Abstracts
A simple, precise, sensitive, rapid, specific and economical spectrophotometric method was developed to determine methyldopa (MTD) content in bulk and pharmaceutical dosage formulations. The proposed method was based on the formation of a colored product from the nitrosation reaction of MTD with sodium nitrite in an acid medium. The resultant nitroso derivative species reacts further with sodium hydroxide and is converted it into a more stable compound. This yellow nitrosation product exhibited an absorption maximum at 430 nm. Beer's Law was obeyed in a concentration range of 6.37 to 82.81 μg mL-1 MTD with an excellent coefficient of determination (R 2 = 0.9998). No interference was observed from common excipients in formulations. The results showed the method to be simple, accurate and readily applied for the determination of MTD in pure form and in pharmaceutical preparations. The analytical results obtained for these products using the proposed method are in agreement with those of the Brazilian Pharmacopoeia procedure at a 95% confidence level.
Methyldopa/spectrophotometric determination; Pharmaceutical formulations/quality control; Spectrophotometry/qualitative analysis
Desenvolveu-se método espectrofotométrico simples, preciso, sensível, rápido, específico e econômico para a determinação do teor de metildopa (MTD) em matéria-prima e em formulações farmacêuticas. O método proposto baseia-se na formação de um produto colorido resultante da reação de nitrosação da MTD com nitrito de sódio em meio ácido. A espécie resultante (nitroso derivado) reage com hidróxido de sódio e é convertida a um composto mais estável de cor amarela. Este produto exibiu máximo de absorção a 430 nm. A lei de Beer foi obedecida na faixa de concentração de 6,37 a 82,81 μg mL-1 de MTD com excelente coeficiente de determinação (R 2 = 0,9998). Não se observou interferência de excipientes comumente encontrados em formulações farmacêuticas comerciais. Os resultados demonstraram que o método proposto apresenta simplicidade, excelentes precisão e exatidão e pode ser aplicado para a determinação de MTD na sua forma pura e em preparações farmacêuticas. Os resultados analíticos obtidos pelo método proposto estão de acordo com aqueles obtidos pelo método oficial descrito na Farmacopéia Brasileira, a um nível de confiança de 95%.
Metildopa/determinação espectrofotométrica; Formulações farmacêuticas/controle de qualidade; Espectrofotometria/análise qualitativa
INTRODUCTION
Methyldopa (MTD), chemically known as α-methyl-3,4-dihydroxyphenylalanine (Figure 1), is a catechol derivative (catecholamine) widely used as an antihypertensive agent. MTD is a centrally acting alpha2-adrenoreceptor agonist, which reduces sympathetic tone and produces a fall in blood pressure (Hoffman, 2005HOFFMAN, B.B. Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists. In: HARDMAN, J.G.; LIMBIRD, L.E.; MOLINOFF, P.B.; RUDDON, R.W.; GILMAN, A.G. (Eds.). Goodman & Gilman's the pharmacological basis of therapeutics. 10.ed. New York: Mac Graw-Hill, 2005 chap.10, p.163-165. ).
Several types of analytical procedures have been employed for the analysis of catechol derivatives in pharmaceutical formulations and/or biological specimens. These procedures include: titrimetric determination (Walash, Abououf, Salem, 1982WALASH, M.I.; ABOUOUF, A.; SALEM, F.B. Spectrophotometric and titrimetric determination of certain adrenergic drugs, using organic brominating agents. J. Assoc. Off. Anal. Chem., v.65, n.6, p.1445-1451, 1982.; Mohamed, Salem, 1984MOHAMED, W.I.; SALEM, F.B. Spectrophotometric and titrimetric determination of certain adrenergic drugs. Anal. Lett., v.17, n.3, p.191-203, 1984.; Amin, 1986AMIN, D. Titrimetric determination of catecholamines and related-compounds via bromine oxidation and substitution. Analyst, v.111, n.2, p.255-257, 1986.; Salem, 1987SALEM, F.B. Spectrophotometric and titrimetric determination of catecholamines. Talanta, v.34, n.9, p.810-812, 1987.; Salem, 1993aSALEM, F.B. Titrimetric and spectrophotometric determination of catecholamines. Anal. Lett., v.26, n.9, p.1959-1966, 1993a.; Farmacopeia Brasileira, 2010FARMACOPEIA Brasileira. 5.ed. v.2. Brasília: ANVISA, 2010. p.1143-1145.; USP, 2013), gas chromatography (Lee, Hong-You, Fowlie, 1989LEE, H.B.; HONG-YOU, R.L.; FOWLIE, P.J.A. Chemical derivatization analysis of phenols: determination of chlorinated phenolics in pulp and paper effluents. J. Assoc. Off. Anal. Chem., v.72, n.6, p.979-984, 1989.; Sharma et al., 1996SHARMA, C.; MOHANTY, S.; KUMAR, S.; RAO, N.J. Gas chromatographic analysis of chlorophenolic, resin and fatty acids in chlorination and caustic extraction stage effluent from kahi-grass. Analyst, v.121, n.12, p.1963-1967, 1996.), kinetic methods (Martinez-Lozano et al., 1991MARTINEZ-LOZANO, C.; PÉREZ-RUIZ, T.; TOMAS, V.; VAL, O. Determination of epinephrine, norepinephrine, dopamine and L-dopa in pharmaceuticals by a photokinetic