abstract
This work aimed to investigate in vitro the influence of monoolein (MO) on progesterone (PG) transdermal delivery and skin retention. Information about the role of MO as an absorption enhancer for lipophilic molecules can help on innovative product development capable of delivering the hormone through the skin in a consistent manner, improving transdermal therapy of hormonal replacement. MO was dispersed in propylene glycol under heat at concentrations of 0% (control), 5% w/w, 10% w/w and 20% w/w. Then, 0.6% of PG (w/w) was added to each formulation. Permeation profile of the hormone was determined in vitro for 48 h using porcine skin in Franz diffusion cells. PG permeation doubled when 5% (w/w) of MO was present in formulation in comparison to both the control and higher MO concentrations (10% and 20% w/w). An equal trend was observed for PG retention in stratum corneum (SC) and reminiscent skin (E+D). PG release rates from the MO formulations, investigated using cellulose membranes, revealed that concentrations of MO higher than 5% (w/w) hindered PG release, which indeed negatively reflected on the hormone permeation through the skin. In conclusion, this work demonstrated the feasibility of MO addition (at 5% w/w) in formulations as a simple method to increase transdermal PG delivery for therapies of hormonal replacement. In contrast, higher MO concentrations (from 10% to 20% w/w) can control active release, and this approach could be extrapolated to other lipophilic, low-molecular-weight molecules.
Uniterms:
Monoolein/influence/transdermal therapy; Progesterone/transdermal delivery; Progesterone/skin retention; Hormonal replacement/transdermal therapy; Skin permeation.
resumo
Este trabalho teve como objetivo investigar in vitro a influência de monooleína (MO) na permeação transdérmica de progesterona (PG), bem como sobre a retenção cutânea desse hormônio a fim de (i) liberar de maneira mais consistente hormônio através da pele para melhorar a terapia transdérmica de reposição hormonal e (ii) trazer mais informações sobre o papel da MO como promotor da absorção cutânea de moléculas lipofílicas, tema ainda pouco explorado na literatura. MO foi dispersa em propilenoglicol, a concentrações de 0% (controle), 5%, 10% e 20% (p/p). Adicionou-se, em seguida, 0,6% (p/p) de PG a cada uma das formulações. O perfil de permeação do hormônio foi então determinado in vitro durante 48 h, utilizando pele de porco em células de difusão do tipo Franz. MO a 5% (p/p) foi capaz de duplicar a permeação de PG em comparação ao controle e às concentrações mais elevadas de MO, assim como a retenção de PG no estrato córneo (SC) e epiderme e derme remanescentes (E+D). A velocidade de liberação de PG a partir das formulações foi investigada usando membranas de celulose e este estudo revelou que concentrações de MO superiores a 5% (p/p) impediram a liberacão de PG, o que de fato refletiu de forma negativa na permeação cutânea do hormônio. Concluindo, este trabalho demonstrou a viabilidade da adição de MO a uma formulação como um método simples para aumentar a permeação transdérmica de PG para uso em terapias de reposição hormonal. Por outro lado, altas concentrações de MO (de 10% a 20% p/p) controlam a liberação de PG e este efeito pode ser extrapolado para outras moléculas lipofílicas de baixa massa molecular.
Uniterms:
Monooleína/influência/permeação transdérmica; Progesterona/permeação transdérmica; Progesterona/retenção cutânea; Reposição hormonal/terapia transdérmica; Permeação cutânea.
INTRODUCTION
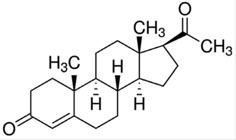
The use of oral progesterone (PG, Figure 1) is approved by the FDA and by other international regulatory agencies and is the most conventional strategy for hormone replacement therapy in menopausal women (Studd, 2014STUDD J. Hormone therapy for reproductive depression in women. Post Reprod. Health, v.20, n.4, p.132-137, 2014.).
Chemical structure of progesterone (PG) (MW = 314.46 g/mol; water solubility = 16.8 mg/L at 25 oC).
