ABSTRACT
The practice of immersion in burn patient has been abandoned in many parts of the world but in Brazil it is still common. The aim of this study was to ascertain if balneotherapy is a risk factor for Pseudomonas aeruginosa colonization in thermally injured patients. Eighteen patients from a Burn Center were studied for 14 weeks for Pseudomonas aeruginosa. Samples were collected by swabbing the exudate of wounds, before and after giving bath to the patients and from balneotherapy table. Pulsed-field gel electrophoresis was used to determine bacterial genetic relatedness. Thirty-seven P. aeruginosa isolates were detected from 292 swabs collected from patients' burn surface area and from the balneotherapy table. Profile analysis of P. aeruginosa DNA fragmentation showed 10 clones among the 37 strains analyzed. Type A is the most prevalent clone, with 23 strains distributed into eight subtypes. These were present in the swabs collected, before and after the patients' bath, from the surface of the bath table, suggesting that there was cross-contamination between the patients in different ways. This work demonstrates that balneotherapy is a risk factor in the Burn Center studied, because the same clone was found among P. aeruginosa isolates collected at various points and times.
Uniterms:
Pseudomonas aeruginosa/colonization; Burn patients; Balneotherapy; PFGE.
RESUMO
A prática de balneotarapia em paciente queimado foi abandonada em muitas partes do mundo, mas no Brasil ainda é comum. O objetivo deste estudo foi verificar se a balneoterapia é um fator de risco para a colonização por Pseudomonas aeruginosa em pacientes queimados. Dezoito pacientes internados em um Centro de Queimadura (CQ) foram acompanhados por 14 semanas. Amostras foram coletadas do exsudato de feridas, antes e depois do banho dos pacientes e também da mesa onde a balneoterapia foi realizada. A relação genética entre as cepas de P. aeruginosa foi determinada pela electroforese em gel de campo pulsado. Trinta e sete cepas foram detectadas a partir de 292 swabs coletados de área de superfície das feridas dos pacientes e da mesa de balneoterapia. Análise de fragmentação do DNA das 37 P. aeruginosa mostrou a existência de 10 clones. O tipo A foi o clone mais prevalente, com 23 cepas distribuídas em oito subtipos. Estas estavam presentes nas lesões dos pacientes antes e após o banho e na mesa onde o banho foi realizado, sugerindo contaminação cruzada inter e intra-pacientes e pacientes e mesa de banho. Este trabalho mostra que a balneoterapia é um fator de risco para colonização por P. aeruginosa, no CQ estudado, pois um mesmo clone da bactéria foi encontrado nos isolados coletados em vários pontos e épocas diferentes.
Unitermos:
Pseudomonas aeruginosa/colonização; Pacientes queimados/tratamento; Balneoterapia; Eletroforese em campo pulsado.
INTRODUCTION
Infection is a major cause of morbidity and mortality of burn patients (Elmanama et al., 2013Elmanama, A.; Laham, N.A.A.; Tayh, G.A. Antimicrobial susceptibility of bacterial isolates from burn units in Gaza. Burns, v.39, p.1612-1618, 2013.; Mahar et al., 2010Mahar, P.; Padiglione, A.; Cleland, H.; Paul, E.; Hinrichs, M.; Wasiak, J. Pseudomonas aeruginosa bacteraemia in burns patients: risk factors and outcomes. Burns, v.36, p.1228-1233, 2010.). These patients are highly susceptible to infections, because by then they have lost the integrity of the skin, the first barrier of protection against infectious agents. Besides, they may require hospitalization for long periods, which increases the risk of infection (Church et al., 2006Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev., v.19, p.403-434, 2006.).
Balneotherapy or hydrotherapy in burn patient can be done in designated areas only that ensure control and prevention of infection. The burn wound is washed and scrubbed with water and degermed by a spray hose. During this procedure, topical agents, necrotic tissue and microorganisms are removed (Kowalske, 2011KOWALSKE, K.J. Burn wound care. Phys. Med. Rehabil. Clin. N. Am., v.22, p.213-227, 2011.). However, the practice of immersion has been abandoned in many parts of the world. Notwithstanding, in some Burn Center (BC) in Brazil it is still used.
