ABSTRACT
Bisphenol-A (BPA) belongs to the family of endocrine disrupting chemicals (EDCs) and it is used in the production of polycarbonate plastic and epoxy resins. The reproductive toxicity of BPA is well documented but it also exerts its toxic effects through multiple pathways especially by inducing a state of oxidative stress and causing damage to the vital organs. In the present study, histopathologic and oxidative damage caused by BPA in liver and kidneys of fresh water cyprinid, Ctenopharyngodon idella was evaluated. LC50 of BPA for Ctenopharyngodon idella was determined by probit regression analysis. Fish were exposed to a sublethal concentration of BPA i.e. 3.2 ppm (1/2 LC50) for 14 days. Histologic studies revealed that BPA caused degenerative changes in liver and kidneys and exposure of sublethal concentration of BPA caused oxidative damage in both organs. Lipid peroxidation significantly increased in liver and kidneys of treated group. Catalase activity and reduced glutathione content significantly decreased in the group exposed to BPA compared to control and glutathione-S-transferase activity increased significantly in both organs exposed to the sublethal concentration of BPA. From this study it is concluded that BPA caused toxic effects in fish species by changing oxidative balance and damaging the vital organs.
Keywords:
Bisphenol-A/effects; Histopathology; Ctenopharyngodon idella/endocrine disrupting chemicals/Oxidative stress
INTRODUCTION
Waterbodies are a major sink for industrial, domestic and other anthropogenic compounds (Canli, Ay, Kalay, 1998 Canli M, Ay Ö, Kalay M. Levels of heavy metals (Cd, Pb, Cu, Cr and Ni) in tissue of Cyprinus carpio, Barbus capito and Chondrostoma regium from the Seyhan River, Turkey. Turk J Zool. 1998;22:149-157.). The aquatic pollution has far reaching impacts on organisms in the recipient environment. Fish as inhabitant of aquatic system cannot avoid the inimical effects of the pollutants. A set of biomarkers is generally used to evaluate the biological effects of pollutants. Such biomarkers act as an early warning of a specific detrimental biological endpoint. Oxidative stress and histopathologic biomarkers are used in ecotoxicology (Pandey et al., 2003 Pandey S, Parvez S, Sayeed I, Haque R, Bin-Hafeez B, Raisuddin S. Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl and Schn.). Sci Total Environ. 2003;309(1-3):105-115.). Histopathology of fish tissues is a reliable monitoring tool, which allows the assessment of the environmental stressor’s effects. It is one of the most reliable indicator of the health impairment induced by the anthropogenic stressors in aquatic organism (Fernandes et al., 2008 Fernandes C, Fontainhas-Fernandes A, Rocha E, Salgado MA. Monitoring pollution in Esmoriz-Paramos lagoon, Portugal: Liver histological and biochemical effects in Liza saliens. Environ Monit Assess. 2008;145(1-3):315-322.; Leonardi, Tarifeno, Vera, 2009 Leonardi M, Tarifeno E, Vera J. Diseases of the chilean flounder, paralichthys adspersus (Steindachner, 1867), as a biomarker of marine coastal pollution near the Itata River (Chile): Part II. Histopathological lesions. Arch Environ Contam Toxicol. 2009;56(3):546-556.). Enzymatic and non-enzymatic antioxidants are important defense mechanism of organisms which provide protection against environmental pro-oxidants by countering the impact of reactive oxygen species (Tabrez, Ahmad, 2009 Tabrez S, Ahmad M. Effect of wastewater intake on antioxidant and marker enzymes of tissue damage in rat tissues: implications for the use of biochemical markers. Food Chem Toxicol. 2009;47(10):2465-2478.). Therefore, antioxidant parameters and oxidative stress indices are considered potential biomarkers and are frequently used as screening tools to assess the impacts of environmental stress. Important antioxidant enzymes are catalase (CAT), superoxide dismutase (SOD), glutathione-S-transferase (GST) and glutathione peroxidase (GPx). In addition, glutathione, vitamins and carotene also help the organism to mitigate the external pollutants and help the protective enzyme system of the organism.
Of many compounds released into the aquatic environment, endocrine disrupting chemicals (EDCs) have gained much attention. Bisphenol-A (BPA) is a well-known EDC. It is the highest volume chemical produced worldwide and mimics the naturally occurring estrogen, estradiol 17β. BPA is used in the production of epoxy resin lining of food and beverage containers, polycarbonate plastic (Vom Saal, Hughes, 2005), as a constituent of dental sealant (Olea et al., 1996Olea N, Pulgar R, Perez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, Pedraza V, Soto AM, Sonnenschein C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996;104(3):298-305.; Vandenberg et al., 2007 Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24(2):139-177.), as a flame retardant precursor and also used in the production of thermal papers and carbonless copy (Suzuki et al., 2000 Suzuki K, Ishikawa K, Sugiyama K, Furuta H, Nishimura F. Content and release of bisphenol A from polycarbonate dental products. Den Mat J. 2000;19(4):389-395.; Debenest et al., 2010 Debenest T, Gagné F, Petit AN, André C, Kohli M, Blaise C. Ecotoxicity of a brominated flame retardant (tetrabromobisphenol A) and its derivatives to aquatic organisms. Comp Biochem Physiol C Toxicol Pharmacol. 2010;152(4):407-412.; Liao, Kannan, 2011Liao C, Kannan K. Widespread occurrence of bisphenol-A in paper and paper products: implications for human exposure. Environ Sci Technol. 2011;45(21):9372-9379.). Several studies suggest that BPA cause acute, short-term and sub-chronic toxicity (Tyl et al., 2002 Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, Brine DR, Veselica MM, Fail PA, Chang TY, Seely JC, Joiner RL, Butala JH, Dimond SS, Cagen SZ, Shiotsuka RN, Stropp GD, Waechter JM. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol Sci. 2002;68(1):121-146.; Tyl, 2008). Bisphenol-A cause tissue injury by forming reactive oxygen species (Kabuto, Amakawa, Shishibori, 2004Kabuto H, Amakawa M, Shishibori T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 2004;74(24):2931-2940.; Hassan et al., 2012Hassan ZK, Elobeid MA, Virk P, Omer SA, ElAmin M, Daghestani MA, AlOlayan EM. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid Med Cell Longev. 2012;2012:194829.; Aboul Ezz, Khadrawy, Mourad, 2015 Aboul Ezz HS, Khadrawy YA, Mourad IM. The effect of bisphenol A on some oxidative stress parameters and acetylcholinesterase activity in the heart of male albino rats. Cytotechnology. 2015;67(1):145-155.)
