slides, inflorescences were dissected, stained with 1% propionic carmine, and analyzed in optical microscope. It was sought to observe cells at different stages of meiotic division, as well as the arrangement of chromosomes. All meiotic abnormalities were considered. For the verification of chromosome pairing, analyses were carried out in at least 20 cells per plant at diakinesis and metaphase I stages (Dahmer et al. 2008, Simioni and Valle 2011Simioni C and Valle CB (2011) Meiotic analysis in induced tetraploids of Brachiaria decumbens Stapf. Crop Breeding and Applied Biotechnology 11: 43-49.).
Pollen viability analysis
Pollen grains viability was estimated in the anthers collected from inflorescences at mature stage, fixed in 3: 1 solution (absolute ethanol: glacial acetic acid), at room temperature for 24 hours, and stored in 70% alcohol until analysis. In the preparation of the slides, pollen grains were extracted from flowers, stained with 1% propionic carmine, and observed in optical microscope. Pollen grains were considered fertile when full and well stained, while those unstained or weakly stained, were considered sterile (not viable unviable) (Singh 1993Singh RJ (1993) Plant cytogenetics. CRC Press, Boca Ratton, 391p.). One thousand mature pollen grains were counted in four flowers per plant, following the protocol already established and widely used (Dahmer et al. 2008Dahmer N, Schifino-Wittmann MT, Dall'Agnol M and Castro B (2008) Cytogenetic data for Paspalum notatum Flugge accessions. Scientia Agricola 65: 381-388. , Guerra et al. 2013Guerra D, Schifino-Wittmann MT, Schwarz SF, Souza PVD and Campos SS (2013) Reproductive characteristics of citrus rootstocks grown under greenhouse and field environments. Crop Breeding and Applied Biotechnology 13: 186-193.).
RESULTS AND DISCUSSION
Mode of reproduction analysis
The duplicated plants WKS 63 and WKS 92 confirmed having sexual mode of reproduction (Table 1), with Polygonum type embryo sac: a single meiotic embryo sac, two polar nuclei, and a cluster of antipodal cells toward the chalazal (Figure 1a). WKS 3 presented modifications in its mode of reproduction after chromosome duplication, and became apomictic. This plant had ovaries with multiple aposporic embryo sacs, which are characterized by the egg cell, one or two synergids, a binucleated central cell, and absence of antipodes (Figure 1b). Quarin et al. (2001Quarin CL, Espinoza F, Martinez EJ, Pessino SC and Bovo OA (2001) A rise of ploidy level induces the expression of apomixis in Paspalum notatum. Sexual Plant Reprodution 13: 243-249.) recorded this phenomenon by analyzing the mode of reproduction of three P. notatum plants artificially duplicated; two of them had facultative apomictic reproduction. Based on these results, the authors state that the apomixis gene is present at the diploid level; however, it is not expressed in the plant. The ploidy-dependence may occur at a locus that controls the apomixis by means of a secondary factor that requires higher dosage of alleles to affect the expression of major locus. It is likely that the expression of apomixis in this duplicated plant is a gene dosage effect. Simioni and Valle (2011Simioni C and Valle CB (2011) Meiotic analysis in induced tetraploids of Brachiaria decumbens Stapf. Crop Breeding and Applied Biotechnology 11: 43-49.) observed the confirmation of the sexual mode of reproduction in three plants obtained by somatic chromosome duplication of sexually reproducing diploid genotype of Brachiaria decumbens.
a) Cytoembryological aspect of a sexual ovary (duplicated plant WKS 63): antipodes (1) and polar nuclei (2); b) Cytoembryological aspect of apomictic ovary (duplicated plant WKS 3), multiple sacs (arrows). Scale: 10 µm.
Number and percentage (%) of meiotic (S), apomictic (A), unidentifiable (U), shriveled (Sh), embryo sacs total analyzed ovaries (T), and mode of reproduction (MR) of the three P. notatum tetraploidized plants
Analyses of the mode of reproduction of parents and progenies are fundamental in breeding programs aimed at enabling intraspecific hybridizations. For the choice of the parents, this evaluation allows identifying the plants that will be used as female parents (plants of sexual reproduction) and as male parents (apomictic plants).
