Abstract
We report the in vitro induction of polyploids from two cassava cultivars by colchicine treatment. Shoot nodal segments, collected from in vitro cultivated plants of Porquinho and Vassourinha cultivars, were used as explants. Explants were treated in liquid media without and with colchicine (0.05, 0.10 or 0.15%), at 90 rpm, in the dark, for 48 or 96 h. Normal-like and putative-polyploid phenotypes were recovered from in vitro culture, for both cultivars. All plants from the putative-polyploid group were confirmed as tetraploids by flow cytometry and root tip chromosome counting, whereas no association was found between ploidy level and phenotype in normal-like plants. Mixoploids were also recovered from both cultivars. Vassourinha was more responsive to colchicine treatment than Porquinho. Tetraploid plants have fewer, but bigger, stomata guard cells than those in diploids. Colchicine at 0.10% for 96 hours induced a high number of tetraploids in both cultivars.
Key words:
Chimera; chromosome doubling; colchicine; Manihot esculenta; polyploidy
INTRODUCTION
Polyploidy is a driving force in plant evolution, and most angiosperms have experienced at least one round of chromosome doubling (Jiao et al. 2011Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis Ps, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J and dePamphilis CW (2011) Ancestral polyploidy in seed plants and angiosperms. Nature 473: 97-100., Madlung 2013Madlung A (2013) Polyploidy and its effect on evolutionary success: old questions revisited with new tools. Heredity 110: 99-104.). Chromosome duplication can occur naturally, generally at low frequencies (Hahn et al. 1992Hahn SK, Bai VK and Asiedu R (1992) Spontaneous somatic tetraploids in cassava. Japan Journal of Breeding 42: 303-308.), and thus, induce phenotypic modifications. Once these phenotypic alterations could be identified in nature, many artificial strategies were developed to increase the frequency of polyploid induction. These new lines often express the gigas effect, a common phenomenon in polyploid plants, and morphologically observed as larger tissues and organs that can be useful in plant breeding and crop production (Dhooghe et al. 2011Dhooghe E, Van Laere K, Eeckhaut T, Leus L and Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell, Tissue and Organ Culture 104: 359-373., Pereira et al. 2012Pereira RC, Davide LC, Techio VH and Timbó ALO (2012) Chromosome doubling of grasses: an alternative to plant breeding. Ciência Rural 42: 1278-1285.).
Examples of artificially induced polyploids expressing interesting phenotypes have been reported in several species, including roses, citrus fruit, orchids, banana and cassava. In roses, artificially induced polyploids had larger flowers with more petals (Kermani et al. 2003Kermani MJ, Sarasan V, Roberts AV, Yokoya K, Wentworth J and Sieber VK (2003) Oryzalin-induced chromosome doubling in Rosa and its effect on plant morphology and pollen viability. Theoretical and Applied Genetics 107: 1195-1200., Khosravi et al. 2008Khosravi P, Kermani MJ, Nematzadeh GA, Bihamta MR and Yokoya K (2008) Role of mitotic inhibitors and genotype on chromosome doubling of Rosa. Euphytica 160: 267-275. ). Artificially induced polyploids in orchids, such as Dendrobium, Epidendrum, Odontioda and Phalaenopsis, have been produced to obtain larger flowers (Miguel and Leonhardt 2011Miguel TP and Leonhardt KW (2011) In vitro polyploid induction of orchids using oryzalin. Scientia Horticulturae 130: 314-319., Vichiato et al. 2014Vichiato MRDM, Vichiato M, Pasqual M, Rodrigues FA and Castro DMD (2014) Morphological effects of induced polyploidy in Dendrobium nobile Lindl. (Orchidaceae). Crop Breeding and Applied Biotechnology 14: 154-159.). In citrus, colchicine-induced autotetraploid pummelos (C. grandis) have been produced to be further used as tetraploid breeding parents to produce superior triploid seedless cultivars (Grosser et al. 2014Grosser JW, Kainth D and Dutt M (2014) Production of colchicine-induced autotetraploids in pummelo (Citrus grandis Osbeck) through indirect organogenesis. HortScience 49: 944-948.), while autotetraploid plants of citrumelo and citranges (all Citrus sp. X Poncirus trifoliata hybrids) were produced to be used directly as rootstocks, because they can produce smaller plants (Guerra et al. 2014Guerra D, Wittmann MTS, Schwarz SF, Souza PVD, Gonzatto MP and Weiler RL (2014) Comparison between diploid and tetraploid citrus rootstocks: morphological characterization and growth evaluation. Bragantia 73: 1-7.).
