ABSTRACT
The aim of this study was to investigate the effect of gels containing the monoterpene borneol in induced oral mucositis using an animal model. Gels were prepared with borneol at 1.2% and 2.4% (w/w). Oral mucositis was induced by administration of three doses of 5-fluorouracil (30 mg/kg, i.p.) and injury with acetic acid (50%, v/v) soaked in filter paper applied to right cheek mucosa for 60s. Four subgroups comprising 12 animals each were formed. Six animals from each group were sacrificed at days seven and fourteen after oral mucositis induction. Mucous samples were processed and stained with hematoxylin-eosin and Masson’s Trichrome. The semiquantitative evaluation involved observation of inflammatory parameters. ImageJ® software was used in the quantitative evaluation. For statistical analyses, Two-way ANOVA, followed by Tukey’s post-test (p <0.05), were employed. Borneol 2.4% gel proved effective in the treatment of oral mucositis with statistically significant differences between groups for angiogenesis control, inflammatory cell count reduction and percentage neoformed collagen increase. The confirmation of anti-inflammatory and healing action of borneol in oral mucositis in rats renders it a good marker for predicting this activity for plant extracts rich in this substance.
Keywords:
Chemotherapy; Oral gel/Borneol monoterpene/effects; Oral gel/Borneol monoterpene/action anti-inflammatory; Oral gel/Borneol monoterpene/healing action; Bornyl alcohol/effects; Palliative care
INTRODUCTION
The clinical manifestations most commonly associated with treatment of cancer of the oral cavity are oral mucositis (OM), osteoradionecrosis, dry mouth, radiation caries, trismus, opportunistic infections and dysphagia, among others. OM is an important complication of oncological treatments such as head and neck radiotherapy, chemotherapy and Hematopoietic Stem Cell Transplantation (HSCT), autologous or allogeneic, having a prevalence of 75%-99%. (Simões, Castro, Cazal, 201126. Simões CA, Castro JFL, Cazal C. Cândida oral como fator agravante da mucosite radioinduzida. Rev Bras Cancer. 2011;57(1):23-29.; Patussi et al., 201421. Patussi C, Sassi LM, Munhoz EC, Zanicotti RTS, Schussel JL. Clinical assessment of oral mucositis and candidiasis compare to chemotherapic nadir in transplanted patients. Braz Oral Res. 2014;28:1-7.). Therefore, the healthcare team should have a broad knowledge of all oral complications of anticancer therapy in order to improve patients´ quality of life (Sera et al., 201325. Sera EAR, Oliveira RV, Mariotto AH, Aquino DR, Scherma AP. Avaliação dos cuidados odontológicos pré e trans tratamento radioterápico. Braz J Oral Sci. 2013;23(3):30-38.).
Among the different oral manifestations, mucositis is the most common and distressing condition that develops as an acute adverse effect of chemotherapy and radiotherapy of the head and neck and is one of the leading causes of disability that limits cancer therapy dose. This complication leads to therapy alteration, dose reduction, delay, interruption or termination and can negatively affect the healing process. It is clinically characterized by erythematous and ulcerative lesions that affect the vermilion of the lips and oral mucosa (Smith et al., 200727. Smith PW, Wilson MJH, Zhang J, Wang Q, Osann K, Chen Z, Wigdor H, Schwartz J, Epstein J. In vivo imaging of oral mucositis in an animal model using optical coherence tomography and optical doppler tomography. Clin Cancer Res. 2007;13(8):2449-2454.).
The use of medicinal plants and herbal medicines represents a good alternative treatment approach for this patient group. Products containing Aloe vera L., Leptospermum scoparium J.R. & G. Forst and Kunzea ericoides A. Rich have been used to treat oral mucositis with satisfactory results (Rodriguez-Caballero et al., 201223. Rodriguez-Caballero A, Torres-Lagares D, Robles-García M, Pachon-Ibañez J, Gonzalez-Padilla D, Gutierrez-Perez JL. Cancer treatment-induced oral mucositis: a critical review. Int J Oral Max Surg. 2012;41(2):225-238.; Freitas, Rodrigues, Gaspi, 201413. Freitas VS, Rodrigues RAF, Gaspi FOG. Propriedades farmacológicas da Aloe vera L. Burm. f. Rev Bras Plantas Med. 2014;16(2):299-307..). Chamomile has been used in the treatment of OM with promising results (Dos Reis et al., 201612. Dos Reis PE, Ciol MA, de Melo NS, Figueiredo PT, Leite AF, Manzi Nde M. Chamomile infusion cryotherapy to prevent oral mucositis induced by chemotherapy: a pilot study. Support Care Cancer. 2016;24(10):4393-4398.; Braga et al., 20157. Braga FT, Santos AC, Bueno PC, Silveira RC, Santos CB, Bastos JK, Carvalho EC. Use of chamomilla recutita in the prevention and treatment of oral mucositis in patients undergoing hematopoietic stem cell transplantation: a randomized, controlled, phase II clinical trial. Cancer Nurs. 2015;38(4):322-329.; Curra et al., 20139. Curra M, Martins MA, Lauxen IS, Pellicioli AC, Sant'Ana Filho M, Pavesi VC, Carrard VC, Martins MD. Effect of topical chamomile on immunohistochemical levels of IL-1ß and TNF-a in 5-fluorouracil-induced oral mucositis in hamsters. Cancer Chemother Pharmacol. 2013;71(2):293-2999.; Pavesi et al., 201122. Pavesi VC, Lopez TC, Martins MA, Sant'Ana Filho M, Bussadori SK, Fernandes KP, Mesquita-Ferrari RA, Martins MD. Healing action of topical chamomile on 5-fluoracil induced oral mucositis in hamster. Support Care Cancer. 2011;19(5):639-646.)
