Abstracts
The hemoglobinopathies are included among the most common genetic diseases in the world. In Brazil, hemoglobinopathies are related to the diversity of racial backgrounds and the degree of interbreeding. The study focused on the prevalence of hemoglobinopathies using conventional and confirmatory laboratory tests in children from public schools in Ribeirão Preto-SP. The study involved the participation of 427 children between six and nine years of age. Hematologic evaluation, hemoglobin electrophoresis on cellulose acetate at alkaline pH, quantification of hemoglobin fractions by high performance liquid chromatography (HPLC) and detection of -α3.7 deletion for α thalassemia by polymerase chain reaction were performed. The results of hemoglobin electrophoresis on cellulose acetate and HPLC of the children studied showed the presence of 30 children (7%) with hemoglobinopathies. Eleven children presented results indicating suspicion of S/β-thalassemia; their parents and/or siblings were evaluated and confirmed the presence of only Hb S. The analysis of deletion -α3.7 to characterize α-thalassemias sampling performed on 207 participants identified 26 children (12.6%) with deletion -α3.7. Thus, 54 (12.6%) of the children studied present this genetic alteration. For the detection of α-thalassemias it is necessary to use confirmatory methods such as molecular analysis and evaluation of family members in doubtful cases to facilitate genetic counseling in families, in which deletion -α3.7 is more frequent in Brazil.
Hemoglobinopathy; Hemoglobin electrophoresis; High performance liquid chromatography/quantitative analysis; Polymerase chain reaction
As hemoglobinopatias estão incluídas nas doenças genéticas mais comuns no mundo. No Brasil, as hemoglobinopatias são relatadas pela diversidade racial e o grau de miscigenação. O estudo focou a prevalência das hemoglobinopatias usando métodos laboratoriais convencionais como a eletroforese de hemoglobina em acetato de celulose em pH alcalino e confirmatório por reação em cadeia de polimerase (PCR) em crianças de escolas públicas de Ribeirão Preto-SP. O estudo envolveu a participação de 427 crianças entre 6-9 anos de idade. Determinaram-se os valores hematológicos, efetuou-se eletroforese de hemoglobina em acetato de celulose em pH alcalino, quantificação das frações de hemoglobina por HPLC e a detecção da deleção -α3,7 pela PCR. Os resultados da eletroforese de hemoglobina em acetato de celulose e do HPLC, nas crianças estudadas, mostraram a presença de 30 crianças (7%) com hemoglobinopatias. Onze crianças apresentaram resultado indicando a suspeita de S/β-talassemia; seus pais e/ou irmãos confirmaram a presença de apenas a Hb S. A análise da deleção -α3,7, uma das alterações que estão presentes na α-talassemia, realizada em 207 participantes, identificou 26 crianças (12,6%) com a deleção -α3,7. Dessa forma, 54 (12,6%) das crianças estudadas apresentam hemoglobinopatias. Para a deleção da α-talassemias é necessário utilizar métodos confirmatórios como as análises moleculares e avaliação de membros da família, em casos duvidosos, facilitando o aconselhamento genético nas famílias, sendo a deleção -α3,7 mais frequente no Brasil.
Hemoglobinopatias; Eletroforese de hemoglobina; Cromatografia líquida de alta eficiência/análise quantitativa; Reação em cadeia de polimerase
INTRODUCTION
The hemoglobinopathies are a group of inherited diseases that are classified based on the presence of structurally abnormal hemoglobin (Hb) such as hemoglobins S, C, D and E, and/or one or more globin chain disabilities, known as thalassemias (Clark, Thein, 2004CLARK, B.E.; THEIN, SL. Molecular diagnosis of haemoglobin disorders. Clin. Lab. Haematol., v.26, n.3, p.159-176, 2004.; Henderson et al., 2009HENDERSON, S.; TIMBS, A.; MCCARTHY, J.; GALLIENNE, A.; MOURIK, M.V. ; MASTERS, G.; MAY, A.; KHALIL, M.S.; SCHUCH, A.; OLD, J. Incidence of haemoglobinopathies in various populations - the impact of immigration. Clin. Biochem., v.42, n.18, p.1745-1756, 2009.). These pathologies are included among the most common genetic diseases in the world, with an estimated prevalence of 7% of the worldwide population (Melo-Reis et al., 2006MELO-REIS, p.R.; NAOUM, p.C.; DINIZ-FILHO, J.A.F.; DIAS-PENNA, K.G.B.; MESQUITA, M.M.; BALESTRA, FA. Prevalência de talassemias e hemoglobinas variantes no estado de Goiás, Brasil. J. Bras. Patol. Med. Lab., v.42, n.6, p.425-430, 2006.; Manca, Masala, 2008MANCA, L.; MASALA, B. Disorders of the synthesis of human fetal hemoglobin. IUBMB Life, v.60, n.2, p.94-111, 2008.).
