Abstracts
The objective of this study was to develop a pharmaceutical O/W emulsion containing plant-derived polyphenol extracts and evaluate its stability and antioxidant activity. O/W emulsions were prepared using ionic surfactant polysorbate 80 (Tween 80®). The odorwas adjusted with few drops of blue sea fragrance. DPPH (1,1-diphenyl-2-picrylhydrazyl) assay was used to evaluate the antioxidant activity of the plant extracts alone and emulsions containing these extracts. Physical stability was assessed by submitting the emulsions to storage at 8 ºC, 25 ºC, 40 ºC and 40 ºC + 70% RH (relative humidity) for two months. Various physical characteristics of emulsions monitored, include color, creaming, liquefaction, centrifugation and pH. Brookfield rotational rheometer was used to determined viscosities and rheological behavior of emulsions. Different types of emulsion were determined microscopically, while pH values of emulsions were measured by a pH meter. Electrical conductivity data confirmed that the outer phase was water. Samples presented an acceptable pH value for an external topical use. Shear thinning behaviour was observed for all emulsions. The polyphenol-rich-plant-derived extracts alone and the extract containing emulsions showed good antioxidant activities. This research confirmed that the method used was suitable for preparing emulsions with Hippophae rhamnoids and Cassia fistula extracts, suggesting that those emulsions are suitable for topical use.
Hippophae rhamnoids; Cassia fistula; Emulsion O; Emulsion O; Emulsion O; Emulsion O; Emulsion O; Emulsion O
O presente estudo objetivou o desenvolvimento de uma emulsão farmacêutica óleo-água contendo extratos de plantas ricos em polifenóis, a comparação à sua formulação-controle e a avaliação de sua estabilidade, assim como de sua capacidade antioxidante. Extrato concentrado de Hippophae rhamnoids e Cassia fistula foi encapsulado no interior da fase oleosa da emulsão O/W. As emulsões foram preparadas usando o tensoativo iônico monooleato sorbital de polioxietileno (Tween 80®). O odor foi ajustado pela adição de algumas gotas de fragrância azul do mar. O ensaio do DPPH (1,1-difenil-2-picrilidrazil) foi utilizado para avaliar a atividade antioxidante dos extratos de plantas sozinhos e nas emulsões contendo os extratos. A estabilidade física foi avaliada submetendo os cremes a diferentes temperaturas de estocagem, como a 8 ºC, 25 ºC e 40 ºC e a 40% + 70% de umidade relativa por um período de 2 meses. As características físicas das emulsões foram monitoradas por 2 meses incluindo cor, cremosidade, liquefação, centrifugação e pH. O reômetro rotacional de Brookfield foi utilizado para determinar a viscosidade e o comportamento reológico das emulsões. O programa Rheocalc Brookfield foi utilizado para análise dos dados. As características organolépticas também foram avaliadas. O tipo de emulsão foi determinado microscopicamente, enquanto o pH das emulsões foi avaliado por meio de um pHmetro. A estabilidade farmacêutica esperada das emulsões foi alcançada dentro dos dois meses de estudo. Os resultados da condutividade elétrica confirmaram que a fase externa da emulsão era composta de água. O pH das amostras estava dentro da normalidade para uso tópico. A emulsão apresentou boa fragrância e pode ser retirada da pele com água após a aplicação, características desejáveis em emulsões O/W. Os extratos vegetais ricos em polifenóis isolados ou nas emulsões apresentaram boa atividade antioxidante. Nossos estudos confirmaram que o método utilizado foi adequado para preparar a emulsão semi-sólida contendo extratos de Hippophae rhamnoids e Cassia fistula. Nossos achados sugerem que emulsões contendo extratos de Hippophae rhamnoids e Cassia fistula são adequados para o uso tópico.
