Abstract
In this study, conditions were optimized for development of a simple RP-HPLC method for simultaneous analysis of gatifloxacin and dexamethasone in different matrices like pharmaceuticals, human serum and urine. Good separation of gatifloxacin and dexamethasone from the induced degradation products was accomplished using C8 as stationary phase; 0.02 M phosphate buffer (pH 3.0) and methanol (42:58 v/v) as mobile phase. The concentration was measured with DAD at 270 nm. Linearity was observed in the range of 0.000040-0.000280 mol/L for gatifloxacin (r2≥0.999) and 0.000013-0.000091 mol/L for dexamethasone (r2≥0.999). Both the analyte peaks were completely separated from the peaks of induced degradation products as indicated by the peak purity index (≥0.9999 for both analytes). The optimized method is recommended to be used for concurrent analysis of gatifloxacin and dexamethasone in different matrices.
Uniterms:
Gatifloxacin; Dexamethasone; High performance liquid chromatography; Validation; Plasma; Degradation products.
INTRODUCTION
Gatifloxacin (Figure 1A), a broad spectrum antibiotic is primarily used to treat bacterial infections like conjunctivitis, keratitis, pre and post-operative (Martindale, 2009). Dexamethasone (Figure 1B), a corticosteroid, used primarily for ocular inflammation conditions and where the risk of bacterial ocular infection or its risk is found (Martindale, 2009). The determination of gatifloxacin has widely been reported using spectrophotometry (Sonali et al., 2006SONALI, P.; LATA, K.; ASHA, T.; DESHPANDE, A.D. Simultaneous spectrophotometric estimation of gatifloxacin and ornidazole in tablet dosage form. Ind. J. Pharm. Sci., v.68, p.819-821, 2006.; Venugopal, Ranendra, 2005VENUGOPAL, K.; RANENDRA, N. New, simple and validated UV-spectrophotometric methods for the estimation of gatifloxacin in bulk and formulations. Farmaco, v.60, p.906-912, 2005.), HPLC (Santoro et al., 2006SANTORO, M.I.; KASSAB, N.M.; SINGH, A.K.; KEDOR-HACKMAM, E.R. Quantitative determination of gatifloxacin, levofloxacin, lomefloxacin and pefloxacin fluoroquinolonic antibiotics in pharmaceutical preparations by high-performance liquid chromatography. J. Pharm. Biomed. Anal., v.40, n.1, p.179-184, 2006. ), atomic absorption spectrophotometry, conductometry, colorimetry (Sheikh, 2008SHEIKH, M. Atomic absorption spectroscopic, conductometric and colorimetric methods for determination of some fluoroquinolone antibacterials using ammonium reineckate. Spectrochim. Acta A., v.69, p.1188-1194, 2008.) and spectrofluorimetry (Ocaña, Barragán, Callejón, 2005OCAÑA, J.A.; BARRAGÁN, F.J.; CALLEJÓN, M. Spectrofluorimetric and micelle-enhanced spectrofluorimetric determination of gatifloxacin in human urine and serum. J. Pharm. Biomed. Anal., v.37, n.2, p.327-332, 2005.). Similarly, the estimation of dexamethasone has also been reported widely by HPLC (Iqbalet al., 2006; Huetos et al., 1999HUETOS, O.; RAMOS, M.; MARTÍN DE POZUELO, M.; SAN ANDRÉS, M.; REUVERS, T.B. Determination of dexamethasone in feed by TLC and HPLC. Analyst, v.124, p.1583-1587, 1999.; Mallinson et al., 1995MALLINSON, E.T.; DREAS, J.S.; WILSON, R.T.; HENRY, A.C. Determination of dexamethasone in liver and muscle by Liquid Chromatography and Gas Chromatography/Mass Spectrometry. J. Agric. Food Chem., v.43, p.140-145, 1995.; Chen et al., 2008CHEN, Q.; ZIELINSKI, D.; CHEN, J.; KOSKI, A.; WERST, D.; NOWAK, S. A validated, stability-indicating HPLC method for the determination of dexamethasone related substances on dexamethasone-coated drug-eluting stents. J. Pharm. Biomed. Anal., v.48, p.732-738, 2008.; Lemus Gallego, Pérez Arroyo, 2002; Cocenza, Mainardes, Gremião, 2009COCENZA, M.C.; MAINARDES, R.M.; GREMIÃO, M.P. Development and validation of HPLC method for analysis of dexamethasone acetate in micro emulsions. J. Pharm. Sci., v.45, p.87-92, 2009.; Kwak, D'Amico, 1995KWAK, H.W.; D'AMICO, D.J. Determination of dexamethasone sodium phosphate in vitreous by high performance liquid chromatography. Korean J. Ophthalmol., v.9, p.79-83, 1995.), GC-MS (Mallinson et al., 1995) and TLC (Huetos et al., 1999).
