abstract
A series of N-substituted 2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamides (8a-w) was synthesized in three steps. The first step involved the sequential conversion of 2-(1H-indol-3-yl)acetic acid (1) to ester (2) followed by hydrazide (3) formation and finally cyclization in the presence of CS2 and alcoholic KOH yielded 5-(1H-indole-3-yl-methyl)-1,3,4-oxadiazole-2-thiol (4). In the second step, aryl/aralkyl amines (5a-w) were reacted with 2-bromoacetyl bromide (6) in basic medium to yield 2-bromo-N-substituted acetamides (7a-w). In the third step, these electrophiles (7a-w) were reacted with 4 to afford the target compounds (8a-w). Structural elucidation of all the synthesized derivatives was done by 1H-NMR, IR and EI-MS spectral techniques. Moreover, they were screened for antibacterial and hemolytic activity. Enzyme inhibition activity was well supported by molecular docking results, for example, compound 8q exhibited better inhibitory potential against α-glucosidase, while 8g and 8b exhibited comparatively better inhibition against butyrylcholinesterase and lipoxygenase, respectively. Similarly, compounds 8b and 8c showed very good antibacterial activity against Salmonella typhi, which was very close to that of ciprofloxacin, a standard antibiotic used in this study. 8c and 8l also showed very good antibacterial activity against Staphylococcus aureus as well. Almost all compounds showed very slight hemolytic activity, where 8p exhibited the least. Therefore, the molecules synthesized may have utility as suitable therapeutic agents.
Uniterms:
1H-indol-3-acetic acid; N-substituted 2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl] sulfanyl}acetamides/antibacterial activity; N-substituted 2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol- 2-yl]sulfanyl} acetmides/hmolytic activity; α-Glicosidase; Butirylcholinesterase; Lipoxygenase
resumo
Uma série de acetamidas 2-{[5-(1H-indol-3-ilmetil)-1,3,4-oxadiazol-2-il]sulfanila} N-substituídas (8a-w) foi sintetizada em três fases. A primeira etapa envolveu a conversão sequencial de ácido 2-(1H-indol-3-il)acético (1) a éster (2), seguido por hidrazida (3) e, finalmente, a e ciclização na presença de CS2 e KOH alcoólico produziu 5-(1H-indol-3-il- metil)-1,3,4-oxadiazole-2-tiol (4). Na segunda etapa, aminas arílicas/aralquílicas(5a-w) reagiram com brometo de 2-bromoacetila (6), em meio básico, para se obter acetamidas 2-bromo-N-substituídas (7a-w). Na terceira etapa, estes eletrófilos (7a- w) reagiram com 4, para se obter os compostos alvo (8a-w). A elucidação estrutural de todos os derivados sintetizados foi realizada por 1H-NMR, IR e técnicas de espectrometria de EI-MS. Além disso, eles foram submetidos a triagem de atividade antibacteriana e hemolítica. Análise da inibição enzimática foi bem apoiada pelos resultados de docking molecular. Por exemplo, o composto 8q exibiu melhor potencial inibitório contra α-glicosidase, e os compostos 8g e 8b exibiram, comparativamente, melhor inibição contra butirilcolinesterase (BChE) elipoxigenase (LOX), respectivamente. Do mesmo modo os compostos 8b e 8c mostraram excelente potencial antibacteriano contra SalmonellaTyphi, semelhante ao do ciprofloxacino, antibiótico padrão usado neste estudo. Os compostos 8c e 8l também mostraram excelente potencial antibacteriano contra Staphylococcus aureus . Quase todos os compostos mostraram pequena atividade hemolítica, sendo que o composto 8p apresentou menor atividade. Assim, as moléculas sintetizadas podem ter a sua utilidade como agentes terapêuticos adequados.
Unitermos:
Ácido 1H-indol-3-acético; Acetamidas 2-{[5-(1H-indol-3-ilmetil)-1,3,4-oxadiazol-2-il]sulfanila}N-substituídas/atividade antibacteriana; Acetamidas 2-{[5-(1H-indol-3-ilmetil)-1,3,4-oxadiazol-2-il]sulfanila}N-substituídas/atividade hemolítica; α-Glicosidase; Butirilcolinesterase; Lipoxigenase
INTRODUCTION
Oxadiazoles comprise four different classes, where 1,3,4-oxadiazoles are more important and are present in a number of biologically active molecules. (Renukadevi, Birada, 1999). Due to the propensity of the 1,3,4-oxadiazole ring to undergo a variety of electrophilic and nucleophilic substitution reactions, a large number of derivatives have been synthesized that have remarkable pharmacological, medicinal, and biological activities such as antimicrobial (Rakesh et al., 2010; Zuhair et al., 2008), anti-inflammatory (Dewangan et al., 2010DEWANGAN, D.; PANDEY, A.; SIVAKUMAR, T.; RAJAVEL, R.; DUBEY, R.D. Synthesis of some novel 2, 5-disubstituted 1, 3, 4-oxadiazole and its analgesic, anti-inflammatory, anti-bacterial and anti-tubercular activity. Int. J. Chem. Tech. Res.v.2, p.1397-1412, 2010.; Omar, Mahfouz, Rahman, 1996), cytotoxic and hypoglycemic (Hasan et al., 2011HASAN, A.; GAPIL, S.; KHAN, I. Synthesis, characterization and antifungal activity of some new 5-Substituted 1,3,4-oxadiazole-2-thiols. Asian J. Chem., v.23, p.2007-2010, 2011. ), anticancer (Bhatnacar et al., 1986BHATNACAR, S.; KAMTHAN, D.; MEHRA, S.C.; TANDAN, S.K. Synthesis of some new N3-substituted hydantions as potential anticonvulsant agents. Ind. J. Pharm., v.18, p.235-238, 1986.), anticonvulsive (Yar, Akhter, 2009YAR, M.S.; AKHTER, M.W. Synthesis and anticonvulsant activity of substituted oxadiazole and thiadiazole derivatives. Acta Pol. Pharm. v.66, n.4, p.393-397, 2009.), antitubercular (Ali, Shaharyar, 2007ALI, M.A.; SHAHARYAR, M. Oxadiazole mannich bases: synthesis and antimycobacterial activity. Bioorg. Med. Chem. Lett., v.17, n.12, p.3314-3316, 2007.), and fungicidal and insecticidal (Zou, Zhang, Jin, 2002ZOU, X.; ZHANG, Z.; JIN, G. Synthesis and biological activity of 1,3,4-Oxadiazole-substituted pyridazinones. J. Chem. Res., v.5, p.228-230, 2002.; Holla et al., 2004HOLLA, B.; PRASNNA, S.C.S.; BOJA, P.; RAO, K.S.; BHATT, S.; GANESH, U. Synthesis and insecticidal activity of some 1,3,4-Oxadiazoles derived from 2-chloropyridine-5-acetic acid. Indian J. Chem. v.43, p.864-8, 2004.). Indole derivatives display a wide range of biological activities. The indole ring is found in the hormones serotonin and melatonin, amino acid tryptophan, the anti- inflammatory drug indomethacin, the psychotropic drug LSD and the antitumor agents vinblastine and vincristine (Murphy et al., 1997MURPHY, J.A.; SCOTT, K.A.; SINCLAIR, R.S.; LEWIS, N. A new synthesis of indoles. Tetrahedron Lett., v.38, p.7295-7298, 1997.). Coronaridine and voacangine are indole alkaloids that have antileishmanial, antitumor, diuretic and hypoglycemic activities (Camila, Paulo, Meireles, 2007). The indole moiety has been incorporated as a core skeleton to access active sites, implicated in enzyme inhibitions (Lednicer, 2007LEDNICER, D. The organic chemistry of drug synthesis. New Jersey: A John Wiley & Sons, 2007. ??? p.). Some indole derivatives are also identified as good inhibitors of 5- lipoxygenase (LOX) (Hutchinson et al., 1995HUTCHINSON, J.H.; CHARLESON, S.; EVANS, J.F.; FALGUEYRET, J.P.; HOOGSTEEN, K., JONES, T.R.; KARGMAN, S.; MACDONALD, D.; McFARLAVNE, C.S.; NICHOLSON, D.W. Thiopyranol[2,3,4-c,d]indoles as inhibitors of 5-lipoxygenase, 5-lipoxygenase-activating protein, and leukotriene C4 synthase. J. Med. Chem., v.38, n.22, p.4538-4547, 1995.; Yar et al., 2014YAR, M.; ASHRAF, M.; NAQVI, S.A.R.; SIDRA, L.R.; NASAR, R.; KHAN, Z.A.; ELENI PONTIKI, E.; MUSHTAQ, N.; KHAN, I.U.; MAHMOOD, N.; SHAHZAD, S.A. Synthesis, in vitro lipoxygenase inhibition, docking study and thermal stability analyses of novel indole derivatives, Non-isothermal kinetic study of potent LOX inhibitor N'-(diphenylmethylene) -2-(1H-indole-3-yl) acetohydrazide. J. Iran. Chem. Soc., v.11, p.369-378, 2014.). LOX is a lipid-peroxidizing enzyme, which shows an essential role in leukotriene biosynthesis and mediates the pathophysiology of allergic disorders, inflammatory reactions and neo-angiogenesis (Bhattacharjee, 2005BHATTACHARJEE, S. Reactive oxygen species and oxidative burst: Roles in stress, senescence and signaltransduction in plants. Curr. Sci., v.89, p.1113-1121, 2005.; Jampilek et al., 2006JAMPILEK, J.; DOLEZAL, M.; OPLETALOVA, V.; HARTL, J. 5-Lipoxygenase, leukotrienes biosynthesis and potential antileukotrienic agents. Curr. Med. Chem., v.13, p.117-129, 2006.; Pommery et al., 2004POMMERY, N.; TAVERNE, T.; TELLIEZ, A.; GOOSSENS, L.; CHARLIER, C. J. ; POMMERY, J.; GOOSSENS, J.F.; HOUSSIN, R.; DURANT, F.; HÉNICHART, J.P. New COX-2/5-LOX inhibitors: apoptosis-inducing agents potentially useful in prostate cancer chemotherapy., J. Med. Chem. v.47, n.25, p.6195-6206, 2004.). Cholinesterases are well-known enzymes that are present in cholinergic and non-cholinergic tissues and in plasma and other body fluids of animals (Ryhanen, 1983). Butrylcholinesterase (BChE) is present in intestine, liver, heart, kidneys and lungs (Dave et al., 2000DAVE, K.R.; SYAL, A.R.; KATYARE, S.S. Tissue cholinesterases. A comparative study of their kinetic properties. Z. Naturforsch. C., v.55, n.1-2, p.100-108, 2000.). BChE hydrolyzes ester-containing drugs and scavenges cholinesterase inhibitors, including potent organophosphorus nerve agents before reaching the synaptic targets (Silver, 1974). Inhibitors of the enzyme α-glucosidase are molecules that are used as oral anti-diabetic drugs in the treatment of type-2 diabetes mellitus. The inhibitors of this enzyme can delay the release of D-glucose from disaccharides and oligosaccharides from dietary carbohydrates and slow down glucose absorption, resulting in reduced postprandial hyperglycemia (Lebovitz, 1997LEBOVITZ, H.E. Alpha-glucosidase inhibitors. Endocrinol. Metab. Clin. North Am., v.26, n.3, p.539-551, 1997.). Therefore, inhibition of α-glucosidase is considered important in managing type-2 diabetes. Cytotoxicity of synthesized compounds in terms of hemolytic activity may be studied to assess the possible toxicity profile of the most active compounds. Shirinzadeh et al. (2010)SHIRINZADEH, H.; EREN, B.; GURER-ORHAN, H.; SUZEN, S.; ÖZDEN, S. Novel indole-based analogs of melatonin: synthesis and in vitro antioxidant activity studies. Moleculesv.15, n.4, p.2187-2202, 2010. studied indole hydrazide/hydrazone derivatives for their inhibitory effect on AAPH-induced hemolysis of human erythrocytes and found most of the compounds had significantly high antioxidant activity, when compared to melatonin. A new series of 2-[[5-alkyl/aralkyl-1,3,4-oxadiazol-2-yl]thio]-N-[4-(4-morpholinyl)phenyl]acetamides was evaluated by Akhtar et al. (2014AKHTAR, M.N.; GUL, S.; REHMAN, A-UR.; ABBASI, M. A.; KHAN, K.M.; NAFEESA, K.; SIDDIQA, A.; SHAHID, M.; SUBHANI, Z. Synthesis, antimicrobial evaluation and hemolytic activity of 2-[[5-alkyl/aralkyl substituted-1,3,4-oxadiazol-2-yl]thio]-N-[4-(4-morpholinyl)phenyl]acetamide derivatives J. Saudi Chem. Soc., in press, 2014.) for antimicrobial and hemolytic activity; most of them were found to be active against the selected microbial species to a variable extent relative to reference standards, and showed less toxicity as well.
