Abstracts
The aim of the present research is to formulate and evaluate the gastroretentive floating drug delivery system of antihypertensive drug, propranolol HCl. Gastroretentive floating tablets (GRFT) were prepared by using a synthetic hydrophilic polymer polyethylene oxide of different grades such as PEO WSR N-12 K and PEO 18 NF as release retarding polymers and calcium carbonate as gas generating agent. The GRFT were compressed by direct compression strategy and the tablets were evaluated for physico-chemical properties, in vitro buoyancy, swelling studies, in vitro dissolution studies and release mechanism studies. From the dissolution and buoyancy studies, F 9 was selected as an optimized formulation. The optimized formulation followed zero order rate kinetics with non-Fickian diffusion mechanism. The optimized formulation was characterised with FTIR studies and observed no interaction between the drug and the polymers.
Gastroretentive floating/ drug delivery system; Gastroretentive floating tablets/in vitro dissolution; Hydrophilic polymers/use in drug delivery system; Polyethylene oxide; Propranolol HCl/delivery system
O objetivo da presente pesquisa é o de formular e avaliar o sistema de liberação de fármaco gastrorretentivo flutuante, contendo o anti-hipertensivo, cloridrato de propranolol. Comprimidos gastrorretentivo flutuantes (GRFT) foram preparados utilizando um polímero hidrofílico sintético, o óxido de polietileno, de diferentes graus, tais como GE WSR N-12 K e GE 18 NF, como polímeros de retardamento de liberação, e carbonato de cálcio, como agente gerador de gases. Os GRFT foram comprimidos por compressão direta e avaliados para determinação das propriedades físico-químicas, flutuabilidade in vitro, estudos de inchamento, de dissolução in vitro e de mecanismo de liberação. Dos testes de dissolução e de flutuabilidade, selecionou-se F 9 como formulação otimizada. A formulação otimizada seguiu cinética de ordem zero, com mecanismo de difusão não-Fickiano. Essa formulação foi caracterizada por estudos de FTIR, não se observando interação entre o fármaco e os polímeros.
Retenção gástrica flutuante/sistema de liberação de fármaco; Comprimidos gastrorretentivo flutuante/dissolução in vitro ; Polímeros hidrofílicos/uso em sistema de liberação de fármaco; Óxido de polietileno; Cloridrato de propranolol/ sistema de liberação
INTRODUCTION
Oral dosage forms have been developed from the past four decades due to their significant therapeutic advantages such as ease of administration, patient compliance and flexibility in formulation (Srikanth et al., 2011aSRIKANTH, M.V.; JANAKI RAM, B.; SUNIL, S.A.; SREENIVASA RAO, N.; RAMANA MURTHY, K.V. Gastroretentive drug delivery systems: novel approaches and its evaluation: a review. Int. J. Pharm. Sci. Rev. Res., v.10, n.1, p.203-216, 2011a.). Nowadays, the trend is going towards the preparation of novel controlled drug delivery systems, in which the active drug can be controlled for a longer period. However, in the controlled drug delivery, the drug absorption is inadequate and highly variable in the individuals due to its physiological variability such as gastrointestinal transit as well as gastric residence time (GRT) of the dosage forms (Srikanth et al., 2011aSRIKANTH, M.V.; JANAKI RAM, B.; SUNIL, S.A.; SREENIVASA RAO, N.; RAMANA MURTHY, K.V. Gastroretentive drug delivery systems: novel approaches and its evaluation: a review. Int. J. Pharm. Sci. Rev. Res., v.10, n.1, p.203-216, 2011a.). Gastroretentive technology is an alternative to overcome this problem. Through this technology, the dosage form can be able to remain in the gastric region for several hours and hence significantly prolonging the gastric residence time of drugs. Prolonged gastric retention improves bioavailability, reduces drug waste, and improves solubility of drugs that are less soluble in a high pH environment. It is also suitable for local drug delivery to the stomach and proximal small intestines (Chawla et al., 2003CHAWLA, G.; GUPTA, P.; KORADIA, V.; BANSAL, A.K. Gastroretention a means to address regional variability in intestinal drug absorption. Pharm. Technol., v.27, n.7, p.50-68, 2003.).