Analysis of meiotic behavior
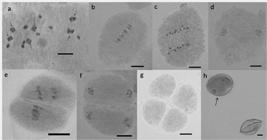
Chromosome associations were observed at the diakinesis and metaphase I stages, which are the best stages for visualization of chromosome configurations (Dahmer et al. 2008Dahmer N, Schifino-Wittmann MT, Dall'Agnol M and Castro B (2008) Cytogenetic data for Paspalum notatum Flugge accessions. Scientia Agricola 65: 381-388. , Simioni and Valle 2011Simioni C and Valle CB (2011) Meiotic analysis in induced tetraploids of Brachiaria decumbens Stapf. Crop Breeding and Applied Biotechnology 11: 43-49.). The three plants confirmed tetraploidy (2n=4x=40), as described by Weiler et al. (2015Weiler RL, Krycki KC, Guerra D, Simioni C and Dall'Agnol M (2015) Chromosome doubling in Paspalum notatum var. saure (cultivar Pensacola). Crop Breeding and Applied Biotechnology 15: 106-111.). Chromosome pairing was typical of tetraploidy, with univalent, bivalent, trivalent and quadrivalent chromosome associations (Table 2). WKS 63 (Figure 2) presented mostly quadrivalent associations. According to Ramsey and Schemske (2002Ramsey J and Schemske DW (2002) Neopolyploid in flowering plants. Annual Review of Ecology and Systematics 33: 589-631.), genotypes with polysomic inheritance have tendency for multivalent formation.
Meiotic aspects of the tetraplidized plant WKS 63. a) Diakinesis. It is observed the presence of quadrivalent associations (arrows). b) Normal metaphase I. c) Normal anaphase I. d) Normal telophase I. e) Anaphase II with asynchrony. f) Normal telophase II. g) Microsporocyte (Tetrad). h) Viable pollen grain (stained; arrow), and unviable pollen grain (non-stained). Scale: 10 µm.
The plants WKS 92 (Figure 3) and WKS 3 (Figure 4) presented less meiotic abnormalities when compared with WKS 63, with most of the chromosomes in bivalent and sporadic quadrivalent associations, showing tendency for regularization of the chromosome pairing and genetic control of the pairing in these newly formed tetraploids. According to Dahmer et al. (2008Dahmer N, Schifino-Wittmann MT, Dall'Agnol M and Castro B (2008) Cytogenetic data for Paspalum notatum Flugge accessions. Scientia Agricola 65: 381-388. ), in the case of apomictic ecotypes, there is the need of meiotic regularity, since they are pseudogamic, so that to it is possible to ensure sufficient pollen fertility to form the endosperm. This is the case of WKS 3 in the present experiment.
Meiotic aspects of the tetraploidized plant WKS 92. a) Diakinesis. It was observed the presence of one quadrivalent association (arrow). b) Normal metaphase. c) Anaphase I with the presence of laggard chromosomes (arrows). d) Normal telophase I. e) Normal metaphase II. f) Normal anaphase II. g) Normal telophase II. h) Microsporocyte (triad and tetrad). Scale: 10 µm.
Meiotic aspects of the tetraploidized plant WKS 3. a) Diakinesis. It was observed the presence of two quadrivalents associations (arrows). b) Metaphase I. Presence of chromosomes in early ascension (arrow). c) Anaphase I with the presence of laggard chromosomes (arrows). d) Normal telophase I. e) Normal anaphase II. f) Anaphase II with asynchrony. It was observed the presence of laggard chromosome (arrow). g) Microsporocyte (polyads with micronuclei). h) Viable pollen grains (stained). Scale: 10 µm.