Some authors consider that colonization of the burned area does not necessarily represent a risk factor for infection (Tredget et al., 2004Tredget, E.; Shankowsky, H.A.; Rennie, R.; Burrell, R.E.; Logsetty, S. Pseudomonas infections in the thermally injured patient. Burns, v.30, p.3-26, 2004.; Wang et al., 2010Wang, X.Q.; Kravchuk, O.; Kimble, R.M. A retrospective review of burn dressings on a porcine burn model. Burns, v.36, p.680-720, 2010.). But, when there is contamination of the living tissue underlying the injury, with clinical signs of inflammatory reaction and browning, one can infer that the burned surface area (BSA) is infected and may lead to a systemic infection (Ulkur et al., 2005Ulkur, E.; Oncul, O.; Karagoz, H.; Yeniz, E.; Celikoz, B. Comparison of silver-coated dressing (Acticoat), chlorhexidine acetate 0.5% (Bactigrass), and fusidic acid 2% (Fucidin) for topical antibacterial effect in methicillin-resistant staphylococci-contaminated, full-skin thickness rat burn wounds. Burns, v.31, p.874-77, 2005.).
In burn patients, particularly those with severe burns, P. aeruginosa can cause a great variety of systemic infections, such as urinary tract infection, respiratory system infection, dermatitis, soft tissue infection, bacteremia, bone and joint infection and gastrointestinal infection (Taneja et al., 2013TANEJA, N.; CHARI, P.; SINGH, M.; SINGH, G.; BISWAL, M.; SHARMA, M. Evolution of bacterial flora in burn wounds: key role of environmental disinfection in control of infection. Int. J. Burns Trauma, v.3, p.102-107, 2013.; Yali et al., 2013Yali, G.; Jing, C.; Chunjiang, L.; Cheng, Z.; Xiaoqiang, L.; Yizhi, P. Comparison of pathogens and antibiotic resistance of burn patients in the burn ICU or in the common burn ward. Burns, v.40, p.402-407, 2013.). Burned patients present an immunosuppression condition; they consequently have higher susceptibility to infections by P. aeruginosa, with a high mortality rate. In addition, as prevention and control of P. aeruginosa infections continue to be serious nosocomial problems worldwide, treatment of P. aeruginosa infections is a serious medical challenge especially in burned patients (Taneja et al., 2013; Yali et al., 2013; Fallah et al., 2013FALLAH, F.; BORHAN, R.S.; HASHEMI, A. Detection of bla(IMP) and bla(VIM) metallo-β-lactamases genes among Pseudomonas aeruginosa strains. Int. J. Burns Trauma, v.3, p.122-124, 2013. ).
Therefore, prophylaxis with antibiotics is used for preventing or reducing the risk of infections in thermally injured patients (Barajas-Nava et al., 2013Barajas-Nava, L.A.; Lopez-Alcalde, J.; RoquÉ I FIGULS, M.; SolÀ, I.; Bonfill COSP, X. Antibiotic prophylaxis for preventing burn wound infection. Cochrane Database Syst. Rev., v.6, art.CD008738, p.1-174, 2013.). Some antibiotics are used locally on the skin (topical treatment), while others are taken systemically.
Silver sulfadiazine (SSD) cream is one of the topical agents that is most commonly used as an adjuvant in the prevention and treatment of wound infections in patients with severe burns. In addition, it has broad antimicrobial spectrum on microorganisms found on BSA, with prolonged action. It is believed that bacteriostatic and bactericidal effect of SSD increases when it is associated with 0.4% cerium nitrate (De Gracia, 2001De Gracia, C.G. An open study comparing topical silver sulfadiazine and topical silver sulfadiazine-cerium nitrate in the treatment of moderate and severe burns. Burns, v.27, p.67-74, 2001.; Eski et al., 2011Eski, M.; Ozer, F.; Firat, C.; Alhan, D.; Arslan, N.; Senturk, T.; Isik, S. Cerium nitrate treatment prevents progressive tissue necrosis in the zone of stasis following burn. Burns, v.38, p.283-289, 2011.).