Chromosome doubling can be induced by a wide range of substances, which usually act by blocking spindle fiber polymerization. However, in recent years, in vitro polyploid induction has mostly been achieved with the use of colchicine and oryzalin (Dhooghe et al. 2011Dhooghe E, Van Laere K, Eeckhaut T, Leus L and Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell, Tissue and Organ Culture 104: 359-373.). Both substances have been evaluated in several species, such as banana, potato and cassava. Given its genotype-dependence effect, some research has indicated that oryzalin was less efficient at chromosome duplication in cassava (Awoleye et al. 1994Awoleye F, Van Duren M, Dolezel J and Novak FJ (1994) Nuclear DNA content and in vitro induced somatic polyploidization cassava (Manihot esculenta Crantz) breeding. Euphytica 76: 195-202.), but more efficient for potato (Sree Ramulu et al. 1991Sree Ramulu K, Verhoeven HA and Dijkhuis P (1991) Mitotic blocking, micronucleation, and chromosome doubling by oryzalin, amiprophos-methyl, and colchicine in potato. Protoplasma 160: 65-71.). Oryzalin was also more efficient than colchicine at producing polyploid bananas (van Duren et al. 1996van Duren M, Morpurgo R, Dolezel J and Afza R (1996) Induction and verification of autotetraploids in diploid banana (Musa acuminata) by in vitro techniques. Euphytica 88: 25-34.).
Manihot esculenta (cassava) belongs to the Euphorbiaceae family. This species has a perennial shrub habit, and is one of the most important root and tuber crops in tropical regions, mainly being produced in developing countries. Despite its high genetic variability in germplasm resources, polyploidy induction is an important tool for cassava breeding due to its capacity to improve anatomical traits (Nassar et al. 2008Nassar NM, Graciano-Ribeiro D, Fernandes SDC and Araujo PC (2008) Anatomical alterations due to polyploidy in cassava, Manihot esculenta Crantz. Genetics and Molecular Research 7: 276-283., Graciano-Ribeiro and Nassar 2012Graciano-Ribeiro D and Nassar NMA (2012) A comparative anatomical study in cassava diploid and tetraploid hybrids. Plant Systematics and Evolution 298: 1771-1721.). Moreover, a proteomic study concluded that there was remarkable variation in photosynthetic activity, HCN content and resistance to salt stress in polyploid plants (An et al. 2014An F, Fan J, Li J, Li QX, Li K, Zhu W, Wen F, Carvalho LJCB and Chen S (2014) Comparison of leaf proteomes of cassava (Manihot esculenta Crantz) cultivar NZ199 diploid and autotetraploid genotypes. PloS One 4: e0085991.).
Natural polyploidy has also been reported for cassava but is mostly restricted to tetraploid and triploid plants (Hahn et al. 1990Hahn SK, Bai KV and Asiedu R (1990) Tetraploids, triploids, and 2n pollen from diploid interspecific crosses with cassava. Theoretical and Applied Genetics 79: 433-439., 1992Hahn SK, Bai VK and Asiedu R (1992) Spontaneous somatic tetraploids in cassava. Japan Journal of Breeding 42: 303-308.), suggesting the occurrence of unreduced 2n-gametes. Induced artificial chromosome doubling is a better way to obtain polyploid lines from interesting superior genetic material. These polyploid lines have the potential to be used directly as new cultivars or integrated into cassava breeding programs as genitors.
The production of artificial polyploids of cassava has been reported using in vivo treatment of nodal segments (Nassar 2004Nassar N (2004) Polyploidy, chimera and fertility of interspecific cassava (Manihot esculenta Crantz) hybrids. The Indian Journal of Genetics and Plant Breeding 64: 317-318.), in vitro treatment of apical and axillar buds (Awoleye et al. 1994Awoleye F, Van Duren M, Dolezel J and Novak FJ (1994) Nuclear DNA content and in vitro induced somatic polyploidization cassava (Manihot esculenta Crantz) breeding. Euphytica 76: 195-202., Carvalho et al. 2016Carvalho MJS, Gomes VB, Souza AS, Aud FF, Santos-Serejo JA and Oliveira EJ (2016) Inducing autotetraploids in cassava using oryzalin and colchicine and their in vitro morphophysiological effects. Genetics and Molecular Research 15: gmr.15028281), and induction of unreduced gametes before fertilization (Lai et al. 2015Lai HG, Chen X, Chen Z, Ye JQ, Li KM and Jin-Ping L (2015) Induction of female 2n gametes and creation of tetraploids through sexual hybridization in cassava (Manihot esculenta). Euphytica 201: 265-273.). Given the importance of cassava as a basic food worldwide and the potential of polyploids in plant improvement, we report herein the results of chromosome doubling in plants of two cassava cultivars using colchicine treatment under in vitro culture.
MATERIAL AND METHODS
Plant material, explant preparation and colchicine treatment
Cassava cultivars ‘Porquinho’ and ‘Vassourinha’ (Manihot esculenta) were used as explant sources. The criteria utilized for cultivar selection in this study took into account their phenotypic diversity, good in vitro response in previous experiments, and their potential for cassava improvement. ‘Porquinho’ is a cultivar for processing. Plants are short in stature and show resistance to bacterial diseases. ‘Vassourinha’ is more appropriate for direct consumption and shows high production of stalks and tuberous roots.