Several studies on tetraploid species and accessions have registered regular meiosis and wide variability of the emergence of several uni, bi, tri and tetravalent chromosome associations. The predominance of bivalent associations were observed in 36 (Dahmer et al. 2008Dahmer N, Schifino-Wittmann MT, Dall'Agnol M and Castro B (2008) Cytogenetic data for Paspalum notatum Flugge accessions. Scientia Agricola 65: 381-388. ) and five (Moraes-Fernandes et al. 1973Moraes-Fernandes MIB, Barreto IL and Salzano FM (1973) Cytogenetic, ecologic and morphologic studies in Brazilian forms of Paspalum notatum. Canadian Journal of Genetics and Cytology 15: 523-531.) P. notatum accessions, 24 accessions of different Paspalum species (Pagliarini et al. 2001Pagliarini MS, Carraro LR, Freitas PM, Adamowski EV, Batista LA and Valls JFM (2001) Cytogenetic characterization of Brazilian Paspalum accessions. Hereditas 135: 27-34. ), 53 Paspalum nicorae accessions (Reis et al. 2008Reis CAO, Schifino-Wittmann MT and Dall'agnol M (2008) Chromosome numbers, meiotic behavior and pollen fertility in a collection of Paspalum nicorae Parodi accessions. Crop Breeding and Applied Biotechnology 8: 212-218.), three polyploidized plants of Brachiaria decumbens (Simioni and Valle 2011Simioni C and Valle CB (2011) Meiotic analysis in induced tetraploids of Brachiaria decumbens Stapf. Crop Breeding and Applied Biotechnology 11: 43-49.), six accessions of different Brachiaria species (Araújo et al. 2005Araújo ACG, Nóbrega JM, Pozzobon MT and Carneiro VTC (2005) Evidence of sexuality in induced tetraploids of Brachiaria brizantha (Poaceae). Euphytica 144: 39-40.), one Brachiaria ruziziensis accession (Risso-Pascotto et al. 2005Risso-Pascotto C, Pagliarini MS and Valle CB (2005) Multiple spindle sand cellularization during microsporogenesis in an artificial induced tetraploid accession of Brachiaria ruziziensis (Gramineae). Plant Cell Reports 23: 522-527. ) and a Paspalum durifolium accession (Quarin 1994Quarin CL (1994) A Tetraploid cytotype of Paspalum durifolium: cytology, reproductive behavior and this relationship to diploid P. intermedium. Hereditas 121: 115-118.). In contrast, other authors reported accessions in which most of the associations were uni- or multivalent: B. brizantha, B. decumbens and B. ruziziensis (Valle and Savidan 1996Valle CB and Savidan YH (1996) Genetics, cytogenetics and reproductive biology of Brachiaria. In Miles JW, Maass BL and Valle CB (eds) Brachiaria: biology, agronomy and improvement. CIAT/EMBRAPA, Cali, p. 147-163. ), B. decumbens cv. Basilisk (Junqueira-Filho et al. 2003Junqueira-Filho RG, Mendes-Bonato MB, Pagliarini MS, Bione NCP, Valle CB and Penteado MIO (2003) Absence of microspore polarity, symmetric divisions and pollen cell fate in Brachiaria decumbens (Gramineae). Genome 46: 83-86.), and Panicum maximum (Caetano et al. 2006Caetano CM, Bonfá BRCN and Canto MW (2006) Autotetraploidia e número cromossômico em uma cultivar de Panicum maximum Jacq (Gramineae/Poaceae). Acta Agronomica 55: 62-66. , Pessim et al. 2010Pessim C, Pagliarini MS, Jank L, Kaneshima MAS and Mendes-Bonato MB (2010) Meiotic Behavior in Panicum maximum Jacq. (Poaceae: Panicoideae: Paniceae): hybrids and their genitors. Acta Scientiarum 32: 417-422.).
Table 3 shows a total of 4082 microsporocytes analyzed for meiotic behavior in the three duplicated plants in this experiment. In 442 cells, there were no irregularities from prophase I to the meiotic products, representing a mean percentage of meiotic abnormalities of 10.83% in the three plants. Tetraploid plants generally have meiotic abnormalities related to irregular chromosomes segregation, which generates genetically unbalanced microspores, and thus hinders the fertility of pollen grains (Pagliarini and Pozzobon 2004Pagliarini MS and Pozzobon MT (2004) Meiose vegetal: um enfoque para a caracterização de germoplasma. In Peñaloza APS (ed) Anais do II curso de citogenética aplicada a recursos genéticos vegetais. EMBRAPA, Brasília, p. 24-41.). Multiple associations in diakinesis and abnormalities related to irregular chromosome segregation (early ascension) were the most frequent abnormalities in both divisions in the three plants. It was also noted laggard chromosomes and asynchrony in the three plants. Few bridges and micronucleus were observed. The meiotic products were mostly normal, with few dyads, triads and polyads, which resulted in excellent pollen viability of these plants.