In Brazil, hemoglobinopathies are related to the diversity of racial backgrounds and the degree of interbreeding, and may be regional. The most frequent hemoglobin variants are S and C of African origin, however, due to interbreeding these hemoglobins came to be found in other ethnic groups (Aigner et al., 2006AIGNER, C.P. ; SANDRINI, F.; DUARTE, E.G.; ANDRADE, M.P. ; LARGURA, M. A.; LARGURA, A. Estudo do perfil de hemoglobinas em 9 189 testes realizados no Álvaro Centro de Análises e Pesquisas Clínicas. Rev. Bras. Anal. Clin., v.38, n.2, p.107-109, 2006.). The impact of population migration increases the combinations of abnormal hemoglobins and the variety of mutations (Henderson et al., 2009HENDERSON, S.; TIMBS, A.; MCCARTHY, J.; GALLIENNE, A.; MOURIK, M.V. ; MASTERS, G.; MAY, A.; KHALIL, M.S.; SCHUCH, A.; OLD, J. Incidence of haemoglobinopathies in various populations - the impact of immigration. Clin. Biochem., v.42, n.18, p.1745-1756, 2009.).
In most abnormal hemoglobins a point mutation, in other words, a single amino acid substitution occurs. Over 1100 hemoglobin variants involving the chains α, β, δ and γ have been described (Wagner et al., 2005WAGNER, S.C.; SILVESTRI, M.C.; BITTAR, C.M.; FRIEDRISCH, J. R.; SILLA, LMR. Prevalence of thalassemias and variant hemoglobin in patients with non-ferropenic anemia. Rev. Bras. Hematol. Hemoter., v.27, n.1, p.37-42, 2005.). In the heterozygous state, production of Hb A and variant hemoglobin occurs without severe clinical manifestations. In the homozygous state, Hb A is absent, thus possibly resulting severe anemia. The interaction of two hemoglobin variants or a combination of a hemoglobin variant and a genetic alteration to thalassemia may still occur (Clark, Thein, 2004CLARK, B.E.; THEIN, SL. Molecular diagnosis of haemoglobin disorders. Clin. Lab. Haematol., v.26, n.3, p.159-176, 2004.).
Different types of thalassemia are found in the population, the most common being the α and β-thalassemias (Galanello et al.,1998GALANELLO, R.; SOLLAINO, C.; PAGLIETTI, E.; BARELLA, S.; PERRA, C.; DONEDDU, I.; PIRRONI, M. G.; MACCIONI, L.; CAO, A. α-Thalassemia carrier identification by DNA analysis in the screening for thalassemia. Am. J. Hematol., v.59, n.4, p.273-278, 1998.; Galanello, Origa, 2010GALANELLO, R.; ORIGA, R. Beta-thalassemia. Orphanet. J. Rare Dis., v.5, n.11, p.2-15, 2010.; Cousens et al., 2010COUSENS, N.E.; GAFF, C.L.; METCALFE, S.A.; DELATYCKI, MB. Carrier screening for beta-thalassaemia: a review of international practice. Eur. J. Hum. Gen., v.18, n.10, p.1077-1083, 2010.). The α-thalassemia is the most common inherited disease in the world. Recent studies show that α-thalassemia affects 5% of the worldwide population and until recently (Vichinsky, 2010VICHINSKY, E. Complexity of alpha thalassemia: growing health problem with new approaches to screening, diagnosis, and therapy. Ann. NY Acad. Sci., v.1202, v.180-187, 2010.), the frequency of this pathology was underestimated due the use of inadequate diagnostic methods. In Brazil α-thalassemia affects mainly Asian and some African groups. Studies show a frequency of 10-12% in some regions and up to 25% in specific groups. For α-thalassemia in the state of São Paulo in Caucasian descendants, the estimated ratio is 3% (Bonini-Domingos, 2004BONINI-DOMINGOS, CR. Thalassemia screening in Brazil: results for 20 years. Rev. Bras. Hematol. Hemoter., v.26, n.4, p.288-289, 2004.).
Mutations or deletions may result in a lack of production of globin (β0 and α0) or decreased production of these (β+ and α+)(Cunninghan, 2010CUNNINGHAN, MJ. Update on thalassemia: clinical care and complications. Hematol. Oncol. Clin. North Am., v.24, n.1, p.215-227, 2010.). Over 95% of cases of α-thalassemia are due to deletions, being -α5.2 and -α 20.5generally causing α0-thalassemia and -α3.7, -α4.2causing α+-talassemia (Weatherall, 2001WEATHERALL, DJ. Phenotype-genotype relationships in monogenic disease: lessons from the thalassaemias. Nat. Rev. Genet., v.2, n.4, p.245-255, 2001.). Molecular analyses indicate a high prevalence of deletion -α3.7 in the state of São Paulo (Sonati et al., 1991SONATI, M.F.; FARAH, S.B.; RAMALHO, A.S.; COSTA, FF. High prevalence of alpha-thalassemia in a black population of Brazil. Hemoglobin, v.15, n.4, p.309-311, 1991.; Borges et al., 2001BORGES, E.; WENNING, M.R.; KIMURA, E.M.; GERVASIO, S.A.; COSTA, F.F.; SONATI, MF. High prevalence of alpha-thalassemia among individuals with microcytosis and hypochromia without anemia. Braz. J. Med. Biol. Res., v.34, n.6, p.759-762, 2001.; Oliveira et al., 2006OLIVEIRA, G.L.V. ; MENDIBURU, C.F.; BONINI-DOMINGOS, CR. Avaliação do perfil hematológico de portadores de talassemia alfa provenientes das regiões Sudeste e Nordeste do Brasil. Rev. Bras. Hematol. Hemoter., v.28, n.2, p.105-109, 2006.).
