Abstracts
The efficacy of a drug in a specific application requires the maintenance of appropriate drug blood level concentration during a prolonged period of time. Controlled release delivery is available for many routes of administration and offers many advantages (as microparticles and nanoparticles) over immediate release delivery. These advantages include reduced dosing frequency, better therapeutic control, fewer side effects, and, consequently, these dosage forms are well accepted by patients. Advances in polymer material science, particle engineering design, manufacture, and nanotechnology have led the way to the introduction of several marketed controlled release products and several more are in pre-clinical and clinical development. The objective of this work is to prepare and evaluate diltiazem HCl loaded albumin microparticles using a factorial design. Albumin (natural polymer) microparticles were prepared by emulsion heat-stabilization method. Selected formulations were characterized for their entrapment efficiency, particle size, surface morphology, and release behavior. Analysis of variance for entrapment efficiency indicates that entrapment efficiency is best fitted to a response surface linear model. Surface morphology was studied by scanning electron microscopy. Scanning electron microscopy of the microparticles revealed a spherical, nonporous and uniform appearance, with a smooth surface. The geometric mean diameter of the microparticles was found to be 2-9 µm, which more than 75% were below 3.5 µm and drug incorporation efficiency of 59.74 to 72.48% (w/w). In vitro release profile for formulations containing diltiazem HCl loaded BSA microparticles with heat stabilization technique shows slow controlled the release of the drug up to 24 hours. The release pattern was biphasic, characterized by an initial burst effect followed by a slow release. All selected microparticles exhibited a prolonged release for almost 24 hours. On comparing regression-coefficient (r²) values for Hixson Crowel, Higuchi and Peppas kinetic models, different batches of microparticles showed Fickian, non-Fickian, and diffusion kinetics. The release mechanism was regulated by D:P ratio. From the statistical analysis it was observed that as the drug:polymer (D:P) ratio increased, there was a significant increase in the encapsulation efficiency. Based on the particle size, entrapment efficiency and physical appearance, DTM-3 formulations were selected for in vivo release study and stability study. The in vivo result of drug loaded microparticles showed preferential drug targeting to liver followed by lungs, kidneys and spleen. Stability studies showed that maximum drug content and closest in vitro release to initial data were found in the formulation stored at 4 ºC. In present study, diltiazem HCl loaded BSA microparticles were prepared and targeted to various organs to satisfactory level and were found to be stable at 4 ºC.
Diltiazem HCl loaded microparticles; Diltiazem HCl loaded microparticles; Diltiazem HCl loaded microparticles; Albumin; Microparticles batches; Cloridrato de diltiazem; Cloridrato de diltiazem
A eficácia terapêutica de um fármaco depende da manutenção de seu nível plasmático adequado em determinado intervalo de tempo. Nesse sentido, a liberação modificada de fármacos está disponível em muitas vias de administração e oferece muitas vantagens (como micropartículas e nanopartículas) quando comparada às formulações de liberação imediata. Essas vantagens incluem reduzida frequência da dosagem, melhor controle terapêutico e menos efeitos colaterais. Assim sendo, esses produtos apresentam maior aceitação pelos pacientes. Os avanços na ciência dos materiais, na engenharia das partículas, em manufatura e em nanotecnologia permitiram a introdução no mercado de vários produtos de liberação modificada e vários outros se encontram em desenvolvimento pré-clínico e clínico. O objetivo do presente trabalho foi preparar e avaliar o fármaco cloridrato de diltiazem associado a micropartículas de albumina utilizando planejamento fatorial. As micropartículas de albumina, um polímero natural, foram preparadas por método de emulsão empregando estabilização por calor. As formulações selecionadas foram caracterizadas no que se refere à sua eficiência de encapsulamento, tamanho médio de partículas, morfologia de superfície e perfil de liberação do fármaco. A análise de variância relativa à eficiência de encapsulamento indicou superfície de resposta linear. Com referência à morfologia superficial, essa foi avaliada empregando microscopia eletrônica de varredura. Essa análise revelou micropartículas esféricas, não porosas e de aparência uniforme, com superfície lisa. O diâmetro médio das micropartículas foi entre 2 e 9 µm, sendo que mais de 75% das micropartículas se apresentaram abaixo de 3,5 µm. Além disso, a eficiência de encapsulamento foi entre 59,74 e 72,48%. Quanto ao ensaio para avaliação do perfil de liberação in vitro do fármaco associado às micropartículas, as formulações apresentaram liberação lenta até 24 horas. O comportamento foi caracterizado por liberação inicial (efeito burst) seguida por liberação lenta. Todas as fórmulas selecionadas apresentaram liberação prolongada por aproximadamente 24 horas. Na comparação entre os valores de coeficientes de regressão (R²), os modelos propostos por Hixson Crowel, Higuchi e Peppas, para diferentes formulações de micropartículas, demonstraram cinética de liberação de acordo com modelo Fickiano e não-Fickiano. O mecanismo de liberação do fármaco foi regulado pela razão entre o fármaco e o polímero. A análise estatística revelou significativo aumento da eficiência de encapsulamento quando essa razão aumentou. As avaliações relativas à análise dimensional das micropartículas, à eficiência de encapsulamento do fármaco e à morfologia permitiram a seleção da formulação DTM-3 para os ensaios de liberação in vivo e para o estudo da estabilidade. O ensaio de liberação in vivo do fármaco associado às micropartículas demonstrou sítio-alvo preferencial no fígado, seguido pelos pulmões rins e baço. No presente estudo, as micropartículas de albumina contendo cloridrato de diltiazem foram adequadamente preparadas e orientadas satisfatoriamente para vários órgãos. Além disso, a formulação selecionada apresentou estabilidade físico-química a 4 ºC.