The gastric retention of the dosage forms can be achieved by several methods such as floatation, mucoadhesion, swellable system, hydrodynamically balanced system, sedimentation, expansion modified shape systems, and so on (Streubel, Siepmann, Bodmeier, 2006STREUBEL, A.; SIEPMANN, J.; BODMEIER, R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr. Opin. Pharmacol., v.6, n.1, p.501-508, 2006.). Out of the techniques, floatation is the convenient and effective method for the gastric retention. Gastroretentive floating drug delivery systems (GRFDDS) can be buoyant in the gastric medium for prolonged period of time due to its lower bulk density compared to the gastric medium. While the system is floating on the gastric contents, the drug will be released constantly at a desired rate from the dosage form and the GRT will be enhance. Due to increase in the GRT of the dosage form, more amount of the drug can be released in the gastric region, so that improves the bioavailability of the drug and also a better control of fluctuations in the plasma drug concentrations (Mayavanshi, Gajjar, 2008MAYAVANSHI, A.V.; GAJJAR, S.S. Floating drug delivery systems to increase gastric retention of drugs: a review. Res. J. Pharm. Technol.., v.1, n.4, p.345-348, 2008. ) is achieved.
In the present investigation propranolol HCl (PPH) was selected as a model drug for the development of gastroretentive floating drug delivery systems. PPH, a type of drug known as a beta-blocker, is used in the treatment of high blood pressure, angina pectoris (chest pain, usually caused by lack of oxygen to the heart due to clogged arteries), changes in heart rhythm, prevention of migraine headache, hereditary tremors, hypertrophic subaortic stenosis (a condition related to exertional angina), and tumors of the adrenal gland (Tripathi, 2003TRIPATHI, K.D. Antihypertensive drugs, essentials of medical pharmacology. 5.ed., New Delhi: Jaypee Brothers, 2003. p.235-236.). Cmax of PPH occurs about 1 to 4 h after an oral dose and its elimination half-life is 3-4 h. Due to its short half-life, conventional tablets have to be administered several times to get an optimum effect or else controlled drug delivery is the alternative. Due to the short half-life and insolubility in intestinal fluids (acid soluble basic drug), PPH has been selected as a drug candidate for developing gastro retentive dosage form (Srikanth et al., 2011bSRIKANTH, M.V.; SREENIVASA RAO, N.; SUNIL, S.A.; SHARMA, G.S.; UHUMWANGHO, M.U.; RAMANA MURTHY, K.V. Formulation and evaluation of Gastro retentive floating drug delivery system of Ofloxacin. Drug Discov. Today., v.3, n.3, p.7-9, 2011b.).
The objective of the present research is to prepare PPH gastroretentive floating drug delivery system with suitable release retarding agents and to evaluate its buoyancy properties. In the present work different grades of polyethylene oxides (PEO) such as PEO WSR N 12 K and PEO 18 NF were used as swelling as well as release retarding polymers. The molecular weight of PEO WSR N 12 K and PEO 18 NF is 1000 000 and 4300 000 respectively. Calcium carbonate was used as gas generating agent.
MATERIAL AND METHODS
Material
Propranolol HCl was provided by Dr Reddy's Laboratories Ltd (Hyderabad, India). PEO grades, calcium carbonate and magnesium stearate were obtained as gift samples from Unichem Laboratories Ltd (Goa, India). All other reagents and chemicals were of analytical grade.
Preparation of gastroretentive floating tablets (GRFT) of propranolol HCl
All the ingredients sufficient for a batch of 100 tablets according to the formulae shown in Table I were accurately weighed and passed through the sieve #40. PPH was geometrically mixed with PEO until a homogeneous blend was achieved. Calcium carbonate was added to the above mixture and mixed for 5 min in a polybag. Blend was lubricated with presifted magnesium stearate (sieve # 60) for 3 min. The lubricated blend was evaluated for flow properties. The final blend was directly compressed into tablets on a 16-station rotary tablet punching machine (M/s. Cadmach Machinery Co. Pvt., Ltd., India) using 8 mm round plain punches at the hardness of 4-6 kg/cm2.
Determination of flow properties of lubricated blend
Angle of repose method
Angle of repose was determined by fixed funnel and free standing cone method. A funnel with the end of the stem cut perpendicular to its axis of symmetry was fixed at a given height (h) above the graph paper placed on a flat horizontal surface. The gum powder was carefully poured through the funnel until the apex of the conical pile just touched the tip of the funnel. The radius of the base ® of the pile was determined and the tangent angle of the repose (θ) was calculated by following equation (Banker, Anderson, 1991BANKER, G.S.; ANDERSON, N.R. Tablets. In: LACHMAN, L.; LIEBERMAN, H. A.; KANIG, J.L. (Eds.) The theory and practice of industrial pharmacy. 3.ed. Bombay: Verghese Publishing House, 1991. p.317.).
Tan θ = h/r
Determination of compressibility index, Hausner´s ratio
Compressibility index (C.I) and Hausner's ratio of the lubricated blend was determined by measuring the Bulk density (BD) and Tapped density (TD) of a powder.
