Abstract
The subfamily Huperzioideae of the Lycopodiaceae includes 41 species in Brazil. The genus Huperzia is represented by a single species, and Phlegmariurus has 40 species. They occur in most habitat types with a humid climate, from tropical lowland forest, to montane forest, and campo vegetation in the highlands. There are 25 endemics, more than half of the species. The present treatment includes nomenclature, descriptions and illustrations of all species, and a key for their identification. Reference collections are cited and summarize the distribution of the species and document their identity. Short discussions deal with problems of species delimitation and compare closely related species.
Key words:
diversity; floristics; lycophytes; phytogeography; taxonomy
Resumo
A subfamilia Huperzioideae de Lycopodiaceae apresenta 41 espécies no Brasil. O gênero Huperzia é representado por uma única species e Phlegmariurus por 40. Estas ocorrem na maioria dos habitats com climas úmidos, desde florestas tropicais de planície a florestas montanas e vegetação de campos de altitude. Há 25 endêmicas, mais da metade do total de espécies. O presente trabalho inclui nomenclatura, descrições e ilustrações de espécies e uma chave para identificação. Coleções de referência são citadas, sumarizando a distribuição e documentando a identidade das espécies. Breves discussões tratam de problemas na delimitação de espécies e comparações com espécies próximas.
Palavras-chave:
diversidade; florística; licófitas; fitogeografia; taxonomia
Introduction
This is the third and final part of a series of treatments of the Brazilian Lycopodiaceae. It deals with the genera Huperzia Bernh. and Phlegmariurus Holub, including a total of 41 species. The first part (Øllgaard & Windisch 2014Øllgaard B (2014) Six new species and some nomenclatural changes in neotropical Lycopodiaceae. Nordic Journal of Botany 33: 186-196.) treated the genera Austrolycopodium Holub, Diphasiastrum Holub, Diphasium C.Presl ex Rothm., and Lycopodium L. (subfamily Lycopodioideae according to Wagner & Beitel 1992Wagner WHJr & Beitel JM (1992) Generic classification of modern North American Lycopodiaceae. Annals Missouri Botanical Garden 79: 676-686.), including 5 species, and gave a general introduction to the family, the history of its study and exploration in Brazil, and keys to the genera of the family. The second part (Øllgaard & Windisch 2016Øllgaard B & Windisch PG (2016) Lycopodiaceae in Brazil. Conspectus of the family. II. The genera Lycopodiella s.str., Palhinhaea, and Pseudolycopodiella. Rodriguésia 67: 691-719.) treated the genera Lycopodiella Holub, Palhinhaea Carv. Vasc. & Franco and Pseudolycopodiella Holub (subfamily Lycopodielloideae of Wagner & Beitel exØllgaard 2014Øllgaard B & Windisch PG (2014) Lycopodiaceae in Brazil. Conspectus of the family. I. The genera Lycopodium, Austrolycopodium, Diphasium and Diphasiastrum. Rodriguésia 65: 261-277.), including 17 species. The present part deals with the subfamily Huperzioideae of Wagner & Beitel (1992)Wagner WHJr & Beitel JM (1992) Generic classification of modern North American Lycopodiaceae. Annals Missouri Botanical Garden 79: 676-686. validated by Øllgaard (2014)Øllgaard B & Windisch PG (2014) Lycopodiaceae in Brazil. Conspectus of the family. I. The genera Lycopodium, Austrolycopodium, Diphasium and Diphasiastrum. Rodriguésia 65: 261-277., by far the largest species group in Brazil.
Nessel (1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535., 1955)Nessel H (1955) Lycopodiaceae. In: Hoehne FC (ed.) Flora Brasílica II: 1-131. included several extralimital species. They are listed and excluded in Øllgaard & Windisch (1987)Øllgaard B (1987) A revised classification of the Lycopodiaceae sensu lato. Opera Botanica 92: 153-178. and are not further treated here.
Material and Methods
The methodology used in this study is the same adopted by the first and second part of this treatment (Øllgaard & Windisch 2014Øllgaard B (2014) Six new species and some nomenclatural changes in neotropical Lycopodiaceae. Nordic Journal of Botany 33: 186-196., 2016Øllgaard B & Windisch PG (2016) Lycopodiaceae in Brazil. Conspectus of the family. II. The genera Lycopodiella s.str., Palhinhaea, and Pseudolycopodiella. Rodriguésia 67: 691-719.). A list of studied collections is included in the descriptions of each species, but in cases of great numbers of collections only a number of reference collections are mentioned to indicate the general distribution. Where only reference collections are given, complete listings are available from the first author.
The cited specimens serve both for identification purposes, and to document general distribution. However, several collections assigned to Glaziou apparently are far out of the range of the species in question, and may serve only for identification.
Where useful published illustrations are available they are cited.
Results
Taxonomic treatment
These two genera can be distinguished from the remaining genera of the Lycopodiaceae in Brazil by the characters presented in the following key:
- Key to the Brazilian genera of the Huperzioideae
-
1. Stems isotomously branched throughout, without elongate, indeterminate main stems, but sometimes heteroblastic, roots usually forming one basal tuft, sporophylls and vegetative leaves alike, or the sporophylls, if smaller, persisting and green, not subpeltate and ephemeral; spores foveolate-fossulate (Huperzioideae).
-
2. Plants terrestrial, usually with gemmiferous (=bulbiferous) lateral branchlets, spores concave between laesurae, with truncated corners and foveolate proximal faces; plants terrestrial, ascending to erect....................... Huperzia
-
2’. Plants terrestrial or epiphytic, lacking gemmiferous branchlets, spores plane between laesurae, with evenly angular corners, proximal surfaces usually plain; plants terrestrial or epiphytic, erect or pendulous. Phlegmariurus
-
-
1’.... Stems anisotomously branched throughout, not gemmiferous, the branches differentiated into elongate, indeterminate, rhizomatous, or creeping, trailing, or climbing main stems, and usually determinate branchlet systems; sporophylls strongly modified, ephemeral, unlike vegetative leaves, peltate or subpeltate, aggregated in compact, terminal strobili. (Lycopodioideae: Austrolycopodium, Diphasiastrum, Diphasium, and Lycopodium s. str.) and (Lycopodielloideae: Lycopodiella s. str., Palhinhaea, and Pseudolycopodiella) (Øllgaard & Windisch 2014Øllgaard B (2014) Six new species and some nomenclatural changes in neotropical Lycopodiaceae. Nordic Journal of Botany 33: 186-196., 2016).
Huperzia Bernh., J. Bot. (Schrader) 1800(2): 126. 1801. - Type: Huperzia selago (L.) Schrank & Mart. (= Lycopodium selago L.).
PlananthusMirbel, in Lamarck & Mirbel, Hist. Nat. Veg. 3: 476. 1802Mirbel CFB (1802) In: Lamarck JBPA, Monet Chevalier & Mirbel CFB (eds.). 1802. Histoire naturelle des vegétaux, classés par familles 3: i-iii, 1-588. . Type: Plananthus selago (L.) P.Beauv. (= Lycopodium selago L.). - Lycopodium subgen. SelagoBaker, Handb. Fern-Allies 8. 1887Baker JG (1887) Handbook of the fern-allies. George Bell & Sons, London. 159p.. - Type: Lycopodium selago L. - Lycopodium subgen. Urostachya Pritzel, Nat. Pflanzenfam. 1 (4): 592. 1901. - Type: Lycopodium selago L. - Urostachys (Pritzel) Herter, Beih. Bot. Centralbl. 39: 249. 1922Herter W (1922) Itinera Herteriana III. Heteropteridophyta austroamericana. (Equisetales Lycopodiales Selaginellales Isoëtales austroamericanae.) Beihefte Botanisches Centralblatt 39: 248-256.. - Type: Urostachys selago (L.) Herter (= Lycopodium selago L.).
Terrestrial, erect or ascending, homophyllous or irregularly seasonally heterophyllous, gemmiferous plants; spores triangular with truncate corners and concave sides between laesura.
Temperate, arctic and alpine regions of the Northern Hemisphere, montane and alpine regions in the Paleotropics, scattered in temperate regions of the southern hemisphere. Huperzia Bernh. (mainly Southeast Asia) has few Neotropical records (Mexico, Greater Antilles, Southern Brazil). Speciation in the group seems associated with a high frequency of hybridization (Beitel 1979Beitel JM (1979) Clubmosses (Lycopodium) in North America. Fiddlehead Forum 6: 1-8., 1984Beitel JM (1984) The Appalachian Firmoss, a new species in the Huperzia selago (Lycopodiaceae) complex in eastern North America. American Journal of Botany 71: 140.; Wagner et al. 1985Wagner WH, Wagner FS & Beitel JM (1985) Evidence for interspecific hybridisation in pteridophytes with subterranean mycoparasitic gametophytes. Proceedings of the Royal Society of Edinburgh 86B: 273-281.).
The formation of gemmae is restricted to this group. The gemmae are interpreted as easily detachable shoot tips of highly anisotomous branchlets, and thus represent a specialized feature in the Lycopodiaceae. Their structure was described by Stevenson (1976)Stevenson DW (1976) Observations on phyllotaxis, stelar morphology, the shoot apex and gemmae of Lycopodium lucidulum Michaux (Lycopodiaceae). Botanical Journal of the Linnean Society 72: 81-100., who also reviewed the earlier gemma studies. Gemma formation is associated with a distinct spore type (the Selago type of Wilce 1972Wilce JH (1972) Lycopod Spores, I. General Spore Patterns and the Generic Segregates of Lycopodium. American Fern Journal 62: 65-79.), in which the spores are distinctly triangular with truncate corners in polar view and have more or less concave, usually deeply pitted proximal faces. This is in contrast to the suborbicular polar view, with evenly rounded or angular corners, and flat, almost smooth proximal faces in Phlegmariurus Holub. The two spore types are easily distinguished in the light microscope (Øllgaard & Windisch 2014Øllgaard B (2014) Six new species and some nomenclatural changes in neotropical Lycopodiaceae. Nordic Journal of Botany 33: 186-196.).