Cloridrato de diltiazem; Cloridrato de diltiazem; Albumina; Formulações de micropartículas
ARTICLES
Biodegradable microparticulate drug delivery system of diltiazem HCl
Manish Kumar GuptaI,* * Correspondence: Manish Kumar Gupta. Institute of Pharmacy, HCPG College, Varanasi - 221002. E-mail: manishg3010@gmail.com; manish_3010@indiatimes.com ; Deepak PrakashI; Brahmeshwar MishraII
IInstitute of Pharmacy, Harish Chandra Post Graduate College, Varanasi, India
IIDepartment of Pharmaceutics, Indian Institute of Technology, Banaras Hindu University, Varanasi, India
ABSTRACT
The efficacy of a drug in a specific application requires the maintenance of appropriate drug blood level concentration during a prolonged period of time. Controlled release delivery is available for many routes of administration and offers many advantages (as microparticles and nanoparticles) over immediate release delivery. These advantages include reduced dosing frequency, better therapeutic control, fewer side effects, and, consequently, these dosage forms are well accepted by patients. Advances in polymer material science, particle engineering design, manufacture, and nanotechnology have led the way to the introduction of several marketed controlled release products and several more are in pre-clinical and clinical development. The objective of this work is to prepare and evaluate diltiazem HCl loaded albumin microparticles using a factorial design. Albumin (natural polymer) microparticles were prepared by emulsion heat-stabilization method. Selected formulations were characterized for their entrapment efficiency, particle size, surface morphology, and release behavior. Analysis of variance for entrapment efficiency indicates that entrapment efficiency is best fitted to a response surface linear model. Surface morphology was studied by scanning electron microscopy. Scanning electron microscopy of the microparticles revealed a spherical, nonporous and uniform appearance, with a smooth surface. The geometric mean diameter of the microparticles was found to be 2-9 µm, which more than 75% were below 3.5 µm and drug incorporation efficiency of 59.74 to 72.48% (w/w). In vitro release profile for formulations containing diltiazem HCl loaded BSA microparticles with heat stabilization technique shows slow controlled the release of the drug up to 24 hours. The release pattern was biphasic, characterized by an initial burst effect followed by a slow release. All selected microparticles exhibited a prolonged release for almost 24 hours. On comparing regression-coefficient (r2) values for Hixson Crowel, Higuchi and Peppas kinetic models, different batches of microparticles showed Fickian, non-Fickian, and diffusion kinetics. The release mechanism was regulated by D:P ratio. From the statistical analysis it was observed that as the drug:polymer (D:P) ratio increased, there was a significant increase in the encapsulation efficiency. Based on the particle size, entrapment efficiency and physical appearance, DTM-3 formulations were selected for in vivo release study and stability study. The in vivo result of drug loaded microparticles showed preferential drug targeting to liver followed by lungs, kidneys and spleen. Stability studies showed that maximum drug content and closest in vitro release to initial data were found in the formulation stored at 4 ºC. In present study, diltiazem HCl loaded BSA microparticles were prepared and targeted to various organs to satisfactory level and were found to be stable at 4 ºC.
Uniterms: Diltiazem HCl loaded microparticles/preparation. Diltiazem HCl loaded microparticles/ release profile/evaluation. Albumin/microparticles. Microparticles batches/diffusion kinetics.
RESUMO
A eficácia terapêutica de um fármaco depende da manutenção de seu nível plasmático adequado em determinado intervalo de tempo. Nesse sentido, a liberação modificada de fármacos está disponível em muitas vias de administração e oferece muitas vantagens (como micropartículas e nanopartículas) quando comparada às formulações de liberação imediata. Essas vantagens incluem reduzida frequência da dosagem, melhor controle terapêutico e menos efeitos colaterais. Assim sendo, esses produtos apresentam maior aceitação pelos pacientes. Os avanços na ciência dos materiais, na engenharia das partículas, em manufatura e em nanotecnologia permitiram a introdução no mercado de vários produtos de liberação modificada e vários outros se encontram em desenvolvimento pré-clínico e clínico. O objetivo do presente trabalho foi preparar e avaliar o fármaco cloridrato de diltiazem associado a micropartículas de albumina utilizando planejamento fatorial. As micropartículas de albumina, um polímero natural, foram preparadas por método de emulsão empregando estabilização por calor. As formulações selecionadas foram caracterizadas no que se refere à sua eficiência de encapsulamento, tamanho médio de partículas, morfologia de superfície e perfil de liberação do fármaco. A análise de variância relativa à eficiência de encapsulamento indicou superfície de resposta linear. Com referência à morfologia superficial, essa foi avaliada empregando microscopia eletrônica de varredura. Essa análise revelou micropartículas esféricas, não porosas e de aparência uniforme, com superfície lisa. O diâmetro médio das micropartículas foi entre 2 e 9 µm, sendo que mais de 75% das micropartículas se apresentaram abaixo de 3,5 µm. Além disso, a eficiência de encapsulamento foi entre 59,74 e 72,48%. Quanto ao ensaio para avaliação do perfil de liberação in vitro do fármaco associado às micropartículas, as formulações apresentaram liberação lenta até 24 horas. O comportamento foi caracterizado por liberação inicial (efeito burst) seguida por liberação lenta. Todas as fórmulas selecionadas apresentaram liberação prolongada por aproximadamente 24 horas. Na comparação entre os valores de coeficientes de regressão (R2), os modelos propostos por Hixson Crowel, Higuchi e Peppas, para diferentes formulações de micropartículas, demonstraram cinética de liberação de acordo com modelo Fickiano e não-Fickiano. O mecanismo de liberação do fármaco foi regulado pela razão entre o fármaco e o polímero. A análise estatística revelou significativo aumento da eficiência de encapsulamento quando essa razão aumentou. As avaliações relativas à análise dimensional das micropartículas, à eficiência de encapsulamento do fármaco e à morfologia permitiram a seleção da formulação DTM-3 para os ensaios de liberação in vivo e para o estudo da estabilidade. O ensaio de liberação in vivo do fármaco associado às micropartículas demonstrou sítio-alvo preferencial no fígado, seguido pelos pulmões rins e baço. No presente estudo, as micropartículas de albumina contendo cloridrato de diltiazem foram adequadamente preparadas e orientadas satisfatoriamente para vários órgãos. Além disso, a formulação selecionada apresentou estabilidade físico-química a 4 ºC.