Molecular methods are often used in epidemiologic studies to monitor the spread of a particular strain in the same patient or environment or between different patients and environments. From such monitoring, it is possible to decipher the transmission routes of the bacteria (Nonaka et al., 2010Nonaka, L.; Inubushi, A.; Shinomiya, H.; Murase, M.; Suzuki, S. Differences of genetic diversity and antibiotics susceptibility of Pseudomonas aeruginosa isolated from hospital, river and coastal seawater. Environ. Microbiol. Rep., v.2, p.465-472, 2010.). Pulse-field gel electrophoresis (PFGE) is a gold standard genotypic technique for P. aeruginosa (Hu et al., 2013Hu, H.; Harmer, C.; Anuj, S.; Wainwright, C.E.; Manos, J.; Cheney, J.; Harbour, C.; Zablotska, I.; Turnbull, L.; Whitchurch, C.B.; Grimwood, K.; Rose, B. FBAL study investigators Type 3 secretion system effector genotype and secretion phenotype of longitudinally collected Pseudomonas aeruginosa isolates from young children diagnosed with cystic fibrosis following newborn screening. Clin. Microbiol. Infect., v.19, p.266-272, 2013.; Nonaka et al., 2010).
The aims of this study were to assess the role of the balneotherapy as a risk factor for burned patient as well to investigate the colonization by P. aeruginosa in the same patients to evaluate the efficacy of balneotherapy in its elimination.
STUDY SETTING
The BC studied belongs to a hospital in Rio de Janeiro city, Brazil. This center has 12 beds, of which eight are for adult patients and four for children. Generally, the patient's hospital stay is prolonged, ranging from 30 to 180 days.
At this center, we conducted an observational study for 14 weeks (September to December 2012) with prospective analysis. The inclusion criteria for the subjects of study were adult patients (+18 years old) admitted to the BC, whose bath samples can be collected at least on two different days.
METHODS
Management of burn patients
At this burn unit, all patients, after disinfecting the burned area with a degerming application, are submitted to a daily balneotherapy session. However, in this paper only one table was studied and approximately 5 to 6 patients received sequential treatments in the immersion table. SSD is applied for topical treatment of both superficial and deep burns. The Human Research Ethics Committee from HUAP at Universidade Federal Fluminense approved this study by number 68538.
Bacterial isolates and identification
Samples from burn surface area (BSA) were collected every Monday from each patient by swabbing the exudate of wounds immediately after removing the SSD dressing, but before giving bath to the patient. After giving bath and before SSD dressing, samples were collected again from the previous sampling points, regardless of the clinical condition of the patients. Prior to sampling, the wound was rinsed with sterile saline 0.9% to eliminate any trace of SSD, which is applied daily for topical treatment.
Swabs also were collected from the table where balneotherapy was realized, before each bath and after the last bath. Between every two baths, a cleaning procedure was performed by the cleaning staff. Water analysis was performed once a month.
All swabs were inoculated into 2 mL of salt solution (0.9%), vortex and 1 mL was introduced into Tryptone Soya Broth (TSB; HIMEDIA, India) in double concentration. The samples were incubated at 35 °C (± 2) for 24 to 48 hours. The tubes that showed turbid medium were screwed onto cetrimide agar (HIMEDIA, India) and incubated again at the same conditions mentioned above. The colonies that could grow in cetrimide agar were submitted to testing for P. aeruginosa, using the standard biochemical tests. All the isolates were kept on crioprotector medium at -20 °C. To confirm detection of P. aeruginosa, polimerase chain reaction (PCR) was carried out according to Spilker et al. (2004Spilker, T.; Coenye, T.; Vandamme, P.; Lipuma, J.J. PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J. Clin. Microbiol., v.142, p.2074-2079, 2004.).
Determination of minimal inhibitory concentration (MIC)
The MIC values were determined by agar microdilution method, using Pseudomonas aeruginosa ATCC 27853 as microorganism control. Muller Hinton agar (MHA) medium (DIFCO LABORATORIES, USA) was prepared with serial diluted concentrations of SSD (PHARMA NOSTRA, India) incorporated into agar. Steers replicator was used to transfer the inoculum to the plate.
Characterization of the isolates by PFGE
Clonal relatedness of the isolates was evaluated by PFGE as described previously (Gautom et al., 1997Gautom, R.K. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol., v.35, p.2977-2980, 1997.), using the SpeI restriction enzyme instead of XbaI performed in a contour-clamped homogeneous-electric-field DRIII apparatus (Bio-Rad Laboratories, Hercules, CA., USA). The clonality was determined by visual analysis using Tenover criterion (Tenover et al., 1995Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed- field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol., v.33, p.2233-2239, 1995.).