Conventional laboratory diagnosis for α-thalassemia can be characterized by precipitation of hemoglobin H, visualized by supravital staining. This diagnosis can be difficult when there is a concomitant hemoglobinopathy such as Hb S, Hb C, Hb E and β-thalassemia, because of the decrease or absence of Hb H and cellular inclusions (Chui et al., 2003CHUI, D.H.K.; FUCHAROEN, S.; CHAN, v. Hemoglobin H disease: not necessarily a benign disorder. Blood, v.101, n.3, p.791-799, 2003.).
In general, the most used method for the identification of hemoglobinopathies is hemoglobin electrophoresis on cellulose acetate at alkaline pH, however its sensitivity is limited, especially in the case of isoform co-migration, such as Hb S, Lepore and Hb D (Chinelato-Fernandes et al., 2003CHINELATO-FERNANDES, A.R.; LEONELI, G.G.; CALDERAN, p.O.; OLIVEIRA, R.B.; SILVA, W.A.; HIDALGO, C.A.; BONINI-DOMINGOS, CR. Avaliação eletroforética, cromatográfica e molecular da Hb D Los Angeles no Brasil. Rev. Bras. Hematol. Hemoter., v.25, n.3, p.161-168, 2003.). Thus, confirmatory tests are required, such as acid agarose gel electrophoresis, although there is controversy regarding the distinction of hemoglobins D and G, and O and E (Ondei et al., 2007ONDEI, L.S.; ZAMARO, p.J.A.; MANGONARO, p.H.; VALÊNCIO, C.R.; BONINI-DOMINGOS, CR. HPLC determination of hemoglobins to establish reference values with the aid of statistics and informatics. Genet. Mol. Res., v.6, n.2, p.453-460, 2007.). High-performance liquid chromatography (HPLC) and molecular techniques such as polymerase chain reaction (PCR) and sequencing the gene are more accurate methods for the diagnosis of hemoglobinopathies (Bertholo, Moreira, 2006BERTHOLO, L.C.; MOREIRA, HW. Focalização isoelétrica na identificação das hemoglobinas. J. Bras. Patol. Med. Lab., v.42, n.3, p.163-168, 2006.; Wenning et al., 2000WENNING, M.R.S.C.; KIMURA, E.M.; COSTA, F.F.; SAAD, S.T.O.; GERVÁSIO, S.; JORGE, S.B.; BORGES, E.; SILVA, n.M.; SONATI, MF. α-globin genes: thalassemic and structural alterations in a Brazilian population. Braz. J. Med. Biol. Res., v.33, n.9, p.1041-1045, 2000.; Bonini-Domingos, 2004BONINI-DOMINGOS, CR. Thalassemia screening in Brazil: results for 20 years. Rev. Bras. Hematol. Hemoter., v.26, n.4, p.288-289, 2004.).
The combined analysis of all methods in the diagnosis of hemoglobinopathies is the most appropriate since each method alone has limitations and can cause a misinterpretation of results (Hughes et al., 2009HUGHES, H.Y.; MCKIE, K.; CARMICHAEL, H.; BORA, K.; KUTLAR, A.; KUTLAR, F. Diagnostic complication and molecular characteristics of Hb SC-Chicago disease with α-thal-2 (α37 deletion): effects of multiple variant on patient's phenotype. Ann. Hematol., v.88, n.11, p.1151-1153, 2009.). Thus, this study aimed to evaluate the prevalence of hemoglobinopathies using conventional and confirmatory laboratory tests such as hemoglobin electrophoresis, quantification of hemoglobin fractions by HPLC, and analysis of the deletion that causes more frequent α-thalassemia (-α3.7) in southeast Brazil (Wenning et al., 2000WENNING, M.R.S.C.; KIMURA, E.M.; COSTA, F.F.; SAAD, S.T.O.; GERVÁSIO, S.; JORGE, S.B.; BORGES, E.; SILVA, n.M.; SONATI, MF. α-globin genes: thalassemic and structural alterations in a Brazilian population. Braz. J. Med. Biol. Res., v.33, n.9, p.1041-1045, 2000.; Bonini-Domingos, 2004BONINI-DOMINGOS, CR. Thalassemia screening in Brazil: results for 20 years. Rev. Bras. Hematol. Hemoter., v.26, n.4, p.288-289, 2004.), also occurring in children from public schools in Ribeirão Preto-SP, by molecular biology.
