Abstracts
Enzyme stability is critical in biotechnology, pharmaceutical and cosmetic industries. Investigations on this subject have drawn attention because of its practical application. Bromelain is a thiol-endopeptidase, obtained from pineapple (Ananas comosus), known for its clinical and therapeutic applications, particularly to selective burn debridement and improvement of antibiotic action and anti-inflammatory activities. To date, the use of bromelain in pharmacological or industrial applications is limited, due to commercial availability, costs, and sensitivity to pH and temperature. Therefore, a better understanding of enzyme stability would be of great interest. The aim of this study was to evaluate bromelain activity and stability in several pH (2.0 to 8.0) and in polyethylene glycol and polyacrylic acid solutions. We observed that bromelain was able to maintain its stability at pH 5.0 for the temperatures studied. PEG solutions increased bromelain stability, but PAA solutions had the opposite effect.
Bromelain/pH stability; Polyethylene glycol/enzyme stability; Polyacrylic acid/enzyme stability; Enzyme/stability
Estabilidade de enzimas é uma questão fundamental em indústrias biotecnológicas, farmacêuticas e cosméticas. As investigações sobre o assunto têm chamado a atenção por sua aplicação prática. A bromelina é uma tiol-endopeptidase, obtida a partir do abacaxi (Ananas comosus). É conhecida por suas aplicações clínicas e terapêuticas, especialmente para desbridamento seletivo de queimaduras, melhoria de ações antibiótica e de atividades anti-inflamatórias. Até o momento, a utilização da bromelina em aplicações farmacológicas industriais é limitada, devido à disponibilidade comercial, os custos, a sensibilidade ao pH e temperatura. Portanto, a maior compreensão da estabilidade desta enzima seria de grande interesse. O objetivo deste estudo foi avaliar a estabilidade da atividade da bromelina em vários pH (2,0 a 8,0) e em soluções de polietilenoglicol e de ácido poliacrílico. Observamos que a bromelina foi capaz de manter a sua estabilidade em pH 5.0, em todas as temperaturas estudadas. Soluções de PEG aumentaram a estabilidade da bromelina, enquanto que soluções de PAA obtiveram efeito oposto.
Bromelina/estabilidade em pH; Polietilenoglicol/estabilidade de enzimas; Ácido poliacrílico/estabilidade de enzimas; Enzimas/estabilidade
INTRODUCTION
Stem-bromelain (EC. 3.4.22.32) is a thiol-endopeptidase with 23.8 kDa and isoelectric point (pI) 9.55, obtained from pineapple (Ananascomosus) stem (Maurer, 2001MAURER, H.R. Bromelain: biochemistry, pharmacology and medical use. Cell. Mol. Life Sci., v.58, n.9, p.1234-1245, 2001.). Bromelain is known for its clinical and therapeutic applications, particularly to modulation of tumor growth, selective burn debridement, and improvement of antibiotic action and anti-inflammatory, antithrombotic, and fibrinolytic activities (Babu, Rastogi, Raghavarao, 2008BABU, B.; RASTOGI, N.; RAGHAVARAO, K. Liquid-liquid extraction of bromelain and polyphenol oxidase using aqueous two-phase system. Chem. Eng. Process., v.47, n.1, p.83-89, 2008.; Maurer, 2001MAURER, H.R. Bromelain: biochemistry, pharmacology and medical use. Cell. Mol. Life Sci., v.58, n.9, p.1234-1245, 2001.; Rosenberg et al., 2012ROSENBERG, L.; KRIEGER, Y.; SILBERSTEIN, E.; ARNON, O.; SINELNIKOV, I.A.; BOGDANOV-BEREZOVISKY, A.; SINGER, A.J. Selectivity of a bromelain based enzymatic debridement agent: a porcine study. Burns, v.38, n.7, p.1035-1040, 2012.). It is also used in meat processing tenderization and as a dietary supplement. This enzyme is used in the United States and Europe as an alternative medication complementary to glucocorticoids, non-steroidal antirheumatics and immunomodulatory agents. Because of its very low toxicity, it has become a suitable tool for controlling chronic inflammatory diseases (Babu, Rastogi, Raghavarao, 2008BABU, B.; RASTOGI, N.; RAGHAVARAO, K. Liquid-liquid extraction of bromelain and polyphenol oxidase using aqueous two-phase system. Chem. Eng. Process., v.47, n.1, p.83-89, 2008.; Hebbar, Sumana, Raghavarao, 2008HEBBAR, H.U.; SUMANA, B.; RAGHAVARAO, K.S.M.S. Use of reverse micellar systems for the extraction and purification of bromelain from pineapple wastes. Bioresour. Technol., v.99, n.11, p.4896-4902, 2008.; Maurer, 2001MAURER, H.R. Bromelain: biochemistry, pharmacology and medical use. Cell. Mol. Life Sci., v.58, n.9, p.1234-1245, 2001.).
Proteolytic activity was completely abrogated when a 10 mg/mL bromelain solution was exposed to simulated murine gastric juice, for 2 h at 37 °C (Hale, 2004HALE, L.P. Proteolytic activity and immunogenicity of oral bromelain within the gastrointestinal tract of mice. Int. Immunopharmacol., v.4, n.2, p.255-264, 2004.). However, in another study (Hale et al., 2005HALE, L.P. ;GREER, P.K.; TRINH, C.T; JAMES, C.L. Proteinase activity and stability of natural bromelain preparations.Int. Immunopharmacol., v.5, n.4, p.783-793, 2005.), it was found that, when at a concentration of 250 mg/mL, bromelain was resistant to inactivation by exposure to pH 2, with <5% loss of activity. These studies suggest that the provision of large bolus concentrations of bromelain may prevent inactivation by low pH and by digestive enzymes, such as trypsin, which could enhance the proteolytic activity of bromelain within the gastrointestinal tract in vivo.
