ABSTRACT
Dioscorea pentaphylla L., a wild tuber is used both as food and medicines among different ethnic groups of Similipal Biosphere Reserve, India. Tubers are used against skin infections. In order to establish and confirm tribal claims, methanol extract was subjected to fractionation. The active fraction (DP1) was subsequently used for further purification and NMR (Nuclear magnetic resonance) characterization. The phytochemical analysis revealed the presence of saponin groups. The antibacterial activity of DP1 was done against selected bacterial strains (Salmonella typhi, Shigella flexneri, Streptococcus pyogenes, Streptococcus mutans and Vibrio cholerae) using DD (disc diffusion), AWD (agar well diffusion) and broth dilution assay. The activity was compared with antibiotics Penicillin and Kanamycin. It was observed that DP1 showed significant inhibitory activity against the tested bacteria. The characterization of DP1 through NMR analysis and presence of proton in carbon position at C-3, C-19, C-18, C-21 and C-27 was same as the known compound “Diosgenin”. Therefore, isolated compound was confirmed to be Diosgenin. The study for the first time showed that, diosgenin present in D. pentaphylla tuber was responsible for antibacterial and antioxidant potential. Present study highlights the importance of Dioscorea species as sources of diverse secondary metabolites for the isolation of active compound(s).
Keywords:
Dioscorea pentaphylla/extract/active fraction; Dioscorea pentaphylla/ antibacterial activity/antioxidant; NMR characterization
INTRODUCTION
The use of natural products with therapeutic properties by common man is as ancient as human civilization and, for long, plant products were the main sources of traditional medicines (Ji, Li, Zhang, 2009Ji HF, Li XZ, Zhang HY. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in fight against cancer and dementia? EMBO Rep. 2009;10(3):194-200.). The industrial revolution and the development of organic chemistry resulted in preference for synthetic products for pharmacological treatment (Rats, 2001Rats SMK. Plant as source of drugs. Toxicon. 2001;39(5):603-613.; Kwik, 2015Kwik GG. US competitiveness in synthetic biology. Health Secur. 2015;13(6):378-389.). Even if we consider the impact of the discovery of the penicillin, obtained from micro-organisms, as on anti-infection therapy, the importance of natural products can never be ignored. About 25% of the drugs prescribed worldwide come from plants, and many such active compounds are in current use (Shu, 1998Shu YZ. Recent natural products based drug development: a pharmaceutical industry perspective. J Nat Prod. 1998;61(8):1053-1071.; Ciddi, 2012Ciddi V. Natural products derived from plant as a source of drugs. J Adv Phar Tech Res. 2012;3(4):200-201.). Most of important drugs obtained from plants are digoxin from Digitalis species, quinine from Cinchona species, vincristrine and vinblastine from Catharanthus roseus (L.) G. Don., atropine from Atropa belladonna L., morphine codeine from Papaver somniferum L., and diosgenin from Dioscorea species etc. Natural compounds from plant sources are being used for designing and formulating new drugs (Rats, 2001; Dittbrenner et al., 2009Dittbrenner A, Mock HP, Borner A, Lohwasser U. Variability of alkaloid content in Papaver somniferum L. J Appl Bot Food Qual. 2009;82:103-107.; Tostmann et al., 2010Tostmann A, Boeree MJ, Aarnoutse RE, Lange WCM, Dekhuijzen R. Antituberculosis drug induced hepatotoxicity: concise up-to-date review. J Gast Hepat. 2010;23(2):192-202.; Cragg, Newman, 2005Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethanopharmacol. 2005;100(1-2):72-9.; Archana, Paul, Tiwari, 2011Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharma Sci. 2011;1(1):98-106.; Heena, Lele, 2012Heena JS, Lele SS. Extraction of Diosgenin, bioactive compounds from Natural Source Dioscorea alata var purpurae. Anal Nioana Tech. 2012;3(4):1-3.; Chavez et al., 2013Chavez GJG, Fose AV, Zavala AJF, Heredia JB, Sepulveda D, Yahia EM, et al. Technologies for extraction and production of bioactive compounds to be used as Nutraceutical and Food Ingredients: an overview. Compreh Rev Food Sci Food Safet. 2013;12(1):5-23.; Lahlou, 2013Lahlou M. The success of natural products in drug discovery. Pharmaco Pharma. 2013;4:17-31.).