It is undeniable, however, that transdermal delivery of PG would be more advantageous than oral therapy, if considered the possibility to obtain a controlled and prolonged hormone delivery to the blood and the avoidance of first-pass metabolism in the liver, which guarantee a more consistent bioavailability of the hormonal substance (Silva et al., 2010SILVA, J.A.; APOLINÁRIO, A.C.; SOUZA, M.S.R.; DAMASCENO, B.P.G.L.; MEDEIROS, A.C.D. Administração cutânea de fármacos: desafios e estratégias para o desenvolvimento de formulações transdérmicas. Rev. Ciênc. Farm. Básica Apl., v.31, n.3, p.125-131, 2010.). In addition, the painless, non-invasive and easy administration of transdermal patches and formulations can improve patient acceptance (Martins, Veiga, 2002MARTINS, M.R.; VEIGA, F. Promotores de permeação para a liberação transdérmica de fármacos: uma nova aplicação para as ciclodextrinas. Braz. J. Pharm. Sci., v.38, n.1, p.33-54, 2002.).
Many papers evaluating transdermal creams and gels containing PG for treating menopausal related symptoms have been published over the years (Chang et al., 1995CHANG, K.J.; LEE, T.T.; LINARES-CRUZ, G.; FOURNIER, S.; DE LIGNIERES, B. Influences of percutaneous administration of estradiol and progesterone on human breast epithelial cell cycle in vivo. Fertil. Steril., v.63, n.4, p.785-791, 1995.; Du et al., 2013DU, J.Y.; SANCHEZ, P.; KIM, L.; AZEN, C.G.; ZAVA, D.T.; STANCZYK, F.Z. Percutaneous progesterone delivery via cream or gel application in postmenopausal women: a randomized cross-over study of progesterone levels in serum whole blood saliva capillary blood. Menopause, v.20, p.1107-1226, 2013.; Leonetti et al., 2005LEONETTI, H.B.; LANDES, J.; STEINBERG, D.; ANASTI, J.N. Transdermal progesterone cream as an alternative progestin in hormone therapy. Altern. Ther. Health Med., v.11, n.6, p.36-38, 2005.). However, PG potential in preventing estrogen-stimulated cell proliferation has not yet been fully explored because current formulations and devices are unable to deliver therapeutically relevant progesterone concentrations (Zava et al., 2014ZAVA, D.T.; GROVES, M.N.; STANCZYK, F.Z. Percutaneous absorption of progesterone. Maturitas, v.77, n.2, p.91-92, 2014.).
In this context, some research groups have devoted their efforts in using many different strategies to overcome the stratum corneum (SC) barrier of skin. Swarnakar et al. (2007SWARNAKAR, N.K.; JAIN, V.; DUBEY, V.; MISHRA, D.; JAIN, N.K. Enhanced oromucosal delivery of progesterone via hexosomes. Pharm. Res., v.24, n.12, p.2223-2230, 2007.), for instance, prepared and characterized lipid-based hexosomes for oral administration of PG, but, to our knowledge, no further study from the group evaluated cutaneous permeation. A more recent study determined the efficacy of a transdermal nanostructured formulation of PG combined with estriol, and preliminary clinical studies demonstrated the system was safe and effective (Botelho et al., 2014BOTELHO, M.A.; QUEIROZ, D.B.; BARROS, G.; GUERREIRO, S.; FECHINE, P.; UMBELINO, S.; LYRA, A.; BORGES, B.; FREITAS, A.; QUEIROZ, D.C.; RUELA, R.; ALMEIDA, J.G.; QUINTANS, L. JR. Nanostructured transdermal hormone replacement therapy for relieving menopausal symptoms: a confocal Raman spectroscopy study. Clinics, v.69, n.2, p.75-82, 2014.).