Also, Hypericum perforatum L. extract, whose phytocomplex contains borneol, has shown anti-inflammatory and healing action in oral mucositis animal models both systemically and locally through oral gel (Tanideh et al., 201429. Tanideh N, Namazi F, Tadbir AA, Ebrahimi H, Koohi-Hosseinabadi O. Comparative assessment of the therapeutic effects of the topical and systemic forms of Hypericum perforatum extract on induced oral mucositis in golden hamsters. Int J Oral Max Surg. 2014;43(10):1286-1292.). Borneol (Figure 1) is a monoterpene, bicyclic aromatic alcohol, present in many essential oils of medicinal plants of the Dipterocarpaceae, Lamiaceae (Rosmarinus officinalis L., Thymus vulgaris L. and Salvia officinalis L.) (Dipterocarpus turbinatus Gaertn.) Valerianaceae (Valeriana officinalis L.) and Asteraceae (Matricaria chamomilla L.) families (Horváthová et al., 200914. Horváthová E, Slamenová D, Marsálková L, Sramková M, Wsólová L. Effects of borneol on the level of DNA damage induced in primary rat hepatocytes and testicular cells by hydrogen peroxide. Food Chem Toxicol. 2009;47(6):1318-1323.; Borges et al., 20126. Borges AM, Pereira J, Cardoso MG, Alves JÁ, Lucena EMP. Determinação de óleos essenciais de alfavaca (Ocimum gratissimum L.), orégano (Origanum vulgare L.) e tomilho (Thymus vulgaris L.). Rev Bras Plantas Med. 2012;14(4):656-665.). In Chinese and Indian folk medicine, borneol is used in the treatment of sore throats, mouth sores, wounds, burns and skin infections (Liu et al., 200918. Liu YM, Zhao GF, Xia XH, Xu QY, Shen Q. Influence of muskiness compounded with borneol on blood brain barrier after ischemic reperfusion injury. J Tradit Chin Med. 2009;28(6):459-462.; Kong et al., 201415. Kong Q, Wu Z, Chu X, Liang R, Xia M, Li L. Study on the anti-cerebral ischemia effect of borneol and its mechanism. Afr J Tradit Complement Altern Med. 2014;11(1):161-164.). Some studies indicate that borneol has analgesic, anti-inflammatory, antioxidant, antibacterial and healing actions (Horváthová et al., 2009; Liu et al., 2009; Barreto, 20135. Barreto RSS. Efeito cicatrizante do (-)-borneol incorporado ao filme bioativo de quitosana em roedores. [tese]. Aracajú: Universidade Federal de Sergipe, 2013.).
Although borneol has been shown to be effective in lesions of the oral cavity, the literature reports no pre-clinical models demonstrating its action in oral mucositis. In order to fill this gap, the present study was conducted investigating the action of gels containing borneol in 5-Fluorouracil-induced oral mucositis using an animal model with Wistar rats.
MATERIAL AND METHODS
Plant Material
(+)-Borneol 97% was obtained in isolated and powder form (420247 - Sigma Aldrich, USA), Synonym: endo-(1R)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol; CAS Number: 464-43-7; Empirical Formula: C10H18O; Molecular Weight 154.25; Lot # BGBB9812V.
Preparation of Aristoflex® gels containing borneol and stability tests
Aristoflex polymer was mixed with methylparaben in water at ambient temperature under stirring at 400 rpm (Heidolph RZR stirrer, Germany) for 30 minutes to complete gelation. The mixture was then incorporated into (+)-borneol powder (Sigma-Aldrich, USA) at concentrations of 1.2% and 2.4%. The gels thus obtained (30 g each, containing borneol at concentrations of 0%, 1.2% and 2.4%) were packaged, labeled and analyzed for organoleptic (appearance, color and odor) and physico-chemical (pH, conductivity and viscosity) parameters and also for both primary and accelerated stability (Oliveira, 200920. Oliveira AZM. Desenvolvimento de formulações cosméticas com ácido hialurônico. [dissertação]. Porto: Universidade do Porto; 2009.).
Animals
Male Wistar rats (300-350 g, 12-16 weeks, n=48) were acclimatized for six days prior to experiments and housed during the experimental period at 22±2 °C under a 12/12h light-dark cycle with access to feed and water ad libitum. After the onset of oral mucositis, the animals were tagged and randomly distributed into four subgroups of 12 animals each: G1 Group (Borneol 0%); G2 Group (Borneol 1.2%); G3 Group (Borneol 2.4%); and G4 group (Control). All animals were weighed at the beginning and end of the experiment for clinical evaluation. Another 24 animals were used in pilot tests for dose and technique adjustments, but not included in the results of the study. The project was submitted to the Research Ethics Committee on the use of animals (CEUA - UNIVASF) and was approved under permit 0001/140814 on June 2nd, 2015.