Grass carp (Ctenopharyngodon idella) is a cosmopolitan species. It is an exotic species introduced in the Pakistani rivers and thrives well in river and pond system. It is now cultured with other major carps. The present study focused on the role of BPA in generating the reactive oxygen species (ROS) and inducing oxidative stress in fish (Ctenopharyngodon idella) liver and kidneys and inducing histopathologic alteration. The effect of bisphenol-A on a biogenic macromolecular peroxidation indicator (thio barbituric acid-reactive substance; TBARS), reactive oxygen species (ROS) regulating enzymes (catalase and GPx), an antioxidant (glutathione) and histology of vital organs was evaluated.
MATERIAL AND METHODS
Juvenile grass carp (Ctenopharyngodon idella hereafter C.idella) (10.5±2.1 cm; 50.3±3.57 g) were purchased from a commercial fish farm. Fish were acclimatized for two weeks in 50 liter well aerated glass aquaria. Physico-chemical parameters of water e.g. dissolved oxygen, temperature and pH were recorded every day. Fish were kept under natural photoperiod and temperature, fed with commercial fish pallet (Oryza Organics, Lahore).
Acute study
For acute studies LC50 (lethal concentration with 50% mortality) was calculated for 96 hours (APHA, 2005) by probit regression analysis (Finney, 1971Finney DJ. Probit analysis. Cambridge: Cambridge University Press; 1971. 333 p.), using IBM SPSS statistics (Version: 20). Fish were divided into 20 groups (6 fish per group) and exposed to graded concentrations of BPA (0.5 ppm to 10 ppm) with the increment of 0.5 ppm. Water was replaced every day and fresh toxicant was added after water renewal for 96 hours. Fish were monitored at regular intervals and dead fish were removed from aquaria.
Sublethal studies
For sublethal studies, 20 fish were divided into two groups (control and treated), having 10 fish per group. Bisphenol-A, 96 hour LC50 for juvenile grass carp (Ctenopharyngodon idella) was 6.42385 ppm. A non-lethal concentration of 3.2 ppm was selected (1/2 LC50) and fish were exposed to 1/2 LC50 concentration for 14 days. After the stipulated time, five fish were randomly selected from the control and treatment group. Fish were anesthetized with clove oil (Kaiser et al., 2006 Kaiser H, Brill G, Cahill J, Collet K, Czypionka K, Green K, Orr P, Pattrick R, Scheepers R, Stonier M, Whitehead A, Yearsley R. Testing clove oil as an anesthetic for long-distance transport of live fish: the case of Lake Victoria chilchid Haplochromis obliquidens. J appl Ichthyol. 2006;22(6):510-514.), length and weight was recorded and fish were dissected humanely. Liver and kidneys were removed, cleaned of extraneous tissue and weighed to the nearest mg. A portion of tissues were preserved in 10% formalin for histologic studies and the remaining portions were washed with 0.9% ice-cold saline solution, blotted on filter paper, snap frozen in liquid nitrogen and stored at -80 oC until further biochemical analysis. All animal handling and experimental protocols were approved by research committee of Department of Zoology, GC University, Lahore.
Histologic studies
The preserved tissues were processed in various grades of ethanol, cleared in xylene and impregnated with wax (mp; 58 oC). Five microns thick sections were cut using rotary microtome (Leica RM 2165). Tissue sections were stained with hematoxylin and eosin (H&E). Stained slides were studied and photographed by high resolution microscope (Leica, Japan) fitted with a digital camera.
Biochemical analysis
Biochemical analysis was done using post mitochondrial supernatant (PMS). The tissues were ground in liquid nitrogen, homogenized in chilled 0.1 M potassium phosphate buffer (pH 7.4) containing 1.17% KCl and centrifuged for 30 mints at 10,500 rpm to obtain the PMS.
Measurement of lipid peroxidation
Lipid peroxidation was determined by using the method of Wright, Colby and Miles (1981 Wright JR, Colby HD, Miles PR. Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys. 1981;206(2):296-304.) with slight modifications. Reaction mixture (3 mL) contained 10% tissue homogenate, 10% TCA and 0.67 % TBA. The reaction mixture was incubated in boiling water for 45 min, cooled and centrifuged at 2500×g for 10 min. Absorbance of the supernatant was taken at 532 nm at 37 oC using Hitachi U-2000 spectrophotometer. Rate of Lipid peroxidation was assessed by measuring thiobarbituric acid reactive substances and expressed as nmol TBARS formed/g tissue at 37 oC by using a molar extinction coefficient of 1.56 x 105/M/ cm.