The BD was determined by three tap method. An amount of powder equivalent to 10 g was accurately weighed, placed in a 100 mL measuring cylinder without compaction. The volume occupied was measured and the initial bulk density was calculated by the following equation (Carr, 1965CARR, R.L. Evaluation of flow properties of solid. Chem. Eng., v.72, n.3, p.163-168, 1965.).
Tapped density (TD) of a powder is the ratio of the mass of the powder to the volume occupied by the powder after a fixed number of taps. The tapped density of the powder represents its random dense packing (Carr, 1965CARR, R.L. Evaluation of flow properties of solid. Chem. Eng., v.72, n.3, p.163-168, 1965.).
An amount of powder equivalent to 10 g was accurately weighed, transferred into a 100 mL measuring cylinder and placed on to the tapped density tester (model C-TDA2, Campbell Electronics, Mumbai, India) and subjected to USP-II method i.e., 250 drops per minute with a drop height of 3 mm±10%. The volume of the powder bed was measured after each increment of 250 drops until the difference between succeeding measurements is less than 2%. The final volume was recorded and the tapped density was calculated by the following equation.
Hausner's ratio was determined by dividing the tapped density (TD) by bulk density (BD), and Carr's compressibility index (CI) was determined using following equation (Carr, 1965CARR, R.L. Evaluation of flow properties of solid. Chem. Eng., v.72, n.3, p.163-168, 1965.).
Evaluation of the tablets
The floating tablets were evaluated for physico chemical parameters like weight variation, hardness, friability & assay, buoyancy characteristics, swelling studies, in vitro dissolution studies and release mechanism studies.
Weight variation
Twenty tablets were selected at randomly and weighed individually for the determination of weight variation of tablets. The mean and standard deviation were determined (Indian Pharmacopoeia, 2007INDIAN PHARMACOPOEIA. Ghaziabad: The Indian Pharmacopoeia Commision, 2007. v.I, p.183.).
Hardness test
Five tablets were selected at random and the hardness of each tablet was measured on Monsanto hardness tester.
Friability test
The friability test was carried out in Roche Friabilator. Twenty tablets were weighed (Xo) initially and put in a rotating drum. They were subjected to 100 falls of 6 inches height (25 rpm for four minutes). After complete rotations the tablets were dedusted by using camel hairbrush and weighed (X). The percent loss in weight or friability (f) was calculated by the formula given in the following equation: (Carr, 1965CARR, R.L. Evaluation of flow properties of solid. Chem. Eng., v.72, n.3, p.163-168, 1965.)
Assay
From each batch, 10 tablets were randomly collected and powdered in a glass mortar. Accurately weighed 80mg of the powder was transferred into a 100 mL volumetric flask. The drug was extracted with 50 mL of 0.1 N HCl with vigorous shaking on a mechanical shaker for 1 h and filtered into a 100 mL volumetric flask through 0.45 μm Millipore nylon filter disc and the filtrate was made up to the mark with 0.1 N HCl. Further appropriate dilutions were made and the absorbance was measured at 289 nm against blank (0.1 N HCl).
Floating characteristics
All the formulated floating tablets were subjected to floating studies and for each batch 5 tablets were used. The in vitro buoyancy was determined by floating lag time, as per the method described by Srikanth et al. (2011c)SRIKANTH, M.V.; UHUMWANGHO, M.U.; SREENIVASA RAO, N.; SUNIL, S.A.; JANKAI RAM, B.; RAMA MURTHY, K.V. Formulation and evaluation of gastro retentive floating drug delivery system for propranolol HCl. J. Pharm. Allied Sci., v.8, n.2, p.1339-1348, 2011c.. The tablets were placed in a 900 mL beaker containing 0.1 N HCl. The time required for the tablet to rise to the surface and float was determined as floating lag time. The duration of time the dosage form constantly remained on the surface of medium was determined as total floating time (Srikanth et al., 2011cSRIKANTH, M.V.; UHUMWANGHO, M.U.; SREENIVASA RAO, N.; SUNIL, S.A.; JANKAI RAM, B.; RAMA MURTHY, K.V. Formulation and evaluation of gastro retentive floating drug delivery system for propranolol HCl. J. Pharm. Allied Sci., v.8, n.2, p.1339-1348, 2011c.).
Swelling studies
The swelling ability of the GRFT was determined in 900 mL of acidic medium (0.1 N HCl) at room temperature. Weighed tablet was immersed in the medium and it was removed periodically from the medium. After draining the free water, the tablets were measured for weight gain. Swelling index (% SI) was expressed by the following equation (Debajyoti, Amresh, 2010DEBAJYOTI, R.; AMRESH, K.P. Designing and in vitro studies of gastric floating tablets of tramadol hydrochloride. Int. J. Appl. Pharm., v.2, n.4, p.12-16, 2010.).