Bromelain demonstrated a high activity in McIlvaine buffer at the average minimum pH value of wine (pH 3.2), but also a significantly higher activity in tartaric buffer, responsible for the interaction of enzyme affinity (Benucci et al., 2011BENUCCI, I.; LIBURDI, K.; GARZILLO, A.M.V.; ESTI, M. Bromelain from pineapple stem in alcoholic-acidic buffers for wine application. Food. Chem., v.124, n.4, p.1349-1353, 2011.).
Investigations on protein-polymer systems have drawn the attention of scientists because of their extensive application to new pharmaceutical technologies and biomedical usage. Some polymers are often used as modifiers of biological macromolecules to improve the biochemical activity of proteins or drug bioavailability (Zielenkiewicz et al., 2006ZIELENKIEWICZ, W.; SWIERZEWSKY, R.; ATTANASIO, F.; RIALDI, G. Thermochemical, volumetric and spectroscopic properties of lysozyme-poly(ethylene) glycol system. J. Therm. Anal. Calorim., v.83, n.3, p.587-595, 2006.).
Polyethylene glycol (PEG) 400 g/mol is capable of inducing a molten globule-like state from acid-denatured stem bromelain; and interestingly, high molecular weight PEG 6000 g/mol and 20000 g/mol, which generally stabilize proteins by a preferential hydration mechanism, were found to play a denaturing role on acid unfolded stem bromelain (Ahmad, Khan, 2006AHMAD, B.; KHAN, R.H. Studies on the acid unfolded and molten globule states of catalytically active stem bromelain: a comparison with catalytically inactive form. J. Biochem., v.140, n.4, p.501-508, 2006.). Fatima and Khan (2007)FATIMA, S.; KHAN, R.H. Effect of polyethylene glycols on the function and structure of thiol proteases. J. Biochem., v.142, n.1, p.65-72, 2007. also worked with PEG 400, 6000 and 20000 g/mol, and found that they lead to destabilization of thiol proteases.
To date, the use of bromelain in pharmacological or industrial applications is limited, due to commercial availability, costs, and sensitivity to pH and temperature. Therefore, a better understanding of enzyme stability would be of interest (Xue et al., 2010XUE, Y.;WU, C.Y.; BRANDFORD-WHITE, C. J.; NING, X.; NIE, H.L.; ZHU, L.M. Chemical modification of stem bromelain with anhydride groups to enhance its stability and catalytic activity. J. Mol. Catal. B: Enzym., v.63, n.3-4, p.188-193, 2010.).
Stability of enzymes is a critical issue in the biotechnology industry. Enzymes are sensitive to minor modifications in environmental conditions, such as temperature, pH, and ionic strength. Both operational and storage stabilities affect the production of enzyme-based products. Stabilizing an enzyme normally means suppressing the unfolding of the protein and retaining the catalytic activity (Rao, Goyal, 2013RAO, T.J.M.; GOYAL, A. Purification, optimization of assay, and stability studies of dextransucrase isolated from Weissella cibaria JAG8. Prep. Biochem. Biotechnol., v.43, n.4, p.329-341, 2013.).
Because of the continuous interest in this protease, for its numerous applications in medicine, pharmacology and in food industry, and considering the high potential of bromelain, polyethylene glycol and polyacrylic acid as protein stabilizer, the stability of bromelain was investigated.
MATERIAL AND METHODS
Material
Stem bromelain (B4882), polyacrylic acid (PAA, sodium salt) 8000 g/mol, and polyethylene glycol (PEG) 2000 g/mol, 4000 g/mol and 6000 g/mol were purchased from Sigma®. All other chemicals were of analytical grade.
Stability studies
Stability studies of 3 mg/ml of stem bromelain were carried out in a thermo regulated bath, at several temperatures (20, 30, 40 e 50 °C) for 7 h in different pH values (2.0 to 8.0). After analyzing the results obtained by the stability study at several pH, bromelain stability was also studied in different polymer solutions, exposing stem bromelain to PEG 2000, 4000 and 6000 g/mol at 5, 10 and 15% (w/w), or PAA 8000g/mol at the same concentrations. The activation energy was calculated with the Arrhenius equation, and the enthalpy (ΔH‡) and entropy (ΔS‡) were determined using the Eyring equation, as described by De Lencastre Novaes et al. (2010)DE LENCASTRE NOVAES, L.C.; MAZZOLA, P.G.; PESSOA JR, A.; PENNA, T.C.V. Effect of polyethylene glycol on the thermal stability of green fluorescent protein. Biotechnol. Prog., v.26, n.1, p.252-256, 2010.. All the experiments were performed in triplicates.
Bromelain activity
Bromelain activity was measured by the methodology described by Kunitz (1947)KUNITZ, M. Crystalline soybean trypsin inhibitor: II general properties. J. Gen. Physiol., v.30, n.4, p.291-310, 1947. and modified by Walter (1984)WALTER, H.E. Proteinases: methods with hemoglobin, casein and azocoll as substrates. In: BERGMEYER, H.U. Methods of enzymatic analysis. Weinheim: Ed. Verlag Chemie, 1984. v.5, p.270-277. , using 2% (w/v) casein as substrate and tyrosine as standard. In short, the methodology consists in allowing bromelain cleaves casein at 37 °C for 10 min, following the addition of trichloroacetic acid. The casein cleavage releases tyrosine residues, which are detected by spectroscopy at 280 nm.