Chemotherapy-induced oral mucositis
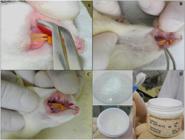
Oral mucositis was induced by administration of the 5-fluorouracil chemotherapy drug (Eurofarma, São Paulo, Brazil) on days 0, 2 and 4 (30 mg/kg, i.p.) according to Lima et al. (201517. Lima GMG, Severo MC, Santana-Melo GF, Carvalho MA, Vilela-Goulart MG, Salgado MA, Gomes MF. Amniotic membrane as a biological dressing for 5-fluoruracil-induced oral mucositis in rats. Int J Oral Max Surg. 2015;44(7):845-851.). On day two, the animals were anesthetized [ketamine hydrochloride 10% (50 mg/kg) and xylazine hydrochloride 2% (5 mg/kg i.p.), 0.4 ml per animal] and right cheek mucous chemically injured by applying 9 mm2 filter paper soaked in 10 µL of solution [glacial acetic acid 96% (50%, v/v) in distilled water] for 60 seconds (Figures 2A, 2B and 2C).
Experimental procedure for testing. A. Placement of filter paper soaked in 50% acetic acid; B. Contact time with cheek mucosa of 60 s; C. Aspect of standardized lesion (9 mm2) after filter paper removal; D. Appearance of gels used in treatments.
Treatment subgroups
The G1, G2 and G3 Groups received a daily application of gels at concentrations of 0%, 1.2% and 2.4%, respectively, using disposable flexible swabs (Figure 2D). The G4 Group (Control) received topical application with distilled water. After the application, all animals were prevented from drinking water or eating for 30 minutes to allow time for the gels to act (Tanideh et al., 201429. Tanideh N, Namazi F, Tadbir AA, Ebrahimi H, Koohi-Hosseinabadi O. Comparative assessment of the therapeutic effects of the topical and systemic forms of Hypericum perforatum extract on induced oral mucositis in golden hamsters. Int J Oral Max Surg. 2014;43(10):1286-1292.). The treatments were administered from the third to 13th day after injury with acetic acid.
Mucous removal and histology procedure
Six animals from each group were sacrificed by deep anesthesia [ketamine hydrochloride 10% (100 mg/kg) and xylazine hydrochloride 2% (10 mg/kg) i.p., 0.5 mLper animal] on days seven and fourteen after injury. Subsequently, right cheek mucous was removed from each animal with surgical scissors and scalpel blade number 15 and placed in 10% neutral buffered formalin solution for 48h (Akgullu et al., 20153. Akgullu C, Huyut MA, Boyacioglu M, Gules O, Eryilmaz U, Hekim T, Dogan E, Zencir C, Güngör H. Nebivolol to attenuate the effects of hyper homocysteinaemia in rats. Atherosclerosis. 2015;240(1):33-99.).
Specimens were dehydrated, diaphonized, embedded in paraffin and cut transversely into 5 µm-thick slices on a microtome (Leica Biosystems, Wetzlar, Germany). Mucous samples were stained with hematoxylin-eosin (HE) and Masson’s trichrome. All sections were mounted on glass slides with cover slips using Entellan (Merck, Darms tadt, Germany) for microscopy.
Histological evaluation
Fields were scanned using a Leica® DM2500 microscope (Leica Biosystems, Wetzlar, Germany) under x4 and x10 objective lenses, coupled to a CELESTRON® Digital Microscope Imager Model 44421 camera (Torrance, California, United States) using the software supplied with the digital camera. The same light intensity was used in all digitalization; images were acquired in RGB format at a resolution of 2048 X 1536 pixels with JPEG extension. For quantitative analysis, the ImageJ® program was employed using the plugin “Cell Counter” and the number of inflammatory cells and amount of blood vessels stained with HE on the slides were measured. To evaluate percentage collagen, the slides stained with Masson’s trichrome were photographed and then processed using ImageJ® and the Threshold plugin to measure percentage collagen at the injury site (Vilela-Goulart et al., 200830. Vilela-Goulart MG, Teixeira RTS, Rangel DC, Niccoli-Filho W, Gomes MF. Homogenous amniotic membrane as a biological dressing for oral mucositis in rats: Histomorphometric analysis. Arch Oral Biol. 2008;53(12):1163-1171.).
