Abstract
The aim of the present research work was to investigate the enzyme inhibitory potential of some new sulfonamides having benzodioxane and acetamide moieties. The synthesis was started by the reaction of N-2,3-dihydrobenzo[1,4]-dioxin-6-amine (1) with 4-methylbenzenesulfonyl chloride (2) in the presence of 10% aqueous Na2CO3 to yield N-(2,3-dihydrobenzo[1,4]-dioxin-6-yl)-4-methylbenzenesulfonamide (3), which was then reacted with 2-bromo-N-(un/substituted-phenyl)acetamides (6a-l) in DMF and lithium hydride as a base to afford various 2-{2,3-dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(un/substituted-phenyl)acetamides (7a-l). All the synthesized compounds were characterized by their IR and 1H-NMR spectral data along with CHN analysis data. The enzyme inhibitory activities of these compounds were tested against a-glucosidase and acetylcholinesterase (AChE). Most of the compounds exhibited substantial inhibitory activity against yeast a-glucosidase and weak against AChE. The in silico molecular docking results were also consistent with in vitro enzyme inhibition data.
Keywords:
Benzodioxane; Acetamide; Spectral analysis; a-Glucosidase; Acetylcholinesterase; Molecular docking
INTRODUCTION
Sulfonamides are chemotherapeutic compounds consisting of a -SO2NH2 functional group that are of biological importance in the fight against pathogenic microbes and they have brought on a revolution in the field of pharmaceutical chemistry. Pathogenic microbes are a great threat to human health and are a cause of growing concern among people across the world. These compounds were firstly used as antibacterial agents (Reddy et al., 2012 Reddy NS, Rao AS, Chari MA, Kumar VR, Jyothy V, Himabindu V. Synthesis and antibacterial activity of sulfonamide derivatives at C-8 alkyl chain of anacardic acid mixture isolated from a natural product cashew nut shell liquid (CNSL). J Chem Sci. 2012;124(3):723-730.; Alsughayer et al., 2011 Alsughayer A, Elassar AZA, Mustafa S, Sagheer FA. Synthesis, structure analysis and antibacterial activity of new potent sulfonamide derivatives. J Biomater Nanobiotech. 2011;2:144-149.; Mahdi et al., 2015 Mahdi MF, Al-Smaism RF, Al-Khaliq ZMA. Synthesis, characterization and antibacterial activity of new series of sulfamethoxazole derivatives. World J Pharm Pharmaceut Sci. 2015;4(10):284-293.). Many sulfa drugs are widely used against various diseases as antifungal (Rathod et al., 2012 Rathod CP, Dhawle SC, Pekamwar SS, Kadam NR, Rekhawar MU. Synthesis, characterization, antimicrobial and antifungal screening of some novel benzene sulfonamide derivatives. Int J Pharm Res School. 2012;1(4):1-4.), antimicrobial (Ajeet, Kumar, 2014 Ajeet, Kumar A. Designing, proposed synthesis and docking analysis of novel sulfonamide derivatives as antibacterial agents. Am J Pharmacol Sci. 2014;2(2):37-41.), antitumor agents (Huang, Lin Huang, 2001 Huang Z, Lin Z, Huang J. A novel kind of antitumour drugs using sulfonamide as parent compound. Eur J Med Chem. 2001;36(11-12):863-872.) and antihypertensive (Bhagwat et al., 2014 Bhagwat AM, Bhat AR, Palled MS, Khade AP, Patil AM. Synthesis and antihypertensive screening of novel substituted 1,2-pyrazoline sulfonamide derivatives. Am J PharmTech Res. 2014;4(2):326-336.) agents. Sulfonamides act as antiviral and antitumor agents and they are used to inhibit cancer cell growth and to block tumor invasion (Huang, Lin, Huang, 2001; Ghorab et al., 2016 Ghorab MM, Alsaid MS, Al-Dosari MS, El-Gazzar MG, Parvez MK. Design, synthesis and anticancer evaluation of novel quinazoline-sulfonamide hybrids. Mol Divers Preserv Int. 2016;21(2):189-193.). Some sulfonamides act as good anti-proliferative agents and show promising broad-spectrum antitumor activity as compared to that of the commonly used anticancer drugs (Kasimogullari et al., 2015). Many sulfonamide derivatives are effective inhibitors of butyrylcholinesterase (BChE), acetylcholinesterase (AChE) for the treatment of Alzheimer’s disease (AD) (Abbasi et al., 2014a) and also show a good inhibitory potential against a-glucosidase and lipoxygenase (Abbasi et al., 2014b).
The compounds that have a 1,4-benzodioxane moiety exhibit a wide range of attractive biological activities, and their synthesis has received considerable attention over the years. Compounds with this moiety have biological activities such as anti-hepatotoxic (Ahmad, Khan, Alam, 2003 Ahmad B, Khan SA, Alam T. Synthesis and antihepatotoxic activity of some heterocyclic compounds containing the 1,4-dioxane ring system. Pharmazie. 2003;58(3):173-176.), α-adrenergic blocking (Chapleo et al., 1983 Chapleo CB, Myers PL, Butler CM, Doxey JC, Roach AG, Smith CFC. Synthesis of some 1,4-benzidioxans as selective presynaptic alpha-2-adrenoreceptor antagonists and potential antidepressants. J Med Chem. 1983;26:823-831.) and anti-inflammatory (Vazquez, Rosell, Pujol, 1997 Vazquez MT, Rosell G, Pujol MD. Synthesis and anti-inflammatory activity of rac-2-(2,3-dihydro-1,4-benzodioxin) propionic acid and its R and S enantiomers. Eur J Med Chem. 1997;32(6):529-534.). The 1,4-benzodioxane ring system is also a part of many pharmaceutically important compounds such as silybin, americanin A6 and haedoxan A7, which show anti-hepatotoxic and insecticidal activities (Irshad et al., 2014 Irshad M, Abbasi MA, Aziz-ur-Rehman, Siddique SZ, Ashraf M, Ejaz SA, et al. Synthesis, characterization and biological screening of some new sulfonamides derivatives of 1,4-benzodioxane-6-amine. J Chem Soc Pak. 2014;36(4):660-673.). Flavonolignans were first discovered in the seeds of Silybum marianum, commonly known as milk thistle. The original flavonolignan to be discovered was silybin, and it is still the most studied flavonolignan and 1,4-benzodioxane lignin (Abouzid, Ahmad, 2013 Abouzid S, Ahmad O. Structure-activity relationship and biosynthesis. Stud Nat Prod Chem. 2013;40:469-484.; Pelter, Hansel, 1968 Pelter A, Hansel R. The structure of silybin (Silybum substance E6), the first flavonolignan. Tetrahedron Lett. 1968;9(25):2911-2916.). Silybin has shown a range of different biological activities including hepatoprotective, anticancer and antioxidant, among others (Kren, Walterova, 2005 Kren V, Walterova D. Silybin and Silymarin-new effects and applications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149(1):29-41.; Gazak, Walterova, Kren, 2007 Gazak R, Walterova D, Kren V. Silybin and silymarin - new and emerging applications in medicine. Curr Med Chem. 2007;14(3):315-338.; Kaspaday et al., 2009).
a-Glucosidase (EC 3.2.1.20) belongs to the family of hydrolases and is located in the brush border surface membrane of small intestinal cells (Chiba, 1997 Chiba S. Molecular mechanism in alpha-glucosidase and glucoamylase. Biosci Biotechnol Biochem. 1997;61(8):1233-1239.). a-Glucosidase inhibitors are used as oral anti-diabetic drugs for patients with type-2 diabetes mellitus (T2DM). Postprandial hyperglycemia plays a vital role in the development of T2DM and nephropathy, neuropathy, microangiopathy, macroangiopathy which are complications associated with this disease (Lebovitz, 1997 Lebovitz HE. Alpha-glucosidase inhibitors. Endocrinol Metab Clin North Am. 1997;26(3):539-551.). The inhibitors of this enzyme can delay the release of D-glucose from oligosaccharides and disaccharides from dietary complex carbohydrates and delay glucose absorption, resulting in reduced postprandial hyperglycemia. Hence, the inhibition of a-glucosidase is an important step in the treatment of T2DM (Chapdelaine, Trembley, Dube, 1978 Chapdelaine P, Trembley RR, Dube JY. p-Nitrophenol-alpha-D-glucopyranoside as substrate for measurement of maltase activity in human semen. Clin Chem. 1978;24(2):208-211.; Abbasi et al., 2016 Abbasi, MA, Islam M, Aziz-ur-Rehman, Rassol S, Rubab K, Hussain G, et. al. Synthesis, characterization, antibacterial, a-glucosidase inhibition and hemolytic studies on some new N-(2,3-dimethylphenyl)benzenesulfonamide derivatives. Trop J Pharm Res. 2016;15(3):591-598.). Several marketed drugs including acarbose and voglobiose are famous inhibitors of a-glucosidase.