RESULTS AND DISCUSSION
First, the stability of stem bromelain in several pH values (2.0 to 8.0) was performed at four different temperatures (20, 30, 40 and 50 ºC). Samples were withdrawn at 0.5; 1; 1.5; 2; 2.5; 3; 5 and 7 hours.
At 20 °C, bromelain was more stable at pH 2.0, remaining with 95.5% of its initial relative activity, followed by pH 8.0, with 79.9% after 7 h. For other pH values, the relative activity remained around 65% after 7 h (Figure 1).
Relative stability of bromelain in several pH at 20 °C. The error bars refer to standard deviation.
Ahmad and Khan (2006)AHMAD, B.; RATHAR, G.M.; VARSHNEY, A.; KHAN, R.H. pH-dependent urea-induced unfolding of stem bromelain: unusual stability against urea at neutral pH. Biochemistry, v.74, n.12, p.1337-1343, 2009. described that bromelain is on a denatured state at pH 2.0, 25 °C, when 80% of the native structure is lost; however, in our study, bromelain activity was detected in all collected samples.
At 30 °C, stem bromelain was more stable at pH 5.0, with 83.9% of remaining relative activity, followed by pH 4, with 75.5% (Figure 2). Godoi (2007)GODOI, P.H. Estudo da atividade enzimática da bromelina pura em solução em diferentes temperaturas e pH. Campinas, 2007. 50 p. [Dissertion of Master degree. Faculty of Chemical Enginneering. Satate University of Campinas]. found that at 35 °C, bromelain in pH 8.0 loses 33.9% of its proteolytic activity; at pH 4.0, the loss was of 13.7%. Despite the 5 °C of difference between our study and Godoi's study (2007)GODOI, P.H. Estudo da atividade enzimática da bromelina pura em solução em diferentes temperaturas e pH. Campinas, 2007. 50 p. [Dissertion of Master degree. Faculty of Chemical Enginneering. Satate University of Campinas]. , we found that, at pH 8.0, the remaining activity after 7 h was 46.1%, and at pH 4.0 was 75.5%.
Relative stability of bromelain in several pH at 30 °C. The error bars refer to standard deviation.
At 40 °C, stem bromelain continued to be more stable at pH 5.0, with 99.8% of relative activity after 7 h (Figure 3). At pH 4.0, after this same period, the remaining activity was 89.4%, and at pH 6.0, 56.7%. At pH 2.0, bromelain lost all the initial activity within 30 min.
Relative stability of bromelain in several pH at 40 °C. The error bars refer to standard deviation.
At 50 °C, pH 5.0 provided the best stability for stem bromelain, with 77.7% of relative activity after 7 h (Figure 4). At pH 4.0 and 6.0, the values of relative activity were 49.6% and 42.4%, respectively. At pH 2.0, bromelain lost all the initial activity within 30 min.
Relative stability of bromelain in several pH at 50 °C. The error bars refer to standard deviation.
Godoi (2007)GODOI, P.H. Estudo da atividade enzimática da bromelina pura em solução em diferentes temperaturas e pH. Campinas, 2007. 50 p. [Dissertion of Master degree. Faculty of Chemical Enginneering. Satate University of Campinas]. found that all bromelain activity is lost when it is below 55 °C at pH 8.0. When pH was closer to 8.0, bromelain was more susceptible to denaturation and the enzymatic activity is lowered. In this study, the remaining activity at pH 8.0 was 5.9%.
At pH 2.0, the higher stability was observed at 20 ºC. The same was observed at pH 3.0, where after 7h, the remaining activity was 65.2%; as well at pH 7.0 (71.7% remaining activity) and pH 8.0 (79.9% remaining activity). At pH 4.0, the higher stability was achieved at 40 ºC, when bromelain lost 10.6% of its initial activity after 7h. However, at 50 °C it lost more than 50% of its initial activity after 7 h. Ahmad and co-workers (2009)AHMAD, B.; RATHAR, G.M.; VARSHNEY, A.; KHAN, R.H. pH-dependent urea-induced unfolding of stem bromelain: unusual stability against urea at neutral pH. Biochemistry, v.74, n.12, p.1337-1343, 2009. described that, at pH 4.5, the unfolding of stem bromelain appeared to follow a two-state mechanism of protein unfolding.
Bromelain maintained 99.8% of its initial activity in pH 5.0 at 40 ºC. In the other temperatures studied, the remaining activities were 75.3%, 83.9% and 77.7% at 20, 30 and 50 ºC, respectively. At pH 6.0, higher stability was observed at 20 and 30ºC, losing 34.4 and 41.4% of initial activity after 7 h, respectively.
According to Anwar; Ahmad; Younus (2007)ANWAR, T.; AHMAD, B.; YOUNUS, H. Cross-linked stem bromelain: a more stabilized active preparation. Biocatal. Biotransfor., v.25, n.6, p.453-458, 2007., bromelain gradually lost its activity after 3h at 60 ºC, pH 7.0, and the remaining activity after incubation was 18%. That means, with the increase in temperature from 50 ºC to 60 ºC, bromelain lose 1.6-fold activity; since in our study, the remaining activity was around 29% at 50 ºC, pH 7.0, after 3 h incubation.