Podio et al. (2012Podio M, Siena LA, Hojsgaard D, Stein J, Quarin CL and Ortiz JPA (2012) Evaluation of meiotic abnormalities and pollen viability in aposporous and sexual tetraploid Paspalum notatum (Poaceae). Plant Systematics and Evolution 298: 1625-1633.) analyzed five natural apomictic tetraploid accessions and three artificially induced sexual tetraploid of P. notatum, and found that, in the apomictics, 55.6% of the cells at anaphase I are normal, and in the sexual accessions, 70.3% of the cells at anaphase I are normal. Abnormalities were mostly laggard chromosomes, chromatin bridges, and the presence of micronuclei, which appeared in 44.3% of the apomictic and in 29.66% of the sexual plants. In telophase I, both apomictic and sexual accessions presented chromosomes clustered in the poles of the cells, as well as small size micronuclei, suggesting they were composed of chromosome fragments. It can be inferred that meiotic behavior is characteristic of each genotype, as previously reported by Stein et al. (2004Stein J, Quarin CL, Martinez EJ, Pessino SC and Ortiz JPA (2004) Tetraploid races of Paspalum notatum show polysomic inheritance and preferential chromosome pairing around the apospory-controlling locus. Theoretical and Applied Genetics 109: 186-191.) for the species.
Pessim et al. (2010Pessim C, Pagliarini MS, Jank L, Kaneshima MAS and Mendes-Bonato MB (2010) Meiotic Behavior in Panicum maximum Jacq. (Poaceae: Panicoideae: Paniceae): hybrids and their genitors. Acta Scientiarum 32: 417-422.) observed high meiotic stability in hybrid genotypes and parents of P. maximum, with abnormalities ranging from 6.7 to 14.2%, such as irregular chromosome segregation, chromosome stickiness, and absence of cytokinesis. However, they did not affect pollen viability.
Analyses of pollen viability
The three duplicated plants showed high pollen viability: WKS 3, WKS 63 and WKS 92 recorded 92.3%, 88.7% and 95.7% of stained pollen grains, respectively. Studies reported pollen viability of ecotypes and of native apomictic accessions of P. notatum in the state of Rio Grande do Sul:Dahmer et al (2008Dahmer N, Schifino-Wittmann MT, Dall'Agnol M and Castro B (2008) Cytogenetic data for Paspalum notatum Flugge accessions. Scientia Agricola 65: 381-388. ) found pollen viability ranging from 81.0 to 91.47% in the ecotype "Bagual", and of 86.0 to 98.0% in the ecotype "André da Rocha". Moraes-Fernandes et al. (1973Moraes-Fernandes MIB, Barreto IL and Salzano FM (1973) Cytogenetic, ecologic and morphologic studies in Brazilian forms of Paspalum notatum. Canadian Journal of Genetics and Cytology 15: 523-531.) reported pollen fertility ranging from 0 to 84.3%; and Reis et al. (2008Reis CAO, Schifino-Wittmann MT and Dall'agnol M (2008) Chromosome numbers, meiotic behavior and pollen fertility in a collection of Paspalum nicorae Parodi accessions. Crop Breeding and Applied Biotechnology 8: 212-218.) found pollen viability ranging from 88.99 to 95.06% in 53 accessions, despite the numerous meiotic irregularities found. The high pollen viability of apomictic plants is expected, since seed formation occurs only if there is fertilization of the polar nuclei of the embryo sac by one of the gametic nuclei of pollen grain, due to pseudogamy, typical in species of Paspalum and Brachiaria, which present this mode of reproduction (Pagliarini and Pozzobon 2004Pagliarini MS and Pozzobon MT (2004) Meiose vegetal: um enfoque para a caracterização de germoplasma. In Peñaloza APS (ed) Anais do II curso de citogenética aplicada a recursos genéticos vegetais. EMBRAPA, Brasília, p. 24-41.).