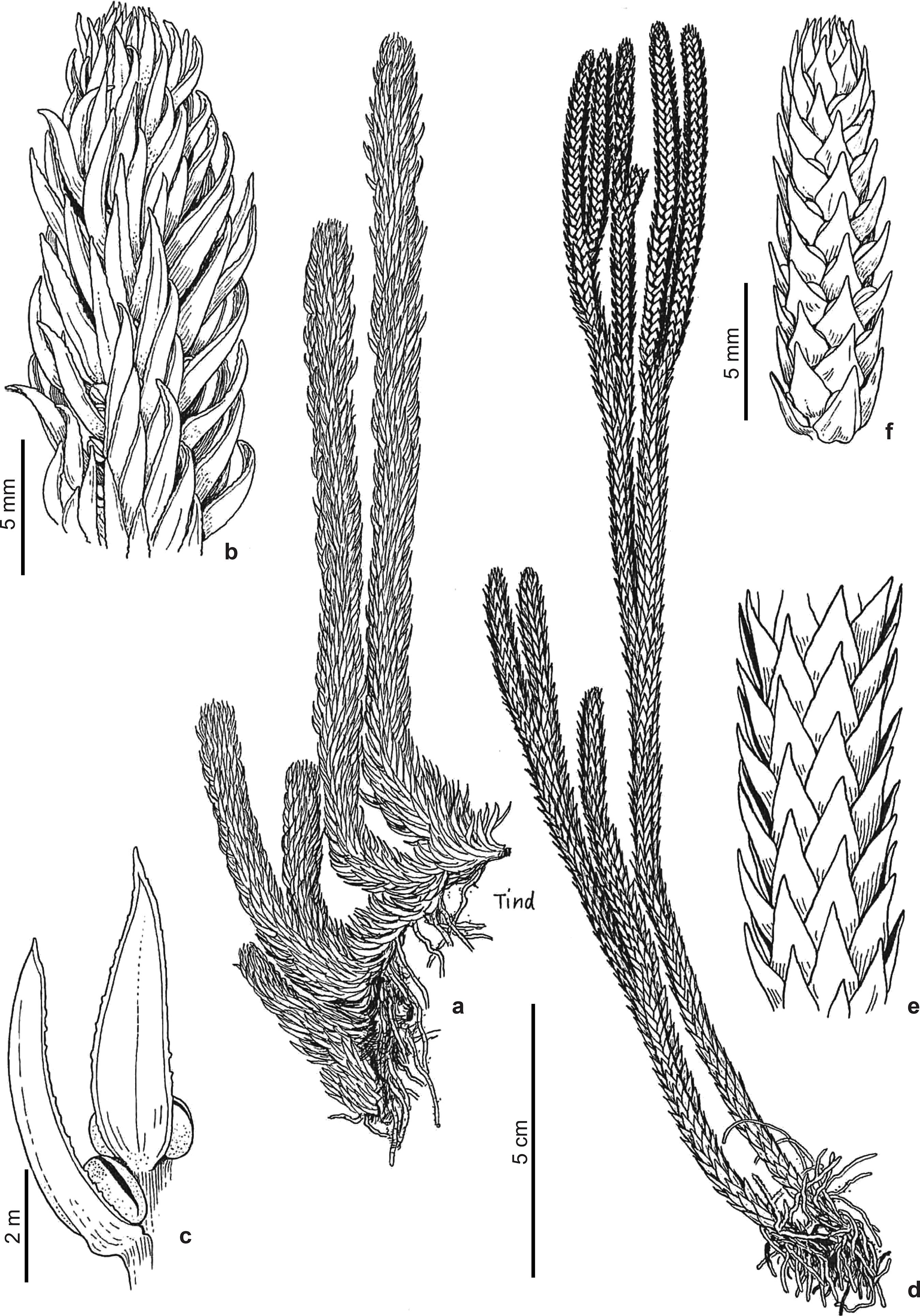
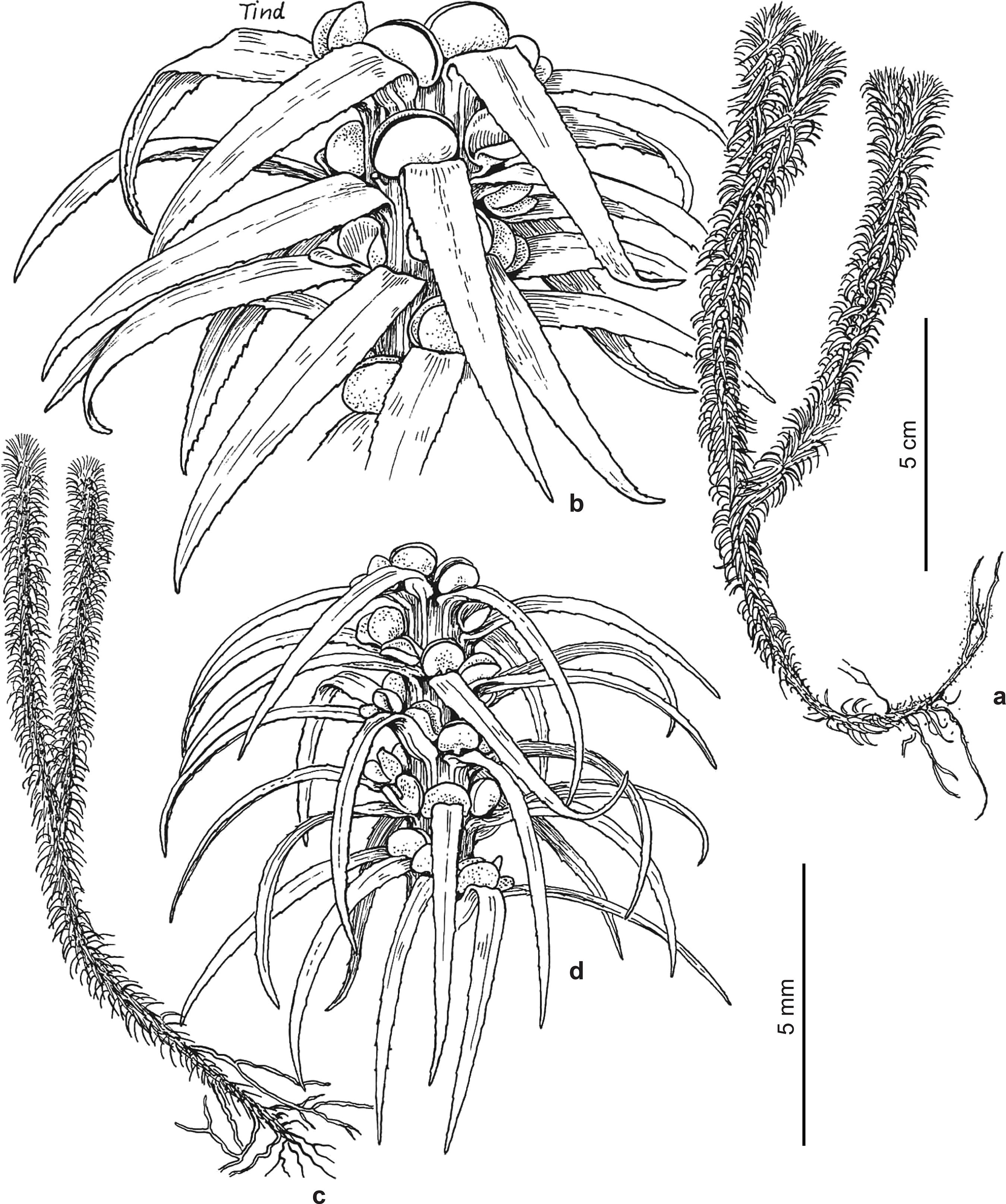
Huperzia catharinae (Christ) Holub, Folia Geobot. Phytotax. 20: 71. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. Fig. 1a-d
a-d. Huperzia catharinae - a. growth habit; b. vegetative leaf; c. distal division with sporangiate leaves and gemmiphores; d. gemmiphore. (Brazil: Santa Catarina, bords de la Serra do Oratorio, Ule 2313 (HBG)).
Lycopodium catharinaeChrist, in Schwacke, Pl. Nov. Mineiras 2: 39. 1900Christ H (1900) Spicilegium Pteridologicum Austro-Brasilense. In: Schwacke W (ed.) Citade de Minas (Ouro Preto), Minas Geraes. Plantas Novas Mineiras 2: 12-42, t. 1-4.. - Urostachys catharinae (Christ) Nessel, Arch. Bot. Est. S. Paulo 1: 387. 1927. - Type: Santa Catarina, bords de la Serra do Oratorio, Feb. 1890, Ule no. 313 (holotype herb. Christ, P). - Ule 2313 (HBG) with the same collection data as the type, and Ule 313 (P) with the same date, and the locality: Am Rande der Serra Geral [of which Serra do Oratorio is a part], - are isotypes. There are several similar examples of renumbered sets of Ule duplicates, in which 2000 was added to the original number.
Plants terrestrial, ascending to erect from a decumbent base, to ca. 15 cm tall or to 20 cm long, sparsely branched, to 4 times dichotomous. Shoots unequally thick, with repeated constrictions along stems due to periodic variation in leaf length and gemmiphore development, 10-27 mm in diam. incl. leaves, sporangiate in separate, seasonally produced zones, from ca. 10-15 cm above the stem base. Stems excl. leaves 1.5-2 mm thick at the base, sometimes tapering to ca. 1-1.5 mm upward, pale greenish white. Leaves borne in more or less regular, often oblique, alternating whorls of 4, these 1-2 mm apart, forming 8 indistinct longitudinal ranks, perpendicular to reflexed, straight, linear to oblanceolate, with a long, narrow, petiole-like base, 7-14 × 1-1.5 mm, flat, with slightly revolute margins, with slightly prominent vein above and below, with almost smooth to serrate or shallowly erose-dentate margins. Sporangiate and vegetative leaves alike. Leaves adjacent to gemmiphores much reduced. Gemmiphores with easily detached gemmae borne seemingly in the place of leaves at shoot constrictions. Sporangia 1.5-2 mm wide.