Unitermos: Cloridrato de diltiazem/associado a micropartículas/preparação. Cloridrato de diltiazem/associado a micropartículas/avaliação do perfil de liberação. Albumina/micropartículas. Formulações de micropartículas/cinética de liberação.
INTRODUCTION
The efficacy of a drug in a specific application requires the maintenance of appropriate drug blood level concentration during a prolonged period of time. However, the conventional administration of drugs, gives a poor control of the concentration of these substances in plasma because of variations in the concentration of the bioactive product, once a specific dose has been administered. The conventional dosage systems can rise to alternative periods of inefficacy or toxicity. These difficulties have been called for the development of new administration techniques for bioactive compounds, directed towards attaining the steady state plasma concentration. In the recent years, considerable attention has been focused on the development of Novel Drug Delivery Systems (NDDS). The reason for this paradigm shift is due to the low development cost and time required for developing a NDDS for the existing drugs rather developing a new drug molecule. In the form of NDDS, existing drug molecule can get a new life, thereby increasing the market value and product patent life. Controlled release, prolonged action, sustained release, extended release, depot dosage forms are terms used to identify these drug delivery systems that are designed to achieve prolonged therapeutic effected by continuously releasing medication over an extended period of time after administration of single dose (Chinna et al., 2010). The therapeutic benefits of new systems include increased efficacy of the site-specific delivery, decreased toxicity/side effects, increased convenience, shorter hospitalizations, variable treatments for previously incurable diseases, potential for prophylactic applications and lower healthcare costs-both long term and better patient compliance (Lee et al., 2000; Lalla, 1991). In a majority of studies the homo and copolymer have been used for drug delivery application because they can be fabricated into a variety of morphologies, including films, rods, and microparticles by compression molding, solvent casting, solvent evaporation technique and phase separation technique (Chinna et al., 2010).
Biodegradable polymers have been of long interest in controlled release technology because of the ability of these polymers to be reabsorbed by the body. This alleviates the need for removal, often surgically, of a drug release device. Knowledge and skill in the field of biodegradable polymer technology is progressing rapidly enough that researchers have at their disposal a substantial number of degradable polymers with a range of degradation rates. Not only the researchers may use a single polymer, copolymer, or blend, but also they may use a combination of polymers. These polymers, which have been prepared as films, microparticles, rods, and other forms, display a bulk erosion hydrolysis (Pappas et al., 1995; Mansour et al., 2010).
While biodegradable microparticles have proven to be useful in a wide range of controlled drug delivery applications, several research groups have investigated opportunities for utilizing biodegradable microparticles in composite or hybrid systems with other biodegradable or non-degradable systems. The release rates and profiles of both hydrophilic drugs and hydrophobic drugs have shown to be significantly changed when the biodegradable microparticles containing these drugs are incorporated into silicone (nondegradable) or gelatin (degradable) films. The drug released from the microparticles within the silicone films does not exhibit the initial high burst of release in vitro from free microparticles (Pappas et al., 1995). Naturally derived polymers are abundant and usually biodegradable. Their principal disadvantages lie in the development of reproducible production methods, because their structural complexity often renders modification and purification difficult. Additionally, significant batch-to-batch variations occur because of their 'biopreparation' in living organisms (Nela et al., 1999).
Microparticulates in the size range of about 1-100 µm, consisting of biodegradable or bioerodible solids with embodied therapeutic agents, represent a prominent class of delivery systems. Their administration is most commonly by local injection of suitable dispersions but applications to the respiratory tract or peroral delivery are equally interesting. In particular, there is much interest currently in the use of biodegradable polymers for the preparation of microparticles containing a wide range of therapeutic agents, which can, of course, be used for parenteral administration. Solid biodegradable microparticles incorporating a drug dispersed throughout the particle matrix have the potential for the controlled release of the drug from this system after i.m. injection. Microparticles designed for parenteral drug delivery can be composed of a variety of materials with different physical characteristics such as biocompatible, biodegradable, injectable, sterile, compatible with diluents, and pharmaceutically stable. Depending on the used microparticulate material, its size distribution and degradation or erosion kinetics, various delivery profiles are feasible (Merkle et al., 2002; Nanjwade et al., 2011).
The therapeutic and economic success of microparticulates as a delivery platform is related closely to the advent of therapeutics from biotechnological sources. For future development, the following prospects for this class of drug delivery systems are envisaged (Merkle et al., 2002):
Innovations for novel biodegradable and biocompatible materials and additives for better control of delivery kinetics;
Improvements of manufacturing techniques in terms of narrow particle size distribution, high loading efficiency, aseptic manufacturing technologies and drug stability;
Development of microparticulates as tools in tissue engineering, for example, to establish localized gradients of mitogenic or morphogenic agents and support organized tissular development;
Exploration of novel therapeutic opportunities through the use of microparticulates for systemic and localized delivery;
Innovations regarding novel materials and surface modifications to improve on biocompatibility and targeting features of microparticulates;
Design of microparticulates for optimized delivery of antigens to professional antigen-presenting cells.