The semiquantitative analysis was performed by a blinded evaluator who observed inflammatory parameters including presence of cellular inflammatory infiltrate, vasodilatation, necrosis, ulceration, abscess, hemorrhage and edema. These parameters were attributed scores of 0-3, as described by Lima et al. (200516. Lima V, Brito GA, Cunha FQ, Reboucas CG, Falcao BA, Augusto RF, Souza ML, Leitao BT, Ribeiro RA. Effects of the tumour necrosis factor-alpha inhibitors pentoxifylline and thalidomide in short-term experimental oral mucositis in hamsters. Eur J Oral Sci. 2005;113:210-217.): Score 0 - normal epithelium and connective tissue without vasodilatation; absence of, or mild, cellular infiltration; absence of hemorrhagic areas, ulcerations or abscesses; Score 1 - mild vascular ingurgitation, re-epithelization areas; mild inflammatory infiltration with mononuclear prevalence; absence of hemorrhagic areas, edema, ulcerations or abscesses; Score 2 - moderate vascular ingurgitation, areas of hydropic epithelial degeneration, inflammatory infiltration with neutrophil prevalence, presence of hemorrhagic areas, edema and some ulcerations, absence of abscesses; Score 3 - severe vascular ingurgitation and dilatation, inflammatory infiltration with neutrophil prevalence, presence of hemorrhagic areas, edema and extensive ulceration and abscesses.
Statistical analysis
Comparisons for all variables were performed independent of day, between days, independent of group and between groups. Quantitative and semiquantitative variables were expressed as means. For comparisons, the two-way ANOVA test, followed by Tukey’s post-test, was used for comparisons. In all tests, p≤0.05 was adopted for the level of rejection of the null hypothesis for significant values.
RESULTS AND DISCUSSION
The formulation developed was stable and transparent with a smooth texture, homogeneous appearance and characteristic aroma. Borneol 0% had pH = 5.0; Conductivity=100.0 mV; Viscosity=58,500 mPa.s; and centrifugation without phase separation. Borneol 1.2% had a pH = 4.7 ± 0.1; Conductivity=120.2 ± 0.5 mV; Viscosity=45,360 mPa.s; and centrifugation without phase separation. Borneol 2.4% had a pH=4.5±0.2; Conductivity=139.0±1.5 mV; Viscosity=55,560 mPa.s; and centrifugation without phase separation. Acid pH values were expected because the Aristoflex® gel base was formulated with a pH of 5.0. In a previous study, the same gel base had a pH of around 5.0, no phase change and good viscosity (Russo, Guimarães, Cardoso, 200824. Russo FC, Guimarães MA, Cardoso LE. Desenvolvimento de um gel anestésico contendo óleo essencial de canela (Cinnamomum zeylanicum Blume). In: 12º Latin American Meeting of Scientific Initiation; 2008; São Paulo: Universidade do Vale do Paraíba, 2008. [cited 2016 May 19]. Available from: http://www.inicepg.univap.br/cd/INIC_2008/anais/arquivosINIC/INIC1300_01_O.pdf.). Furthermore, borneol is also considered an acid pH substance (Xiao-Fei et al., 200832. Xiao-Fei J, Jia-Li Z, Yue-Mei Y, Law FCP, Yan-Jiang Q, Mei-Cun Y. Preliminary study: biotransformation of borneol to camphor in mice, rats, and rabbits. Mod Tradit Chin Med Mater Med. 2008;10(3):27-36.). Dantas et al. (201610. Dantas MGB, Reis SAGB, Damasceno CMD, Rolim LA, Rolim-Neto PJ, Carvalho FO, Quintans-Junior LJ, Almeida JRGS. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. Scient W J. 2016;2016:1-4.) used a topical gel formulation with 5% borneol which had a pH of 3.95.
At day seven, a statistically significant difference (p <0.05) in scores was found for the G3 group (Borneol 2.4%) compared with both the G1 (Borneol 0%) and G4 (Control) groups. This result indicated better healing for Borneol 2.4% (Figure 3). G1 Group (Borneol 0%) exhibited extensive ulcers and hydropic degeneration, spongiosis and exocytosis. Connective tissue (CT) showed moderate and diffuse presence of polymorphonuclear cells (PMNs) with areas of necrosis and bleeding. The G2 Group (Borneol 1.2%) showed ulcerated areas, hydropic degeneration, spongiosis and exocytosis. CT showed areas of necrosis, presence of some congested blood vessels, as well as moderate and diffuse presence of PMNs. The G3 Group (Borneol 2.4%) had discrete hydropic degeneration, spongiosis, and partial reepithelialization in five animals. CT showed PMNs and the presence of few congested blood vessels. The G4 Group (Negative control) exhibited ulcerated areas, hydropic degeneration, spongiosis and intense exocytosis. CT had edema and necrosis, moderate congested blood vessels, as well as moderate and diffuse presence of PMNs (Figure 4).
Cicatrization score according to groups and days of experimentation. p<0,05: Control vs. Borneol 2.4% (7 days) ; p<0,05: Borneol 0% vs. Borneol 2.4% (7 days). p<0,05: Control vs. Borneol 2.4% (14 days). p<0,05: Borneol 0% vs. Borneol 2.4% (14 days). (Mann-Whitney test).
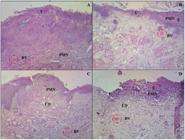
Microscopic aspects of groups at seven days of experimentation. A Group treated with gel base (Borneol 0%); B Group treated with gel (Borneol 1.2%); C Group treated with gel (Borneol 2.4%); and D Group (Negative control). PMN=Polymorphonuclear cells; BV=Blood vessel; U=Ulcerated area; E=Exocytosis; ED=edema. Magnification x10. HE staining.