Endemic. Known only from the type collection in the state of Santa Catarina.
A closer comparison of Huperzia catharinae and Asiatic material of the group of Huperzia serrata (Thunb. ex Murray) Trevisan (type from Japan) may show that they are, indeed, closely related. The Brazilian material differs from other Neotropical material we have seen referred to H. serrata, and most of the Asiatic material we have seen, by the narrower and less coarsely serrate leaves. Because of these differences, H. catharinae is maintained.
PhlegmariurusHolub, Preslia 36: 21. 1964. - Type: Lycopodium phlegmaria L. (=Phlegmariurus phlegmaria (L.) T.Sen & U.Sen.
HuperziaBernhardi, J. Bot. (Schrader) 1800Bernhardi DII (1801) Tentamen alterum filices in genera redigendi. Journal für die Botanik (Schrader) 1800: 121-136, t. 1-2.(2): 126. 1801, pro parte.
Plants epiphytic or terrestrial, pendulous, recurved, erect, or ascending, isotomously branched throughout, or sometimes sprouting from the rooting base of the plant. Roots arising from the stem stele, descending through the cortex to the stem base, here emerging as one basal tuft. Shoots homophyllous or gradually to abruptly heterophyllous, the constriction of distal divisions of heterophyllous species associated or not with presence of sporangia. Sporophylls and vegetative leaves alike or sporophylls shorter, not peltate, persisting and green after sporangium dehiscence. Sporangia axillary, reniform, isovalvate, with a short slender stalk; side and inner walls of sporangium epidermis cells sinuate, thickened and lignified. Spores foveolate or fossulate. Gametophytes usually subterranean or deep in epiphytic substrate, mycorrhizal, cylindrical with radial or bilateral symmetry, with pluricellular, uniseriate hairs among the gametangia.
Distribution: Perhaps 300 species worldwide, 40 in Brazil of which 24 are endemic.
Phlegmariurus Holub is pantropical and with few temperate species. Species diversity is highest throughout the tropics in evergreen montane forests, and in the wet Andean grass and shrublands in South America.
The genus Phlegmariurus Holub until fairly recently was generally included in the genus Huperzia Bernh.. However, Phlegmariurus Holub is distinct from Huperzia Bernh. with regard to spore type and the lack of gemmae. The species of Huperzia Bernh. are entirely terrestrial whereas the majority of Phlegmariurus Holub species are epiphytic. There are no known intergeneric hybrids. Whereas Huperzia Bernh. appears to be entirely terrestrial, the terrestrial species in Phlegmariurus Holub have been derived from epiphytic elements in the genus according to Wikström et al. (1999)Wikström N, Kenrick P & Chase M (1999) Epiphytism and terrestrialization in tropical Huperzia (Lycopodiaceae). Plant Systematics and Evolution 218: 221-243. . There is phylogenetic evidence supporting the two genera as separate lineages, e.g., Field et al. (2016)Field AR, Testo W, Bostock PD & Waycott M (2016) Molecular phylogenetics and the morphology of the Lycopodiaceae subfamily Huperzioideae supports three genera: Huperzia, Phlegmariurus and Phylloglossum. Molecular Phylogenetics and Evolution 94: 635-657..
In Phlegmariurus Holub the sporangia are situated in the axils of sporophylls that may be similar to the vegetative leaves, or reduced in size and occupy major parts of constricted distal divisions in pendulous epiphytes. These are commonly referred to as strobili, but we prefer to restrict the term strobilus to the homologous structures in Lycopodioideae and Lycopodielloideae. In these subfamilies, the sporophylls occupy the strobili entirely, are ephemeral, and wither during or just after spore release. In contrast, in Phlegmariurus Holub, the sporophylls commonly occupy only part of the constricted divisions, and zones with sporophylls may alternate seasonally with vegetative leaves of the same aspect, and the sporophylls remain green indefinitely after spore release. We prefer the term sporangiate division or sporangiate branch for these structures.
- Key to the species of Phlegmariurus Holub in Brazil
-
1. Plants erect, or ascending to erect, terrestrial or epiphytic; shoot apices erect. Leaf margins entire, denticulate or ciliolate.
-
2. Plants heteroblastic, with creeping and often rooting horizontal to prostrate-ascending, basal, rejuvenating shoots, these producing erect, simple or sparsely branched aerial branches (Fig. 4a).............................................................. Phlegmariurus badinianus
Figure 2
a-c. Phlegmariurus acerosus - a. growth habit; b. leaves of proximal division; c. distal sporangiate division. d-f. Phlegmariurus comans - d. growth habit; e. leaves of proximal division; f. distal sporangiate division. (a-c. Ecuador, Quinindé, Holdridge 1652 (US); d-f. Brazil, Serra do Itatiaia, Ule 3537 (HBG)).
Figure 3
Phlegmariurus aqualupianus - growth habit. (Brazil, Minas Gerais: Santa Maria do Salto, Distrito de Talismã, Faz. Duas Barras, 800 m, A. Salino et al. 9192 (BHCB))
Figure 4
a-c. Phlegmariurus badinianus - a. growth habit; b. apex of erect branch; c. two sporangiate leaves. d-f. Phlegmariurus itambensis - d. growth habit; e. part of proximal division; f. sporangiate shoot apex. (a-c. Brazil, Minas Gerais, Serra do Caparaó, Windisch et al. 4971 (AAU); d-f. Brazil, Minas Gerais Serra do Espinhaço, Summit of Pico do Itambé, Anderson et al. 35783 (NY)).
-
2’. Plants homoblastic, shoots not differentiated into creeping horizontal and erect aerial shoots.
-
3...... Leaf margins denticulate (at least of some leaves, sometimes minutely and remotely denticulate) by pointed teeth, or ciliolate.
-
4...... Leaves distinctly convex abaxially, at least in the upper divisions.
-
5...... Leaves 5-8 × 1.2-1.8 mm (Fig. 7e-g)....................................... Phlegmariurus christii
Figure 5
a-c. Phlegmariurus biformis - a. growth habit; b. expanded leaves of proximal division; c. distal sporangiate division. d-f. Phlegmariurus erythrocaulos - d. growth habit; e. expanded leaves of proximal division; f. distal sporangiate division. (a-c. Brazil, Santa Catarina, Morro do Cambirela, parte W, Pahoça, Bresolin 447 (FLOR); d-f. Brazil, Rio de Janeiro, Planalto of Itatiaia, vicinity of Agulhas Negras, near Piedra Atar, Tryon 6686 (AAU)).
Figure 6
a-b. Phlegmariurus dichotomus - a. growth habit; b. terminal sporangiate division. c-d. Phlegmariurus capillaris - c. growth habit; d. distal sporangiate division. (a-b. Ecuador, Quinindé, Holdridge 1654 (GH); c-d. Colombia, Popoyán, Hartweg 1464 (P)).
Figure 7
a-b. Phlegmariurus deminuens - a. growth habit; b. distal sporangiate division. c-d. Phlegmariurus rostrifolius - c. growth habit; d. distal sporangiate division. e-g. Phlegmariurus christii - e. growth habit; f. middle sporangiate division; g. sporangiate leaf. (a-b. Holotype, Brazil, Minas Geraes, A. de Saint Hilaire D 248 (P); c-d. Brazil, Minas Gerais, Serra da Papagaio, A. Silveira 2605 (P); e-g. Brazil, Rio de Janeiro, Serra dos Orgãos, Pedra do Sini, Brade 16526 (RB)).
-
5’.... Leaves 3-5 × ca. 1 mm (Fig. 7c,d)..................................... Phlegmariurus rostrifolius
-
-
4’..... Leaves abaxially flat or concave sometimes with a prominent vein.
-
6...... Leaves 8-11 × 1.5-2 mm, linear-lanceolate, usually 8-10-seriate (Fig. 11a,b).......................... Phlegmariurus hemleri
Figure 8
a-d. Phlegmariurus flexibilis - a. growth habit; b. leaves from proximal division; c. growth habit of fertile plant; d. sporangiate leaves from distal division. e-g. Phlegmariurus loefgrenianus - e. growth habit; f. leaves from proximal division; g. sporangiate leaves of distal division. (a-d. Brazil, Paraná, Jacarehy, Dusén 14762 (S); e-g. Brazil, São Paulo, Cidade Jardim, São Paulo, Gehrt s.n.; Bonn-Nessel 368a (SP-27049)).
Figure 9
a-d. Phlegmariurus quadrifariatus - a. growth habit; b. proximal division with reduced leaves; c. Brazil, Rio de Janeiro, Morro da Fazenda, Tijuca, Glaziou 5219 (P), growth habit, proximal divisions with expanded leaves; d. distal division with sporangiate leaves. e-h. Phlegmariurus fontinaloides - e. growth habit of plant with expanded leaves in proximal divisions; f. Brazil, Paraná, Itaiacoca, 1904, Dusén s.n. (P), growth habit of plant with constricted proximal divisions; g. constricted proximal division; h. sporangiate distal division. (a-d. Brazil, Rio de Janeiro, Itatiaia, Rio Campo Belo, 900 m, Brade 21455 (AAU); e-h. Brazil, Rio de Janeiro: Pico de Papagaio, forest of Tijuca, Brade 8615 (AAU)).
Figure 10
a-c. Phlegmariurus friburgensis - a. growth habit; b. distal sporangiate division; c. middle sporangiate division. d-e. Phlegmariurus nudus - d. growth habit, the proximal part with two new sprouting shoots; e. sporangiate division. [a-b. Glaziou 4476 (P); c. A.P. Duarte 13961 (AAU); d-e. Luederwaldt s.n. (SP18.082) ].