MATERIAL AND METHODS
The study group included 427 children between six and nine years of age of both genders, recruited from state and municipal schools in the city of Ribeirão Preto-SP, namely: Centro Municipal de Educação Infantil Virgílio Salata, Escola Estadual Antônio Diederichsen, Escola Estadual Dr. Tomas Alberto Whatelly, EMEFEM Dom Luiz Do Amaral Mousinho, and Escola Estadual Dom Alberto José Gonçalves. Participation in this study was at the consent endorsed by their parents. This study was approved by the Ethics Committee of the Faculty of Dentistry of Ribeirão Preto, University of São Paulo (case no. 2006.1.797.58.5).
Ten milliliters (mL) of blood were collected and placed in a tube containing dipotassium ethylenediaminetetraacetic acid (K2 EDTA) 10%, which was used in the following determinations: hematologic evaluation using automatic counter Micros 45- Horiba ABX(r); hemoglobin electrophoresis on cellulose acetate at alkaline pH, according to Naoum (1999)NAOUM, pC. Hemoglobinopatias. 2.ed. São Paulo: Livraria Editora Santos, 1999. p. 57-100.; quantification of hemoglobin fractions by HPLC Variant II Bio-Rad(r) automated system (β-thalassemia Short Program kit); and extraction of genetic material to perform the PCR.
For detection of deletion -α3.7 the specific primers (Dodé et al., 1993): 5' CCA TGC CTG GCA CGT TTG CTG AGG 3'(C9 primer); 5' GAT GCA CCC ACT GGA CTC CT 3'(C10 primer) were used. The PCR reaction was processed in a thermocycler (Eppendorf Mastercycle), 30 cycles being performed at 94 oC/2 min., 56 oC/1 min., 72 oC/2 min., preceded by an initial heat-denaturation step at 94 oC/5 min. and followed by a final extension step at 72 oC/10 min. The mixture was subjected to horizontal electrophoresis on a 1% agarose gel with ethidium bromide, with a running time of 1 hour and 20 minutes at 90 volts, 220 mA. Then the gel was exposed to UV light for visualization of the presented bands.
Thirteen parents and two brothers participated in the study in order to confirm the results of children who showed the presence of Hb S and increased Hb A2, suggestive of hemoglobinopathy HbS/β-thalassemia. Five mL of blood were collected and placed into a tube containing K2 EDTA, which was used for the identification and quantification of hemoglobin fractions by HPLC.
RESULTS AND DISCUSSION
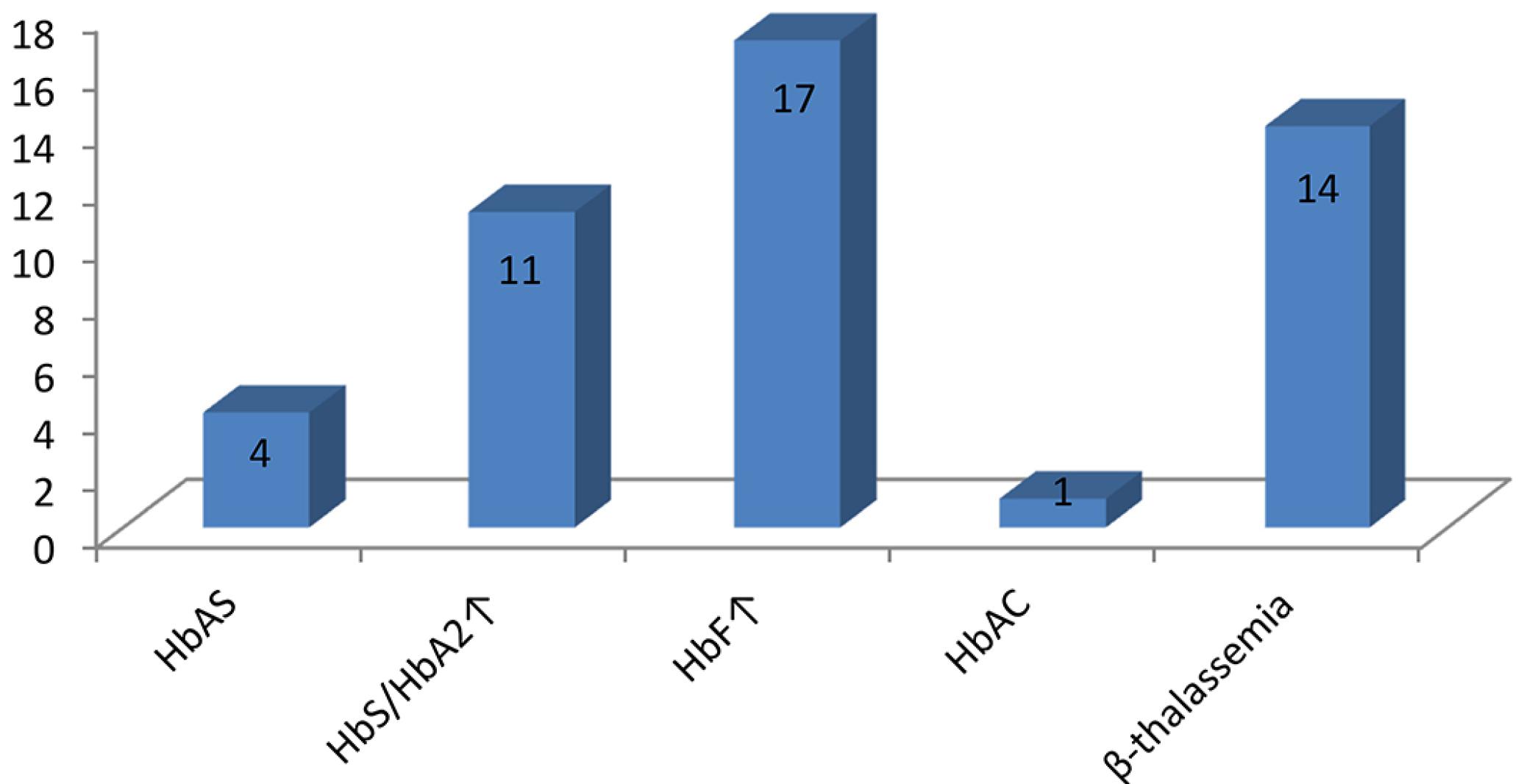