method. Analyst, v.116, n.8, p.857-859, 1991.), fluorimetry (Salem, 1993bSALEM, F.B. Spectrophotometric and fluorometric determination of catecholamines. Anal. Lett., v.26, n.2, p.281-294, 1993b.), chemiluminescence (Nozaki et al., 1996NOZAKI, O.; IWAEDA, T.; KATO, Y. Amines for detection of dopamine by generation of hydrogen peroxide and peroxyoxalate chemiluminescence. J. Biolumin. Chemilumin., v.11, n.6, p.309-314, 1996.; Nozaki et al., 1999NOZAKI, O.; IWAEDA, T.; MORIYAMA, H.; KATO, Y. Chemiluminescent detection of catecholamines by generation of hydrogen peroxide with imidazole. Luminescence, v.14, n.3, p.123-127, 1999.), amperometry (Garrido, Lima, Delerue-Mattos, 1997GARRIDO, M.E.; LIMA, J.L.F.C.; DELERUE-MATTOS, C. Flow injection amperometric determination of L-dopa, epinephrine or dopamine in pharmaceutical preparations. J. Pharm. Biomed. Anal., v.15, n.6, p.845-849, 1997.), high-performance liquid chromatography (Tsuchiya et al., 1997TSUCHIYA, M.; SATO, M.; KATO, H.; OKUBO, T.; JUNEJA, L.R.; KIM, M. Simultaneous determination of catechins in human saliva by high-performance liquid chromatography. J. Chromatogr. B, v.703, n.1-2, p.253-258, 1997.; Parsons, Kerr, Weiss, 1998PARSONS, L.R.; KERR, T.M.; WEISS, F. Simple microbore high-performance liquid chromatographic method for the determination of dopamine and cocaine from a single in vivo brain microdialysis sample. J. Chromatogr. B, v.709, n.1, p.35-39, 1998.), voltammetric determination (Kozminski et al., 1998KOZMINSKI, K.D.; GUTMAN, D.A.; DAVILA, V.; SULZER, D.; EWING, A.G. Voltammetric and pharmacological characterization of dopamine release from single exocytotic events at rat pheochromocytoma (PC12) cells. Anal. Chem., v.70, n.15, p.3123-3130, 1998.) and diffuse reflectance spectroscopy (Ribeiro, Pezza, Pezza, 2006RIBEIRO, P.R.S.; PEZZA, L.; PEZZA, H.R. Determination of methyldopa in pharmaceutical formulations by combined spot test-diffuse reflectance spectroscopy. J. Braz. Chem. Soc., v.17, n.4, p.674-679, 2006.). Some of these methods are complex for routine analysis and either require expensive or sophisticated instruments or involve procedures entailing rigorous control of the experimental conditions. Most of the titrimetric methods reported were indirect titrations and based on reduction reactions, which suffer interferences from unsaturated organic compounds. The official method reported in the Brazilian Pharmacopoeia (Farmacopeia brasileira, 2010FARMACOPEIA Brasileira. 5.ed. v.2. Brasília: ANVISA, 2010. p.1143-1145.) and in USP (2013) describes a nonaqueous titration for the assay of MTD.
Spectrophotometric methods have been proposed for the determination of catecholamines, such as MTD. A differential UV spectrophotometric procedure has also been used for the determination of MTD in pharmaceutical formulations in the presence of germanium dioxide at 292 nm (Davidson, 1984DAVIDSON, A.G. Difference spectrophotometric assay of 1,2-diphenolic drugs in pharmaceutical formulations. 2. Germanium dioxide reagent. J. Pharm. Sci., v.73, n.11, p.1582-1584, 1984.). MTD has been determined in the visible region after reaction with 2,3,5-triphenyltetrazolium chloride (El-Rabbat, Omar, 1978EL-RABBAT, N.A.; OMAR, N.M. Colorimetric determination of catecholamines by 2,3,5-triphenyltetrazolium chloride. J. Pharm. Sci., v.67, n.6, p.779-781, 1978.), potassium bromate (Mohamed, Salem, 1984MOHAMED, W.I.; SALEM, F.B. Spectrophotometric and titrimetric determination of certain adrenergic drugs. Anal. Lett., v.17, n.3, p.191-203, 1984.), vanillin (Salem, 1985SALEM, F.B. Colorimetric determination of certain sympathomimetic amines. Anal. Lett., v.18, n.9, p.1063-1075, 1985.), p-dimethylaminocinnamaldehyde (Walash, Abououf, Salem, 1985WALASH, M.I.; ABOUOUF, A.; SALEM, F.B. Colorimetric determination of sympathomimetic amines methyldopa and noradrenaline. J. Assoc. Off. Anal. Chem., v.68, n.1, p.91-95, 1985.), molybdophosphoric acid in sulphuric acid medium (Issopoulos, 1989ISSOPOULOS, P.B. Spectrophotometric determination of microquantities of carbidopa, levodopa and α-methyldopa using molybdophosphoric acid. Pharm. Acta Helv., v.64, n.3, p.82-85, 1989.), Fe(III), o-phenanthroline (Issopoulos, 1990ISSOPOULOS, P.B. High-sensitivity spectrophotometric determination of trace amounts of levodopa, carbidopa and α-methyldopa. Fresen. J. Anal. Chem., v.336, n.2, p.124-128, 1990.), ferric chloride (Zivanovic, Vasiljevic, Radulovic, 1991ZIVANOVIC, L.; VASILJEVIC, S.; RADULOVIC, D. Colorimetric assay of methyldopa bulk drug and tablets as Fe(III) complex. Boll. Chim. Farm., v.130, n.5, p.162-165, 1991.), neotetrazolium chloride (Issopoulos, Economou, 1993ISSOPOULOS, P.B.; ECONOMOU, P.T. A new high-sensitivity spectrophotometric method for the determination of microconcentrations of α-methyldopa. Farmaco, v.48, n.1, p.127-135, 1993.), metaperiodate (Nevado, Gallego, Laguna, 1995NEVADO, J.J.B.; GALLEGO, J.M.L.; LAGUNA, P.B. Spectrophotometric determination of