Measurement of reduced glutathione
Reduced glutathione (GSH) was measured following Jollow et al. (1974 Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR. Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzeneoxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151-69.) as described by Haque et al. (2003 Haque R, Bin-Hafeez B, Parvez S, Pandey S, Sayeed I, Ali M, Raisuddin S. Aqueous extract of walnut (Juglans regia L.) protects mice against cyclophosphamide induced biochemical toxicity. Hum Exp Toxicol. 2003;22(9):473-480.). One ml of PMS (10%) was incubated on ice with equal volume of 4 % sulphosalicylic acid (4%). After one hour the mixture was centrifuged for 15 min (1200 rpm, 4 oC). Supernatant was filtered. The final reaction mixture (3 mL) contained filtered aliquot, 0.1 M phosphate buffer and 10 mM DTNB. Absorbance was recorded at 412 nm. The results were expressed as nmol GSH/g of tissue using molar extinction coefficient of 1.36x104/M/cm.
Catalase activity
The catalase (CAT) activity was measured using the method of Claiborne (1985 Claiborne A. Catalase activity. In: Greenwald RA, editor. CRC handbook of methods in oxygen radical research. Boca Raton: CRC Press; 1985. p. 283-84.) as described by Haque et al. (2003 Haque R, Bin-Hafeez B, Parvez S, Pandey S, Sayeed I, Ali M, Raisuddin S. Aqueous extract of walnut (Juglans regia L.) protects mice against cyclophosphamide induced biochemical toxicity. Hum Exp Toxicol. 2003;22(9):473-480.). The reaction mixture consisted of 0.09 M H2O2, 0.1 M phosphate buffer and PMS (10%) in a total volume of 3 ml. Change in absorbance was recorded after every 30 seconds at 240 nm in a double beam spectrophotometer (Hitachi U-2000). Catalase activity was calculated in terms of nmol H2O2 consumed/min/mg protein.
Glutathione-S-transferase activity
The glutathione-S-transferase activity was measured kinetically using 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate. Briefly, the reaction mixture (2ml) contained 0.1M phosphate buffer, GSH (1 mM), CDNB (1 mM) and PMS (10%). The change in absorbance was recorded at 340 nm and the enzyme activity was calculated as nmol CDNB conjugates formed/min/mg protein (Habig, Pabst, Jakoby, 1974 Habig WH, Pabst MJ, Jakoby WB. Glutathione S transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130-39.).
Protein estimation
Protein was estimated according to method by Lowry et al. (1951 Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193(1):265-275.) using bovine serum albumin (BSA) as standard.
Statistical analysis
The statistical analysis was performed using IBM SPSS (version 20). Data for glutathione-S-transferase and lipid peroxidation were log transformed and all data are expressed as mean ± S.E.M. significant difference between control and treated group was computed using Student’s t-test. p<0.05.
RESULTS
No mortality was observed during the sublethal studies. However, as the time passed, the fish showed altered swimming pattern, erratic swimming and loss of balance. The 96 hours LC50 value of BPA according to the probit-regression analysis was 6.323 ppm with 95% lower and upper confidence limit as 6.801 and 5.683 ppm respectively.
Effect of BPA on liver and kidneys histology
Fish liver from the control group did not show any alteration in structure. Hepatocytes have central nuclei arranged around the central vein. Cords of hepatocytes are separated by sinusoids (Figure 1). The liver sections of fish exposed to sublethal concentration of BPA for 14 days showed various histopathologic changes including ruptured central vein, lipid like vacuolization, macrophage and lymphocytes infiltration, ruptured and degenerated hepatocytes (Figure 1). Photomicrograph of C. idella kidneys from the control group showed normal structure, the brush border of proximal tubules and the lumen of distal tubules were normal in appearance (Figure 2). Kidney sections from the group exposed to sublethal concentration of BPA showed damaged renal tubules, shrinkage of tubules and tubule lumen and degeneration of tubules and hematopoietic tissue (Figure 2).
Liver tissue of C. idella from control and treated group exposed to the sublethal concentration of BPA for 14 days. (C) central vein; (E) epithelial layer; (H) hepatocyte; (V) lipid type vacuolization; (rC) ruptured central vein; (L) lymphocyte infiltration. H&E stain, 40X.
Kidney tissue of C. idella from control and treated group exposed to the sublethal concentration of BPA for 14 days. (H) hematopoietic tissue; (Dt) distal tubules; (L) tube lumen; (D) degeneration of tubules; (S) shrinkage of tube lumen. H & E stain, 40X.
Effect of BPA on enzyme activity
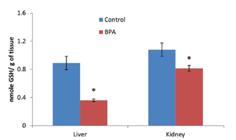
A significant decrease in liver and kidney catalase activity (p<0.05) was observed in the bisphenol-A treated groups compared to control (Figure 3). Significant enhancement in the level of lipid peroxidation was recorded in liver and kidney (p<0.05) of fish exposed to BPA in comparison with the control group (Figure 4).
Catalase activity in the liver and kidneys of C.idella (n=5), exposed to the sublethal concentration of BPA for 14 days. The values given are mean ± S.E.M. *= p<0.05.
Lipid peroxidation in the liver and kidneys of C. idella (n=5), exposed to the sublethal concentration of BPA for 14 days. Values given are mean ±S.E.M. * = p<0.05.