In vitro dissolution studies
The release of propranolol HCl from floating tablets was determined by using Dissolution Tester USP XXIII (LABINDIA, Disso 200). The dissolution test was performed using 900 mL 0.1 N HCl solution at 37 ± 0.5 °C and the paddles were rotated at 50 rpm. At appropriate time interval, 5 mL of aliquot was withdrawn from the dissolution medium and it was replaced with fresh medium to maintain the volume constant. The samples were filtered and diluted to suitable concentrations with 0.1 N HCl. The absorbance of the solutions were measured at 289 nm for propranolol HCl with a UV-Visible double beam spectrophotometer (Elico SL210, India). Cumulative percentage drug release was calculated using an equation obtained from standard curve. The dissolution experiments were done in triplicate.
Release kinetics
There are number of kinetic models are available to describe the overall release of drug from the dosage forms. The dissolution profiles of all the batches were fitted to zero order, first order, Higuchi, Hixon-Crowell (erosion) and Korsemeyer-Peppas to ascertain the kinetic modelling of drug release (Lazarus, Cooper, 1961LAZARUS, J.; COOPER, J. Absorption, testing, and clinical evaluation of oral prolonged-action drugs. J. Pharm. Sci., v.50, n.9, p.715-720, 1961.; Wagner, 1969WAGNER, J.G. Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets and capsules. J. Pharm. Sci., v.58, n.10, p.1253, 1969.; Higuchi, 1963HIGUCHI, T. Mechanism of sustained action medication: Theoretical analysis of rate release of solid drugs dispersed in solid matrices. J. Pharm. Sci., v.52, n.12, p.1145-1149, 1963.; Korsmeyer, Gurny, Peppas, 1983KORSMEYER, R.; GURNY, R.; PEPPAS, N. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm., v.15, p.25-35, 1983.; Hixson, Crowell, 1931HIXSON, A.W.; CROWELL, J.H. Dependence of reaction velocity upon surface and agitation (I) theoretical consideration. Ind. Eng. Chem., v.23, n.8, p.923-31, 1931.).
The order of drug release from matrix systems was described by using zero order kinetics or first orders kinetics. The mechanism of drug release from matrix systems was studied by using Higuchi or erosion equation. The 'n' value is obtained as a slope for different batches of matrix tablets by plotting log percent drug dissolved against log time. If the value of n = 0.45 indicates Fickian (case I) release; >0.45 but <0.89 for non-Fickian (anomalous) release; and > 0.89 indicates super case II transport. Case II generally refers to the erosion of the polymeric chain and non-Fickian diffusion refers to a combination of both diffusion and erosion mechanism from the controlled drug release tablets.
Characterization of the optimized formulation
Formulations were optimized based on the 12 h drug retarding property, minimal polymer quantity and well buoyancy properties. Optimized formulation was further characterized with Fourier transformation-infrared spectroscopy (FTIR) for interaction studies.
Fourier transformation-infrared spectroscopy (FTIR)
FTIR was used to identify if there is any drug excipient interaction. FTIR studies were performed on drug, polymer and optimized formulation. Samples were analyzed by potassium bromide pellet method in an IR spectrophotometer (Shimadzu, FTIR 8700) in the region between 3500-500 cm-1.
RESULTS AND DISCUSSION
Pre-compression studies
Direct compression technique was used for the preparation of the floating tablets. Flow characteristics of the material being compressed are important parameter and hence studies were undertaken for the evaluation of flow characteristics of the lubricated blend used in the present study. The results of flow properties of the prepared lubricated blends are shown in Table II. The angle of repose values were within the range of 30-34° and 29-34° for PEO WSR N-12 K and 18 NF based formulations respectively indicated good flow properties. The compressibility index and Hausner's ration of all the formulations were in the range of 12-14 and 1.11 to 1.15 respectively indicated that flow properties of the blends were good.
Tableting properties
The results of the weight uniformity, hardness, friability as well as drug content are presented in Table III. It was observed all the formulations of PPH prepared using selected polymers PEO WSR N-12 K and PEO 18 NF complied with compendia standard for uniformity of weight. The hardness for all the formulations was found to be in the range of 4-6 kg/cm2. The assay of the drug was >99%. The percentage weight loss in the friability test was found to be <0.5%. Thus, all the formulations were found to be of good quality fulfilling all the official requirements.