Cholinesterases such as AChE (EC 3.1.1.7) and BChE (EC 3.1.1.8) belong to the serine hydrolase class. These are the best potential targets for the symptomatic treatment of AD and its related disorders. The specificities of the enzymes for their substrates and inhibitors depend on differences in the amino acid residues of the active sites of the two esterases. AChE catalyzes the hydrolysis of the neurotransmitter acetylcholine, terminating the nerve impulse at cholinergic synapses (Tougu, 2001 Tougu V. Acetylcholinesterase: Mechanism of catalysis and inhibition. Curr Med Chem. 2001;1(2):155-170.; Gautheir, 2001).
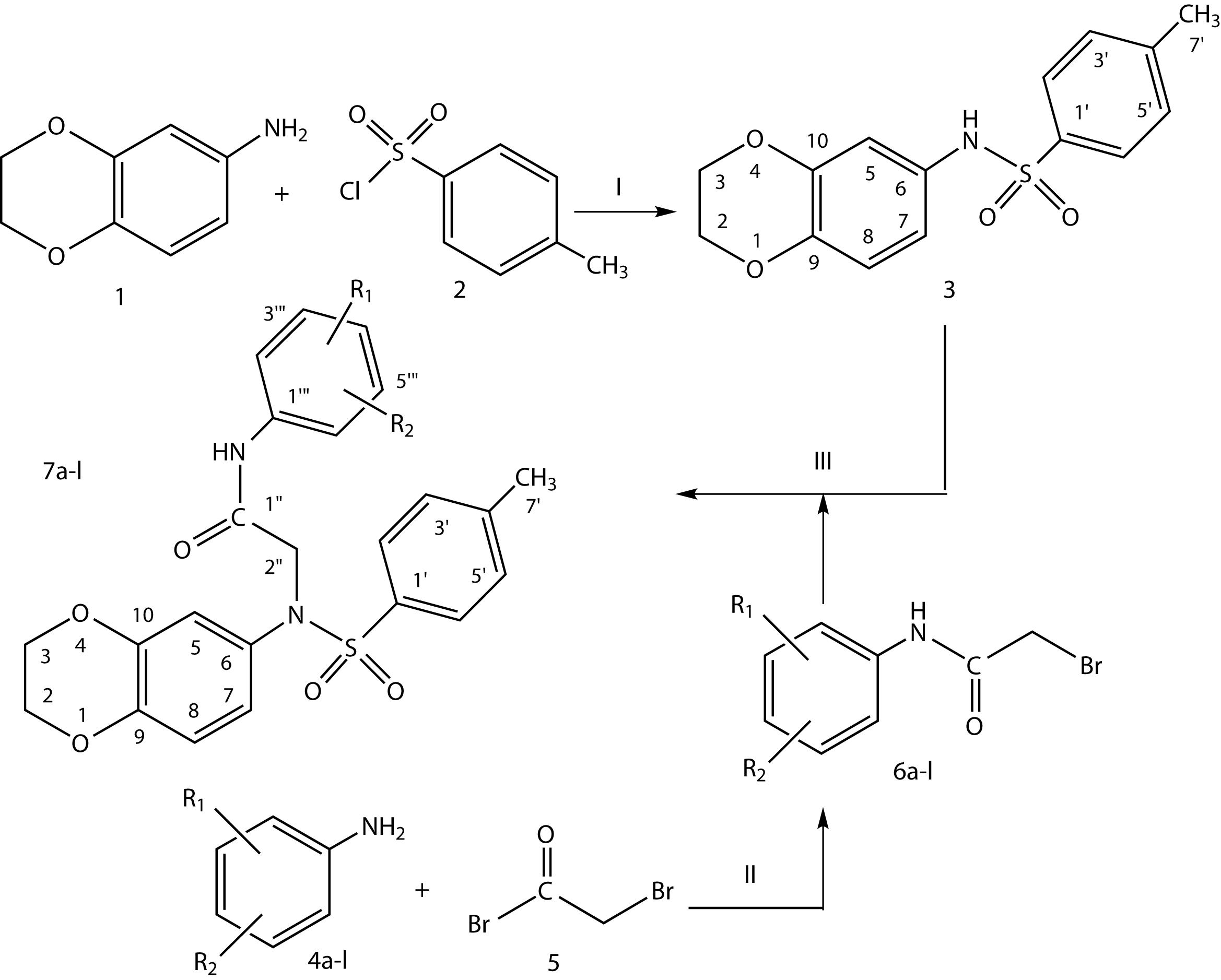
The revolutionary importance of sulfonamides in the field of pharmacology encouraged us to synthesize new sulfonamide-acetamide derivatives using 1,4-benzodioxane as central moiety. In the present work, a number of N-substituted derivatives starting from 2,3-dihydro-1,4-benzodioxin-6-amine were synthesized. The first, parent sulfonamide N-(2,3-dihydrobenzo[1,4]-dioxin-6-yl)-4-methylbenzenesulfonamide (3) was prepared by reacting 2,3-dihydro-1,4-benzodioxin-6-amine (1) with 4-methylbenzenesulfonyl chloride (2) by stirring in aqueous alkaline medium at room temperature, and it was then further derivatized with different 2-bromo-N-(un/substituted-phenyl)acetamides (6a-l) to get targeted molecules (7a-l). These newly synthesized molecules were then screened against a-glucosidase and acetylcholinesterase enzymes to explore their therapeutic potential for T2DM and AD.
MATERIAL AND METHODS
General
The chemicals utilized were procured from Sigma Aldrich/Alfa Aesar. The solvents used in reactions were of analytical grade and used as such. Pre-coated TLC silica gel G-25-UV254 plates were used to monitor the reactions using various percentages of n-hexane and ethyl acetate as solvent system. Melting points of compounds were recorded using a Gallenkamp melting point apparatus with open capillary tube. A MIDAC M2000 photon spectrometer was used to record the FTIR spectra in KBr (υ, cm-1). A Burker spectrometer operating at 25 °C at 400/600 MHz was used to record the 1H NMR spectra in CDCl3. The coupling constant (J) is given in Hz and chemical shift (d) in ppm. The abbreviations used in the interpretation of 1H NMR spectra are as follows: s, singlet; d, doublet; dd, doublet of doublets; t, triplet; br.t, broad triplet; q, quartet; quin, quintet; sex, sextet; sep, septet; m, multiplet.
Synthesis
Synthesis of N-(2,3-dihydrobenzo[1,4]-dioxin-6-yl)-4-methylbenzenesulfonamide (3)
N-(2,3-Dihydrobenzo[1,4]-dioxin-6-amine (1mL; 0.002 mol; 1) was suspended in 25 mL distilled water and stirred for half an hour. Next, 10% aqueous Na2CO3 was added to maintain the pH at 9-10, and the reaction mixture stirred for half an hour. 4-Methylbenzenesulfonyl chloride (0.47 g; 0.002 mol; 2) was then added to the mixture along with gradual stirring, which was further stirred for several hours until completion of the reaction, which was monitored by TLC until a single spot was obtained. The product was precipitated at pH 2 using conc. HCl and filtered out, washed with distilled water, and air-dried to afford N-(2,3-dihydrobenzo[1,4]-dioxin-6-yl)-4-methylbenzenesulfonamide (3): a light brown amorphous powder; yield 80%; m.p. 129-130 °C; molecular formula: C15H15O4NS; molecular weight: 305.367 gmol-1; IR: 3248 (N-H stretching), 3045 (C-H stretching of aromatic ring), 2926 (-CH2 stretching), 1633 (C=C stretching of aromatic ring), 1383 (-SO2 stretching); 1H-NMR: d (ppm) 10.12 (s, 1H, NHSO2), 7.57 (d, J= 7.8 Hz, 2H, H-2’ & H-6’), 7.27 (d, J =7.8 Hz, 2H, H-3’ & H-5’), 6.62 (d, J= 8.4 Hz, 1H, H-8), 6.56 (d, J =2.4 Hz, 1H, H-5), 6.47 (dd, J= 2.4,8.4 Hz, 1H, H-7), 4.15 (br.s, 4H, CH2-2, CH2-3), 2.43 (s, 3H, CH3-7’).