Hippophae rhamnoids; Cassia fistula; Emulsão óleo-água; Emulsão óleo-água; Emulsão óleo-água
ARTICLE
Development, characterization and antioxidant activity of polysorbate based O/W emulsion containing polyphenols derived from Hippophae rhamnoides and Cassia fistula
Barkat Ali KhanI; Naveed AkhtarI; Haroon KhanII; Valdir de Andrade BragaIII
IDepartment of Pharmacy, Faculty of Pharmacy and Alternative Medicine, The Islamia University of Bahawalpur, Pakistan
IIDepartment of Pharmaceutical Chemistry, Gomal University, Pakistan
IIIDepartment of Biotechnology, Biotechnology Center, Federal University of Paraiba, Brazil
Correspondence
ABSTRACT
The objective of this study was to develop a pharmaceutical O/W emulsion containing plant-derived polyphenol extracts and evaluate its stability and antioxidant activity. O/W emulsions were prepared using ionic surfactant polysorbate 80 (Tween 80®). The odorwas adjusted with few drops of blue sea fragrance. DPPH (1,1-diphenyl-2-picrylhydrazyl) assay was used to evaluate the antioxidant activity of the plant extracts alone and emulsions containing these extracts. Physical stability was assessed by submitting the emulsions to storage at 8 ºC, 25 ºC, 40 ºC and 40 ºC + 70% RH (relative humidity) for two months. Various physical characteristics of emulsions monitored, include color, creaming, liquefaction, centrifugation and pH. Brookfield rotational rheometer was used to determined viscosities and rheological behavior of emulsions. Different types of emulsion were determined microscopically, while pH values of emulsions were measured by a pH meter. Electrical conductivity data confirmed that the outer phase was water. Samples presented an acceptable pH value for an external topical use. Shear thinning behaviour was observed for all emulsions. The polyphenol-rich-plant-derived extracts alone and the extract containing emulsions showed good antioxidant activities. This research confirmed that the method used was suitable for preparing emulsions with Hippophae rhamnoids and Cassia fistula extracts, suggesting that those emulsions are suitable for topical use.
Uniterms:Hippophae rhamnoids/pharmacognosy. Cassia fistula/pharmacognosy. Emulsion O/W/topical use. Emulsion O/W/conductivity. Emulsion O/W/rheology.
RESUMO
O presente estudo objetivou o desenvolvimento de uma emulsão farmacêutica óleo-água contendo extratos de plantas ricos em polifenóis, a comparação à sua formulação-controle e a avaliação de sua estabilidade, assim como de sua capacidade antioxidante. Extrato concentrado de Hippophae rhamnoids e Cassia fistula foi encapsulado no interior da fase oleosa da emulsão O/W. As emulsões foram preparadas usando o tensoativo iônico monooleato sorbital de polioxietileno (Tween 80®). O odor foi ajustado pela adição de algumas gotas de fragrância azul do mar. O ensaio do DPPH (1,1-difenil-2-picrilidrazil) foi utilizado para avaliar a atividade antioxidante dos extratos de plantas sozinhos e nas emulsões contendo os extratos. A estabilidade física foi avaliada submetendo os cremes a diferentes temperaturas de estocagem, como a 8 ºC, 25 ºC e 40 ºC e a 40% + 70% de umidade relativa por um período de 2 meses. As características físicas das emulsões foram monitoradas por 2 meses incluindo cor, cremosidade, liquefação, centrifugação e pH. O reômetro rotacional de Brookfield foi utilizado para determinar a viscosidade e o comportamento reológico das emulsões. O programa Rheocalc Brookfield foi utilizado para análise dos dados. As características organolépticas também foram avaliadas. O tipo de emulsão foi determinado microscopicamente, enquanto o pH das emulsões foi avaliado por meio de um pHmetro. A estabilidade farmacêutica esperada das emulsões foi alcançada dentro dos dois meses de estudo. Os resultados da condutividade elétrica confirmaram que a fase externa da emulsão era composta de água. O pH das amostras estava dentro da normalidade para uso tópico. A emulsão apresentou boa fragrância e pode ser retirada da pele com água após a aplicação, características desejáveis em emulsões O/W. Os extratos vegetais ricos em polifenóis isolados ou nas emulsões apresentaram boa atividade antioxidante. Nossos estudos confirmaram que o método utilizado foi adequado para preparar a emulsão semi-sólida contendo extratos de Hippophae rhamnoids e Cassia fistula. Nossos achados sugerem que emulsões contendo extratos de Hippophae rhamnoids e Cassia fistula são adequados para o uso tópico.
Unitermos: Hippophae rhamnoids/farmacognosia. Cassia fistula/farmacognosia. Emulsão óleo-água/uso tópico. Emulsão óleo-água/condutividade. Emulsão óleo-água/reologia.
INTRODUCTION
Emulsions show potential applications in paint, food, cosmetic and pharmaceutical industries. There has been given special attention to the emulsion as a vehicle for carrying drugs to the body as they increase the bioavailability of those compounds (Herbert et al., 1988). Oil-in-water or O/W emulsions are commonly used as water-washable drug bases and for general cosmetic purposes (Magdy, 2004). Additional value can be given to those formulations by including active ingredients with specific cosmetic effects. Particularly advantageous cosmetic emulsion preparations are obtained when antioxidants are used as active ingredients (Bleckmann et al., 2006). There is a growing attention in natural antioxidants present in plants. Many antioxidant compounds are isolated from natural herbs and extracts and used as potential antioxidants in cosmetics (Naveed, 2001). Owing to their beneficial and therapeutic properties, natural plant extracts and their derived products have frequently been included in the form of emulsions in current pharmaceutical and cosmetics formulations and preparations.