The aim of present study was to design and synthesize some novel poly-functional compounds having 1,3,4-oxadiaozole, indole and substituted acetamide moieties together in one molecule, which may prove to be potent antibacterial and anti-enzymatic agents and which could be studied further for pharmacological purposes.
MATERIAL AND METHODS
General
The chemicals employed in this study were of analytical grade and purchased from Merck and Alfa Aesar. All solvents were distilled before use. Melting points of the synthesized compounds were recorded on a Gallenkamp melting point apparatus by open capillary tube and were uncorrected. To observe the progress of the reaction, thin layer chromatography (TLC) was carried out on pre-coated silica gel G-25-UV254 plates using ethyl acetate and n-hexane in various proportions as mobile phase. Spots were visualized at 254 nm under a UV lamp. IR spectra were recorded using the KBr pellet method with a HYPER IR spectrometer, and wave number was given in cm-1. Mass spectra (EI-MS) were recorded using a JOEL JMS 600H spectrometer, with a data system. Nuclear magnetic resonance spectra were recorded in DMSO-d6 on a Bruker spectrometer operating at 300-500 MHz, and chemical shifts (δ) were given in ppm.
Synthesis
Preparation of ethyl 2-(1H-indol-3-yl)acetate (2)
2-(1H-Indol-3-yl)acetic acid (20.0 g; 0.11 mol; 1) in absolute ethanol (60 mL) and catalytic amount of concentrated sulfuric acid (10 mL; 0.18 mol) were added to a 500-mL round-bottomed flask (RB), which was refluxed for 8 h until completion of the reaction. The reaction mixture was neutralized with 20 mL of 10% aqueous sodium carbonate. The product was isolated by the solvent extraction technique using chloroform (50x3 mL). The organic phase containing the ester was decanted off and washed a number of times with distilled water and dried using anhydrous MgSO4.
Preparation of 2-(1H-indol-3-yl)acetohydrazide (3)
Ethyl 2-(1H-indol-3-yl)acetate (19.0 mL; 2) in 30 mL of methanol and hydrazine hydrate (80%; 25 mL) was added to a 500-mL RB flask. The reaction mixture was stirred for 3 h at room temperature. After absolute conversion, the acid hydrazide was obtained by distilling methanol from the reaction mixture. The precipitates were filtered, washed with n-hexane and air-dried to yield pure 2-(1H-indol-3-yl)acetohydrazide (3).
Preparation of 5-(1H-indole-3-yl-methyl)-1,3,4-oxadiazole-2-thiol (4)
2-(1H-Indol-3-yl)acetohydrazide (20.0 g, 0.11 mol; 3) in absolute ethanol (30 mL) was added to a 500-mL RB flask. Potassium hydroxide (6.3 g, 0.11 mol) was added to the solution followed by the addition of carbon disulfide (14.0 mL; 0.22 mol) and the mixture refluxed with stirring for 6 h. Progress of the reaction was monitored by TLC, and on completion, it was diluted with distilled water (50 mL) and acidified with dilute hydrochloric acid to pH 5-6. The precipitates formed were filtered, washed with water and re-crystallized from ethanol to obtain pure 5-(1H-indole-3-yl-methyl)-1,3,4-oxadiazole-2-thiol (4) in good yield.
Preparation of N-substituted 2-bromoacetamide electrophiles (7a-w)
Preparation of N-substituted 2-bromoacetamide derivatives was carried out by reaction of various aryl/aralkyl amines (5a-w) with 2-bromoacetyl bromide (6) in equimolar quantities (0.001m) and shaking manually in 10% aqueous Na2CO3. Solid precipitates were formed after 10-20 min, filtered and washed with cold distilled water to obtain the desired electrophiles (7a-w).
Preparation of N-substituted 2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl} acetamides (8a-w)
Equimolar quantities of 5-(1H-indol-3-yl-methyl)-1,3,4-oxadiazole-2-thiol (0.001 mol; 4) and 2-bromo-N-substituted acetamides (7a-w); in N-N-dimethylformamide (7 mL) and NaH (0.002 mol) were reacted in a 25-mL RB flask. The reaction mixture was stirred for 8 h at 35 oC. After absolute conversion, the reaction mixture was poured on crushed ice; precipitates thus formed were filtered, washed with distilled water and dried to afford pure N-substituted 2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamides (8a-w) in good yields. The synthetic pathway is illustrated in Figure 1 and Table I (Sabahat et al., 2013).
Outline for synthesis of N-substituted 2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamides (8a-w).
Spectral characterization
Ethyl 1H-indole-3-acetate (2)
Brownish liquid; Yield: 79%; Molecular formula: C12H13N2O; Molecular Weight: 203 g/mol; IR (KBr) υmax: 3415 (N-H), 3037 (C-H Ar), 1633 (C=O), 1534 (Ar C=C); 1H-NMR (400 MHz): δ 10.9 (s, 1H, NH-1ʹ), 7.48 ( br.d, J = 8.0 Hz, 1H, H-4'), 7.34 (br.d, J = 8.0 Hz, 1H, H-7'), 7.23 (br.s, 1H, H-2'), 7.06 (t, J = 7.6 Hz, 1H, H-5'), 6.97 (t, J = 7.6 Hz, 1H, H-6'), 4.16 (q, J= 7.2, 2H, -OCH2CH3), 3.71 (s, 2H, CH2-10ʹ), 1.17 (t, J = 7.2 Hz, 3H, -OCH2CH3); EIMS: m/z 203 (C12H13N2O)˙+ [M]+, 158 (C10H8NO)+, 130 (C9H8N)+, 59 (C3H5O2)+.
2-(1H-Indol-3-yl)acetohydrazide (3)
Brownish crystals; Yield: 89%; m.p. 113 °C; Molecular formula: C10H11N3O; Molecular Weight: 189 g/mol; IR (KBr) υmax: 3431 (N-H), 3032 (C-H Ar), 1630 (C=O), 1529 (Ar C=C); 1H-NMR (400 MHz): δ 10.8 (s, 1H, NH-1ʹ), 9.08 (s, 1H, NHNH2), 7.55 ( br.d, J = 7.6 Hz, 1H, H-4'), 7.31 (br.d, J = 8.0 Hz, 1H, H-7'), 7.16 (br.s, 1H, H-2'), 7.04 (t, J = 7.2 Hz, 1H, H-5'), 6.95 (t, J = 7.6 Hz, 1H, H-6'), 4.16 ( br.s,1H, NHNH2) 3.43 (s, 2H, CH2-10ʹ); EIMS: m/z 189 (C10H11N3O)˙+ [M]+, 158 (C10H8NO)+, 130 (C9H8N)+, 59 (C3H5O2)+.
2-(1H-Indol-3-yl-methyl)-1,3,4-oxadiazole-5-thiol (4)
Dark brown powder; Yield: 76%; m.p. 125 °C; Molecular formula: C11H9N3OS; Molecular Weight: 231 gmol-1; IR (KBr) υmax: 3337 (N-H, stretching), 3085 (C-H, str. of aromatic ring), 1562 (C=C, aromatic str.), 1666 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 638 (C-S bond str.); 1H-NMR (400 MHz): δ 11.0 (s, 1H, NH-1ʹ), 7.49 ( br.d, J = 7.6 Hz, 1H, H-4'), 7.37 (br.d, J = 8.0 Hz, 1H, H-7'), 7.34 (br.s, 1H, H-2'), 7.09 (t, J = 7.6 Hz, 1H, H-5'), 7.00 (t, J = 7.6 Hz, 1H, H-6'), 4.20 (s, 2H, CH2-10ʹ). EIMS: m/z 233 (C11H9N3OS+2)˙+ [M+2]+, 231 (C11H9N3OS)˙+ [M]+, 158 (C10H8NO)+, 156 (C10H8N2)+, 130 (C9H8N)+.