Most of the wild plants have been reported to have antimicrobial activity (Mikayel, Margarit, Armen, 2017Mikayel G, Margarit P, Armen T. Antimicrobial activity of some plant materials used in Armenian traditional medicine. BMC Compl Alter Med. 2017;17:50.). Still number of wild unexplored / neglected plants are available in the forest having both food as well as medicinal values. Among the indigenous forest food plants, the tubers and roots are the most important wild medicinal foods after grains (Birgitta, Gullick, 1999Birgitta GK, Gullick C. Exploring the potential of indigenous wild food plants in Southern Sudan. Proceeding of a workshop of Lokichoggio. In: Proceedings of a Workshop Held in Lokichoggio. 1999 Jun 3-5. Kenya: The Mitchell Group; 1999. p. 3-5.). Many of these tubers are used for preparation of medicines against diseases by the rural and tribal people (Tabassum, Hamdani, 2014Tabassum N, Hamdani M. Plants used to treat skin diseases. Pharmaco Rev. 2014;8(15):52-60.). Therefore, there is an urgent need for screening primary and secondary metabolites in many such wild plants species. Among such wild tubers, India harbours a rich genetic diversity of tropical root and tuberous plants such as “Yams” (Bān Aālu), aroids and several others like ginger, arrowroot, zedoary, ginger lily, wild turmeric and some orchids. In Similipal Biosphere Reserve (Figure 1), Odisha, India, the tuberous edible medicinal plant D. pentaphylla is one such tuber that acclaims unique importance. It is locally known as “Panja Aalu” or “Panja Sānga” (Kumar et al., 2017Kumar S, Das G, Seung SH, Patra JK. Dioscorea spp. (A wild edible tuber): a study on its Ethnopharmacological potential and traditional use by the local people of Similipal Biosphere Reserve, India. Front Pharma. 2017;8:Article 52.). Tribal communities use different preparations out of this tuber as medicine, particularly against inflammation, anti-aging and diseases caused by microbial pathogens. They attribute antioxidant activity and antimicrobial activities due to the expression of browning properties and presence of secondary metabolites in them.
Keeping this in mind, in the present study an attempt has been made to study the browning properties and antioxidant of tuber extracts and to characterize the constituents present in active fraction of the tuber extract of D. pentaphylla (Figure 2) against tested bacterial strains. The antioxidant and antimicrobial assay of D. pentaphylla extracts were carried out. TLC (Thin Layer Chromatography) profiling of active extract and fractionation was done. NMR (Nuclear Magnetic Resonance) analysis of the spot of active fraction was done to identify the bioactive compound(s) present in the tubers of D. pentaphylla.
MATERIAL AND METHODS
Selection and collection of experimental plant (tuber)
The experimental plant was collected from the Padampur village, in the peripheral area of Similipal Biosphere Reserve, Odisha, India and was kept in poly bags tagged with the botanical name as per standard sampling procedure and passport description (Hawkes, 1980Hawkes JG. Crop genetic resources: field collection manual. England: International Board of Plant Genetic Resources, University of Birmingham; 1980. p.1-30.; Christian, Brigitte, 2004Christian RV, Brigitte VL. Tools and methods for data collection in ethnobotanical studies of homegardens. Field Method. 2004;16(3):285-306.). The collected germplasm of experimental plant was propagated and grown in the gene bank of Department of Botany, Ravenshaw University, Cuttack, India for further experimental work.
Browning values and tuber extract preparation
Browning values were qualitatively estimated with the rate of colour changing. Soxhlet method was adopted (Tiwari et al., 2011Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharma Sci. 2011;1(1):98-106.) to obtain the methanol extract of D. pentaphylla tuber. The tubers were collected and dried at room temperature under shade and were powdered after drying using mechanical devices. The powdered material of the experimental plant was kept in thimble and extraction was carried out using the Soxhlet apparatus. The residues were collected and left for air drying and dried crude extracts were stored in refrigerator for further experimental work.
Estimation of antioxidant activity
In order to study the antioxidant activity of experimental plant extracts, the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay and metal chelating activity were evaluated. The standard methods were adopted for the said scavenging activity. DPPH was carried out followed by Cao, Sofic and Prior (1997Cao G, Sofic E, Prior LR. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radical Biol Med. 1997;22(5):749-760.) and metal chelating activity was done using Gouda et al. (2014Gouda S, Moharana RR, Das G, Patra JK. Free radical scavenging potential of extracts of Gracilaria verrucosa (L) (Harvey): An economically important seaweed from Chilika lake. Int J Pharm Pharma Sci. 2014;6(1):707-710.). The DPPH activity was expressed as EC50 values (effective concentration showing 50% of inhibition activity). DPPH was carried out using 5.0 mL of dilutions (100 µg/mL) of the experimental compounds and standard were mixed with 1 mL of a 0.001 % ethanolic solution of DPPH. DPPH solution was freshly prepared in each experiments and was stored in dark at 4± 2 °C. The compounds were incubated for 20-30 minutes in the dark at 30±2 °C. After incubation, Spectrophotometer readings were taken at 517 nm. All determination was performed in triplicate for better documentation. The Metal Chelating Activity of the plant extracts was determined using Gouda et al. (2014). About 1ml of plant extract added to a solution of 0.5 mL ferrous chloride (0.2mM), and then about 0.2 mL. of Ferozin (5 mM) was added to it and incubated at room temperature for 10 minutes. The absorbance of the solution was then measured at 562 nm.