General procedure for the synthesis of 2-bromo-N-(un/substituted-phenyl)acetamides ( 6a-l )
2-Bromo-N-(un/substituted-phenyl)acetamides (6a-l) were prepared by vigorous manual shaking of un/substituted anilines (0.16 g; 0.55 mmol; 4a-l, one in each reaction) with bromoacetyl bromide (0.2 g; 0.50 mmol; 5). The pH of the solution was maintained by using 10% aqueous Na2CO3. The completion of the reaction was monitored by TLC till single spot. Afterwards, the reaction mixture was poured on crushed ice. The precipitated products were filtered, washed and dried to acquire the pure products (6a-l).
General procedure for the synthesis of 2-{2,3-dihydro-1,4-benzodioxin-6-yl[(4- methylphenyl)- sulfonyl]amino}-N-(un/substituted-phenyl)acetamides ( 7a-l )
N-(2,3-Dihydrobenzo[1,4]-dioxin-6-yl)-4-methylbenzenesulfonamide (0.2 g; 0.57 mmol; 3) in 10 mL N,N-dimethyl formamide (DMF) was placed into a 50-mL round-bottomed flask along with lithium hydride (0.004 g) and the reaction mixture was stirred for 30 min at 25 °C. 2- Bromo-N-(un/substituted-phenyl)acetamides (0.60 mmol; 6a-l) were added to the reaction mixture, which was further stirred for 3-4 h. The reaction was monitored by TLC until a single spot. After completion, the reaction mixture was poured onto crushed ice and precipitated products were filtered out, washed and air dried to obtain the pure products (7a-l).
Spectral characterization
2-{2,3-Dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-phenylacetamide (7a)
Light pink amorphous powder; yield 82%; mp 177-178 °C; molecular formula C23H22O5N2S; molecular weight 438 g mol-1; IR (KBr) υ (cm-1): 3249 (N-H stretching), 3047 (C-H stretching of aromatic ring), 2929 (-CH2 stretching), 1715 (C=O stretching), 1635 (C=C stretching of aromatic ring), 1387 (-SO2 stretching); 1H-NMR: d (ppm) 8.31 (s, 1H, NHCO), 7.64 (d, J= 8.0 Hz, 2H, H-2’ & H-6’), 7.52 (m, 2H, H-2’’’ & H-6’’’), 7.33-7.28 (m, 4H, H-3’, H-5’, H-3’’’ & H-5’’’), 7.11 (br.t, J= 7.2 Hz, 1H, H-4’’’), 6.75 (d, J= 8.4 Hz, 1H, H-8), 6.66 (d, J= 2.4 Hz, 1H, H-5), 6.53 (dd, J =2.4, 8.4 Hz, 1H, H-7), 4.22-4.21 (m, 4H, CH2-2 & CH2-3), 4.19 (br.s, 2H, CH2-2’’), 2.43 (s, 3H, CH3-7’). Anal. Calc. for C23H22O5N2S (438.12): C, 63.00; H, 5.06; N, 6.39. Found: C, 63.31; H, 5.19; N, 6.53.
2-{2,3-Dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(2-methoxyphenyl)- acetamide (7b)
Amorphous powder; yield 78%; m.p. 119-120 °C; molecular formula C24H24O6N2S; molecular weight 468 g mol-1. IR (KBr) υ (cm-1): 3247 (N-H stretching), 3049 (C-H stretching of aromatic ring), 2930 (-CH2 stretching), 1713 (C=O stretching), 1637 (C=C stretching of aromatic ring), 1383 (-SO2 stretching); 1H-NMR: d (ppm) 8.23 (s, 1H, NHCO), 7.57 (d, J= 7.8 Hz, 2H, H-2’ & H-6’), 7.27 (d, J= 7.8 Hz, 2H, H-3’ & H-5’), 6.96 (m, 2H, H-3’’’ & H-5’’’), 6.70 (br.t, J= 7.2 Hz, 1H, H-4’’’), 6.62 (d, J= 8.4 Hz, 1H, H-8), 6.56 (d, J= 2.4 Hz, 1H, H-5), 6.47 (dd, J= 2.4,8.4 Hz, 1H, H-7), 6.14 (br.d, J= 8.5, 1H, H-6’’’), 4.25 (br.s, 2H, CH2-2’’), 4.15 (br.s, 4H, CH2-2 & CH2-3), 3.87 (s, 3H, 2-OCH3), 2.40 (s, 3H, CH3-7’). Anal. Calc. for C15H15O4NS (468.14): C, 61.52; H, 5.16; N, 5.98. Found: C, 61.74; H, 5.27; N, 6.19.
2-{2,3-Dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(4-ethoxyphenyl)-acetamide (7c)
Pinkish amorphous powder; yield 80%; m.p. 156-157 °C; molecular formula C25H26N2O6S; molecular weight 482 gmol-1; IR (KBr) υ (cm-1): 3251 (N-H stretching), 3047 (C-H stretching of aromatic ring), 2927 (-CH2 stretching), 1717 (C=O stretching), 1635 (C=C stretching of aromatic ring), 1387 (-SO2 stretching); 1H NMR: d (ppm) 8.25 (s, 1H, NHCO), 7.55 (d, J= 8.4 Hz, 2H, H-2’ & H-6’), 7.35 (d, J= 8.4 Hz, 2H, H-3’ & H-5’), 7.32 (d, J= 9.0 Hz, 2H, H-2’’’ & H-6’’’), 6.82 (d, J= 9.0 Hz, 2H, H-3’’’ & H-5’’’), 6.72 (d, J= 2.4 Hz, 1H, H-5), 6.70 (d, J= 8.4 Hz, 1H, H-8), 6.58 (dd, J= 2.4, 8.4 Hz, 1H, H-7), 4.32 (br.s, 2H, CH2-2’’), 4.20-4.18 (m, 4H, CH2-2 & CH2-3), 3.98 (q, J= 7.2 Hz, 2H, 4-OCH2CH3), 2.43 (s, 3H, CH3-7’), 1.34 (t, J= 7.2 Hz, 3H, 4-OCH2CH3). Anal. Calc. for C25H26O6N2S (482.15): C, 62.23; H, 5.43; N, 5.81. Found: C, 62.41; H, 5.57; N, 5.93.
2-{2,3-Dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(2-methylphenyl)- acetamide (7d)
Dark brown amorphous powder; yield 80%; m.p. 95-96 °C; molecular formula C24H24N2O5S; molecular weight 452 gmol-1; IR (KBr) υ (cm-1): 3257 (N-H stretching), 3043 (C-H stretching of aromatic ring), 2930 (-CH2 stretching), 1719 (C=O stretching), 1640 (C=C stretching of aromatic ring), 1389 (-SO2 stretching); 1H-NMR: d (ppm) 8.13 (s, 1H, NHCO), 7.54 (d, J= 8.5 Hz, 2H, H-2’ & H-6’), 7.36 (d, J= 8.5 Hz, 2H, H-3’ & H-5’), 7.27 (br.d, J= 7.0 Hz, 1H, H-6’’’), 7.18 (br.d, J= 7.5 Hz, 1H, H-3’’’), 7.15-7.13 (m, 1H, H-5’’’), 7.12-7.10 (m, 1H, H-4’’’), 6.75 (br.s, 1H, H-5), 6.73 (d, J= 6.5 Hz, 1H, H-8), 6.60 (dd, J= 2.5-8.5 Hz, 1H, H-7), 4.34 (br.s, 2H, CH2-2’’), 4.22-4.19 (m, 4H, CH2-2 & CH2-3), 2.43 (s, 3H, CH3-7’), 2.08 (s, 3H, CH3-2’’’). Anal. Calc. for C24H24N2O5S (452.14): C, 63.70; H, 5.35; N, 6.19. Found: C, 63.83; H, 5.44; N, 6.31.