Statistical analysis
Descriptive statistics was used for exploratory analysis of the variables involved in balneotherapy, and McNemar, a non-parametric test, was employed for risk factors analysis.
RESULTS
The results concerning the balneotherapy of 18 patients, for 14 weeks, were analyzed. Seventy baths, with a mean of 5 (± 0.6) patients per day bath, were monitored. The average time spent by a patient in each balneotherapy session was 29 minutes.
From the 292 swabs studied, 37 strains were identified as P. aeruginosa; of these, 28 strains were collected from BSA and nine from the balneotherapy table surface (BTS). Nine patients (50%) showed no bacterial colonization at any time of the study. Ages of the patients varied from 19 to 77 years (mean age=41, sd=18), but no extreme age patient was present in the group where P. aeruginosa was identified.
The gender distribution of the 18 patients tested was equitable (50% males and 50% females). Despite the isolates being few, it is remarkable that six out of nine patients, who tested positive for P. aeruginosa, were females. No relationship was observed between the percentages of BSA and colonization by P. aeruginosa, because patients with both 8.5% and 80% BSA were colonized by this microorganism. In addition, of the 28 P. aeruginosa isolates found in BSA, 20 were detected in female patients (Table I).
No P. aeruginosa isolates were detected in the water used for bathing the patients or in the material used to clean the table. Temporality assessment of P. aeruginosa colonization reveals an increase in bacteria during the last weeks of the study.
The first P. aeruginosa detected in this study was isolated from BSA and obtained on the fourth bath-day (day which the swab was collected) from the third patient before being submitted to the procedure.
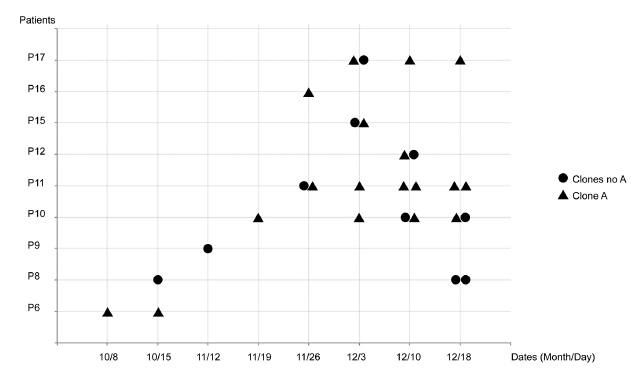
The analysis of the total DNA fragmentation profile by PFGE demonstrated the existence of 10 clones among 37 strains of P. aeruginosa strains analyzed. Clone A was the most prevalent with 23 strains (62%), distributed into 08 subtypes (A1-A8). The majority of the patients were colonized by clone A in different moments of the study, except the patient 8 and 9 (P8 and P9; Figure 1). This clone was also found on the table surface after the balneotherapy sessions 13 and 14 (Table I).
Other PFGE patterns were also found and designated as C, D, E, F, G, H, I and J. Profiles classified as C, D, G, H and I were detected only in the BSA of patients, and H and J on the table (Table I). Three patients (P10, P11 and P17) had P. aeruginosa identified several times during the study (Figure 1). PFGE pattern could not be determined by SpeI in two samples (Table I).
The temporal analysis suggested the ineffectiveness of the balneotherapy (Figure 1), although the number of samples did not allow the identification of a significant statistical difference neither for the bath (odds ratio=1.200; 0.305 < CI 95% < 4.971; p = 1.0000) nor for the table (odds ratio= 0.333; 0.006< CI 95% < 4.151; p = 0.6171) as a risk factor.
In addition, this analysis could also show as P. aeruginosa was spread to different patients. The subtype A2 was found in different patients (P6, P10, P11 and P16) and in different moments. Similarly, the subtype A3 was found after and before the P11 bath. These examples suggest the hydrotherapy was not effective (Table I).
Because silver sulfadiazine was extensively used in the treatment of burned patients, MIC for this antimicrobial was determined for 37 P. aeruginosa isolated in this study. The results demonstrated that MIC values ranged from 32 mg/L to 128 mg/L;
32 mg/L in eight isolates, 64 mg/L in four and 128 mg/L in most isolates (Table I).