The applications of topical emulsions need both their efficacy as well as nominal risk of skin irritation/skin sensitization. This is prejudiced by their formulation, nature of their use and quantity and quality of ingredients. The shelf life is the time period for which a drug can be hoard before it turns out to be unfit for use due to chemical decay/physical deterioration. Hippophae rhamnoides juice is a significant source of several precious chemicals such as ascorbic acid, toco-pherolmacrotrients, organic acids and polyunsaturated fatty acids (Akhtar et al., 2010). Polyphenols (flavonoids) are present in Hippophae rhamnoides. The chief flavonoids in Hippophae rhamnoides are isorhamnetin, quercitin, myricetin and kaempferol. Hippophae rhamnoides has been used for the treatment of photo damage, inflammation and burns in Chinese folk medicines as well as in the treatment of skin ailments such as psoriasis, eczema, lupus erythematosus and dermatosus (Barkat et al., 2011). Cassia fistula usually well-known as Indian Laburnum, is scattered in Asia, Mauritius, South Africa, Mexico, China, East Africa and Brazil as an ornamental tree for its beautiful bunches of yellow flowers and it is recognized by the British pharmacopoeia. The key constituents present in Cassia fistula are tannins, fatty acids, isoflavonoids, flavonoids, glycosides, anthraquinones and phenolic compounds (Barkat, Naveed, 2012).
Numerous plant polyphenols are value-adding chemicals and are used as constituents in cosmetics, foods and pharmaceuticals. The prospective use of phenolic compounds for the introduction of new skin care cosmetics (creams/emulsions) has been underlined (Kiken, Cohen, 2002). Plant polyphenols can be used as sunscreen, whitening and anti-aging agents (González et al., 2008).
Emulsion stability is an important factor governing the shelf life of products. Predominantly, emulsions are thermodynamically unstable systems and they are predisposed to break over time due to various physicochemical mechanisms such as creaming, flocculation, coalescence, phase inversion and/or Ostwald ripening. Stability in emulsions must be considered. The present research involved the formulation development of emulsions, systematic study of the antioxidant properties of extracts alone and in emulsions and stabilization of emulsions.
MATERIAL AND METHODS
Chemicals
Folin Ciocalteu reagent and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma Chemical Co., Ltd (Saint Louis, USA). Polysorbate 80, Span 20, Liquid paraffin, Stearic acid, Bees wax and Cetomacrogol were taken from Merck Germany.
Plant Materials
Cassia fistula samples were collected from old campus (Abbasia campus), the Islamia University of Bahawalpur, Pakistan, while Hippophae rhamnoides berries were purchased from Pak Sea Buckthorn International, Skardo, Pakistan. The identification was performed at Cholistan Institute of Desert Studies (CIDS), The Islamia University of Bahawalpur. The voucher specimens were deposited at the herbarium of Pharmacognosy Section, Faculty of Pharmacy and Alternative Medicine, The Islamia University of Bahawalpur Pakistan.
Apparatus
Centrifuge Machine ( Hettich EBA 20, Germany), Cold Incubator (Sanyo MIR-153, Japan), Conductivity-Meter (WTW COND-197i, Germany), Digital Humidity Meter (TES Electronic Corp, Taiwan), Electrical Balance (Precisa BJ-210, Switzerland), Homogenizer (Euro-Star, IKA D 230, Germany), Hot Incubator (Sanyo MIR-162, Japan), PH-Meter (WTW pH-197i, Germany), Refrigerator (Dawlance, Pakistan), Rotary evaporator (Eyela, Co. Ltd. Japan).
METHODS
Preparation of the Plant Extracts
Extraction of plants is described by Barkat and Naveed (2012) and shortly describe here. 150 mg of Cassia fistula leaves was extracted by 70% methanol for 72 h at room temperature in a 5 liters beaker in a dark room. 320 mg of Hippophae rhamnoides berries were compressed and successively macerated in a mixture of 2 liters of methanol and distilled water in a ratio of 1:1. The macerated plant material was filtered through 16 layers of muslin cloth for coarse filtration. The coarse filtrate was then filtered through a Whatman No. 1 filter paper in order to get particle free extracts. The filtrate was evaporated under reduced pressure at 40 ºC in a rotary vacuum evaporator and stored in amber container and under refrigeration (-18 ºC) until used for further analyses.