A simpler approach for PG delivery would consist on the use of absorption enhancers incorporated in PG transdermal formulations. Absorption enhancers reversibly decrease skin barrier resistance, allowing the drug to penetrating viable tissues to act locally and/or reach the systemic circulation (Alexander et al., 2012ALEXANDER, A.; DWIVEDI S.; AJAZUDDIN; GIRI, T.K.; SARAF, S.; SARAF, S.; TRIPATHI, D.K. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J. Control. Release, v.164, n.1, p.26-40, 2012.). Monoolein (MO, Figure 2) is one of the most studied absorption enhancer (Simonetti et al., 2009SIMONETTI, L.D.; GELFUSO, G.M.; BARBOSA, J.C.; LOPEZ, R.F. Assessment of the percutaneous penetration of cisplatin: the effect of monoolein and the drug skin penetration pathway. Eur. J. Pharm. Biopharm. v.73, n.1, p.90-94, 2009.; Herai et al., 2007HERAI, H.; GRATIERI, T.; THOMAZINE, J.A.; BENTLEY, M.V.L.B.; LOPEZ, R.F.V. Doxorubicin skin penetration from monoolein-containing propylene glycol formulations., Int. J. Pharm. v.329, n.1-2, p.88-93, 2007.; Steluti et al., 2001STELUTI, R.; DE ROSA, F.S.; COLLET, J.H.; BENTLEY, M.V.L.B. Influence of monoolein on 384 in vivo protoporphyrin IX accumulation in hairless mouse skin induced by 5- 385 aminolevulinic acid. In: VI PHARMATECH: ANUAL MEETING OF THE SBTF, 6., 2001, Recife. Proceedings. Recife: SBTF, 2001.; Lopes, Collett, Bentley, 2005LOPES, L.B.; COLLETT, J.H.; BENTLEY, M.V.L.B. Topical delivery of cyclosporin A: an in vitro study using monoolein as a penetration enhancer. Eur. J. Pharm. Biopharm., v.60, n.1, p.25-30, 2005.; Pereira et al., 2002PEREIRA, G.R.; COLETT, J.H.; GARCIA, S.B., THOMAZINI, J.A.; BENTLEY, M.V. Glycerol monooleate/solvents systems for progesterone transdermal delivery: in vitro permeation and microscopic studies, Braz. J. Pharm. Sci. v.38, n.1, p.55-62, 2002.; Puglia et al., 2013PUGLIA, C.; CARDILE, V.; PANICO, A.M.; CRASCÌ, L.; OFFERTA, A.; CAGGIA, S.; DRECHSLER, M.; MARIANI, P.; CORTESI, R.; ESPOSITO, E. Evaluation of monooleine aqueous dispersions as tools for topical administration of curcumin: characterization, in vitro and ex-vivo studies. J. Pharm. Sci., v.102, n.1, p.2349-2361, 2013.). It is pharmacologically inert, non-toxic, immediate and reversible in action, non-irritating, non-allergenic, odorless, colorless and chemically and physically compatible with many drugs and pharmaceutical excipients (Hadgraft, 1999HADGRAFT, J. Passive enhancement strategies in topical and transdermal drug delivery. Int. J. Pharm., v.184, n.1, p.1-6, 1999.; Qiu, Caffrey, 2000QIU, H.; CAFFREY, M. The phase diagram of the monoolein/water system: metastability and equilibrium aspects. Biomaterials, v.21, n.3, p.223-234, 2000.). MO is able to interact with phospholipid bilayers of SC lipid matrix and destabilize its structure momentarily (Pereira et al., 2002PEREIRA, G.R.; COLETT, J.H.; GARCIA, S.B., THOMAZINI, J.A.; BENTLEY, M.V. Glycerol monooleate/solvents systems for progesterone transdermal delivery: in vitro permeation and microscopic studies, Braz. J. Pharm. Sci. v.38, n.1, p.55-62, 2002.). However, due to its high lipophilicity, MO is rarely incorporated in formulations containing lipophilic active agents, such as PG, since high interaction with the absorption enhancer could hinder drug release, hindering drug transdermal permeation.