The glutathione-S-transferase activity increased significantly in both tissues of fish exposed to sublethal BPA compared to the control (Figure 5). Bisphenol-A exposure for 14 days caused a significant decrease in reduced glutathione content (p<0.05) both in liver and kidney (Figure 6).
Glutathione-S-transferase activity in the liver and kidneys of C.idella (n=5), exposed to the sublethal concentration of BPA for 14 days. The values given are mean ± S.E.M. *= p<0.05.
Reduced glutathione content in the liver and kidneys of C. idella (n=5), exposed to the sublethal concentration of BPA for 14 days. The values give are mean ±S.E.M. * = p< 0.05.
DISCUSSION
Various anthropogenic materials are released into bodies of water which affect aquatic life especially fish. Histopathology and enzyme status are useful to determine the effects of anthropogenic pollutants on the organisms (Khoshnood et al., 2010Khoshnood Z, Khodabandeh S, Mosafer S, Khoshnood R. Effects of cortisol on gill chloride cells in Persian sturgeon, Acipenser persicus fry. Yakhteh Med J. 2010;11(4):424-431.). The present study revealed that bisphenol-A administration induced a state of oxidative stress and changed the liver and kidney histology of freshwater fish, C. idella.
Exposure to the sublethal concentration of bisphenol-A changed the normal architecture of liver, increase lipid like vacuolization and inflammation in sinusoids. Similar changes were recorded in the liver of Catla catla exposed to 1-4 ppm BPA (Faheem, Jahan, Lone, 2016 Faheem M, Jahan N, Lone KP. Histopathological effects of bisphenol-A on liver, kidneys and gills of Indian major carp, Catla catla (Hamilton, 1822). J Anim Plant Sci. 2016;26(2):514-522.). Dilated sinusoids and change in liver normal structure may be due to the loss of structural proteins after the toxicant exposure. One of the nonspecific responses of fish in toxic conditions is hepatocyte vacuolation (Roberts, 1978 Roberts RJ. Fish pathology. London: Bailliere Tindall; 1978. 466 p.) this vacuolization is typical of lipid accumulation in liver. Similar changes in liver were reported by Peixoto et al. (2013 Peixoto FP, Carrola J, Coimbra AM, Fernandes C, Teixeira P, Coelho L, Conceição I, Oliveira MM, Fontaínhas-Fernandes A. Oxidative stress responses and histological hepatic alterations in barbel, barbus bocagei, from Vizela River, Portugal. Rev Int Contam Ambie. 2013;29(1):29-38.) in Barbus bocagei caught from Vizela River, Portugal. Ameur et al. (2012 Ameur WB, de Lapuente J, El Megdiche Y, Barhoumia B, Trabelsi 0S, Camps L, Serret J, Ramos-López D, Gonzalez-Linares J, Driss MR, Borràs M. Oxidative stress, genotoxicity and histopathology biomarker responses in mullet (Mugil cephalus) and sea bass (Dicentrarchus labrax) liver from Bizerte Lagoon (Tunisia). Mar Poll Bull. 2012;64(2):241-251.) found similar histopathologic changes in liver of Mugil cephalus and Dicentrarchus labrax from Bizerte Lagoon, Tunisia. Kidney sections of fish exposed to BPA showed necrosis, degeneration of renal tubules, inflammation in hematopoietic tissue, shrinkage of tube lumen, and hemorrhage. Similar results were reported in Cyprinus carpio exposed to deltamethrin (Cengiz, 2006Cengiz EI. Gill and kidney histopathology in the freshwater fish Cyprinus carpio after acute exposure to deltamethrin. Environ Toxicol Pharm. 2006;22(2):200-204.) and in Oreochromis niloticus exposed to heavy metals (Khidr, Mekkawy, 2008Khidr MB, Mekkawy IAA. Effect of separate and combined lead and selenium on the liver of the cichlid fish Oreochromis niloticus: ultrastructural study. Egypt J Zoo. 2008;50:89-119.).
Chemicals possessing the properties of endocrine disruption, e.g. lindane, phthalates, dioxins and bisphenol A are reported to cause oxidative stress in brain, kidney, testis and liver of rodents (Latchoumycandane, Chitra, Mathur, 2002Latchoumycandane C, Chitra KC, Mathur PP. The effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the antioxidant system in mitochondrial and microsomal fractions of rat testis. Toxicology. 2002;171(2-3):127-135.; Bindhumol, Chitra, Mathur, 2003 Bindhumol V, Chitra KC, Mathur PP. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology. 2003;188(2-3):117-124.; Chitra, Latchoumycandane, Mathur, 2003; Kabuto, Amakawa, Shishibori, 2004Kabuto H, Amakawa M, Shishibori T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 2004;74(24):2931-2940.). Bisphenol-A and other bisphenols reduce mitochondrial function (Nakagawa, Toyama, 2000Nakagawa Y, Toyama S. Metabolism and cytotoxicity of bisphenol A and other bisphenols in isolated rat hepatocytes. Arch Toxicol. 2000;74(2):99-105.) and induce generation of reactive oxygen species in epididymal sperms of rat (Kabuto, Amakawa, Shishibori, 2004). Corresponding studies on fish are scare and very little studies could be traced from the literature.