dopamine and methyldopa with metaperiodate by flow injection analysis. Fresen. J. Anal. Chem., v.353, n.2, p.221-223, 1995.), barbituric acid (Aman et al., 1998AMAN, T.; KHAN, I.U.; ASLAM, N.; AHMED, I. Spectrophotometric determination of methyldopa in pure and pharmaceutical preparations. Anal. Lett., v.31, n.6, p.1007-1020, 1998.), isoniazid in the presence of N-bromosuccinimide (Nagaraja et al., 1998NAGARAJA, P.; MURTHY, K.C.S.; RANGAPPA, K.S.; GOWDA, N.M.M. Spectrophotometric methods for the determination of certain catecholamine derivatives in pharmaceutical preparations. Talanta, v.46, n.1, p.39-44, 1998.), polyphenol oxidase enzyme (Vieira, Fatibello Filho, 1998VIEIRA, I.C.; FATIBELLO FILHO, O. Spectrophotometric determination of methyldopa and dopamine in pharmaceutical formulations using a crude extract of sweet potato root (Ipomoea batatas (L.) lam.) as enzymatic source. Talanta, v.46, n.4, p.559-564, 1998.), diazotized sulfanilamide in the presence of molybdate (Nagaraja, Vasantha, Sunitha, 2001aNAGARAJA, P.; VASANTHA, R.A.; SUNITHA, K.R. A sensitive and selective spectrophotometric estimation of catechol derivatives in pharmaceutical preparations. Talanta, v.55, n.6, p.1039-1046, 2001a.), semicarbazide hydrochloride in the presence of potassium persulfate (Nagaraja et al.,2001bNAGARAJA, P.; VASANTHA, R.A.; MURTHY, K.C.S.; RANGAPPA, K.S. Spectrophotometric determination of some aromatic vic-diols in the pharmaceutical formulations. Chem. Anal. (Warsaw), v.46, n.4, p.569-577, 2001b.), ammonium molybdate (Ribeiro et al., 2005aRIBEIRO, P.R.S.; PEZZA, L.; PEZZA, H.R. Spectrophotometric determination of methyldopa in pharmaceutical formulations. Eclét. Quím., v.30, n.3, p.23-28, 2005b.; Ribeiro, Pezza, Pezza, 2005bRIBEIRO, P.R.S.; GOMES NETO, J.A.; PEZZA, L.; PEZZA, H.R. Flow-injection spectrophotometric determination of methyldopa in pharmaceutical formulations. Talanta, v.67, n.1, p.240-244, 2005a.), 2,2-diphenyl-picrylhydrazyl (Matos, Silva, Ribeiro, 2012MATOS, O.R.; SILVA, F.C.; RIBEIRO, P.R.S. A new, simple and sensitive analytical method for determination of methyldopa in pharmaceutical formulations using the 2,2-diphenyil-picrylhydrazyl. Lat. Am. J. Pharm., v.31, n.2, p.190-194, 2012.) and ferric chloride/nitroso-R-salt (Al Abachi, Hadi, 2013AL ABACHI, M.Q.; HADI, H. New, simple and validated kinetics spectrophotometric method for determination of methyldopa in its pharmaceutical formulations. Int. J. Recent Sci. Res., v.4, n.4, p.320-324, 2013.). However, most of these methods suffer from several disadvantages, such as long-waiting times or a heating step for reaction development, instability of the colored species, complex procedure, requirement for nonaqueous media, poor detection limit, or lack of previous application to pharmaceutical formulations.
The present study reports a simple, precise, sensitive, rapid, specific and economical spectrophotometric method developed to determine the (MTD) content in bulk and pharmaceutical dosage formulations. The proposed method was based on the formation of a nitrous derivative of MTD. The yellow-colored product had maximum absorption at 430 nm. The proposed method has none of the disadvantages of interference from the excipients normally found with MTD in tablet dosage formulations and involves no extraction or heating steps. The method was used to determine MTD in pharmaceutical formulations. The results obtained by applying the proposed method showed relatively good agreement with those obtained using the standard procedure reported in the Brazilian Pharmacopoeia (Farmacapeia Brasileira, 2010) at a 95% confidence level.
MATERIAL AND METHODS
Apparatus
A Femto Model CIRRUS 80ST spectrophotometer with 1 cm matched silica cells was used for all absorbance measurements. Volume measurements were made with a plunger-operated pipetter (100-1000 μL) and Metrohm model 665 automatic burettes. All experiments were performed in a thermostatically-controlled room (25±1) ºC. A Hanna Model Pack pH 21 digital pH-meter, calibrated with standard buffer solutions, was used for pH measurements.
Chemicals and reagents
All of the reagents used were of analytical reagent grade. Deionized water was used throughout the experiments.
A stock solution (MTDS - 318.4 μg ml-1) of MTD standard (Pharma Nostra - São Paulo, Brazil, purity grade > 99.2%) was prepared daily by dissolving 15.9 mg of the reference substance in water and diluting to the mark in a 50 ml volumetric flask. Working standard solutions were obtained by appropriate dilution of this stock solution with the same solvent and were standardized using the standard procedure reported in the Brazilian Pharmacopoeia (Farmacopeia Brasileira, 2010FARMACOPEIA Brasileira. 5.ed. v.2. Brasília: ANVISA, 2010. p.1143-1145.).
Aqueous solutions of sodium nitrite [0.6 mol L-1] (Merck), sodium hydroxide [5.0 x 10-2 mol L-1] (Merck), and hydrochloric acid [1.6 x 10-2 mol L-1] (Merck) were prepared and used.