DISCUSSION
P. aeruginosa, an important source of nosocomial infections, can be acquired in a community or hospital setting. It is the most common opportunistic pathogen to infect hospitalized burn patients (Fallah et al., 2013FALLAH, F.; BORHAN, R.S.; HASHEMI, A. Detection of bla(IMP) and bla(VIM) metallo-β-lactamases genes among Pseudomonas aeruginosa strains. Int. J. Burns Trauma, v.3, p.122-124, 2013. ; Ackerman et al., 2013Ackerman, B.H.; Reigart, C.L.; Stair-Buchmann, M.; Haith, L.R.; Patton, M.L.; Guilday, R.E. Use of nebulized antimicrobial agents in burned and mechanically ventilated patients with persistent Acinetobacter baumannii, Pseudomonas aeruginosa, or Enterobacteriacea. Burns, v.39, n.5, p.885-891, 2013.). According to Ackerman et al (2013), patients with severe burns have immunosuppression, and consequently, increased susceptibility to nosocomial infections by P. aeruginosa, resulting in high mortality rates, mainly because of their high intrinsic resistance to many antibiotics.
In this study, 37 P. aeruginosa isolates were detected from the BSA of 9 out of 18 patients. In addition, this microorganism was identified on the table surface where balneotherapy occurred.
Although in this study we observed P. aeruginosa colonizing more female patients, we could not investigate the role of gender as a risk factor, owing to the small number of patients with Pseudomonas colonization. Moreover, some authors find no significant statistical difference between the results of patient cultures and their genders (Al Laham et al., 2013Al Laham, N.; Elmanama, A.A.; Tayh, G.A. Possible risk factors associated with burn wound colonization in burn units of Gaza strip hospitals, Palestine. Ann. Burns Fire Disasters, v.26, n.2, p.68-75, 2013.).
Molecular typing methods using PFGE are important tools for epidemiological studies (Ballarini et al., 2012Ballarini, A.; Scalet, G.; Kos, M.; Cramer, N.; Wiehlmann, L.; Jousson, O. Molecular typing and epidemiological investigation of clinical populations of Pseudomonas aeruginosa using an oligonucleotide-microarray. BMC Microbiol., v.27, p.152, 2012.; Mudau et al., 2013Mudau, M.; Jacobson, R.; Minenza, N.; Kuonza, L.; Morris, V.; Engelbrecht, H.; Nicol, M.P.; Bamford, C. Outbreak of multi-drug resistant Pseudomonas aeruginosa bloodstream infection in the haematology unit of a South African Academic Hospital. PLoS One, v.8, e55985, 2013.). The similarity between strains allows determination of the source of an outbreak, and facilitates implementation of effective control measures (Mudau et al., 2013).
In this work, we detected one clone (named A) as a major P. aeruginosa clone spread during all period of the study (Figure I). This clone was found on the surface of the table among multiple procedures and on BSA patient suggesting transmission of the isolate from table to patient (Table I).