2-{2,3-Dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(3-methylphenyl)- acetamide (7e)
Amorphous powder; yield 80%; m.p. 114-115 °C; molecular formula C24H24N2O5S; molecular weight 452 gmol-1: IR (KBr) υ (cm-1): 3251 (N-H stretching), 3045 (C-H stretching of aromatic ring), 2933 (-CH2 stretching), 1717 (C=O stretching), 1641 (C=C stretching of aromatic ring), 1390 (-SO2 stretching); 1H-NMR: d (ppm) 8.19 (s, 1H, NHCO), 7.55 (d, J= 8.4 Hz, 2H, H-2’ & H-6’), 7.35 (d, J= 8.4 Hz, 2H, H-3’ & H-5’), 7.30 (br.s, 1H, H-2’’’), 7.26 (br.d, J= 7.8 Hz, 1H, H-6’’’), 7.15 (br.d, J= 7.8 Hz, 1H, H-5’’’), 6.91 (br.d, J= 7.2 Hz, 1H, H-4’’’), 6.71 (d, J= 9.0 Hz, 1H, H-8), 6.56 (d, J= 2.4 Hz, 1H, H-5), 6.47 (dd, J= 2.4,8.4 Hz, 1H, H-7), 4.34 (br.s, 2H, CH2-2’’), 4.20-4.18 (m, 4H, CH2-2 & CH2-3), 2.29 (s, 3H, CH3-3’’’), 2.43 (s, 3H, CH3-7’). Anal. Calc. for C24H24N2O5S (452.14): C, 63.70; H, 5.35; N, 6.19. Found: C, 63.88; H, 5.41; N, 6.27.
2-{2,3-Dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(4-methylphenyl)- acetamide (7f)
Of white amorphous powder; yield 80%; m.p. 134-135 °C; molecular formula C24H24O5N2S; molecular weight 452 gmol-1; IR (KBr) υ (cm-1): 3258 (N-H stretching), 3047 (C-H stretching of aromatic ring), 2936 (-CH2 stretching), 1711 (C=O stretching), 1643 (C=C stretching of aromatic ring), 1385 (-SO2 stretching); 1H-NMR: d (ppm) 8.29 (s, 1H, NHCO), 7.51 (d, J= 8.0 Hz, 2H, H-2’ & H-6’), 7.36 (d, J= 8.5 Hz, 2H, H-3’ & H-5’), 7.28 (d, J= 8.0, 2H, H-2’’’ & H-6’’’), 7.11 (d, J= 8.0 Hz, 2H, H-3’’’ & H-5’’’), 6.75 (d, J= 8.5 Hz, 1H, H-8), 6.66 (d, J= 2.5 Hz, 1H, H-5), 6.53 (dd, J= 2.5,8.5 Hz, 1H, H-7), 4.22-4.19 (m, 4H, CH2-2 & CH2-3), 4.18 (br.s, 2H, CH2-2’’), 2.43 (s, 3H, CH3-7’), 2.29 (s, 3H, CH3-4’’’). Anal. Calc. for C24H24N2O5S (452.14): C, 63.70; H, 5.35; N, 6.19. Found: C, 63.81; H, 5.46; N, 6.23.
2-{2,3-Dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(2,3-dimethylphenyl)- acetamide (7g)
Of white amorphous powder; yield 80%; mp 84-85 °C; molecular formula C25H26N2O5S; molecular weight 466 gmol-1; IR (KBr) υ (cm-1): 3261 (N-H stretching), 3053 (C-H stretching of aromatic ring), 2940 (-CH2 stretching), 1718 (C=O stretching), 1647 (C=C stretching of aromatic ring), 1389 (-SO2 stretching); 1H-NMR: d (ppm) 8.25 (s, 1H, NHCO), 7.64 (br.t, J= 7.8 Hz, H-5’’’), 7.50 (d, J= 8.4 Hz, 2H, H-2’ & H-6’), 7.28 (d, J= 8.0 Hz, 2H, H-3’ & H-5’), 6.98-6.96 (m, 2H, H-4’’’ & H-6’’’), 6.76 (d, J= 8.4 Hz, 1H, H-8), 6.67 (d, J= 2.4 Hz, 1H, H-5), 6.53 (dd, J= 2.4,8.4 Hz, 1H, H-7), 4.22-4.21 (m, 6H, CH2-2, CH2-3 & CH2-2’’), 2.43 (s, 3H, CH3-7’), 2.26 (s, 3H, CH3-3’’’), 2.24 (s, 3H, CH3-2’’’). Anal. Calc. for C25H26N2O5S (466.16): C, 64.36; H, 5.62; N, 6.00. Found: C, 64.44; H, 5.71; N, 6.19.
2-{2,3-Dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(2,4-dimethylphenyl)- acetamide (7h)
Light pink amorphous powder; yield 80%; m.p. 150-151 °C; molecular formula C25H26N2O5S; molecular weight 466 gmol-1; IR (KBr) υ (cm-1): 3265 (N-H stretching), 3055 (C-H stretching of aromatic ring), 2937 (-CH2 stretching), 1721 (C=O stretching), 1649 (C=C stretching of aromatic ring), 1383 (-SO2 stretching); 1H-NMR: d (ppm) 8.27 (s, 1H, NHCO), 7.48 (d, J= 8.4 Hz, 2H, H-2’ & H-6’), 7.26 (d, J= 8.4 Hz, 2H, H-3’ & H-5’), 6.78 (d, J= 8.4 Hz, 1H, H-8), 6.73 (s, 1H, H-3’’’), 6.69 (d, J= 2.4 Hz, 1H, H-5),6.58 (d, 2H, H-5’’’ & H-6’’’), 6.55 (dd, J= 2.4, 8.4 Hz, 1H, H-7), 4.22-4.21 (m, 6H, CH2-2, CH2-3 & CH2-2’’), 2.43 (s, 3H, CH3-7’), 2.29 (s, 3H, CH3-4’’’), 2.23 (s, 3H, CH3-2’’’). Anal. Calc. for C25H26N2O5S (466.16): C, 64.36; H, 5.62; N, 6.00. Found: C, 64.49; H, 5.68; N, 6.23.
2-{2,3-Dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(2,5-dimethylphenyl)- acetamide (7i)
Light purple amorphous powder; yield 80%; m.p. 115-116 °C; molecular formula C25H26N2O5S; molecular weight 466 gmol-1; IR (KBr) υ (cm-1): 3255 (N-H stretching), 3057 (C-H stretching of aromatic ring), 2937 (-CH2 stretching), 1721 (C=O stretching), 1650 (C=C stretching of aromatic ring), 1391 (-SO2 stretching); 1H NMR: d (ppm) 8.31 (s, 1H, NHCO), 7.51 (d, J= 8.4 Hz, 2H, H-2’ & H-6’), 7.28 (d, J= 8.4 Hz, 2H, H-3’ & H-5’), 6.76 (d, J= 8.4 Hz, 1H, H-8), 6.68 (d, J= 2.4 Hz, 1H, H-5), 6.54 (dd, J= 2.4, 8.4 Hz, 1H, H-7), 4.23-4.22 (m, 6H, CH2-2, CH2-3 & CH2-2’’), 2.43 (s, 3H, CH3-7’), 2.28 (s, 3H, CH3-5’’’), 2.16 (s, 3H, CH3-2’’’). Anal. Calc. for C25H26N2O5S (466.16): C, 64.36; H, 5.62; N, 6.00. Found: C, 64.47; H, 5.77; N, 6.22.
2-{2,3-Dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(2,6-dimethylphenyl)- acetamide (7j)
Amorphous powder; yield 80%; m.p. 86-87 °C; molecular formula C25H26N2O5S; molecular weight 466 gmol-1; IR (KBr) υ (cm-1): 3253 (N-H stretching), 3055 (C-H stretching of aromatic ring), 2939 (-CH2 stretching), 1719 (C=O stretching), 1647 (C=C stretching of aromatic ring), 1388 (-SO2 stretching); 1H NMR: d (ppm) 7.93 (s, 1H, NHCO), 7.50 (d, J= 8.5 Hz, 2H, H-2’ & H-6’), 7.28 (d, J= 8.0 Hz, 2H, H-3’ & H-5’), 7.08 (br.t, J= 7.5 Hz, 1H, H-4’’’), 6.78 (d, J= 8.5 Hz, 1H, H-8), 6.70 (d, J= 2.5 Hz, 1H, H-5), 6.57 (dd, J= 2.5,8.5 Hz, 1H, H-7), 4.27 (br.s, 2H, CH2-2’’), 4.25-4.22 (m, 4H, CH2-2 & CH2-3), 2.43 (s, 3H, CH3-7’), 2.09 (s, 6H, CH3-2’’’ & CH3-6’’’). Anal. Calc. for C25H26N2O5S (466.16): C, 64.36; H, 5.62; N, 6.00. Found: C, 64.46; H, 5.75; N, 6.24.