In vitro buoyancy studies
All the formulations were evaluated for in vitro buoyancy properties and results are mentioned in Table III. Floating lag time of PEO WSR N 12 K and PEO 18 NF based formulations were found to be in the range of 8-12 min and 1-6 min respectively. Total floating times of same based formulations were in the range of 4-12 h and 4-14 h respectively. From the buoyancy properties, it was observed that calcium carbonate is essential for the floating. Calcium carbonate liberates carbon dioxide, when it contact with acidic medium. The gas generated is trapped and protected within the gel formed by hydration of the PEO, thus decreasing the density of the tablet below 1 g/mL, and the tablet becomes buoyant (Srikanth et al., 2012aSRIKANTH, M.V.; SREENIVASA RAO, N.; SUNIL, S.A.; JANAKI RAM, B.; RAMANA MURTHY, K.V. A statistical experimental approach for the development of propranolol HCl gastric floating tablets and its evaluation. Acta Pharm. Sin. B, v.2, n.1, p.60-69, 2012a.). Both grades of PEO are readily swellable polymers; this made the tablets buoyant in less time. PEO WSR 18 NF based formulations floated rapidly than PEO WSR N- 12 K.
Swelling studies
The swelling studies of the floating tablets were conducted for 12 h. Swelling index of the all formulations was exhibited in the range of 74-102 %. From the swelling results, it is confirmed that swelling index is depended upon the quantity of the polymer and exposure time of the tablet to the medium. The swelling of the floating tablet has been increased gradually along with the time and it reached to saturation at particular time depends upon the quantity of the polymer it has (Swarna et al., 2012). Results showed that the swelling index increases with increased in concentration of PEO (Figure 1).
In vitro dissolution studies
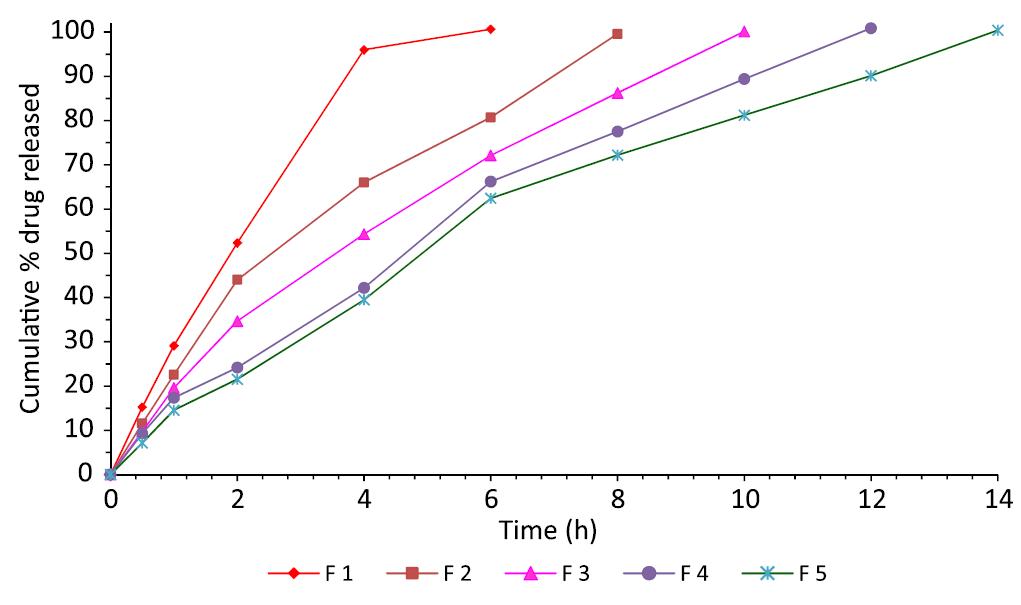
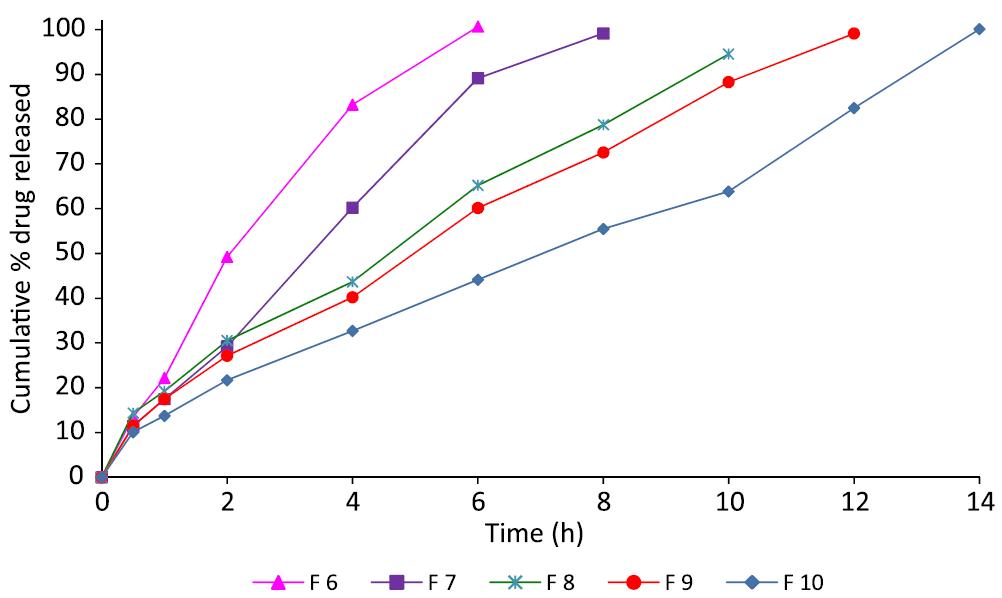
The results of dissolution studies of formulation F 1-F 5 and F 6-F 10 containing increased concentrations of PEO WSR N-12 K and PEO 18 NF respectively were shown in Figures 2 and 3, respectively. PEO WSR N-12 K based formulations (F 1-5) containing drug to polymer ratios 1:1, 1:2, 1:3, 1:3.5 and 1:4 released 29.44, 22.13, 19.74, 17.32 and 14.56% of the active drug at the end of 1h and retarded the drug up to 6, 8, 10, 12 and 14 h respectively. Another grade PEO polymer PEO 18 NF, having