-
6’.... Leaves 4-8 × 0.5-1 mm, linear-subulate or linear-lanceolate, usually 10-14-seriate (Fig. 20a-d) Phlegmariurus reflexus
Figure 12
a-c. Phlegmariurus martii - a. growth habit; b. leaves of proximal division; c. distal sporangiate division. - d-f. Phlegmariurus heterocarpos - d. growth habit.; e. leaves of proximal division; f. distal sporangiate division. (a-c. Brazil, Espirito Santo, Mun. Castelo, Braço do Sul, Brade 19311 (SP); d-f. Brazil, Sao Paulo, Dúvidas, road Mayrink-Santos, Gehrt SP 27499 (SP))
Figure 13
a-e. Phlegmariurus hexastichus - a. growth habit of entirely constricted plant; b. constricted proximal division; c. growth habit of plants with expanded leaves in proximal divisions; d. basal division with expanded leaves; e. distal sporangiate division. (a-b. Brazil, Rio de Janeiro, Glaziou 2794 (BR); c-e. Brazil, Faz. do Tacoaral, en montant aux Campos Brejos, Glaziou 7494 (C)).
Figure 14
a-b. Phlegmariurus hippurideus - a. growth habit; b. sporangiate division. c-d. Phlegmariurus mandiocanus - c. growth habit; d. sporangiate division; d1. schematic transverse section of leaf. e-f. Phlegmariurus wilsonii - e. growth habit; f. sporangiate division. (a-b. Colombia, Cauca, Sneidern 2196 (S); c-d. Langsdorff Mandioca, Brazil (P); e-f. Ecuador, Carchi, Maldonado, Holm-Nielsen et al. 6091 (AAU)).
Figure 15
a-c. Phlegmariurus huberi - a. growth habit; b. vegetative leaf of lower division; c. sporangiate division. (Venezuela, Bolívar, Macizo de Chimantá, Apacará-tepui, Steyermark et al. 128412 (AAU)).
Figure 16
a-b. Phlegmariurus intermedius - a. growth habit; b. sporangiate division. c-d. Phlegmariurus pungentifolius - c. growth habit; d, sporangiate division. (a-b. Brazil, Bahia, Serra dos Lençois, Serra da Larguinha, (Capão Grande), Harley et al. 22599 (AAU); c-d. Brazil, Minas Gerais, Serra do Espinhaço, Serra da Caraça, Irwin et al. 29233 (P).
Figure 17
a-c. Phlegmariurus linifolius var. jenmanii - a. growth habit; b. leaves of proximal division; c. sporangiate distal division. - d-f. Phlegmariurus mollicomus - d. growth habit; e. young plant; f. distal sporangiate division. - g-i. Phlegmariurus taxifolius - g. growth habit; h. leaves of proximal division; i. distal sporangiate division. (a-c. Ecuador, Napo, Río Guëpí, Brandbyge & Azanza 30596 (AAU); d-f. Brazil, Mun. Petropolis, Road Fazenda Inglesa-Pati dos Alferes, Plowman & Martinelli 10128 (AAU); g-i. Peru, prope Olleras et Aipate, Humboldt (BONN-herb. Nessel)).
Figure 18
a-b. Phlegmariurus mooreanus - a. growth habit; b. distal sporangiate division. c-e. Phlegmariurus ruber - c. growth habit; d. distal vegetative division; e. distal sporangiate division. (a-b. Brazil, Bahia, Gipfel der Serra das Almas, Lützelburg 18871 (M); c-e. Brazil, Minas Gerais, Summit of Carapuça, Glaziou 15801 (P)).
Figure 19
a-c. Phlegmariurus recurvifolius - a. growth habit; b. proximal vegetative division; c. distal sporangiate division. (Venezuela, Bolívar, Gran Sabana, Arautá-parú, Steyermark & Dunsterville 104163 (GB)).
Figure 20
a-d. Phlegmariurus reflexus - a. growth habit; b. sporangiate division; c. growth habit; d. sporangiate division. (a-b. Brazil, Rio de Janeiro, Itaipava-Theresópolis, km 27, Pabst 7150 p.p. (HB), large form; c-d. Brazil, Rio de Janeiro, Nova Friburgo, road to Picos da Salina, Windisch 4977 (AAU), small form).
-
-
-
3’.... Leaf margins smooth, not denticulate or ciliate.
-
7...... Leaves ascending to appressed.
8...... Leaves distinctly convex abaxially, at least in distal divisions.
9...... Leaves of upper divisions sharply carinate, plants not red (Fig. 4d-f)............................. Phlegmariurus itambensis
9’..... Upper divisions with leaves rounded to somewhat conduplicate abaxially, plants green or red.
10.... Plants green, leaves in whorls of 6-8 (Fig. 7a,b).......................................................... Phlegmariurus deminuens
10’.. Plants distinctly red, leaves in whorls of 4-5 (Fig. 18c-e).......................................... Phlegmariurus ruber
8’.... Leaves distinctly flattened abaxially, or flattened with prominent vein.
11.... Leaves in whorls of 8-10, 16-20-seriate (Fig. 22a-c)............................................................ Phlegmariurus treitubensis
Figure 21
a-b. Phlegmariurus sellowianus - a. growth habit; b. sporangiate division. c-e. Phlegmariurus silveirae - c. growth habit; d. leaves of proximal divisions; e. sporangiate division. (a-b. Brazil, Rio de Janeiro, Alto Macahé, Glaziou 4468 (P); c-e. Brazil, Sao Paulo, Alto da Serra, Brade 5848 (HB)).
Figure 22
a-c. Phlegmariurus treitubensis - a. growth habit; b. young plant with swollen base; c. sporangiate division. d-e. Phlegmariurus regnellii - d. growth habit of fully developed plant; e. sporangiate division. (a-c. Brazil, Minas Gerais, Mun. Baependi, São Tomé das Letras Brade 20402 (P); d-e. Brazil, Minas Gerais, Serra de Caldas, Mosén 4654 (S).
11’... Leaves in whorls of 6-7, 12-14-seriate (Fig. 22d,e).............................................................. Phlegmariurus regnellii
-
7’..... Leaves patent or recurved.
-
12.... Leaves filiform to narrowly linear, 1 mm or less wide.
-
13.... Longest leaves 6 mm long or shorter.
-
14.... Basal half of leaf ascending, from there strongly recurved and hook-like, branching angles divaricate (Fig. 16a,b).................................................................. Phlegmariurus intermedius
-
14’.. Leaves recurved from a patent base, branching angles narrow (Fig. 10a-c) Phlegmariurus friburgensis
-
-
13’... Longest leaves 8-17 mm long.
-
15.... Leaves linear to filiform, canaliculate and involute, or bisulcate.
-
16.... Leaves bisulcate above, with prominently tumid margins and vein, leaf bases with widening, prominently decurrent margins and median veinal ridge, usually bright red (Fig. 14c,d) .................................................................................................................................... Phlegmariurus mandiocanus
-
16’... Leaves canaliculate to involute, often with prominent vein abaxially near the base, decurrent leaf base usually not wider than the lamina base (Fig. 14e,f) Phlegmariurus wilsonii
-
-
15’.. Leaves linear, flat or with slightly revolute margins
17.... Plants usually epiphytic, leaf lamina usually twisted at the base to a vertical position (Fig. 6a,b) Phlegmariurus dichotomus
17’.. Plants terrestrial or rupestral, leaf lamina not twisted, leaves patent to reflexed.
-
-
18.... Leaves 6-13 × 0.6-0.8 mm, stems 1.3-2 mm thick at the base (dried) (Fig. 10d,e)............................ Phlegmariurus nudus
18’... Leaves 11-19 × 0.8-1.3 mm, stems 2.5-4 mm thick at the base (dried) (Fig. 14a,b).......................... Phlegmariurus hippurideus
-
-
-
-
-
12’. Leaves narrowly to broadly lanceolate, more than 1 mm wide.
-
19.... Leaves subdecussate, rupestral or epiphytic plants, scrambling, usually densely branched and divaricate, usually with red stems (Fig. 5d-f)............................................................................... Phlegmariurus erythrocaulos
-
19’.. Leaves borne in whorls of (3-)4-10.
-
20.... Longest leaves less than 7 mm long, strongly recurved, rigid and hook-like, 16-20-seriate (Fig. 18a,b) Phlegmariurus mooreanus
-
20’... Longest leaves 8-20 mm long, straight to recurved, (6-)8-16 seriate.
-
21.... Leaves usually 5-9 mm long, 1-1.3 mm wide, coriaceous, patent to reflexed (Fig. 16c,d) Phlegmariurus pungentifolius
-
21’.. Leaves usually 10-20 mm long, herbaceous to subcoriaceous, patent to softly recurved.
-
22.... Leaves borne in whorls of (3-)4, 2.5-3.5 mm wide (Fig. 21a,b).......................................... Phlegmariurus sellowianus
-
22’... Leaves borne in whorls of 5-8, less than 2 mm wide.
-
23.... Leaves of distal divisions usually terminating in a short very thin whip-like, usually curved or twisted tip (Fig. 19a-c)......................................................................................................................... Phlegmariurus recurvifolius
-
23’.. Leaves of distal division without a whip-like tip.
-
-
-
-
-
-
-
1’. Plants pendulous, or initially erect with nodding to pendulous shoot apices, usually epiphytic; leaf margins smooth, not denticulate or erose. Plants epiphytic or rupestral.
-
25. Shoots with more or less sharply dimorphic leaves; proximal divisions with long expanded leaves; distal divisions constricted, with appressed, short, often decussate leaves; - or the entire plant covered by imbricate, short, broad, leaves.
-
26.... Shoots with more or less sharply dimorphic leaves; proximal divisions extensive and usually branched, with long expanded leaves; distal sporangiate divisions constricted, with appressed, short, often decussate leaves.
-
27.... Expanded leaves elliptic-oblong, 6-11 × 2.8-4 mm, usually continuously overlapping in pressed specimens (Fig. 3)................................................................................................... Phlegmariurus aqualupianus
-
27’... Expanded leaves linear or lanceolate to linear-lanceolate or narrowly ovate, 1-2(-3) mm wide, not continuously overlapping.
-
28.... Expanded leaves linear, the lamina usually twisted at the leaf base to a vertical position, ± falcately curved, to 1 mm wide, 8-12-seriate (Fig. 12a-c).......................................................................................................... Phlegmariurus martii
-
28’.. Expanded leaves ovate-lanceolate to linear-lanceolate, 1.3-2(-3) mm wide, subdecussate or in irregular whorls of 3.