2-{2,3-Dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(3,4-dimethylphenyl)- acetamide (7k).
Light pink amorphous powder; yield 80%; mp 159-160 °C; molecular formula C25H26O5N2S; molecular weight 466 gmol-1; IR (KBr) υ (cm-1): 3257 (N-H stretching), 3050 (C-H stretching of aromatic ring), 2937 (-CH2 stretching), 1713 (C=O stretching), 1645 (C=C stretching of aromatic ring), 1389 (-SO2 stretching); 1H-NMR: d (ppm) 8.23 (s, 1H, NHCO), 7.51 (d, J= 8.0 Hz, 2H, H-2’ & H-6’), 7.28 (d, J= 8.4 Hz, 2H, H-3’ & H-5’), 7.24 (merged in the signal of CDCl3, 2H, H-2’’’ & H-6’’’), 7.05 (br.d, J= 8.0 Hz, 1H, H-5’’’), 6.75 (d, J= 8.8 Hz, 1H, H-8), 6.65 (d, J= 2.4 Hz, 1H, H-5), 6.53 (dd, J= 2.8, 8.4 Hz, 1H, H-7), 4.23-4.19 (m, 4H, CH2-2, CH2-3), 4.18 (br.s, 2H, CH2-2’’), 2.43 (s, 3H, CH3-7’), 2.23 (s, 3H, CH3-3’’’), 2.20 (s, 3H, CH3-4’’’). Anal. Calc. for C25H26N2O5S (466.16): C, 64.36; H, 5.62; N, 6.00. Found: C, 64.39; H, 5.76; N, 6.12.
2-{2,3-Dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(3,5-dimethylphenyl)- acetamide (7l).
Light pink amorphous powder; yield 80%; mp 130-131 °C; molecular formula C25H26N2O5S; molecular weight 466 gmol-1; IR (KBr) υ (cm-1): 3260 (N-H stretching), 3058 (C-H stretching of aromatic ring), 2935 (-CH2 stretching), 1715 (C=O stretching), 1648 (C=C stretching of aromatic ring), 1390 (-SO2 stretching); 1H-NMR: d (ppm) 8.24 (s, 1H, NHCO), 7.51 (d, J= 8.0 Hz, 2H, H-2’ & H-6’), 7.28 (d, J= 8.0 Hz, 2H, H-3’ & H-5’), 7.12 (br.s, 2H, H-2’’’ & H-6’’’), 6.76-6.74 (m, 2H, H-8, H-4’’’), 6.66 (d, J= 2.4 Hz, 1H, H-5), 6.53 (dd, J= 2.4,8.4 Hz, 1H, H-7), 4.22-4.21 (m, 4H, CH2-2 & CH2-3), 4.17 (br.s, 2H, CH2-2’’), 2.43 (s, 3H, CH3-7’), 2.28 (s, 6H, CH3-3’’’ & CH3-5’’’). Anal. Calc. for C25H26N2O5S (466.16): C, 64.36; H, 5.62; N, 6.00. Found: C, 64.41; H, 5.73; N, 6.14.
Enzyme inhibition assays
a-Glucosidase assay
The enzyme inhibition activity against α-glucosidase was performed according to a reported method (Chapdelaine, Trembley, Dube, 1978 Chapdelaine P, Trembley RR, Dube JY. p-Nitrophenol-alpha-D-glucopyranoside as substrate for measurement of maltase activity in human semen. Clin Chem. 1978;24(2):208-211.). The enzyme assay was optimized for the given time at temperature to maintain the linearity of the enzyme reaction wherein the concentration of the substrate was not limiting (data not shown). The reaction mix consisted of phosphate buffer (50 mM, pH 6.8, 70 µL), test compound (0.5 mM, 10 µL) and 10 µL (0.057 U) of enzyme, (Sigma Inc., Cat No. G5003, Type-I from Saccharomyces cerevisiae) to make a total volume of 100 µL, which was mixed well and pre-incubated for 10 min at 37 ºC and pre-read at 400 nm. Next, 10 µL of 0.5 mM substrate, p-nitrophenyl-D-glucopyranoside, was added to start the reaction, and incubation continued for another 30 min, followed by reading at 400 nm with a microplate reader (Epoch, BioTek, USA). The change in absorbance was used as an index for the measurement of percentage inhibition. Acarbose was used as the positive control. All the experiments were performed in triplicates. Inhibition (%) was calculated by the following equation:
EZ-Fit Enzyme Kinetics Software (Perrella Scientific Inc. Amherst, USA) was used to calculate IC50 values of the compounds. Active compounds were serially diluted to suitable concentrations of 0.25, 0.125, 0.0625, 0.03125, 0.0156 mM and their percentage inhibition was determined, and the data were used for the calculations of IC50 values.
Acetylcholinesterase assay
The enzyme inhibition assay against AChE was performed according to a reported method (Ellman et al., 1961 Ellman FL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88-95.). The reaction mixture was prepared with 50 mM Na2HPO4 buffer (pH 7.7, 60 µL), test compound (0.5 mM, 10 µL well-1), followed by the addition 10 µL of 0.005 U Electrophorus electricus AChE (Sigma Inc.) to make a total volume of 100 µL. The contents were mixed, pre-incubated for 10 minutes at 37ºC, and pre-read at 405 nm. Next, 10 µL of 0.5 mM the substrate acetylthiocholine iodide and 10 µL of 0.5 mM DTNB were added and reaction was started. The reaction contents were incubated for 15 minutes at 37ºC and absorbance was measured at 405 nm. These experiments were carried out in triplicate. Eserine (0.5 mM well-1) was employed as a positive control. The percentage inhibition and IC50 values were calculated by the same method as described for α-glucosidase assay.
Molecular docking
To predict the bioactive conformations, various ligands were docked into the binding pockets of the enzymes by using the default parameters of MOE-Dock program.
Ligand preparation
The three-dimensional (3D) structures of the synthesized compounds were made by using ChemDraw Ultra 12.0 (Cambridge Soft, 2001 Cambridge Soft. Chemdraw Ultra. Cambridge Soft Corporation, Cambridge, Massachusetts, USA; 2001.) and saved in MDL Mol file format which were then opened in Molecular Operating Environment (MOE 2009-2010). The energies of the compounds were minimized by using the default parameter of MOE energy minimization algorithm (gradients: 0.05, force field: MMFF94X). A database was created in which all the compounds were saved in the mdb file format for the next step of docking.
Receptor protein preparation
The 3D structures of receptor protein molecules of yeast α-glucosidase (PDB Code: 3NO4) and AChE (PDB Code: 1GQR) were retrieved from Protein Data Bank (Tan et al., 2010 Tan K, Tesar C, Wilton R, Keighr L, Babingg G, Jaochimiak A. The crystal structure of the a-Glucosidase (Family-31) from Ruminococcus. FASEB J. 2010;24:3939-3949.; Bar-on et al., 2002 Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Kinetic and structural studies on the interaction of cholinesterase with the anti-Alzheimer drug rivastigmine. Biochemistry 2002;41(11):3555-64.). All water molecules were released from the receptor proteins and 3D protonation was carried out by using Protonate 3D Option (Tan et al., 2010; Bar-on et al., 2002). Protein molecules were energy minimized by using the default parameters of MOE 2009-10 energy minimization algorithm (gradient: 0.05, Force Field: MMFF94X). By using default parameters of MOE-Dock Program, all compounds were docked into binding pockets of the above proteins. The re-docking procedure was also used to increase the validity of docking protocol (Boström, Greenwood, Gottfries, 2003).
Statistical analysis
All the experiments were carried out in triplicate. Statistical analysis was performed using Microsoft Excel 2010 and the results are provided as mean ± SEM.