N-Cyclohexyl-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamide (8a)
Light brown amorphous; Yield: 83%; m.p. 139 °C; Molecular formula C19H22N4O2S; Molecular weight: 370 g/mol; IR (KBr) υmax: 3341 (N-H, stretching), 3083 (C-H, str. of aromatic ring), 1658 (C=O str.), 1558 (C=C, aromatic str.), 1667 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 638 (C-S bond str.); 1H-NMR (300 MHz): δ 11.04 (s, 1H, NH-1'), 8.11 (d, J = 10.8 Hz, 1H, NH-1''''), 7.48 (d, J = 8.1 Hz, 1H, H-4'), 7.35 (br. d, J = 8.1 Hz, 1H, H-7'), 7.30 ( br. s, 1H, H-2'), 7.08 ( t, J = 6.9 Hz, 1H, H-5'), 6.98 ( t, J = 6.9 Hz, 1H, H-6'), 4.30 (s, 1H, H-10') 3.94 (s, 2H, H-2''), 3.02 ( br.s, 1H, H-1'''), 1.16-1.09 (m, 10H, H-2''' to H-6'''); EIMS: m/z 372 (C19H22N4O2S+2)˙+ [M+2]+, 370 (C19H21N4O2S)˙+ [M]+, 242 (C10H14N3O2S+2)+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 140 (C8H14NO)+, 130 (C9H8N)+, 126 (C7H12NO)+, 98 (C7H12N)+, 83 (C6H11)+.
N-Phenyl-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamide (8b)
Brown amorphous powder; Yield: 80%; m.p. 153 °C; Molecular formula: C19H16N4O2S; Molecular weight: 364 g/mol IR (KBr) υmax: 3339 (N-H, stretching), 3086 (C-H, str. of aromatic ring), 1654 (C=O str.), 1559 (C=C, aromatic str.), 1669 (C=N, str. of oxadiazole ring), 1067 (C-O-C bond str.), 638 (C-S bond str.); 1H-NMR (400 MHz): δ 11.03 (s, 1H, NH-1'), 8.77 ( s,1H, NH-1''''), 7.54 (br.d, J = 8.0 Hz, 2H, H-2''' & H-6'''), 7.49 (d, J = 8.0 Hz, 1H, H-4'), 7.34 (br.d, J = 8.0 Hz, 1H, H-7'), 7.32 ( br.s, 1H, H-2'), 7.31-7.23 (m, 3H, H-3''' to H-5'''), 7.07 ( t, J = 7.6 Hz, 1H, H-5'), 6.98 ( t, J = 6.8 Hz, 1H, H-6'), 4.30 (s, 1H, H-10'), 4.26 ( br.s, 1H, H-2''); EIMS: m/z 366 (C19H16N4O2S)˙+ [M+2]+, 364 (C19H16N4O2S)˙+ [M]+, 362 (C10H8N3O2S)+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 134 (C8H8NO)+, 130 (C9H8N)+, 120 (C8H10N)+, 92 (C6H6N)+, 77 (C6H5)+.
N-Benzyl-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamide (8c)
Light brown amorphous powder; Yield: 90%; m.p. 148 °C; Molecular formula: C20H18N4O2S; Molecular weight: 378 g/mol; IR (KBr) υmax: 3343 (N-H, stretching), 3088 (C-H, str. of aromatic ring), 1657 (C=O str.), 1560 (C=C, aromatic str.), 1669 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 637 (C-S bond str.); 1H-NMR (400 MHz): δ 11.03 (s, 1H, NH-1'), 8.74 (t, J = 5.6 Hz, 1H, NH-1''''), 7.49 (d, J = 8.0 Hz, 1H, H-4'), 7.36 (br.d, J = 8.0 Hz, 1H, H-7' ), 7.30 ( br.s, 1H, H-2'), 7.28-7.26 (m, 2H, H-2''' & H-6'''), 7.23-7.20 (m, 3H, H-3''', H-4''' & H-5'''), 7.08 ( t, J = 7.6 Hz, 1H, H-5'), 6.97 ( t, J = 6.9 Hz, 1H, H-6'), 4.30 (s, 1H, H-10'), 4.26 ( br.s, 1H, H-2''), 4.05 ( br.s, 2H, H-7'''); EIMS: m/z 380 (C20H18N4O2S+2)˙+ [M+2]+, 378 (C20H18N4O2S)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 148 (C9H10NO)+, 134 (C8H8NO)+, 130 (C9H8N)+, 106 C7H8NO)+, 91 (C7H7)+.
N-Phenylethyl-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamide (8d)
Brown colored and amorphous powder; Yield: 85%; m.p. 101 °C; Molecular formula: C21H20N4SO2; Molecular weight: 392 g/mol; IR (KBr) υmax: 3338 (N-H, stretching), 3086 (C-H, str. of aromatic ring), 1657 (C=O str.), 1562 (C=C, aromatic str.), 1668 (C=N, str. of oxadiazole ring), 1063 (C-O-C bond str.), 633 (C-S bond str.); 1H-NMR (400 MHz): δ 11.03 (s, 1H, NH-1'), 8.74 (t, J = 5.6 Hz, 1H, NH-1''''), 7.48 (d, J = 8.0 Hz, 1H, H-4'), 7.38 (br.d, J = 8.0 Hz, 1H, H-7'), 7.33 ( br.s, 1H, H-2'), 7.29-7.26 (m, 2H, H-3''' & H-5'''), 7.23-7.19 (m, 3H, H-2''', H-6''' & H-4'''), 7.08 ( t, J = 7.6 Hz, 1H, H-5'), 6.97 ( t, J = 6.9 Hz, 1H, H-6'), 4.30 (s, 1H, H-10'), 4.26 ( br.s, 1H, H-2''), 4.05 ( t, J = 8.4 Hz, 2H, H-8'''), 4.05 ( t, J = 8.0 Hz, 1H, H-7'''); EIMS: m/z 394 (C21H20N4SO2+2)˙+ [M+2]+, 392 (C21H20N4SO2)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 162 (C10H12NO)+, )+, 158 (C10H8NO)+, 156 (C10H8N2)+, 148 (C9H10NO)+, 130 (C9H8N)+, 120 (C7H8N)+, 105 (C8H9)+, 91 (C7H7)+.
N-(2-Methylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamide (8e)
Brownish grey colored amorphous powder; Yield: 79%; m.p. 155 °C; Molecular formula: C20H18N4O2S; Molecular weight: 378 g/mol; IR (KBr) υmax: 3339 (N-H, stretching), 3085 (C-H, str. of aromatic ring), 1651 (C=O str.), 1565 (C=C, aromatic str.), 1663 (C=N, str. of oxadiazole ring), 1069 (C-O-C bond str.), 639 (C-S bond str.); 1H-NMR (300 MHz): δ 11.04 (s, 1H, NH-1'), 9.69 (s, 1H, NH-1''''), 7.49 (d, J = 7.8 Hz, 1H, H-4'), 7.38 (br.d, J = 7.8 Hz, 1H, H-7'), 7.37-7.34 (m, 2H, H-3''' & H-6'''), 7.32 ( br.s, 1H, H-2'), 7.18 ( t, J = 7.5 Hz, 1H, H-5'), 7.10-7.05 (m, 2H, H-4''' & H-5'''), 6.97 ( t, J = 7.5 Hz, 1H, H-6'), 4.32 (s, 1H, H-10'), 4.24 (s, 2H, H-2''), 2.16 (s, 3H, CH3-2'''); EIMS: m/z 380 (C20H18N4SO2+2)˙+ [M+2]+, 378 (C20H18N4SO2)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 148 (C9H10NO)+, 130 (C9H8N)+, 106, (C7H8N)+, 91 (C7H7)+.
N-(3-Methylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamide (8f)
Dark brown and sticky; Yield: 68%; Molecular formula: C21H20N4O2S; Molecular weight: 378 g/mol; IR (KBr) υmax: 3338 (N-H, stretching), 3086 (C-H, str. of aromatic ring), 1658 (C=O str.), 1568 (C=C, aromatic str.), 1663 (C=N, str. of oxadiazole ring), 1067 (C-O-C bond str.), 633 (C-S bond str.); 1H-NMR (400 MHz): δ 11.03 (s, 1H, NH-1'), 8.89 ( s, 1H, NH-1''''), 7.48 (d, J = 8.0 Hz, 1H, H-4'), 7.37 (br.d, J = 8.0 Hz, 1H, H-7'), 7.35 (br.s, 1H, H-2'''), 7.34 (br.d, J = 8.0 Hz, 1H, H-6'''), 7.30 (br.s, 1H, H-2'), 7.17 (t, J = 7.6 Hz, 1H, H-5'''), 7.09 ( t, J = 7.6 Hz, 1H, H-5'), 7.03 ( t, J = 6.9 Hz, 1H, H-6'), 7.00 (br.d, J = 8.0 Hz, 1H, H-4'''), 4.31 (s, 1H, H-10'), 4.27 ( br.s, 1H, H-2''), 2.30 (s, 3H, CH3-3'''); EIMS: m/z 380 (C20H18N4SO2)˙+ [M+2]+, 378 (C20H18N4SO2+2)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 148 (C9H10NO)+, 134 (C8H8NO)+, 130 (C9H8N)+, 106, (C7H8N)+, 91 (C7H7)+.
N-(4-Methylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamide (8g)
Light brown amorphous; Yield: 88%; m.p. 142 °C; Molecular formula: C21H20N4O2S; Molecular weight: 378 g/mol; IR (KBr) υmax: 3339 (N-H, stretching), 3085 (C-H, str. of aromatic ring), 1655 (C=O str.), 1560 (C=C, aromatic str.), 1667 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 637 (C-S bond str.); 1H-NMR (500 MHz): δ 11.03 (s, 1H, NH-1'), 10.24 (s, 1H, NH-1''''), 7.50 (d, J = 8.0 Hz, 1H, H-4'), 7.48 (d, J = 8.5 Hz, 2H, H-2''' & H-6'''), 7.37 (br.d, J = 8.5 Hz, 1H, H-7'), 7.32 ( br.s, 1H, H-2'), 7.12 (d, J = 7.5 Hz, 2H, H-3''' & H-5'''), 7.09 ( t, J = 7.6 Hz, 1H, H-5'), 6.99 ( t, J = 6.9 Hz, 1H, H-6'), 4.30 (s, 1H, H-10'), 4.19 ( br.s, 1H, H-2''), 2.25 (s, 1H, CH3-4'''); EIMS: m/z 380 (C20H18N4SO2)˙+ [M+2]+, 378 (C20H18N4SO2+2)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 148 (C9H10NO)+, 134 (C8H8NO)+, 130 (C9H8N)+, 106, (C7H8N)+, 91 (C7H7)+.