-
29.... Leaves of terminal divisions variable, often with complete reduction series, and recurrent to expanded shape, decussate or subdecussate, continuously or discontinuously sporangiate, Expanded leaves of basal divisions 1.5-3 mm wide (Fig. 23a,b).......................................... Phlegmariurus myrsinites
-
29’... Leaves of terminal divisions usually uniformly constricted, with appressed, short, often decussate leaves.
30.... Flaccidly pendulous epiphytes, to 70 cm long, stems not red (Fig. 5a-c)................................................... Phlegmariurus biformis
30’.. Rupestral or epiphytic, scrambling to hanging, to 25 cm long, usually densely branched and divaricate, usually with red stems (Fig. 5d-f).......................................................................................... Phlegmariurus erythrocaulos
-
-
-
-
26’. Entire plant (or at least the terminal half) covered by imbricate, short, broad, decussate leaves, or the plants sometimes with proximal divisions short and unbranched, and sometimes with few expanded leaves at the very base.
-
31.... Leaves decussate and imbricate throughout, or rarely with a few expanded, oblong or ovate, opposite or spiralled leaves at the very base of the stem.
-
32.... Constricted shoots sharply quadrangular throughout, 2-3 mm in diam. including leaves, the leaves sharply carinate, expanded leaves sometimes present at the very base of the plant, narrowly oblong (Fig. 9a-d) Phlegmariurus quadrifariatus
-
32’... Constricted shoots rounded abaxially throughout, 0.7-1.5 mm in diam. including leaves, the leaves carinate, expanded leaves sometimes present at the very base of the plant, ovate to obovate (Fig. 9f-h) Phlegmariurus fontinaloides
-
-
31’.. Leaves of proximal divisions in whorls of 3, forming 6 ranks, imbricate, upward decussate, or sometimes with narrowly oblong expanded leavesat the very base of the plant, (Fig. 13a-e)............................................................................ Phlegmariurus hexastichus
-
-
-
25’. Leaves uniform and expanded throughout, or gradually reduced upward, leaves of sporangiate divisions usually in whorls of 3 or more.
-
33. Leaves, at least of proximal portions inserted singly (not in whorls) or occasionally some paired.
-
34.... Stem strongly flexuous, bending at each leaf insertion (Fig. 8a-d)... Phlegmariurus flexibilis
-
34’.. Stem not or very slightly flexuous.
-
35.... Leaves of proximal divisions 13-25 × 1-2 mm (Fig. 17a-c)................................................................... Phlegmariurus linifolius var. jenmanii
-
35’... Leaves of proximal divisions 5-10 × 0.4-0.7(-1) mm (Fig. 6c,d).......................................................... Phlegmariurus capillaris
-
-
-
33’. Leaves borne in ± regular whorls of 3 or more.
-
36.... Plants very slender, stems of proximal divisions less than 1 mm thick excluding leaves (dried), leaves acicular to linear-subulate, less than 1 mm wide.
-
37.... Leaves of proximal divisions linear to filiform, 7-10 mm long, not widened at the base, with prominent vein abaxially and slightly revolute margins (Fig. 17d-f)....................................................................................................... Phlegmariurus mollicomus
-
37’... Leaves of proximal divisions acicular-linear, 3-7 mm long, slightly widened at the base, convex below.
-
38.... Flaccidly pendulous epiphytes or sometimes rupestral, leaves 3-5 mm long, softly herbaceous, sporangia 0.7-1 mm (Fig. 2a-c).................................................................................. Phlegmariurus acerosus
-
38’.. Slender, scrambling or hanging, rupestral or sometimes epiphytic, often with strongly diverging ramification, leaves 4-7 mm long, subcoriaceous, sporangia 1-1.3 mm (Fig. 2d-f) Phlegmariurus comans
-
-
-
36’.. Plants slender to robust, stems of proximal divisions 1-2.5 mm thick excl. leaves (dried).
-
39.... Leaves of proximal divisions convex abaxially and canaliculate adaxially.
-
40.... Leaves of proximal divisions 10-20 × 1-1.5 mm, abaxially evenly rounded (Fig. 12d-f) Phlegmariurus heterocarpos
-
40’.. Leaves of proximal divisions 3- 6(-13) × 1-1.5 mm, abaxially subcarinate to carinate, especially in distal divisions (Fig. 8e-g)........................................................................ Phlegmariurus loefgrenianus
-
-
39’... Leaves of proximal divisions flat, or slightly convex abaxially with flat margins, or slightly convex adaxially.
-
41.... Leaves of proximal divisions 6-12 × 0.7-1 mm (Fig. 12a-c)......................................................... Phlegmariurus martii
-
41’.. Leaves of proximal divisions 11-20 × 1.2-3 mm.
42. Leaves spreading to ascending or somewhat appressed, often twisting the lamina to vertical position, sporophylls ascending to appressed, usually not twisted (Fig. 17g-i)........................................................... Phlegmariurus taxifolius
42’. Leaves usually spreading to nearly perpendicular, the lamina twisted to vertical position, sporophylls usually spreading and twisted (Fig. 21c-e)................................................................................................................... Phlegmariurus silveirae
-
-
-
-
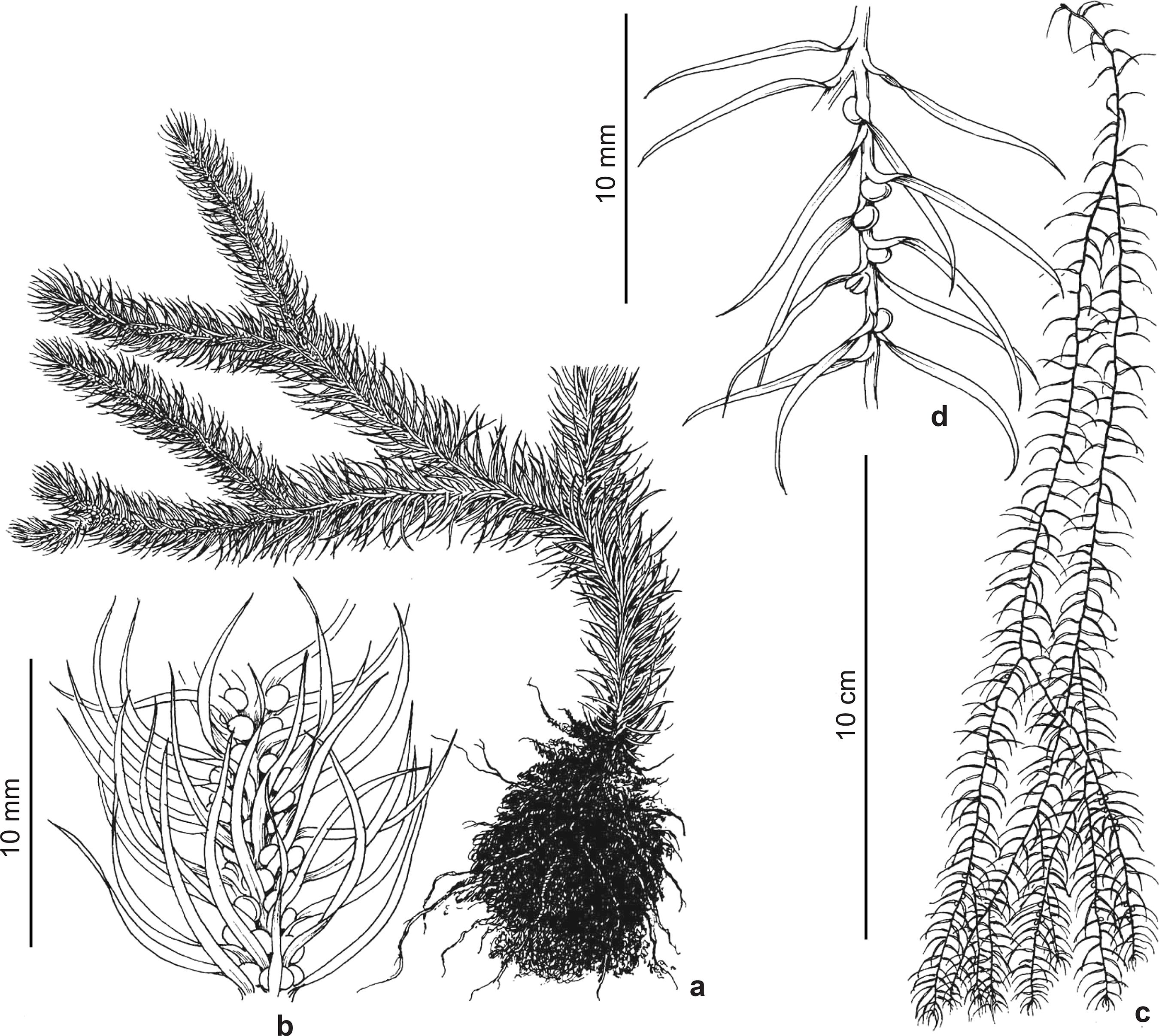
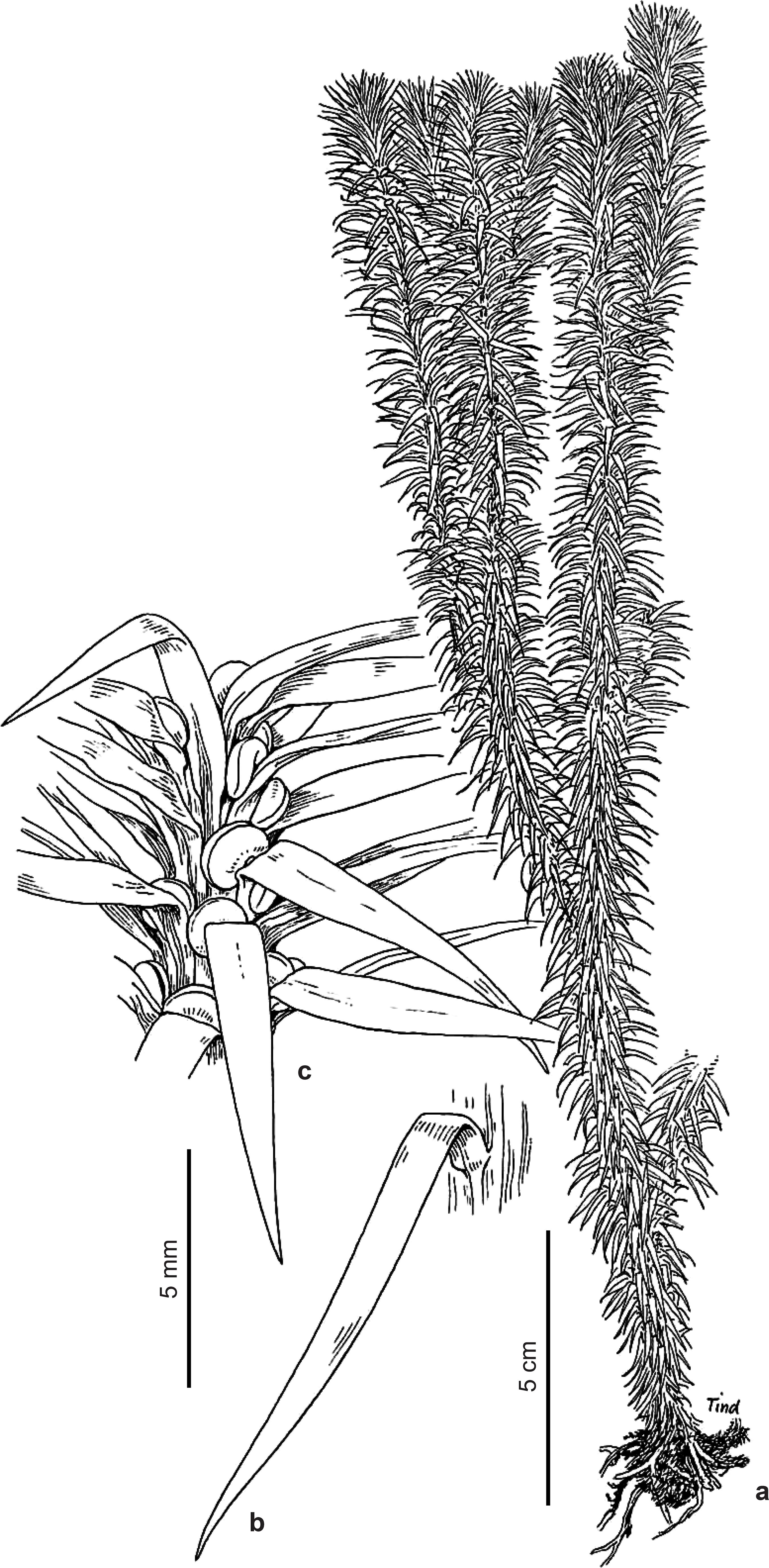
Phlegmariurus acerosus (Sw.) B. Øllg. Rodriguésia 63(2): 480. 2012. Fig. 2a-c