At day 14, a statistically significant difference (p <0.05) in scores was found for the G3 group (Borneol 2.4%) compared with both the G1 (Borneol 0%) and G4 (Control) groups. This result indicated better healing for Borneol 2.4% (Figure 3). G1 Group (Borneol 0%) showed hyperkeratosis and full reepithelialization. CT had congested blood vessels, as well as moderate and diffuse presence of Mononuclear (MN) cells. The G2 Group (Borneol 1.2%) showed reepithelialization but with more blood vessels in G3. CT showed numerous clogged blood vessels and a diffuse mild chronic inflammatory process. The G3 Group (Borneol 2.4%) had a normal aspect and full reepithelialization. CT showed normal features, in addition to granulation tissue with fibroblasts, congested blood vessels and discrete, diffuse presence of MN. The G4 Group (Negative Control) had acanthosis (thickened spinous layer) with loss of integrity of basal membrane and exocytosis. Epithelialization occurred with different morphology in four animals. The CT was found to contain a large amount of granulation tissue with fibroblasts, congested blood vessels and chronic inflammatory infiltrate (Figure 5).
Microscopic aspects of groups at fourteen days of experimentation. A Group treated with gel base (Borneol 0%); B Group treated with gel (Borneol 1.2%); C Group treated with gel (Borneol 2.4%); and D Group (Control). RE-=reepithelialization; HK=Hyperkeratinization; BV=Blood Vessel; MN=Mononuclear; AC=acanthosis. Magnification x10. HE staining.
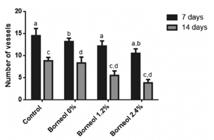
At seven days, the group treated with Borneol 2.4% showed a lower number of vessels than the other groups (G1, G2 and G4). The G3 Group showed statistical significance (p<0.05) when compared to the three other groups. The G2 Group (Borneol 1.2%) showed statistically significant differences when compared to both the G1 Group (Borneol 0%) and Control Groups. There were no differences between the G1 and Control Groups. At 14 days, there was statistical significance between all cross comparisons except the G1 Group and Control Group (Figure 6).
Vessel count according to group and day of experimentation on anti-inflammatory activity test of oral gel containing different concentrations of borneol. ap<0.05: Control vs. Borneol 1.2% and Borneol 2.4% (7 days) ; bp<0.05: Borneol 0% vs. Borneol 1.2% and Borneol 2.4% (7 days). cp<0.05: Control vs. Borneol 1.2% and Borneol 2.4% (14 days). dp<0.05: Borneol 0% vs. Borneol 1.2% and Borneol 2.4% (14 days) (Two-Way Analysis of Variance ANOVA, followed by Tukey’s post-test).
While healing depends on the neoformation of blood vessels, persistent or excessive vessels may denote the existence of chronic inflammation or fibrosis (Abbas et al., 20101. Abbas AK, Fausto N, Kumar V, Cotran RS, Aster JC, Robbins SL. Robbins & Cotran: patologia: bases patológicas das doenças. 8th ed. Rio de Janeiro: Elsevier; 2010. 1458 p.). On histological analysis of the blood vessels, the Control Group showed a greater number of blood vessels in the final process of wound healing compared to the other groups (G2 and G3). This indicates that the healing process in the Control Group required a greater angiogenesis for the formation of granulation tissue (Abruceze, 20132. Abruceze LHB. Avaliação da eficácia dos biocurativos em ratos Wistar com lesões de pele causadas por Queimaduras. [dissertação]. Botucatu: Faculdade de Medicina de Botucatu, Universidade Estadual Paulista; 2013.).
The proliferation of blood vessels is stimulated by vascular endothelial growth factor (VEGF). Some factors stimulate the expression of VEGF in angiogenesis and ischemia, while increases in certain growth factors are responsible for the elevated VEGF expression in chronic inflammation and wound healing. The inflamed tissue is generally in hypoxia, where inflammation may promote angiogenesis by inducing the release of angiogenic factors leading to fibrosis (Nagy, Dvorak, Devorak, 200719. Nagy JA, Dvorak AM, Devorak HF. VEGF-A and the induction of Pathological Angiogenesis. An Rev Pathol Mech Dis. 2007;2:251-275.). According to a study by Dai et al. (200911. Dai JP, Chen J, Bei YF, Han BX, Wang S. Influence of borneol on primary mice oral fibroblasts: a penetration enhancer may be used in oral submucous fibrosis. J Oral Pathol Med. 2009;38(3):276-281.), borneol showed anti-fibrotic action.
Regarding inflammatory cell count, the gels containing borneol at concentrations of 1.2% and 2.4% exhibited anti-inflammatory action at seven and 14 days, reducing the number of these cells at the injury site. The Control Group and Borneol 0% groups, at seven days, showed a much more intense inflammatory process than the treated groups (Borneol 1.2% and Borneol 2.4%), but no differences were observed between the two borneol concentrations. Cell infiltrates were less intense in all groups at 14 days. Differences were observed between the G1 Group and G3 Group and also between the two gels (Borneol 1.2% and Borneol 2.4%) compared to the Control Group, but no differences between the two concentrations were evident (Figure 7).