In the present study, the catalase activity was significantly decreased both in liver and kidney. Catalase is an active and first enzyme that shows alteration, following oxidative stress (Jin et al., 2010Jin YX, Zhang YX, Shu LJ, Chen LF, Sun LW, Qian HF, Liu WP, Fu ZW. Oxidative stress response and gene expression in adult Zebrafish (Danio rario). Chemosphere. 2010;78(7):846-852.). The reason of decrease catalase activity may be the inactivation of enzyme by overproduction of ROS (Pigeolet et al., 1990 Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels DC, Raes M, Zachary D, Ramacle J. Glutathione peroxidase, superoxide dismutase and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev. 1990;51(3):283-390.). Sayeed et al. (2003 Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Haque R, Raisuddin S. Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol Environ Saf. 2003;56(2):295-301.) reported a significant decrease in catalase activity in all the organs of Channa punctatus exposed to deltamethrin. Chitra and Maiby (2014 Chitra KC, Sajitha R. Effect of bisphenol-A on the antioxidant defense system and its impact on the activity of succinate dehydrogenase in the gill of freshwater fish, Oreochromis mossambicus. J Cell Tissue Res. 2014;14(2):4219-4226.) studied decrease in catalase activity in the liver of fresh water fish Oreochromis mossambicus exposed to the sublethal concentration of BPA which is in accordance with the present study. Faheem et al. (2012 Faheem M, Sulehria AQK, Tariq M, Khadija I, Saeed M. Effect of sub-lethal dose of cadmium chloride on biochemical profile and catalase activity in fresh water fish Oreochromis niloticus. Biologia (Pakistan). 2012;58(1-2):73-78.) reported a decrease in the catalase activity in liver of Oreochromis niloticus exposed to the sublethal concentration of cadmium. Wu et al. (2011 Wu M, Xu H, Shen Y, Qiu W, Yang M. Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol-A, nonylphenol, and their mixture. Environ Toxicol Chem. 2011;30(10):2335-2341.) reported a significantly reduced catalase activity in zebrafish exposed to graded concentration (0.1-1000μg/l) of BPA. A similar decrease was observed in gills of Oreochromis mossambicus exposed to 1 ppm BPA for 10 and 20 days (Chitra, Sajhita, 2014). A decrease in catalase activity was reported by Li et al. (2016 Li D, Chen Q, Cao J, Chen H, Li L, Cedergreen N, Xie H, Xie L. The chronic effects of lignin-derived bisphenol and bisphenol A in Japanese medaka Oryzias latipes. Aquat Toxicol. 2016;170:199-207.) in all vital organs of Japanese medaka exposed to 1.5 mg/l BPA. Contrary to these findings, male medaka exposed to 10μg/l BPA showed 5-20 fold upregulation of catalase mRNA (Qiu et al., 2016 Qiu W, Chen J, Li Y, Chen Z, Jiang L, Yang M, Wu M. Oxidative stress and immune disturbance after long-term exposure to bisphenol A in juvenile common carp (Cyprinus carpio). Ecotoxicol Environ Saf. 2016;130:93-102.). This may indicate that at lower concentrations fish respond quickly to remove BPA but as the exposure concentration increases the tissue systems of fish undergoes exhaustive necrosis thus causing a decrease in activity of enzymes. We have already reported above damage to the liver and kidney system of C. idella exposed to sublethal concentration of BPA (Figure 1 and 2).
The reactive oxygen species (ROS) produced during oxidative stress reacts with unsaturated fatty acids that are present in membranes and cause lipid peroxidation. Therefore, increased lipid peroxidation is an indication of high level of ROS (Thiele et al., 1995Thiele JJ, Freisleben HJ, Fuchs J, Ochsendorf FR. Ascorbic acid and urate in human seminal plasma: determination and interrelationships with chemiluminescence in washed semen. Hum Reprod. 1995;10(1):110-115.) and used extensively to study oxidative stress (Huggett et al., 1992 Huggett RJ, Kimerle RA, Mehrle PM, Bergman HL. Biochemical, physiological and histological markers of anthropogenic stress. Boca Raton, FL: Lewis Publishers; 1992. 347 p.). A significant increase (p< 0.05) in lipid peroxidation was recorded in C. idella after 14 days exposure of bisphenol-A compared to control group. Chitra, Latchoumycandane and Mathur (2003 Chitra KC, Latchoumycandane C, Mathur PP. Induction of oxidative stress by bisphenol-A in the epididymal sperm of rats. Toxicology. 2003;185(1-2):119-127.) reported similar findings in rats orally administered with BPA for 45 days. Wu et al. (2011 Wu M, Xu H, Shen Y, Qiu W, Yang M. Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol-A, nonylphenol, and their mixture. Environ Toxicol Chem. 2011;30(10):2335-2341.) found a concentration dependent increase in LPO activity in zebrafish embryo exposed to graded concentration (0.1-1000μg/L) of BPA. Increased level of lipid peroxidation was recorded in common carp (Cyprinus carpio) exposed to 1 ppm BPA (Qiu et al., 2016 Qiu W, Chen J, Li Y, Chen Z, Jiang L, Yang M, Wu M. Oxidative stress and immune disturbance after long-term exposure to bisphenol A in juvenile common carp (Cyprinus carpio). Ecotoxicol Environ Saf. 2016;130:93-102.). The results of present study indicate that fish exposed to bisphenol-A experienced oxidative stress because of the lipid peroxidation in response to generation of ROS. Lipid peroxidation level may also increase when antioxidant defense is no longer capable of coping with increased ROS.