Lycopodium acerosum Sw., Flora Indiae Occidentalis 3: 1575. 1806. - Urostachys acerosus (Sw.) Nessel, Arch. Bot. Est. S. Paulo 1: 399. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia acerosa (Sw.) Holub, Folia Geobot. Phytotax. 20: 70. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Lectotype (designated by Proctor 1977Proctor GR (1977) Pteridophyta. In: Howard RA (ed.). Flora of the Lesser Antilles, Leeward and Windward Islands 2: 1-414.): Plumier, Traité Foug. Amér. t. 166 (B) (1705Plumier C (1705) Traité des fougères de l’Amérique, Paris. 146p., t. 1-170.); an authentic specimen possibly in Herb. Suriani no. 635 (P, n. v.).
Published illustrations: Øllgaard 1988Øllgaard B (1988) Lycopodiaceae. In: Harling G & Andersson L (eds.) Flora of Ecuador 33: 1-155.: p. 91, figure 18 C.
Plants epiphytic, slender, flaccidly pendulous, at least to 70 cm long. Shoots usually gradually heterophyllous, sometimes homophyllous, 3-6 mm in diam. incl. the leaves in proximal divisions, tapering to 1-3(-6) mm in distal divisions. Stems excl. leaves 0.6-1 mm thick at the base, tapering to ca. 0.3-0.5 mm upward, somewhat concealed by the leaves, pale greenish to stramineous, at least to 10 times dichotomous, usually densely, unilaterally or omnilaterally sporangiate in separate, periodically produced zones of the distal divisions, or continuously sporangiate from 15-30 cm above the base and upward. Leaves gradually modified along the stems. Leaves of proximal divisions densely crowded, borne in irregular alternating whorls of 6-7, these 0.5-2 mm apart, forming 12-14 indistinct longitudinal ranks, ascending and upward curved to appressed or somewhat secund, acicular-filiform, narrowly and prominently decurrent, 3.5-5 mm long, 0.2-0.4 mm wide just above the widened base, soft herbaceous to subcoriaceous, convex below, canaliculate above, with involute margins. Vegetative leaves of distal constricted divisions borne in irregular whorls of 4-6, these 0.5-2 mm apart, forming 8-12 indistinct longitudinal ranks, ascending to closely appressed, acicular-filiform to linear-lanceolate, 2-4 mm long, otherwise conform. Sporophylls borne in irregular, alternating whorls of 3-5, conform, or shorter and wider, linear-lanceolate and long acuminate to lanceolate, usually appressed, 1.5-4 × 0.3-0.8 mm, rounded to subcarinate abaxially. Sporangia 0.7-1 mm in diam.
Distribution and habitats: West Indies, Guatemala, Costa Rica, Panamá, northern South America, south to Ecuador and Bolivia and southeastern Brazil, with one record for Amazonia [Prov Rio Negro ad Egam passim, 1819, Martius s.n. (M)].
Habitats: Epiphytic and sometimes epilithic in montane and cloud forest in the states of Amazonas, Bahia, Minas Gerais, Rio de Janeiro, São Paulo, Paraná, Rio Grande do Sul, and Santa Catarina, alt. ca. 300-2,600 m, the low altitudes in the southern part of the range.
Notes: Phlegmariurus acerosus has often been referred to Lycopodium verticillatum L. f. or Lycopodium setaceum Lam., both of which are based on material from Réunion Island, and are considered synonymous. The plants from the Old World referred to Lycopodium verticillatum resemble P. acerosus, but differ in a more robust growth habit, usually longer and more crowded leaves, and usually more densely and omnilaterally sporangiate distal divisions. However, molecular data (Wikström & Kenrick 2000Wikström N & Kenrick P (2000) Phylogeny of epiphytic Huperzia (Lycopodiaceae): paleotropical and neotropical clades corroborated by rbcl sequences. Nordic Journal of Botany 20: 165-171.) indicate that the group of P. acerosus belongs to a purely American clade, and the group of Lycopodium verticillatum is purely Old World.
Phlegmariurus acerosus is variable with respect to leaf size and direction. In some collections, the shoots are very narrow with short, appressed leaves, and in others they are wider with longer and patent-ascending leaves. It rather closely resembles shaded forms of P. comans (Nessel) B. Øllg., that differ being generally larger, shorter, and more robust, apparently as a response more exposed habitats.
Reference specimens (more than 80 collections studied): BRAZIL. BAHIA: Serra Larga, W of Lençois, near Caeté-Açu, 1,400 m, Harley et al. in CFCR 7264 (AAU, SPF). Ilhéos, Max. Neovid (BR). MINAS GERAIS: Aiuruoca, P.E. Serra do Papagaio, trail by Pico do Papagaio from Truta do Vale do Matutu, and Pico do Papagaio, 1,400-2,020 m, Salino & Almeida 10456 (BHCB photo AAU). Caldas, 18.II.1855, Regnell I 493 (AAU, BR, GH, K, P, S, UC, US, Z). Parque Nacional do Caparaó, Vale Verde, Krieger. et al. 752, (AAU, CESJ 23524). PARANÁ: Campina Grande do Sul, road Rio Taquari-Rio Divisa, Hatschbach 6364 (L, US). Jaguaraiva, Dusén 17060 B (F, GH, S). Laranjeiras do Sul, Rio Iguaçu, Salto Osorio, 29.IX.1968, Hatschbach et al. 19829 (F, MBM, UC). Morretes, Pilão da Pedra, 5.III.1961, Hatschbach 7858 (MBM, US). Piraquara, Roça Nova, 22.VII.1980, Hatschbach & Oliveira 43026 (AAU, MBM). Roça Nova, 3.IV.1909, Dusén 8343 (K, S, US). São José dos Pinhais, Purgatório, 19.VII.1967, Hatschbach 16713 (L, NY, US). RIO DE JANEIRO: Serra dos Orgãos, Rio Paquequer, 1,000 m, 17.VIII.1940, Brade 16407 (AAU, RB). Serra do Itatiaia, 2,600 m, IX.1913, Brade 6604 (HB). Serra do Macahé, 1,000 m, Ule (HBG). RIO GRANDE DO SUL: Santa Cruz, Sete Lagoas, Herval do Paredão, 650 m, Jürgens 16 (S). SANTA CATARINA: Araranguá, Serra da Pedra, 1,000 m, Reitz C325 (RB). Joinville, 5.III.1906, Schmalz 153 VIII (F, S). Lages, Spanagel ex Rosenst. Fil. austrobras. 416 (M). Papanduva, Serra do Espigão, 1,000 m, Reitz & Klein 13050 (HBR). SÃO PAULO: Serra da Bocaina: 1,700 m, 5.V.1951, Brade 20861 (AAU, MO, RB). Iguape, Serra Paranapiacaba, 800 m, Brade 8489 (HB). Campos do Jordão, Umuarama, Kuhlmann, SP 32264 (HB, SP, US).