Antibacterial activity of D. pentaphylla tuber extracts
Antibacterial activity using Disc diffusion assay was done with the 6 mm of disc prepared from Whatman filter paper (Amanda et al., 2012Amanda JD, Bhat N, Ruth AK, Katherine LO, David RM. Disc diffusion bioassays for the detection of antibiotic activity in body fluids: applications for the pneumonia etiology research for child health project. Clin Inf Dis. 2012;54(Suppl 2):S159-164.). Each extract was dissolved in dimethyl sulfoxide. The sets of three dilutions (0.5, 1.0 and 2.0 mg/mL) of crude extracts and standard drugs were prepared. 6 mm of discs were kept in the drugs for 12 h before placing on the agar plates. The zones of growth inhibition around the disks were measured after 18 to 24 hrs of incubation at 37 °C for bacteria. The sensitivities of the microbial species to the plant extracts were determined by measuring the sizes of inhibitory zones (including the diameter of disk) on the agar surface around the disks, and values less than 8 mm were considered as not active against microorganisms.
Fractionation of methanolic extract of D. pentaphylla tuber
First author already reported that the methanol extract showed highest antibacterial activity against two Gram-positive bacteria Streptococcus mutans (MTCC 497) and Streptococcus pyogenes (MTCC 1926); and three Gram-negative bacteria Vibrio cholerae (MTCC 3906), Shigella flexneri (MTCC 1457) and Salmonella typhi (MTCC 1252) (Kumar, Behera, Jena, 2013Kumar S, Behera SP, Jena PK. Validation of tribal claims on Dioscorea pentaphylla L. through phytochemical screening and evaluation of antibacterial activity. Plant Sci Res. 2013;35(1-2):55-61.; Kumar, Jena, 2014). Therefore, a combination of preparative TLC and column chromatography (Raman 2006) were used for the initial fractionation of the crude extracts and isolation of the active compounds. The dry powder extract was dissolved in respective solvent (acetone, methanol and aqueous). 10 µL of the extract was applied as a drop at the origin of the preparative TLC plate with pre determined mobile phase (Bhatanagar et al., 2012Bhatanagar S, Sahoo S, Mohapatra AK, Behera DR. Phytochemical analysis, antioxidant and cytotoxic activity of medicinal plant Combretum roxburghii (Family: Comberataceae). Int J Drug Dev Res. 2012;4(1):193-202.). The active well visualized bands were confirmed.
The bands that are to be analyzed were marked and the silica containing the compounds was scraped off repeatedly and collected in closed container. The collected samples were centrifuged with respective solvent. The supernatant was collected and kept for further experiments. Normal column chromatography was performed with the use of silica gel powder (60-120 mesh, Merck- 0.040-0.063 mm, and code-61806205001730) on a 45 cm glass column of 1.4 cm diameter. The previously collected supernatant was loaded on the packed column. A gradient mobile phase, composed of different ratios of chloroform and methanol, was used in order to elute the compounds over the range of polarities. The used mobile phase for active visualized bands on TLC was also used in column. Fractions were collected and monitored on TLC again with respective mobile phase and confirmed the spot at same Rf (retention factor) (Puspa, Weeraddana, 2011Puspa DA, Weeraddana CDS. Screening of petroleum ether, chloroform, ethyl acetate, ethanol and water extracts of medicinal plant, AvicenniaMarina for antibacterial activity against antibiotic resistant bacteria species, Staphylococcus and Proteus. J Pharma Biomed Sci. 2011;11(18):1-4.; Kuete et al., 2012Kuete V, Teponno RB, Mbaveng AT, Tapondjou LA, Meyer JM, Barboni L, et al. Antibacterial activities of the extracts, fractions and compounds from Dioscorea bulbifera. BMC Comp Alternat Med. 2012;12:1472-6882.; Sathiavelu, Arunachalam, 2012Sathiavelu M, Arunachalam S. High performance thin layer chromatography profile of Cassythafiliformis. Asian Pacific J Trop Biomed. 2012;2(3 Suppl):S1431-S1435.). The antibacterial activity of the fraction is reported (Kumar, Jena, 2014Kumar S, Jena PK. Chromatographic, antibacterial and FT-IR analysis of Dioscorea pentaphylla L. tuber extracts. Plant Sci Res. 2014;35(1-2):55-62.) and the confirmed fraction was concentrated. The concentrated fractions were dissolved in a small volume of respective solvent and subjected to phytochemical analysis, NMR analysis and antibacterial activity (Present study).