Methyl-2-[(2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetyl)amino]benzoate (8h)
Brown amorphous solid; Yield: 86%; m.p. 98 °C; Molecular formula: C21H18N4O2S; Molecular weight: 422g/mol; IR (KBr) υmax: 3339 (N-H, stretching), 3085 (C-H, str. of aromatic ring), 1654 (C=O str.), 1561 (C=C, aromatic str.), 1667 (C=N, str. of oxadiazole ring), 1067 (C-O-C bond str.), 637 (C-S bond str.); 1H-NMR (500 MHz): δ 11.02 (s, 1H, NH-1'), 8.20 (d, J = 8.5 Hz, 1H, H-3'''), 7.90 (d, J = 7.5 Hz, 1H, H-6'''), 7.61 (t, J = 7.0 Hz, 1H, H-4'''), 7.74 (d, J = 8.0 Hz, 1H, H-4'), 7.36 (d, J = 8.0 Hz, 1H, H-7'), 7.31 ( br.s, 1H, H-2'), 7.22 (t, J = 7.0 Hz, 1H, H-5'''), 7.08 ( t, J = 7.0 Hz, 1H, H-5'), 6.95 ( t, J = 7.0 Hz, 1H, H-6'), 4.32 (s, 1H, H-10'), 4.25 ( br.s, 1H, H-2''), 3.75 (s, 3H, COOCH3-2'''); EIMS: m/z 424 (C21H18N4O4S)˙+ [M+2]+, 422 (C21H18N4O4S+2)˙+ [M]+, 393 (C20H15N4O3S+2)+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 192 (C10H10NO3)+, 178(C9H8NO3)+, 158 (C10H8NO)+ 156 (C10H8N2)+, 150 (C8H8NO2)+, 135 (C8H7O2)+, 130 (C9H8N)+, 104 (C7H6N)+.
N-(2-Ethoxyphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl} acetamide (8i)
Dark brown sticky solid ; Yield: 74%; Molecular formula: C21H20N4O3S; Molecular weight: 408 g/mol; IR (KBr) υmax: 3338 (N-H, stretching), 3088 (C-H, str. of aromatic ring), 1658 (C=O str.), 1565 (C=C, aromatic str.), 1666 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 630 (C-S bond str.); 1H-NMR (400 MHz): δ 11.04 (s, 1H, NH-1'), 9.94 ( s, 1H, NH-1''''), 7.46 (d, J = 7.2 Hz, 1H, H-4'), 7.36 (br.d, J = 7.6 Hz, 1H, H-7'), 7.30 ( br. s, 1H, H-2'), 7.29 (d, J = 7.6 Hz, 1H, H-6'''),7.08 ( t, J = 7.6 Hz, 1H, H-5'), 7.04 ( t, J = 7.6 Hz, 1H, H-6'), 7.01-6.89 (m, 3H, H-3''' to H-5'''), 4.31 (s, 1H, H-10'), 4.26 ( br.s, 1H, H-2''), 4.01 (q, J = 7.2 Hz, 2H, OCH2-2'''), 1.44 (t, J = 6.8 Hz, 3H, OCH2-CH3-2'''); EIMS: m/z 410 (C21H20N4O3S)˙+ [M+2]+, 408 (C21H20N4O3S)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 178 (C10H12NO2)+, 164 (C9H10NO2)+, 158 (C10H8NO)+ 156 (C10H8N2)+ 136 (C9H10NO)+, 130 (C9H8O)+, 121 (C8H9O)+.
N-(4-Ethoxyphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl} acetamide (8j)
Light brown amorphous solid; Yield: 87%; m.p. 118 °C; Molecular formula: C21H20N4O3S; Molecular weight: 408 g/mol; IR (KBr) υmax: 3340 (N-H, stretching), 3080 (C-H, str. of aromatic ring), 1658 (C=O str.), 1560 (C=C, aromatic str.), 1667 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 633 (C-S bond str.); 1H-NMR (500 MHz): δ 11.03 (s, 1H, NH-1' ), 10.19 ( s,1H, NH-1''''), 7.50 (d, J = 7.5 Hz, 1H, H-4' ), 7.45 (d, J = 7.5 Hz, 1H, H-2''' & H-6'''), 7.37 (br.d, J = 8.5 Hz, 1H, H-7' ), 7.32 ( br.s, 1H, H-2'), 7.09 ( t, J = 8.0 Hz, 1H, H-5'), 6.99 ( t, J = 7.2 Hz, 1H, H-6'), 6.87 (d, J = 7.5 Hz, 2H, H-3''' & H-5'''), 4.33 (s, 1H, H-10'), 4.21(s, 2H, H-2''''), 3.98 (quart. 2H, J = 7.0 Hz, -OCH2- 4'''), 1.31 (t, J = 7.0 Hz, 3H, OCH2-CH3-4'''); ( br.s, 1H, H-2''), 4.01 (q, J = 7.2 Hz, 2H, OCH2-2'''), 1.44 (t, J = 6.8 Hz, 3H, OCH2-CH3-2'''); EIMS: m/z 410 (C21H20N4O3S)˙+ [M+2]+, 408 (C21H20N4O3S)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 178 (C10H12NO2)+, 164 (C9H10NO2)+, 158 (C10H8NO)+ 156 (C10H8N2)+ 136 (C9H10NO)+, 130 (C9H8O)+, 121 (C8H9O)+.
N-(2-Ethylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl} acetamide (8k)
Light brown amorphous solid; Yield: 85%; m.p. 143 °C; Molecular formula: C21H20N4O2S; Molecular weight: 392 g/mol; IR (KBr) υmax: 3339 (N-H, stretching), 3084 (C-H, str. of aromatic ring), 1657 (C=O str.), 1562 (C=C, aromatic str.), 1665 (C=N, str. of oxadiazole ring), 1064 (C-O-C bond str.), 637 (C-S bond str.); 1H-NMR (500 MHz,): δ 11.04 (s, 1H, NH-1'), 9.50 (s, 1H, NH-1''''), 7.51 (d, J = 7.5 Hz, 1H, H-4'), 7.38 (br.d, J = 8.0 Hz, 1H, H-7'), 7.32-7.31 ( m, 2H, H-2', H-6'''), 7.23 ( dist. t, J = 6.0 Hz, 1H, H-5'''), 7.18-7.15 (m, 2H, H-3''', H-4'''), 7.10 ( t, J = 7.0 Hz, 1H, H-5'), 6.99 ( t, J = 7.5 Hz, 1H, H-6'), 4.34 (s, 1H, H-10'), 4.25 ( br.s, 1H, H-2''), 2.58 (q, J = 7.5 Hz, 2H, -CH2-2'''), 1.43 (t, J = 7.5 Hz, 3H, -CH2-CH3-2'''); EIMS: m/z 394 (C21H20N4SO2+2)˙+ [M+2]+, 392 (C21H20N4SO2)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 162 (C10H12NO)+, )+, 158 (C10H8NO)+, 156 (C10H8N2)+, 148 (C9H10NO)+, 130 (C9H8N)+, 120 (C7H8N)+, 105 (C8H9)+, 79 (C6H7)+.
N-(4-Ethylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl} acetamide (8l)
Brown amorphous solid; Yield: 68%; m.p. 124 °C; Molecular formula: C21H20N4O2S; Molecular weight: 392 g/mol; IR (KBr) υmax: 3332 (N-H, stretching), 3079 (C-H, str. of aromatic ring), 1660 (C=O str.), 1563 (C=C, aromatic str.), 1667 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 630 (C-S bond str.); 1H-NMR (400 MHz): δ 11.02 (s, 1H, NH-1'), 9.96 ( s, 1H, NH-1''''), 7.48 (d, J = 8.0 Hz, 1H, H-4'), 7.36 (br.d, J = 8.0 Hz, 1H, H-7'), 7.32 ( br.s, 1H, H-2'), 7.30 (d, J = 8.8 Hz, 1H, H-2''' & H-6'''), 7.08 ( t, J = 7.6 Hz, 1H, H-5'), 6.98 ( t, J = 7.6 Hz, 1H, H-6'), 6.90 (d, J = 8.4 Hz, 2H, H-3''' & H-5'''), 4.30 (s, 1H, H-10'), 4.21-3.87 (m-overlapped, 4H, CH2-2'' & -CH2-4'''), 1.36 (t, J = 7.2 Hz, 3H, -CH2-CH3-4'''); EIMS: m/z 394 (C21H20N4SO2+2)˙+ [M+2]+, 392 (C21H20N4SO2)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 162 (C10H12NO)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 148 (C9H10NO)+, 130 (C9H8N)+, 120 (C7H8N)+, 105 (C8H9)+, 79 (C6H7)+.
N-(2-Methoxyphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl} acetamide (8m)
Dark brown amorphous solid; Yield: 90%; m.p. 127 °C; Molecular formula: C20H18N4O3S; Molecular weight: 394 g/mol; IR (KBr) υmax: 3338 (N-H, stretching), 3085 (C-H, str. of aromatic ring), 1658 (C=O str.), 1567 (C=C, aromatic str.), 1665 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 632 (C-S bond str.); 1H-NMR (400 MHz): δ 11.02 (s, 1H, NH-1'), 9.96 ( s, 1H, NH-1''''), 7.48 (d, J = 8.0 Hz, 1H, H-4'), 7.36 (br.d, J = 8.0 Hz, 1H, H-7'), 7.30 ( br.s, 1H, H-2'), 7.28 (d, J = 7.6 Hz, 1H, H-6'''), 7.08 ( t, J = 7.6 Hz, 1H, H-5'), 7.01-6.89 (m, 3H, H-3''' to H-5'''), 6.98 ( t, J = 6.9 Hz, 1H, H-6'), 4.30 (s, 1H, H-10'), 4.26 ( br.s, 1H, H-2''), 4.01 (s, 3H, OCH3-2'''); EIMS: m/z 396 (C20H18N4O3S+2)˙+ [M+2]+, 394 (C20H18N4O3S)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 164 (C9H10NO2)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 150 (C8H8NO2)+, 130 (C9H8N)+, 122 (C7H8NO)+, 107 (C7H7O)+.