high molecular weight, was used for the gastric retention of the active drug. As the concentration of 18 NF increased, the drug retardation is also increased. The formulations F6, F7, F8, F9 and F10 showed maximum drug release at 6, 8, 10, 12 and 14 h respectively. The formulation F 9 showed excellent buoyancy properties and retarding properties than all other formulations.
Dissolution profile of propranolol HCl GRFT prepared by PEO 18 NF. PEO: polyethylene oxide.
From all the above results it was concluded that the drug retardation is mainly depends up on the concentration of the polymer as well as swelling property of the polymer. Molecular weight of the polymer was also played a major role in the drug retardation (Srikanth et al, 2012bSRIKANTH, M.V.; SUNIL, S.A.; JANAKI RAM, B.; RAMANA MURTHY, K.V. Design and in vitro evaluation of effervescent gastric floating drug delivery systems of propranolol HCl. Invest. Clin., v.53, n.1, p.60-70, 2012b.). It was observed that polymer with high molecular weight retarded drug efficiently than the polymer with lower molecular weight. The order of the drug retarding capacity of the polymer and their drug-polymer ratio was as follows PEO NF (1:1.5) > PEO WSR N-12 K (1:3.5). Even though positive results were obtained by F 4 formulated with PEO WSR N 12 K, F 9 formulated with PEO 18 NF was selected as an optimized formulation as the same desired results were obtained with less quantity of the polymer besides its good buoyancy properties.
Release kinetics
All the formulations made with PEO WSR N-12 K followed zero order kinetics with erosion mechanism. All PEO WSR 18 NF based formulations followed zero order rate kinetics except the F 6 formulation which followed first order rate kinetics. Formulations F 6 and F 7 followed erosion mechanism with higher regression values of 0.9970 and 0.9853 respectively and remaining all formulations followed non Fickian diffusion mechanism. From the results, it was observed that as the concentration of the polymer increases, the release mechanism changed from erosion to diffusion. (Table IV).
Optimization
Based on the low polymer concentration, good buoyancy properties and drug retardation property up to 12 h, the formulation F 9 was selected as an optimized. PEO 18 NF was selected as a suitable polymer for the development of gastro retentive floating drug delivery system of propranolol HCl.