Plant material was collected from an experimental field at the Fundação Estadual de Pesquisa Agropecuária do Estado do Rio Grande do Sul (Fepagro), Brazil. Stocks were planted in Piracicaba, SP, Brazil, and cultivated for explant collection. Plant shoots of both cultivars were collected and disinfested in sodium hypochlorite solution (1%) for 1 minute, rinsed in tap water, cut into 15-cm-long pieces, and placed in containers with a soil:vermiculite (4:1) mixture for sprouting.
Shoots that developed after 30 days of culture, and were about 20-30 mm long, were collected and disinfested in an ethanol solution (50%) for 1 minute, and then in a sodium hypochlorite solution (0.25%) containing one drop of detergent (Tween 20) for 15 minutes. Under aseptic conditions, this material (plant shoots) was then rinsed three times in sterile distilled water (1 minute per rinse). Shoots were cut into 10 to 15 mm nodal segments and introduced into Murashige and Skoog (1962Murashige T and Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497.) culture media, modified by the addition of pentahydrate cuprous sulfate (0.5 mg L-1), and supplemented with NAA (0.02 mg L-1), BAP (0.04 mg L-1) and GA3 (0.05 mg L-1), at pH 5.8 (Souza et al. 2009Souza AS, Souza FVD, Santos-Serejo JA, Junghans TG, Paz OP, Montarroyos AVV, Santos VS and Morais LS (2009) Preservação de germoplasma vegetal, com ênfase na conservação in vitro de variedades de mandioca. Embrapa, Cruz das almas, 24p. (Circular Técnica 90).). Explants were incubated at 25 ± 2 oC in a 16 h photoperiod (35 µmol m-2 s-1).
After three subcultures, in vitro shoots, about 1-2 cm long, were treated in liquid media of the same composition, without or with colchicine (0.05, 0.10, 0.15%), in a rotary shaker (90 rpm), at 25 ± 2 oC, in the dark, for 48 or 96 h. Each treatment comprised 50 nodal segments, with two replicates (except controls, which comprised 25 nodal segments), resulting in a total of 700 explants per cultivar. After colchicine treatment, explants were transferred to liquid culture media without colchicine for 24 h, and then transferred again to semi-solid media with the same composition. Experiments were terminated when regenerated plants from in vitro culture were sampled for analyses after three subcultures, i.e., before plant acclimatization. Each subculture had a duration of approximately 30 days. Plants were ready for acclimatization approximately 6 months after starting the experiments.
Flow cytometry
Leaves from in vitro regenerated plants were collected for flow cytometry analyses. Nuclei suspensions were prepared according to the following procedure: Leaf samples (50 mg) were chopped 30-50 times in a Petri dish with 200 µL nuclei extraction buffer solution (Cy-UV stain - Partec Gmbh, Germany). After one minute, 0.8 mL DAPI solution (Cy-UV stain - Partec Gmbh, Germany) was added and the mixture was filtered through a nylon screen (30 µm). The filtered solution was analyzed in a UV-LED Partec flow cytometer (CyFlow Ploidy Analyser, Partec Gmbh, Germany) with light emission at 365 nm, adjusted to fluorescence optical detection to Gain = 597 and Low Level (LL) of 0.64. More than 5,000 nuclei were assessed in each sample. Nuclear DNA histograms were constructed using CyView software (Partec Gmbh, Germany).
Chromosome counting
Root tips of regenerated plants (with and without treatment) ranging from 1-1.5 cm were harvested, pretreated in an 8-hydroxyquinoline solution (0.002 M) for 3 hours, fixed in Carnoy solution (3 parts of ethanol and 1 part of acetic acid) at room temperature for 24 h and then kept at 4 oC. Chromosome staining was carried out using the Feulgen method (Cuco et al. 2003Cuco SM, Mondin M, Vieira MLC and Aguiar-Perecin ML (2003) Técnicas para a obtenção de preparações citológicas com alta frequência de metáfases mitóticas em plantas: Passiflora (Passifloraceae) e Crotalaria (Leguminosae). Acta Botanica Brasilica 17: 363-370., Mondin et al. 2007Mondin M, Santos-Serejo JA and Aguiar-Perecin ML (2007) Karyotype characterization of Crotalaria juncea (L.) by chromosome banding and physical mapping of 18S-5.8 S-26S and 5S rRNA gene sites. Genetics and Molecular Biology 30: 65-72.). In brief, root tips were rinsed two times for 5 minutes and then hydrolyzed in HCl solution (1 M) at 60 oC for 8 minutes, and rinsed again to remove the acid. Hydrolyzed root tips were incubated in Schiff reactive solution in the dark for 45 minutes, for color development. For slide preparation, stained root tips were rinsed twice in citrate buffer (0.01 M) for 5 minutes and digested by a mixture of cellulase (9.2 U mL-1) and pectinase (14.7 U mL-1), for 15 minutes. After digestion, the samples were rinsed again and kept in cold citrate buffer solution till slide preparation. One root tip was macerated on a slide by adding one drop of 1% acetic carmine solution and squashed to spread the chromosomes. The coverslip was removed in liquid-nitrogen, air-dried and mounted in Entellan. Metaphase plates with well-spread chromosomes were digitalized using a CCD camera coupled to an Axiophot 2 - Zeiss microscope using the IKAROS Metasystems software.