N-(2,3-Dimethylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl} acetamide (8n)
Dark brown amorphous solid; Yield: 98%; m.p. 118 °C; Molecular formula: C21H20N4O2S; Molecular weight: 392.48 g/mol; IR (KBr) υmax: 3343 (N-H, stretching), 3078 (C-H, str. of aromatic ring), 1649 (C=O str.), 1566 (C=C, aromatic str.), 1660 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 634 (C-S bond str.); 1H-NMR (400 MHz): δ 11.02 (s, 1H, NH-1'), 9.94 ( s, 1H, NH-1''''), 7.48 (d, J = 8.4 Hz, 1H, H-4'), 7.36 (br.d, J = 8.4 Hz, 1H, H-7'), 7.34 ( br.s, 1H, H-2'), 7.31 (br.d, J = 7.6 Hz, 1H, H-6'''), 7.09 ( t, J = 7.6 Hz, 1H, H-5'), 7.06 (br.t, J = 7.2 Hz, 1H, H-5'''),6.98 ( t, J = 7.2 Hz, 1H, H-6'), 6.96-6.94 (m, 1H, H-4'''), 4.30 (s, 1H, H-10'), 4.28 ( br.s, 1H, H-2''), 2.25 (s, 3H, CH3-2'''), 2.14 (s, 3H, CH3-3'''); EIMS: m/z 394 (C21H20N4O2S)˙+ [M+2]+, 392 (C21H20N4O2S)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 162 (C10H12NO)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 148 (C9H10NO)+, 130 (C9H8N)+, 120 (C8H10N)+, 105 (C8H9)+.
N-(2,4-Dimethylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl} acetamide (8o)
Dark brown amorphous solid; Yield: 88%; m.p. 153 °C; Molecular formula: C21H20N4O2S; Molecular weight: 392.48 g/mol; IR (KBr) υmax: 3339 (N-H, stretching), 3085 (C-H, str. of aromatic ring), 1657 (C=O str.), 1561 (C=C, aromatic str.), 1667 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 637 (C-S bond str.); 1H-NMR (400 MHz): δ 11.03 (s, 1H, NH-1'), 9.97 ( s, 1H, NH-1''''), 7.49 (d, J = 8.0 Hz, 1H, H-4'), 7.36 (br.d, J = 7.6 Hz, 1H, H-7'), 7.31 ( br.s, 1H, H-2'), 7.29 (br.d, J = 9.2 Hz, 1H, H-6'''), 7.09 ( t, J = 7.6 Hz, 1H, H-5'), 7.07 (br.d, J = 8.4 Hz, 1H, H-5'''), 7.02 (s, 1H, H-3'''), 6.98 ( t, J = 6.8 Hz, 1H, H-6'), 4.30 (s, 1H, H-10'), 4.26 ( br.s, 1H, H-2''), 2.30 (s, 3H, CH3-2'''), 1.52 (s, 3H, CH3-4'''); EIMS: m/z 394 (C21H20N4O2S)˙+ [M+2]+, 392 (C21H20N4O2S)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 162 (C10H12NO)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 148 (C9H10NO)+, 130 (C9H8N)+, 120 (C8H10N)+, 105 (C8H9)+.
N-(2,5-Dimethylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl} acetamide (8p)
Dark brown amorphous solid; Yield: 86%; m.p. 126 °C; Molecular formula: C21H20N4O2S; Molecular weight: 392.48 g/mol; IR (KBr) υmax: 3336 (N-H, stretching), 3088 (C-H, str. of aromatic ring), 1657 (C=O str.), 1560 (C=C, aromatic str.), 1667 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 636 (C-S bond str.); 1H-NMR (400 MHz): δ 11.04 (s, 1H, NH-1'), 9.98 ( s, 1H, NH-1''''), 7.47 (d, J = 7.6 Hz, 1H, H-4'), 7.36 (br.d, J = 7.6 Hz, 1H, H-7'), 7.31 ( br.s, 1H, H-2'), 7.29 (s, 1H, H-6'''), 7.09 ( t, J = 7.6 Hz, 1H, H-5'), 7.07 (br.d, J = 8.0 Hz, 1H, H-3'''), 7.02(br.d, J = 8.4 Hz, 1H, H-4'''), 6.98 ( t, J = 6.8 Hz, 1H, H-6'), 4.30 (s, 1H, H-10'), 4.26 ( br.s, 1H, H-2''), 2.30 (s, 3H, CH3-2'''), 1.52 (s, 3H, CH3-5'''); EIMS: m/z 394 (C21H20N4O2S)˙+ [M+2]+, 392 (C21H20N4O2S)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 162 (C10H12NO)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 148 (C9H10NO)+, 130 (C9H8N)+, 120 (C8H10N)+, 105 (C8H9)+.
N-(2,6-Dimethylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl} acetamide (8q)
Brown amorphous solid; Yield: 90%; m.p. 146 °C; Molecular formula: C21H20N4O2S; Molecular weight: 394.48 g/mol; IR (KBr) υmax: 3339 (N-H, stretching), 3088 (C-H, str. of aromatic ring), 1653 (C=O str.), 1560 (C=C, aromatic str.), 1661 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 630 (C-S bond str.); 1H-NMR (400 MHz): δ 11.02 (s, 1H, NH-1'), 9.96 ( s, 1H, NH-1''''), 7.48 (d, J = 8.0 Hz, 1H, H-4'), 7.36 (br.d, J = 8.0 Hz, 1H, H-7'), 7.30 ( br.s, 1H, H-2'), 7.08 ( t, J = 7.6 Hz, 1H, H-5'), 7.05-7.03 (m, 3H, H-3''' to H-5'''), 6.98 ( t, J = 6.9 Hz, 1H, H-6'), 4.30 (s, 1H, H-10'), 4.26 (br.s, 1H, H-2''), 2.16 (s, 6H, CH3-2''' & CH3-6'''); EIMS: m/z 394 (C21H20N4O2S)˙+ [M+2]+, 392 (C21H20N4O2S)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 162 (C10H12NO)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 148 (C9H10NO)+, 130 (C9H8N)+, 120 (C8H10N)+, 105 (C8H9)+.
N-(3,4-Dimethylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl} acetamide (8r)
Dark brown amorphous solid; Yield: 81%; m.p. 140°C; Molecular formula: C21H20N4O2S; Molecular weight: 392.48 g/mol; IR (KBr) υmax: 3342 (N-H, stretching), 3084 (C-H, str. of aromatic ring), 1659 (C=O str.), 1559 (C=C, aromatic str.), 1667 (C=N, str. of oxadiazole ring), 1069 (C-O-C bond str.), 637 (C-S bond str.); 1H-NMR (400 MHz): δ 11.03 (s, 1H, NH-1'), 10.16 ( s, 1H, NH-1''''), 7.50 (d, J = 7.5 Hz, 1H, H-4'), 7.35 (br.d, J = 8.5 Hz, 1H, H-7'), 7.31 (s, 1H, H-2'''), 7.30 ( br.s, 1H, H-2'), 7.24 (br.d, J = 8.0 Hz, 1H, H-6'''), 7.08 ( t, J = 8.0 Hz, 1H, H-5'), 7.04 (br.d, J = 8.0 Hz, 1H, H-5'''), 6.97 ( t, J = 8.0 Hz, 1H, H-6'), 4.30 (s, 1H, H-10'), 4.18 ( br.s, 1H, H-2''), 2.16 (s, 3H, CH3-3'''), 2.12 (s, 3H, CH3-4'''); EIMS: m/z 394 (C21H20N4O2S)˙+ [M+2]+, 392 (C21H20N4O2S)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 162 (C10H12NO)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 148 (C9H10NO)+, 130 (C9H8N)+, 120 (C8H10N)+, 105 (C8H9)+.
N-(3,5-Dimethylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl} acetamide (8s)
Brownish grey amorphous solid; Yield: 79%; m.p. 99 °C; Molecular formula: C21H20N4O2S; Molecular weight: 392.48 g/mol; IR (KBr) υmax: 3340 (N-H, stretching), 3087 (C-H, str. of aromatic ring), 1658 (C=O str.), 1560 (C=C, aromatic str.), 1669 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 636 (C-S bond str.); 1H-NMR (400 MHz): δ 11.03 (s, 1H, NH-1'), 9.98 ( s, 1H, NH-1''''), 7.49 (d, J = 8.0 Hz, 1H, H-4'), 7.36 (br.d, J = 8.0 Hz, 1H, H-7'), 7.30 ( br.s, 1H, H-2'), 7.16 (br.s, 2H, H-2''' & H-6'''), 7.08 ( t, J = 7.2 Hz, 1H, H-5'), 6.98 ( t, J = 7.6 Hz, 1H, H-6'), 6.72 (br.s, 1H, H-4'''), 4.32 (s, 1H, H-10'), 4.25 ( br.s, 1H, H-2''), 2.93 (s, 3H, CH3-3'''), 2.85 (s, 3H, CH3-5'''); ); EIMS: m/z 394 (C21H20N4O2S)˙+ [M+2]+, 392 (C21H20N4O2S)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 162 (C10H12NO)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 148 (C9H10NO)+, 130 (C9H8N)+, 120 (C8H10N)+, 105 (C8H9)+.
N-(5-Chloro-2-methylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadizol-2yl]sulfanyl} acetamide (8t)
Brown amorphous solid; Yield: 78%; m.p. 110 °C; Molecular formula: C20H17N4SO3Cl; Molecular weight: 428.5 g/mol; HR-MS: IR (KBr) υmax: 3333 (N-H, stretching), 3087 (C-H, str. of aromatic ring), 1659 (C=O str.), 1566 (C=C, aromatic str.), 1670 (C=N, str. of oxadiazole ring), 1060 (C-O-C bond str.), 637 (C-S bond str.); 1H-NMR (500 MHz): δ 11.11 (s, 1H, NH-1'), 9.96 (s, 1H, NH-1''''), 8.11-7.95 (m, 2H, H-6''', H-4'''), 7.50 (d, J = 7.5 Hz, 1H, H-4'), 7.37 (d, J = 8.0 Hz, 1H, H-7'), 7.33 ( br. s, 1H, H-2'), 7.13 (d, J = 8.5 Hz, 1H, H-3'''), 7.11 ( t, J = 7.5 Hz, 1H, H-5'), 6.98 ( t, J = 7.5 Hz, 1H, H-6'), 4.33 (s, 1H, H-10'), 3.84 (s, 2H, H-2''), 2.89 (s, 3H, -OCH32'''); EIMS: m/z 432 (C20H17N4O3SCl+4)˙+ [M+4]+, 430 (C20H17N4O3SCl+2)˙+ [M+2]+, 428 (C20H17N4O3SCl)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 200 (C9H9NO2Cl+2)+, 198 (C9H9NO2Cl)+, 186 (C8H7NO2Cl+2)+, 184 (C8H7NO2Cl)+, 158 (C10H8NO)+, 158 (C7H7NOCl+2)+, 156 (C7H7NOCl)+, 156 (C10H8N2)+, 143(C7H6OCl+2)+, 141(C7H6OCl)+, 130 (C9H8N)+.