Pharmaceutical formulations (tablets) of four commercial brands were analyzed. These tablets were purchased from local drugstores and all were tested prior to their respective listed expiry dates. All pharmaceuticals studied were package labeled to contain 250 or 500 mg of MTD per tablet.
The excipients used in the interference study were of pharmaceutical grade. Thus, sucrose, glucose, talc, fructose, lactose, polyethylene glycol, microcrystalline cellulose, croscarmellose sodium, starch, polyvinylpyrrolidone and magnesium stearate were purchased from Sigma (St. Louis, MO, p. a.).
Methodology
Determination of wavelength of maximum absorption
A reference substance stock solution (MTDS) (50.94 μg mL-1 of MTD) was transferred into a 5 mL calibrated flask. In this flask, 1.0 mL of hydrochloric acid (1.6 x 10-2 mol L-1) and 1.0 mL of sodium nitrite (0.6 mol L-1) were added and kept aside for 5 min; then 1.0 mL of sodium hydroxide (5.0 x 10-2 mol L-1) was added to the solution. After five minutes, the volume was then made up with deionized water. A spectroscopic scan (400-800 nm) was carried out with this solution to determine the λmax for the detection of MTD, against the corresponding reagent blank.
Linearity and range
Aliquots of reference substance stock solution ranging from 6.37 to 82.81 μg mL-1 MTD were transferred to a series of 5.0 mL volumetric flasks. To each flask, 1.0 mL of hydrochloric acid (1.6 x 10-2 mol L-1)and 1.0 mL of sodium nitrite (0.6 mol L-1) were added and kept aside for 5 min; then 1.0 mL of sodium hydroxide (5.0 x 10-2 mol L-1) was added to the solution. After five minutes, the volume was then made up with deionized water. The absorbance was measured at 430 nm against the corresponding reagent blank. Calibration graphs were prepared by plotting absorbance against drug concentration. These graphs or the corresponding linear least squares equations were used to convert absorbance into MTD concentration, for any given analyzed sample.
Stoichiometric relationship
Job's method (Foster, 1969FOSTER, R. Organic charge transfer complexes. London: Academic Press, 1969. 136 p.) was applied by placing 0.50 to 2.25 mL of 318.4 μg mL-1 MTD solution into a series of 10.0 mL volume flasks; this was followed by placing 2.25 to 0.50 mL of 1.5 × 10-3 mol L-1 sodium nitrite, and 1.0 mL of hydrochloric acid (1.6 x 10-2 mol L-1) into the flasks which were kept aside for 5 min; then 1.0 mL of sodium hydroxide (5.0 x 10-2 mol L-1) was added to the solution. After five minutes, the volume was then made up with deionized water. The absorbance was measured at 430 nm against the corresponding reagent blank. The results were plotted as shown in Figure 5, indicating the existence of 1:1 (MTD: sodium nitrite).
Stability
The stability of the product formed under the above-mentioned optimum conditions was investigated. For this study, a reference substance stock solution (50.94 μg mL-1 of MTD) was transferred into a 5 mL calibrated flask and analyzed according to the recommended procedure for the calibration curve. This solution was kept for 24 h at room temperature and subsequently analyzed to test for short-term stability.
Intra-day precision (repeatability) and inter-day precision (intermediate precision) studies
MTD tablets were finely powdered and a sample stock solution (MTDP) of 318.4 μg mL-1 was prepared following the same dilution pattern of MTDS. Three different aliquots of MTDP were then diluted to obtain the concentrations of 31.84, 44.58 and 57.31 μg mL-1 and analyzed according to the recommended procedure for the calibration curve. The quantity per tablet was calculated from the standard calibration graph. This procedure was repeated on the two proceeding days.
Specificity in the presence of excipients
To assess the usefulness of the proposed method, the effect of the common components (additives, adjuvants and excipients) which often accompany MTD in tablet dosage formulations (silicon dioxide, sodium laureth sulfate, mannitol, sodium metabisulfite, polyethylene glycol, citric acid, lactose, microcrystalline cellulose, croscarmellose sodium, talc, starch and magnesium stearate) were investigated using the developed method. The ratios of the concentrations of MTD (44.58 μg mL-1) to the excipient substances were fixed at 1.0 and 10.0.
Procedure for the assay of MTD in pharmaceutical samples
Four market brands of MTD tablets purchased from local drugstores were randomly selected and analyzed using the newly developed and validated method. For the determination of MTD in pharmaceutical samples, the average tablet weight was calculated from the contents of 20 tablets that were finely powdered and weighed. A portion of this powder, equivalent to ca. 15.8 mg of MTD was accurately weighed and dissolved in 25 ml of deionized water by shaking for 15 min in a mechanical shaker. The resulting mixture was filtered and transferred into 50.0 mL graduated flasks, and the volume completed with deionized water. Aliquots from this solution, containing the equivalent of 44.58 μg mL-1 of MTD, were transferred into 5.0 mL graduated flasks and analyzed according to the recommended procedure for the calibration curve. The quantity per tablet was calculated from the standard calibration graph.
Accuracy/recovery studies
To study the accuracy of the proposed method and determine the interference from the excipients used in the dosage forms, recovery experiments were carried out by the standard addition method. This study was performed by addition of known amounts (17.19; 19.10; 21.01 and 22.92 μg mL-1, corresponding to levels of 90; 100; 110 and 120%, respectively) of the standard substance (pure drug - MTD) to a known concentration of the previously analyzed commercial tablets (samples A, B, C and D). The resulting mixtures were analyzed according to the recommended procedure for the calibration curve. The recovery of drug was calculated by comparing the concentration obtained from the spiked mixtures with those of the pure drug.