Stomatal morphometry
Stomatal analysis was carried out using molds produced through the application of nitrocellulose layers on the abaxial surface of developed leaves, collected from regenerated plants. Layers were dried and the plastic film was removed and transferred to distilled water, and then to a glass slide (Hamill et al. 1992Hamill SD, Smith MK and Dodd WA (1992) In vitro induction of banana autotetraploids by colchicine treatment of micropropagated diploids. Australian Journal of Botany 40: 887-896.). Analysis was performed in a Zeiss Axiophot microscope (400x magnification) equipped with an AVT-Horn color video camera connected to a computer, and ToupView 3.7 software. About 25 stomata per leaf were analyzed (five stomata in five aleatory fields), in their largest diameter (length), and perpendicular to the ostiole (width), as well as the number of stomata per leaf area (stomatal frequency per mm2).
Statistical analyses
The polyploid induction experiment was conducted using a randomized design with two cultivars, three colchicine concentrations and two exposure times, and 50 explants per treatment (except for control, with 25), and was repeated twice. Data were subjected to analysis of variance, using the F test (P<0.05). Stomatal morphometry (stomata length, width, and density) was analyzed by Wilcoxon-Mann-Whitney test (P<0.001).
RESULTS
In vitro regenerated plants were phenotypically distinct and could be divided into two different groups. One group comprised plants with a similar phenotype to the original cultivar, which were referred to as ‘normal-like plants’, characterized by digitate leaves, a pale-green color, with long internodes and petioles, and thinner and apparently longer stems and roots (Figure 1). The other group comprised plants exhibiting a distinct phenotype from diploid plants and these were referred to as ‘putative-polyploid plants’. They were characterized by tri-lobate leaves, a dark-green color, with shorter internodes and petioles, and thicker stems and roots, demonstrating a phenomenon widely known as the gigas effect (Figure 1). Normal-like and putative-polyploid phenotypes were observed for both the Porquinho and Vassourinha cultivars. All plants from both phenotypes were analyzed by flow cytometry to estimate ploidy level and were compared with original cultivars.
Phenotype of putative-polyploid (left) and normal-like (right) plants of Manihot esculenta, after colchicine treatment, indicating differences in plant size and leaf morphology.
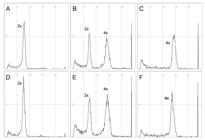
The three main classes of plants identified were diploid, mixoploid (chimeras with diploid and tetraploid sectors) and tetraploid (Figure 2). Histograms generated by flow cytometry analysis demonstrated that the peak corresponding to the diploid genome was located close to 74, while the peak corresponding to the tetraploid genome was duplicated, at around 146 (Figure 2c, f).
Flow cytometry results showing in vitro diploid (A and D), mixoploid (B and E) and polyploid (C and F) plants for both Porquinho (first row) and Vassourinha (second row) cultivars. Each cytological type shows a typical peak in flow cytometry analysis; diploid (2x) and tetraploid (4x) plants shows a unique peak, while mixoploid plants show both peaks for 2x and 4x genomes.
Two different phenotype groups, referred to as normal-like and putative-polyploid, were observed during in vitro culture. There was no association between phenotype and ploidy level in the normal-like group of plants with the observation of diploid, mixoploid and tetraploid plants in this group. On the other hand, all plants classified as putative-polyploid were confirmed as tetraploids.
The Vassourinha cultivar was significantly more responsive to tetraploid induction by colchicine treatment (P = 0.04), at least two-fold more so than Porquinho (Table 1). Vassourinha produced tetraploids in all treatments while the highest alkaloid concentration (0.15%) did not produce any tetraploid plants from Porquinho. Colchicine concentration and time of exposure did not significantly affect in vitro tetraploid induction (P > 0.05).
Colchicine at 0.10% for 96 hours induced a large number of tetraploids for both cultivars (Table 1), while colchicine treatment at 0.05% for 96 hours in Vassourinha showed similar efficiency to the best treatment tested in Porquinho (0.10% for 96 hours). Treatment with colchicine at 0.05% for 48 hours was the second most efficient treatment for Porquinho (Table 1). Shoot survival rate was not affected by cultivar, colchicine concentration or time of exposure. In all cases, shoot survival rate ranged from 14% to 26% in treatments and from 64% to 78% in controls, indicating the toxic effect of colchicine (Table 1).