Several techniques can be used to prepare polymeric microparticles providing controlled drug delivery. Most commonly, organic solvent evaporation and/or extraction methods are applied. Depending on the solubility of the drug simple or multiple emulsion technique, oil-in-water (o/w) and water-in-oil-in-water (w/o/w) methods are used. In the first case, a lipophilic drug is dissolved together with the polymer in an organic phase, which is dispersed into outer aqueous phase. Upon contact, the organic solvent diffuses into the external water phase and evaporates at its surface. Consequently, the polymer precipitates and entraps the drug. If a drug hydrophilic is to be incorporated, it can generally not be dissolved within organic phase and drug loss into the external aqueous phase tends to be remarkable, resulting in low encapsulation efficiencies. To overcome this restriction w/o/w techniques can be applied, avoiding the direct contact of the drug containing phase with the outer water phase. An aqueous solution of the drug is emulsified into an organic phase containing the dissolved polymer. This w/o emulsion is dispersed into an outer aqueous phase. Upon solvent diffusion/evaporation, the polymer precipitates and incorporates the drug (Perez et al., 2003).
Albumin microparticles are biodegradable particles that can be produced in a size range of 1 to 200 µm in diameter, by either physical or chemical solidification of an albumin emulsion in an organic phase. Bovine serum albumin (BSA) is widely used for microparticles preparation because it is non-antigenic, biodegradable, free from toxicity, able to control the physicochemical characteristics of the microparticles produced, and readily available (Mathew et al., 2007).
Microparticles of bovine serum albumin (BSA) may be prepared using an emulsion of aqueous BSA in oil. Sufficient elevation of the temperature of the emulsion will set the BSA microparticles or polymerization of polymer by using cross-linking agents. Any drug may be included, either in solution along with the BSA or in suspension, for water-insoluble drugs. The microparticles are cooled and then washed with ether (or another appropriate solvent) and collected usually by filtration or centrifugation (Birnbaum et al., 2003).
Diltiazem hydrochloride, a calcium channel blocker, is widely used for the treatment of angina pectoris, hypertension and arrhythmias. It is administered orally (tablets, capsules, sustained release tablets/capsules) and parenterally (intravenous). Diltiazem hydrochloride is a water-soluble cardiovascular drug. The usual dose of diltiazem is 90-120 mg. The conventional tablet and capsule is administered 3 or 4 times a day due to its short biological half-life of about 6 h. The problems of frequent administration and variable low bioavailability (30-45%) after oral administration of conventional tablet or capsules have been attenuated by designing diltiazem in the form of sustained release tablet or capsules. The sustained release forms are administered two times a day due to its limited residence time in the gastrointestinal tract. These limitations of diltiazem hydrochloride in conventional dosage form, sustained release tablet or capsules can be overcome by administrating diltiazem hydrochloride by other route of administration (Das et al., 2008).
The purpose of the present study is to prepare diltiazem hydrochloride microparticles using bovine serum albumin (biodegradable natural polymer) by emulsification-heat stabilization technique and to investigate the effect of microparticle structure and drug release from them.
MATERIAL AND METHODS
Material
Diltiazem hydrochloride BP was supplied by Neuland Laborotaries Ltd., Hyderabad (A.P., India) and Bovine serum albumin (BSA fraction V) was purchased from LOBA Chemie Pvt. Ltd. (Mumbai, India). Cottonseed oil was purchased from Acros organics (New Jersey, USA).
Formulation of microparticles of diltiazem HCl (Dandagi et al., 2006; Kristina et al., 2001; Tuncay et al., 2000; Arshady, 1990)
Table I represents the design of various formulations developed. BSA microparticles containing diltiazem HCl were prepared by emulsification-heat stabilization technique (Figure 1: show the systemic diagram of emulsification-heat stabilization technique). One hundred fifty milligrams BSA was dissolved in 3 mL deionised water containing 0.1% (v/v) Tween 80, to which 100 mg diltiazem hydrochloride was added and used as the aqueous phase. The oil phase comprised of 3 mL cottonseed oil and 1 mL petroleum ether with 1% (v/v) Span 80 (as emulsifier), which mixed together and allowed to stir for 15 min at 1000 rpm on a magnetic stirrer. The aqueous phase was added drop wise to the oil phase and stirred for 45 min to form the primary emulsion. This primary emulsion was then added to 100 mL of cottonseed oil preheated to 115-120 ºC using 21 No. needle and stirred at 1200 rpm for 1 hours to allow the formation and solidification of microparticles. The suspension was then allowed to cool to room temperature with continuous stirring using a magnetic stirrer for 3 hours. On cooling, 150 mL of anhydrous ether was added. The suspension containing the microparticles was centrifuged at 3500 rpm for 15 min and the settled microparticles were washed three times with ether to remove traces of oil on microparticles surfaces. The obtained microparticles were then vacuum dried in a desiccator overnight and stored at 4 ºC in dark. Four batches of microparticles were prepared by the above-mentioned methods and labeled as DTM-1, DTM-2, DTM-3, and DTM-4. (Table I).
Characterization of microparticles
Calculation of percentage yield of microparticles (Salem et al., 2011; Yeo et al., 2004)
The yield of each formula was calculated by dividing the final weight of the microparticles produced by the weight of the BSA added. The yield was calculated according to this equation:
% Yield = (WM / WBSA+ Drug)× 100
where, the WM is the final weight of the microparticles after drying whilst WBSA+ Drug is the sum of initial weight of BSA and drug.