RESULTS AND DISCUSSION
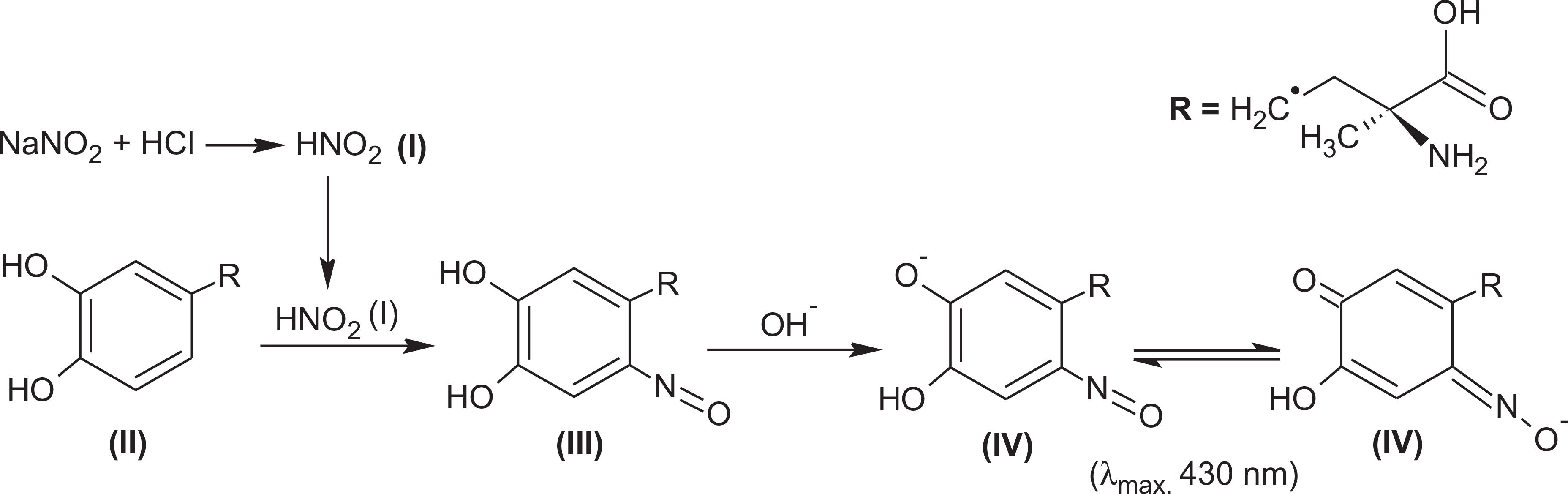
Sodium nitrite and hydrochloric acid react to produce nitrous acid (Figure 2, compound I). Owing to the presence of phenolic hydroxy groups in compounds (such as MTD - Figure 1), the reaction of this acid leads to the formation of nitroso derivatives (yellow-colored products - Figure 2, compound III) which are unstable (Suarez, Vieira, Fatibello Filho, 2005SUAREZ, W.T.; VIEIRA, H.J.; FATIBELLO FILHO, O. Determinação de paracetamol em produtos farmacêuticos empregando um sistema de análise por injeção em fluxo com geração de ácido nitroso. Eclét. Quím., v.30, n.1, p.23-28, 2005.). The products formed in acid media are unstable but can be stabilized by the interaction with an alkaline reagent to form the highly stable compounds (Figure 2, compound IV). This indicates that the alkalinisation of the medium gives rise to the formation of a bathochromic shift, together with a hyperchromic effect (Belal et al., 1979BELAL, S.F.; EL SAYED, M.A.H.; EL WALILY, A.; ABDINE, H. Spectrophotometric determination of acetaminophen and salicylamide through nitrosation and subsequent chelation. Analyst, v.104, n.1, p.919-927, 1979.).
The absorption spectrum of the colored product shows maximum absorption at 430 nm (Figure 3). Thus, the probable mechanism for the reaction between MTD (catecholamine) and the sodium nitrite in an acid medium involves the formation of nitroso derivatives as shown in FigureFemto Model CIRRUS 2. This type of formation is probably due to the contribution of an extra pair of unshared electrons in the interaction with the aromatic nucleus (Daveu et al., 1997DAVEU, C.; SERVY, C.; DENDANE, M.; MARIN, P.; DUCROCQ, C. Oxidation and nitration of catecholamines by nitrogen oxides derived from nitric oxide. Nitric Oxide-Biol. Chem., v.1, n.3, p.234-243, 1997.; d'Ischia, Costantini, 1995D'ISCHIA, M.; COSTANTINI, C. Nitric oxide-induced nitration of catecholamine neurotransmitters: a key to neuronal degeneration? Bioorg. Med. Chem., v.3, n.7, p.923-927, 1995.).
Method development and optimization
The effect of different volumes of the reagent used was investigated to obtain optimum results for the determination of MTD. The optimum conditions were established based on the development of maximum color intensity and stability upon variation of parameters affecting the nitrosation reaction of MTD by the sodium nitrite in an acid medium.
Using different volumes of HCl and of NaOH, it was found that maximum color intensity and stability were obtained by developing the reactions in 1.0 mL of hydrochloric acid (1.6 ×10-2 mol L-1) and 1.0 mL of sodium hydroxide (5.0 ×10-2 mol L-1), as described in the recommended procedure. The adopted concentration of sodium nitrite (0.6 mol L-1) was found to be sufficient for providing maximum and repeatable color intensity, but when the concentration of reagent fell below this concentration the absorbance decreased while at higher concentrations the absorbance was not increased.
The order of addition of the reactants recommended in the general procedure produced quantitative results. Any other order was found to produce deviant results and reduced color intensity.