Although gatifloxacin and dexamethasone fixed dose combination (FDC) is available for sale, however not officially adopted by any pharmacopeia. Scientific literature was also found scarce of any stability indicating method for this FDC. In order to fill this knowledge gap, the present study was designed to optimize the chromatographic conditions in order to estimate them in different matrices (pharmaceutical formulations, human urine and serum). In the past few years, different FDC have already been reported by our research group after developing suitable analytical methods (Ashfaq et al., 2013ASHFAQ, M.; HAMAD, A.; KHAN, I.U.; MUSTAFA, G. LC determination of rosuvastatin and ezetimibe in human plasma. J. Chil. Chem. Soc., v.58, n.4, p.2177-2181, 2013.; 2014; Khan et al., 2013KHAN, I.U.; ASHFAQ, M.; RAZZAQ, S.N.; MARIAM, I. Simultaneous determination of piroxicam and paracetamol in pharmaceutical formulations using stability indicating HPLC method. J. Liq. Chrom. Rel. Technol., v.36, p.1437-1450, 2013.; 2014; Razzaq et al., 2013RAZZAQ, S.N.; ASHFAQ, M.; MARIAM, I.; KHAN, I.U.; RAZZAQ, S.S. Simultaneous RP-HPLC determination of sparfloxacin and dexamethasone in pharmaceutical formulations. Braz. J. Pharm. Sci., v.49, p.301-309, 2013.; 2014; John et al., 2015aJOHN, P.; AZEEM, W.; ASHFAQ, M.; KHAN, I.U.; RAZZAQ, S.N. Stability indicating RP-HPLC method for simultaneous determination of piroxicam and ofloxacin in binary combination. Pak. J. Pharm. Sci, v.28, n.5, p.1713-1721, 2015a., b; Saleem et al., 2015SALEEM, A.; ANWAR, S.; HUSSAIN, T.; AHMAD, R.; MUSTAFA, G.; ASHFAQ, M. Simultaneous determination of acetaminophen, pamabrom and pyrilamine maleate in pharmaceutical formulations using stability indicating HPLC assay method. J. Mex. Chem. Soc. v.59, p.93-98, 2015.).
MATERIAL AND METHODS
Chemicals and Reagents
Gatifloxacin (99.7%) and dexamethasone (99.2%) reference standards were obtained as gift samples from a pharmaceutical laboratory located at Lahore, Pakistan. GATE DX and GATIBLU-D ophthalmic preparations (3 mg/mL gatifloxacin and 1 mg/mL dexamethasone) were analyzed during the applications of the method. All other chemicals and reagents were of highest analytical grades available in the market. For filtration of all solutions including mobile phase, 0.45 mm nylon filters (Millipore, USA) were used.
Equipment and Chromatographic Conditions
Liquid chromatography as well as integration of peak areas and chromatographic parameter were calculated using Shimadzu LC-20A system (Kyoto, Japan) at 270 nm. Hypersil C8 column (0.250 m X 4.6 mm, 5 mm) was used as stationary phase while 0.02 M phosphate buffer pH 3.0 (adjusted by 1 mL triethylamine and phosphoric acid) and methanol (42:58 v/v) were used as mobile phase at 1.5 mL/min.
Preparation of Standard Solution (A) and Working Standard Solution (B)
Stock solution (A) of gatifloxacin (0.0040 mol/L) and dexamethasone (0.00130 mol/L) was prepared by dissolving 150 mg gatifloxacin and 50 mg dexamethasone in few mL of methanol in 100 mL measuring flask, sonicated for few minutes and then diluted up to the mark with mobile phase. Solution was then subsequently diluted to get working solution (B) of gatifloxacin (0.000160 mol/L) and dexamethasone (0.000052 mol/L).