N-(2-Ethoxy-6-methylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2yl]sulfanyl} acetamide (8u)
Light brown amorphous solid; Yield: 88%; m.p. 138 °C; Molecular formula: C22H22N4O3S; Molecular weight: 422 g/mol; HR-MS: IR (KBr) υmax: 3338 (N-H, stretching), 3084 (C-H, str. of aromatic ring), 1654 (C=O str.), 1563 (C=C, aromatic str.), 1669 (C=N, str. of oxadiazole ring), 1068 (C-O-C bond str.), 637 (C-S bond str.); 1H-NMR (300 MHz,): δ 11.04 (s, 1H, NH-1'), 9.64 (s, 1H, NH-1''''), 7.49 ( br.d, J = 7.8 Hz, 1H, H-4'), 7.36 (br.d, J = 8.1 Hz, 1H, H-7'), 7.32 ( br.s, 1H, H-2'), 7.08 ( t, J = 6.9 Hz, 1H, H-5'), 7.05-7.03 (m, 3H, H-3''' to H-5'''), 6.98 ( t, J = 7.5 Hz, 1H, H-6'), 4.32 (s, 1H, H-10'), 4.22 (s, 2H, H-2''), 2.49-2.45( m. 2H,merged in signal of DMSO-d6, -OCH2-CH3-2'''), 2.06 (s, 3H, CH3-6''') 1.01 (t, 3H, -OCH2-CH3-2''' ); EIMS: m/z 408 (C22H22N4O2S+2)˙+ [M+2]+, 406 (C22H22N4O2S)˙+ [M], 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 178 (C10H12NO2)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 135 (C9H12N)+, 130 (C9H8O)+, 119 (C9H11)+, 104 (C7H6N)+, 79 (C7H6N)+.
N-(2-Methyl-6-nitrophenyl)-2-{[5-(1H-Indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamide (8v)
Light brown amorphous powder; Yield: 92%; m.p. 192 °C; Molecular formula: C20H17N5O4S Molecular weight: 423 g/mol; IR (KBr) υmax: 3332 (N-H, stretching), 3085 (C-H, str. of aromatic ring), 1655 (C=O str.), 1561 (C=C, aromatic str.), 1667 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 637 (C-S bond str.); 1H-NMR (300 MHz,): δ 11.02 (s, 1H, NH-1'), 10.26 (s, 1H, NH-1''''), 7.74 (d, J = 8.0 Hz, 1H, H-5'''), 7.59 ( br.d, J = 7.6 Hz, 1H, H-4'), 7.48 (br.d, J = 7.6 Hz, 1H, H-7' ), 7.39 (d, J = 8.0 Hz, 1H, H-3'''), 7.35 (d, J = 8.0 Hz, 1H, H- H-4'''), 7.31 ( br.s, 1H, H-2'), 7.08 ( t, J = 6.9 Hz, 1H, H-5'), 6.97 ( t, J = 7.5 Hz, 1H, H-6'), 4.32 (s, 1H, H-10'), 4.22 (s, 2H, H-2''), 2.24 ( s, 3H, -CH3-2'''); EIMS: m/z 425 (C20H17N5SO4+2)˙+ [M+2]+, 423 (C20H17N5SO4)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 193 (C9H9N2O3)+, 179 (C8H7N2O3)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 151 (C7H7N2O2)+, 130 (C9H8N)+, 136 (C7H6NO2)+.
N-(4-methyl-2-pyridinyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl} acetamide (8w)
Dark purple amorphous powder; Yield: 83%; m.p. 206 °C; Molecular formula: C19H17N5O2S; Molecular weight: 379 g/mol; IR (KBr) υmax: 3345 (N-H, stretching), 3081 (C-H, str. of aromatic ring), 1657 (C=O str.), 1563 (C=C, aromatic str.), 1669 (C=N, str. of oxadiazole ring), 1065 (C-O-C bond str.), 637 (C-S bond str.); 1H-NMR (400 MHz): δ 11.02 (s, 1H, NH-1'), 9.96 ( s, 1H, NH-1''''), 8.01 (d, J = 7.2 Hz, 1H, H-6'''), 7.48 (d, J = 8.4 Hz, 1H, H-4'), 7.36 (br.d, J = 8.0 Hz, 1H, H-7'), 7.30 (br.s, 1H, H-2'), 7.14 (s, 1H, H-3'''), 7.09 (d, J = 7.2 Hz, 1H, H-5'''), 7.08 ( t, J = 7.6 Hz, 1H, H-5'), 6.98 (t, J = 6.9 Hz, 1H, H-6'), 4.30 (s, 1H, H-10'), 4.26 ( br.s, 1H, H-2''), 2.40 (s, 3H, CH3-4'''); EIMS: m/z 381 (C19H17N5O2S+2)˙+ [M+2]+, 379 (C19H17N5O2S)˙+ [M]+, 233 (C11H9N3OS+2)+, 231 (C11H9N3OS)+, 158 (C10H8NO)+, 156 (C10H8N2)+, 149 (C8H9N2O)+, 135 (C7H7N2O)+, 130 (C9H8N)+, 107 (C6H7N2)+.
Biological screening
Lipoxygenase (LOX) assay
Lipoxygenase activity was assayed according to the reported methods (Tappel, 1953TAPPEL, A.L. The mechanism of the oxidation of unsaturated fatty acid catalyzed by hematin compounds. Arch. Biochem. Biophys., v.44, n.2, p.378-395, 1953.; Evans, 1987EVANS, A.T. Actions of cannabis constituents on enzymes of arachidonate metabolism: anti inflammatory potential, Biochem. Pharmacol. v.36, n.12, p.2035-2037, 1987.; Baylac, Racine, 2003BAYLAC, S.; RACINE, P. Inhibition of 5-lipoxygenase by essential oils and other natural fragrant extracts. Int. J. Aromather. v.13, p.138-142, 2003.).
Glucosidase assay
α-Glucosidase inhibitory activity was evaluated according to the cited method (Pierre et al., 1978PIERRE, C.; ROLAND, R.; TREMBLAY, R.R.; DUBE, J.Y p-Nitrophenol-α-D-glucopyranoside as substrate for measurement of maltase activity in human semen. Clin. Chem.v.24, n.2, p.208-211, 1978.).
Butyrylcholinesterase (BChE) assay
The BChE inhibition activity was performed according to the reported method (Ellman et al., 1961ELLMAN, G.L.; COURTNEY, K.D.; ANDRES, V.; FEATHERSTONE, R.M. A new and rapid calorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol., v.7, p.88-95, 1961.).
Antibacterial activity
The antibacterial activity was performed in sterile 96-wells micro plates under aseptic environments. The method is based on the principle that microbial cell number increases as the microbial growth proceeds in a log phase of growth which results in increased absorbance of broth medium (Kaspady et al., 2009KASPADY, M.; NARAYANASWAMY, V.K.; RAJU, M.; RAO, G.K. Synthesis, antibacterial activity of 2,4-disubstituted oxazoles and thiazoles as bioestersLett. Drug. Des. Discov., v.6, p.21-28, 2009.; Yang et al., 2006YANG, C.R.; ZANG, Y.; JACOB, M.R.; KHAN, S.I.; ZHANG, YJ.; LI, X.C. Antifungal activity of C-27 steroidal saponins. Antimicrob. Agents Chemother., v.50, n.5, p.1710-1714, 2006.).
Hemolytic activity assay
Hemolytic activity of the compounds was performed by the reported methods (Shahid et al., 2013SHAHID, M.; BUKHARI, S.A.; GUL, Y.; MUNIR, H.; ANJUM, F.; ZUBER, M.; JAMIL, T.; ZIA, K.M. Graft polymerization of guar gum with acryl amide irradiated by microwaves for colonic drug delivery. Int. J. Biol. Macromol. v.62, p.172-179, 2013.; Zuber et al., 2014ZUBER, M.; TABASUM, S.; JAMIL, T.; SHAHID, M.; HUSSAIN, R.; FERAS, K.; BHATTI, K. Biocompatibility and microscopic evaluation of polyurethane-poly(methyl methacrylate)-titanium dioxide based composites for dental applications. J. Appl. Polym. Sci.v.131, n.3, p.39806.1-39806.9, 2014.).
Statistical analysis
All the measurements were carried out in triplicate and statistical analysis was performed by Microsoft Excel 2010. The results are presented as mean ± SEM with 90% CL.
Molecular docking methodology
To predict the bioactive conformations, various compounds (ligands) were docked into the binding pockets of the selected proteins (enzymes) by using the default parameters of MOE-Dock program.
Prior to docking, the protein molecules of α-glucosidase, lipoxygenase and butyrylcholinesterase were retrieved from Protein Data Bank having PDB ID codes of 3NO4 (Resolution: 2.02Å), 1IK3 (Resolution: 2.0Å) and 1POP (Resolution: 2.0Å) respectively. All the water molecules were removed from receptor proteins and 3D protonation was carried out by using the Protonate 3D Option. The energies of protein molecules were minimized by using the default parameters of MOE 2009-10 energy minimization algorithm (gradient: 0.05, Force Field: MMFF94X). Then all the ligands were docked into the binding pockets (selective residues/amino acids as shown in the docking images) of the above mentioned proteins using Triangular Matching docking method. Re-docking procedure was also applied to validate the docking protocol. Each complex was analyzed for the type of interactions; bond distances and their 3D images were taken.