Total Phenolics determination
Yafang et al. (2011) method was adopted with slight modification. 200 µL of plant extract were oxidized with Folin-Ciocalteu reagent (1 mL; 0.5 N) and the reaction was then neutralized with 1 mL of the saturated sodium carbonate (75 g/L). The absorbance of the resulting blue color solution was measured at 760 nm with spectrophotometer after incubation for 2 h at room temperature. Quantification was done on the basis of the standard curve of gallic acid. Results were expressed as milligram of gallic acid equivalent (mg GAE/g).
DPPH free radical scavenging activity
Sharma et al. (2011) method with slight modification was adopted for free radical scavenging activity of both, extracts alone and emulsions containing extracts. Stock solution of DPPH (33 mg in 1 L) was prepared in methanol, which gave initial absorbance of 0.493. 5 mL. Stock solutions were added to 1 mL of sample solution at different dilutions (250-1500 µg/mL). After 30 min, absorbance was measured at 517 nm spectophotometrically. Percentage scavenging activity was calculated using the following formula:
where A0= absorbance of the control and A1= absorbance of the sample. Scavenging activity was compared with ascorbic acid.
Preparation of emulsions
O/W emulsions (control formulation and formulation containing the extracts) were prepared by the addition of the aqueous phase to the oily phase with continuous agitation. The emulsions were stabilized by Polysorbate 80 (Tween 80®) (Table I). Oily phase was heated to 70±1 ºC. At the same time, aqueous phase was heated to 75±1 ºC and extracts were added to the preparation. Next, aqueous phase was added to the oil phase in a drop-to-drop basis. Mixing was carried out at 41.9 g by the mechanical mixer for 20 minutes until complete aqueous phase was added. Finally, 2 to 3 drops of fragrance were added during this stirring time to give good odor to the formulations. Control formulation was also prepared by the same above method but without plant extracts (the active ingredient) and served as control.
Characterization of emulsions
Emulsions were examined organoleptically (odor, color, thickness, look, feel) and physically (creaming/sedimentation and phase separation). Type of emulsions was determined microscopically. pH and electrical conductivity values of freshly prepared emulsions and emulsions kept at different storage conditions were determined by a digital pH-meter and digital conductivity-meter respectively. Centrifugal tests were performed for emulsions immediately after preparation. The centrifugal tests were repeated for emulsions after 24 hours, 7, 14, 21, 28 and 60 days of preparation. The centrifugal tests were performed at 25 ºC and at 4192 g for 10 minutes by placing 5g of sample in disposable stoppered centrifugal tubes. Rheological parameters and viscosity of the emulsions was determined using Brookfield DV III ultra V6.0 RV cone and plate rheometer (Brookfield Engineering Laboratories) using spindle # CPE40 at 25 ± 0.5 ºC. Rheogram curves constructed with ascendant and descendant segments were obtained with rotation speeds increasing progressively (0.1 to 0.4 g) and gradually decreasing (0.4 to 0.1 g).
Pharmaceutical stability tests
Stability tests were performed on samples kept at 8 ± 0.1ºC (in refrigerator), 25 ± 0.1 ºC, 40 ± 0.1 ºC and 40 ± 0.1 ºC (in incubator) with 75% relative humidity (RH) for 2 months.
Mathematical analysis
The percentage changes for the individual values of pH and conductivity, taken every week, were calculated by the following formula;
Percentage Change = [(A - B) / B]*100
Here; A = Individual value of any parameter of 1st, 2nd, 3rd, 4th or 8th week
B = Zero hour value of that parameter (freshly prepared emulsions)
Statistical analysis
Statistical analysis was performed by using SPSS 12.0 on the PC computer. The ANOVA (analysis of variance) test was applied to determine difference of all the parameters studied at the initial and different time intervals at all storage conditions. Statistically, a significant difference was considered at a p value of less than 0.05 (5%).
Rheocalc software
Rheological parameters (flow index and % confidence of fit) of emulsions were analyzed by using the Brookfield software, Rheocalc.
RESULTS
Total phenolics contents
Total phenolic contents (TPC) determined spectophotometrically showed adequate amount of these important contents present in extracts of Hippophae rhamnoides and Cassia fistula. Total phenolic content is directly linked with antioxidant activity. Total phenolic content, articulated in mg GAE/g of Hippophae rhamnoides and Cassia fistula are shown in Table II.
DPPH free radical scavenging activity
The change in absorbency produced by reduced DPPH was used to evaluate the antioxidant ability of the plant extract and emulsions containing plant extracts. The antioxidant activity of plant extract and after addition of plant extract to the emulsions is shown in Table III.
Type of emulsions
The amaranth red dye was mixed with the emulsions and a drop of the emulsion was placed on the microscopic slide, covered it with a cover slip, and examined it under a microscope. The disperse globules appeared red and the ground colorless, indicated O/W emulsions.