In this way, this work aimed to investigate in vitro MO influence on PG transdermal delivery and skin retention, which could bring more information about the role of MO as absorption enhancer for lipophilic molecules, a still little explored matter in scientific literature.
MATERIAL AND METHODS
Material
PG was kindly provided by "Farmacotécnica Farmácia de Manipulação" (Brasília, Brazil). MO (gliceril oleate, ≥ 99%) was purchased from Sigma-Aldrich (Steinheim, Germany). Propylene glycol used to prepared formulations, as well as to be placed in receptor solution, was purchased from Dinâmica Química Ltda. (São Paulo, Brazil). Monobasic and dibasic sodium phosphate (Vetec, Rio de Janeiro, Brazil), and sodium chloride (Serva, Rio de Janeiro, Brazil) were all used in buffer preparation, and sodium hydroxide (Dinâmica Química Ltda., São Paulo, Brazil) was used to adjust the pHs of the buffer and formulations. Regenerated Cellulose Dialysis Tubing (Dialysis Tubing MWCO 12000-14000, Fisherbrand) used in release studies was purchased from Fisher Scientific (Leicestershire, United Kingdom). Methanol and acetonitrile, used for extraction and chromatographic analyses, were of HPLC grade and purchased from Tedia Brazil Ltda. (Rio de Janeiro, Brazil). The water used in all preparations was of Milli Q grade (Millipore, France).
Skin
Porcine skin was kindly provided by the Frigorific Bonasa (Brasília, Brazil). Porcine ears were obtained soon after animal sacrifice, before the scalding process. The whole skin was removed from the outer region of the ear, separated from its underlying layer with scissors and stored frozen at -4 °C for a maximum of 1 month before use.
Formulations
Formulations were prepared by adding MO at 5%, 10% or 20% (w/w) to propylene glycol and heated to 40 °C for complete homogenization. After cooling, 0.6% (w/w) of PG was incorporated to each formulation and the pH adjusted to 5 with an aqueous solution of sodium hydroxide at 1 M. A control formulation was prepared without MO, i.e., simply dissolving 0.6% (w/w) of PG in propylene glycol and adjusting the pH to 5.
In vitro skin permeation
Permeation studies were carried out in modified "Franz" diffusion cells, mounted with small pieces (2 cm × 2 cm) of porcine ear skin separating the donor and receptor compartments. The receptor chamber was filled with a solution prepared with phosphate buffer (pH 7.4) and propylene glycol in the ratio of 60:40 (v/v). The skin was hydrated for 30 minutes by adding 1 mL of buffer to the donor chamber prior the beginning of the experiments. After draw off the buffer, it was added the same volume for each of the formulations to be tested or the control formulation to the donor compartment, which was closed with Parafilm(r) to minimize formulation evaporation. The receptor solution was continuously stirred at 500 rpm for 48 h. Samples were collected in defined time intervals of 1 h, 3 h, 6 h, 12 h, 24 h, 30 h, 36 h and 48 h. A minimum of four replicates was performed for each formulation.
PG recovery from SC and reminiscent epidermis and dermis (E+D)
At the end of each permeation experiment, tape-stripping technique was performed to determine and differentiate PG penetration in SC from that in E+D. The skin was removed from the diffusion cell and placed onto a flat surface with the SC facing up. That part of skin, which had been in contact with the PG donor formulation, was cleaned with deionized water (water used to clean the skin was discarded). A plastic template with 1.7 cm2 of exhibition area was placed on the skin in order to leave exposed only the drug transport area. Then, the skin was tape-stripped 15 times, using Scotch Book Tape (3 M, St Paul, Minnesota). PG was determined after extraction of the drug from the tapes with methanol over a 12 h period. The remaining skin was cut into small pieces and placed in plastic tube along with methanol also over a period of 12 h for drug extraction. The resulting suspensions were filtered on 0.22 μm filters and quantified by HPLC.