Linearity and range
The analytical curve (Figure 4) was obtained by the method of least squares from seven points, each of which was the average of three determinations. The proposed analytical method was validated by evaluating linear dynamic range, precision, limit of detection (LOD), and limit of quantitation (LOQ). Under the experimental described, Beer's law was obeyed in the concentration range from 6.37 to 82.81 μg mL-1 MTD with an excellent coefficient of determination (R 2 = 0.9998). The absorbance values for this concentration range were adjusted by the equation: A=5.92×10-3+20.79×10-3 °C, where C is the concentration of MTD in μg mL-1 (molar absorptivity (ε): 4.423 ×103 mol-1 cm-1). The LOD (3.SDblank/slope of curve) and LOQ (10.SDblank/slope of curve) were 2.52 ×10-2 μg mL-1 and 7.68 ×10-2 μg mL-1 of MTD, respectively (ICH, 2005INTERNATIONAL CONFERENCE ON HARMONIZATION. ICH Q2 (R1): validation of analytical procedures-text and methodology. Geneva: ICH, 2005. 15 p.).
Determination of stoichiometric relationships
Job's method of continuous variations (Foster, 1969FOSTER, R. Organic charge transfer complexes. London: Academic Press, 1969. 136 p.) was employed for the determination of stoichiometric relationships: equimolar concentrations of MTD and sodium nitrite was found to be 1:1 (Figure 5).
Intra-day and inter-day precision
Assay precision was defined by determining intra-day and inter-day precision, expressed as relative standard deviation (RSD). The inter-day variation was evaluated over 3 days. The intra-day and inter-day precision studies (Table I) of the developed method confirmed adequate sample stability and method reliability, where all the RSDs were < 2.0%.
Intra-day and inter-day precision determined for three different concentrations of MTD (n = 3)
Stability
The stability of the product formed under the above-mentioned optimum conditions was investigated. The data given in Table II show that full color development is immediate at room temperature (25 °C) and the values of absorbance of the product formed were found to remain unchanged after standing for 24 hours at room temperature. This product was stable in the temperature range of 20 60 oC. However, a temperature of 25 °C was chosen for the absorbance measurements.
Specificity in the presence of excipients
Since the aim of this study was to determine MTD in pharmaceuticals, the effects of the most commonly used excipients were carefully examined. The excipients studied were silicon dioxide, sodium laureth sulfate, mannitol, sodium metabisulfite, polyethylene glycol, citric acid, lactose, microcrystalline cellulose, croscarmellose sodium, talc, starch and magnesium stearate. For this study, solutions containing MTD and each of the excipients taken separately at concentrations greater than or equal to 10 times that of MTD, were stirred with water in a magnetic mixer for 15 minutes, diluted, filtered when necessary, and analyzed under the same conditions described in the preparation of analytical curve.
The effect of each excipient was considered interference when the absorbance signal showed an error greater than or equal to 3.0% in the determination of the drug. The percentage of MTD found in these solutions ranged from 99.0 to 100.5%, with coefficient of variation values of less than 2.0% for three replicates, indicating that no interferences were observed under the studied conditions.
Content of MTD in marketed brands
The samples were prepared using the developed method. The proposed method was successfully applied for the determination of MTD in four tablet formulations. The results given in Table III compared favorably with the official method (Farmacopeia Brasileira, 2010FARMACOPEIA Brasileira. 5.ed. v.2. Brasília: ANVISA, 2010. p.1143-1145.), at 95% confidence level, confirming the applicability of the proposed method for the determination of MTD in pharmaceutical dosage forms (tablets).
The results were subjected to a paired comparison test (Miller, Miller, 1993MILLER, J.C.; MILLER, J.N. Estadística para química analítica, 2. ed. Delaware: Addison-Wesley Iberoamericana, 1993. 209 p.); the data of t and F ratios show no significant differences between the results of the proposed and the official methods, at a 95% confidence level. The RSD values obtained for the samples ranged from 0.7 to 3.8%, as shown in Table III. According to Horwitz (1982)HORWITZ, W. Evaluation of analytical methods used for regulation of foods and drugs. Anal. Chem., v.54, n.1, p.67A-76A, 1982., the maximum RSD value acceptable for the working level of the analyte (48.58 μg mL-1) is 8.0%. The AOAC (1993)ASSOCIATION OF OFFICIAL ANALYTICAL CHEMISTS. AOAC. Peer verified methods program: manual on policies and procedures. Arlington: AOAC International, 1993. 17 p. set the maximum acceptable RSD value at 5.3% for the same analyte level. Therefore, the spectrophotometric method for determination of MTD in pharmaceutical formulations reported in this paper is precise, accurate, and suitable for use in routine analysis.
Accuracy/recovery and repeatability studies
In order to investigate the presence of matrix effects and to check the accuracy and precision of the developed method a recovery study was also carried out. The results of the recovery tests are given in Table IV. The mean recovery values for all samples within the 100.0-101.0% range and RSD within 0.9-1.6% confirm an accurate and precise method for application to pharmaceutical dosage forms. Thus, the results indicate no interference from any of the excipients present in tablets.
In the repeatability study, the RSD was 2.0; 1.1; 1.4 and 0.9% for solution (samples A, B, C and D, respectively) containing an equivalent of 44.58 μg mL-1 of MTD (n = 10). These results reveal good precision of the proposed method.
CONCLUSION
The proposed method represents a very simple, cheap, rapid, precise, accurate, highly sensitive and environmentally-friendly (low consumption of reagents/solvents) analytical method for determining MTD in commercial pharmaceutical preparations with satisfactory recovery. Additionally, the approach fulfils all the main requirements of routine analysis as it is robust, has a low instrumentation and operational cost in comparison to chromatographic methods, and involves no pre-treatment of the sample.