Stability studies
Organoleptic tests
The emulsions (control and active formulations) were divided into four samples, which were kept at 8 ºC in refrigerator, at 25 ºC, 40 ºC and at 40 ºC + 75% RH (Relative Humidity) in thermal incubators. They were organoleptically evaluated to note alteration in odor, color, liquefaction and phase separation for a period of 2 months at definite time intervals. No differences were observed in the organoleptic properties among emulsions for the two months of the experiments under different conditions as mentioned above.
Centrifugation
Centrifugation tests were performed at 4192 g for 10 minutes to check phase separation for 2 months at different time intervals. There was no phase separation found after centrifugation in any of the samples of emulsions.
Electrical conductivity
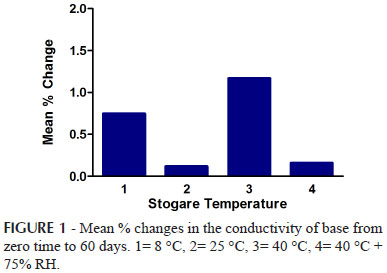
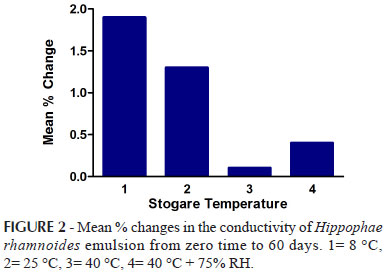
The conductivity of the prepared emulsions were measured for all the samples kept at 8 ºC, 25 oC, 40 oC and 40 oC + 75% RH immediately after preparation and then repeated after 12, 24, 36, 48, 72 hours and 7, 14, 21, 28 and 60 days. The test was performed in triplicate for each sample. The percentage changes in the conductivity values are presented in the Figures 1, 2 and 3.
pH Tests of emulsions
There were no significant changes in pH values of emulsions at all storage temperatures as a function of time. Minor changes of pH values as a function of time and storage temperatures indicated that the examined emulsions were stable. pH values of the emulsions kept at different storage conditions for 2 months have been determined and reported in Table IV as mean and SEM.
Rheological parameters
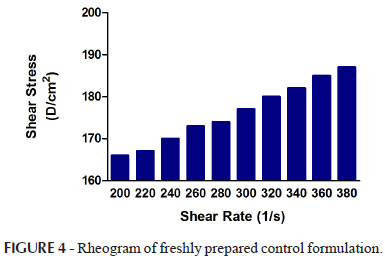
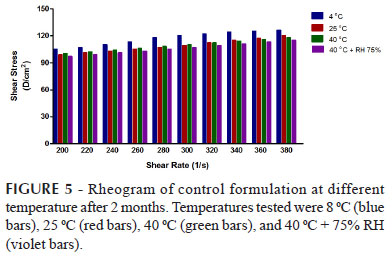
Rheological parameters (flow index and % confidence of fit) of emulsions reserved at various storage conditions up to two months were observed and have been given in Tables V while Rheograms of the emulsions have been given in Figures 4, 5, 6, 7, 8 and 9.
Viscosity studies
Mean Viscosity (cP) ± standard deviations (SD) of the emulsions studied for 2 months stored at various storage conditions are presented in Table VI.
DISCUSSION
Topical emulsions (cosmeceuticals) are prepared to reduce wrinkles (skin aging), fight acne/melasma and to control/normalize skin sebum secretion. For various types of skin complaints, topical formulations like sunscreen, skin protective, anti-acne, anti-melasma, anti-wrinkle and anti-aging are developed using either natural or synthetic materials. For example, Mahmood and Akhtar have documented that a combination of lotus and green tea emulsions had improved skin parameters (Mahmood and Akhtar, 2013). In addition, Casagrande et al. (2006) have described that topical formulations containing quercetin present protective effect against UVB-induced oxidative stress in hairless mice. The designing procedure for topical formulation needs quality attributes. The eminence of a formulation must satisfy the consumer's need in terms of its out-put. The plant extracts used in topical preparation have diversity of properties like antioxidant, antiseptic, anti-inflammatory, emollient, anti-seborrhatic and anti-kerolytic activity. Topical products with plant extracts argue to have fewer side effects as compared to products containing synthetic agents. In this study, we formulated an O/W emulsion for facial use. Therefore, the water content in formula contains 50% by weight of the emulsion. Generally, the type of emulsions is determined by the phase ratio of oil and water if these numbers are high (>3). For example, in this study with 40.0% of oil and 60.0% of water (an o:w phase ratio of 2:3), the emulsion was expected to be O/W (Friberg, 1988). Microscopic examinations exposed that all emulsions were O/W. When color amaranth was used, the emulsion background was pinkish with colorless globules. However, with oil soluble color, Sudan red globules were reddish against a colorless background. Confirmation of emulsion type was carried out by the electrical conductivity test, where all emulsions conducted electricity and thus confirmed to be O/W.