Persistence of clones of P. aeruginosa in the hospital environment has been discussed by several authors (Tredget et al., 2004Tredget, E.; Shankowsky, H.A.; Rennie, R.; Burrell, R.E.; Logsetty, S. Pseudomonas infections in the thermally injured patient. Burns, v.30, p.3-26, 2004.; Wolska et al., 2012Wolska, K.; Kot, B.; Jakubczak, A. Phenotypic and genotypic diversity of Pseudomonas aeruginosa strains isolated from hospitals in Siedlce (Poland). Braz. J. Microbiol., v.43, p.274-282, 2012.; Johansson el al., 2014Johansson, E.; Welinder-Olsson, C.; Gilljam, M. Genotyping of Pseudomonas aeruginosa isolates from lung transplant recipients and aquatic environment-detected in-hospital transmission. APMIS, v.122, p.85-91, 2014.). Analysis of table surface demonstrates possible cross-transmission of the microorganism during balneotherapy procedure (Table I). This microorganism is known for its ability to adhere to both biological cell membranes and inert surfaces, mediated through the pili and by the production of large amounts of exopolysaccharides (Pier et al., 1987Pier, G.B.; Ames, P.; Edwards, M.S.; Edwards, M.S.; Auerbach, H.; Goldfarb, J.; Speert, D.P.; Hurwitch, S. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. N. Engl. J. Med., v.317, p.793-798, 1987.). In addition, this microorganism has strong capability to develop multidrug resistance to many drugs (Ackerman et al., 2013Ackerman, B.H.; Reigart, C.L.; Stair-Buchmann, M.; Haith, L.R.; Patton, M.L.; Guilday, R.E. Use of nebulized antimicrobial agents in burned and mechanically ventilated patients with persistent Acinetobacter baumannii, Pseudomonas aeruginosa, or Enterobacteriacea. Burns, v.39, n.5, p.885-891, 2013.; Shanthi et al., 2013Shanthi, J.; Shanthi, J.; Pazhanimurugan, R.; Gopikrishnan, V.; Balagurunathan, R. Mechanism of drug resistance, characterization of plasmid-borne determinants and transformation study in P. aeruginosa from burn and ICU units-its susceptibility pattern. Burns, v.39, p.643-649, 2013.). Additionally, Tredget et al. (2004) demonstrated that P. aeruginosa clinical isolates, belonging to the same clone, were present in both the patients and the sink trap. In this study, transmissions from patient to patient and patient to balneotherapy table were verified. It seems that some isolates have greater ability to persist and fit better in the environment at the expense of others (Elmanama et al., 2013Elmanama, A.; Laham, N.A.A.; Tayh, G.A. Antimicrobial susceptibility of bacterial isolates from burn units in Gaza. Burns, v.39, p.1612-1618, 2013.). In this study, although only a few isolates were detected, we can verify the predominant clone persisted for 14 weeks (Figure 1). Possibly, the clone is better adapted to the conditions of the study.
Analysis of the frequency of subtype A2 demonstrates the transmission of the isolate between two patients (Table I), besides confirming the isolate's ability to persist. A3 subtype isolate demonstrates the ineffectiveness of balneotherapy for decolonization, because the same isolate was found before and after balneotherapy of the same patient.
Regardless of clonality, evaluating contamination by P. aeruginosa as a risk factor might deserve attention. Although no statistically significant relationship could be observed among balneotherapy procedures, probably because of fewer patients and fewer events, the fact that six patients, who were not contaminated before bathing, became contaminated after bathing, suggests that bathing can be considered a clinically risk factor. In addition, eight patients who were found as contaminated before balneotherapy remained so even after bathing (Figure 1). This leads one to believe that neither the procedure nor the degermant allowed decontamination. This observation is reinforced by the genetic profile analysis of DNA bacteria showing similarity among isolates collected at points and different moments.
The risks associated with the ways the injury is taken care of, such as daily bath, can be related to the source of water (tap, hose or shower), which is frequently contaminated with microorganisms of the environment, such as bodies of other patients (Tredget et al., 2004Tredget, E.; Shankowsky, H.A.; Rennie, R.; Burrell, R.E.; Logsetty, S. Pseudomonas infections in the thermally injured patient. Burns, v.30, p.3-26, 2004.). For this study, besides the bath table, the water used was also evaluated and no contamination by P. aeruginosa was observed.
Silver sulfadiazine serves better to prevent wounds colonization/infection and therefore is the best choice to reduce the risk of sepsis, a serious complication and a major threat to burn victims with large burns (Shanthi et al., 2014; Hajska et al., 2014Hajska, M.; Slobodnikova, L.; Hupkova, H.; Koller, J. In vitro efficacy of various topical antimicrobial agents in different time periods from contamination to application against 6 multidrug-resistant bacterial strains isolated from burn patients. Burns, v.40, n.4, p.713-718, 2014.). At the BC studied, SSD agent was used as a dressing reference standard therapy. It is important to note that in this study, 64% of the isolates tested showed MIC of 128 mg/L (Table I). This suggests decreased susceptibility of the bacteria in comparison to the findings of a study conducted in 1973, in New York, which reports that 100% of the strains of P. aeruginosa infection of skin had MIC of 50 mg/L and 60% of the isolates tested showed MIC of 6.25 mg/L (Carr et al., 1973Carr, H.S.; Wlodkowski, T.J.; Rosenkranz, H.S. Silver sulfadiazine: in vitro antibacterial activity. Antimicrob. Agents Chemother., v.4, n.5, p.585-587, 1973.). We suggest that the wide use of this topical agent could select more resistant strains to this antimicrobial.