The experiment produced 47 mixoploid plants of Vassourinha, and 43 of Porquinho. Treatment with colchicine 0.05% for 96 hours produced a large number of mixoploids of both cultivars. However, this treatment also showed the lowest efficiency for producing tetraploid plants in Porquinho.
Some mixoploid plants presented large tetraploid sectors, often with a similar size to the diploid sectors (Figure 2b, e). This result indicates that these plants could be vegetatively propagated in order to obtain a higher number of stable (solid) tetraploid plants.
Chromosome counting was carried out to confirm the results shown by flow cytometry, especially for plants considered as tetraploids. Control plants were 2n=2x=36 (Figure 3a) while all tetraploid plants were 2n=4x=72 (Figure 3b). Mixoploid plants were not considered for this analysis. Stomata morphometric analysis showed that tetraploid plants had fewer guard cells per leaf area, with a reduction of around 50% (Table 2), and larger cells (~30% larger) than those observed in diploids (Figure 3c, d).
Chromosomes of Vassourinha cultivar, diploid metaphase, from a control plant showing 2n=2x=36 (Bar=10 µm) (A), and tetraploid metaphase with 2n=4x=72 (Bar=10 µm) (B). Stomatal morphology, density and morphometry for Porquinho variety, diploid (C), and polyploid (D). Note the increase in cell size and reduction in stomatal density of tetraploid plant.
DISCUSSION
Polyploidy induction by colchicine and other substances is a well-known approach to chromosome doubling that can be used to produce polyploid lines, to explore the gigas effect and to produce seedless fruits in triploid lines (Zeng et al. 2006Zeng SH, Chen CW, Hong L, Liu JH and Deng XX (2006) In vitro induction, regeneration and analysis of autotetraploids derived from protoplasts and callus treated with colchicine in Citrus. Plant Cell, Tissue and Organ Culture 87: 85-93, Dhooghe et al. 2011Dhooghe E, Van Laere K, Eeckhaut T, Leus L and Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell, Tissue and Organ Culture 104: 359-373., Vichiato et al. 2014Vichiato MRDM, Vichiato M, Pasqual M, Rodrigues FA and Castro DMD (2014) Morphological effects of induced polyploidy in Dendrobium nobile Lindl. (Orchidaceae). Crop Breeding and Applied Biotechnology 14: 154-159., Lai et al. 2015Lai HG, Chen X, Chen Z, Ye JQ, Li KM and Jin-Ping L (2015) Induction of female 2n gametes and creation of tetraploids through sexual hybridization in cassava (Manihot esculenta). Euphytica 201: 265-273.) and even to enhance active principle production in medicinal plants (Yan et al. 2016Yan HJ, Xiong Y, Zhang HY and He ML (2016) In vitro induction and morphological characteristics of octoploid plants in Pogostemon cablin. Breeding Science 66: 169-174.). In the specific case of Manihot esculenta, polyploid lines have been used to explore the gigas effect, especially to increase root size, to increase starch and vitamin contents, and to improve photosynthetic activity and salt tolerance (Nassar et al. 2008Nassar NM, Graciano-Ribeiro D, Fernandes SDC and Araujo PC (2008) Anatomical alterations due to polyploidy in cassava, Manihot esculenta Crantz. Genetics and Molecular Research 7: 276-283., An et al. 2014An F, Fan J, Li J, Li QX, Li K, Zhu W, Wen F, Carvalho LJCB and Chen S (2014) Comparison of leaf proteomes of cassava (Manihot esculenta Crantz) cultivar NZ199 diploid and autotetraploid genotypes. PloS One 4: e0085991., Lai et al. 2015Lai HG, Chen X, Chen Z, Ye JQ, Li KM and Jin-Ping L (2015) Induction of female 2n gametes and creation of tetraploids through sexual hybridization in cassava (Manihot esculenta). Euphytica 201: 265-273.). The efficiency of polyploid induction by colchicine or oryzalin is often low, and has been reported in other species, such as pummelo (Citrus grandis) (Kainth and Grosser 2010Kainth D and Grosser JW (2010) Induction of autotetraploids in pummelo (Citrus grandis L. Osbeck) through colchicine treatment of meristematically active seeds in vitro. Proceedings of the Florida State Horticultural Society 123: 44-48.), Dendobrium, Epidendrum, Odontia and Phalaenopsis (Miguel and Leonhardt 2011Miguel TP and Leonhardt KW (2011) In vitro polyploid induction of orchids using oryzalin. Scientia Horticulturae 130: 314-319.) and cassava (Carvalho et al. 2016Carvalho MJS, Gomes VB, Souza AS, Aud FF, Santos-Serejo JA and Oliveira EJ (2016) Inducing autotetraploids in cassava using oryzalin and colchicine and their in vitro morphophysiological effects. Genetics and Molecular Research 15: gmr.15028281).