Entrapment efficiency: (Dandagi et al., 2006; Yeo et al., 2004; Thakkar et al., 2005; Gupta et al., 2009)
Unentrapped drug: Ten milliliter of pH 7.4 phosphate buffer saline (PBS) was added to 10 mg of microparticles and this mixture was kept in an ultrasonicator for 1 minute. After centrifugation, the supernatant was filtered through a 0.45 µm filter and the filtrate was suitably diluted with buffer solution. The absorbance of resulting solution was measured at 237 nm by using UV-visible spectrophotometer. The standard calibration curve of diltiazem HCl was carried out by using UV-visible spectrophotometer and HPTLC. (Figure 2)
Entrapped drug: The residue obtained on centrifugation was mixed with 5 mL 0.1 N hydrochloric acid containing 2% w/v pepsin and allowed to stand for 24 hours. The dispersion was then shaken with methylene chloride to extract diltiazem HCl. The organic extract was evaporated to dryness and the residue dissolved in methanol. The absorbance of the resulting solution was measured at 240 nm on a UV-visible spectrophotometer to determine the amount of Diltiazem HCl present in the microparticles.
Drug entrapment efficiency = (M actual / M theoretical) x 100%
Particle size distribution and morphology of microparticles (Mathew et al., 2007; Dandagi et al., 2006; Yeo et al., 2004; Gupta et al., 2009)
Size and morphology (shape and surface characteristics) were characterized by scanning electron microscopy using JEOL-T330A scanning microscope (Japan). Dry microparticles were placed on an electron microscope brass stub and coated with gold in an ion sputter. Pictures of microparticles were taken by random scanning of the stub. The diameter of about 100 microparticles was measured from optical microscope of each batch. Finally, average mean diameters were obtained (Table I).
In vitro drug release studies (Salem et al., 2011; Gupta et al., 2009; Oner et al.,1993; Shenoy et al., 1997)
Drug-loaded microparticles equivalent to 50 mg of drug were weighed and transferred in to a 100 mL conical flask. To this 50 mL of 7.4 pH PBS was added, then the flasks were kept in a metabolic shaker and the shaker was adjusted to 50 horizontal shakes/min at 37±0.5 ºC. One milliliter of the drug releasing media was withdrawn at various time intervals of 15 min, 1, 2, 4, 6, 8, 12, 16, 20 and 24 h and replaced by the same volume of phosphate buffer saline (PBS). These samples were filtered though 0.45 µm membrane filter. The filtrate was diluted suitably. The drug was estimated in each batch by UV-Vis spectrophotometer at 237 nm. For batch DTM-1, the drug release study was carried by HPTLC method for comparing the results, which are coming from UV-Visible spectroscopy.
In vivo drug distribution studies (Dandagi et al., 2006; Gupta et al., 2009; Oner et al.,1993; Shenoy et al., 1997)
This study was carried out to compare the targeting efficiency of drug-loaded microparticles and controlled release with that of the free drug in terms of percentage increase in targeting to various organs of reticule endothelial system like liver, lungs, kidney and spleen.
Thirty healthy adult Albino mice weighing 35-40 g were selected, a constant day and night cycle was maintained and they were fasted for 12 h. The animals were divided into six groups each containing five mice. Groups I, II and III received microparticles equivalent to 700 mg of diltiazem HCl intravenously in the tail vein after re-dispersing in sterile phosphate buffer saline solution. Microparticles from batch DTM-3 were selected for the study. Groups IV and V received 700 mg of pure diltiazem HCl drug intravenously and Group VI was kept as solvent control and received only saline phosphate buffer.
After 1 h the Groups I, IV and VI mice were sacrificed and their liver, lungs, kidney and spleen were isolated. The Group V was sacrificed after 2 h and Groups II and III were sacrificed after12 and 24 h, respectively. The individual organs of each mice were homogenized with adding small quantity of phosphate saline buffer pH 7.4 and 0.1 N HCl, then kept in fridge for 12 h to precipitate protein and then centrifuged at 15,000 rpm to obtain the supernatant. The supernatant was filtered though an ultrafilter membrane of pore size 0.2 µm and drug content was estimated using UV spectrophotometer at 237 nm.
Stability study (Oliva et al., 1999; Kulkarni et al., 2004)
All the eight batches of Diltiazem HCl microparticles were tested for stability. All the preparations were divided into three sets and were stored at 4 ºC (refrigerator), ambient temperature/humidity and 40 ºC (thermostatic oven). After 15, 30 and 60 days, drug content of all the formulations was determined by the method discussed previously in entrapment efficiency section. In vitro release study was also carried out of the best one formulation.
RESULTS
Microparticles based on the biodegradable polymer bovine serum albumin (BSA) have been extensively investigated as controlled drug delivery system because of their excellent biocompatibility and biodegradability. In recent years, a continued interest in BSA microparticles has been triggered by their application for the controlled release of different variety of drugs.
In the present study BSA microparticles encapsulated with Diltiazem HCl were prepared by emulsification-heat stabilization and emulsification-polymerization technique. When the primary emulsion is unstable, encapsulation efficiency is low because the internal aqueous phase tends to merge with the neighboring aqueous continuous phase. Stability of the primary emulsion can be enhanced by including emulsifying agents such as PVA, Tween-80, or Span-80 either in the internal aqueous phase or in the polymer phase.
The percentage practical yield value of microparticles prepared with Bovine Serum Albumin was in range of 65.46% to 72.82% (Table I). The yield value increased as the amount of polymer added to each formulation increased. Maximum yield was found to be 72.82% (w/w) in DTM-4.