Phlegmariurus aqualupianus (Spring) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 3
Lycopodium aqualupianumSpring, Bull. Acad. Roy. Sci. Bruxelles. 8: 518. 1841Spring A (1841) Enumeratio Lycopodinearum, quas in ejusdem plantarum ordinis monographia mox edenda descripsit A. Spring. Bulletins de l’Académie Royale des Sciences et Belle-Lettres de Bruxelles 8: 511-523.. -Lycopodium guadalupianum Spring exFée, Hist. Foug. Ant. 131, t. 33, 1. 1866Fée ALA (1866) Histoire des Fougères et des Lycopodiacées des Antilles. Onzième et dernière mémoire sur la famille des fougères, Strasbourg. 164p.. nom. superfl. - Urostachys aqualupianus (Spring) Herter, Repert. Spec. Nov. Regni Veg. 19: 166. 1923Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.. - Huperzia aqualupiana (Spring) Rothm., Feddes Repert. 54: 62. 1944. - Lectotype: Guadeloupe, L’herminier (lectotype LG; isolectotypes BM, K, P) designated by Badré (1983: 4)Badré F (1983) Antoine Frédéric Spring (1814-1872) et son herbier. Lejeunia Nouvelle 109: 1-14..
Plants epiphytic, pendulous, flaccid, 3-4 times dichotomous. Stems stramineous, angular, 15-50 cm long, thin, usually less than 1 mm thick excl. leaves. Shoots dimorphic, the proximal divisions with wide expanded leaves, the distal divisions abruptly narrowed, with small imbricate leaves. Proximal expanded divisions with uniform leaves, 10-17 mm wide incl. the leaves, sometimes slightly tapering distally, usually continuously overlapping in pressed specimens. Expanded leaves decussate or in whorls of 3, forming 4-6 longitudinal rows, regularly inserted, ascending to patent, softy herbaceous, elliptic-oblong, 6-11 × 2.8-4 mm, with entire margins, slightly apiculate, with decurrent base. Constricted divisions quadrangular, with extensive non-sporangiate zones proximally. Leaves of constricted divisions imbricate, regularly decussate, ovate-acuminate, carinate, 3-4.5 × 1.5 mm. Sporangia ca. 1 mm wide, completely concealed by sporophyll bases.
Distribution and habitats: West Indies, Colombia, Venezuela, Guyana, Brazil; wet premontane and cloud forest, alt. 500-1,800 m. Brazil, know only from Minas Gerais state.
Specimens studied: BRAZIL. MINAS GERAIS: Santa Maria do Salto, Distrito de Talismã, Fazenda Duas Barras, close to divisa with state of Bahia, 750-850 m, 9.X.2003, Salino et al. 9192 (BHCB); dense humid montane forest, epiphyte at 1.5 m; 850-1,000 m, Salino et al. 10052 (BHCB).
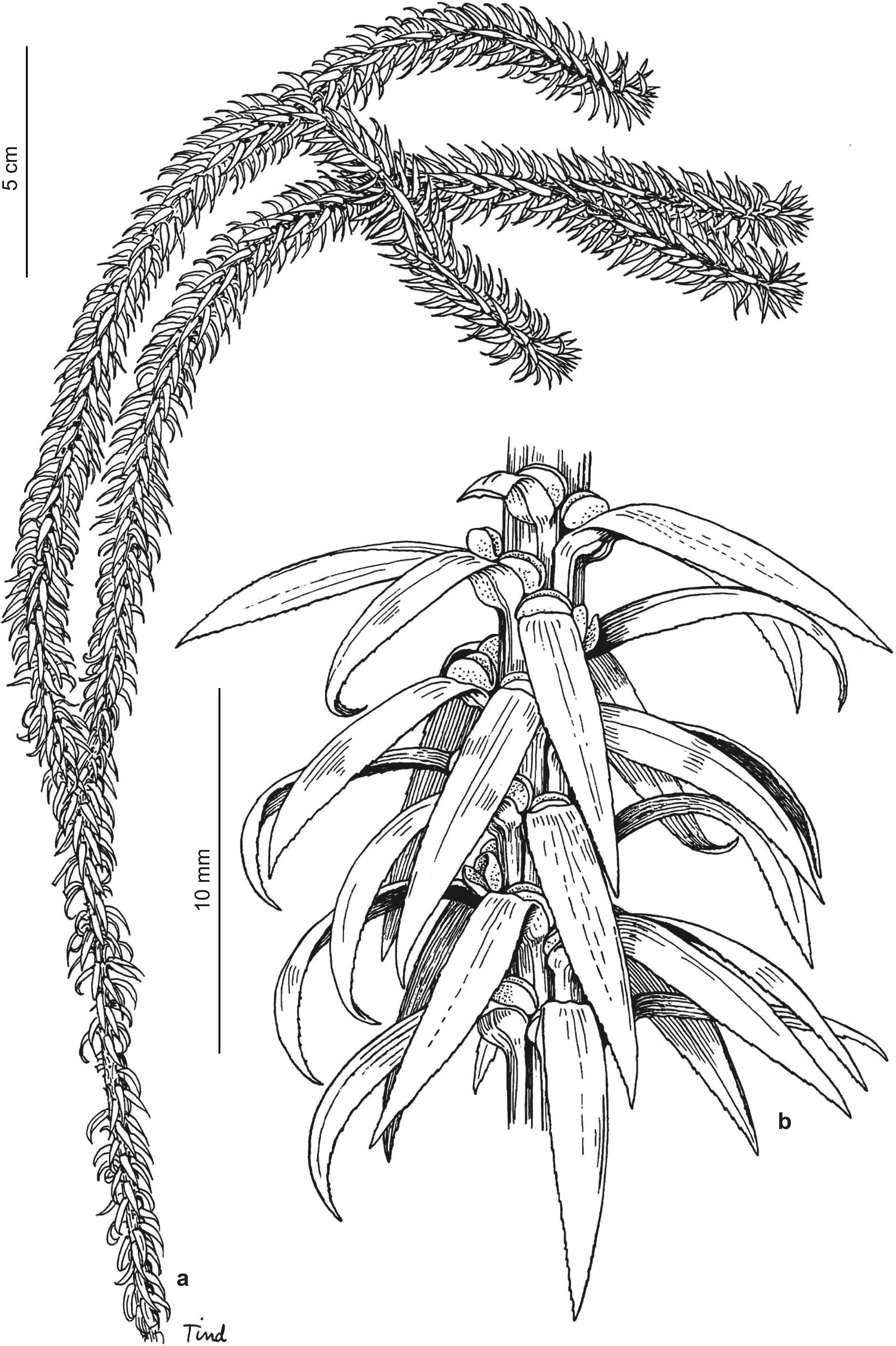
Phlegmariurus badinianus (B. Øllg. & P.G. Windisch) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 4a-c
Huperzia badiniana B. Øllg. & P.G. Windisch, Bradea 5: 7, fig. 1A. 1987. - Type: Brazil: estado Minas Gerais: Serra do Caparaó, campo, ca. 2570 alt., 8 Feb 1987, Windisch et al. 4971 (holotype HB, isotypes AAU, GH, SP).
Published illustrations: Øllgaard & Windisch 1987Øllgaard B (1987) A revised classification of the Lycopodiaceae sensu lato. Opera Botanica 92: 153-178., fig. 1A.
Plants terrestrial, with short prostrate juvenating basal shoots, from which stiffly erect, well spaced to somewhat aggregated, simple or to twice forked, to 25 cm tall, finger-like, aerial shoots arise. Aerial shoots homophyllous or almost so, sometimes with gradually shorter leaves upward, gradually tapering from 6-20 mm in diam. incl. leaves at the base, to 4-10 mm near the apex. Stem of aerial branches excl. leaves 2-3 mm thick throughout, almost completely concealed by leaves. Leaves of aerial shoots borne in irregular alternating whorls of 4-6, these 1.5-3 mm apart, forming 8-12 indistinct or sometimes regular longitudinal ranks, spreading to arcuate-appressed at the base, upward appressed, slightly to strongly arcuate, linear-lanceolate to lanceolate, (4-)5-9 × 1.3-2 mm, abaxially convex and evenly rounded, or with a slightly prominent veinal ridge, with or without a slightly prominent basal swelling (air sac), with sclerified, minutely evenly rugose to erose-verruculate margins, green, soft-herbaceous to subcoriaceous, dull to somewhat lustrous. Sporangia ca. 2 mm wide.
Distribution and habitats: Endemic. Restricted to few high points in Southeastern Brazil: Serra do Caparaó, Itatiaia, Papagaio, and Campo de Capivare, Serra Geral.
Related to Phlegmariurus saururus (Lam.) B. Øllg., from which it may be distinguished by its more slender growth habit, smaller dimensions, the very loosely aggregated aerial shoots without squeezed and etiolated leaves at the base of the aerial shoots. Also the leaf margins are more irregularly uneven than usual for P. saururus.
Reference specimens (14 collections studied): BRAZIL. MINAS GERAIS: Serra do Caparaó, Pico do Cristal, 2,600 m, Brade 17011 (AAU, F, GH, MO, R, SP, UB). Serra do Papagaio, Silveira 182 (BONN-Herb. Nessel 130, R). RIO DE JANEIRO: Serra do Itatiaia, Agulhas Negras, 2,400 m, Ule 3535 (HBG). Estrada Nova km 15, 2,300 m, Brade 20291 (AAU, RB). SANTA CATARINA: Serra Geral, Campo Capivaras, am Abhange eines Baches, Ule 2333 (HBG). Urubici, Serra, ca. 1,700 m alt., solo humoso, sobre rocha, Windisch et al. 11045 (HB, photo AAU).
Phlegmariurus biformis (Hook.) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 5a-c
Lycopodium biforme Hook., Icon. Pl. 3: t. 228. 1839 [1840]. - Urostachys biformis (Hook.) Herter, Index Lyc. 52. 1949Herter W (1949) Index Lycopodiorum, Montevideo. Pp. 1-120.. - Huperzia biformis (Hook.) Holub, Folia Geobot. Phytotax. 20: 71. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Organ Mountains, alt. ca. 5000 ft., Gardner 80, 2nd Fern Coll. (holotype K, isotypes BM, P).