Studies on the effect of alkaline pH on stem bromelain have reported no conformational changes in protein in a pH range from 7.0 to 10.0, as well as no significant changes in physical parameters were detected in this pH region (Haq, Rasheedi, Khan, 2002HAQ, S.K.; RASHEEDI, S.; KHAN, R.H. Characterization of a partially folded intermediate of stem bromelain at low pH. Eur. J. Biochem., v.269, n.1, p.47-52, 2002.).
Despite the fact that bromelain does not show a great stability at pH 7.0, several authors describe this pH as the best for the proteolytic measurement of bromelain activity (Campos, 2007CAMPOS, E.S. Purificação e caracterização de bromelina a partir do extrato bruto de ananas comosus por adsorção em leito expandido. Campinas, 2007. 74 p. [Dissertion of Master degree. Faculty of Chemical Enginneering. Satate University of Campinas]. ; Kunitz, 1947KUNITZ, M. Crystalline soybean trypsin inhibitor: II general properties. J. Gen. Physiol., v.30, n.4, p.291-310, 1947.; Walter, 1984WALTER, H.E. Proteinases: methods with hemoglobin, casein and azocoll as substrates. In: BERGMEYER, H.U. Methods of enzymatic analysis. Weinheim: Ed. Verlag Chemie, 1984. v.5, p.270-277. ; Xue et al., 2010XUE, Y.;WU, C.Y.; BRANDFORD-WHITE, C. J.; NING, X.; NIE, H.L.; ZHU, L.M. Chemical modification of stem bromelain with anhydride groups to enhance its stability and catalytic activity. J. Mol. Catal. B: Enzym., v.63, n.3-4, p.188-193, 2010.).
The study of thermodynamics parameters of bromelain activity at several pH showed a higher energy of activation (Ea) obtained at pH 2.0, and lower at pH 5.0. A similar pattern was found for ΔH and ΔS, indicating that stem bromelain was more stable at pH 5.0 (Table I). The thermal stability of enzymes is often related to a decrease in entropy (ΔS) and an increase in Gibbs free energy (ΔG). An increase in ΔS indicates that there is an increase in the number of proteins