Antibacterial activity of active spot (DP-1)
The methanol fraction of D. pentaphylla tuber was screened for antibacterial activity against two Gram-positive bacteria Streptococcus mutans (MTCC 497) and Streptococcus pyogenes (MTCC 1926); and three Gram-negative bacteria Vibrio cholerae (MTCC 3906), Shigellaflexneri (MTCC 1457) and Salmonella typhi (MTCC 1252). All of the used MTCC (Microbial Type Culture Collection) bacterial strains were collected from Institute of Microbial Technology (IMTECH), Chandigarh. Antibacterial activity was done using slight modification of standard methods of Agar Well Diffusion assay (Allen, Molan, Reid, 1991Allen KL, Molan PC, Reid GM. A survey of the antibacterial activity of some New Zealand honeys. J Pharm Pharma. 1991;43(12):817-822.), Disc Diffusion method (Scorzoni et al., 2007Scorzoni L, Benaducci T, Almeida AMF, Silva DHS, Bolzani VS, Mendes MJS. Comparative study of disc diffusion and microdilution methods for evaluation of antifungal activity of natural compounds against medical yeasts Candida spp and Cryptococcus sp. Rev Ciênc Farm Básica Apl. 2007;28(1):25-34.; Amanda et al., 2012Amanda JD, Bhat N, Ruth AK, Katherine LO, David RM. Disc diffusion bioassays for the detection of antibiotic activity in body fluids: applications for the pneumonia etiology research for child health project. Clin Inf Dis. 2012;54(Suppl 2):S159-164.; Zare et al., 2012Zare Z, Majid A, Sattari TN, Iranbaksh A, Mehrabian S. Antimicrobial activity of leaf and flower extracts of Lippia nodiflora L. (Verbenaceae). J Plant Prot Res. 2012;52(4):401-403.; Thompson et al., 2013Thompson A, Meah D, Ahmed N, Jenkins RC, Chileshe E, Phillips CO, et al. Comparison of the antibacterial activity of essential oils and extracts of medicinal and culinary herbs to investigate potential new treatments for irritable bowel syndrome. BMC Comp Alt Med. 2013;13:1-19.) and Broth dilution assay (Rai et al., 2010Rai US, Arun M, Isloor Shetty P, Vijesh AM, Prabhu N, Isloor S, et al. Novel Chromeno (2,3-b)-pyrimidine derivatives as potential anti-microbial agents. Euro J Med Chem. 2010;45(6):2695-2699.).
Phytochemical assays of DP1
Phyto-chemical analysis of DP1 was carried out using standard procedure to identify the possible bioactive compound(s) (Harborne, 1973Harborne JB. Phytochemical methods. London: Chapman and Hall; 1973. p. 49-188.; Trease, Evans, 1989Trease GE, Evans WC. Pharmacognosy. London: WB Scanders Company; 1989. p. 89-300.; Sofowara, 1993).
Test of Tannin: The powder DP1 was boiled in 10 mL of distilled water and filtered using “whatman filter paper” of filter grade 42. 2 mL of filtrate was taken in a test tube and 3-5 drops of 0.1 % ferric chloride solution was added. The brownish green or blue black colouration indicated the presence of tannins.
Test for Saponin: The powder DP1 was boiled in 15 mL of distilled water and filtered using Whatman filter paper of filter grade 42. 5 mL of filtrate was mixed with 2 mL of normal distilled water and shaken vigorously. The stable persistent froth indicated the presence of saponins.
Test of Flavonoids: 6 mL of dilute ammonium solution was added to a portion of the aqueous filtrate of DP1 followed by addition of concentrated sulphuric acid. A yellow colouration indicated the presence of flavonoids.
Test of Terpenoids: DP1 was mixed with 1 mL of methanol and 2.5 mL of chloroform and 3 mL of concentrated sulphuric acid was added. A reddish-brown colouration of interface indicated the presence of terpenoids.
Test of Glycosides: DP1 powder was treated with 1 % ferric chloride solution and was put into water bath for 5 minutes at 100 °C. The mixture was cooled, and equal volume of benzene was added. The benzene layer was separated, and 5 mL of ammonia solution was added. Formation of rose pink colour indicated the presence of glycosides.
Test of Phenolic compounds: DP1 powder was treated with 3-5 drops of 1% ferric chloride solution. Formation of bluish black colouration indicated the presence of phenolic compounds.
Test for Reducing Sugar: DP1 powder was dissolved with distilled water and filtered. The filtrate was boiled with 2 drops of Fehling’s solution A and B for 5 minutes. An orange-red precipitate was obtained, which indicated the presence of reducing sugar.
Test for Steroids: DP1 was dissolved in 2 mL of methanol and again dissolved in 5 mL chloroform and then 5 mL of concentrated sulphuric acid was added. Formation of 2 phases (upper red and lower yellow with green fluorescence) indicated the presence of steroids.
Test for Alkaloids: DP1 powder was mixed with 5 mL of 1% aqueous HCl on water bath and then filtered. 2-5 drops of Dragendorff’s reagent were added in the filtrate. The occurrence of orange-red precipitate indicated the presence of alkaloids in the sample extract.
1 H NMR analysis for estimation of active spot / bands
1H NMR analysis of spot DP1 found in the active fraction of methanol extract of D. pentaphylla tuber was recorded on a NMR-400 MHz and also the chemicals shifts were recorded (Singh et al., 2014Singh M, Hamid AA, Prakash O, Khan F, Kumar A, Aiyelaagbe OO, et al. Synthesis of diosgenin analogues as potential anti-inflammatory agents. J Steroid Biochem Mol Biol. 2014;143:323-333.). The results were compared with reference graph and number of protons present in C position (Ghosh et al., 2014Ghosh S, More P, Derle A, Patil AB, Markad P, Asok A, et al. Diosgenin from Dioscorea bulbifera: Novel hit for treatment of type II diabetes mellitus with inhibitory activity against alpha-amylase and alpha-glucosidase. PLoS One. 2014;9(9):e106039.).
