Abstracts
In recent decades, there has been a significant increase in the incidence of fungal diseases. Certain fungal diseases cause cutaneous lesions and in the usual treatment, generally administred orally, the drug reaches the site of action with difficulty and its concentration is too low. An approach much explored in recent years is the development of nanotechnology-based drug delivery systems, and microemulsions (ME) and liquid crystals (LC) are promising. ME and LC were developed with oleic acid or copaiba oil as the oil phase, propoxyl (5OP) ethoxyl (20 OE) cetyl alcohol as surfactant and water. An analytical method to assess the incorporation of fluconazole (FLU) in the systems under study was validated according to guidelines of the International Conference on Harmonization (ICH) guidelines and the Brazilian Food, Drug and Sanitation Agency (ANVISA). The method was conducted on a C18-RP column (250 × 4.6 mm i.d.), maintained at room temperature. The mobile phase consisted of acetonitrile and water (50:50, v/v), run at a flow rate of 1.0mL/min and using ultraviolet detection at 210nm. The chromatographic separation was obtained with a retention time of 6.3min, and was linear in the range of 20-400 µg/mL (r2=0.9999). The specificity showed no interference of the excipients. The accuracy was 100.76%. The limits of detection and quantitation were 0.057 and 0.172 µg.mL-1, respectively. Moreover, method validation demonstrated satisfactory results for precision and robustness. The proposed method was applied for the analysis of the incorporation of FLU in ME and LC, contributing to improve the quality control and to assure the therapeutic efficacy.
Nanotehcnology; Microemulsions; Liquid crystals; High-performance liquid chromatography/qualitative analysis; Fluconazole
Nas últimas décadas, houve aumento significativo na incidência de doenças fúngicas. Certas doenças fúngicas provocam lesões cutâneas, sendo que no tratamento usual, geralmente administrado por via oral, o medicamento chega ao local de ação com dificuldade, em concentração muito baixa. Uma abordagem muito explorada nos últimos anos é o desenvolvimento de sistemas de administração de fármacos baseados em nanotecnologia, como as microemulsões (ME) e cristais líquidos (LC). ME e LC foram desenvolvidos com o ácido oleico ou óleo de copaíba como fase oleosa, álcool cetílico propoxilado (5 OP) e etoxilado (20 OE) como tensoativo e água. Método analítico para avaliar a incorporação de fluconazol (FLU) nos sistemas em estudo foi validado de acordo com as diretrizes da Conferência Internacional de Harmonização (ICH) e Agência Nacional de Vigilância Sanitária (ANVISA). O método foi desenvolvido empregando coluna C18-RP (250 x 4,6 mm id), mantida à temperatura ambiente. A fase móvel consistiu de acetonitrila e água (50:50, v/v), executado a uma taxa de fluxo de 1,0 mL/min e com detecção ultravioleta a 210 nm. A separação cromatográfica foi obtida com o tempo de retenção de 6,3min, e mostrou-se linear no intervalo de 20-400 µg/mL (r2=0,9999). Pelo estudo de especificidade, observou-se não interferência dos excipientes. A precisão foi 100,76%. Os limites de detecção e de quantificação foram 0,057 e 0,172 µg.mL-1, respectivamente. Além disso, a validação do método demonstrou resultados satisfatórios para a precisão e robustez. O método proposto foi aplicado para a análise da incorporação do FLU em ME e cristais líquidos, contribuindo para aumentar o controle de qualidade e garantir a eficácia terapêutica.
Nanotecnologia; Microemulsões; Cristais líquidos; Cromatografia líquida de alta eficiência/análise qualitativa; Fluconazol
INTRODUCTION
Fungal infections are considered a major problem for public health. A significant group of such infections are the dermatomycoses, involving the skin, especially the stratum corneum, and appendages such as nails, hair and mucous membranes (oral and vaginal) (Negroni, 2010NEGRONI, R. Historical aspects of dermatomycoses. Clin. Dermatol., v.28, p.125-132, 2010.; Erchiga, Fiorencio, 2005ERCHIGA, V.C.; FLORENCIO, V.D. Micosis cutáneas. Med. Clin., v.125, p.467-474, 2005.; Bachhav, Patrale, 2009BACHHAV, Y.G.; PATRAVALE, V.B. Microemulsion based vaginal gel of fluconazole: Formulation, in vitro and in vivo evaluation. Int. J. Pharm., v.365, p.175-179, 2009.).