The PG recovery from SC and E+D was previously validated and showed error percentages within the limits accepted for validation of methods involving extractive processes (± 15%) (FDA, 2000FOOD AND DRUG ADMINISTRATION. FDA. Guidance for Industry: analytical procedures and methods validation: chemistry, manufacturing, and controls documentation. Rockville: US Food and Drug Administration, 2000. 33 p.).
In vitro release
These studies were performed using the same modified Franz-type diffusion cells, but mounted with synthetic hydrophilic membranes of cellulose acetate separating the donor and receptor compartments instead of skin. The receptor compartment was filled with 15 mL of a receptor solution prepared with phosphate buffer (pH 7.4) and propylene glycol in the ratio of 60:40 (v/v). The receptor solution was continuously stirred by means of a spinning bar magnet, at 500 rpm. 1 mL of each tested formulation was placed on donor compartment and, from this time, samples from receptor compartment were collected every hour for 12 h. At the end of the experiment, the amount of the drug released across the membrane, i.e., the amount of PG released in the receptor solution, was determined analytically as described below, and release kinetics of PG from each MO formulation was determined.
Analytical analysis
PG was quantified by an analytical method using a high performance liquid chromatograph (HPLC) Shimadzu LC-20 AD, composed by two pumps (model LC 20-AT), automatic gun (model 9SIL-20AD) and oven (model CTO - 20th century), coupled to a spectrophotometric detector (model SPD-M20A) and a computer equipped with the chromatographic analysis program Shimadzu LC. A reverse-phase column (Dionex 4.0 × 125 mm, 5 µm) was used as stationary phase, and mobile phase consisted of a mixture of water: acetonitrile (70: 30) (v/v). The mobile phase flow rate was 1 mL/min, the sample injection volume was 50 μL, the oven was used at 40 ºC and detection was made at 244 nm. The method was validated in terms of linearity, precision, accuracy, specificity/selectivity and limit of quantification, according to current legislation (FDA, 2000FOOD AND DRUG ADMINISTRATION. FDA. Guidance for Industry: analytical procedures and methods validation: chemistry, manufacturing, and controls documentation. Rockville: US Food and Drug Administration, 2000. 33 p.).
Data analyses
Three to five replicates of each transport experiment were performed. Results are presented as mean ± standard deviation (SD) and expressed in terms of the quantity of PG per unit area of skin (μg/cm2). Linear regressions were obtained with Microsoft Excel 2007. Statistical analyses were performed with the program GraphPad Prism. Statistical significance was fixed at p < 0.05.
RESULTS AND DISCUSSION
PG is a lipophilic, relatively small and non-charged molecule. It presents log P = 3.5, features which are generally required for proper SC permeation. However, its potency is not high enough to assure high blood concentrations for long time periods (Zava et al., 2014ZAVA, D.T.; GROVES, M.N.; STANCZYK, F.Z. Percutaneous absorption of progesterone. Maturitas, v.77, n.2, p.91-92, 2014.). This study aimed to investigate the feasibility of MO to be used as permeation enhancer in formulations incorporating PG for enhancing its transdermal delivery. For that, MO was dispersed in propylene glycol in three different concentrations and PG permeation from these formulations were evaluated relatively to a control (without MO addition).
Figure 3 shows PG in vitro permeation profiles for 48 h as a function of MO concentration in the formulations.
PG permeation profile through porcine skin from formulations containing 0% w/w (Control) (■), 5% w/w (●), 10% w/w (▲) and 20% w/w (×) of MO. Each point represents the average value for 4-5 determinations and the vertical bars represent the standard deviation (± SD).
Although PG does permeate the skin passively, permeated levels up to the first 24 h of experiment were under method's limit of quantification (LOQ = 500 ng/mL). Even until 36 h, PG levels were near the LOQ for control formulation and formulations with 10% and 20% of MO.