The encapsulation (drug loading) efficiency for the formulated microparticles are summarized in Table I. the amount of drug bound per 10 mg microparticles was determined in the prepared all four batches. The maximum drug loading was found in DTM-4 (72.48%).
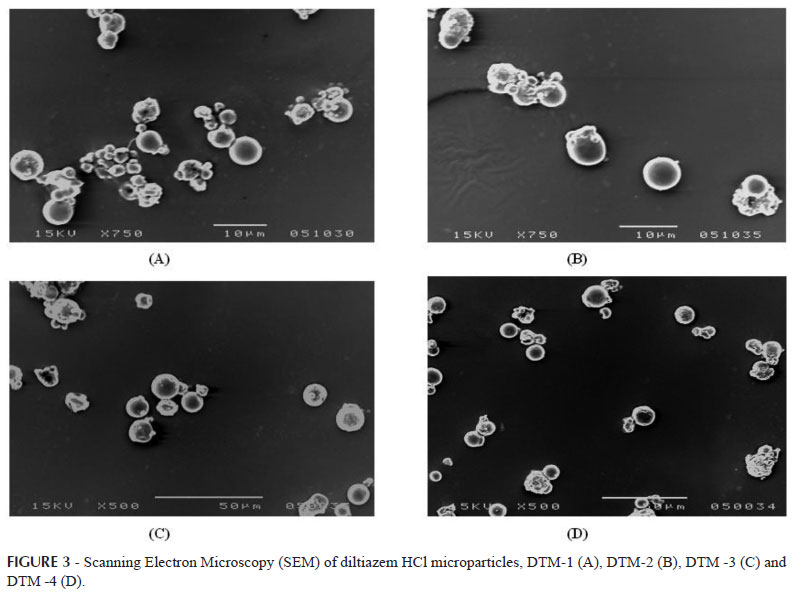
In the present investigation, the particle size distribution of the microparticles prepared with BSA could be changed by simple altering the drug to polymer ratio. When related factors such as emulsification stirring speed, heating time, solvent to polymer ratio and surfactant/stabilizer concentration were kept constant, an increasing in polymer loading elicited a change in microparticles size distribution. Scanning electron photomicrographs of all the four formulations are shown in Figure 3. An increase in the albumin concentration from led to significant increase in the particle size. Average particle size of bovine serum albumin microparticles of diltiazem HCl was found to be 2.17, 2.34, 2.48 and 8.25 µm for DTM-1, DTM-2, DTM-3 and DTM-4 respectively. (Table I). The SEM photomicrographs (Figure 3) of the microparticles reveal that they are spherical, nonporous, and uniform with a smooth surface, which was prepared by heat stabilization technique. The particles appeared to be aggregate in nature without evidence of any collapsed particles.
The cumulative percent drug release of pure drug was found to be 97.95% at 2 hours. Cumulative percent of drug release after 24 hours was 89.42%, 87.48%, 76.65%, and 74.83% for DTM-1, DTM-2, DTM-3 and DTM-4 respectively by using UV spectroscopy. It was observed that the drug release from the formulations decreased with increase in the amount of polymer added in each formulation (Figure 4). The in vitro release of all the batches of microparticles showed a bi-phasic release with an initial burst effect. The drug release from BSA microparticles can usually be divided into an initial release (burst) phase followed by a slower continuous release phase. The initial release, which plays an important role in the therapeutic efficacy of microparticles, is normally defined as the amount of drug released during the first hour. The initial release is commonly attributed to the release of drug located close to the surface of microparticles. It is related to the microstructure (porosity) of the microparticles. In the first hour, drug release was 24.82%, 26.67%, 27.28% and 28.92% for DTM-1, DTM-2, DTM-3 and DTM-4 formulations respectively. Afterwards the drug release followed a steady pattern approximating zero order release. An alternative method to estimate the drug release concentration of formulation DTM-1 was carried out by HPTLC. The graph of HPTLC is shown in Figure 5.
The regression coefficient for formulations DTM-1, DTM-2, DTM-3, and DTM-4 of zero-order plot was found to be 0.9339, 0.9551, 0.9633 and 0.9973 respectively. The regression coefficient for formulations DTM-1 to DTM-4 of first-order plot was found to be -0.9882, -0.9933, -0.9901 and -0.9767 respectively. The regression coefficient for formulations DTM-1 to DTM-4 of Higuchi Matrix plot was found to be 0.9871, 0.9929, 0.9959 and 0.9871 respectively. Hixson Crowell plot regressions coefficient of formulations DTM-1 to DTM-4 was found to be -0.9775, -0.9908, -0.9935 and -0.9808 respectively. The slope 'n' values in Peppas model for DTM-1 to DTM-4 were 0.355, 0.324, 0.296 and 0.242 respectively, which is less than 0.45.
The average drug distribution efficiency of drug-loaded microparticles was found to be 15.22% in liver, 7.86% in lungs, 6.50% in spleen and 4.47% in kidneys after 24 hours, whereas accumulation of pure drug in liver was 5.89%, in lungs it was 3.48%, in spleen 2.06% and in kidneys 1.43% of the injected dose after 2 hours (Table II).
In vitro release studies revealed that the formulation (DTM-3) stored at 4 ºC showed 76.84% release, the one which was stored at ambient temperature/humidity showed 78.27% and 40 ºC batch showed 78.68% release after 24 hours.
DISCUSSION
In the present study, an attempt is made to formulate diltiazem HCl as micro-particulate drug delivery system in order to localize drug at the absorption site, enhances its bioavailability, reduces dose, thereby improving patient compliance. Microparticulate system of diltiazem HCl was formulated using BSA (natural polymer) as carrier by emulsification-heat stabilizing method. Prior to formulation, preformulation studies were carried out in order to establish compatibility between drug and polymer by FTIR spectroscopy.