Preparation of Sample Solution
An appropriate volume of commercial ophthalmic solution was diluted with mobile phase to get 0.000160 mol/L gatifloxacin and 0.000052 mol/L dexamethasone.
Preparation of Human Urine Samples
1250 mL of solution A was mixed with equal volume of human urine for 2 minutes, followed by its centrifugation for 10 minutes at 4000 rpm. The supernatant (200 mL) was added in 2300 mL of the mobile phase. The final concentration of solution thus obtained was equal to (0.000160 mol/L of gatifloxacin and 0.000052 mol/L of dexamethasone).
Preparation of Human Serum Samples
Solucion A, 50 mL, was mixed with 250 mL human serum, followed by adding 950 mL mobile phase. The mixture was centrifuged for 10 minutes at 4000 rpm and the supernatant was shifted in polypropylene tubes having concentration equal to 0.000160 mol/L for gatifloxacin and 0.000052 mol/L for dexamethasone.
Linearity
Linearity was established by analyzing each solution in triplicate in the range of 0.000040-0.000280 mol/L (0.000040, 0.000080, 0.000120, 0.000160, 0.000200, 0.000240 and 0.000280 mol/L) of gatifloxacin and 0.000013-0.000091 mol/L of dexamethasone (0.000013, 0.000026, 0.000039, 0.000052, 0.000065, 0.000078 and 0.000091 mol/L).
Accuracy
Synthetic mixtures of both analytes were analyzed in order to evaluate accuracy. The synthetic mixture was prepared by mixing appropriate amounts of gatifloxacin, dexamethasone, benzalkonium chloride and sodium chloride in 1L purified water. The solution was then diluted to get solutions of three different concentrations (50-150%) of working solution concentration (0.000160 mol/L gatifloxacin and 0.000052 mol/L dexamethasone). These solutions were further used to test recovery studies.
Precision
Precision was evaluated by calculating % RSD of the samples analyzed within one day for five times and for sample analyzed for three days. Three different concentration of solutions were used for both within-day and between-day precision.
Specificity (Stress Testing)
Acid Degradation Studies
To four separate measuring flasks, each contained 1 mL solution A, 5 M hydrochloric acid (1 mL) was mixed and kept in chamber at 40 oC/75% RH for 1, 8, 16 and 23 h followed by neutralization and completing to the mark.
Base, Oxidative and Thermal Degradation Studies
Same procedure was used for base, oxidative and thermal stress studies except using 5 M NaOH instead of 5 M HCl in basic stress, 6% H2O2 for oxidative stress (1, 8,23 and 70 h) and 1, 16, 24 and 70 h time for thermal stress.
Photolytic Degradation Studies
Three separate 25 mL measuring flasks containing 1 mL of solution A were exposed to direct sunlight for 2, 4 and 6 h, followed by marking the volume.
RESULTS AND DISCUSSION
Reverse phase (RP) chromatography has been used widely in the last two decades for the separation of many organic molecules particularly pharmaceuticals because of containing major non-polar groups as well as containing unsaturated π electrons. As both the investigating pharmaceuticals have this common feature in their structure, so in this study, an RP-HPLC was developed for simultaneous analysis of gatifloxacin and dexamethasone in different matrices like pharmaceuticals, human serum and urine.
Optimization of Mobile Phase and Stationary Phase
Both dexamethasone and gatifloxacin have been analyzed individually in previous methods (Razzaq et al., 2013RAZZAQ, S.N.; ASHFAQ, M.; MARIAM, I.; KHAN, I.U.; RAZZAQ, S.S. Simultaneous RP-HPLC determination of sparfloxacin and dexamethasone in pharmaceutical formulations. Braz. J. Pharm. Sci., v.49, p.301-309, 2013.; 2014; Khan et al., 2014KHAN, I.U.; RAZZAQ, S.N.; MARIAM, I.; ASHFAQ, M.; RAZZAQ, S.S. Stability-indicating RP-HPLC method for simultaneous determination of gatifloxacin and flurbiprofen in binary combination. Quím. Nova, v.37, p.349-354, 2014.). In order to develop a method that can quantify both of these simultaneously, we took advantage of the problems faced during the previous studies, where dexamethasone peak showed high tailing on C18, Phenyl-2, and Cyano columns but symmetrical/good peak on C8 (Razzaq et al., 2013; 2014; Khan et al., 2014). So we chose C8 column for this FDC. Some minor changes in the composition of organic and aqueous phase as well as pH were good enough to resolve both these compounds from each other as well as from induced degradation products. The final composition of the mobile phase thus used was, methanol: phosphate buffer 0.02 M, pH 3.0 (58:42, v/v) that resulted in good/symmetrical peaks.