It is clear from Figure 3 that 5% (w/w) of MO in formulation was sufficient to increase in approximately 2-fold PG permeation when compared to control. Pereira et al. (2002PEREIRA, G.R.; COLETT, J.H.; GARCIA, S.B., THOMAZINI, J.A.; BENTLEY, M.V. Glycerol monooleate/solvents systems for progesterone transdermal delivery: in vitro permeation and microscopic studies, Braz. J. Pharm. Sci. v.38, n.1, p.55-62, 2002.) studied transdermal delivery of PG from MO dispersed in mineral oil (at 20%) and observed a 3-fold enhance in PG delivery over control (240 and 80 µg/cm2, respectively, after 48 h of experiment). However, authors used mice skin as a model, whose SC is much thinner than porcine and human skin, compromising the comparison with the data presented in this paper.
Despite of the increase in PG permeation provided by 5% (w/w) MO in formulation, 20% (w/w) of MO dramatically decreased (in about 50%) PG permeation through porcine skin (P > 0.05). Steluti et al. (2001STELUTI, R.; DE ROSA, F.S.; COLLET, J.H.; BENTLEY, M.V.L.B. Influence of monoolein on 384 in vivo protoporphyrin IX accumulation in hairless mouse skin induced by 5- 385 aminolevulinic acid. In: VI PHARMATECH: ANUAL MEETING OF THE SBTF, 6., 2001, Recife. Proceedings. Recife: SBTF, 2001.) observed an increase in permeation of aminolevulinic acid (ALA) through mouse skin from all formulations containing the same range of MO concentrations (5%, 10%, and 20%), while Simonetti et al. (2009SIMONETTI, L.D.; GELFUSO, G.M.; BARBOSA, J.C.; LOPEZ, R.F. Assessment of the percutaneous penetration of cisplatin: the effect of monoolein and the drug skin penetration pathway. Eur. J. Pharm. Biopharm. v.73, n.1, p.90-94, 2009.), evaluating the effect MO on cisplatin (CIS) permeation, showed an increase in drug flux with the increase, up to 10%, of the enhancer. It is interesting to notice that, as molecule hydrosolubility decreases, (ALA>CIS>PG), a progressive annulment of MO enhancement effect is observed. A possible reason for this is that high MO concentrations increase formulation lipophilicity, hindering drug release.
To test the above-mentioned hypothesis, release profile of PG from MO formulations was assessed using cellulose membrane, and the results are presented in Figure 4.
PG release profiles of the PG in propylene glycol formulations (0.6% m/m) containing different concentrations of MO: Control (■); 5% (●), 10% (▲) and 20% (×). Each point represents the average of 4 experiments and the vertical bars represent the standard deviation of the mean (± SD).
As expected, only 5% (w/w) of MO in the formulation did not increase lipophilicity highly enough to influence PG release in comparison with control (P > 0.05). Instead, MO at 10% or 20% (w/w) hindered the hormone release (P < 0.05). As it can be seen in Figure 3, this effect had a significant influence on the permeation of PG through the skin.
The data presented in Table I shows clearer that MO at 5% (w/w) was not able to influence PG release kinetics, while at 10% and 20% (w/w) decreased by 2 and 2.5-fold the hormone release flux.
Similar to our results, Herai et al. (2007HERAI, H.; GRATIERI, T.; THOMAZINE, J.A.; BENTLEY, M.V.L.B.; LOPEZ, R.F.V. Doxorubicin skin penetration from monoolein-containing propylene glycol formulations., Int. J. Pharm. v.329, n.1-2, p.88-93, 2007.) observed that the addition of 5% (w/w) of MO in formulation did not alter significantly the release rate of the chemotherapeutic agent, doxorubicin, while higher MO concentrations did. Herai et al. attributed this effect to an increase in lipophilicity or viscosity provided by MO in the vehicle (Herai et al., 2007HERAI, H.; GRATIERI, T.; THOMAZINE, J.A.; BENTLEY, M.V.L.B.; LOPEZ, R.F.V. Doxorubicin skin penetration from monoolein-containing propylene glycol formulations., Int. J. Pharm. v.329, n.1-2, p.88-93, 2007.). Taken the data together, the effect of lipophilicity provided by MO must be much more pronounced than any viscosity increment it could provide to the formulation, since Simonetti et al. (2009SIMONETTI, L.D.; GELFUSO, G.M.; BARBOSA, J.C.; LOPEZ, R.F. Assessment of the percutaneous penetration of cisplatin: the effect of monoolein and the drug skin penetration pathway. Eur. J. Pharm. Biopharm. v.73, n.1, p.90-94, 2009.) demonstrated that the addition of MO did not alter release rate of the more hydrophilic CIS, in any concentration it was tested.