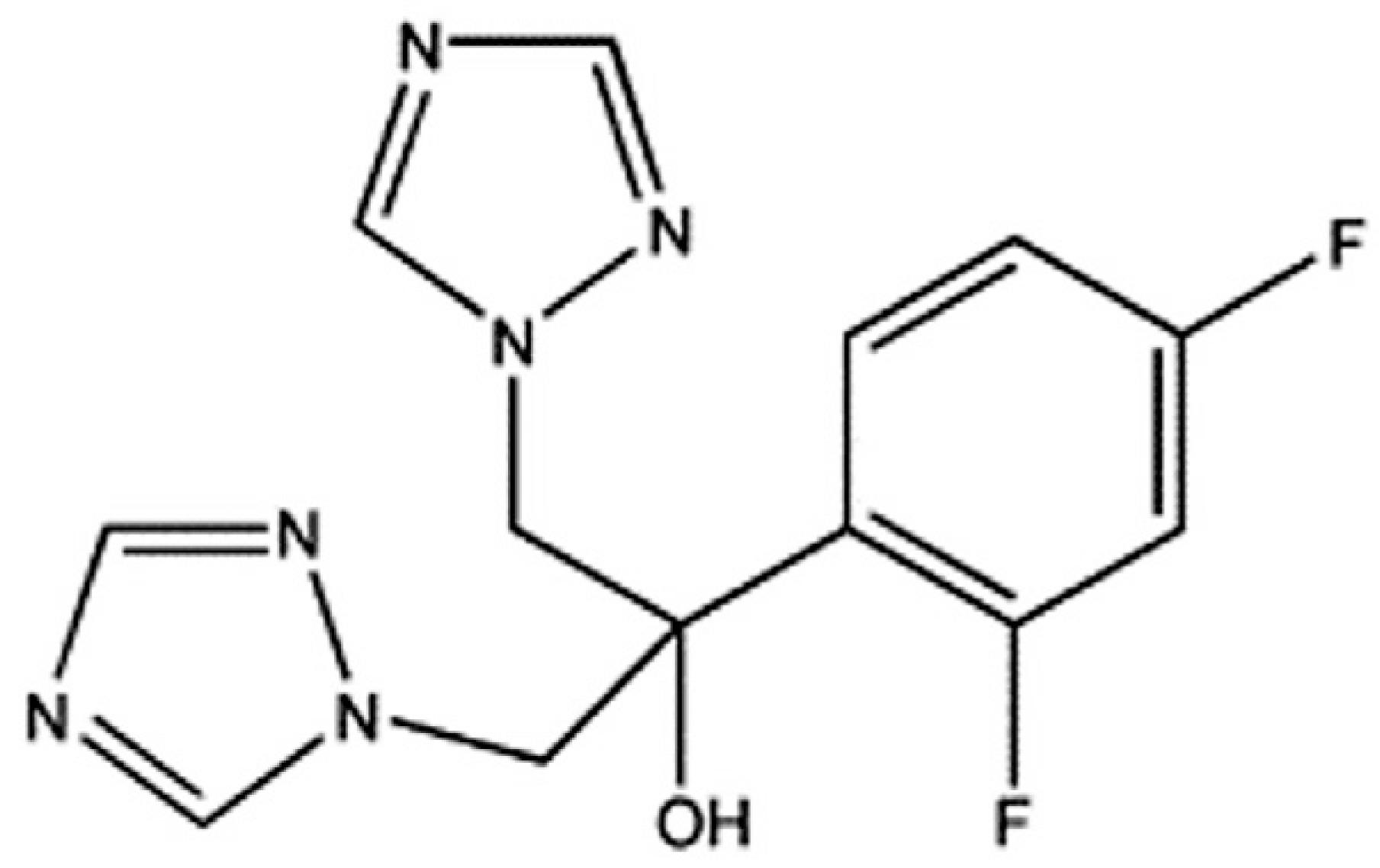
Fluconazole - FLU (Figure 1) is a white, slightly soluble in water, soluble in ethanol, methanol and acetone and very slightly soluble in toluene. The presence of two triazole rings in its structure is responsible for its reduced lipophilicity (Dash, Elmquist, 2001DASH, A.K.; ELMQUIST, W.F. Fluconazole. In: HARRY, G.B. (Ed.) Analytical profiles of drug substances and excipients. London: Academic Press, 2001. v.27, p.67-113. ). Its mechanism of action, like that of the other azoles, is related to its ability to alter the membrane permeability of yeasts and other fungi by inhibiting the synthesis of ergosterol. Inhibition of P-450 lanosterol 14-alpha-demethylase dependent causes accumulation of sterols methylated depletion ergosterol and inhibition of cell growth (Alnaim et al., 2007ALNAIM, L.; ABOU ALSOUD, N.; ZAGHLOUL, I.; AL-JASER, M. Effects of fluconazole on the pharmacokinetics and pharmacodynamics of antimony in cutaneous leishmaniasis-infected hamsters. Int. J. Antimicrob. Agents., v.29, p.728-732, 2007.; Jovanović et al., 2005JOVANOVIĆ, D.; KILIBARDA, V.; ĆIRIĆ, B.; VUČINIĆ, S.; SRNIĆ, D.; VEHABOVIĆ, M.; POTOGIJA, N. A randomized, open-label pharmacokinetic comparison of two oral formulations of fluconazole 150 mg in healthy adult volunteers. Clin. Ther., v.27, p.1588-1595, 2005.; Pore et al., 2006PORE, V.S.; AHER, N.G.; KUMAR, M.; SHUKLA, P.K. Mycotic keratitis: an overview of diagnosis and therapy. Micoses, v.62, p.11178-11186, 2006.).
Given the growing cost of fungal infections to the public health system, and highlighting the important role of azole antifungal agents, it is necessary to seek alternative administration route for these drugs in the treatment of fungal diseases, so as to increase their bioavailability efficiency and thus their therapeutic and to provide clinicians and patients with a greater diversity of treatment.
Strategies currently being investigated to overcome these limitations include the design and the development of drug delivery systems that can improve the efficacy of existing drugs. Topical drug delivery systems may contribute to the enhancement of the current antifungal therapy. Nanotechnology-based drug delivery systems such as liposomes, solid lipid nanoparticles, micro and nanoparticles, microemulsions (ME) and liquid crystals (LC) can modify the drug release profile, improve drug stability, increase the residence time of the drug at the site of action and the ready solubilization of drugs in these materials (Gupta, Vyas, 2012GUPTA, M.; VYAS, S.P. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem. Phys. Lipids, v.165, p.454-461, 2012.; El-Nesr et al., 2010EL-NESR, O.H.; YAHIYA, S.A.; EL-GAZAYERLY, O.N. Effect of formulation design and freeze-drying on properties of fluconazole multilamellar liposomes. Saudi Pharm. J., v.18, p.217-224, 2010.; Gabboun et al., 2001GABBOUN, N.H.; NAJIB, N.M.; IBRAHIM, H.G.; ASSAF, S. Release of salicylic acid and diclofenac acid salts from isotropic and anisotropic nonionic surfactant systems across rat skin. Int. J. Pharm., v.212, p.73-80, 2001.; Peira et al., 2001PEIRA, E.; SCOLARI, P.; GASCO, M.R. Transdermal permeation of apomorphine through hairless mouse skin from microemulsions. Int. J. Pharm., v.226, p.47-51, 2001.).