Inflammatory cell count according to group and day of experimentation. ap<0.05: Control vs. Borneol 0%, Borneol 1.2% and Borneol 2.4% (7 days) ; bp<0.05: Borneol 0% vs. Borneol 1.2% and Borneol 2.4% (7 days). cp<0.05: Control vs. Borneol 1.2% and Borneol 2.4% (14 days) (Two-Way Analysis of Variance ANOVA, followed by Tukey’s post-test).
The findings for number of inflammatory cells corroborate the results of the study by Almeida et al. (20134. Almeida JRGS, Souza GR, Silva JC, Saraiva SRGL, Oliveira-Júnior, RG, Quintans JSS, Barreto RSS, Bonjardim LR, Cavalcanti SCH, Quintans-Junior LJ. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Scient W J. 2013;2013:1-5.), in which the authors confirmed the action of monoterpene and suggested that the substance has therapeutic potential in painful and inflammatory diseases. Tanideh et al. (201429. Tanideh N, Namazi F, Tadbir AA, Ebrahimi H, Koohi-Hosseinabadi O. Comparative assessment of the therapeutic effects of the topical and systemic forms of Hypericum perforatum extract on induced oral mucositis in golden hamsters. Int J Oral Max Surg. 2014;43(10):1286-1292.) tested the action of Hypericum perforatum L. extract for treating induced oral mucositis in animals. The extract of this plant contains Borneol (CIV, 2012) and was administered by gavage (systemically) and locally via an oral gel. The extract showed anti-inflammatory and healing action, especially systemically.
In the present study, percentage collagen at seven days showed statistical significance on comparison of the G1 Group (Borneol 0%) with the G3 Group (Borneol 2.4%) and also on comparisons of the G2 Group and G3 Group with the G4 Group (Control). Comparison of the G2 and G3 Groups with the G4 Group also proved significant at 14 days (Figure 8).
Percentage collagen according to group. ap<0.05: Control vs. Borneol 1.2% and Borneol 2.4% (7 days) ; bp<0.05: Borneol 0% vs. Borneol 2.4% (7 days). cp<0.05: Control vs. Borneol 1.2% and Borneol 2.4% (14 days). dp<0.05: Borneol 0% vs. Borneol 2.4% (14 days) (Two-Way Analysis of Variance ANOVA, followed by Tukey’s post-test).
Percentage collagen in the G2 and G3 Groups on slides stained with Masson’s trichrome was higher than in both G1 and G4 Groups at 14 days. This was due to the healing effect of borneol which promoted greater formation of fibroblasts with better collagen and fibronectin production in local lesions (Figure 9). Tanideh et al. (201429. Tanideh N, Namazi F, Tadbir AA, Ebrahimi H, Koohi-Hosseinabadi O. Comparative assessment of the therapeutic effects of the topical and systemic forms of Hypericum perforatum extract on induced oral mucositis in golden hamsters. Int J Oral Max Surg. 2014;43(10):1286-1292.) also observed increased stimulation of collagen production by topical gel Hypericum perforatum L., which helped repair the wounds, closing areas of damaged oral mucosa in the animals. This effect may be associated with the bactericidal action of borneol, which prevents secondary infection by fungi and bacteria, promoting reepithelialization and standardization of collagen fiber content (Tabanca et al., 200128. Tabanca N, Kirimer N, Demirci B, Demirci F, Baser KH. Composition and antimicrobial activity of the essential oils of Micromeria cristata subsp. phrygia and the enantiomeric distribution of borneol. J Agric Food Chem. 2001;49:4300-4303.; Wenqiang et al., 200631. Wenqiang G, Shufen L, Ruixiang Y, Yanfeng H. Comparison of composition and antifungal activity of Artemisia argyi Levl. et Vant inflorescence essential oil extracted by hydrodistillation and supercritical carbon dioxide. Nat Prod Res. 2006;20(11):992-998.).
Microscopic aspects of groups at fourteen days of experimentation. G1 Group treated with gel base (Borneol 0%); G2 Group treated with gel (Borneol 1.2%); G3 Group treated with gel (Borneol 2.4%); and G4 Group (Control). Note the greater quantity of newly formed collagen in treated groups (G2 and G3). Magnification x10. Masson’s trichrome.
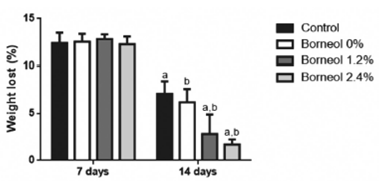
All animals lost weight throughout the experiment due to the side effects of 5-fluorouracil, oral mucositis and reduction in water and feed intake. At 14 days of experimentation, the Borneol 2.4% Group had a lower percentage of weight loss relative to the other three groups (Borneol 0%, Borneol 1.2% and Control), probably due to the greater action of Borneol 2.4% in reducing inflammation and promoting wound healing (Figure 10).
Percentage weight loss according to group and day of experimentation. ap<0.05: Control vs. Borneol 1.2% and Borneol 2.4% (14 days). bp<0.05: Borneol 0% vs. Borneol 1.2% and Borneol 2.4% (14 days) (Two-Way Analysis of Variance ANOVA, followed by Tukey’s post-test).