RESULTS AND DISCUSSION
Nutraceutical foods are very important for an increasing global population. Biodiversity is the hub of uncountable such foods, yet we only make use of a few. Among them, Dioscorea species play a vital role in supplementing the requirement of food and medicines to rural and tribal people. The results of present study revealed that the tuber of D. pentaphylla showed high rate of browning. The browning properties (Figure 3) are directly proportional to the antioxidant activities. The estimation of antioxidant revealed that the organic extracts of tubers showed excellent activity with standards. It was observed that acetone extract showed highest (89 µg/mL EC50 values for DPPH and 86 µg/mL EC50 values for Metal chelating) antioxidant activity (Table I) while Bhandari et al. (2003Bhandari MR, Kasai T, Kawabata J. Nutritional evaluation of wild yam (Dioscorea spp.) tubers of Nepal. Food Chem. 2003;82(4):619-623.) documented the browning values of D. bulbifera, D. Deltoids, D. triphylla and D. versicolor along with the total phenol content (mg/100g) in D. bulbifera, D. deltoida, D. triphylla and D. versicolor and Lubag et al. (2008Lubag AJM, Laurena AC, Tecson-Mendoza EM. Antioxidants of Purple and White Greater Yam (Dioscorea alata L.) Varieties from the Philippines. Philippine J Sci. 2008;137(1):61-67.) reported the antioxidant activity of D. alata (61 %) using the relative lipid peroxidation. In the year of 2011, Roy et al. (2011Roy A, Sitalakshmi T, Geetha RV, Lakshmi T, Vishnu Priya V. In Vitro antioxidant and free radical scavenging activity of the ethanolic extract of Dioscorea villosa (Wild Yam) Tubers. Drug Invention Today. 2011;3(9):214-215.) documented the free radical scavenging activity using DPPH assay of ethanolic extract of D. villosa (87.72%) while Lincy and Mohan (2013Lincy MP, Mohan VR. Antioxidant activity of Dioscorea spicata ROTH of using various in vitro assay models. Pharma Sci Monitor. 2013;4(2):3751-3767.) studied the antioxidant activity of D. spicta using DPPH assay (57.51% at 1000 µg/mL), hydroxyl radical scavenging activity (49.33 % at 1000 µg/mL).
Researches of the recent years have emphasized that there is an urgent need of research for new antimicrobial agents or drugs in the light of antibiotic resistance offered by pathogenic microbes. Keeping these in view, the extracts of the D. pentaphylla were investigated for their anti-microbial values using disc diffusion assay. The results revealed that methanol extract showed highest zone of inhibition followed by acetone and aqueous extracts at all taken concentrations against all tested bacterial strains. It was also noted that the highest inhibition was exhibited by methanol extract of D. pentaphylla tuber against S. pyogenes at all the used concentrations (Table II). The results were in conformity with the traditional uses among the aboriginals of SBR as reported by the tribal of Padampur, Hatibadi, Durdura. They use the tuber against skin infections, against cut, wounds and microbial infections (Kumar, Behera, Jena, 2013Kumar S, Behera SP, Jena PK. Validation of tribal claims on Dioscorea pentaphylla L. through phytochemical screening and evaluation of antibacterial activity. Plant Sci Res. 2013;35(1-2):55-61.). All the three extracts were having excellent inhibitory effects, so the tuber extracts might be quite effective in controlling the diseases caused by V. cholerae, S. typhi, S. flexnerii, S. mutans and S. pyogenes.
As the methanol extract of D. pentaphylla (tuber) showed highest zone of inhibition, therefore it was taken with eight different mobile phases for TLC and column chromatography analysis. TLC analysis showed that the methanol extract showed significant number of visible bands (Kumar, Jena, 2014Kumar S, Jena PK. Chromatographic, antibacterial and FT-IR analysis of Dioscorea pentaphylla L. tuber extracts. Plant Sci Res. 2014;35(1-2):55-62.). The column chromatography of D. pentaphylla revealed that there are six fractions named F1, F2, F3, F4, F5 and F6 which were collected. The antibacterial activity of fractions showed that only F6 exhibited the zone of inhibition against all tested bacterial strains (Kumar, Jena, 2014). Kuete et al. (2012Kuete V, Teponno RB, Mbaveng AT, Tapondjou LA, Meyer JM, Barboni L, et al. Antibacterial activities of the extracts, fractions and compounds from Dioscorea bulbifera. BMC Comp Alternat Med. 2012;12:1472-6882.) also documented the antibacterial activity of methanol extract and fractions from the bulbils of D. bulbifera to be active against E. coli, M. tuberculosis, E. aerogenes, K. pneumoniae and P. aeruginosa. The experiment was further subjected to get the active spot / band using F6 on TLC. It was observed that same spot appeared 23 times out of 25 times of experiments at Rf: 0.82 with respective mobile phase with F6 (Kumar, Jena, 2014). The spot having Rf: 0.82 was named as DP-1 (present study) and results confirmed the spot (DP-1) at the said Rf. The present antibacterial analysis of DP-1 using Agar well Diffusion and Disc Diffusion assay was done and results revealed that it showed significant inhibitory activity against S. pyogenes followed by S. flexneri, S. mutans, V. cholerae and S. typhi at used concentrations (Table III).