Percentage practical yield was found to be maximum in formulation DTM-4. Percentage practical yield increased as the amount of polymer added to each formulation increased, although it may not be dependent upon drug concentration in the formulation. Particle size of the drug-loaded microparticles revealed that the particles were in micron range. The particle size of the albumin microparticles is 2-9 µm. The microparticles describes in this study were spherical, smooth surfaced and without porous which prepared by heat stabilization technique. The microparticles appeared to be aggregate in nature without evidence of any collapsed particles. Drug entrapment efficiency was found to be maximum in DTM-4. It is observed that drug entrapment efficiency increased with increase drug-polymer ratio.
Release of diltiazem HCl from BSA microparticles was prolonged with a slight burst release in the first hours (Figure 4). The release from microparticles continued for at least 24 hours and fit a zero order release pattern. Within 24 hours, about 90% of loaded drug in microparticles was released. Cumulative percent of drug release with respect to time was found to be highest for formulation DTM-1 and the lowest for formulation DTM-4. Based on the regression coefficient values, the best-fit model for DTM-1, DTM-2 and DTM-3 was first order and DTM-4 followed zero-order release. Results of Hixson Crowell plot indicated that DTM-3 and DTM-4 appear to fit this model. It was also observed that DTM-3 followed Higuchi matrix suggesting drug release by diffusion. The 'n' values of Peppas suggest Fickian release and Hixson Crowell regression data show that formulations also appear to release drug by erosion mechanism and the release is drug dissolution limited.
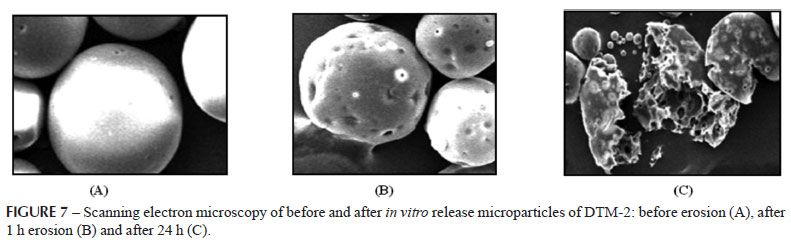
Figure 7 shows degradation of the microparticles by erosion and hydrolysis mechanism. The photograph on 0 hour (A) shows the appearance of microparticle before erosion. The surface of microparticles becomes irregular and porous during the erosion (B) after first hour. It was noticed in photograph on 24 hours (C) that microparticles had broken down to small fragments due to erosion.
Overall, the curve fitting into various mathematical models was found to be good and the in vitro release, of formulations, best fitted into the Peppas model followed by Higuchi's and Hixson Crowell model (Figure 6). On the basis of drug content, particle size morphology, in vitro release kinetics, formulation DTM-3 was selected as an optimum formulation for in vivo and stability studies.
Figure 5, 3D view of HPTLC of in vitro release profile of DTM-1 formulation. First four tracks for standard calibration peak, tracks 5-13 are drug release samples and last track for maximum drug concentration in the formulation. The fifth track of the figure indicates burst effect and 6th to 13th track indicate zero order release pattern.
The present study shows that the targeting efficiency of drug-loaded microparticles over free drug was higher, which may provide increased therapeutic efficacy. Moreover, higher concentration of drug targeted to various organs may help in reduction of dose required for the therapy and thereby dose related side effects could be minimized. The in vivo drug targeting studies revealed the following order of targeting (tissue distribution): liver > lungs > kidneys > spleen.
Drug content data of stability studies indicate that maximum drug was retained by formulation DTM-3 when stored at 4 ºC. Drug content of the formulation DTM-3 stored at various temperature conditions reduced in the following order: 4 ºC > RT > 40ºC. In vitro release data of stability studies indicate that very less variation in release was found at 4 ºC followed by room temperature and 40 ºC.
CONCLUSION
We showed that microparticles of biodegradable polymer were promising as a controlled-release drug delivery system of diltiazem HCl. Bovine serum albumin which are biocompatiable and biodegradable, have studied for the controlled release of many drugs. From the experimental results obtained with respect to particle size and prolonged drug release, it may be concluded that the developed bovine serum albumin (biodegradable) microparticles could be useful for once-a-day intravenous (IV) administration of diltiazem HCl. Further, pharmacokinetic and drug release model studies are required to confirm the application of these microparticles for IV administration. From the above studies, it can be concluded that 4 ºC is the most suitable temperature for storage of diltiazem HCl microparticles.
ACKNOWLEDGEMENTS
Authors wish to thank Neuland Laboratories Ltd, AP, India for providing gift samples of diltiazem HCl BP, Principal, Institute of Pharmacy, HCPG College, Varanasi and HOD, Department of Pharmaceutics, IIT, BHU, Varanasi, for providing necessary facilities.
Received for publication on 26th October 2011
Accepted for publication on 28th August 2012
- ARSHADY, R. Albumin microspheres and microcapsules: methodology of manufacturing techniques. J. Control. Release, v.14, n.2, p.111-131, 1990.
- BIRNBAUM, D.T.; PEPPAS, L.B. Microparticle drug delivery systems. In: BROWN, D. M. (Ed.). Drug delivery systems in cancer therapy Totowa: Humana Press, 2003. p.117-136.
- CHINNA, G.; SHYAM, S.; VARMA, V.K.; SLEEVA, R.; SAI, K. Formulation and Evaluation of Indomethacin Microspheres using natural and synthetic polymers as Controlled Release Dosage Forms. Int. J. Drug Discovery, v.2, n.1, p.8-16, 2010.