RESULTS AND DISCUSSION
We report herein the synthesis of some new N-substituted derivatives of 2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamides. These synthesized compounds were evaluated for their antibacterial and hemolytic activity. Enzyme inhibition activity was well supported by molecular docking results. The reaction sequence leading to the preparation of the desired heterocyclic compounds is outlined in Scheme 1. 5-(1H-Indole-3-yl-methyl)-1,3,4-oxadiazole-2-thiol was prepared by converting 2-(1H-Indol-3-yl)acetic acid (1) to its corresponding ester 2 in ethanol, which was used in excess as solvent to shift this reversible reaction in the forward direction, where H2SO4 was used as catalyst. Aqueous Na2CO3 was used to neutralize the catalyst and unreacted acid (1). Ester 2, on further reaction with hydrazine monohydrate, yielded acid hydrazide 3 after 3 h stirring at room temperature. This reaction readily occurred, otherwise a few drops of glacial acetic acid was added to speed up this reaction. The hydrazide (3) was cyclized to 5-(1H-indole-3-yl-methyl)-1,3,4-oxadiazole-2-thiol (4) in the presence of CS2 and alcoholic KOH with refluxing for 6 h. CS2 is volatile and is used in double quantity compared to 4 to favor product formation. KOH was used to facilitate the delocalization of negative charge during intramolecular cyclization to form 4. A series of N-substituted 2-bromoacetmaide derivatives were also synthesized by manually shaking equimolar amounts of bromoacetyl bromide (VI) with different substituted amines (Va-w) in 10% aqueous Na2CO3 (pH 8-9). Amines with electron-donating groups were more active in nucleophilic reactions than those with electron-withdrawing groups. These N-substituted 2-bromoacetmaide derivatives (7a-w) were reacted further with 5-(1H-indole-3-yl-methyl)-1,3,4-oxadiazole-2-thiol (4) in NaH as weak base and DMF as aprotic medium to produce 2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamides (8a-w), where complete conversion was achieved within almost 8 h by stirring with 68-98% yields, as outlined in Scheme 1 (Sabahat et al., 2013). The products were isolated by adding ice-cold water to the reaction mixture and filtering off the precipitated solid. In some cases, the compound was obtained with the solvent extraction method using chloroform/ethyl acetate. The structure of the parent compound and its derivatives were confirmed by IR, 1H-NMR, and EI-MS data, as described in the experimental section.
Compound 8g was obtained as a light-brown amorphous powder with 88% yield and m.p. 142 °C. The molecular formula C21H20N4O2S was ascertained by EI-MS data which revealed an [M]+ ion peak at m/z 378 and by the assigned protons in the 1H-NMR spectrum. IR spectrum further revealed characteristic peaks at 3339 cm-1 for N-H stretch of the indole moiety, 3085 cm-1 for aromatic C-H stretching, 1667 cm-1 for C=N stretching due to the presence of the oxadiazole ring, 1560 cm-1 for stretching of C=C phenyl rings of indole and N- substituted aromatic ring, 1655 cm-1 for stretching of C=O, 1065 cm-1 for C-O-C bond stretching of oxadiazole ring and 637 cm-1 for C-S stretching. EIMS data further supported the structure by revealing a base peak at m/z 130 for (C9H8N)+ of the indole moiety and of major fragments given in Figure 2. In 1H-NMR, spectrum signals of the indole moiety appeared at δ 11.03 (s, 1H, NH-1'), 7.50 (d, J = 8.0 Hz, 1H, H-4'), 7.37 (br.d, J = 8.5 Hz, 1H, H-7'), and two ortho coupled triplets with integration of one proton each appeared at δ 7.09 for H-5' and δ H-6', respectively, In the aromatic region, a broad singlet appeared at δ 7.32 of H-2', and the last signal for the indole moiety appeared in the aliphatic region with integration of two protons at δ 4.30 of H-10'. The acetamide part of the molecule gave a deshielded singlet of -NH of amide at δ 10.24 for NH-1'''', doublet in aromatic region at δ 7.48 of H-2''' & H-6''' and two other protons of this ring gave a doublet at δ 7.12 of H-3''' & H-5'''. Two more singlets appeared in the aliphatic region with integration of two protons at δ 4.19 of H-2" and with three protons at δ 2.25 for CH3-4"' (Figure 3). On the basis of this spectral evidence, the structure was determined as N-(4-methylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamide (8g). Similarly, the structures of other derivatives of 5-(1H-indole-3-ylmethyl)-1,3,4-oxadiazole-2-thiol were elucidated in the same way, as described in the experimental section.
Mass fragmentation pattern of N-(3-methylphenyl)-2-{[5-(1H-indol-3-ylmethyl)-1,3,4- oxadiazol-2-yl]sulfanyl}acetamide (8g)
Biological screening
Enzyme inhibition activity
In vitro evaluation of the enzyme inhibitory potential of N-substituted 2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamides (8a-w) against α-glucosidase, BChE and LOX revealed that these molecules exhibited variable inhibitory potential as shown by their % inhibition and IC50 values (Table II). Compound 8q showed better inhibition of α-glucosidase with IC50 of 49.71 ± 0.19 µM as compared to standard acarbose (38.25 ± 0.12 µM); 8d, 8p and 8t also exhibited comparable inhibition, and other compounds showed weak or no inhibitory effect against α-glucosidase. Compounds 8g and 8h showed almost similar inhibitory potential against BChE, with IC50 values of 31.62 ± 0.16 and 33.70 ± 0.95 µM, respectively, which were better than other compounds of the series. Against LOX, compound 8b revealed maximum inhibition at at 99.30 ± 2.89 µM, which was almost five times higher than that for the standard baicalein (22.4 ± 1.30 µM). The other compounds showed weak or no inhibition of this enzyme.
Enzyme Inhibition activity of N-substituted 2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamides (8a-w)
Antibacterial activity
All synthesized N-substituted 2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamides (8a-w) were screened for antibacterial activity against two Gram-positive, Bacillus subtilis and Staphylococcus aureus, and three Gram-negative strains, Salmonella typhi, Escherichia coli and Pseudomonas aeruginosa. Most of the synthesized compounds exhibited very good inhibitory potential, as depicted by their MIC values. Ciprofloxacin was used as reference standard in this study (Table III). Compounds 8c and 8d showed very good inhibitory potential against all the bacterial strains used in this study, especially compound 8c with MIC values of 9.75 ± 3.57 and 9.15 ± 4.12 µM against S. typhi and S. aureus, respectively, which were very close to the standard, ciprofloxacin. Compound 8b also revealed very good antibacterial activity (MIC of 9.84 ± 2.99 µM) against S. typhi, while 8d, 8n and 8p showed good inhibitory effect against E. coli with MIC values of 10.45 ± 1.80, 10.48 ± 2.00 and 10.50 ± 2.65 µM respectively. Compounds 8c and 8f also exhibited good inhibition against P. aeruginosa. Against B. subtilis, 8f, 8g, 8o and 8v showed MIC values of 10.78 ± 1.08, 10.33 ± 3.00, 10.56 ± 2.90, and 10.97 ± 2.14 µM respectively, while 8c and 8l again showed very good antibacterial potential against S. aureus, with MIC values very close to that for ciprofloxacin (9.15 ± 4.12, and 9.96 ± 3.58 µM, respectively). The other compounds showed moderate antibacterial potential as a whole.
Hemolytic activity
The lowest hemolytic activity (Table IV) was exhibited by 8p (1.67%), 8t (2.16%) and 8u (2.93%). The highest hemolytic activity was shown by 8j (48.66%) which was lower than the positive control (Triton-X-100). Other compounds mostly showed low hemolytic activity. PBS was used as negative control. On the basis of the antibacterial and enzyme inhibition activities observed, we infer that the compounds synthesized, with mostly little toxicity, may prove to be suitable drug candidates for further improvements to address different targets.
Hemolytic activity of N-substituted 2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamides (8a-w)
Computational docking
To determine the validity of accuracy, the co-crystallized ligands of the following enzymes were extracted and then re-docked into the binding pockets of the receptors. In all these cases, RMSD values between docked and co-crystallized ligands were less than 2 A°, indicating the reliability of the docking method, and thus, our protocol can be used for further studies.
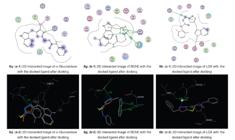
Almost all the derivatives synthesized were computationally docked against α-glucosidase, BChE and LOX to explore the binding modes of all ligands (Figure 4). The results were very much in favor of the experimental work. For example, compound 8q showed interactions with three active site amino acid residues of the target protein. Amino acid Asp197 showed a strong hydrogen bond acceptor (polar) interaction with the five-member ring of the indole moiety residue, with a bond distance of 2.23 Å, while Trp271 showed a strong hydrogen bond donor interaction with the nitrogen atom of the oxadiazole ring, with a bond distance of 2.28 Å. Arg404 showed an arene cation interaction with the oxadiazole ring, with a bond length of 3.92 Å. The other residues in the nearby vicinity of the ligand were Asp73, Trp169, Trp417 and Ala480 (Figure 4, a).Two amino acid residues interacted with compound 8g; His438 interacted strongly with the indole moiety through a couple of arene cation interactions showing bond lengths of 4.62 and 4.83 Å, while Trp82 showed arene-arene interaction with the oxadiazole ring, with a bond distance of 3.33 Å. Other residues that were in close contact with the ligand were Ser79, Met437, Gly116, Gln119 etc. (Figure 4, b). Strong metallic interactions were shown by compound 8b, with amino acid residues Ile857 and Asn713, via one nitrogen atom of the oxadiazole ring through the iron atom, giving bond distances of 1.81 and 2.07 Å, respectively, while His518 showed a hydrogen bond donor interaction with another nitrogen atom of the oxadiazole ring, with a bond length of 2.93 Å. Other residues that existed in the near most region of the ligand were Gln716, Ile557 and Leu773 (Figure 4, c).
(a; 1-2) Binding modes of compound 8q against α-glucosidase, (b; 1-2) Binding modes of compound 8g against BChE, (c; 1-2) Binding modes of compound 8b against LOX.
CONCLUSION
In conclusion, this paper reports the synthesis of N-substituted 2-{[5-(1H-indol-3-ylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}acetamides (8a-w) as potential antibacterial agents, in view of MIC values. Compounds 8b (9.84 ± 2.99 µM against S. typhi), 8c (9.75 ± 3.57 µM against S. typhi and 9.15 ± 4.12 µM against S. aureus), and 8l (9.96 ± 3.58 µM against S. aureus) showed strong antibiotic activity, which was very close to that of the reference standard. These results indicate that our synthesized molecules can be considered as suitable drug candidates. Moreover, the enzyme inhibition analysis was also supported by computational docking, and there was only low to moderate hemolytic activity. Compound 8q showed comparatively good inhibitory potential against α-glucosidase with an IC50 of 49.71 ± 0.19 µM, and 8g showed moderate inhibition of BChE, with an IC50 of 31.62 ± 0.16 µM, while all other compounds synthesized showed weak or no inhibition of LOX. In this regard, some of the compounds synthesized are potential candidates for future drug development studies.