ME are thermodynamically stable systems and isotropic systems, composed of an oil phase, an aqueous phase and a surfactant and/or co-surfactant, their properties facilitate the solubilization of lipophilic drugs, enabling their incorporation, if desired, at concentrations higher than that required at the target (Wei et al., 2012WEI, J.; SU, B.; XING, H. BAO, Z.; YANG, Y.; REN, Q. Effect of ionic liquids on temperature-induced percolation behavior of AOT Microemulsions. Colloid. Surface. A, v.396, p.213-218, 2012.; Warisnoicharoen et al., 2000WARISNOICHAROEN, W.; LANSLEY, A.B.; LAWRENCE, M.J. Noionic oil-in-water microemulsion effect of oil type on phase behavior. Int. J. Pharm., v.198, p.7-27, 2000.; Holmberg et al., 2002HOLMBERG, K.; JONSSON, B.; KRONBERG, B.; LINDMAN, B. Surfactants and polymers in aqueous solution. West Sussex: John Wiley & Sons, 2002. 545 p.; Kreilgaard, 2002KREILGAARD, M. Influence of microemulsions on cutaneous drug delivery. Adv. Drug Deliver. Rev., v.54, p.S77-S98, 2002.). The interaction of the drug with the system also provides controlled drug release and targeting of the site of action; ME are a type of formulation that can increase penetration through the stratum corneum (Huang et al., 2008HUANG, Y.B.; LIN, Y.H.; LU, T.M.; WANG, R.J.; TSAI, Y.H.; WU, P.C. Transdermal delivery of capsaicin derivative-sodium nonivamide acetate using microemulsions as vehicles. Int. J. Pharm., v.349, p.206-211, 2008.; Alvarez-Figuereo, Blanco-Méndez, 2001). This can occur by the following mechanisms: the composition and structure of these systems allow greater quantities of drug to be incorporated than other, conventional, topical formulations such as lotions, creams and gels. It can increase the solubility of drugs poorly soluble in water, via the oil phase, it can enhance drug stability, modify the diffusion barrier of the skin and promote drug release into the skin, without leading to significant irreversible changes in the skin barrier function (Baroli et al., 2000BAROLI, B.; LÓPEZ-QUINTELA, M.A.; DELGADO-CHARRO, M.B.; FADDA, A.M.; BLANCO-MÉNDEZ, J. Microemulsions for topical delivery of 8-methoxsalen. J Control. Release, v.69, p.209-218, 2000.; Hoeller et al., 2008HOELLER, S.; KLANG, V.; VALENTA, C. Skin-compatible lecithin drug delivery systems for fluconazole: effect of phosphatidylethanolamine and oleic acid on skin permeation. J. Pharm. Pharmacol., v.60, p.587-591, 2008.; Heuschkel et al., 2008HEUSCHKEL, S.; GOEBEL, A.; NEUBERT, R.H.H. Microemulsions-modern colloidal carrier for dermal and transdermal. J. Pharm. Sci., v.97, p.603-631, 2008.).
LC are thermodynamically stable, optically isotropic, transparent and condensed structures that are both solid and liquid; thus, they are also known as mesophases (Mainardes et al., 2011MAINARDES, R.M.; GREMIÃO, M.P.D.; CARVALHO, F.C. Exploring the nanotechnology-based drug delivery systems for AIDS Treatment. In: KASENGA, F.G. (Ed.). Understanding HIV/AIDS management and care - pandemic approaches in the 21st century. Croatia: Intech, 2011. p.367-384.; Formariz et al., 2005FORMARIZ, T.P.; URBAN, M.C.C.; SILVA JÚNIOR, A.A.; GREMIÃO, M.P.D.; OLIVEIRA, A.G. Microemulsões e fases líquidas cristalinas como sistemas de liberação de fármacos. Rev. Bras. Ciênc. Farm., v.41, p.301-313, 2005.; Müller-Goymann, 2004MÜLLER-GOYMANN, C.C. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur. J. Pharm. Biopharm., v.58, p.343-356, 2004.). These systems combine the properties of both liquid and solid states and can be formed from the addition of solvents (Müller-Goymann, 2004MÜLLER-GOYMANN, C.C. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur. J. Pharm. Biopharm., v.58, p.343-356, 2004.). Their stability can be checked by varying the temperature (thermotropic liquid crystals) (Chorilli et al., 2011CHORILLI, M.; PRESTES, P.S.; RIGON, R.B.; LEONARDI, G.R.; CHIAVACCI, L.A.; SARMENTO, V.H.V.; OLIVEIRA, A.G.; SCARPA, M.V. Structural characterization and in vivo evaluation of retinyl palmitate in non-ionic lamellar liquid crystalline system. Colloids Surf. B Biointerfaces, v.85, p.182-188, 2011.). The types of liquid crystals that can be formed are (liquid crystal) lamellar, hexagonal or cubic phases (Alam, Shrestha, Aramaki, 2009ALAM, M.M.; SHRESTHA, L.K.; ARAMAKI, K. Glycerol effects on the formation and rheology of cubic phase and related gel emulsion. J. Colloid Interf. Sci., v.329, p.366-371, 2009.). The lamellar phase is formed by parallel layers of surfactant, aqueous and oil phases, which form one-dimensional networks. In the hexagonal phase, cylinders of surfactant molecules are formed and the oil phase may be present either inside the cylinders or in the continuous phase of the system. The cubic phase is the most viscous and has been observed mainly in water/surfactant or surfactant-like lipid systems or water/surfactant/oil ternary systems (Alam, Shrestha, Aramaki, 2009ALAM, M.M.; SHRESTHA, L.K.; ARAMAKI, K. Glycerol effects on the formation and rheology of cubic phase and related gel emulsion. J. Colloid Interf. Sci., v.329, p.366-371, 2009.). Some authors have experimented with four different organizational arrangements of lamellar liquid crystal phases and three different arrangements of the cubic phases (Mannock et al., 2007MANNOCK, D.A.; COLLINS, M.D.; KREICHBAUM, M.; HARPER, P.E.; GRUNER, S.M.; MCELHANEY, R.N. The thermotropic phase behaviour and phase structure of a homologous series of racemic β-d-galactosyl dialkylglycerols studied by differential scanning calorimetry and X-ray diffraction. Chem. Phys. Lipids, v.148, p.26-50, 2007.; Chorilli et al., 2011CHORILLI, M.; PRESTES, P.S.; RIGON, R.B.; LEONARDI, G.R.; CHIAVACCI, L.A.; SARMENTO, V.H.V.; OLIVEIRA, A.G.; SCARPA, M.V. Structural characterization and in vivo evaluation of retinyl palmitate in non-ionic lamellar liquid crystalline system. Colloids Surf. B Biointerfaces, v.85, p.182-188, 2011.).