DP-1 was also excellent in inhibiting the growth at concentration of 10 µg/disc against V. cholerae, S. typhi, S. flexneri, S. pyogenes and S. mutans. Using both assay, DP-1 showed the highest inhibitory activity against MTCC 1926 S. pyogenes. The above results indicated that the DP-1, Kanamycin and neomycin are more effective against Gram-positive bacteria S. pyogenes. It was also examined that ampicillin is more effective against Gram-negative bacteria. Results confirmed that the compounds might be responsible to formulate drugs against S. pyogenes too. (Table III). For further confirmation of the antibacterial activity, broth dilution method was performed for assessment of MIC (Minimum Inhibitory Concentration) values compared to antibiotics. The results revealed that DP-1 showed the lowest MIC values against S. pyogenes and S. mutans as compared to used antibiotics (Table IV).
The above results encouraged for the qualitative tests of phytochemical screening. The phytochemical screening of DP-1 showed the presence of saponin (Table V). Previous reports have revealed that Dioscorea is rich with Diosgenin (Ghosh et al., 2014Ghosh S, More P, Derle A, Patil AB, Markad P, Asok A, et al. Diosgenin from Dioscorea bulbifera: Novel hit for treatment of type II diabetes mellitus with inhibitory activity against alpha-amylase and alpha-glucosidase. PLoS One. 2014;9(9):e106039.), a steroid sapogenin. The sugar-free diosgenin is used for the commercial synthesis of cortisone, pregnenolone, progesterone, and other steroid products (Dierassi, 1992Dierassi C. Steriod research at syntax: "the pill" and cortisone. Steroids. 1992;57:631-641.). The antibacterial activity of saponin / diosgenin reports are documented in literature against Gram-positive and Gram-negative bacterial strains (Karimi, Jaafar, Ahmad, 2011Karimi E, Jaafar HZ, Ahmad S. Phytochemical analysis and antimicrobial activities of methanolic extract of leaf, stem and root from different varieties of Labisa pumila Benth. Molecule. 2011;16(6):4438-4450.).
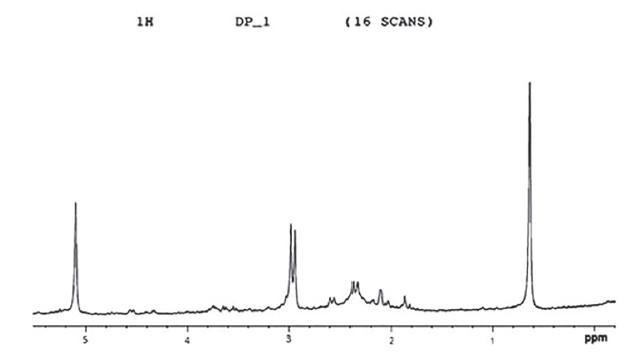
All the above experimental results and findings from literature justified that the active component DP-1 might possess some active compound/group of compounds which is/are responsible for antibacterial activities and this might be dosgenin or its analogue. Keeping this in view, NMR analysis for DP-1 was done to analyze the possible functional groups. The results showed Carbon position at C-3, C-19, C-18, C-21 and C-27 were equal to the known compound Diosgenin. The characterization of DP1 is (C27H42O3) (m/z 414 [M]+), mp 201-203 °C, Rf 0.82, silica gel, n-hexane: ethyl acetate (5:2) 1H NMR (CDCl3,300 MHz): 0.8 (C-18 methyl), 0.83 (C-27 methyl), 0.81 (C-21 methyl), 1.02 (C-19 methyl), 3.38 (C-26), 3.49 (C-3 ), 4.21 (C-1) and 5.02 (C-6 H) (Figure 4). The number of protons were same at said carbon position, therefore the active compound was proved to be diosgenin.
CONCLUSION
The results of present investigations highlight the antioxidants potentials of D. pentaphylla tuber extracts. The antioxidant properties can generate further interest in studying the under-exploited tuber crops for proving the efficacy of these plants as nutraceutical and pharmaceutical foods. The consumption of these crops might play a vital role in preventing human diseases in which free radicals are involved, such as cancer, cardiovascular disease and ageing. The antibacterial potential, TLC and Column Chromatography analysis show its importance in the formulation of new antibacterial drugs to fight against Antimicrobial resistance problem. The antibacterial activity of scraped spot (DP-1) showed first report against the tested bacterial strains and tuber of D. pentaphylla might be effective to cure the disease caused by the used bacterial strains. The characterization of DP1 proved that the compound present in the scraped spot to be Diosgenin. The present study, for the first time established that diosgenin present in D. pentaphylla tuber was more effective against Streptococcus pyogenes and Streptococcus mutans along with strong antioxidant potential. The study emphasize upon further investigation, to isolate the active compounds present in this tuber for formulation of new drugs against bacterial infections.