Fourier transformation-infrared spectroscopy (FTIR)
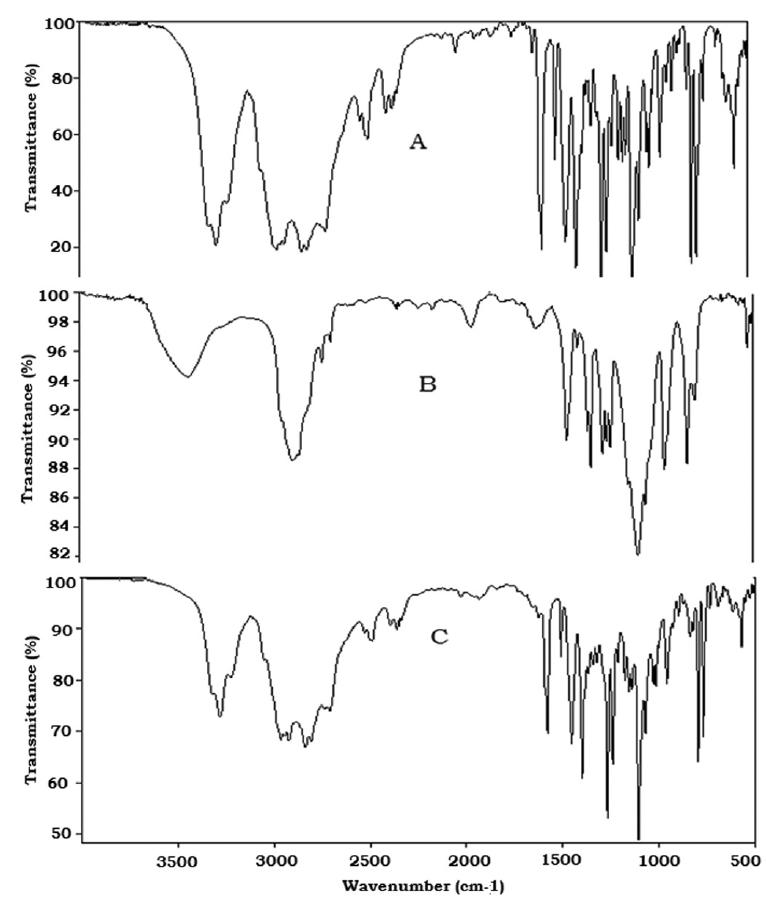
The FTIR spectrum of PPH, PEO 18 NF and F 9 was shown in the Figure 4. The drug PPH showed characteristic secondary amine -N-H stretching at 3278 cm-1, C-H stretching at 2959 cm-1, Aryl C=C stretching at 1584 cm 1, Aryl 0-CH2 asymmetric stretching at 1236 cm-1 , Aryl O-CH2 symmetric stretching at 1034 cm-1 and the peak at 794 cm-1 due to α-substituted naphathalene (Venkata Srikanth et al., 2012VENKATA SRIKANTH,M.; NALI, S.R.; SONGA, A.S.; BATTU, J.R.; KOLAPALLI, V.R.M. Statistical Optimization of a Novel Excipient (CMEC) based gastroretentive floating tablets of propranolol HCl and it's in vivo buoyancy characterization in healthy human volunteers. DARU. J. Pharm. Sci., v.20, n.21, p.1-12, 2012.) (Figure 4). The FTIR spectrum of PEO 18 NF showed the characteristic alcoholic -OH stretching at 3431 cm-1, -C-O-C asymmetric stretching at 1251 cm-1 and -C-O-C symmetric stretching at 1048 cm-1.
Optimized PEO 18 NF based formulation (F 9) showed all the characteristic peaks of PPH with minor shifts in its FTIR spectrum. This spectrum showed secondary amine -N-H stretching at 3274 cm-1, C-H stretching at 2952 cm-1 , Aryl C=C stretching at 1577 cm-1 , Aryl 0-CH2 asymmetric stretching at 1241 cm-1 , Aryl 0-CH2 symmetric stretching at 1028 cm-1 and the peak at 797 cm-1 due to α-substituted naphathalene (Figure 4). The results showed no significant change in the spectrum, indicating no interaction between the polymer and drug.
CONCLUSION
The gastroretentive floating drug delivery is a promising approach to achieve in vitro buoyancy by using hydrophilic polymers of polyethylene oxide grades such as PEO WSR N 12 K, PEO 18 NF and gas-generating agent calcium carbonate. The results concluded that PEO WSR N-12 K and PEO 18 NF based formulations at the drug: polymer ratio 1:4 and 1:1.5 respectively retarded the drug release promptly than all other formulations. High molecular weight PEO grade exhibited higher retarding property and good buoyancy properties. The optimized formulation gives the best result in terms of the required floating lag time and total floating time. Optimized formulation (F 9) when characterized with FTIR studies showed no interactions between drug and polymer. Hence, it can be concluded that, PEO is a suitable polymer for the development of gastroretentive floating drug delivery systems.
ACKNOWLEDGEMENT
The author M.V.Srikanth is thankful to Unichem Laboratories, Goa for providing the gift samples. One of the authors, M.V.Srikanth, is thankful to Murali Mohan Babu GV, Srinivasa Rao Nali, Sunil Songa and Janaki Ram for providing valuable information to carry out the research work.
REFERENCES
- BANKER, G.S.; ANDERSON, N.R. Tablets. In: LACHMAN, L.; LIEBERMAN, H. A.; KANIG, J.L. (Eds.) The theory and practice of industrial pharmacy. 3.ed. Bombay: Verghese Publishing House, 1991. p.317.
- CARR, R.L. Evaluation of flow properties of solid. Chem. Eng., v.72, n.3, p.163-168, 1965.