Our results showed a tetraploid induction efficiency ranging from 4.0 to 47.4% of in vitro plant recovery (Table 1). Cassava plants of Colombia 22 cultivar showed a similar level of efficiency after treatment with different colchicine concentrations (2.5 to 10 mM = 0.1 to 0.4%) while oryzalin was extremely inefficient (Awoleye et al. 1994Awoleye F, Van Duren M, Dolezel J and Novak FJ (1994) Nuclear DNA content and in vitro induced somatic polyploidization cassava (Manihot esculenta Crantz) breeding. Euphytica 76: 195-202.). For another cassava cultivar, BRS Formosa, a similar procedure using both substances was inefficient at producing tetraploid plants (Carvalho et al. 2016Carvalho MJS, Gomes VB, Souza AS, Aud FF, Santos-Serejo JA and Oliveira EJ (2016) Inducing autotetraploids in cassava using oryzalin and colchicine and their in vitro morphophysiological effects. Genetics and Molecular Research 15: gmr.15028281). However, in Hebe 'Oratia Beauty', an efficiency of 45.7% was recorded using oryzalin and 28.6% using colchicine (Gallone et al. 2014Gallone A, Hunter A and Douglas GC (2014) Polyploid induction in vitro using colchicine and oryzalin on Hebe ‘Oratia Beauty’: Production and characterization of the vegetative traits. Scientia Horticulturae 179: 59-66.).
Many parameters can affect the efficiency of polyploid induction (Dhooghe et al. 2011Dhooghe E, Van Laere K, Eeckhaut T, Leus L and Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell, Tissue and Organ Culture 104: 359-373.). In this study, we found that tetraploid induction seemed highly associated with genotype as Vassourinha was significantly more responsive to colchicine in most treatments (P < 0.05) (Table 1). Given the results for Colombia 22 (Awoleye et al. 1994Awoleye F, Van Duren M, Dolezel J and Novak FJ (1994) Nuclear DNA content and in vitro induced somatic polyploidization cassava (Manihot esculenta Crantz) breeding. Euphytica 76: 195-202.) and BRS Formosa (Carvalho et al. 2016Carvalho MJS, Gomes VB, Souza AS, Aud FF, Santos-Serejo JA and Oliveira EJ (2016) Inducing autotetraploids in cassava using oryzalin and colchicine and their in vitro morphophysiological effects. Genetics and Molecular Research 15: gmr.15028281) the hypothesis of a genotype response to polyploidy induction in cassava is highly feasible and is certainly true for other plant species. For example, Khosravi et al. (2008Khosravi P, Kermani MJ, Nematzadeh GA, Bihamta MR and Yokoya K (2008) Role of mitotic inhibitors and genotype on chromosome doubling of Rosa. Euphytica 160: 267-275. ) showed that in rose the response to chromosome doubling induction is quite genotype dependent.
Interestingly, the best performance in terms of chromosome doubling induction for both genetic materials was obtained using the same treatment (0.10%, 96 h exposure). It is important to note that the higher concentration (0.15%) was less effective, probably by inducing greater phytoxicity. Similar results were reported by Awoleye et al. (1994Awoleye F, Van Duren M, Dolezel J and Novak FJ (1994) Nuclear DNA content and in vitro induced somatic polyploidization cassava (Manihot esculenta Crantz) breeding. Euphytica 76: 195-202.) and Carvalho et al. (2016Carvalho MJS, Gomes VB, Souza AS, Aud FF, Santos-Serejo JA and Oliveira EJ (2016) Inducing autotetraploids in cassava using oryzalin and colchicine and their in vitro morphophysiological effects. Genetics and Molecular Research 15: gmr.15028281) in cassava, and by Thompson et al. (2010Thompson DI, Anderson NO and Van Staden J (2010) Colchicine-induced somatic polyploids from in vitro-germinated seeds of South African Watsonia species. HortScience 45: 1398-1402.) in Watsonia.
The higher ratio between mixoploid and tetraploid in vitro plant recovery in Porquinho suggests a stronger resistance to the effect of colchicine on the spindle fiber polymerization process, or greater difficulty of colchicine infiltration into the meristematic region of the apexes, or lower capacity to increase tetraploid sectors during subsequent vegetative in vitro propagation of plants of this genotype.