Cytological analysis are important tools in breeding programs for the selection of compatible and fertile parents which do not present meiotic abnormalities that may hinder gametes viability (Simioni and Valle 2011Simioni C and Valle CB (2011) Meiotic analysis in induced tetraploids of Brachiaria decumbens Stapf. Crop Breeding and Applied Biotechnology 11: 43-49.). This work allowed observing satisfactory meiotic regularity of the three duplicated plants, and made them viable as parents in the breeding program.
Guerra et al (2016Guerra D, De Souza PVD, Schwarz SF, Schifino-Wittmann MT, Werlang CA and Veit PA (2016) Genetic and cytological diversity in cherry tree accessions (Eugenia involucrata DC) in Rio Grande do Sul. Crop Breeding and Applied Biotechnology 16: 219-225.) also recorded cytological stability of 35 cherry trees accessions (Eugenia involucrata DC) collected in the state of Rio Grande do Sul; the average of meiotic cells considered normal was 82.12% and the average pollen viability was 92.44%. The authors concluded that such native access can be used directly in commercial orchards, and also as male parents in directed crosses in breeding programs such as those presented in this work.
With the study of the mode of reproduction, it was possible to direct the plants for intraspecific crosses: WKS 3, of apomictic reproduction, was used as pollen donor, and WKS 63 and WKS and 92 were used as female parents in the hybridizations. In further stages of the program, the hybrid progeny will be evaluated in agronomic trials under field conditions, in order to select genotypes that meet the demands of increased forage yield and that can contribute to the preservation of southern fields, preventing their degradation. This work represents a progress for the development of the Brazilian southern farming, with the use of well-adapted materials, diversifying forage production.
ACKNOWLEDGMENTS
The authors thank SULPASTO, CNPq and CAPES for the financial support, and the Scientific Initiation students Miguel Godinho Verran and Marília Wieser Paz.
REFERENCES
- Acuña CA, Blount AR, Quesenberry KH, Hanna WW and Kenworthy KE (2007) Reproductive characterization of bahiagrass germplasm. Crop Science 47: 1711-1717.
- Araújo ACG, Nóbrega JM, Pozzobon MT and Carneiro VTC (2005) Evidence of sexuality in induced tetraploids of Brachiaria brizantha (Poaceae). Euphytica 144: 39-40.
- Burton GW and Forbes I (1961) Cytology of diploids, natural and induced tetraploids, and intraspecies hybrids of bahiagrass,Paspalum notatum Flugge. Crop Science 1: 402-406.
- Caetano CM, Bonfá BRCN and Canto MW (2006) Autotetraploidia e número cromossômico em uma cultivar de Panicum maximum Jacq (Gramineae/Poaceae). Acta Agronomica 55: 62-66.
- Dahmer N, Schifino-Wittmann MT, Dall'Agnol M and Castro B (2008) Cytogenetic data for Paspalum notatum Flugge accessions. Scientia Agricola 65: 381-388.
- Guerra D, Schifino-Wittmann MT, Schwarz SF, Souza PVD and Campos SS (2013) Reproductive characteristics of citrus rootstocks grown under greenhouse and field environments. Crop Breeding and Applied Biotechnology 13: 186-193.
- Guerra D, De Souza PVD, Schwarz SF, Schifino-Wittmann MT, Werlang CA and Veit PA (2016) Genetic and cytological diversity in cherry tree accessions (Eugenia involucrata DC) in Rio Grande do Sul. Crop Breeding and Applied Biotechnology 16: 219-225.
- Junqueira-Filho RG, Mendes-Bonato MB, Pagliarini MS, Bione NCP, Valle CB and Penteado MIO (2003) Absence of microspore polarity, symmetric divisions and pollen cell fate in Brachiaria decumbens (Gramineae). Genome 46: 83-86.
- Macedo MCM (2009) Integração lavoura e pecuária: o estado da arte e inovações tecnológicas. Revista Brasileira de Zootecnia 38: 133-146.
- Moraes-Fernandes MIB, Barreto IL and Salzano FM (1973) Cytogenetic, ecologic and morphologic studies in Brazilian forms of Paspalum notatum Canadian Journal of Genetics and Cytology 15: 523-531.