CONCLUSIONS
The Borneol 2.4% Group was statistically superior to the Borneol 1.2%, Borneol 0% and Control Groups in the treatment of induced oral mucositis in rats, promoting angiogenesis control, inflammatory cell count reduction at the injury site and percentage neoformed collagen increase in reepithelialization. The G3 group (Borneol 2.4%) was statistically superior to the other three groups on the semiquantitative evaluation, promoting improvement in reepithelialization and healing of chemotherapy-induced oral mucositis.
The confirmation of anti-inflammatory and healing action of borneol (especially Borneol 2.4%) in oral mucositis in rats renders it a good marker for predicting this activity for plant extracts rich in this substance. The study results showed that borneol has anti-inflammatory and healing action in induced oral mucositis in cheek mucosa of Wistar rats.
ACKNOWLEDGMENTS
The authors extend their thanks to the Pharmacist Dr. Emanuel Jair Gonçalves Souza Carvalho (FARMACE) for help in the production of gels and to the FACEPE for financing the study and awarding doctoral fellowships.
REFERENCES
-
1Abbas AK, Fausto N, Kumar V, Cotran RS, Aster JC, Robbins SL. Robbins & Cotran: patologia: bases patológicas das doenças. 8th ed. Rio de Janeiro: Elsevier; 2010. 1458 p.
-
2Abruceze LHB. Avaliação da eficácia dos biocurativos em ratos Wistar com lesões de pele causadas por Queimaduras. [dissertação]. Botucatu: Faculdade de Medicina de Botucatu, Universidade Estadual Paulista; 2013.
-
3Akgullu C, Huyut MA, Boyacioglu M, Gules O, Eryilmaz U, Hekim T, Dogan E, Zencir C, Güngör H. Nebivolol to attenuate the effects of hyper homocysteinaemia in rats. Atherosclerosis. 2015;240(1):33-99.
-
4Almeida JRGS, Souza GR, Silva JC, Saraiva SRGL, Oliveira-Júnior, RG, Quintans JSS, Barreto RSS, Bonjardim LR, Cavalcanti SCH, Quintans-Junior LJ. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Scient W J. 2013;2013:1-5.
-
5Barreto RSS. Efeito cicatrizante do (-)-borneol incorporado ao filme bioativo de quitosana em roedores. [tese]. Aracajú: Universidade Federal de Sergipe, 2013.
-
6Borges AM, Pereira J, Cardoso MG, Alves JÁ, Lucena EMP. Determinação de óleos essenciais de alfavaca (Ocimum gratissimum L.), orégano (Origanum vulgare L.) e tomilho (Thymus vulgaris L.). Rev Bras Plantas Med. 2012;14(4):656-665.
-
7Braga FT, Santos AC, Bueno PC, Silveira RC, Santos CB, Bastos JK, Carvalho EC. Use of chamomilla recutita in the prevention and treatment of oral mucositis in patients undergoing hematopoietic stem cell transplantation: a randomized, controlled, phase II clinical trial. Cancer Nurs. 2015;38(4):322-329.
-
8COSMETIC INGREDIENT REVIEW. CIV. Safety assessment of hypericum perforatum derived ingredients as used in cosmetics. CIR Expert Panel Meeting; 2012. [cited 2016 May 19]. Available from: >http://www.cir-safety.org/sites/default/files/hyperic.pdf
» >http://www.cir-safety.org/sites/default/files/hyperic.pdf -
9Curra M, Martins MA, Lauxen IS, Pellicioli AC, Sant'Ana Filho M, Pavesi VC, Carrard VC, Martins MD. Effect of topical chamomile on immunohistochemical levels of IL-1ß and TNF-a in 5-fluorouracil-induced oral mucositis in hamsters. Cancer Chemother Pharmacol. 2013;71(2):293-2999.
-
10Dantas MGB, Reis SAGB, Damasceno CMD, Rolim LA, Rolim-Neto PJ, Carvalho FO, Quintans-Junior LJ, Almeida JRGS. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. Scient W J. 2016;2016:1-4.
-
11Dai JP, Chen J, Bei YF, Han BX, Wang S. Influence of borneol on primary mice oral fibroblasts: a penetration enhancer may be used in oral submucous fibrosis. J Oral Pathol Med. 2009;38(3):276-281.
-
12Dos Reis PE, Ciol MA, de Melo NS, Figueiredo PT, Leite AF, Manzi Nde M. Chamomile infusion cryotherapy to prevent oral mucositis induced by chemotherapy: a pilot study. Support Care Cancer. 2016;24(10):4393-4398.
-
13Freitas VS, Rodrigues RAF, Gaspi FOG. Propriedades farmacológicas da Aloe vera L. Burm. f. Rev Bras Plantas Med. 2014;16(2):299-307.
-
14Horváthová E, Slamenová D, Marsálková L, Sramková M, Wsólová L. Effects of borneol on the level of DNA damage induced in primary rat hepatocytes and testicular cells by hydrogen peroxide. Food Chem Toxicol. 2009;47(6):1318-1323.