LC-based drug delivery systems have been shown to increase the permeation of drugs because of the high concentrations of surfactants. The surfactants are able to destabilize the lipid layer of the skin, forming small pores in this structure (Kreilgaard, 2002KREILGAARD, M. Influence of microemulsions on cutaneous drug delivery. Adv. Drug Deliver. Rev., v.54, p.S77-S98, 2002.). Höller, Valenta (2007)HÖLLER, S.; VALENTA, C. Effect of selected fluorinated drugs in a "ringing" gel on rheological behavior and skin permeation. Eur. J. Pharm. Biopharm., v.66, p.120-126, 2007. studied the solubility, rheological behaviour and permeation of several drugs from LC of the cubic phase, including FLU. The results showed that the incorporation of FLU in the system did not alter the rheological behavior of the formulation, and the drug was retained longer in the skin after skin permeation test.
Analytical methods employed in the quantitative determination of drug delivery systems
play an important role in the assessment of pharmaceutical preparations, HPLC being the
most frequently used method. To guarantee the quality of commercial products, the
development and validation of analytical methods are crucial for the pharmaceutical
industry (Apostol et al., 2012APOSTOL, I; KRULL, I; KELNER, D. Analytical method validation for
biopharmaceuticals. In: KRULL, I.S. (Ed.) Analytical chemistry. Intech, p.115-134,
2012 .).
The methods for quantitation and detection must be robust, precise and rapid and are
essential in the development, process monitoring and quality control of pharmaceuticals.
Analytical validation is necessary for the routine quality control of products, so that
reliable results can be satisfactorily interpreted (Shabir, 2003SHABIR, G.A. Validation of high performance liquid chromatography
methods for pharmaceutical analysis. Understanding the difference and similarities
between validation requirements of the US Food and Drug Administration, the US
Pharmacopeia and the International Conference on Harmonization. J. Chromatogr. A,
v.987, p.57-66, 2003.; ICH-Q2B, 2005INTERNATIONAL CONFERENCE ON HARMONIZATION. ICH. Technical Requirements
for Registration of Pharmaceutical Human Use Geneva. ICH-Q2B: Switzerland, 2005.
Available at: www.ich.org Accessed on: 25th July 2013.
www.ich.org...
).
The validation of an analytical methodology is useful to demonstrate that the method is
suitable for its intended purpose, to guarantee that every future measurement will be
close enough to the unknown true value, for the content range of the analyte in the
sample, and to ensure that the method meets the requirements of analytical methods,
producing reliable results (Carvalho et
al., 2009CARVALHO, F.C.; BARBI, M.; GREMIÃO, M.P.D. LC evaluation of in vitro
release of AZT from microemulsions. Chromatographia, v.69, p.207-211,
2009.; Cinto et
al., 2009CINTO, P.O.; SOUZA, A.L.R.; LIMA, A.C.; CHAUD, M.V.; GREMIÃO, M.P.D. LC
evaluation of intestinal transport of praziquantel. Chromatographia, v.69, p.213-217,
2009.). Assays based on HPLC are very commonly used to measure
the quality of drugs and, according to ICH, certain parameters must be checked to
validate the analytical method: linearity, specificity, accuracy, precision (inter and
intraday), limit of detection (LOD), limit of quantitation (LOQ) and robustness (ICH-Q2B, 2005INTERNATIONAL CONFERENCE ON HARMONIZATION. ICH. Technical Requirements
for Registration of Pharmaceutical Human Use Geneva. ICH-Q2B: Switzerland, 2005.
Available at: www.ich.org Accessed on: 25th July 2013.
www.ich.org...
; González, Herrador, 2007GONZÁLEZ, A.G.; HERRADOR, M.A. A practical guide to analytical method
validation, including measurement uncertainty and accuracy profiles. Trends Anal.
Chem., v.26, p.227-238, 2007.; Hofer et
al., 2007HOFER, J.D.; OLSEN, B.A.; RICKARD, E.C. Is HPLC assay for drug substance
a useful quality control attribute?. J. Pharm. Biomed. Anal., v.44, p.906-913,
2007.).
Sasongko et al. (2003)SASONGKO, L.; WILLIAMS, K.M.; DAY, R.O.; MCLACHLAN, A.J. Human subcutaneous tissue distribution of fluconazole: comparison of microdialysis and suction blister techniques. Br. J. Clin. Pharmacol., v.56, p.551-561, 2003. developed a method that used HPLC to assay in a form taken FLU orally. Ayub et al. (2007)AYUB, A.C.; GOMES, A.D.M.; LIMA, M.V.C.; VIANNA-SOARES, C.D.; FERREIRA, L.A.M. Topical delivery of fluconazole: in vitro skin penetration and permeation using emulsions as dosage forms. Drug Dev. Ind. Pharm., v.33, p.273-280, 2007. developed and validated an assay to measure the permeation of FLU from emulsions applied to the skin. Bachhav, Patravale (2009)BACHHAV, Y.G.; PATRAVALE, V.B. Microemulsion based vaginal gel of fluconazole: Formulation, in vitro and in vivo evaluation. Int. J. Pharm., v.365, p.175-179, 2009. validated a method of assaying FLU in released from microemulsions for vaginal use. However, no methodology has been developed to quantify fluconazole incorporated into ME or LC for cutaneous administration.