RESULTS AND DISCUSSION
Chemistry
The designed 2-{2,3-dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(un/substituted-phenyl)acetamides (7a-l) were synthesized according to scheme 1 and Table I. The procedures and conditions of the reactions are discussed in the experimental section. For the synthesis of the target derivatives, the first step of the reaction was carried between N-2,3-dihydrobenzo[1,4]-dioxin-6-amine (1) and 4-methylbenzenesulfonyl chloride (2) in aqueous alkaline medium. The reaction mixture was stirred for 4-5 hours at room temperature yielding the parent N-(2,3-dihydrobenzo[1,4]-dioxin-6-yl)-4-methylbenzenesulfonamide (3). The parent compound was obtained as a light brown amorphous powder in good yield by the acidification of the reaction mixture with concentrated HCl to adjust pH 2-3. In a parallel reaction, different amines (4a-l, Table I) were reacted, one by one, with bromoacetyl bromide (5) to obtain respective electrophiles, 2-bromo-N-(un/substituted-phenyl)acetamides (6a-l). In the final step, parent, 3, was coupled with different electrophiles (6a-l), in polar aprotic solvent, i.e. DMF using LiH as a base to afford the targeted N-2-{2,3-dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(un/substituted-phenyl)acetamides (7a-l). The structures of these molecules were deduced by their IR and 1H-NMR spectral data, and CHN analysis also supported the assignment.
Outline for the synthesis of 2-{2,3-dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(un/substituted-phenyl)acetamides (7a-l). Reagents & Conditions: (1) 2,3-dihydrobenzo[1,4]dioxin-6-amine (1)/aq. Na2CO3 soln./pH 9-10/4-methylbenzenesulfonyl chloride (2)/stirring at RT for 3-4 hours. (2) Un/substituted anilines (4a-l)/aq. Na2CO3 soln./pH 9-10/stirring/bromoacetyl bromide (5)/vigorous manual shaking. (3) N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-methylbenzenesulfonamide(3)/DMF/LiH/stirring for half an hour/addition of un/substituted electrophiles, 4a-l, followed by stirring for 4-5 hours at room temperature.
The structural analysis of one of the compounds is discussed hereby in detail as a representative molecule. The molecule, 7f, was obtained as an off-white amorphous powder with 88% yield, having a melting point of 134-135 oC. The molecular formula (C24H24O5N2S) of this compound was predicted by counting the number of protons in its 1H-NMR spectrum, and it was also supported by its CHN analysis data. Various functional groups in this molecule were determined by its IR data. The absorption band at 3258 cm-1 was peculiar for N-H stretching (amide). The other bands were observed at 3047 (C-H stretching of aromatic ring), 2936 (-CH2 stretching), 1711 (C=O stretching), 1643 (C=C stretching of aromatic ring), and 1385 (-SO2 stretching). In its 1H NMR spectrum, a singlet in highly deshielded region at d 8.29 accounted for an acetamidic proton (-NHCO). The 4-methylbenzenesulfonyl moiety in this molecule was characterized by an A2B2 spin system in the aromatic region, represented by two ortho-coupled doublets at d 7.51 (2H, H-2’ & H-6’) and d 7.36 (2H, H-3’ & H-5’) along with a methyl singlet in the aliphatic region at d 2.43. Similarly, another A2B2 spin system was observed for the aromatic protons of 4-methylphenyl moiety substituted on nitrogen atom. This moiety was characterized by two ortho-coupled doublets at d 7.28 (2H, H-2’’’ & H-6’’’), and d 7.11 (2H, H-3’’’ & H-5’’’) along with a methyl signal at d 2.29 as singlet. An AMX spin system for a 6-amino-benzodioxane moiety in the molecule, was corroborated by an ortho-coupled doublet at δ 6.75 (1H, H-8, J= 8.5 Hz), a meta-coupled doublet at d 6.66 (1H, H-5, J= 2.5 Hz), and a reciprocal doublet of doublet at d 6.53 (1H, H-7, J= 2.5, 8.5 Hz). A multiplet at d 4.22-4.19 with integration of four protons was deduced for two symmetrical methylene groups (CH2-2 & CH2-3) in 1,4-benzodioxane moiety. A very close lying broad singlet at d 4.18 was assignable to a methylene group (2H, CH2-2’’) of an acetamido unit attached by its carbon atom with the sulfonamidic nitrogen atom in the molecule. Thus, on the basis of the above cumulative spectral evidence, the structure of 7f was designated 2-{2,3-dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(4-methylphenyl)acetamide. Similarly, the structures of all other synthetic derivatives were characterized, and their spectral data is given in experimental section.
Enzyme inhibition, molecular docking (in silico) and SAR study
In search for new suitable therapeutic agents for the control of type 2 Diabetes mellitus and for the treatment of Alzheimer’s disease, these synthesized molecules, 7a-l, were screened against a-glucosidase and AChE, respectively. In general, these compounds exhibited moderate to high inhibitory potential against a-glucosidase and weak inhibition against AChE, which was evident from there IC50 values as compared to the standard acarbose and serine, respectively (Table II).
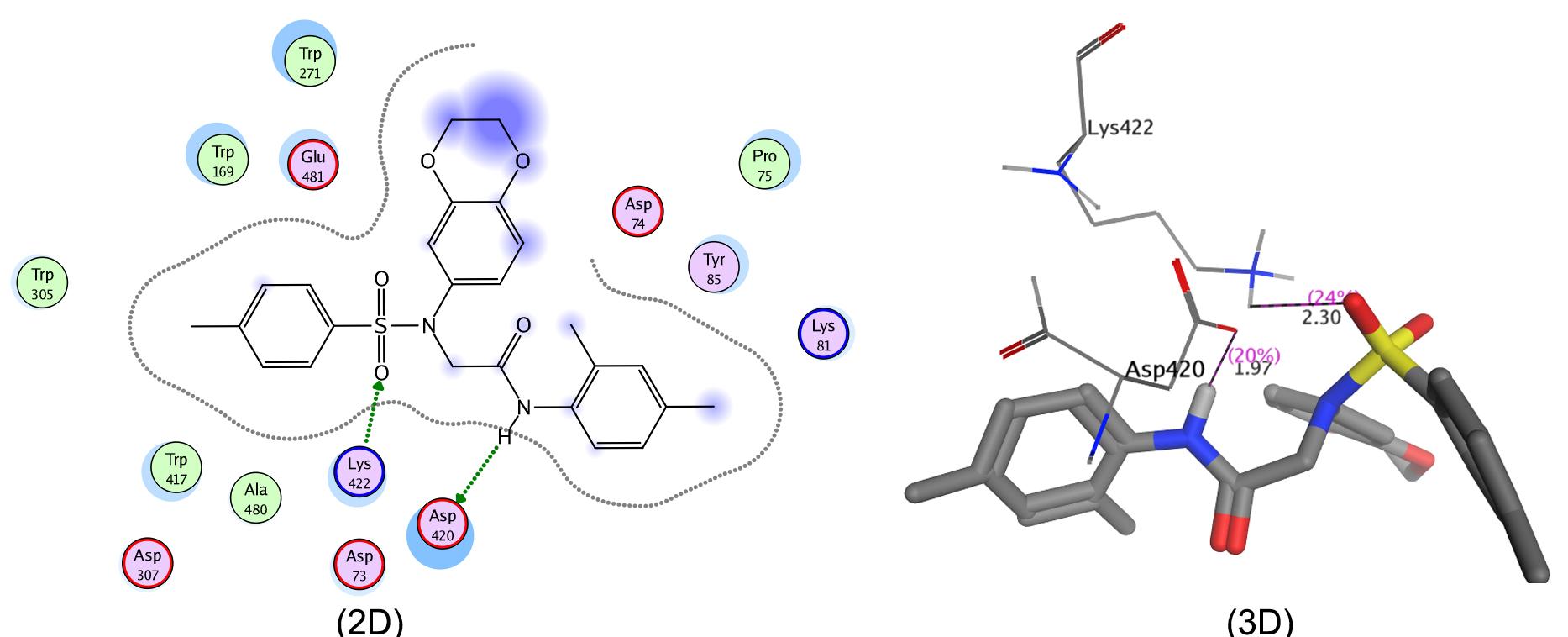
Compound 7h showed potent inhibition against a-glucosidase with an IC50 of 34.21 ± 0.12 µM, which was better than acarbose, which had an IC50 of 37.38 ± 0.12 µM. The enhanced inhibitory potential of this molecule against this enzyme might be attributed to the incorporation of a 2,4-dimethylphenyl moiety in this molecule. Similarly, molecules 7g and 7k, with 2,3-dimethylphenyl and 3,4-dimethylphenyl moieties, respectively, also showed excellent activity. The IC50 values of these molecules were 47.23 ± 0.14 μM and 52.45 ± 0.14 μM, respectively. All molecules showed weak inhibitory profiles against AChE. Molecule 7g exhibited an IC50 of 213.47 ± 0.14 μM which was the lowest among the studied molecules. Its possibly weak inhibitory potential may be due to the amalgamation of 2,3-dimethylphenyl in the core sulfonamide bearing a benzodioxane entity.