- DANDAGI, P.M.; MASTIHOLIMATH, V.S.; PATIL, M.B.; GUPTA, M.K. Biodegradable microparticulate system of Captopril. Int. J. Pharm, v.307, n.1, p.83-88, 2006.
- DAS, M.K.; MAURYA, D.P. Evaluation of Diltiazem HCl-Loaded Mucoadhesive microspheres prepared by emulsification-internal gelation technique. Acta Pol. Pharm. Drug Res., v.65, n.2, p.249-259, 2008.
- GUPTA, M.K.; MISHRA, B.; PRAKASH, D.; RAI, S.K. Nanoparticulate drug delivery system of cyclosporine. Int. J. Pharm. Pharm. Sci, v.1, n.2, p.81-92, 2009.
- KRISTINA, M.; LJILJANA, K.; EMILIJA, F.K.; MAJA, G.D.; EMILIJA, J.I.; KATERINA, G. Crosslinked gelatin microspheres containing BSA as a vaccine formulation: biodegradation and drug release control in the presence of trypsin. Bull. Chem. Technol. Macedonia, v.20, n.2, p.151-156, 2001.
- KULKARNI, G.T.; GOWTHAMARAJAN, K.; SURESH, B. Stability testing of pharmaceutical products: an overview. Indian J. Pharm. Educ., v.38, n.11, p.194-202, 2004.
- LALLA, J.K. Introduction to controlled release and oral controlled drug delivery systems. East. Pharm., v.45, n.7, p.25-28, 1991.
- LEE, T.W.; ROBINSON, J.R. Controlled release drug delivery systems. In: GENNARO, A.R. 20.ed. Remington: the science and practice of pharmacy. Philadelphia: Lippincott Williams and Wilkinsi, 2000. v.l, p.903-928.
- MANSOUR, H.M.; SOHN, M.; AL-GHANANEEM, A.; DELUCA, P.P. Materials for pharmaceutical dosage forms: molecular pharmaceutics and controlled release drug delivery aspects. Int. J. Mol. Sci., v.11, n.9, p.3298-3322, 2010.
- MATHEW, S.T.; DEVI, S.G.; SANDHYA, K.V. Formulation and evaluation of ketorolac tromethamine-loaded albumin microspheres for potential intramuscular administration. AAPS Pharm. Sci. Tech., v.8, n.1, p.71-79, 2007.
- MERKLE, H.P.; GANDER, B.; MEINEL, L.; WALTER, E. Novel opportunities of microparticulates for the delivery of therapeutics and vaccines London: Touch Health Sciences, 2002. p.1-6.
- NANJWADE, B.K.; BECHRA, H.M.; NANJWADE, V.K.; DERKAR, G.K.; MANVI, F.V. Formulation and characterization of hydralazine hydrochloride biodegraded microspheres for intramuscular administration. J. Bioanal. Biomed., v.3, n.1, p.32-37, 2011.
- NELA, A.; DAVID, H. Rationalizing the design of polymeric biomaterials. Tibtech., v.17, n.10, p.409-421, 1999.
- OLIVA, A.; SANTOVENA, A.; LLABREWS, M.; FARINA, J.B. Stability study of human serum albumin pharmaceutical preparations. J. Pharm. Pharmacol., v.51, n.4, p.385-392, 1999.
- ONER, L.; GROVES, M.J. Properties of human albumin microparticles prepared by a chilled cross linking technique. J. Pharm. Pharmacol., v.45, n.10, p.866-870, 1993.
- PEPPAS, B.L. Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int. J. Pharm, v.116, n.1, p.1-9, 1995.
- PEREZ, M.H.; SIEPMANN, J.; ZINUTTI, C.; LAMPRECHTC, A.; UBRICHA, N.; HOFFMANA, M.; BODMEIERB, R.; MAINCENTA, P. Non-degradable microparticles containing a hydrophilic and/or a lipophilic drug: preparation, characterization and drug release modeling. J. Control. Rel., v.88, n.3, p.413-428, 2003.
- RAHIMNEJAD, M.; JAHANSHAHI, M.; NAJAFPOUR, G.D. Production of biological nanoparticles from bovine serum albumin for drug delivery. African J. Biotechnol., v.5, n.20, p.1918-1923, 2006.
- SALEM, H.F.; FAHMY, A.; ALI, A.M.A. Extended immunization of rats using microencapsulated cobra venom. Br. J. Pharmacol. Toxicol, v.2, n.1, p.43-50, 2011.
- SHENOY, B.D.; KINI, D.; UDUPA, N. Formulation and in vitro evaluation of centchoman-loaded biodegradable microspheres. Indian J. Pharm. Sci., v.60, n.1, p.41-44, 1997.
- THAKKAR, H.; SHARMA, R.K.; MISHRA, A.K.; CHUTTANI, K.; MURTHY, R.R. Albumin microspheres as carriers for the antiarthritic drug celecoxib. AAPS Pharm. Sci Tech., v.6, n.1, p.65-73, 2005.
- TUNCAY, M.; CALIS, S.; KAS, H.S.; ERCAN, M.T.; PEKSOY, I.; HINCAL, A.A. In vitro and in vivo evaluation of Diclofenac sodium loaded albumin microspheres. J. Microencapsul., v.17, n.2, p.145-155, 2000.
- YEO, Y.; PARK, K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch. Pharm. Res, v.27, n.1, p.1-12, 2004.
Publication Dates
-
Publication in this collection
14 Feb 2013 -
Date of issue
Dec 2012
History
-
Received
26 Oct 2011 -
Accepted
28 Aug 2012