When applied to the assay of various tablet dosage forms, it offers the advantage of not requiring removal of usual excipients since these did not interfere with the determination of MTD. Therefore, the method can be useful in routine quality control analysis of MTD.
ACKNOWLEDGEMENTS
We would like to thank the FAPEMA, CNPq and INCUBEM/PROEX/UFMA Foundations (Brazil) for financial support.
REFERENCES
- AL ABACHI, M.Q.; HADI, H. New, simple and validated kinetics spectrophotometric method for determination of methyldopa in its pharmaceutical formulations. Int. J. Recent Sci. Res., v.4, n.4, p.320-324, 2013.
- AMAN, T.; KHAN, I.U.; ASLAM, N.; AHMED, I. Spectrophotometric determination of methyldopa in pure and pharmaceutical preparations. Anal. Lett., v.31, n.6, p.1007-1020, 1998.
- AMIN, D. Titrimetric determination of catecholamines and related-compounds via bromine oxidation and substitution. Analyst, v.111, n.2, p.255-257, 1986.
- ASSOCIATION OF OFFICIAL ANALYTICAL CHEMISTS. AOAC. Peer verified methods program: manual on policies and procedures. Arlington: AOAC International, 1993. 17 p.
- BELAL, S.F.; EL SAYED, M.A.H.; EL WALILY, A.; ABDINE, H. Spectrophotometric determination of acetaminophen and salicylamide through nitrosation and subsequent chelation. Analyst, v.104, n.1, p.919-927, 1979.
- FARMACOPEIA Brasileira. 5.ed. v.2. Brasília: ANVISA, 2010. p.1143-1145.
- DAVEU, C.; SERVY, C.; DENDANE, M.; MARIN, P.; DUCROCQ, C. Oxidation and nitration of catecholamines by nitrogen oxides derived from nitric oxide. Nitric Oxide-Biol. Chem., v.1, n.3, p.234-243, 1997.
- DAVIDSON, A.G. Difference spectrophotometric assay of 1,2-diphenolic drugs in pharmaceutical formulations. 2. Germanium dioxide reagent. J. Pharm. Sci., v.73, n.11, p.1582-1584, 1984.
- D'ISCHIA, M.; COSTANTINI, C. Nitric oxide-induced nitration of catecholamine neurotransmitters: a key to neuronal degeneration? Bioorg. Med. Chem., v.3, n.7, p.923-927, 1995.
- EL-RABBAT, N.A.; OMAR, N.M. Colorimetric determination of catecholamines by 2,3,5-triphenyltetrazolium chloride. J. Pharm. Sci., v.67, n.6, p.779-781, 1978.
- FOSTER, R. Organic charge transfer complexes. London: Academic Press, 1969. 136 p.
- GARRIDO, M.E.; LIMA, J.L.F.C.; DELERUE-MATTOS, C. Flow injection amperometric determination of L-dopa, epinephrine or dopamine in pharmaceutical preparations. J. Pharm. Biomed. Anal., v.15, n.6, p.845-849, 1997.
- HOFFMAN, B.B. Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists. In: HARDMAN, J.G.; LIMBIRD, L.E.; MOLINOFF, P.B.; RUDDON, R.W.; GILMAN, A.G. (Eds.). Goodman & Gilman's the pharmacological basis of therapeutics. 10.ed. New York: Mac Graw-Hill, 2005 chap.10, p.163-165.
- HORWITZ, W. Evaluation of analytical methods used for regulation of foods and drugs. Anal. Chem., v.54, n.1, p.67A-76A, 1982.
- INTERNATIONAL CONFERENCE ON HARMONIZATION. ICH Q2 (R1): validation of analytical procedures-text and methodology. Geneva: ICH, 2005. 15 p.
- ISSOPOULOS, P.B. Spectrophotometric determination of microquantities of carbidopa, levodopa and α-methyldopa using molybdophosphoric acid. Pharm. Acta Helv., v.64, n.3, p.82-85, 1989.
- ISSOPOULOS, P.B. High-sensitivity spectrophotometric determination of trace amounts of levodopa, carbidopa and α-methyldopa. Fresen. J. Anal. Chem., v.336, n.2, p.124-128, 1990.
- ISSOPOULOS, P.B.; ECONOMOU, P.T. A new high-sensitivity spectrophotometric method for the determination of microconcentrations of α-methyldopa. Farmaco, v.48, n.1, p.127-135, 1993.
- KOZMINSKI, K.D.; GUTMAN, D.A.; DAVILA, V.; SULZER, D.; EWING, A.G. Voltammetric and pharmacological characterization of dopamine release from single exocytotic events at rat pheochromocytoma (PC12) cells. Anal. Chem., v.70, n.15, p.3123-3130, 1998.
- LEE, H.B.; HONG-YOU, R.L.; FOWLIE, P.J.A. Chemical derivatization analysis of phenols: determination of chlorinated phenolics in pulp and paper effluents. J. Assoc. Off. Anal. Chem., v.72, n.6, p.979-984, 1989.
- MARTINEZ-LOZANO, C.; PÉREZ-RUIZ, T.; TOMAS, V.; VAL, O. Determination of epinephrine, norepinephrine, dopamine and L-dopa in pharmaceuticals by a photokinetic method. Analyst, v.116, n.8, p.857-859, 1991.
- MATOS, O.R.; SILVA, F.C.; RIBEIRO, P.R.S. A new, simple and sensitive analytical method for determination of methyldopa in pharmaceutical formulations using the 2,2-diphenyil-picrylhydrazyl. Lat. Am. J. Pharm., v.31, n.2, p.190-194, 2012.
- MILLER, J.C.; MILLER, J.N. Estadística para química analítica, 2. ed. Delaware: Addison-Wesley Iberoamericana, 1993. 209 p.