Figure 5 summarizes the data of PG recovered from SC, E+D and receptor solution after 48 h of skin treatment with formulations containing 0%, 5%, 10% or 20% (w/w) of MO.
PG recovered from the SC, reminiscent E+D and receptor solution after 48 h of treatment with formulations containing MO at different concentrations. Bars represent the average of 4-5 experiments and vertical bars represent the standard deviation of the mean (± SD). *Significant difference from control in SC samples (P < 0.05); #Significant difference from control in E + D samples (P < 0.05); $Significant difference from control in receptor samples (P < 0.05).
The formulation containing 5% MO (w/w) not only doubled the PG amount permeated, as it also increased hormone retention in reminiscent skin (E + D). Interestingly, 10% of MO (w/w), even restricting PG release, was still capable of retaining a great amount of PG in E + D, even though this high skin retention was not able to guarantee higher transdermal flux of the hormone.
CONCLUSION
In conclusion, the data presented in this work demonstrated the feasibility of MO addition as a simple method to increase transdermal delivery of PG for the therapy of hormonal replacement. It was shown that, depending on the concentration added to the topical and transdermal formulation, MO could cause opposite effects, i.e., while small concentration of MO (5% w/w) can enhance about 2-fold transdermal delivery of the lipophilic molecule, higher concentrations (from 10% to 20% w/w) of MO significantly reduces PG skin permeation as a result of a more pronounced controlling effect in drug release. Such conclusion is corroborated by the 2-fold reduction in PG release observed at higher MO concentrations.
ACKNOWLEDGEMENTS
The authors would like to thank the Brazilian Funding Agencies CAPES, CNPq and FAP-DF, and the University of Brasília for supporting this research.
REFERENCES
- ALEXANDER, A.; DWIVEDI S.; AJAZUDDIN; GIRI, T.K.; SARAF, S.; SARAF, S.; TRIPATHI, D.K. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J. Control. Release, v.164, n.1, p.26-40, 2012.
- BOTELHO, M.A.; QUEIROZ, D.B.; BARROS, G.; GUERREIRO, S.; FECHINE, P.; UMBELINO, S.; LYRA, A.; BORGES, B.; FREITAS, A.; QUEIROZ, D.C.; RUELA, R.; ALMEIDA, J.G.; QUINTANS, L. JR. Nanostructured transdermal hormone replacement therapy for relieving menopausal symptoms: a confocal Raman spectroscopy study. Clinics, v.69, n.2, p.75-82, 2014.
- CHANG, K.J.; LEE, T.T.; LINARES-CRUZ, G.; FOURNIER, S.; DE LIGNIERES, B. Influences of percutaneous administration of estradiol and progesterone on human breast epithelial cell cycle in vivo. Fertil. Steril., v.63, n.4, p.785-791, 1995.
- DU, J.Y.; SANCHEZ, P.; KIM, L.; AZEN, C.G.; ZAVA, D.T.; STANCZYK, F.Z. Percutaneous progesterone delivery via cream or gel application in postmenopausal women: a randomized cross-over study of progesterone levels in serum whole blood saliva capillary blood. Menopause, v.20, p.1107-1226, 2013.
- FOOD AND DRUG ADMINISTRATION. FDA. Guidance for Industry: analytical procedures and methods validation: chemistry, manufacturing, and controls documentation. Rockville: US Food and Drug Administration, 2000. 33 p.
- HADGRAFT, J. Passive enhancement strategies in topical and transdermal drug delivery. Int. J. Pharm., v.184, n.1, p.1-6, 1999.