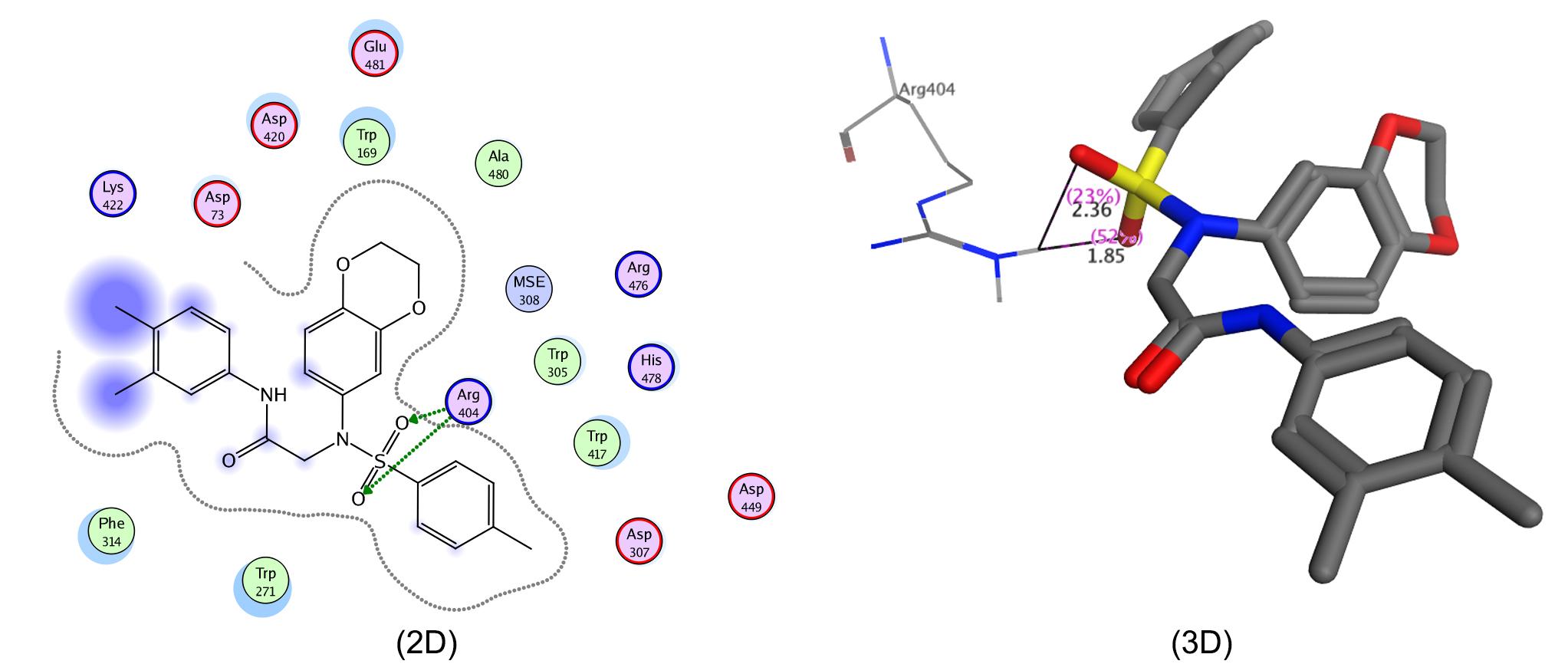
The in silico molecular docking data of these molecules was also in agreement with their in vitro enzyme inhibition data. Molecular docking was performed with selected residues of the active pockets of the enzymes. It is shown (Figure 1; 2D & 3D) that compound 7h was deeply bound in the binding pocket of α-glucosidase by making two strong interactions. Asp420 created polar interaction with the amino proton of the ligand giving a bond length of 1.97 Å while Lys422 made an acidic interaction with sulfonyl oxygen showing a bond distance of 2.30 Å. Similarly, molecule 7g also made four interactions. Arg440 showed a couple of strong acidic interactions with both the sulfonyl oxygen with a bond length of 2.02 and 2.69 Å. Lys422 also made two arene-cation interactions with bond distances of 3.25 and 4.31 Å as shown in 2-D and 3-D (Figure 2). Likewise, 7k showed two acidic interactions between Arg404 and both with the sulfonyl oxygen with bond distances of 1.85Å and 2.36Å (Figure 3; 2D & 3D).
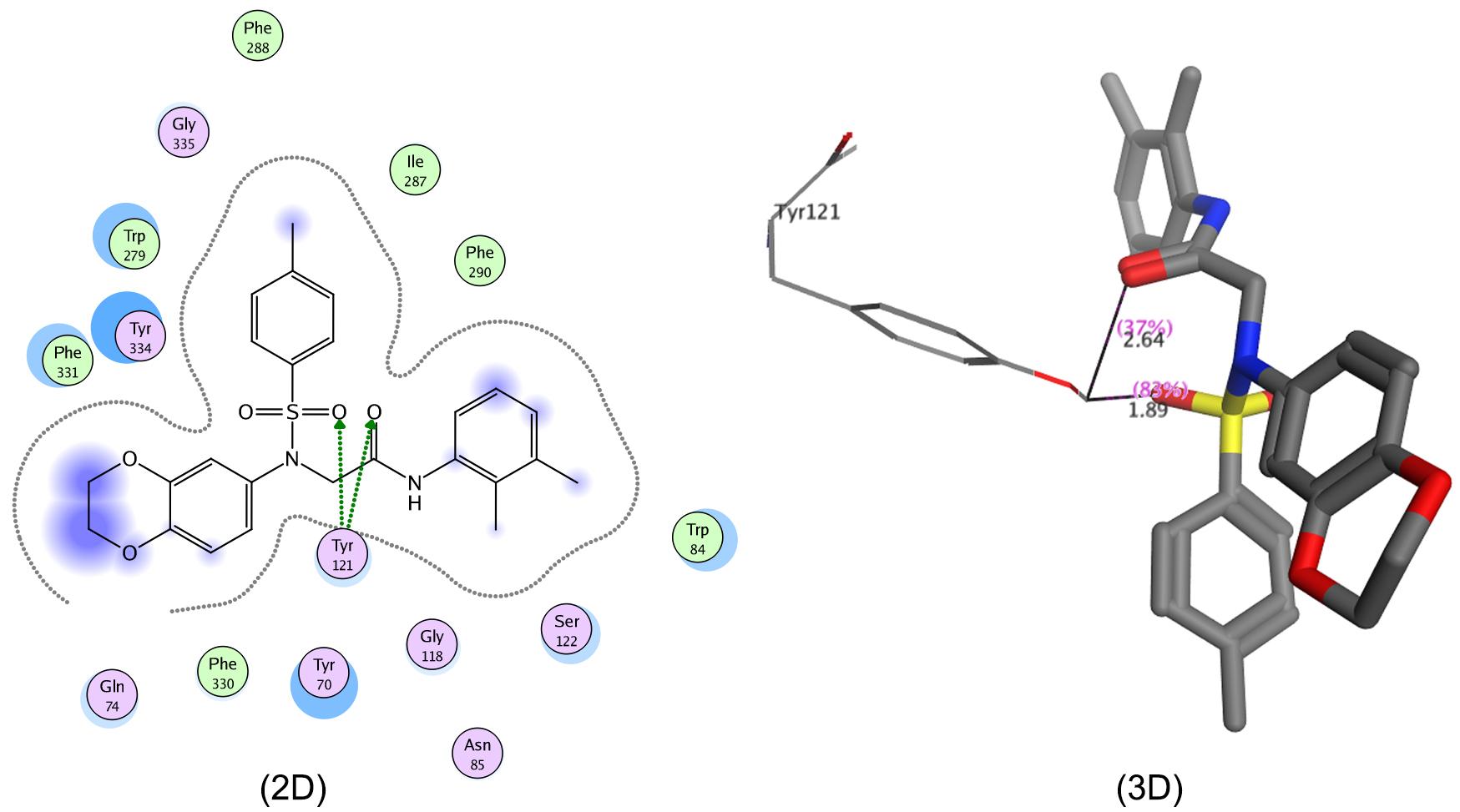
The molecular docking of 7g into the active pocket of AChE indicated two polar interactions, i.e., one between Tyr121 and sulfonyl oxygen (bond length: 1.89Å) while the second with carbonyl oxygen (bond length: 2.64Å) as depicted (Figure 4; 2D & 3D). The strength of the bond was clear from the bond distances of ligand and amino acid residues of protein, i.e., the shorter the bond distance, the stronger the interaction, and vice versa.