in the transition to activated stage (Xue et al., 2010XUE, Y.;WU, C.Y.; BRANDFORD-WHITE, C. J.; NING, X.; NIE, H.L.; ZHU, L.M. Chemical modification of stem bromelain with anhydride groups to enhance its stability and catalytic activity. J. Mol. Catal. B: Enzym., v.63, n.3-4, p.188-193, 2010.). Sriwatanapongse and collaborators (2000)SRIWATANAPONGSE, A.; BALABAN, M.; TEIXEIRA, A. Thermal inactivation kinetics of bromelain in pineapple juice. Trans. ASAE, v.43, n.6, p.1703-1708, 2000. found an Ea of 3.26 × 105 J/mol for bromelain present in the juice of Smooth Cayenne pineapples.
Energy of activation (Ea), enthalpy (?H) and entropy (?S) of stem bromelain in the studied pH values
From the results, we concluded that stem bromelain has higher stability at pH 5.0 in all studied temperatures, since it showed a smaller loss of relative activity. Therefore, for stability studies of bromelain in PEG and PAA solutions, 5%, 10% and 15% of these polymers were prepared in McIlvaine buffer pH 5.0.
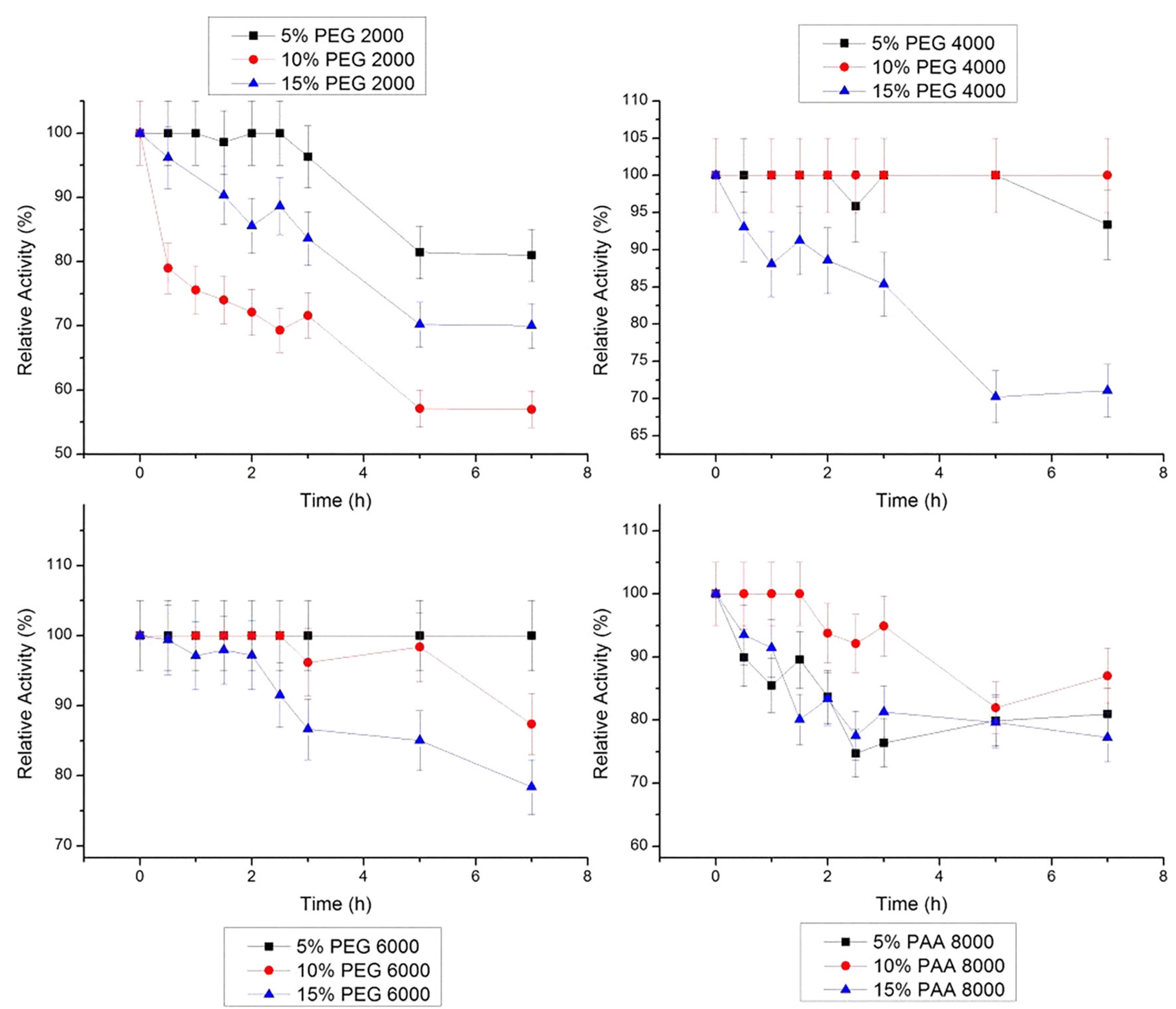
Stem bromelain maintained its activity at 20 °C in all concentrations of PEG 4000 g/mol and in 10% PAA 8000 g/mol, for 7 h (Figure 5). In PEG 6000g/mol, there was a small decrease in relative activity of stem bromelain, with values of remaining relative activity of 99.4%, 98.2% and 84.47% in concentrations of 5, 10 e 15%, respectively, after 7 h.
Relative stability of bromelain at 20 ºC in PEG 2000, 4000, 6000 g/mol and PAA 8000 g/mol at three concentrations (5, 10 and 15% w/w). The error bars refer to standard deviation.
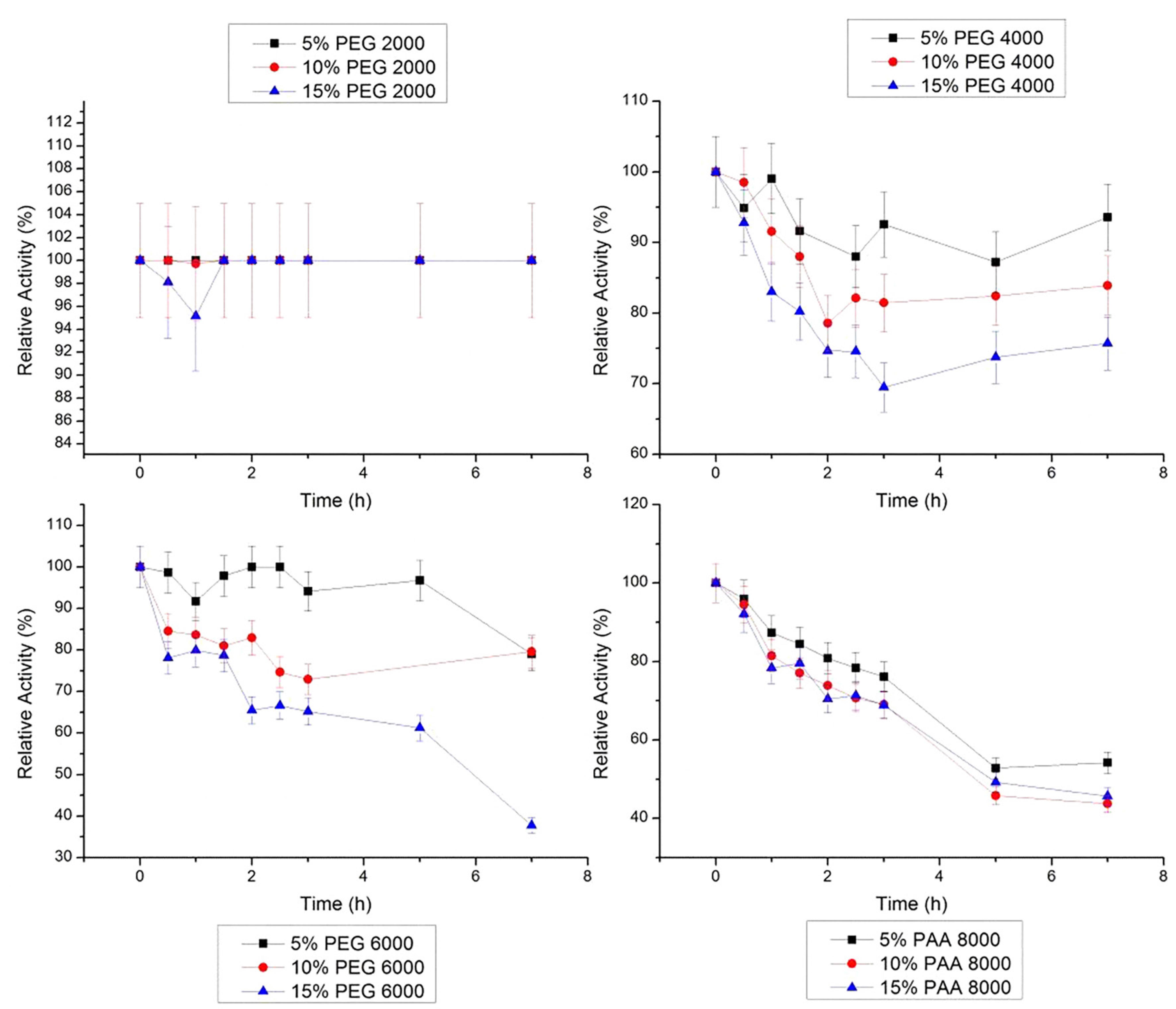
At 30 °C, bromelain was stable in 10% PEG 4000 g/mol and in 5% PEG 6000 g/mol, for the 7 h of study (Figure 6). In the presence of 10% PEG 2000 g/mol, bromelain shows the lowest activity (57.1%) at 30 °C. In 10% PAA 8000 g/mol, the bromelain activity remained stable for 1.5 h, decaying for 87% after 7 h.