Glutathione-S-transferase (GST) is an important phase II biotransformation enzyme and used as biomarker of exposure to pollutants in an aquatic system (Livingstone, 1998 Livingstone DR. The fate of organic xenobiotics in aquatic ecosystems: quantitative and qualitative differences in bio-transformation by invertebrates and fish. Comp Biochem Physiol A. 1998;120(1):43-49.). In the present study, there was an increase in glutathione-S-transferase activity in liver and kidneys of C. idella. The increased GST activity may be to detoxify BPA because GST adds GSH-group to xenobiotics or their metabolites, making them more water- soluble and, thus, excreted more easily (Moorhouse, Casida, 1992 Moorhouse KG, Casida JE. Pesticides as activators of mouse liver microsomal glutathione-S-transferase. Pestic Biochem Physiol. 1992;44:83-90.). Increased liver GST activity has been demonstrated many times in various fish species as the result of exposure to PCBs (Gadagbui, Goksoyr, 2001Gadagbui BKM, Goksoyr A. CYP1A and other biomarker responses to effluents from a textile mill in the Volta River (Ghana) using caged tilapia (Oreochromis niloticus) and sediment-exposed mudfish (Clarias anguillaris). Biomarkers. 2001;1(4):252-261.) and other pollutants (Ahmad et al., 2000Ahmad I, Hamid T, Fatima M, Chand HS, Jain SK, Athar M, Raisuddin S. Induction of hepatic antioxidants in fresh water cat fish (Channa punctatus Bloch) is a biomarker of paper mill effluent exposure. Biochimia et Biophysica Acta. 2000;1523(1):37-48.; Ansari, Ansari 2014 Ansari S, Ansari BA. Temporal variations of CAT, GSH, and LPO in gills and livers of zebrafish, Danio rerio, exposed to dimethoate. Arch Pol Fish. 2014;22:101-109.). Increase in GST activity was observed in zebrafish embryo exposed to very low (0.1μg/l) BPA (Wu et al., 2011 Wu M, Xu H, Shen Y, Qiu W, Yang M. Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol-A, nonylphenol, and their mixture. Environ Toxicol Chem. 2011;30(10):2335-2341.). Olsvik et al. (2009 Olsvik PA, Lie KK, Sturve J, Hasselberg L, Andersen OK. Transcriptional effects of nonylphenol, bisphenol A and PBDE-47 in liver of juvenile Atlantic cod (Gadus morhua). Chemosphere. 2009;75(3):360-367.) found non-significant increase in gst mRNA in liver of Atlantic cod when exposed to 50µg/L of BPA for three weeks. Increase in GST activity was also observed in hepatocytes of pearl mullet exposed to BPA (Kaya, Kaptaner, 2016Kaya Ö, Kaptaner B. Antioxidant defense system parameters in isolated fish hepatocytes exposed to bisphenol A - Effect of vitamin C. Acta Biol Hung. 2016;67(3):225-35.) similar increase in GST activity was reported by Li et al. (2016) in liver and gills of Japanese medaka exposed to 1.5 mg/L BPA.
Reduced glutathione (GSH) is the major non-protein thiol and plays a pivotal role in cell viability protecting cells against lipid peroxidation either alone or in conjugation with other proteins (Anjum et al., 2011 Anjum S, Rahman S, Kaur M, Ahmad F, Rashid H, Ahmad RA, Raisuddin S. Melatonin ameliorates bisphenol A-induced biochemical toxicity in testicular mitochondria of mouse. Food Chem Toxicol. 2011;49(11):2849-2854.). In our study, we found a significant decrease in the amount of reduced GSH in liver and kidney of C.,idella exposed to sublethal concentration of BPA. This decrease may be due to GSH being used to scavenge the free radicals and ROS generated by bisphenol-A. Yazdani, Andresen and Gjoen (2016 Yazdani M, Andresen AM, Gjøen T. Short-term effect of bisphenol-A on oxidative stress responses in Atlantic salmon kidney cell line: a transcriptional study. Toxicol Mech Methods. 2016;26(4):295-300.) reported a significant decrease in gsh mRNA level in Atlantic salmon kidney cell lines exposed to 100μM BPA. The reduced GSH content observed in the present study may be due to its lower transcription. Decreased GSH content was also observed in hepatocytes of pearl mullet exposed to 200 μM BPA for 24 hours (Kaya, Kaptaner, 2016Kaya Ö, Kaptaner B. Antioxidant defense system parameters in isolated fish hepatocytes exposed to bisphenol A - Effect of vitamin C. Acta Biol Hung. 2016;67(3):225-35.).
The results of the present study revealed that the fish like other animals, undergo oxidative stress and respond by changes in activity of the antioxidant enzymes and morphological alterations in liver and kidney tissues. Therefore, the results obtained and reported here are the first for this EDC on C. idella, a cosmopolitan species, and will be useful for future work in elucidating the detailed effects of BPA in this species and other carp species.
ACKNOWLEDGEMENTS
Authors are thankful to Oryza Organcis, Lahore for providing fish diet and Himalaya Fish Hatchery for providing fish specimens.
REFERENCES
- Aboul Ezz HS, Khadrawy YA, Mourad IM. The effect of bisphenol A on some oxidative stress parameters and acetylcholinesterase activity in the heart of male albino rats. Cytotechnology. 2015;67(1):145-155.
- Ahmad I, Hamid T, Fatima M, Chand HS, Jain SK, Athar M, Raisuddin S. Induction of hepatic antioxidants in fresh water cat fish (Channa punctatus Bloch) is a biomarker of paper mill effluent exposure. Biochimia et Biophysica Acta. 2000;1523(1):37-48.
- American Public Health Association. APHA. American Water Works Association. AWWA. Standard methods for the examination of water and Wastewater 2005. 21st edition. Washington, DC: American Public Health Association; 2005.