The results of hemoglobin electrophoresis on cellulose acetate and HPLC of the 427 children studied indicated the presence of 30 children (7%) with hemoglobinopathies, being 14 with increased Hb A2, consistent with the phenotype of β-thalassemia (β+ or β0); 11 with increased Hb A2 and the presence of Hb S, consistent with the phenotype of Hb S/β-thalassemia; 4 with the presence of Hb S carriers of sickle cell trait and 1 with the presence of Hb C, indicating heterozygosity for the Hb C disease (Table I, Figure 1).
Distribution of hemoglobinopathies in the studied children utilizing hemoglobin electrophoresis on cellulose acetate and HPLC methodology
Distribution of hemoglobinopathies in the 427 studied children utilizing hemoglobin electrophoresis on cellulose acetate and HPLC methodology.
The determination of the levels of Hb A2 of 11 participants with suspected Hb S/β-thalassemia was performed by HPLC and the fractions obtained by elution in electrophoresis on cellulose acetate. The median Hb A2 levels determined by HPLC and elution were 6.3% (± 1.1) and 7.0% (± 1.6), respectively, with no significant statistical difference (p > 0.05). A survey of quantitative and qualitative changes of hemoglobin fractions in 11 parents and/or siblings by HPLC only detected the presence of Hb S, discarding the suspicion S/β-thalassemia in children.
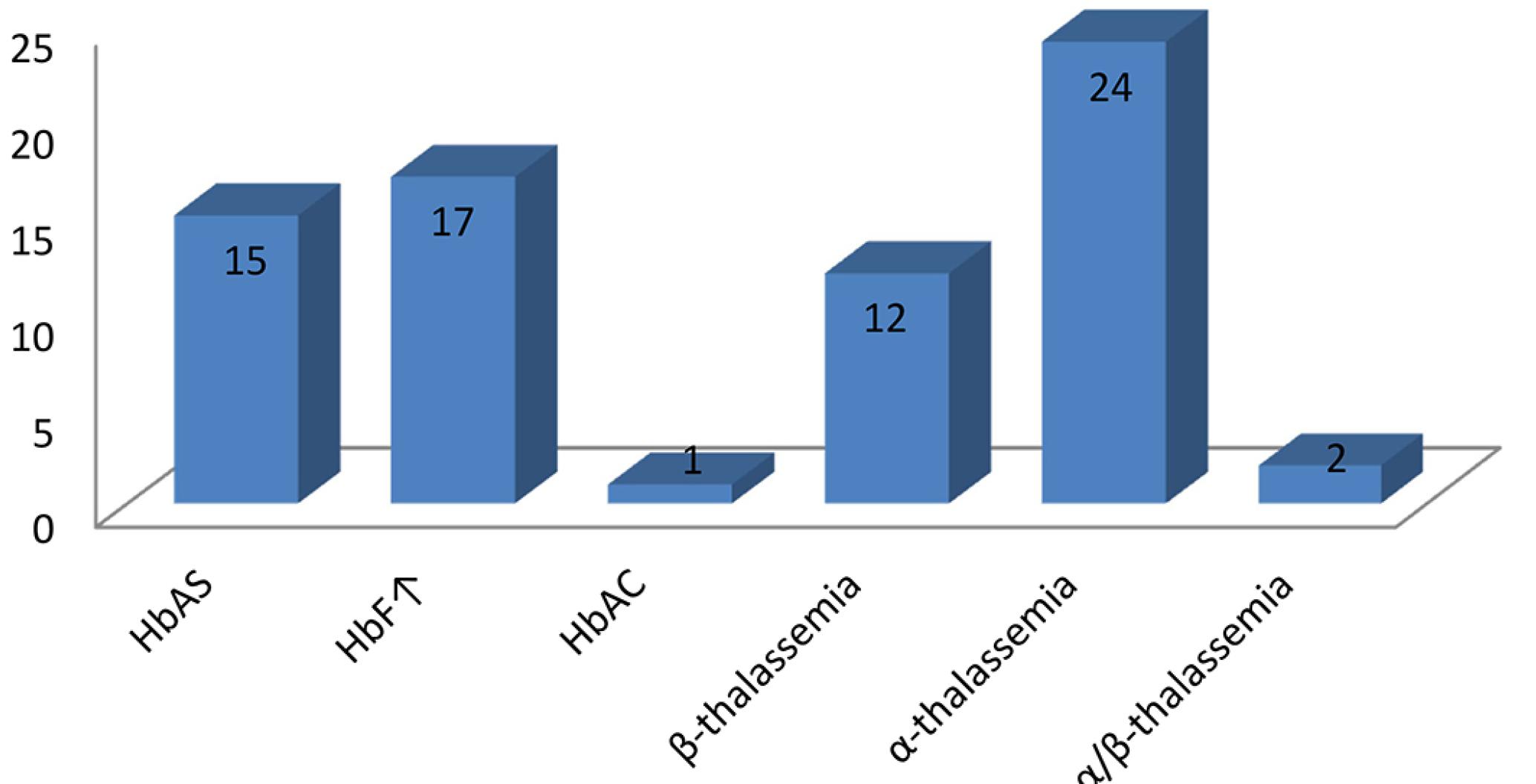
The analysis of deletion -α3.7, featuring α-thalassemia was performed in 207 study participants verifying the presence of 26 children (12.6%) carrying the deletion, being 24 heterozygous and 2 homozygous.
Together the analyses showed a total of 54 children, 12.6% of the children studied, showing some qualitative or quantitative change of the globin chains, namely: 15 (3.5%) women with Hb S, 1 (0.24 %) Hb C and 14 (3.3%) with β-thalassemia minor (Table II, Figure 2).
Distribution of hemoglobinopathies in the studied children utilizing hemoglobin electrophoresis on cellulose acetate, HPLC and PCR methodology
Frequencies of hemoglobinopathies in the 427 studied children utilizing hemoglobin electrophoresis on cellulose acetate and HPLC, and the 207 studied children utilizing PCR.
Among patients with hemoglobinopathies, 27 (50%) were anemic according to WHO criteria, i.e., Hb less than 11.5 g/dL for children under 12 years of age of both genders, 8 (14.8%) of them showed only mild hypochromia and the remainder (35.2%) showed no significant changes.