Relative stability of bromelain at 30 °C in PEG 2000, 4000, 6000 g/mol and PAA 8000 g/mol at three concentrations (5, 10 and 15% w/w). The error bars refer to standard deviation
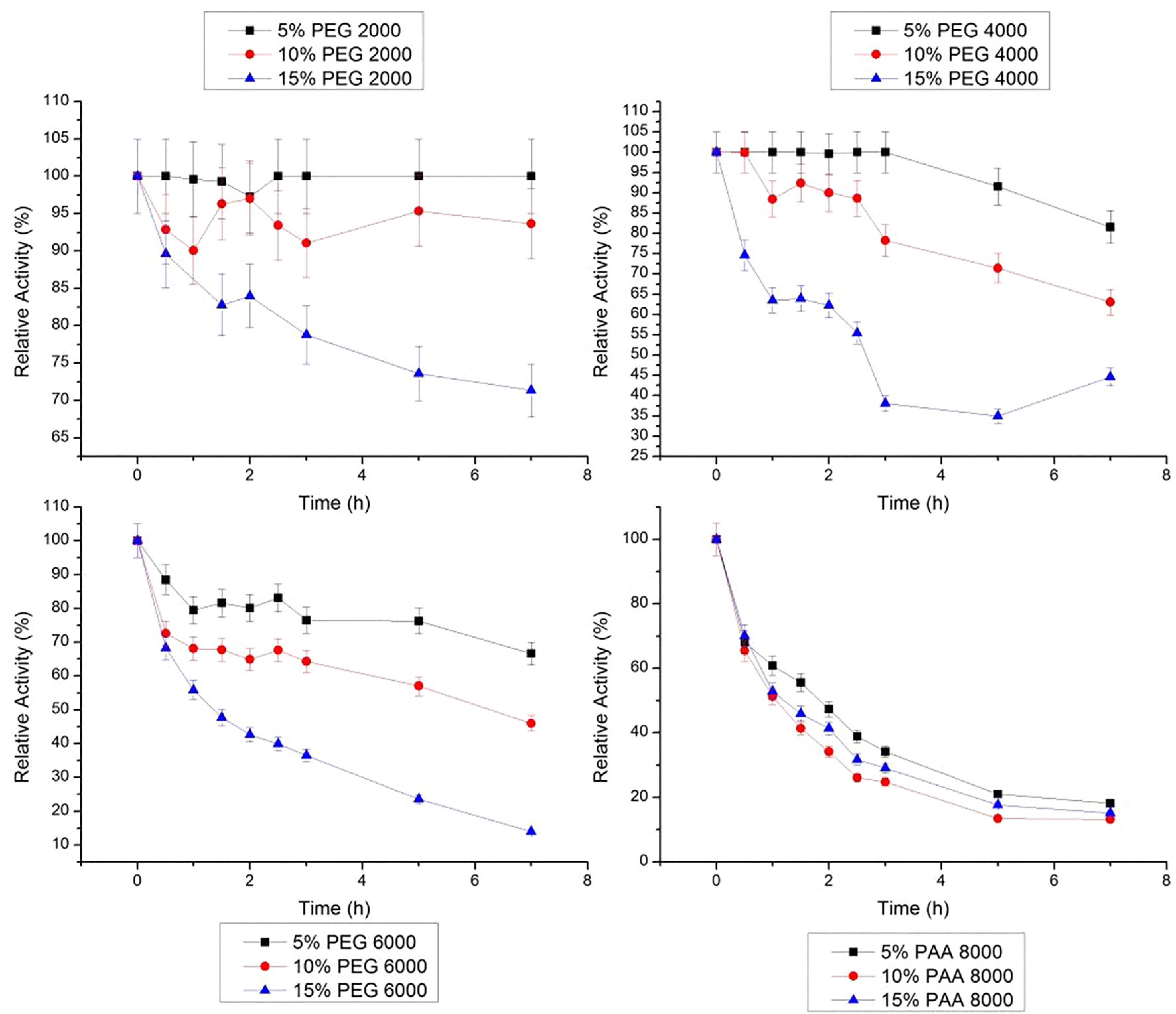
Data obtained at 40 ºC (Figure 7) shows an inverse behavior as showed at 30 °C. In all concentrations of PEG 2000 g/mol, stem bromelain maintained its initial activity during 7 h. In PEG 4000 g/mol, the relative activity loss of bromelain after 7 h was 6.4 to 24.3%, and in PEG 6000 g/mol was 21.0 to 62.2%. The same was observed for the three studied concentrations of PAA 8000 g/mol, and after 7 h, the activities were 54.2, 43.7 and 45.7% in 5, 10 and 15%, respectively.