In light of this, the aim of this work was to validate an analytical methodology by HPLC to determine the amount of FLU incorporated in ME and LC.
MATERIAL AND METHODS
Equipment
The equipment used was a Varian HPLC with a ProStar 330 UV-VIS PDA detector and Rheodyne VS 125 injector with a Varian ProStar® 410 autosample.
Chemicals
To formulate the ME, two copaiba oil were utilized, first copaiba oil (CO) was bought from cooperative in Brazil, the second from Sigma (OS). Fluconazole was purchased from Purifarma and oleic acid (OA) from Synth. Propoxyl (5OP) ethoxyl (20 OE) cetyl alcohol (PROC, Croda) was used as surfactant and the water was purified with a Milli Q Plus.
Development of microemulsion (ME) and liquid crystal (LC) to incorporation of FLU
In order to investigate the ME and LC regions, a ternary phases diagram was constructed, containing as oil phase copaiba oil obtained of cooperatives of Acre (Brazil) (CO), copaiba oil purchased from Sigma (OS) or oleic acid (OA). The second component was the surfactant propoxyl (5OP) ethoxyl (20 OE) cetyl alcohol (PROC) and the third was water. A fixed concentration of FLU (1%) was used. The compositions chosen for of the nanostructured systems are shown in Table I.
Incorporation of FLU in formulations
The capacity of formulations to incorporate FLU was investigated by adding an excess of FLU powder (100 mg/g) to the formulations being tested. After shaking for 48 h at 25 ± 0.1 oC, samples were centrifuged at 360 g for 20 min (Du Pont-Sorvall TC 6 centrifuge), to eliminate undissolved FLU. The supernatants were diluted with mobile phase and the FLU dissolved in it was measured by HPLC method.
Method validation
The analytical method was validated by HPLC, to determine the following parameters:
linearity, specificity, accuracy, inter and intraday precision, limit of detection
(LOD), limit of quantitation (LOQ) and robustness, following the guidelines of the
International Conference on Harmonization (ICH) guidelines (ICH-Q2B, 2005INTERNATIONAL CONFERENCE ON HARMONIZATION. ICH. Technical Requirements
for Registration of Pharmaceutical Human Use Geneva. ICH-Q2B: Switzerland, 2005.
Available at: www.ich.org Accessed on: 25th July 2013.
www.ich.org...
) and the Brazilian Food, Drug and Sanitation Agency
(ANVISA) (Brasil, 2003BRASIL. Ministério da Saúde. Política Nacional de Assistência
Farmacêutica. Resolução nº899, de 29 de maio de 2003. Diário Oficial da União,
Brasília (DF). Acessed on: 27 July 2013.).
Linearity
In order to assess the validity of the assay, 100 mg fluconazole was dissolved in acetonitrile/water 1:1 (mobile phase) in 100 mL volumetric flask (1 mg.mL-1). Appropriate aliquots of this solution were diluted with the mobile phase, yielding concentrations of 20.0, 40.0, 50.0, 100.0, 150.0, 200.0, 250.0, 300.0, 350.0 and 400.0 µg.mL-1. Triplicate preparations of each concentration were performed. The results were subjected to regression analysis by a least-squares method to calculate the calibration equation and determination coefficient.
Specificity
The specificity was determined by measuring the total purity of the chromatographic peaks in samples of phosphate buffer pH 7.4 containing FLU in the concentration at 150 µg.mL-1 and in the formulations containing oleic acid (OA), copaiba oil (CO) bought locally or from Sigma (OS), respectively, with or without FLU. This assay was performed in triplicate.
Precision
Precision data for this validation were determined as recommended by ICH-Q2B (2005)INTERNATIONAL CONFERENCE ON HARMONIZATION. ICH. Technical Requirements
for Registration of Pharmaceutical Human Use Geneva. ICH-Q2B: Switzerland, 2005.
Available at: www.ich.org Accessed on: 25th July 2013.
www.ich.org...
. Repeatability was calculated by
assaying samples of 20.0, 200.0 and 400.0 mg mL1 intra- and inter-day in
three different days; results must be less than 5%. The RSD of peak areas (n = 3) was
evaluated.
Accuracy
To confirm the accuracy of the proposed method, a sample of fluconazole working solution was prepared in triplicate at three different concentrations, 20, 40 and 200 µg.mL-1, within the analytical curve.
Limit of detection (LOD), limit of quantitation (LOQ)
To determine the LOD and LOQ, three analytical curves were prepared at concentrations near the presumed LOQ. The LOD and LOQ were calculated from equations (1) and (2) as described by IHC-Q2B (2005).
LOD= 3 (S.D ./a) (1)
LOQ = 10(S.D ./a) (2)
where S.D is the standard deviation of the y-intercepts and S is the slope of the calibration curve.
Robustness
Robustness was assessed against the intervention of some variables related to quantitation and recovery of the drug like column (Phenomenex and Varian), HPLC (Varian and HP) and detector (DAD and UV). The chromatographic parameters monitored were capacity factor, tailing factor (peak symmetry) and theoretical plates.
Statistical analysis
The ANOVA and Tukey tests were carried out using the BioEstat 5.0 software. Statistical relevance was assumed at p < 0.05.
RESULTS AND DISCUSSION
Method validation
Linearity
Fluconazole eluted from the column was analyzed in the mobile phase (acetonitrile and water 1:1) by measuring absorbance at 210 nm, and the area under the peak was calculated. The analytical curve was obtained by averaging three curves and performing in triplicate for each dilution curve.