In our study the number of carriers of the sickle cell trait (3.5%) was apparently higher than reported in a review by Murao and Ferraz (2007)MURAO, M.; FERRAZ, MHC. Sickle cell trait: heterozygous for the hemoglobin S. Rev. Bras. Hematol. Hemoter., v.29, n.3, p.223-225, 2007., in which the authors estimate 1.9% of patients with Hb S in the state of Sao Paulo. A prevalence of approximately 3% of patients with β-thalassemia in the state of São Paulo is estimated (Bonini-Domingos, 2004BONINI-DOMINGOS, CR. Thalassemia screening in Brazil: results for 20 years. Rev. Bras. Hematol. Hemoter., v.26, n.4, p.288-289, 2004.). In our study a prevalence of 3.3%, very close to the above, was observed. There was the presence of 12.6% of α-thalassemia, results close to the literature (Head et al., 2004HEAD, C. E.; CONROY, M.; JARVIS, M.; PHELAN, L.; BAIN, B J. Some observations on the measurement of haemoglobin A2 and S percentages by high performance liquid chromatography in the presence and absence of α-thalassemia. J. Clin. Pathol., v.57, n.3, p.276-280, 2004.), which indicates prevalence of between 10 and 20% in our population. These numbers are quite significant and justify the need for more extensive research in the city of Ribeirão Preto to characterize the prevalence in the population.
On these results the necessity of conducting prenatal testing and research of family cases of hemoglobinopathies in our population is evident.
Neonatal screening programs follow the standards of the National Program for Newborn Screening (Ministry of Health decree No. 822/01) that recommends the use of two presumptive methods for determining the electrophoretic profile. Currently, screening programs replaced the traditional methods by using isoelectric focusing electrophoresis and HPLC. Despite new presumptive methods having better sensitivity and specificity, they are susceptible to interference and in some cases such as in the diagnosis of α-talassemia the use of molecular methodology is required (Hughes et al., 2009HUGHES, H.Y.; MCKIE, K.; CARMICHAEL, H.; BORA, K.; KUTLAR, A.; KUTLAR, F. Diagnostic complication and molecular characteristics of Hb SC-Chicago disease with α-thal-2 (α37 deletion): effects of multiple variant on patient's phenotype. Ann. Hematol., v.88, n.11, p.1151-1153, 2009.). Our study proved this since the number of patients with hemoglobinopathies rose 5.6% after performing PCR for detection of α-thalassemia.