REFERENCES
- ALI, M.A.; SHAHARYAR, M. Oxadiazole mannich bases: synthesis and antimycobacterial activity. Bioorg. Med. Chem. Lett, v.17, n.12, p.3314-3316, 2007.
- AKHTAR, M.N.; GUL, S.; REHMAN, A-UR.; ABBASI, M. A.; KHAN, K.M.; NAFEESA, K.; SIDDIQA, A.; SHAHID, M.; SUBHANI, Z. Synthesis, antimicrobial evaluation and hemolytic activity of 2-[[5-alkyl/aralkyl substituted-1,3,4-oxadiazol-2-yl]thio]-N-[4-(4-morpholinyl)phenyl]acetamide derivatives J. Saudi Chem. Soc, in press, 2014.
- BAYLAC, S.; RACINE, P. Inhibition of 5-lipoxygenase by essential oils and other natural fragrant extracts. Int. J. Aromather. v.13, p.138-142, 2003.
- BHATNACAR, S.; KAMTHAN, D.; MEHRA, S.C.; TANDAN, S.K. Synthesis of some new N3-substituted hydantions as potential anticonvulsant agents. Ind. J. Pharm, v.18, p.235-238, 1986.
- BHATTACHARJEE, S. Reactive oxygen species and oxidative burst: Roles in stress, senescence and signaltransduction in plants. Curr. Sci, v.89, p.1113-1121, 2005.
- PEREIRA, C.G.; ROSA, P.T.V.; MEIRELES, M.A.A. Extraction and isolation of indol alkaloids from Tabernaemontana catharinensis: technical and economical analysis. J. Supercrit. Fluids, v.40, n.2, p.232-238, 2007.
- CHAWLA, R.; ARORA, A.; KUMAR M.; SHARMA, P.C.; MICHAEL, S.T.; RAVI, K. Synthesis of novel 1,3,4-oxadiazole derivatives as potential antimicrobial agents. Acta Pol. Pharm. v67, n.3, p.247-253, 2010.
- DAVE, K.R.; SYAL, A.R.; KATYARE, S.S. Tissue cholinesterases. A comparative study of their kinetic properties. Z. Naturforsch. C, v.55, n.1-2, p.100-108, 2000.
- DEWANGAN, D.; PANDEY, A.; SIVAKUMAR, T.; RAJAVEL, R.; DUBEY, R.D. Synthesis of some novel 2, 5-disubstituted 1, 3, 4-oxadiazole and its analgesic, anti-inflammatory, anti-bacterial and anti-tubercular activity. Int. J. Chem. Tech. Res.v.2, p.1397-1412, 2010.
- ELLMAN, G.L.; COURTNEY, K.D.; ANDRES, V.; FEATHERSTONE, R.M. A new and rapid calorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol, v.7, p.88-95, 1961.
- EVANS, A.T. Actions of cannabis constituents on enzymes of arachidonate metabolism: anti inflammatory potential, Biochem. Pharmacol v.36, n.12, p.2035-2037, 1987.
- HASAN, A.; THOMAS, N.F.; GAPIL, S. Synthesis, characterization and antifungal evaluation of 5-Substituted-4-Amino-1,2,4-Triazole-3-Thioesters. Molecules., v.16, n.2, p.1297-1309, 2011.
- HASAN, A.; GAPIL, S.; KHAN, I. Synthesis, characterization and antifungal activity of some new 5-Substituted 1,3,4-oxadiazole-2-thiols. Asian J. Chem., v.23, p.2007-2010, 2011.
- HOLLA, B.; PRASNNA, S.C.S.; BOJA, P.; RAO, K.S.; BHATT, S.; GANESH, U. Synthesis and insecticidal activity of some 1,3,4-Oxadiazoles derived from 2-chloropyridine-5-acetic acid. Indian J. Chem. v.43, p.864-8, 2004.
- HUTCHINSON, J.H.; CHARLESON, S.; EVANS, J.F.; FALGUEYRET, J.P.; HOOGSTEEN, K., JONES, T.R.; KARGMAN, S.; MACDONALD, D.; McFARLAVNE, C.S.; NICHOLSON, D.W. Thiopyranol[2,3,4-c,d]indoles as inhibitors of 5-lipoxygenase, 5-lipoxygenase-activating protein, and leukotriene C4 synthase. J. Med. Chem, v.38, n.22, p.4538-4547, 1995.
- JAMPILEK, J.; DOLEZAL, M.; OPLETALOVA, V.; HARTL, J. 5-Lipoxygenase, leukotrienes biosynthesis and potential antileukotrienic agents. Curr. Med. Chem., v.13, p.117-129, 2006.
- KASPADY, M.; NARAYANASWAMY, V.K.; RAJU, M.; RAO, G.K. Synthesis, antibacterial activity of 2,4-disubstituted oxazoles and thiazoles as bioestersLett. Drug. Des. Discov, v.6, p.21-28, 2009.
- LEBOVITZ, H.E. Alpha-glucosidase inhibitors. Endocrinol. Metab. Clin North Am., v.26, n.3, p.539-551, 1997.
- LEDNICER, D. The organic chemistry of drug synthesis. New Jersey: A John Wiley & Sons, 2007. ??? p.
- MUHI-ELDEEN, Z.; JUMA'A, G.; AL-KAISSI, E.; NOURI, L. Antimicrobial activity of some new oxadiazole derivatives. Jordan J. Chem. v.3, p.233-243, 2008.
- MURPHY, J.A.; SCOTT, K.A.; SINCLAIR, R.S.; LEWIS, N. A new synthesis of indoles. Tetrahedron Lett, v.38, p.7295-7298, 1997.
- OMAR, F.A.; MAHFOUZ, N.M.; RAHMAN, M.A. Design, synthesis and antiinflammatory activity of some 1,3,4-oxadiazole derivatives. Eur., J. Med. Chem v.31, n.10, p.819-825, 1996.
- PIERRE, C.; ROLAND, R.; TREMBLAY, R.R.; DUBE, J.Y p-Nitrophenol-α-D-glucopyranoside as substrate for measurement of maltase activity in human semen. Clin. Chem.v.24, n.2, p.208-211, 1978.
- POMMERY, N.; TAVERNE, T.; TELLIEZ, A.; GOOSSENS, L.; CHARLIER, C. J. ; POMMERY, J.; GOOSSENS, J.F.; HOUSSIN, R.; DURANT, F.; HÉNICHART, J.P. New COX-2/5-LOX inhibitors: apoptosis-inducing agents potentially useful in prostate cancer chemotherapy., J. Med. Chem v.47, n.25, p.6195-6206, 2004.
- RENUKADEVI, P.; BIRADA, J.S. Synthesis and antimicrobial activity of 3,5-disubstituted-2-[1'-phenyl-5'-thioalkyl-s-triazol-2'-yl]indoles and 3,5-disubstituted-2-[1'-substituted amino methyl-4'-phenyl-5',4'-phenyl-5'(4'H)-thion-S-triazol-3-yl]indoles. Indian J. Heter. Chem, v.9, p.107-112, 1999.
- SIDDIQUI, S.Z.; AZIZ-UR-REHMAN; ABBASI, M.A.; ABBAS, N.; KHAN, K.M.; ASHRAF, M.; EJAZ, S.A. Synthesis, characterization and biological screening of N-substituted derivatives of 5-benzyl-1,3,4-oxadiazole-2yl-2ꞌꞌ-sulfanyl acetamide. Pak. J. Pharm. Sci, v.26, n.3, p.455-463, 2013.
- SHAHID, M.; BUKHARI, S.A.; GUL, Y.; MUNIR, H.; ANJUM, F.; ZUBER, M.; JAMIL, T.; ZIA, K.M. Graft polymerization of guar gum with acryl amide irradiated by microwaves for colonic drug delivery. Int. J. Biol. Macromol. v.62, p.172-179, 2013.
- SILVER, A. The biology of cholinesterases. New York: Elsevier Agricultural Research Council Institute, 1974. 596 p. (Frontiers of biology, v.36).
- SHIRINZADEH, H.; EREN, B.; GURER-ORHAN, H.; SUZEN, S.; ÖZDEN, S. Novel indole-based analogs of melatonin: synthesis and in vitro antioxidant activity studies. Moleculesv.15, n.4, p.2187-2202, 2010.
- TAPPEL, A.L. The mechanism of the oxidation of unsaturated fatty acid catalyzed by hematin compounds. Arch. Biochem. Biophys, v.44, n.2, p.378-395, 1953.
- YANG, C.R.; ZANG, Y.; JACOB, M.R.; KHAN, S.I.; ZHANG, YJ.; LI, X.C. Antifungal activity of C-27 steroidal saponins. Antimicrob. Agents Chemother., v.50, n.5, p.1710-1714, 2006.
- YAR, M.S.; AKHTER, M.W. Synthesis and anticonvulsant activity of substituted oxadiazole and thiadiazole derivatives. Acta Pol. Pharm. v.66, n.4, p.393-397, 2009.
- YAR, M.; ASHRAF, M.; NAQVI, S.A.R.; SIDRA, L.R.; NASAR, R.; KHAN, Z.A.; ELENI PONTIKI, E.; MUSHTAQ, N.; KHAN, I.U.; MAHMOOD, N.; SHAHZAD, S.A. Synthesis, in vitro lipoxygenase inhibition, docking study and thermal stability analyses of novel indole derivatives, Non-isothermal kinetic study of potent LOX inhibitor N'-(diphenylmethylene) -2-(1H-indole-3-yl) acetohydrazide. J. Iran. Chem. Soc, v.11, p.369-378, 2014.
- ZOU, X.; ZHANG, Z.; JIN, G. Synthesis and biological activity of 1,3,4-Oxadiazole-substituted pyridazinones. J. Chem. Res, v.5, p.228-230, 2002.
- ZUBER, M.; TABASUM, S.; JAMIL, T.; SHAHID, M.; HUSSAIN, R.; FERAS, K.; BHATTI, K. Biocompatibility and microscopic evaluation of polyurethane-poly(methyl methacrylate)-titanium dioxide based composites for dental applications. J. Appl. Polym. Sci.v.131, n.3, p.39806.1-39806.9, 2014.
Publication Dates
-
Publication in this collection
Oct-Dec 2015
History
-
Received
07 Dec 2014 -
Accepted
28 Apr 2015