- HERAI, H.; GRATIERI, T.; THOMAZINE, J.A.; BENTLEY, M.V.L.B.; LOPEZ, R.F.V. Doxorubicin skin penetration from monoolein-containing propylene glycol formulations., Int. J. Pharm. v.329, n.1-2, p.88-93, 2007.
- LEONETTI, H.B.; LANDES, J.; STEINBERG, D.; ANASTI, J.N. Transdermal progesterone cream as an alternative progestin in hormone therapy. Altern. Ther. Health Med., v.11, n.6, p.36-38, 2005.
- LOPES, L.B.; COLLETT, J.H.; BENTLEY, M.V.L.B. Topical delivery of cyclosporin A: an in vitro study using monoolein as a penetration enhancer. Eur. J. Pharm. Biopharm., v.60, n.1, p.25-30, 2005.
- MARTINS, M.R.; VEIGA, F. Promotores de permeação para a liberação transdérmica de fármacos: uma nova aplicação para as ciclodextrinas. Braz. J. Pharm. Sci., v.38, n.1, p.33-54, 2002.
- PEREIRA, G.R.; COLETT, J.H.; GARCIA, S.B., THOMAZINI, J.A.; BENTLEY, M.V. Glycerol monooleate/solvents systems for progesterone transdermal delivery: in vitro permeation and microscopic studies, Braz. J. Pharm. Sci. v.38, n.1, p.55-62, 2002.
- PUGLIA, C.; CARDILE, V.; PANICO, A.M.; CRASCÌ, L.; OFFERTA, A.; CAGGIA, S.; DRECHSLER, M.; MARIANI, P.; CORTESI, R.; ESPOSITO, E. Evaluation of monooleine aqueous dispersions as tools for topical administration of curcumin: characterization, in vitro and ex-vivo studies. J. Pharm. Sci., v.102, n.1, p.2349-2361, 2013.
- QIU, H.; CAFFREY, M. The phase diagram of the monoolein/water system: metastability and equilibrium aspects. Biomaterials, v.21, n.3, p.223-234, 2000.
- STUDD J. Hormone therapy for reproductive depression in women. Post Reprod. Health, v.20, n.4, p.132-137, 2014.
- SILVA, J.A.; APOLINÁRIO, A.C.; SOUZA, M.S.R.; DAMASCENO, B.P.G.L.; MEDEIROS, A.C.D. Administração cutânea de fármacos: desafios e estratégias para o desenvolvimento de formulações transdérmicas. Rev. Ciênc. Farm. Básica Apl., v.31, n.3, p.125-131, 2010.
- SIMONETTI, L.D.; GELFUSO, G.M.; BARBOSA, J.C.; LOPEZ, R.F. Assessment of the percutaneous penetration of cisplatin: the effect of monoolein and the drug skin penetration pathway. Eur. J. Pharm. Biopharm. v.73, n.1, p.90-94, 2009.
- STELUTI, R.; DE ROSA, F.S.; COLLET, J.H.; BENTLEY, M.V.L.B. Influence of monoolein on 384 in vivo protoporphyrin IX accumulation in hairless mouse skin induced by 5- 385 aminolevulinic acid. In: VI PHARMATECH: ANUAL MEETING OF THE SBTF, 6., 2001, Recife. Proceedings. Recife: SBTF, 2001.
- SWARNAKAR, N.K.; JAIN, V.; DUBEY, V.; MISHRA, D.; JAIN, N.K. Enhanced oromucosal delivery of progesterone via hexosomes. Pharm. Res., v.24, n.12, p.2223-2230, 2007.
- ZAVA, D.T.; GROVES, M.N.; STANCZYK, F.Z. Percutaneous absorption of progesterone. Maturitas, v.77, n.2, p.91-92, 2014.
Publication Dates
-
Publication in this collection
Oct-Dec 2015
History
-
Received
25 Nov 2014 -
Accepted
28 May 2015