Relative stability of bromelain at 40 ºC in PEG 2000, 4000, 6000 g/mol and PAA 8000 g/mol at three concentrations (5, 10 and 15% w/w). The error bars refer to standard deviation.
At 50 ºC, only 5% PEG 2000 g/mol relative activity of stem bromelain was stable during the 7 h of study (Figure 8). In other PEG molar weights studied, the loss of bromelain activity was greater, reaching up to 86.0% (15% PEG 6000 g/mol). Different concentrations of PAA showed similar behavior of those observed at 40 ºC (after 7 h): bromelain activity was under 20%, and the lower was in 10% PAA 8000 g/mol, with 13.2%.
Relative stability of bromelain at 50 ºC in PEG 2000, 4000, 6000g/mol and PAA 8000 g/mol at three concentrations (5, 10 and 15% w/w). The error bars refer to standard deviation.
The higher energy of activation was observed in 10% PEG 4000g/mol, while the lowest was observed in 5% PEG 2000 g/mol. The same pattern was observed for ΔH and ΔS, indicating that bromelain were more stable in 5% PEG 2000 g/mol (Table II).
Energy of activation (Ea), enthalpy (?H) and entropy (?S) of stem bromelain in the studied polymers.
PEG increased the stability of stem bromelain activity when compared with the results obtained in buffered solutions. The best condition for stability of bromelain activity in several concentrations and different molar weights was achieved with 5% PEG 2000 g/mol, while the lowest stability was reached with 10% PEG 4000 g/mol. The increase of stability by PEG have been attributed to steric exclusion of the polymer from the vicinity of the protein, promoting a preferential hydration of the protein, although PEG can interact, and possibly bound, with nonpolar residues of protein surface (Azevedo et al., 2004AZEVEDO, A.M.; CABRAL, J.M.S.; PRAZERES, D.M.F.; GIBSON, T.D.; FONSECA, L.P. Thermal and operational stabilities of Hansenula polymorpha alcohol oxidase. J. Mol. Catal. B: Enzym., v.27, n.1, p.37-45, 2004.; Hancock, Hsu, 1996HANCOCK, T.J.; HSU, J.T. Thermal stability studies of a globular protein in aqueous poly(ethylene glycol) by (1)H NMR. Biotechnol. Bioeng., v.51, n.4, p.410-421, 1996.).
Fatima and Khan (2007)FATIMA, S.; KHAN, R.H. Effect of polyethylene glycols on the function and structure of thiol proteases. J. Biochem., v.142, n.1, p.65-72, 2007. showed that PEG 400, 6000 and 20000 g/mol destabilized thiol proteases such as bromelain. In our study, PEG 6000 g/mol also did not promote the stability of bromelain.
This effect could be explained based on the hypothesis that PEG of high molecular weight assumes a compact structure, caused by intramolecular hydrophobic interactions, which leads to a lower solvent interaction than the extended polymers and allows the interaction with protein (Fatima, Khan, 2007FATIMA, S.; KHAN, R.H. Effect of polyethylene glycols on the function and structure of thiol proteases. J. Biochem., v.142, n.1, p.65-72, 2007.). Thus, decrease in preferential exclusion of PEG 6000 g/mol might lead to an increase in interaction of PEG with the protein surface.
The presence of PAA did not increase the stability of bromelain in the evaluated conditions. At 40 and 50ºC, the activity stability was reduced when compared with the results found in buffered bromelain. Charged polymers stabilize or inactivate proteins depending on their mode of interaction. Since proteins are poly-ampholytic molecules, they can interact with polymers or polyelectrolytes via long-range Coulombic forces (Ohtake, Kita, Arakawa, 2011OHTAKE, S.; KITA, Y.; ARAKAWA, T. Interactions of formulation excipients with proteins in solution and in the dried state. Adv. Drug Deliv. Rev., v.63, n.13, p.1053-1073, 2011.). The effect of charged polymers, such as PAA, on protein stability is rather protein specific. Probably, electrostatic and hydrophobic forces were more intense in those temperatures, disfavoring the maintenance of the activity of stem bromelain.
CONCLUSIONS
Bromelain has been used in the food industry for decades, but its uses in cosmetic and pharmaceutical formulations are restricted due to the lack of information regarding its stability, since those processes include a variety of solutes, several pH and, in some cases, the heating for achieving the final product. This study aimed to observe the profile of bromelain stability in several pH values (2.0 to 8.0) and in the most common polymer used by pharmaceutical and cosmetic industry, the polyethylene glycol, as well as its behavior in a charged polymer solution. We observed that bromelain was able to maintain its stability at pH 5.0 for a longer period of time at all temperatures studied. PEG solutions increased bromelain stability, but PAA solutions had the opposite effect.
This study successfully shows that PEG can increase bromelain stability, which represents an important first step towards the development of a cost-effective pharmaceutical or cosmetic product lined up with the application of a major industry residue.
ACKNOWLEDGMENTS
This research was supported by grants from the Coordination for the Improvement of Higher Level Personnel (Capes), National Council for Scientific and Technological Development (CNPq) and State of São Paulo Research Foundation (Fapesp), Brazil.
REFERENCES
- AHMAD, B.; RATHAR, G.M.; VARSHNEY, A.; KHAN, R.H. pH-dependent urea-induced unfolding of stem bromelain: unusual stability against urea at neutral pH. Biochemistry, v.74, n.12, p.1337-1343, 2009.
- AHMAD, B.; KHAN, R.H. Studies on the acid unfolded and molten globule states of catalytically active stem bromelain: a comparison with catalytically inactive form. J. Biochem., v.140, n.4, p.501-508, 2006.