The presence of some in vitro tetraploids with normal-like phenotype resulted in no association between phenotype and ploidy level in normal-like group of plants. Awoleye et al. (1994Awoleye F, Van Duren M, Dolezel J and Novak FJ (1994) Nuclear DNA content and in vitro induced somatic polyploidization cassava (Manihot esculenta Crantz) breeding. Euphytica 76: 195-202.) associated distinct phenotypic characteristics with polyploidy during in vitro regeneration of cassava plants. Therefore, our results indicate that in vitro screening using morphological traits is not efficient for screening polyploid plants; and that cytogenetics approaches are more indicated for the precise selection of polyploids, using flow cytometry and chromosome counting. In fact, chromosome counting is laborious, but is considered the most precise method for polyploidy determination, while flow cytometry is the best alternative for rapid and high throughput screening (Dhooghe et al. 2011Dhooghe E, Van Laere K, Eeckhaut T, Leus L and Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell, Tissue and Organ Culture 104: 359-373.).
The gigas effect is not frequently observed during in vitro culture of most species, mainly because the short period of in vitro culture is not long enough to develop such differences in plants. Some authors have reported a high correlation between increased ploidy of cells of auto and allopolyploid plants with morpho-anatomical changes, including an increase in the size of organs (leaves and flowers), changes in cambium cells, and increased viability of germ cells, among others (Hahn et al. 1990Hahn SK, Bai KV and Asiedu R (1990) Tetraploids, triploids, and 2n pollen from diploid interspecific crosses with cassava. Theoretical and Applied Genetics 79: 433-439., 1992Hahn SK, Bai VK and Asiedu R (1992) Spontaneous somatic tetraploids in cassava. Japan Journal of Breeding 42: 303-308., Awoleye et al. 1994Awoleye F, Van Duren M, Dolezel J and Novak FJ (1994) Nuclear DNA content and in vitro induced somatic polyploidization cassava (Manihot esculenta Crantz) breeding. Euphytica 76: 195-202., Nassar et al. 2008Nassar NM, Graciano-Ribeiro D, Fernandes SDC and Araujo PC (2008) Anatomical alterations due to polyploidy in cassava, Manihot esculenta Crantz. Genetics and Molecular Research 7: 276-283., Graciano-Ribeiro and Nassar 2012Graciano-Ribeiro D and Nassar NMA (2012) A comparative anatomical study in cassava diploid and tetraploid hybrids. Plant Systematics and Evolution 298: 1771-1721.). In this study, such changes were also observed in tetraploid plants, mainly larger stomatal size and lower stomatal density in leaves.
In conclusion, we report herein the in vitro production of tetraploids and mixoploids of two different cassava cultivars. Future developments in tetraploid production should take these results into account, in particular the influence of genotype, dose and exposure time of colchicine as parameters for the best in vitro culture performance.
ACKNOWLEDGMENTS
The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support, and Dr. Sidia Maria Callegari Jacques for help with statistical analyses.
REFERENCES
- An F, Fan J, Li J, Li QX, Li K, Zhu W, Wen F, Carvalho LJCB and Chen S (2014) Comparison of leaf proteomes of cassava (Manihot esculenta Crantz) cultivar NZ199 diploid and autotetraploid genotypes. PloS One 4: e0085991.
- Awoleye F, Van Duren M, Dolezel J and Novak FJ (1994) Nuclear DNA content and in vitro induced somatic polyploidization cassava (Manihot esculenta Crantz) breeding. Euphytica 76: 195-202.
- Carvalho MJS, Gomes VB, Souza AS, Aud FF, Santos-Serejo JA and Oliveira EJ (2016) Inducing autotetraploids in cassava using oryzalin and colchicine and their in vitro morphophysiological effects. Genetics and Molecular Research 15: gmr.15028281
- Cuco SM, Mondin M, Vieira MLC and Aguiar-Perecin ML (2003) Técnicas para a obtenção de preparações citológicas com alta frequência de metáfases mitóticas em plantas: Passiflora (Passifloraceae) e Crotalaria (Leguminosae). Acta Botanica Brasilica 17: 363-370.
- Dhooghe E, Van Laere K, Eeckhaut T, Leus L and Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro Plant Cell, Tissue and Organ Culture 104: 359-373.
- Gallone A, Hunter A and Douglas GC (2014) Polyploid induction in vitro using colchicine and oryzalin on Hebe ‘Oratia Beauty’: Production and characterization of the vegetative traits. Scientia Horticulturae 179: 59-66.
- Graciano-Ribeiro D and Nassar NMA (2012) A comparative anatomical study in cassava diploid and tetraploid hybrids. Plant Systematics and Evolution 298: 1771-1721.
- Grosser JW, Kainth D and Dutt M (2014) Production of colchicine-induced autotetraploids in pummelo (Citrus grandis Osbeck) through indirect organogenesis. HortScience 49: 944-948.
- Guerra D, Wittmann MTS, Schwarz SF, Souza PVD, Gonzatto MP and Weiler RL (2014) Comparison between diploid and tetraploid citrus rootstocks: morphological characterization and growth evaluation. Bragantia 73: 1-7.