The freshly prepared control was white while the active formulations were pale yellow in color. There was no change occurred in color up to the observation period of 2 months. This showed that the emulsions were stable at different storage conditions i.e. 8 ºC, 25 ºC, 40 ºC and 40 ºC + 75% RH throughout 2 months study period. No change in the color may be attributed to different factors contributing the emulsions stability. As plant extracts contain anti-bacterial components which protect the emulsion components from microbial growth and thus might produce such substances which are able to change the color of the formulation during the storage time. In addition, since emulsions and other water containing dosage forms should be preserved from microbial contamination, a preservative mixture of methyl and propyl paraben was used.
It is important to highlight that both total phenolic content and the antioxidant activity measured by DPPH were preserved in our emulsions. This is desired for the emulsions preparation, since most of the desired effects are mediated by antioxidants. Our data is in accordance to what is reported by Kumar et al (2005), in which a methanol extract of Bauhinia racemosa preserved its antioxidant and total phenolic content after processing.
After formulation of emulsions, time and temperature-dependent phenomenon occur to effect its separation leading to decline in viscosity which results in increased liquefaction (Herbert et al., 1988). No liquefaction was observed in any of the sample of control and active formulation of both plant extract stored at 8 ºC and 25 ºC during 2 months study period. However, liquefaction was observed in the sample from 21st day to 60th day kept at 40 ºC and 28th day to 60 day kept at 40 ºC + 75% RH.
Creaming occurs because of the differences in density of two phases under the influence of gravity which results in phase separation (Derrick, 2000). There was no phase separation observed in any of the samples kept at 8 ºC, 25 ºC, 40 ºC and 40 ºC+ 75% RH up to observation period of 2 months with naked eye. It showed that the emulsions were stable at all storage conditions considering phase separation as a parameter of stability.
Centrifugation is an extremely helpful means for assessing and predicting the shelf life of emulsions (Herbert et al., 1988). In this study centrifugation test was performed for emulsions stored at different storage conditions up to a period of 2 months at definite time intervals. No phase separation on centrifugation was seen in any of the samples. It showed the proper homogenization speed during emulsion formulation prevented them from breakage during stress testing as described by Abdurrahman and Rosli (2006).
The electrical conductivity test was carried out as a confirmatory test of emulsion type as well as a stability test. According to James et al. (2000), conductivity differences occur when an emulsion creams and the oil proportion increases in the upper part of emulsion and the water proportion increases in the lower part of emulsion. In addition, according to Garti et al. (1982), subjection of emulsions to conductivity test before and after stress will indicate their stability. The extent of the conductivity differential between the two interpretations will show the degree of instability.
In this study, conductivity test was performed for all the samples of emulsions kept at different storage conditions up to a period of 2 months at definite time intervals. The conductivity of emulsions stored at 8 ºC, 25 ºC showed insignificant difference from zero hour to 2 months study period which indicate that the emulsions were stable as well as the two phases had suitable oil to water ratios which has been generally observed with stable emulsions whereas the conductivity of emulsions stored at 40 ºC and 40 ºC + 75% RH showed decline (as shown in figure 1-3) which indicated instability according to Martin (2007).
The pH is an important parameter for the effectiveness of topical emulsions. The human has a pH range of 4.5 to 6.0 as defined by Jennifer et al.(2003) and 5.5 is believed to be the average pH of the skin. Therefore, the formulations proposed for application to human skin should have pH in this range.
In this study, the pH of freshly prepared control was 5.7 whereas the pH of Hippophae rhamnoides and Cassia fistula was 5.2 and 5.53 respectively, which is within the range of skin pH. The pH values of control kept at different storage conditions i.e. 8, 25, 40 and 40 ºC+ 75% RH was found to be increasing gradually in the 1st week and then it started to decline continuously till 60th day with some variations. At the end of study, pH of control at 8, 25, 40 and 40 ºC+ 75% RH was 5.4, 5.9, 5.3 and 5.8 respectively. Whereas pH of Hippophae rhamnoides and Cassia fistula emulsions, kept at 8, 25, 40 and 40 ºC + 75% RH showed gradual reduction with slight variations with time. The pH values of Hippophae rhamnoides emulsions were 5.34, 5.50, 5.25 and 5.4 whereas the pH values of Cassia fistula emulsions were 5.5, 5.43, 5.35 and 5.25 at 60th day respectively.