The method was evaluated by the determination coefficient and intercept, calculated in the corresponding statistical study (ANOVA; p<0.05). The curve showed a good linearity at these concentrations, the mean (RSD) slope and y-intercept being 0.0005 and 0.0012, with a determination coefficient (r2) of 0.9999. R2 values > 0.999 and intercepts very close to zero confirmed the good linearity of the method (Brasil, 2003BRASIL. Ministério da Saúde. Política Nacional de Assistência Farmacêutica. Resolução nº899, de 29 de maio de 2003. Diário Oficial da União, Brasília (DF). Acessed on: 27 July 2013.).
It can be seen that the coefficient of variation between the samples was less than 2.119% (Table II), fulfilling ANVISA requirement of lower than 5% in these analyses (Brasil, 2003BRASIL. Ministério da Saúde. Política Nacional de Assistência Farmacêutica. Resolução nº899, de 29 de maio de 2003. Diário Oficial da União, Brasília (DF). Acessed on: 27 July 2013.).
Precision
Tests of repeatability (intra-day) and intermediate (inter-day), precision were carried out at three concentration levels, begin low (20 µg.mL-1), medium (200 µg.mL-1) and high (400 µg.mL-1). Table III shows the results for repeatability.
In the repeatability test, the CV among samples was calculated and the highest 1%,
for the lowest concentration. This is an acceptable value. According to ANVISA and
ICH the CV must not exceed 5% in these assays, as in the test of linearity (ICH-Q2B, 2005INTERNATIONAL CONFERENCE ON HARMONIZATION. ICH. Technical Requirements
for Registration of Pharmaceutical Human Use Geneva. ICH-Q2B: Switzerland, 2005.
Available at: www.ich.org Accessed on: 25th July 2013.
www.ich.org...
; Brasil, 2003BRASIL. Ministério da Saúde. Política Nacional de Assistência
Farmacêutica. Resolução nº899, de 29 de maio de 2003. Diário Oficial da União,
Brasília (DF). Acessed on: 27 July 2013.). In Table IV, the
results of the inter-day precision test, done on three consecutive days, are
shown.
Again, the values of coefficient of variation are within the ANVISA and ICH limit of
5% (ICH-Q2B, 2005INTERNATIONAL CONFERENCE ON HARMONIZATION. ICH. Technical Requirements
for Registration of Pharmaceutical Human Use Geneva. ICH-Q2B: Switzerland, 2005.
Available at: www.ich.org Accessed on: 25th July 2013.
www.ich.org...
; Brasil, 2003BRASIL. Ministério da Saúde. Política Nacional de Assistência
Farmacêutica. Resolução nº899, de 29 de maio de 2003. Diário Oficial da União,
Brasília (DF). Acessed on: 27 July 2013.). Therefore, the analytical method was sufficiently
precise in both tests.
Accuracy
In order to check the accuracy, the same concentrations as in the precision test were used and the results were analyzed as percent recovery of the sample. The results are shown in Table V.
The percent recovery values are shown in Table
VI and they were all found to be between 99 and 102%. The results are well
within the interval of recovery values approved by ANVISA, namely 80-120%. Moreover,
the CV was less that 1%, being greatest at a concentration of 200 µg.mL-1.
The values coefficient of variation are thus ANVISA and ICH limit of 5% (ICH-Q2B, 2005INTERNATIONAL CONFERENCE ON HARMONIZATION. ICH. Technical Requirements
for Registration of Pharmaceutical Human Use Geneva. ICH-Q2B: Switzerland, 2005.
Available at: www.ich.org Accessed on: 25th July 2013.
www.ich.org...
; Brasil, 2003BRASIL. Ministério da Saúde. Política Nacional de Assistência
Farmacêutica. Resolução nº899, de 29 de maio de 2003. Diário Oficial da União,
Brasília (DF). Acessed on: 27 July 2013.).
Limits of detection (LOD) and quantitation (LOQ)
The detection limit is the smallest amount of the analyte that can be detected but
not necessarily quantified, while the limit of quantitation is the smallest content
of the analyte that can be analyzed with accuracy and precision (ICH-Q2B, 2005INTERNATIONAL CONFERENCE ON HARMONIZATION. ICH. Technical Requirements
for Registration of Pharmaceutical Human Use Geneva. ICH-Q2B: Switzerland, 2005.
Available at: www.ich.org Accessed on: 25th July 2013.
www.ich.org...
). The values of LOD and LOQ were
0.057 and 0.172 µg.mL-1, respectively, indicating the excellent
sensitivity of the analytical method.
Specificity and Selectivity
Figure 2 shows the chromatogram of a single injection of buffer containing FLU. It can be seen that the buffer did not interfere with in the analysis, as it did not affect the drug peak region. This assay was performed in triplicate.
Chromatogram relating injection phosphate buffer pH 7.4 containing fluconazole (150µg.mL-1).
The analysis of the formulations containing fluconazole (Figure 3) also showed no interference in the drug peak region.
The specificity of an analytical method can be assessed by determining the chromatographic peak purity using a diode array detector (Brasil, 2003BRASIL. Ministério da Saúde. Política Nacional de Assistência Farmacêutica. Resolução nº899, de 29 de maio de 2003. Diário Oficial da União, Brasília (DF). Acessed on: 27 July 2013.), which shows graphically and numerically the similarity of the spectrum of the peak of the substance of interest. The results indicate purity index of 0.99 in both cases, indicating the similarity of the peaks, that is, the chromatographic peak refers to the substance of interest, without interference.
Robustness
In this assay, the interference of certain parameters in the analytical results was
assessed. It was found that changing the column make, type of HPLC or detector did
not interference with the peak retention time or the drug recovery. The system
suitability method acceptance criteria set in each validation run were: capacity
factor < 2.0, tailing factor ≥ 2.0 and theorical plates > 2000. In all cases,
the relative standard deviation (RSD) for the anlayte peak area for two consecutive
injections was < 2.0%. The values obtained are in agreement with the literature
(ICH-2QB, 2005INTERNATIONAL CONFERENCE ON HARMONIZATION. ICH. Technical Requirements
for Registration of Pharmaceutical Human Use Geneva. ICH-Q2B: Switzerland, 2005.