- Ameur WB, de Lapuente J, El Megdiche Y, Barhoumia B, Trabelsi 0S, Camps L, Serret J, Ramos-López D, Gonzalez-Linares J, Driss MR, Borràs M. Oxidative stress, genotoxicity and histopathology biomarker responses in mullet (Mugil cephalus) and sea bass (Dicentrarchus labrax) liver from Bizerte Lagoon (Tunisia). Mar Poll Bull. 2012;64(2):241-251.
- Ansari S, Ansari BA. Temporal variations of CAT, GSH, and LPO in gills and livers of zebrafish, Danio rerio, exposed to dimethoate. Arch Pol Fish. 2014;22:101-109.
- Anjum S, Rahman S, Kaur M, Ahmad F, Rashid H, Ahmad RA, Raisuddin S. Melatonin ameliorates bisphenol A-induced biochemical toxicity in testicular mitochondria of mouse. Food Chem Toxicol. 2011;49(11):2849-2854.
- Bindhumol V, Chitra KC, Mathur PP. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology. 2003;188(2-3):117-124.
- Canli M, Ay Ö, Kalay M. Levels of heavy metals (Cd, Pb, Cu, Cr and Ni) in tissue of Cyprinus carpio, Barbus capito and Chondrostoma regium from the Seyhan River, Turkey. Turk J Zool. 1998;22:149-157.
- Cengiz EI. Gill and kidney histopathology in the freshwater fish Cyprinus carpio after acute exposure to deltamethrin. Environ Toxicol Pharm. 2006;22(2):200-204.
- Chitra KC, Latchoumycandane C, Mathur PP. Induction of oxidative stress by bisphenol-A in the epididymal sperm of rats. Toxicology. 2003;185(1-2):119-127.
- Chitra KC, Maiby S. Oxidative stress of Bisphenol-A and its adverse effects on liver of fresh water fish Oreochromis mossambicus. IJSR. 2014;3(7):221-224.
- Chitra KC, Sajitha R. Effect of bisphenol-A on the antioxidant defense system and its impact on the activity of succinate dehydrogenase in the gill of freshwater fish, Oreochromis mossambicus. J Cell Tissue Res. 2014;14(2):4219-4226.
- Claiborne A. Catalase activity. In: Greenwald RA, editor. CRC handbook of methods in oxygen radical research. Boca Raton: CRC Press; 1985. p. 283-84.
- Debenest T, Gagné F, Petit AN, André C, Kohli M, Blaise C. Ecotoxicity of a brominated flame retardant (tetrabromobisphenol A) and its derivatives to aquatic organisms. Comp Biochem Physiol C Toxicol Pharmacol. 2010;152(4):407-412.
- Faheem M, Jahan N, Lone KP. Histopathological effects of bisphenol-A on liver, kidneys and gills of Indian major carp, Catla catla (Hamilton, 1822). J Anim Plant Sci. 2016;26(2):514-522.
- Faheem M, Sulehria AQK, Tariq M, Khadija I, Saeed M. Effect of sub-lethal dose of cadmium chloride on biochemical profile and catalase activity in fresh water fish Oreochromis niloticus. Biologia (Pakistan). 2012;58(1-2):73-78.
- Fernandes C, Fontainhas-Fernandes A, Rocha E, Salgado MA. Monitoring pollution in Esmoriz-Paramos lagoon, Portugal: Liver histological and biochemical effects in Liza saliens. Environ Monit Assess. 2008;145(1-3):315-322.
- Finney DJ. Probit analysis. Cambridge: Cambridge University Press; 1971. 333 p.
- Gadagbui BKM, Goksoyr A. CYP1A and other biomarker responses to effluents from a textile mill in the Volta River (Ghana) using caged tilapia (Oreochromis niloticus) and sediment-exposed mudfish (Clarias anguillaris). Biomarkers. 2001;1(4):252-261.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130-39.
- Haque R, Bin-Hafeez B, Parvez S, Pandey S, Sayeed I, Ali M, Raisuddin S. Aqueous extract of walnut (Juglans regia L.) protects mice against cyclophosphamide induced biochemical toxicity. Hum Exp Toxicol. 2003;22(9):473-480.
- Hassan ZK, Elobeid MA, Virk P, Omer SA, ElAmin M, Daghestani MA, AlOlayan EM. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid Med Cell Longev. 2012;2012:194829.
- Huggett RJ, Kimerle RA, Mehrle PM, Bergman HL. Biochemical, physiological and histological markers of anthropogenic stress. Boca Raton, FL: Lewis Publishers; 1992. 347 p.
- Jin YX, Zhang YX, Shu LJ, Chen LF, Sun LW, Qian HF, Liu WP, Fu ZW. Oxidative stress response and gene expression in adult Zebrafish (Danio rario). Chemosphere. 2010;78(7):846-852.
- Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR. Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzeneoxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151-69.
- Kabuto H, Amakawa M, Shishibori T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 2004;74(24):2931-2940.
- Kaiser H, Brill G, Cahill J, Collet K, Czypionka K, Green K, Orr P, Pattrick R, Scheepers R, Stonier M, Whitehead A, Yearsley R. Testing clove oil as an anesthetic for long-distance transport of live fish: the case of Lake Victoria chilchid Haplochromis obliquidens. J appl Ichthyol. 2006;22(6):510-514.
- Kaya Ö, Kaptaner B. Antioxidant defense system parameters in isolated fish hepatocytes exposed to bisphenol A - Effect of vitamin C. Acta Biol Hung. 2016;67(3):225-35.