By using statistical technique ANOVA at 5% level of significance, it was found that the change in pH of control was insignificant at different time levels and temperature but there was significant difference in pH change of active formulations at different levels of time and temperature. When LSD (least significant difference) test was applied to check the individual average effects of pH of control at different temperatures with the passage of time by taking average pH values of zero hour at different temperatures as standard, it showed insignificant changes except 3rd and 4th week where differences were significant. Again when LSD test was applied to check the individual average effect of the pH of active formulations at different temperatures with the passage of time by taking average pH values of zero hour at different temperatures as standard, it showed significant changes from 48th hour till the end of study period except the 7th day.
LSD test showed that there was insignificant change in pH of control at different storage conditions but significant changes were noted in pH of active formulations at different storage conditions with the passage of time. The reduction in pH of the formulations at different storage conditions might be due to the oxidation of paraffin oil which generates aldehydes and organic acids. The other reason may be because of the production of acidic species as the plant extracts are very acidic and have high concentration of organic acids such as quinic acid as described by us and others (Barkat, 2010; Raymond et al., 2003).
The flow characteristics of an emulsion are clearly among some of its more important physical features in either technical or aesthetic terms. Hence the ability to measure, adjust and, if possible, forecast such characteristics is very important.
The shear stress causes strain in solids and liquids; the solid deforms and liquid flows. Rearrangement takes place inside the material due to stress application. In a purely viscous material, all the energy required to produce the deformation is dissipated as heat. On the contrary, in a purely elastic material, all the energy required to produce the deformation is stored.
Emulsions dispersed phase affects the rheology of the emulsion by its globule size, volume concentration viscosity and chemical constituents (Naveed, 2000). Rheological analysis allows the characterization of emulsions, to follow changes in emulsion induced by aging shear and temperature and to predict their stability.
Rheological parameters were performed at 25 ºC. Changes in viscosity were noted when shear stress were applied. Rheogram of shear stress versus shear rate were obtained of all the formulations. Viscosities were found to decrease in parallel to increase in shear stress. Viscosities were also found to decrease in emulsions kept at different storage conditions especially at 40 ºC. It was observed from the different study that when temperature was increased, the flow molecules through interface are also increased. The flow molecule correlated with viscosities. The viscosity is very sensitive to the temperature hence; the increment temperature caused reduction of emulsion viscosity (Lim et al., 2011).
The Flow index values indicated that emulsions act as a non Newtonian fluid (Pseudoplastic fluid). The result were in agreement with Saravacos et al. that emulsions show a marked non-newtonian behavior and most fruit and vegetable fluids and pastes are pseudoplastic, where the flow behavior index varies between 0 to 1 (Saravacos, 1995; Pal, 1992). The rheograms of all emulsions showed non-Newtonian behavior, with flow index less than 1 which was an agreeable rheological property reflecting their pseudoplastic tendency. Emulsions with pseudoplastic flow properties cause the formation of a coherent film covering the skin surface. This characteristic is valuable and critical for a better phenolic antioxidant fortification of the skin surface.
CONCLUSION
In conclusion, we report that the emulsions containing Hippophae rhamnoides and Cassia fistula extracts present high antioxidant activity and desirable pharmaceutical stability. Those emulsions could be considered for preparing topical formulations for used in skin care.
ACKNOWLEDGEMENTS
Financial support was given by Higher Education Commission of Pakistan. The authors thank to the Chairman, Department of Pharmacy, The Islamia University of Bahawalpur for his support to complete this research. Dr. Valdir A. Braga was funded by Capes and CNPq in brazil.
Received for publication on 24th November 2012
Accepted for publication on 08th July 2013
- ABDURAHMAN, H.N.; ROSLI, M.Y. Stability investigation of water-in-crude oil emulsion. J. Appl. Sci., v.6, p.2895-2900, 2006.
- AKHTAR, N.; KHAN, B.A.; MAHMOOD, T.; PARVEEN, R.; QAYUM, M.; ANWAR, M.; ZAMAN, S.U.; FAROOQ, M. Formulation and evaluation of antisebum secretion effects of sea buckthorn w/o emulsion. J. Pharm. Biol. Sci., v.2, p.13-17, 2010.
- BARKAT, A.K.; NAVEED, A.; TARIQ, M.; QAYUM, M.; SHAHIQ, U.Z. Formulation and pharmaceutical evaluation of a W/O emulsion of Hippophae rhamnoides. J. Pharm. Res, v.6, p.342-344, 2010.