Available at: www.ich.org Accessed on: 25th July 2013.
www.ich.org...
). Therefore, the method may
be considered robust.
Incorporation of FLU in the formulations
Analyses were performed in triplicate and results expressed as the content of FLU in the formulation, in mg.g 1, as shown in Table VI. The concentration was calculated from the peak area by the equation and analytical curve obtained in the test of linearity: y=0.0005 x + 0.0012.
The statistical analysis showed that the difference between the incorporation of FLU into CO1, CO2, CO3, CO4, OS1, OS2, OS3 and OS4 was not statistically significant (p>0.05). On the other hand, OA1, OA2, OA3 and OA4 exhibited values of FLU incorporation significantly higher (p<0.05) than CO1, CO2, CO3, CO4, OS1, OS2, OS3 and OS4.
It was observed that the formulations with CO or CS as oil phase presented lower quantity of FLU incorporated when compared with formulations with OA as oil phase. This may be due to the complex composition of these oils, which contain various nonpolar components and other polar chains that hinder the solubilization of drugs such as FLU.
Veiga Junior, Pinto, and Maciel et al. (2005)VEIGA JUNIOR, V.F.; PINTO, A.C.; MACIEL, M.A.M. Plantas medicinais: cura segura? Quím. Nova, v.28, p.519-28, 2005. claim that copaiba oil may vary in relation to the concentration and nature of diterpenes and sesquiterpenes present variations according to species, biological or abiotic factors. Thus, although the difference in the amount of FLU incorporated was not statistically significant in copaiba oils from different sources tested, since they have different amounts of terpenes, this could result in different affinities by drugs with different degrees of lipophilicity.
CONCLUSIONS
All validation parameters were satisfactory; the results showed that the method is suitable for quantitative analysis of FLU, consistent with the requirements of ICH and ANVISA, and thus providing a reliable analytical method. The proposed method was accurate in measuring the concentration of FLU in ME and LC.
ACKNOWLEDGMENTS
We would like to thank Timothy John C. Roberts for English language review. Financial support provided by FAPESP (Fundação de Amparo a Pesquisa), PADC (Programa de Apoio ao Desenvolvimento Científico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) is acknowledged.
REFERENCES
- ALAM, M.M.; SHRESTHA, L.K.; ARAMAKI, K. Glycerol effects on the formation and rheology of cubic phase and related gel emulsion. J. Colloid Interf. Sci., v.329, p.366-371, 2009.
- ALNAIM, L.; ABOU ALSOUD, N.; ZAGHLOUL, I.; AL-JASER, M. Effects of fluconazole on the pharmacokinetics and pharmacodynamics of antimony in cutaneous leishmaniasis-infected hamsters. Int. J. Antimicrob. Agents., v.29, p.728-732, 2007.
- ALVAREZ-FIGUEROA, M.J.; BLANCO-MÉNDEZ, J. Transdermal delivery of methotrexate: iontophoretic delivery from hydrogels and passive delivery from microemulsions. Int. J. Antimicrob. Agents, v.215, p.57-65, 2001.
- APOSTOL, I; KRULL, I; KELNER, D. Analytical method validation for biopharmaceuticals. In: KRULL, I.S. (Ed.) Analytical chemistry. Intech, p.115-134, 2012 .
- AYUB, A.C.; GOMES, A.D.M.; LIMA, M.V.C.; VIANNA-SOARES, C.D.; FERREIRA, L.A.M. Topical delivery of fluconazole: in vitro skin penetration and permeation using emulsions as dosage forms. Drug Dev. Ind. Pharm., v.33, p.273-280, 2007.
- BACHHAV, Y.G.; PATRAVALE, V.B. Microemulsion based vaginal gel of fluconazole: Formulation, in vitro and in vivo evaluation. Int. J. Pharm., v.365, p.175-179, 2009.
- BAROLI, B.; LÓPEZ-QUINTELA, M.A.; DELGADO-CHARRO, M.B.; FADDA, A.M.; BLANCO-MÉNDEZ, J. Microemulsions for topical delivery of 8-methoxsalen. J Control. Release, v.69, p.209-218, 2000.
- BRASIL. Ministério da Saúde. Política Nacional de Assistência Farmacêutica. Resolução nº899, de 29 de maio de 2003. Diário Oficial da União, Brasília (DF). Acessed on: 27 July 2013.
- CARVALHO, F.C.; BARBI, M.; GREMIÃO, M.P.D. LC evaluation of in vitro release of AZT from microemulsions. Chromatographia, v.69, p.207-211, 2009.
- CHORILLI, M.; PRESTES, P.S.; RIGON, R.B.; LEONARDI, G.R.; CHIAVACCI, L.A.; SARMENTO, V.H.V.; OLIVEIRA, A.G.; SCARPA, M.V. Structural characterization and in vivo evaluation of retinyl palmitate in non-ionic lamellar liquid crystalline system. Colloids Surf. B Biointerfaces, v.85, p.182-188, 2011.
- CINTO, P.O.; SOUZA, A.L.R.; LIMA, A.C.; CHAUD, M.V.; GREMIÃO, M.P.D. LC evaluation of intestinal transport of praziquantel. Chromatographia, v.69, p.213-217, 2009.
- DASH, A.K.; ELMQUIST, W.F. Fluconazole. In: HARRY, G.B. (Ed.) Analytical profiles of drug substances and excipients. London: Academic Press, 2001. v.27, p.67-113.
- EL-NESR, O.H.; YAHIYA, S.A.; EL-GAZAYERLY, O.N. Effect of formulation design and freeze-drying on properties of fluconazole multilamellar liposomes. Saudi Pharm. J., v.18, p.217-224, 2010.
- ERCHIGA, V.C.; FLORENCIO, V.D. Micosis cutáneas. Med. Clin., v.125, p.467-474, 2005.