The use of HPLC has shown to be more sensitive and specific, and with a better reproducibility for the determination of the Hb A2 method. However it is argued that the percentage of Hb A2 measured by HPLC can be affected by the presence of hemoglobin S (HbS) in heterozygous individuals for β-thalassemia. This is due to reduced affinity of the βS to α chains, which leads to the free α to match δ chains, increasing Hb A2, and co-elution of Hb with Hb A2, falsely elevating its percentage (Head et al., 2004HEAD, C. E.; CONROY, M.; JARVIS, M.; PHELAN, L.; BAIN, B J. Some observations on the measurement of haemoglobin A2 and S percentages by high performance liquid chromatography in the presence and absence of α-thalassemia. J. Clin. Pathol., v.57, n.3, p.276-280, 2004.; Kalleas et al., 2007KALLEAS, C.; TENTES, I.; MARGARITIS, D.; et al. Effect of HbS in the determination of HbA2 with the Biorad Variant II analyzer. Clin. Biochem., v.40, n.9-10, p.744-746, 2007.). No significant differences were observed in quantifying the levels of Hb A2, performed by the methods of electrophoresis on cellulose acetate for elution and HPLC. To confirm suggestive cases of Hb S/β-thalassemia, parents and/or siblings of these children were evaluated, verifying in all cases only the presence of HbS and normal levels of Hb A2, thus inferring that the children had only the sickle cell trait. Probably the increase in Hb A2 was found in these children due to the combination of free α and δ chains and not presenting mutations that compromise the synthesis of β chains.
CONCLUSION
We conclude that in order to detect hemoglobinopathies, the use of confirmatory methods such as molecular analysis for the search of α-thalassemia and the investigation of relatives of the doubtful cases are required. Although it has been investigated only for the most frequent deletion for α-thalassemia, α3.7 it is now possible to state the importance of confirmatory methods. Only then we can ensure accurate diagnosis and facilitate genetic counseling in families where there are carriers of these pathologies.
ACKNOWLEDGMENT
The authors wish to thank CNPq for financial support.
REFERENCES
- AIGNER, C.P. ; SANDRINI, F.; DUARTE, E.G.; ANDRADE, M.P. ; LARGURA, M. A.; LARGURA, A. Estudo do perfil de hemoglobinas em 9 189 testes realizados no Álvaro Centro de Análises e Pesquisas Clínicas. Rev. Bras. Anal. Clin., v.38, n.2, p.107-109, 2006.
- BERTHOLO, L.C.; MOREIRA, HW. Focalização isoelétrica na identificação das hemoglobinas. J. Bras. Patol. Med. Lab., v.42, n.3, p.163-168, 2006.
- BONINI-DOMINGOS, CR. Thalassemia screening in Brazil: results for 20 years. Rev. Bras. Hematol. Hemoter., v.26, n.4, p.288-289, 2004.
- BORGES, E.; WENNING, M.R.; KIMURA, E.M.; GERVASIO, S.A.; COSTA, F.F.; SONATI, MF. High prevalence of alpha-thalassemia among individuals with microcytosis and hypochromia without anemia. Braz. J. Med. Biol. Res., v.34, n.6, p.759-762, 2001.
- CLARK, B.E.; THEIN, SL. Molecular diagnosis of haemoglobin disorders. Clin. Lab. Haematol., v.26, n.3, p.159-176, 2004.
- CHINELATO-FERNANDES, A.R.; LEONELI, G.G.; CALDERAN, p.O.; OLIVEIRA, R.B.; SILVA, W.A.; HIDALGO, C.A.; BONINI-DOMINGOS, CR. Avaliação eletroforética, cromatográfica e molecular da Hb D Los Angeles no Brasil. Rev. Bras. Hematol. Hemoter., v.25, n.3, p.161-168, 2003.
- CHUI, D.H.K.; FUCHAROEN, S.; CHAN, v. Hemoglobin H disease: not necessarily a benign disorder. Blood, v.101, n.3, p.791-799, 2003.
- COUSENS, N.E.; GAFF, C.L.; METCALFE, S.A.; DELATYCKI, MB. Carrier screening for beta-thalassaemia: a review of international practice. Eur. J. Hum. Gen., v.18, n.10, p.1077-1083, 2010.
- CUNNINGHAN, MJ. Update on thalassemia: clinical care and complications. Hematol. Oncol. Clin. North Am., v.24, n.1, p.215-227, 2010.
- DODÉ, C.; KRISHNAMOORTHY, R.; LAMB, J.; ROCHETTE, J. Rapid analysis of -α3 7 thalassaemia and ααα anti 3.7 triplication by enzymatic amplification analysis.Br. J. Haematol., v.83, n.1, p.105-111, 1993.
- GALANELLO, R.; SOLLAINO, C.; PAGLIETTI, E.; BARELLA, S.; PERRA, C.; DONEDDU, I.; PIRRONI, M. G.; MACCIONI, L.; CAO, A. α-Thalassemia carrier identification by DNA analysis in the screening for thalassemia. Am. J. Hematol., v.59, n.4, p.273-278, 1998.