Analytical Method Validation
Validation parameters like linearity, accuracy, precision, robustness, specificity, limit of detection and quantitation as recommended by ICH (International Conference on Harmonization, 1996) were evaluated when validating this method.
Linearity was established by analyzing each solution in triplicate in the range of 0.000040-0.000280 mol/L (0.000040, 0.000080, 0.000120, 0.000160, 0.000200, 0.000240 and 0.000280 mol/L) of gatifloxacin and 0.000013-0.000091 mol/L of dexamethasone (0.000013, 0.000026, 0.000039, 0.000052, 0.000065, 0.000078 and 0.000091 mol/L). The linear regression equation for gatifloxacin was found to be Y= 46312 X + 4983 with correlation coefficient ≥0.999 whereas for dexamethasone it was Y= 94196 X + 1566 with correlation coefficient ≥0.999.
Limit of detection (LOD) was found to be 0.0000008 mol/L and 0.0000003 mol/L (Figure 3) for gatifloxacin and dexamethasone, respectively (Figure 6). LOQ was found to be 0.0000027 mol/L and 0.0000001 mol/L for gatifloxacin and dexamethasone, respectively.
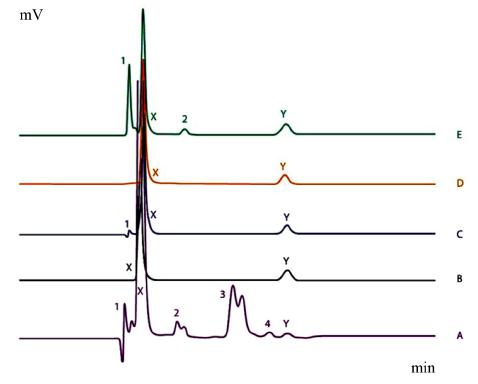
A typical chromatogram of gatifloxacin and dexamethasone under acidic, basic, thermal, photolytic and oxidative stress conditions. Where (X) is gatifloxacin peak, (Y) is dexamethasone peak, (1, 2, 3, and 4) are degradation/impurities peaks, (A) chromatogram of basic stress, (B) chromatogram of thermal stress, (C) chromatogram of acidic stress, (D) chromatogram of photolytic stress and (E) chromatogram of oxidative stress.
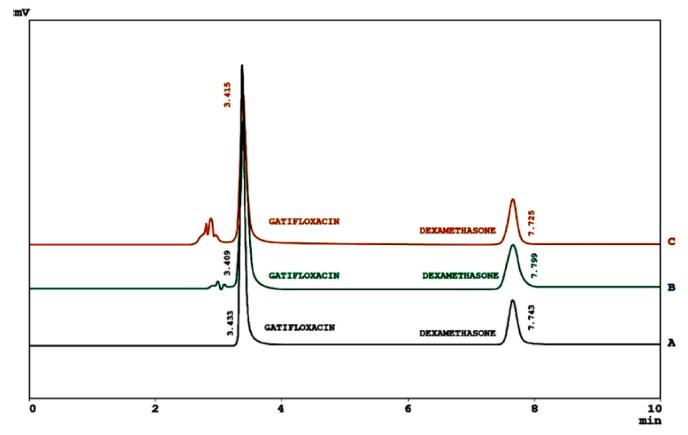
Chromatograms of gatifloxacin and dexamethasone in Pharmaceutical Formulations (A), Human Serum (B) and Urine (C).
Synthetic mixtures of both analytes were analyzed to evaluate accuracy. Solutions of three different concentrations (50-150%) of working solution concentration (0.000160 mol/L gatifloxacin and 0.000052 mol/L dexamethasone) were used to test recovery. The results are presented in (Table I), indicating good recovery.