- Hahn SK, Bai KV and Asiedu R (1990) Tetraploids, triploids, and 2n pollen from diploid interspecific crosses with cassava. Theoretical and Applied Genetics 79: 433-439.
- Hahn SK, Bai VK and Asiedu R (1992) Spontaneous somatic tetraploids in cassava. Japan Journal of Breeding 42: 303-308.
- Hamill SD, Smith MK and Dodd WA (1992) In vitro induction of banana autotetraploids by colchicine treatment of micropropagated diploids. Australian Journal of Botany 40: 887-896.
- Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis Ps, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J and dePamphilis CW (2011) Ancestral polyploidy in seed plants and angiosperms. Nature 473: 97-100.
- Kainth D and Grosser JW (2010) Induction of autotetraploids in pummelo (Citrus grandis L. Osbeck) through colchicine treatment of meristematically active seeds in vitro Proceedings of the Florida State Horticultural Society 123: 44-48.
- Kermani MJ, Sarasan V, Roberts AV, Yokoya K, Wentworth J and Sieber VK (2003) Oryzalin-induced chromosome doubling in Rosa and its effect on plant morphology and pollen viability. Theoretical and Applied Genetics 107: 1195-1200.
- Khosravi P, Kermani MJ, Nematzadeh GA, Bihamta MR and Yokoya K (2008) Role of mitotic inhibitors and genotype on chromosome doubling of Rosa Euphytica 160: 267-275.
- Lai HG, Chen X, Chen Z, Ye JQ, Li KM and Jin-Ping L (2015) Induction of female 2n gametes and creation of tetraploids through sexual hybridization in cassava (Manihot esculenta). Euphytica 201: 265-273.
- Madlung A (2013) Polyploidy and its effect on evolutionary success: old questions revisited with new tools. Heredity 110: 99-104.
- Miguel TP and Leonhardt KW (2011) In vitro polyploid induction of orchids using oryzalin. Scientia Horticulturae 130: 314-319.
- Mondin M, Santos-Serejo JA and Aguiar-Perecin ML (2007) Karyotype characterization of Crotalaria juncea (L.) by chromosome banding and physical mapping of 18S-5.8 S-26S and 5S rRNA gene sites. Genetics and Molecular Biology 30: 65-72.
- Murashige T and Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497.
- Nassar N (2004) Polyploidy, chimera and fertility of interspecific cassava (Manihot esculenta Crantz) hybrids. The Indian Journal of Genetics and Plant Breeding 64: 317-318.
- Nassar NM, Graciano-Ribeiro D, Fernandes SDC and Araujo PC (2008) Anatomical alterations due to polyploidy in cassava, Manihot esculenta Crantz. Genetics and Molecular Research 7: 276-283.
- Pereira RC, Davide LC, Techio VH and Timbó ALO (2012) Chromosome doubling of grasses: an alternative to plant breeding. Ciência Rural 42: 1278-1285.
- Souza AS, Souza FVD, Santos-Serejo JA, Junghans TG, Paz OP, Montarroyos AVV, Santos VS and Morais LS (2009) Preservação de germoplasma vegetal, com ênfase na conservação in vitro de variedades de mandioca. Embrapa, Cruz das almas, 24p. (Circular Técnica 90).
- Sree Ramulu K, Verhoeven HA and Dijkhuis P (1991) Mitotic blocking, micronucleation, and chromosome doubling by oryzalin, amiprophos-methyl, and colchicine in potato. Protoplasma 160: 65-71.
- Thompson DI, Anderson NO and Van Staden J (2010) Colchicine-induced somatic polyploids from in vitro-germinated seeds of South African Watsonia species. HortScience 45: 1398-1402.
- van Duren M, Morpurgo R, Dolezel J and Afza R (1996) Induction and verification of autotetraploids in diploid banana (Musa acuminata) by in vitro techniques. Euphytica 88: 25-34.
- Vichiato MRDM, Vichiato M, Pasqual M, Rodrigues FA and Castro DMD (2014) Morphological effects of induced polyploidy in Dendrobium nobile Lindl. (Orchidaceae). Crop Breeding and Applied Biotechnology 14: 154-159.
- Yan HJ, Xiong Y, Zhang HY and He ML (2016) In vitro induction and morphological characteristics of octoploid plants in Pogostemon cablin Breeding Science 66: 169-174.
- Zeng SH, Chen CW, Hong L, Liu JH and Deng XX (2006) In vitro induction, regeneration and analysis of autotetraploids derived from protoplasts and callus treated with colchicine in Citrus Plant Cell, Tissue and Organ Culture 87: 85-93
Publication Dates
-
Publication in this collection
Apr-Jun 2018 -
Date of issue
Apr 2018
History
-
Received
18 Sept 2017 -
Accepted
18 Dec 2017