- MOHAMED, W.I.; SALEM, F.B. Spectrophotometric and titrimetric determination of certain adrenergic drugs. Anal. Lett., v.17, n.3, p.191-203, 1984.
- NAGARAJA, P.; MURTHY, K.C.S.; RANGAPPA, K.S.; GOWDA, N.M.M. Spectrophotometric methods for the determination of certain catecholamine derivatives in pharmaceutical preparations. Talanta, v.46, n.1, p.39-44, 1998.
- NAGARAJA, P.; VASANTHA, R.A.; SUNITHA, K.R. A sensitive and selective spectrophotometric estimation of catechol derivatives in pharmaceutical preparations. Talanta, v.55, n.6, p.1039-1046, 2001a.
- NAGARAJA, P.; VASANTHA, R.A.; MURTHY, K.C.S.; RANGAPPA, K.S. Spectrophotometric determination of some aromatic vic-diols in the pharmaceutical formulations. Chem. Anal. (Warsaw), v.46, n.4, p.569-577, 2001b.
- NEVADO, J.J.B.; GALLEGO, J.M.L.; LAGUNA, P.B. Spectrophotometric determination of dopamine and methyldopa with metaperiodate by flow injection analysis. Fresen. J. Anal. Chem., v.353, n.2, p.221-223, 1995.
- NOZAKI, O.; IWAEDA, T.; KATO, Y. Amines for detection of dopamine by generation of hydrogen peroxide and peroxyoxalate chemiluminescence. J. Biolumin. Chemilumin., v.11, n.6, p.309-314, 1996.
- NOZAKI, O.; IWAEDA, T.; MORIYAMA, H.; KATO, Y. Chemiluminescent detection of catecholamines by generation of hydrogen peroxide with imidazole. Luminescence, v.14, n.3, p.123-127, 1999.
- PARSONS, L.R.; KERR, T.M.; WEISS, F. Simple microbore high-performance liquid chromatographic method for the determination of dopamine and cocaine from a single in vivo brain microdialysis sample. J. Chromatogr. B, v.709, n.1, p.35-39, 1998.
- RIBEIRO, P.R.S.; GOMES NETO, J.A.; PEZZA, L.; PEZZA, H.R. Flow-injection spectrophotometric determination of methyldopa in pharmaceutical formulations. Talanta, v.67, n.1, p.240-244, 2005a.
- RIBEIRO, P.R.S.; PEZZA, L.; PEZZA, H.R. Spectrophotometric determination of methyldopa in pharmaceutical formulations. Eclét. Quím., v.30, n.3, p.23-28, 2005b.
- RIBEIRO, P.R.S.; PEZZA, L.; PEZZA, H.R. Determination of methyldopa in pharmaceutical formulations by combined spot test-diffuse reflectance spectroscopy. J. Braz. Chem. Soc., v.17, n.4, p.674-679, 2006.
- SALEM, F.B. Colorimetric determination of certain sympathomimetic amines. Anal. Lett., v.18, n.9, p.1063-1075, 1985.
- SALEM, F.B. Spectrophotometric and titrimetric determination of catecholamines. Talanta, v.34, n.9, p.810-812, 1987.
- SALEM, F.B. Titrimetric and spectrophotometric determination of catecholamines. Anal. Lett., v.26, n.9, p.1959-1966, 1993a.
- SALEM, F.B. Spectrophotometric and fluorometric determination of catecholamines. Anal. Lett., v.26, n.2, p.281-294, 1993b.
- SHARMA, C.; MOHANTY, S.; KUMAR, S.; RAO, N.J. Gas chromatographic analysis of chlorophenolic, resin and fatty acids in chlorination and caustic extraction stage effluent from kahi-grass. Analyst, v.121, n.12, p.1963-1967, 1996.
- SUAREZ, W.T.; VIEIRA, H.J.; FATIBELLO FILHO, O. Determinação de paracetamol em produtos farmacêuticos empregando um sistema de análise por injeção em fluxo com geração de ácido nitroso. Eclét. Quím., v.30, n.1, p.23-28, 2005.
- TSUCHIYA, M.; SATO, M.; KATO, H.; OKUBO, T.; JUNEJA, L.R.; KIM, M. Simultaneous determination of catechins in human saliva by high-performance liquid chromatography. J. Chromatogr. B, v.703, n.1-2, p.253-258, 1997.
- UNITED STATES PHARMACOPEIA. 36.ed. Rockville: United States Pharmacopeial Convention, 2013. v.3, p.2889-2890.
- VIEIRA, I.C.; FATIBELLO FILHO, O. Spectrophotometric determination of methyldopa and dopamine in pharmaceutical formulations using a crude extract of sweet potato root (Ipomoea batatas (L.) lam.) as enzymatic source. Talanta, v.46, n.4, p.559-564, 1998.
- WALASH, M.I.; ABOUOUF, A.; SALEM, F.B. Spectrophotometric and titrimetric determination of certain adrenergic drugs, using organic brominating agents. J. Assoc. Off. Anal. Chem., v.65, n.6, p.1445-1451, 1982.
- WALASH, M.I.; ABOUOUF, A.; SALEM, F.B. Colorimetric determination of sympathomimetic amines methyldopa and noradrenaline. J. Assoc. Off. Anal. Chem., v.68, n.1, p.91-95, 1985.
- ZIVANOVIC, L.; VASILJEVIC, S.; RADULOVIC, D. Colorimetric assay of methyldopa bulk drug and tablets as Fe(III) complex. Boll. Chim. Farm., v.130, n.5, p.162-165, 1991.
Publication Dates
-
Publication in this collection
Jul-Sep 2014
History
-
Received
24 July 2013 -
Accepted
04 Oct 2013