- ANWAR, T.; AHMAD, B.; YOUNUS, H. Cross-linked stem bromelain: a more stabilized active preparation. Biocatal. Biotransfor., v.25, n.6, p.453-458, 2007.
- AZEVEDO, A.M.; CABRAL, J.M.S.; PRAZERES, D.M.F.; GIBSON, T.D.; FONSECA, L.P. Thermal and operational stabilities of Hansenula polymorpha alcohol oxidase. J. Mol. Catal. B: Enzym., v.27, n.1, p.37-45, 2004.
- BABU, B.; RASTOGI, N.; RAGHAVARAO, K. Liquid-liquid extraction of bromelain and polyphenol oxidase using aqueous two-phase system. Chem. Eng. Process., v.47, n.1, p.83-89, 2008.
- BENUCCI, I.; LIBURDI, K.; GARZILLO, A.M.V.; ESTI, M. Bromelain from pineapple stem in alcoholic-acidic buffers for wine application. Food. Chem., v.124, n.4, p.1349-1353, 2011.
- CAMPOS, E.S. Purificação e caracterização de bromelina a partir do extrato bruto de ananas comosus por adsorção em leito expandido. Campinas, 2007. 74 p. [Dissertion of Master degree. Faculty of Chemical Enginneering. Satate University of Campinas].
- DE LENCASTRE NOVAES, L.C.; MAZZOLA, P.G.; PESSOA JR, A.; PENNA, T.C.V. Effect of polyethylene glycol on the thermal stability of green fluorescent protein. Biotechnol. Prog., v.26, n.1, p.252-256, 2010.
- FATIMA, S.; KHAN, R.H. Effect of polyethylene glycols on the function and structure of thiol proteases. J. Biochem., v.142, n.1, p.65-72, 2007.
- GODOI, P.H. Estudo da atividade enzimática da bromelina pura em solução em diferentes temperaturas e pH. Campinas, 2007. 50 p. [Dissertion of Master degree. Faculty of Chemical Enginneering. Satate University of Campinas].
- HALE, L.P. Proteolytic activity and immunogenicity of oral bromelain within the gastrointestinal tract of mice. Int. Immunopharmacol., v.4, n.2, p.255-264, 2004.
- HALE, L.P. ;GREER, P.K.; TRINH, C.T; JAMES, C.L. Proteinase activity and stability of natural bromelain preparations.Int. Immunopharmacol., v.5, n.4, p.783-793, 2005.
- HANCOCK, T.J.; HSU, J.T. Thermal stability studies of a globular protein in aqueous poly(ethylene glycol) by (1)H NMR. Biotechnol. Bioeng., v.51, n.4, p.410-421, 1996.
- HAQ, S.K.; RASHEEDI, S.; KHAN, R.H. Characterization of a partially folded intermediate of stem bromelain at low pH. Eur. J. Biochem., v.269, n.1, p.47-52, 2002.
- HEBBAR, H.U.; SUMANA, B.; RAGHAVARAO, K.S.M.S. Use of reverse micellar systems for the extraction and purification of bromelain from pineapple wastes. Bioresour. Technol., v.99, n.11, p.4896-4902, 2008.
- KUNITZ, M. Crystalline soybean trypsin inhibitor: II general properties. J. Gen. Physiol., v.30, n.4, p.291-310, 1947.
- MAURER, H.R. Bromelain: biochemistry, pharmacology and medical use. Cell. Mol. Life Sci., v.58, n.9, p.1234-1245, 2001.
- OHTAKE, S.; KITA, Y.; ARAKAWA, T. Interactions of formulation excipients with proteins in solution and in the dried state. Adv. Drug Deliv. Rev., v.63, n.13, p.1053-1073, 2011.
- RAO, T.J.M.; GOYAL, A. Purification, optimization of assay, and stability studies of dextransucrase isolated from Weissella cibaria JAG8. Prep. Biochem. Biotechnol., v.43, n.4, p.329-341, 2013.
- ROSENBERG, L.; KRIEGER, Y.; SILBERSTEIN, E.; ARNON, O.; SINELNIKOV, I.A.; BOGDANOV-BEREZOVISKY, A.; SINGER, A.J. Selectivity of a bromelain based enzymatic debridement agent: a porcine study. Burns, v.38, n.7, p.1035-1040, 2012.
- SRIWATANAPONGSE, A.; BALABAN, M.; TEIXEIRA, A. Thermal inactivation kinetics of bromelain in pineapple juice. Trans. ASAE, v.43, n.6, p.1703-1708, 2000.
- WALTER, H.E. Proteinases: methods with hemoglobin, casein and azocoll as substrates. In: BERGMEYER, H.U. Methods of enzymatic analysis. Weinheim: Ed. Verlag Chemie, 1984. v.5, p.270-277.
- XUE, Y.;WU, C.Y.; BRANDFORD-WHITE, C. J.; NING, X.; NIE, H.L.; ZHU, L.M. Chemical modification of stem bromelain with anhydride groups to enhance its stability and catalytic activity. J. Mol. Catal. B: Enzym., v.63, n.3-4, p.188-193, 2010.
- ZIELENKIEWICZ, W.; SWIERZEWSKY, R.; ATTANASIO, F.; RIALDI, G. Thermochemical, volumetric and spectroscopic properties of lysozyme-poly(ethylene) glycol system. J. Therm. Anal. Calorim., v.83, n.3, p.587-595, 2006.
Publication Dates
-
Publication in this collection
Apr-Jun 2014
History
-
Received
29 July 2013 -
Accepted
17 Dec 2013