Precision was evaluated by calculating % RSD of the samples analyzed within one day for five times and for sample analyzed for three days. Three different concentration of solutions were used for both within-day and between-day precision. The results are presented in (Table II).
Slight variation in the chromatographic conditions were made in order to evaluate robustness. Results presented in (Table III and Table IV) showed little effect on chromatographic parameters.
The chromatograms involving forced stresses are shown in (Figure 2), whereas the results describing percentage degradation and extent of degradation are provided (Table V).
Both the drugs were degraded with ease under most of the stress conditions (except thermal and oxidative). When comparing the vulnerability of both drugs, it was observed that dexamethasone was easy to degrade compared to gatifloxacin (31% and 95 % degradation under acidic and basic conditions). Up to 4 degradation products were observed during the mentioned stresses.
The developed method was finally used to determine gatifloxacin and dexamethasone in commercially available ophthalmic solutions (Table VI), spiked human serum and urine (Figure 3). Excellent results were obtained from these experiments.
CONCLUSION
In this study, an RP-HPLC method was developed for simultaneous analysis of gatifloxacin and dexamethasone in different matrices like pharmaceuticals, human serum and urine. Method validation was performed as recommended by ICH. The optimized method is recommended to be used for concurrent analysis of gatifloxacin and dexamethasone in different matrices because of its excellent results with inexpensive reagents.
REFERENCES
- ASHFAQ, M.; AKHTAR, T.; MUSTAFA, G.; DANISH, M.; RAZZAQ, S.N.; NAZAR, M.F. Simultaneous estimation of rosuvastatin and amlodipine in pharmaceutical formulations using stability indicating HPLC method. Braz. J. Pharm. Sci., v.50, p.629-638, 2014.
- ASHFAQ, M.; HAMAD, A.; KHAN, I.U.; MUSTAFA, G. LC determination of rosuvastatin and ezetimibe in human plasma. J. Chil. Chem. Soc., v.58, n.4, p.2177-2181, 2013.
- CHEN, Q.; ZIELINSKI, D.; CHEN, J.; KOSKI, A.; WERST, D.; NOWAK, S. A validated, stability-indicating HPLC method for the determination of dexamethasone related substances on dexamethasone-coated drug-eluting stents. J. Pharm. Biomed. Anal., v.48, p.732-738, 2008.
- COCENZA, M.C.; MAINARDES, R.M.; GREMIÃO, M.P. Development and validation of HPLC method for analysis of dexamethasone acetate in micro emulsions. J. Pharm. Sci., v.45, p.87-92, 2009.
- HUETOS, O.; RAMOS, M.; MARTÍN DE POZUELO, M.; SAN ANDRÉS, M.; REUVERS, T.B. Determination of dexamethasone in feed by TLC and HPLC. Analyst, v.124, p.1583-1587, 1999.
- INTERNATIONAL CONFERENCE ON HARMONIZATION (ICH). Guidance for Industry: (Q2B) Note for guidance on validation of analytical procedures: methodology. Geneva: IFPMA, 1996. p.6-13.
- IQBAL, M.S.; SHAD, M.A.; ASHRAF, M.W.; BILAL, M.; SAEED, M. Development and validation of an HPLC method for the determination of dexamethasone, dexamethasone sodium phosphate and chloramphenicol in presence of each other in pharmaceutical preparations. Chromatographia, v.64, p.219-222, 2006.
- JOHN, P.; AZEEM, W.; ASHFAQ, M.; KHAN, I.U.; RAZZAQ, S.N. Stability indicating RP-HPLC method for simultaneous determination of piroxicam and ofloxacin in binary combination. Pak. J. Pharm. Sci, v.28, n.5, p.1713-1721, 2015a.
- JOHN, P.; AZEEM, W.; ASHFAQ, M.; KHAN, I.U.; RAZZAQ, S.N.; KHAN, S.U.D. Stability-indicating RP-HPLC method for simultaneous determination of methoxsalen and p-aminobenzoic acid in binary combination. Bull. Chem. Soc. Ethiop, v.29, p.27-39, 2015b.