- FORMARIZ, T.P.; URBAN, M.C.C.; SILVA JÚNIOR, A.A.; GREMIÃO, M.P.D.; OLIVEIRA, A.G. Microemulsões e fases líquidas cristalinas como sistemas de liberação de fármacos. Rev. Bras. Ciênc. Farm., v.41, p.301-313, 2005.
- GABBOUN, N.H.; NAJIB, N.M.; IBRAHIM, H.G.; ASSAF, S. Release of salicylic acid and diclofenac acid salts from isotropic and anisotropic nonionic surfactant systems across rat skin. Int. J. Pharm., v.212, p.73-80, 2001.
- GONZÁLEZ, A.G.; HERRADOR, M.A. A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. Trends Anal. Chem., v.26, p.227-238, 2007.
- GUPTA, M.; VYAS, S.P. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem. Phys. Lipids, v.165, p.454-461, 2012.
- HEUSCHKEL, S.; GOEBEL, A.; NEUBERT, R.H.H. Microemulsions-modern colloidal carrier for dermal and transdermal. J. Pharm. Sci., v.97, p.603-631, 2008.
- HOELLER, S.; KLANG, V.; VALENTA, C. Skin-compatible lecithin drug delivery systems for fluconazole: effect of phosphatidylethanolamine and oleic acid on skin permeation. J. Pharm. Pharmacol., v.60, p.587-591, 2008.
- HOFER, J.D.; OLSEN, B.A.; RICKARD, E.C. Is HPLC assay for drug substance a useful quality control attribute?. J. Pharm. Biomed. Anal., v.44, p.906-913, 2007.
- HÖLLER, S.; VALENTA, C. Effect of selected fluorinated drugs in a "ringing" gel on rheological behavior and skin permeation. Eur. J. Pharm. Biopharm., v.66, p.120-126, 2007.
- HOLMBERG, K.; JONSSON, B.; KRONBERG, B.; LINDMAN, B. Surfactants and polymers in aqueous solution. West Sussex: John Wiley & Sons, 2002. 545 p.
- HUANG, Y.B.; LIN, Y.H.; LU, T.M.; WANG, R.J.; TSAI, Y.H.; WU, P.C. Transdermal delivery of capsaicin derivative-sodium nonivamide acetate using microemulsions as vehicles. Int. J. Pharm., v.349, p.206-211, 2008.
- INTERNATIONAL CONFERENCE ON HARMONIZATION. ICH. Technical Requirements for Registration of Pharmaceutical Human Use Geneva. ICH-Q2B: Switzerland, 2005. Available at: www.ich.org Accessed on: 25th July 2013.
» www.ich.org - JOVANOVIĆ, D.; KILIBARDA, V.; ĆIRIĆ, B.; VUČINIĆ, S.; SRNIĆ, D.; VEHABOVIĆ, M.; POTOGIJA, N. A randomized, open-label pharmacokinetic comparison of two oral formulations of fluconazole 150 mg in healthy adult volunteers. Clin. Ther., v.27, p.1588-1595, 2005.
- KREILGAARD, M. Influence of microemulsions on cutaneous drug delivery. Adv. Drug Deliver. Rev., v.54, p.S77-S98, 2002.
- MAINARDES, R.M.; GREMIÃO, M.P.D.; CARVALHO, F.C. Exploring the nanotechnology-based drug delivery systems for AIDS Treatment. In: KASENGA, F.G. (Ed.). Understanding HIV/AIDS management and care - pandemic approaches in the 21st century. Croatia: Intech, 2011. p.367-384.
- MANNOCK, D.A.; COLLINS, M.D.; KREICHBAUM, M.; HARPER, P.E.; GRUNER, S.M.; MCELHANEY, R.N. The thermotropic phase behaviour and phase structure of a homologous series of racemic β-d-galactosyl dialkylglycerols studied by differential scanning calorimetry and X-ray diffraction. Chem. Phys. Lipids, v.148, p.26-50, 2007.
- MÜLLER-GOYMANN, C.C. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur. J. Pharm. Biopharm., v.58, p.343-356, 2004.
- NEGRONI, R. Historical aspects of dermatomycoses. Clin. Dermatol., v.28, p.125-132, 2010.
- PEIRA, E.; SCOLARI, P.; GASCO, M.R. Transdermal permeation of apomorphine through hairless mouse skin from microemulsions. Int. J. Pharm., v.226, p.47-51, 2001.
- PORE, V.S.; AHER, N.G.; KUMAR, M.; SHUKLA, P.K. Mycotic keratitis: an overview of diagnosis and therapy. Micoses, v.62, p.11178-11186, 2006.
- SASONGKO, L.; WILLIAMS, K.M.; DAY, R.O.; MCLACHLAN, A.J. Human subcutaneous tissue distribution of fluconazole: comparison of microdialysis and suction blister techniques. Br. J. Clin. Pharmacol., v.56, p.551-561, 2003.
- SHABIR, G.A. Validation of high performance liquid chromatography methods for pharmaceutical analysis. Understanding the difference and similarities between validation requirements of the US Food and Drug Administration, the US Pharmacopeia and the International Conference on Harmonization. J. Chromatogr. A, v.987, p.57-66, 2003.
- VEIGA JUNIOR, V.F.; PINTO, A.C.; MACIEL, M.A.M. Plantas medicinais: cura segura? Quím. Nova, v.28, p.519-28, 2005.
- WARISNOICHAROEN, W.; LANSLEY, A.B.; LAWRENCE, M.J. Noionic oil-in-water microemulsion effect of oil type on phase behavior. Int. J. Pharm., v.198, p.7-27, 2000.
- WEI, J.; SU, B.; XING, H. BAO, Z.; YANG, Y.; REN, Q. Effect of ionic liquids on temperature-induced percolation behavior of AOT Microemulsions. Colloid. Surface. A, v.396, p.213-218, 2012.
Publication Dates
-
Publication in this collection
Apr-Jun 2014
History
-
Received
05 Aug 2013 -
Accepted
16 Dec 2013