- HEAD, C. E.; CONROY, M.; JARVIS, M.; PHELAN, L.; BAIN, B J. Some observations on the measurement of haemoglobin A2 and S percentages by high performance liquid chromatography in the presence and absence of α-thalassemia. J. Clin. Pathol., v.57, n.3, p.276-280, 2004.
- GALANELLO, R.; ORIGA, R. Beta-thalassemia. Orphanet. J. Rare Dis., v.5, n.11, p.2-15, 2010.
- HENDERSON, S.; TIMBS, A.; MCCARTHY, J.; GALLIENNE, A.; MOURIK, M.V. ; MASTERS, G.; MAY, A.; KHALIL, M.S.; SCHUCH, A.; OLD, J. Incidence of haemoglobinopathies in various populations - the impact of immigration. Clin. Biochem., v.42, n.18, p.1745-1756, 2009.
- HUGHES, H.Y.; MCKIE, K.; CARMICHAEL, H.; BORA, K.; KUTLAR, A.; KUTLAR, F. Diagnostic complication and molecular characteristics of Hb SC-Chicago disease with α-thal-2 (α37 deletion): effects of multiple variant on patient's phenotype. Ann. Hematol., v.88, n.11, p.1151-1153, 2009.
- KALLEAS, C.; TENTES, I.; MARGARITIS, D.; et al. Effect of HbS in the determination of HbA2 with the Biorad Variant II analyzer. Clin. Biochem., v.40, n.9-10, p.744-746, 2007.
- MANCA, L.; MASALA, B. Disorders of the synthesis of human fetal hemoglobin. IUBMB Life, v.60, n.2, p.94-111, 2008.
- MELO, L.M.S.; SIQUEIRA, F.A.M.; CONTE, A.C.F.; BONINI-DOMINGOS, CR. Rastreamento de hemoglobinas variantes e talassemias com associação de métodos diagnósticos. Rev. Bras. Hematol. Hemoter., v.30, n.1, p.12-17, 2008.
- MELO-REIS, p.R.; NAOUM, p.C.; DINIZ-FILHO, J.A.F.; DIAS-PENNA, K.G.B.; MESQUITA, M.M.; BALESTRA, FA. Prevalência de talassemias e hemoglobinas variantes no estado de Goiás, Brasil. J. Bras. Patol. Med. Lab., v.42, n.6, p.425-430, 2006.
- MURAO, M.; FERRAZ, MHC. Sickle cell trait: heterozygous for the hemoglobin S. Rev. Bras. Hematol. Hemoter., v.29, n.3, p.223-225, 2007.
- NAOUM, pC. Hemoglobinopatias. 2.ed. São Paulo: Livraria Editora Santos, 1999. p. 57-100.
- OLIVEIRA, G.L.V. ; MENDIBURU, C.F.; BONINI-DOMINGOS, CR. Avaliação do perfil hematológico de portadores de talassemia alfa provenientes das regiões Sudeste e Nordeste do Brasil. Rev. Bras. Hematol. Hemoter., v.28, n.2, p.105-109, 2006.
- ONDEI, L.S.; ZAMARO, p.J.A.; MANGONARO, p.H.; VALÊNCIO, C.R.; BONINI-DOMINGOS, CR. HPLC determination of hemoglobins to establish reference values with the aid of statistics and informatics. Genet. Mol. Res., v.6, n.2, p.453-460, 2007.
- SONATI, M.F.; FARAH, S.B.; RAMALHO, A.S.; COSTA, FF. High prevalence of alpha-thalassemia in a black population of Brazil. Hemoglobin, v.15, n.4, p.309-311, 1991.
- VICHINSKY, E. Complexity of alpha thalassemia: growing health problem with new approaches to screening, diagnosis, and therapy. Ann. NY Acad. Sci., v.1202, v.180-187, 2010.
- WAGNER, S.C.; SILVESTRI, M.C.; BITTAR, C.M.; FRIEDRISCH, J. R.; SILLA, LMR. Prevalence of thalassemias and variant hemoglobin in patients with non-ferropenic anemia. Rev. Bras. Hematol. Hemoter., v.27, n.1, p.37-42, 2005.
- WEATHERALL, DJ. Phenotype-genotype relationships in monogenic disease: lessons from the thalassaemias. Nat. Rev. Genet., v.2, n.4, p.245-255, 2001.
- WENNING, M.R.S.C.; KIMURA, E.M.; COSTA, F.F.; SAAD, S.T.O.; GERVÁSIO, S.; JORGE, S.B.; BORGES, E.; SILVA, n.M.; SONATI, MF. α-globin genes: thalassemic and structural alterations in a Brazilian population. Braz. J. Med. Biol. Res., v.33, n.9, p.1041-1045, 2000.
Publication Dates
-
Publication in this collection
Apr-Jun 2015
History
-
Received
25 Mar 2014 -
Accepted
20 Oct 2014