-
15Kong Q, Wu Z, Chu X, Liang R, Xia M, Li L. Study on the anti-cerebral ischemia effect of borneol and its mechanism. Afr J Tradit Complement Altern Med. 2014;11(1):161-164.
-
16Lima V, Brito GA, Cunha FQ, Reboucas CG, Falcao BA, Augusto RF, Souza ML, Leitao BT, Ribeiro RA. Effects of the tumour necrosis factor-alpha inhibitors pentoxifylline and thalidomide in short-term experimental oral mucositis in hamsters. Eur J Oral Sci. 2005;113:210-217.
-
17Lima GMG, Severo MC, Santana-Melo GF, Carvalho MA, Vilela-Goulart MG, Salgado MA, Gomes MF. Amniotic membrane as a biological dressing for 5-fluoruracil-induced oral mucositis in rats. Int J Oral Max Surg. 2015;44(7):845-851.
-
18Liu YM, Zhao GF, Xia XH, Xu QY, Shen Q. Influence of muskiness compounded with borneol on blood brain barrier after ischemic reperfusion injury. J Tradit Chin Med. 2009;28(6):459-462.
-
19Nagy JA, Dvorak AM, Devorak HF. VEGF-A and the induction of Pathological Angiogenesis. An Rev Pathol Mech Dis. 2007;2:251-275.
-
20Oliveira AZM. Desenvolvimento de formulações cosméticas com ácido hialurônico. [dissertação]. Porto: Universidade do Porto; 2009.
-
21Patussi C, Sassi LM, Munhoz EC, Zanicotti RTS, Schussel JL. Clinical assessment of oral mucositis and candidiasis compare to chemotherapic nadir in transplanted patients. Braz Oral Res. 2014;28:1-7.
-
22Pavesi VC, Lopez TC, Martins MA, Sant'Ana Filho M, Bussadori SK, Fernandes KP, Mesquita-Ferrari RA, Martins MD. Healing action of topical chamomile on 5-fluoracil induced oral mucositis in hamster. Support Care Cancer. 2011;19(5):639-646.
-
23Rodriguez-Caballero A, Torres-Lagares D, Robles-García M, Pachon-Ibañez J, Gonzalez-Padilla D, Gutierrez-Perez JL. Cancer treatment-induced oral mucositis: a critical review. Int J Oral Max Surg. 2012;41(2):225-238.
-
24Russo FC, Guimarães MA, Cardoso LE. Desenvolvimento de um gel anestésico contendo óleo essencial de canela (Cinnamomum zeylanicum Blume). In: 12º Latin American Meeting of Scientific Initiation; 2008; São Paulo: Universidade do Vale do Paraíba, 2008. [cited 2016 May 19]. Available from: http://www.inicepg.univap.br/cd/INIC_2008/anais/arquivosINIC/INIC1300_01_O.pdf.
-
25Sera EAR, Oliveira RV, Mariotto AH, Aquino DR, Scherma AP. Avaliação dos cuidados odontológicos pré e trans tratamento radioterápico. Braz J Oral Sci. 2013;23(3):30-38.
-
26Simões CA, Castro JFL, Cazal C. Cândida oral como fator agravante da mucosite radioinduzida. Rev Bras Cancer. 2011;57(1):23-29.
-
27Smith PW, Wilson MJH, Zhang J, Wang Q, Osann K, Chen Z, Wigdor H, Schwartz J, Epstein J. In vivo imaging of oral mucositis in an animal model using optical coherence tomography and optical doppler tomography. Clin Cancer Res. 2007;13(8):2449-2454.
-
28Tabanca N, Kirimer N, Demirci B, Demirci F, Baser KH. Composition and antimicrobial activity of the essential oils of Micromeria cristata subsp. phrygia and the enantiomeric distribution of borneol. J Agric Food Chem. 2001;49:4300-4303.
-
29Tanideh N, Namazi F, Tadbir AA, Ebrahimi H, Koohi-Hosseinabadi O. Comparative assessment of the therapeutic effects of the topical and systemic forms of Hypericum perforatum extract on induced oral mucositis in golden hamsters. Int J Oral Max Surg. 2014;43(10):1286-1292.
-
30Vilela-Goulart MG, Teixeira RTS, Rangel DC, Niccoli-Filho W, Gomes MF. Homogenous amniotic membrane as a biological dressing for oral mucositis in rats: Histomorphometric analysis. Arch Oral Biol. 2008;53(12):1163-1171.
-
31Wenqiang G, Shufen L, Ruixiang Y, Yanfeng H. Comparison of composition and antifungal activity of Artemisia argyi Levl. et Vant inflorescence essential oil extracted by hydrodistillation and supercritical carbon dioxide. Nat Prod Res. 2006;20(11):992-998.
-
32Xiao-Fei J, Jia-Li Z, Yue-Mei Y, Law FCP, Yan-Jiang Q, Mei-Cun Y. Preliminary study: biotransformation of borneol to camphor in mice, rats, and rabbits. Mod Tradit Chin Med Mater Med. 2008;10(3):27-36.
Publication Dates
-
Publication in this collection
2017
History
-
Received
13 Oct 2016 -
Accepted
13 Feb 2017