ACKNOWLEDGEMENTS
The authors are grateful to the HOD, Department of Botany and Prof. Pradipta Kumar Mohapatra, Ravenshaw University, Cuttack for providing the facilities for the present study. Authors are also thankful to the Dr. R. C. Misra, National Bureau of Plant Genetic Resources, Base Centre, Cuttack for the identification of the experimental plant during field survey at Similipal Biosphere Reserve, India; Director, National Institute of Science Education and Research, Bhubaneswar for the characterization of scraped spot through official facilities of experimental work; Prof. K. Pramanik, National Institute of Technology, Rourkela & Mr. S. Gouda for the help in the estimation of antioxidant activities.
REFERENCES
- Allen KL, Molan PC, Reid GM. A survey of the antibacterial activity of some New Zealand honeys. J Pharm Pharma. 1991;43(12):817-822.
- Amanda JD, Bhat N, Ruth AK, Katherine LO, David RM. Disc diffusion bioassays for the detection of antibiotic activity in body fluids: applications for the pneumonia etiology research for child health project. Clin Inf Dis. 2012;54(Suppl 2):S159-164.
- Archana JS, Paul R, Tiwari A. Indian medicinal plants: a rich source of Natural Immuno-modulator. Int J Pharma. 2011;7:198-205.
- Bhandari MR, Kasai T, Kawabata J. Nutritional evaluation of wild yam (Dioscorea spp.) tubers of Nepal. Food Chem. 2003;82(4):619-623.
- Bhatanagar S, Sahoo S, Mohapatra AK, Behera DR. Phytochemical analysis, antioxidant and cytotoxic activity of medicinal plant Combretum roxburghii (Family: Comberataceae). Int J Drug Dev Res. 2012;4(1):193-202.
- Birgitta GK, Gullick C. Exploring the potential of indigenous wild food plants in Southern Sudan. Proceeding of a workshop of Lokichoggio. In: Proceedings of a Workshop Held in Lokichoggio. 1999 Jun 3-5. Kenya: The Mitchell Group; 1999. p. 3-5.
- Cao G, Sofic E, Prior LR. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radical Biol Med. 1997;22(5):749-760.
- Chavez GJG, Fose AV, Zavala AJF, Heredia JB, Sepulveda D, Yahia EM, et al. Technologies for extraction and production of bioactive compounds to be used as Nutraceutical and Food Ingredients: an overview. Compreh Rev Food Sci Food Safet. 2013;12(1):5-23.
- Christian RV, Brigitte VL. Tools and methods for data collection in ethnobotanical studies of homegardens. Field Method. 2004;16(3):285-306.
- Ciddi V. Natural products derived from plant as a source of drugs. J Adv Phar Tech Res. 2012;3(4):200-201.
- Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethanopharmacol. 2005;100(1-2):72-9.
- Dierassi C. Steriod research at syntax: "the pill" and cortisone. Steroids. 1992;57:631-641.
- Dittbrenner A, Mock HP, Borner A, Lohwasser U. Variability of alkaloid content in Papaver somniferum L. J Appl Bot Food Qual. 2009;82:103-107.
- Ghosh S, More P, Derle A, Patil AB, Markad P, Asok A, et al. Diosgenin from Dioscorea bulbifera: Novel hit for treatment of type II diabetes mellitus with inhibitory activity against alpha-amylase and alpha-glucosidase. PLoS One. 2014;9(9):e106039.
- Gouda S, Moharana RR, Das G, Patra JK. Free radical scavenging potential of extracts of Gracilaria verrucosa (L) (Harvey): An economically important seaweed from Chilika lake. Int J Pharm Pharma Sci. 2014;6(1):707-710.
- Harborne JB. Phytochemical methods. London: Chapman and Hall; 1973. p. 49-188.
- Hawkes JG. Crop genetic resources: field collection manual. England: International Board of Plant Genetic Resources, University of Birmingham; 1980. p.1-30.
- Heena JS, Lele SS. Extraction of Diosgenin, bioactive compounds from Natural Source Dioscorea alata var purpurae. Anal Nioana Tech. 2012;3(4):1-3.
- Ji HF, Li XZ, Zhang HY. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in fight against cancer and dementia? EMBO Rep. 2009;10(3):194-200.
- Karimi E, Jaafar HZ, Ahmad S. Phytochemical analysis and antimicrobial activities of methanolic extract of leaf, stem and root from different varieties of Labisa pumila Benth. Molecule. 2011;16(6):4438-4450.