- Khidr MB, Mekkawy IAA. Effect of separate and combined lead and selenium on the liver of the cichlid fish Oreochromis niloticus: ultrastructural study. Egypt J Zoo. 2008;50:89-119.
- Khoshnood Z, Khodabandeh S, Mosafer S, Khoshnood R. Effects of cortisol on gill chloride cells in Persian sturgeon, Acipenser persicus fry. Yakhteh Med J. 2010;11(4):424-431.
- Latchoumycandane C, Chitra KC, Mathur PP. The effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the antioxidant system in mitochondrial and microsomal fractions of rat testis. Toxicology. 2002;171(2-3):127-135.
- Leonardi M, Tarifeno E, Vera J. Diseases of the chilean flounder, paralichthys adspersus (Steindachner, 1867), as a biomarker of marine coastal pollution near the Itata River (Chile): Part II. Histopathological lesions. Arch Environ Contam Toxicol. 2009;56(3):546-556.
- Liao C, Kannan K. Widespread occurrence of bisphenol-A in paper and paper products: implications for human exposure. Environ Sci Technol. 2011;45(21):9372-9379.
- Li D, Chen Q, Cao J, Chen H, Li L, Cedergreen N, Xie H, Xie L. The chronic effects of lignin-derived bisphenol and bisphenol A in Japanese medaka Oryzias latipes. Aquat Toxicol. 2016;170:199-207.
- Livingstone DR. The fate of organic xenobiotics in aquatic ecosystems: quantitative and qualitative differences in bio-transformation by invertebrates and fish. Comp Biochem Physiol A. 1998;120(1):43-49.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193(1):265-275.
- Moorhouse KG, Casida JE. Pesticides as activators of mouse liver microsomal glutathione-S-transferase. Pestic Biochem Physiol. 1992;44:83-90.
- Nakagawa Y, Toyama S. Metabolism and cytotoxicity of bisphenol A and other bisphenols in isolated rat hepatocytes. Arch Toxicol. 2000;74(2):99-105.
- Olea N, Pulgar R, Perez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, Pedraza V, Soto AM, Sonnenschein C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996;104(3):298-305.
- Olsvik PA, Lie KK, Sturve J, Hasselberg L, Andersen OK. Transcriptional effects of nonylphenol, bisphenol A and PBDE-47 in liver of juvenile Atlantic cod (Gadus morhua). Chemosphere. 2009;75(3):360-367.
- Pandey S, Parvez S, Sayeed I, Haque R, Bin-Hafeez B, Raisuddin S. Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl and Schn.). Sci Total Environ. 2003;309(1-3):105-115.
- Peixoto FP, Carrola J, Coimbra AM, Fernandes C, Teixeira P, Coelho L, Conceição I, Oliveira MM, Fontaínhas-Fernandes A. Oxidative stress responses and histological hepatic alterations in barbel, barbus bocagei, from Vizela River, Portugal. Rev Int Contam Ambie. 2013;29(1):29-38.
- Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels DC, Raes M, Zachary D, Ramacle J. Glutathione peroxidase, superoxide dismutase and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev. 1990;51(3):283-390.
- Qiu W, Chen J, Li Y, Chen Z, Jiang L, Yang M, Wu M. Oxidative stress and immune disturbance after long-term exposure to bisphenol A in juvenile common carp (Cyprinus carpio). Ecotoxicol Environ Saf. 2016;130:93-102.
- Roberts RJ. Fish pathology. London: Bailliere Tindall; 1978. 466 p.
- Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Haque R, Raisuddin S. Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol Environ Saf. 2003;56(2):295-301.
- Suzuki K, Ishikawa K, Sugiyama K, Furuta H, Nishimura F. Content and release of bisphenol A from polycarbonate dental products. Den Mat J. 2000;19(4):389-395.
- Tabrez S, Ahmad M. Effect of wastewater intake on antioxidant and marker enzymes of tissue damage in rat tissues: implications for the use of biochemical markers. Food Chem Toxicol. 2009;47(10):2465-2478.
- Thiele JJ, Freisleben HJ, Fuchs J, Ochsendorf FR. Ascorbic acid and urate in human seminal plasma: determination and interrelationships with chemiluminescence in washed semen. Hum Reprod. 1995;10(1):110-115.
- Tyl RW, Myers CB, Marr MC, Sloan CS, Castillo NP, Veselica MM, Seely JC, Dimond SS, Van Miller JP, Shiotsuka RN, Beyer D, Hentges SG, Waechter JM. Two-generation reproductive toxicity study of dietary bisphenol A (BPA) in CD-1 (Swiss) mice. Toxicol Sci. 2008;104(2):362-384.
- Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, Brine DR, Veselica MM, Fail PA, Chang TY, Seely JC, Joiner RL, Butala JH, Dimond SS, Cagen SZ, Shiotsuka RN, Stropp GD, Waechter JM. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol Sci. 2002;68(1):121-146.
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24(2):139-177.
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113(8):926-933.
- Wright JR, Colby HD, Miles PR. Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys. 1981;206(2):296-304.
- Wu M, Xu H, Shen Y, Qiu W, Yang M. Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol-A, nonylphenol, and their mixture. Environ Toxicol Chem. 2011;30(10):2335-2341.
- Yazdani M, Andresen AM, Gjøen T. Short-term effect of bisphenol-A on oxidative stress responses in Atlantic salmon kidney cell line: a transcriptional study. Toxicol Mech Methods. 2016;26(4):295-300.
Publication Dates
-
Publication in this collection
2017
History
-
Received
11 Jan 2017 -
Accepted
21 Feb 2017