CONCLUSIONS
The targeted 2-{2,3-dihydro-1,4-benzodioxin-6-yl[(4-methylphenyl)sulfonyl]amino}-N-(un/substituted-phenyl)acetamides (7a-l) were synthesized in good yields. Some of the molecules exhibited promising inhibition against α-glucosidase which was also supported by molecular docking studies. So, it was concluded that some of the molecules might be considered as suitable therapeutic agents for type 2 diabetes.
Acknowledgements
The authors are very thankful to Higher Education Commission (HEC) of Pakistan for providing a financial grant for this study. Dr. A. Leyva provided English editing of the manuscript.
References
- Abbasi, MA, Islam M, Aziz-ur-Rehman, Rassol S, Rubab K, Hussain G, et. al. Synthesis, characterization, antibacterial, a-glucosidase inhibition and hemolytic studies on some new N-(2,3-dimethylphenyl)benzenesulfonamide derivatives. Trop J Pharm Res. 2016;15(3):591-598.
- Abbasi MA, Saeed A, Aziz-ur-Rehman, Khan KM, Ashraf M, Ejaz SA. Synthesis of brominated 2-phenitidine derivatives as valuable inhibitors of cholinesterases for the treatment of Alzheimer's disease. Iran J Pharm Res. 2014a;13(1):87-94.
- Abbasi MA, Raza N, Aziz-ur-Rehman, Rasool S, Khan KM, Ashraf M, et al. In vitro enzyme inhibition studies on new sulfonamide derivatives of 4-tosyl chloride. World J Pharm Sci. 2014b;2(2):161-169.
- Abouzid S, Ahmad O. Structure-activity relationship and biosynthesis. Stud Nat Prod Chem. 2013;40:469-484.
- Ahmad B, Khan SA, Alam T. Synthesis and antihepatotoxic activity of some heterocyclic compounds containing the 1,4-dioxane ring system. Pharmazie. 2003;58(3):173-176.
- Ajeet, Kumar A. Designing, proposed synthesis and docking analysis of novel sulfonamide derivatives as antibacterial agents. Am J Pharmacol Sci. 2014;2(2):37-41.
- Alsughayer A, Elassar AZA, Mustafa S, Sagheer FA. Synthesis, structure analysis and antibacterial activity of new potent sulfonamide derivatives. J Biomater Nanobiotech. 2011;2:144-149.
- Bhagwat AM, Bhat AR, Palled MS, Khade AP, Patil AM. Synthesis and antihypertensive screening of novel substituted 1,2-pyrazoline sulfonamide derivatives. Am J PharmTech Res. 2014;4(2):326-336.
- Bostrom J, Greenwood JR, Gottfries J. Assessing the performance of omega with respect to retrieving bioactive conformations. J Mol Graph Model. 2003;21(5):449-462.
- Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Kinetic and structural studies on the interaction of cholinesterase with the anti-Alzheimer drug rivastigmine. Biochemistry 2002;41(11):3555-64.
- Chapdelaine P, Trembley RR, Dube JY. p-Nitrophenol-alpha-D-glucopyranoside as substrate for measurement of maltase activity in human semen. Clin Chem. 1978;24(2):208-211.
- Chapleo CB, Myers PL, Butler CM, Doxey JC, Roach AG, Smith CFC. Synthesis of some 1,4-benzidioxans as selective presynaptic alpha-2-adrenoreceptor antagonists and potential antidepressants. J Med Chem. 1983;26:823-831.
- Chiba S. Molecular mechanism in alpha-glucosidase and glucoamylase. Biosci Biotechnol Biochem. 1997;61(8):1233-1239.
- Cambridge Soft. Chemdraw Ultra. Cambridge Soft Corporation, Cambridge, Massachusetts, USA; 2001.
- Ellman FL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88-95.
- Gauthier S. Cholinergic adverse effects of cholinesterase inhibitors in Alzheimer's disease. Drug Aging. 2001;18(11):853-862.
- Gazak R, Walterova D, Kren V. Silybin and silymarin - new and emerging applications in medicine. Curr Med Chem. 2007;14(3):315-338.
- Ghorab MM, Alsaid MS, Al-Dosari MS, El-Gazzar MG, Parvez MK. Design, synthesis and anticancer evaluation of novel quinazoline-sulfonamide hybrids. Mol Divers Preserv Int. 2016;21(2):189-193.
- Huang Z, Lin Z, Huang J. A novel kind of antitumour drugs using sulfonamide as parent compound. Eur J Med Chem. 2001;36(11-12):863-872.
- Irshad M, Abbasi MA, Aziz-ur-Rehman, Siddique SZ, Ashraf M, Ejaz SA, et al. Synthesis, characterization and biological screening of some new sulfonamides derivatives of 1,4-benzodioxane-6-amine. J Chem Soc Pak. 2014;36(4):660-673.
- Kasimogullarik R, Duran H, Yaglioglu AS, Mert S, Demirtas I. Design, synthesis, characterization and antiproliferative activity of novel pyrazole-3-carboxylic acid derivatives. Monatsh Chem. 2015;146(10):1743-1749.
- Kren V, Walterova D. Silybin and Silymarin-new effects and applications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149(1):29-41.
- Kaspady M, Narayanaswamy VK, Raju M, Rao GK. Synthesis, antibacterial activity of 2,4-disubstitutedd oxazoles ans thiazoles and bioisosteres. Lett Drug Discov. 2009;6(1):21-28.
- Lebovitz HE. Alpha-glucosidase inhibitors. Endocrinol Metab Clin North Am. 1997;26(3):539-551.
- Mahdi MF, Al-Smaism RF, Al-Khaliq ZMA. Synthesis, characterization and antibacterial activity of new series of sulfamethoxazole derivatives. World J Pharm Pharmaceut Sci. 2015;4(10):284-293.
- Molecular Operating Environment (MOE) 2010.10, C.C.G.I.M., Quebec, Canada. 2010.
- Pelter A, Hansel R. The structure of silybin (Silybum substance E6), the first flavonolignan. Tetrahedron Lett. 1968;9(25):2911-2916.
- Rathod CP, Dhawle SC, Pekamwar SS, Kadam NR, Rekhawar MU. Synthesis, characterization, antimicrobial and antifungal screening of some novel benzene sulfonamide derivatives. Int J Pharm Res School. 2012;1(4):1-4.
- Reddy NS, Rao AS, Chari MA, Kumar VR, Jyothy V, Himabindu V. Synthesis and antibacterial activity of sulfonamide derivatives at C-8 alkyl chain of anacardic acid mixture isolated from a natural product cashew nut shell liquid (CNSL). J Chem Sci. 2012;124(3):723-730.
- Tougu V. Acetylcholinesterase: Mechanism of catalysis and inhibition. Curr Med Chem. 2001;1(2):155-170.
- Tan K, Tesar C, Wilton R, Keighr L, Babingg G, Jaochimiak A. The crystal structure of the a-Glucosidase (Family-31) from Ruminococcus. FASEB J. 2010;24:3939-3949.
- Vazquez MT, Rosell G, Pujol MD. Synthesis and anti-inflammatory activity of rac-2-(2,3-dihydro-1,4-benzodioxin) propionic acid and its R and S enantiomers. Eur J Med Chem. 1997;32(6):529-534.
Publication Dates
-
Publication in this collection
15 Aug 2019 -
Date of issue
2019
History
-
Received
20 Jan 2017 -
Accepted
20 Mar 2018