- Kuete V, Teponno RB, Mbaveng AT, Tapondjou LA, Meyer JM, Barboni L, et al. Antibacterial activities of the extracts, fractions and compounds from Dioscorea bulbifera. BMC Comp Alternat Med. 2012;12:1472-6882.
- Kumar S, Behera SP, Jena PK. Validation of tribal claims on Dioscorea pentaphylla L. through phytochemical screening and evaluation of antibacterial activity. Plant Sci Res. 2013;35(1-2):55-61.
- Kumar S, Das G, Seung SH, Patra JK. Dioscorea spp. (A wild edible tuber): a study on its Ethnopharmacological potential and traditional use by the local people of Similipal Biosphere Reserve, India. Front Pharma. 2017;8:Article 52.
- Kumar S, Jena PK. Chromatographic, antibacterial and FT-IR analysis of Dioscorea pentaphylla L. tuber extracts. Plant Sci Res. 2014;35(1-2):55-62.
- Kwik GG. US competitiveness in synthetic biology. Health Secur. 2015;13(6):378-389.
- Lahlou M. The success of natural products in drug discovery. Pharmaco Pharma. 2013;4:17-31.
- Lincy MP, Mohan VR. Antioxidant activity of Dioscorea spicata ROTH of using various in vitro assay models. Pharma Sci Monitor. 2013;4(2):3751-3767.
- Lubag AJM, Laurena AC, Tecson-Mendoza EM. Antioxidants of Purple and White Greater Yam (Dioscorea alata L.) Varieties from the Philippines. Philippine J Sci. 2008;137(1):61-67.
- Mikayel G, Margarit P, Armen T. Antimicrobial activity of some plant materials used in Armenian traditional medicine. BMC Compl Alter Med. 2017;17:50.
- Puspa DA, Weeraddana CDS. Screening of petroleum ether, chloroform, ethyl acetate, ethanol and water extracts of medicinal plant, AvicenniaMarina for antibacterial activity against antibiotic resistant bacteria species, Staphylococcus and Proteus. J Pharma Biomed Sci. 2011;11(18):1-4.
- Rai US, Arun M, Isloor Shetty P, Vijesh AM, Prabhu N, Isloor S, et al. Novel Chromeno (2,3-b)-pyrimidine derivatives as potential anti-microbial agents. Euro J Med Chem. 2010;45(6):2695-2699.
- Rats SMK. Plant as source of drugs. Toxicon. 2001;39(5):603-613.
- Roy A, Sitalakshmi T, Geetha RV, Lakshmi T, Vishnu Priya V. In Vitro antioxidant and free radical scavenging activity of the ethanolic extract of Dioscorea villosa (Wild Yam) Tubers. Drug Invention Today. 2011;3(9):214-215.
- Sathiavelu M, Arunachalam S. High performance thin layer chromatography profile of Cassythafiliformis. Asian Pacific J Trop Biomed. 2012;2(3 Suppl):S1431-S1435.
- Scorzoni L, Benaducci T, Almeida AMF, Silva DHS, Bolzani VS, Mendes MJS. Comparative study of disc diffusion and microdilution methods for evaluation of antifungal activity of natural compounds against medical yeasts Candida spp and Cryptococcus sp. Rev Ciênc Farm Básica Apl. 2007;28(1):25-34.
- Shu YZ. Recent natural products based drug development: a pharmaceutical industry perspective. J Nat Prod. 1998;61(8):1053-1071.
- Singh M, Hamid AA, Prakash O, Khan F, Kumar A, Aiyelaagbe OO, et al. Synthesis of diosgenin analogues as potential anti-inflammatory agents. J Steroid Biochem Mol Biol. 2014;143:323-333.
- Sofowora A. Screening plants for bioactive agents. Medicinal plants and traditional medicinal in Africa. 2nd ed. Ibadan, Nigeria: Spectrum Books Ltd, Sunshine House; 1993. p. 134-156.
- Tabassum N, Hamdani M. Plants used to treat skin diseases. Pharmaco Rev. 2014;8(15):52-60.
- Thompson A, Meah D, Ahmed N, Jenkins RC, Chileshe E, Phillips CO, et al. Comparison of the antibacterial activity of essential oils and extracts of medicinal and culinary herbs to investigate potential new treatments for irritable bowel syndrome. BMC Comp Alt Med. 2013;13:1-19.
- Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharma Sci. 2011;1(1):98-106.
- Tostmann A, Boeree MJ, Aarnoutse RE, Lange WCM, Dekhuijzen R. Antituberculosis drug induced hepatotoxicity: concise up-to-date review. J Gast Hepat. 2010;23(2):192-202.
- Trease GE, Evans WC. Pharmacognosy. London: WB Scanders Company; 1989. p. 89-300.
- Zare Z, Majid A, Sattari TN, Iranbaksh A, Mehrabian S. Antimicrobial activity of leaf and flower extracts of Lippia nodiflora L. (Verbenaceae). J Plant Prot Res. 2012;52(4):401-403.
Publication Dates
-
Publication in this collection
2017
History
-
Received
14 Jan 2017 -
Accepted
13 Mar 2017