- KHAN, I.U.; ASHFAQ, M.; RAZZAQ, S.N.; MARIAM, I. Simultaneous determination of piroxicam and paracetamol in pharmaceutical formulations using stability indicating HPLC method. J. Liq. Chrom. Rel. Technol., v.36, p.1437-1450, 2013.
- KHAN, I.U.; RAZZAQ, S.N.; MARIAM, I.; ASHFAQ, M.; RAZZAQ, S.S. Stability-indicating RP-HPLC method for simultaneous determination of gatifloxacin and flurbiprofen in binary combination. Quím. Nova, v.37, p.349-354, 2014.
- KWAK, H.W.; D'AMICO, D.J. Determination of dexamethasone sodium phosphate in vitreous by high performance liquid chromatography. Korean J. Ophthalmol., v.9, p.79-83, 1995.
- LEMUS GALLEGO, J.M.; PÉREZ ARROYO, J. Simultaneous determination of dexamethasone and trimethoprim by liquid chromatography. J. Pharm. Biomed. Anal., v.30, n.4, p.1255-1261, 2002.
- MALLINSON, E.T.; DREAS, J.S.; WILSON, R.T.; HENRY, A.C. Determination of dexamethasone in liver and muscle by Liquid Chromatography and Gas Chromatography/Mass Spectrometry. J. Agric. Food Chem., v.43, p.140-145, 1995.
- MARTINDALE: the extra pharmacopoeia. 36 ed. London: Pharmaceutical Press, 2009. p.281, 1526.
- OCAÑA, J.A.; BARRAGÁN, F.J.; CALLEJÓN, M. Spectrofluorimetric and micelle-enhanced spectrofluorimetric determination of gatifloxacin in human urine and serum. J. Pharm. Biomed. Anal., v.37, n.2, p.327-332, 2005.
- RAZZAQ, S.N.; ASHFAQ, M.; KHAN, I.U.; MARIAM, I.; RAZZAQ, S.S.; AZEEM, W. Simultaneous determination of dexamethasone and moxifloxacin in pharmaceutical formulations using stability indicating HPLC method. Arab. J. Chem., 2014. DOI: <http://dx.doi.org/10.1016/j.arabjc.2014.11.016>. (In press).
» http://dx.doi.org/10.1016/j.arabjc.2014.11.016 - RAZZAQ, S.N.; ASHFAQ, M.; MARIAM, I.; KHAN, I.U.; RAZZAQ, S.S. Simultaneous RP-HPLC determination of sparfloxacin and dexamethasone in pharmaceutical formulations. Braz. J. Pharm. Sci., v.49, p.301-309, 2013.
- SALEEM, A.; ANWAR, S.; HUSSAIN, T.; AHMAD, R.; MUSTAFA, G.; ASHFAQ, M. Simultaneous determination of acetaminophen, pamabrom and pyrilamine maleate in pharmaceutical formulations using stability indicating HPLC assay method. J. Mex. Chem. Soc. v.59, p.93-98, 2015.
- SANTORO, M.I.; KASSAB, N.M.; SINGH, A.K.; KEDOR-HACKMAM, E.R. Quantitative determination of gatifloxacin, levofloxacin, lomefloxacin and pefloxacin fluoroquinolonic antibiotics in pharmaceutical preparations by high-performance liquid chromatography. J. Pharm. Biomed. Anal., v.40, n.1, p.179-184, 2006.
- SHEIKH, M. Atomic absorption spectroscopic, conductometric and colorimetric methods for determination of some fluoroquinolone antibacterials using ammonium reineckate. Spectrochim. Acta A., v.69, p.1188-1194, 2008.
- SONALI, P.; LATA, K.; ASHA, T.; DESHPANDE, A.D. Simultaneous spectrophotometric estimation of gatifloxacin and ornidazole in tablet dosage form. Ind. J. Pharm. Sci., v.68, p.819-821, 2006.
- VENUGOPAL, K.; RANENDRA, N. New, simple and validated UV-spectrophotometric methods for the estimation of gatifloxacin in bulk and formulations. Farmaco, v.60, p.906-912, 2005.
Publication Dates
-
Publication in this collection
2017
History
-
Received
08 Sept 2015 -
Accepted
16 Nov 2016