- BARKAT, A.K.; NAVEED, A. Phytochemical analysis and acute toxicity tests of two medicinal plant extracts. J. Med. Plants Res, v.6, p.3545-3548, 2012.
- CASAGRANDE, R.; GEORGETTI, S.R.; VERRI JR., W.A.; DORTA, D.J.; DOS SANTOS, A.C.; FONSECA, M.J. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B, v.84, p.21-27. 2006.
- DERRİCK, R. Fat crystals and emulsion stability, a review. Food Res. Int, v.3, p.3-14, 2000.
- FRIBERG, S.E.; GOLDSMITH, L.B.; HILTON, M.L. Theory of emulsions. In: LIEBERMAN, H.A.; RIEGER, M.M.; BANKER, G.S. (Eds.). Pharmaceutical dosage forms: disperse systems. New York: Marcel Dekker Inc., 1988. v.1, p.49-91.
- GARTI, N.; MAGDASSI, S.; RUBEINSTEIN, A. Tropical semisolids. Drug Dev. Ind. Pharm., v.8, p.475-482, 1982.
- GONZÁLEZ, S.; FERNÁNDEZ-LORENTEY, M.; GILABERTE-CALZADA. The latest on skin photoprotection. Clin. Dermatol, v.26, p.614-626, 2008.
- HERBERT, A.L.; MARTIN, M.R.; GILBERT, S.B. Pharmaceutical emulsions Pharmaceutical dosage forms: disperse systems. New York: Marcel Dekker, 1988. v.1, p.285-288.
- JAMES, S.; JOSEPH, T.R.; ORAPİN, P.R. Remington: the science and practice of pharmacy, coarse dispersion. 20.ed. New York: Lippincott Williams and Wilkins, 2000. p.316-334.
- JENNIFER, L.M.; KAREN, L.C.; IBULAIMU, K.; PHILIP, F.S.; DAVID, J.S. Evaluation of the effect of pH on in vitro growth of Malassezia pachydermatis. Can. J. Vet. Res, v.67, p.56-59, 2003.
- KIKEN, D.A.; COHEN, D.E. Contact dermatitis to botanical extracts. Am. J. Contact. Dermat, v.3, p.148-52, 2002.
- KUMAR, R.S.; SIVAKUMAR, T.; SUNDERAM, R.S.; GUPTA, M.; MAZUMDAR, U.K.; GOMATHI, P.; RAJESHWAR, Y.; SARAVANAN, S.; KUMAR, M.S.; MURUGESH, K.; KUMAR, K.A. Antioxidant and antimicrobial activities of Bauhinia racemosa L. stem bark. Braz. J. Med. Biol. Res, v.38, p.1015-1024, 2005.
- LIM, C.J.; MAHIRAN, B.; DZOLKHIFLI, O. Physicochemical characterization of nonionic surfactants in oil-in-water (O/W) nano-emulsions for new pesticide formulations. Int. J. Appl. Sci. Technol., v.1, p.131-142, 2011.
- MAGDY, I.M. Optimization of chlorphenesin emulgel formulation. AAPS J, v.6, p.1-7, 2004.
- MARTIN, A.N.; SWARBRICK, J.; CAMMARATA, A. Physical pharmacy 2ed. Philadelphia: Lea and Febiger, p.525-537, 1970.
- MAHMOOD T, AKHTAR N. Combined topical application of lotus and green tea improves facial skin surface parameters. Rejuvenation Res v.16, p.91-97, 2013.
- NAVEED, A. Formulation and evaluation of a cosmetic multiple emulsion system containing macademia nut oil and two antiaging agents. Anadolu, 2001. p.104-107. [Thesis of PhD degree. Department of Pharmaceutical Technology, Anadolu University]
- RAJINDER, P. Rheology of polymer-thickened emulsions. J. Rheol, v.36, p.1245-1261, 1992.
- RAYMOND, C.R.; PAUL, J.S.; PAUL, J.W. Dimethicone, mineral oil, wax white; wax yellow. In: Handbook of pharmaceutical excipients. 4.ed. London: The PhP, pharmaceutical press, 2003. p.687-690.
- SARAVACOS, G.D.; KOSTAROPOULOS, A.E. Transport properties in processing of fruits and vegetables. Food Technol, v.33, p.49-59, 1995.
- YAFANG, S.Z.; JINSONG, B. Total phenolic content and antioxidant capacity of rice grains with extremely small size. Afr. J. Agr. Res., v.6, p.2289-2293, 2011.
Correspondence:
Publication Dates
-
Publication in this collection
11 Mar 2014 -
Date of issue
Dec 2013
History
-
Received
24 Nov 2012 -
Accepted
08 July 2013