CONCLUSION
The temporal analysis of balneotherapy in decolonization of SCQ by strains of P. aeruginosa shows that this procedure is not effective for decontamination of patients and table. On the surface of the table where the baths were realized, clones of the same subtype of P. aeruginosa were found, suggesting that the table was not being adequately disinfected. In addition, clones of the same subtype of P. aeruginosa were found, before and after bathing, in different burn areas of the same patient. This suggests cross-contamination between patients.
The MIC values obtained in this study for silver sulfadiazine are rather higher in comparison to those reported in different decades, suggesting that other techniques must be used for topical treatment of burned patients.
ACKNOWLEDGEMENTS
The authors thank the staff and the patients of the Burn Center studied. The study was funded in by research grant from the CNPq (476064/2011-2) , from the FAPERJ (E-26/110.279/2012) and from the PROPPi-UFF; Brazil. There are no conflicts of interest to report for this study.
REFERENCES
- Ackerman, B.H.; Reigart, C.L.; Stair-Buchmann, M.; Haith, L.R.; Patton, M.L.; Guilday, R.E. Use of nebulized antimicrobial agents in burned and mechanically ventilated patients with persistent Acinetobacter baumannii, Pseudomonas aeruginosa, or Enterobacteriacea. Burns, v.39, n.5, p.885-891, 2013.
- Al Laham, N.; Elmanama, A.A.; Tayh, G.A. Possible risk factors associated with burn wound colonization in burn units of Gaza strip hospitals, Palestine. Ann. Burns Fire Disasters, v.26, n.2, p.68-75, 2013.
- Ballarini, A.; Scalet, G.; Kos, M.; Cramer, N.; Wiehlmann, L.; Jousson, O. Molecular typing and epidemiological investigation of clinical populations of Pseudomonas aeruginosa using an oligonucleotide-microarray. BMC Microbiol., v.27, p.152, 2012.
- Barajas-Nava, L.A.; Lopez-Alcalde, J.; RoquÉ I FIGULS, M.; SolÀ, I.; Bonfill COSP, X. Antibiotic prophylaxis for preventing burn wound infection. Cochrane Database Syst. Rev., v.6, art.CD008738, p.1-174, 2013.
- Carr, H.S.; Wlodkowski, T.J.; Rosenkranz, H.S. Silver sulfadiazine: in vitro antibacterial activity. Antimicrob. Agents Chemother., v.4, n.5, p.585-587, 1973.
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev., v.19, p.403-434, 2006.
- De Gracia, C.G. An open study comparing topical silver sulfadiazine and topical silver sulfadiazine-cerium nitrate in the treatment of moderate and severe burns. Burns, v.27, p.67-74, 2001.
- Elmanama, A.; Laham, N.A.A.; Tayh, G.A. Antimicrobial susceptibility of bacterial isolates from burn units in Gaza. Burns, v.39, p.1612-1618, 2013.
- Eski, M.; Ozer, F.; Firat, C.; Alhan, D.; Arslan, N.; Senturk, T.; Isik, S. Cerium nitrate treatment prevents progressive tissue necrosis in the zone of stasis following burn. Burns, v.38, p.283-289, 2011.
- FALLAH, F.; BORHAN, R.S.; HASHEMI, A. Detection of bla(IMP) and bla(VIM) metallo-β-lactamases genes among Pseudomonas aeruginosa strains. Int. J. Burns Trauma, v.3, p.122-124, 2013.
- Gautom, R.K. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol., v.35, p.2977-2980, 1997.
- Hajska, M.; Slobodnikova, L.; Hupkova, H.; Koller, J. In vitro efficacy of various topical antimicrobial agents in different time periods from contamination to application against 6 multidrug-resistant bacterial strains isolated from burn patients. Burns, v.40, n.4, p.713-718, 2014.