- Nabinger C and Dall'Agnol M (2008) Principais gramíneas nativas do RS: características gerais, distribuição e potencial forrageiro. In Dall'Agnol M, Nabinger C and Santos RJ (eds) Anais do 3( simpósio de forrageiras e produção animal. UFRGS, Porto Alegre, p.7-54.
- Pagliarini MS and Pozzobon MT (2004) Meiose vegetal: um enfoque para a caracterização de germoplasma. In Peñaloza APS (ed) Anais do II curso de citogenética aplicada a recursos genéticos vegetais. EMBRAPA, Brasília, p. 24-41.
- Pagliarini MS, Carraro LR, Freitas PM, Adamowski EV, Batista LA and Valls JFM (2001) Cytogenetic characterization of Brazilian Paspalum accessions. Hereditas 135: 27-34.
- Pessim C, Pagliarini MS, Jank L, Kaneshima MAS and Mendes-Bonato MB (2010) Meiotic Behavior in Panicum maximum Jacq. (Poaceae: Panicoideae: Paniceae): hybrids and their genitors. Acta Scientiarum 32: 417-422.
- Podio M, Siena LA, Hojsgaard D, Stein J, Quarin CL and Ortiz JPA (2012) Evaluation of meiotic abnormalities and pollen viability in aposporous and sexual tetraploid Paspalum notatum (Poaceae). Plant Systematics and Evolution 298: 1625-1633.
- Quarin CL (1992) The nature of apomixis and its origin in Panicoid grasses. Apomixis Newslett 5: 7-15.
- Quarin CL (1994) A Tetraploid cytotype of Paspalum durifolium: cytology, reproductive behavior and this relationship to diploid P. intermedium Hereditas 121: 115-118.
- Quarin CL, Espinoza F, Martinez EJ, Pessino SC and Bovo OA (2001) A rise of ploidy level induces the expression of apomixis in Paspalum notatum Sexual Plant Reprodution 13: 243-249.
- Ramsey J and Schemske DW (2002) Neopolyploid in flowering plants. Annual Review of Ecology and Systematics 33: 589-631.
- Reis CAO, Schifino-Wittmann MT and Dall'agnol M (2008) Chromosome numbers, meiotic behavior and pollen fertility in a collection of Paspalum nicorae Parodi accessions. Crop Breeding and Applied Biotechnology 8: 212-218.
- Risso-Pascotto C, Pagliarini MS and Valle CB (2005) Multiple spindle sand cellularization during microsporogenesis in an artificial induced tetraploid accession of Brachiaria ruziziensis (Gramineae). Plant Cell Reports 23: 522-527.
- Simioni C and Valle CB (2011) Meiotic analysis in induced tetraploids of Brachiaria decumbens Stapf. Crop Breeding and Applied Biotechnology 11: 43-49.
- Singh RJ (1993) Plant cytogenetics. CRC Press, Boca Ratton, 391p.
- Stein J, Quarin CL, Martinez EJ, Pessino SC and Ortiz JPA (2004) Tetraploid races of Paspalum notatum show polysomic inheritance and preferential chromosome pairing around the apospory-controlling locus. Theoretical and Applied Genetics 109: 186-191.
- Valle CB and Savidan YH (1996) Genetics, cytogenetics and reproductive biology of Brachiaria In Miles JW, Maass BL and Valle CB (eds) Brachiaria: biology, agronomy and improvement. CIAT/EMBRAPA, Cali, p. 147-163.
- Weiler RL, Krycki KC, Guerra D, Simioni C and Dall'Agnol M (2015) Chromosome doubling in Paspalum notatum var. saure (cultivar Pensacola). Crop Breeding and Applied Biotechnology 15: 106-111.
- Young BA, Sherwood RT and Bashaw EC (1979) Cleared-pistil and thick-sectioning techniques for detecting aposporus apomixis in grasses. Canadian Journal of Botany 57: 1668-1672.
Publication Dates
-
Publication in this collection
Dec 2016
History
-
Received
07 Apr 2015 -
Accepted
20 May 2016