- CHAWLA, G.; GUPTA, P.; KORADIA, V.; BANSAL, A.K. Gastroretention a means to address regional variability in intestinal drug absorption. Pharm. Technol., v.27, n.7, p.50-68, 2003.
- DEBAJYOTI, R.; AMRESH, K.P. Designing and in vitro studies of gastric floating tablets of tramadol hydrochloride. Int. J. Appl. Pharm., v.2, n.4, p.12-16, 2010.
- HIGUCHI, T. Mechanism of sustained action medication: Theoretical analysis of rate release of solid drugs dispersed in solid matrices. J. Pharm. Sci., v.52, n.12, p.1145-1149, 1963.
- HIXSON, A.W.; CROWELL, J.H. Dependence of reaction velocity upon surface and agitation (I) theoretical consideration. Ind. Eng. Chem., v.23, n.8, p.923-31, 1931.
- INDIAN PHARMACOPOEIA. Ghaziabad: The Indian Pharmacopoeia Commision, 2007. v.I, p.183.
- KORSMEYER, R.; GURNY, R.; PEPPAS, N. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm., v.15, p.25-35, 1983.
- LAZARUS, J.; COOPER, J. Absorption, testing, and clinical evaluation of oral prolonged-action drugs. J. Pharm. Sci., v.50, n.9, p.715-720, 1961.
- MAYAVANSHI, A.V.; GAJJAR, S.S. Floating drug delivery systems to increase gastric retention of drugs: a review. Res. J. Pharm. Technol.., v.1, n.4, p.345-348, 2008.
- SRIKANTH, M.V.; JANAKI RAM, B.; SUNIL, S.A.; SREENIVASA RAO, N.; RAMANA MURTHY, K.V. Gastroretentive drug delivery systems: novel approaches and its evaluation: a review. Int. J. Pharm. Sci. Rev. Res., v.10, n.1, p.203-216, 2011a.
- SRIKANTH, M.V.; SREENIVASA RAO, N.; SUNIL, S.A.; JANAKI RAM, B.; RAMANA MURTHY, K.V. A statistical experimental approach for the development of propranolol HCl gastric floating tablets and its evaluation. Acta Pharm. Sin. B, v.2, n.1, p.60-69, 2012a.
- SRIKANTH, M.V.; SREENIVASA RAO, N.; SUNIL, S.A.; SHARMA, G.S.; UHUMWANGHO, M.U.; RAMANA MURTHY, K.V. Formulation and evaluation of Gastro retentive floating drug delivery system of Ofloxacin. Drug Discov. Today., v.3, n.3, p.7-9, 2011b.
- SRIKANTH, M.V.; SUNIL, S.A.; JANAKI RAM, B.; RAMANA MURTHY, K.V. Design and in vitro evaluation of effervescent gastric floating drug delivery systems of propranolol HCl. Invest. Clin., v.53, n.1, p.60-70, 2012b.
- SRIKANTH, M.V.; UHUMWANGHO, M.U.; SREENIVASA RAO, N.; SUNIL, S.A.; JANKAI RAM, B.; RAMA MURTHY, K.V. Formulation and evaluation of gastro retentive floating drug delivery system for propranolol HCl. J. Pharm. Allied Sci., v.8, n.2, p.1339-1348, 2011c.
- STREUBEL, A.; SIEPMANN, J.; BODMEIER, R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr. Opin. Pharmacol., v.6, n.1, p.501-508, 2006.
- SWARNA KAMALA, C.H.; SRINIVAS REDDY, K.; HADASSAH, M.; HEPSIBHA, E METILDA.; SRIDEVI, S. Formulation and evaluation of gastroretentive floating tablets of gabapentin using effervescent technology. Int. J. Pharm. Biomed. Res., v.3, n.4, p.202-208, 2012.
- TRIPATHI, K.D. Antihypertensive drugs, essentials of medical pharmacology. 5.ed., New Delhi: Jaypee Brothers, 2003. p.235-236.
- VENKATA SRIKANTH,M.; NALI, S.R.; SONGA, A.S.; BATTU, J.R.; KOLAPALLI, V.R.M. Statistical Optimization of a Novel Excipient (CMEC) based gastroretentive floating tablets of propranolol HCl and it's in vivo buoyancy characterization in healthy human volunteers. DARU. J. Pharm. Sci., v.20, n.21, p.1-12, 2012.
- WAGNER, J.G. Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets and capsules. J. Pharm. Sci., v.58, n.10, p.1253, 1969.
Publication Dates
-
Publication in this collection
Apr-Jun 2014
History
-
Received
13 Nov 2013 -
Accepted
23 Jan 2013