- Hu, H.; Harmer, C.; Anuj, S.; Wainwright, C.E.; Manos, J.; Cheney, J.; Harbour, C.; Zablotska, I.; Turnbull, L.; Whitchurch, C.B.; Grimwood, K.; Rose, B. FBAL study investigators Type 3 secretion system effector genotype and secretion phenotype of longitudinally collected Pseudomonas aeruginosa isolates from young children diagnosed with cystic fibrosis following newborn screening. Clin. Microbiol. Infect., v.19, p.266-272, 2013.
- Johansson, E.; Welinder-Olsson, C.; Gilljam, M. Genotyping of Pseudomonas aeruginosa isolates from lung transplant recipients and aquatic environment-detected in-hospital transmission. APMIS, v.122, p.85-91, 2014.
- KOWALSKE, K.J. Burn wound care. Phys. Med. Rehabil. Clin. N. Am., v.22, p.213-227, 2011.
- Mahar, P.; Padiglione, A.; Cleland, H.; Paul, E.; Hinrichs, M.; Wasiak, J. Pseudomonas aeruginosa bacteraemia in burns patients: risk factors and outcomes. Burns, v.36, p.1228-1233, 2010.
- Mudau, M.; Jacobson, R.; Minenza, N.; Kuonza, L.; Morris, V.; Engelbrecht, H.; Nicol, M.P.; Bamford, C. Outbreak of multi-drug resistant Pseudomonas aeruginosa bloodstream infection in the haematology unit of a South African Academic Hospital. PLoS One, v.8, e55985, 2013.
- Nonaka, L.; Inubushi, A.; Shinomiya, H.; Murase, M.; Suzuki, S. Differences of genetic diversity and antibiotics susceptibility of Pseudomonas aeruginosa isolated from hospital, river and coastal seawater. Environ. Microbiol. Rep., v.2, p.465-472, 2010.
- Pier, G.B.; Ames, P.; Edwards, M.S.; Edwards, M.S.; Auerbach, H.; Goldfarb, J.; Speert, D.P.; Hurwitch, S. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. N. Engl. J. Med., v.317, p.793-798, 1987.
- Shanthi, J.; Shanthi, J.; Pazhanimurugan, R.; Gopikrishnan, V.; Balagurunathan, R. Mechanism of drug resistance, characterization of plasmid-borne determinants and transformation study in P. aeruginosa from burn and ICU units-its susceptibility pattern. Burns, v.39, p.643-649, 2013.
- Spilker, T.; Coenye, T.; Vandamme, P.; Lipuma, J.J. PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J. Clin. Microbiol., v.142, p.2074-2079, 2004.
- TANEJA, N.; CHARI, P.; SINGH, M.; SINGH, G.; BISWAL, M.; SHARMA, M. Evolution of bacterial flora in burn wounds: key role of environmental disinfection in control of infection. Int. J. Burns Trauma, v.3, p.102-107, 2013.
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed- field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol., v.33, p.2233-2239, 1995.
- Tredget, E.; Shankowsky, H.A.; Rennie, R.; Burrell, R.E.; Logsetty, S. Pseudomonas infections in the thermally injured patient. Burns, v.30, p.3-26, 2004.
- Ulkur, E.; Oncul, O.; Karagoz, H.; Yeniz, E.; Celikoz, B. Comparison of silver-coated dressing (Acticoat), chlorhexidine acetate 0.5% (Bactigrass), and fusidic acid 2% (Fucidin) for topical antibacterial effect in methicillin-resistant staphylococci-contaminated, full-skin thickness rat burn wounds. Burns, v.31, p.874-77, 2005.
- Wang, X.Q.; Kravchuk, O.; Kimble, R.M. A retrospective review of burn dressings on a porcine burn model. Burns, v.36, p.680-720, 2010.
- Wolska, K.; Kot, B.; Jakubczak, A. Phenotypic and genotypic diversity of Pseudomonas aeruginosa strains isolated from hospitals in Siedlce (Poland). Braz. J. Microbiol., v.43, p.274-282, 2012.
- Yali, G.; Jing, C.; Chunjiang, L.; Cheng, Z.; Xiaoqiang, L.; Yizhi, P. Comparison of pathogens and antibiotic resistance of burn patients in the burn ICU or in the common burn ward. Burns, v.40, p.402-407, 2013.
Publication Dates
-
Publication in this collection
Mar 2016
History
-
Received
23 Oct 2014 -
Accepted
22 Dec 2015