Plants epiphytic, delicate, pendulous, at least to 70 cm long. Shoots heterophyllous. Proximal divisions, to 15-30 cm from the base, ca. 12-25 mm in diam. incl. the expanded leaves. Distal divisions abruptly constricted to ca. 1 mm in diam. incl. the imbricate, reduced leaves, to 25 cm long, subterete. Stems excl. leaves 0.5-0.8(-1) mm thick at the base, upward tapering to ca. 0.3-0.5 mm, greenish, rarely reddish tinged, at least to 10 times dichotomous. Expanded leaves of proximal divisions decussate, subdecussate or alternate, the leaf pairs 1.5-5 mm apart, widely spaced in alternate-leaved stem portions, perpendicular to the stem to falcately ascending, lanceolate to linear-lanceolate, usually widest just above the leaf base to ca. one-third from the base, straight to slightly falcately curved, 7-15 × 1.3-2.3 mm, softly to firmly herbaceous, with flat to slightly revolute margins, the lamina twisted to vertical position from the base. Leaves of constricted distal divisions decussate or subdecussate, the leaf pairs ca. 1-2 mm apart, usually densely sporangiate throughout, appressed and clasping with their margins, abaxially rounded to indistinctly carinate, widely ovate to almost orbicular, acute to mucronate, 1-1.5(-2) × 1(-1.5) mm, equalling or slightly exceeding the sporangia, with smooth to slightly uneven margins. Sporangia 0.7-1(-1.3) mm in diam.
Distribution and habitats: Endemic, but closely related to the Andean Phlegmariurus phylicifolius (Poiret) B. Øllg. Also related to Phlegmariurus erythrocaulos (Fée) B. Øllg., see there for comparison. A slender epiphyte from sheltered situations in wet montane forest, alt. 850-2,400 m. States of Bahia, Minas Gerais, Espírito Santo, Rio de Janeiro, São Paulo, Paraná, and Santa Catarina.
Reference specimens (61 collections studied): BRAZIL. BAHIA: Abaíra, Tijuquinho, 1,700 m, Harley et al. 51218 (K). ESPÍRITO SANTO: Castelo, Braço Sul, Brade 19299 (AAU, RB). MINAS GERAIS: Serra da Araponga, Faz. Neblina, 1,200 m, 20º43’S 42º29’W, Leoni 3057 (BHCB, photo AAU). Catas Altas, RPPN Santuario do Caraça, Pico do Sol, 1,865 m, Oliveira et al. 515 (BHCB, photo AAU). Delfim Moreira, Serra da Mantiqueira, Faz. Boa Esperança, trilha dos Romeiros, 1,558 m, Fernandes 892 (BHCB, photo AAU). Serra de Ibitipoca, Krieger 2665 (photo AAU ex CESJ). Viçosa, Serra da Grama, 22.III.1941, Carmo (SP 45911, HB). Serra do Caparaó, 2,100 m, Brade 17029 (AAU, RB). Alto Caparaó, Cachoeira da Farofa, 1,937 m, Souza et al. 1292 (BHCB, photo AAU). Caldas, Pedra Branca, Regnell III 1501 (BR, S). Aiuruoca, RPPN do Matutu, region of Macieira, Salino 10440 (BHCB, photo AAU). PARANÁ: Piraquara, Rio Taquary, Hatschbach 2550 (RB). Guaratuba, Serra da Araçatuba, 1,350 m, Hatschbach 6584 (MBM, US). RIO DE JANEIRO: Serra dos Orgãos, Sete Quedas, 1,700 m, Brade 9253 (HB). Pedra do Frade, 1,700 m, Brade 16444 (S, US). Serra do Macahé, I.1900, Ule (HBG). Nova Friburgo, Morro Caledonia, Leite 4346 (US). Santa Maria Madalena, Alto do Desengano, 2,000 m, Santos Lima & Brade 13187 (RB); Alto da Republica, Santos Lima 348 (AAU). SANTA CATARINA: Blumenau, Spitzkopf, Viereck 119 (M). Itajaí, Morro do Baú, 850 m, Reitz C2091 (RB, S). São Francisco do Sul, Morro do Campo Alegre, 1,200 m, Reitz & Klein 9772 (HBR). SÃO PAULO: Campos do Jordão, São José dos Alpes, 9-12 km along road to Horto Florestal, 1,900 m, Windisch et al. 4990 (AAU, HB). Serra da Bocaina, Fazenda Lageado, 1,650 m, Brade 20642 (AAU, MO).
Phlegmariurus capillaris (Sodiro) B. Øllg., Rodriguésia 63 (2): 480. 2012. Fig. 6c-d
Lycopodium capillareSodiro, Recensio Crypt. Vasc. Prov. Quitenses 90. 1883Sodiro A (1883) Recensio cryptogamarum vascularium provinciæ Quitensis. Republicæ Æquatorianæ. I-VII. Typis curiae ecclesiasticae, Quito. Pp. 1-114. . - Lycopodium sarmentosum Spring var. capillare (Sodiro) Sodiro, Crypt. Vasc. Quit. 573. 1893. - Urostachys capillaris (Sodiro) Herter, Index Lyc. 54. 1949Herter W (1949) Index Lycopodiorum, Montevideo. Pp. 1-120.. - Huperzia capillaris (Sodiro) Holub, Folia Geobot. Phytotax. 20: 71. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Ecuador, (Prov. Pichincha), Hda Guanaxilla, 480 m, Sodiro s.n.; (holotype K). The type locality is probably in the area NE of Santo Domingo de los Colorados.
Lycopodium sarmentosumSpring var. rubescens Spring, Mém. Acad. Roy. Sci. Belgique 24 [Mon. Lyc. 2]: 13. 1849Spring A (1849) Monographie de la famille des Lycopodiacées, seconde partie. Mémoires de l’Académie Royale des Sciences, Lettres et Beaux Arts de Belgique 24: 1-358.. - Urostachys rubescens (Spring) Herter, Index Lyc. 79. 1949Herter W (1949) Index Lycopodiorum, Montevideo. Pp. 1-120.. - Lectotype: Colombia, prov. Popoyan, Hartweg 1464 (K with Spring’s annotation, isotypes B, P, NY fragm.), designated by Øllgaard (1988: 93)Øllgaard B (1988) Lycopodiaceae. In: Harling G & Andersson L (eds.) Flora of Ecuador 33: 1-155..
Lycopodium underwoodianumMaxon, Contr. U. S. Natl. Herb. 13: 41. 1909Maxon WR (1909) Studies of tropical American Ferns nº. 2. Contributions from the United States National Herbarium 13: 1-43, t. 1-9.. - Urostachys underwoodianus (Maxon) Herter, Index Lyc. 87. 1949Herter W (1949) Index Lycopodiorum, Montevideo. Pp. 1-120.. - Huperzia underwoodiana (Maxon) Holub, Folia Geobot. Phytotax. 20: 77. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Costa Rica, vicinity of Coliblanco, about 1,950 m, Maxon 213 (holotype US, isotypes BM, C, GH, NY, P).
Lycopodium guatemalenseMaxon, Contr. U. S. Natl. Herb. 17: 177, pl. 9. 1913Maxon WR (1913) Studies of tropical American Ferns, nº 4. Contributions from the United States National Herbarium 17: 133-179, t. 1-10.. - Urostachys guatemalensis (Maxon) Herter, Repert. Spec. Nov. Regni Veg. 19: 165. 1923Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.. - Type: Guatemala, Pansamalá, Tuerckheim, ed. Donn.-Sm. 957 (holotype US, isotype UC).
Published illustrations: Lellinger, 1989Lellinger DB (1989) The ferns and fern-allies of Costa Rica, Panama, and the Chocó. (Part 1: Psilotaceae through Dicksoniaceae). Pteridologia 2A: 1-364.: p. 30 fig. 17; Øllgaard, 1988Øllgaard B (1988) Lycopodiaceae. In: Harling G & Andersson L (eds.) Flora of Ecuador 33: 1-155.: p. 95 fig. 19C.
Plants epiphytic, pendulous, with flaccidly hanging divisions, very delicate, 9-50 cm long. Shoots homophyllous, rarely gradually heterophyllous, 7-15 mm in diam. incl. leaves in proximal divisions. Stems excl. leaves 0.3-0.5 (-0.8) mm thick near the base, slightly tapering upward, straight or somewhat flexuous, sometimes bright red, discontinuously sporangiate from 6-25 cm above the base and upward, to 6(-13) times dichotomous. Leaves almost uniform throughout, borne apparently randomly, spirally arranged, occasionally in pairs or whorls of 3, these 1-3(-5) mm apart, spreading, straight to falcately curved, with strongly twisted, often deflexed lamina base, linear-lanceolate, widest in the middle or the basal half, 5-10(-12) × 0.4-0.7(-1) mm, almost flat, or slightly convex adaxially, soft-herbaceous, with obscure to somewhat prominent vein above. Lamina base, or sometimes the entire leaf tinged with red. Sporangia 0.7-1 mm in diam.
Distribution and habitats: Pendulous epiphyte in wet forest. Central America, Northern Tropical South America. South to Bolivia. In Brazil known only from the Territory of Roraima.
Apparently closely related to Phlegmariurus linifolius (L.) B. Øllg. Differs in the more delicate growth habit. Stem coloration is variable, the red colour lacking in some collections. Only one Brazilian collection known.
Specimen studied: BRAZIL. RORAIMA: Rio Uraricoeara, vicinity of Uaicá airstrip, 3.XII.1973, Prance et al. 20006 (NY).
Phlegmariurus christii (Silveira) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 7e-g
Lycopodium christiiSilveira, Bol. Comm. Geogr. Geol. Minas Geraes 2, 5: 117, t. 1. 1898Silveira A (1898) Novae species Lycopodiacearum civitatis Minas Geraes (Brazil). Boletim da Commissão Geográfica e Geológica do Estado Minas Geraes 5: 117-145, t. 1-12. . - Urostachys christii (Silveira) Nessel, Arch. Bot. Est. S. Paulo 1: 379. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia christii (Silveira) Holub, Folia Geobot. Phytotax. 20: 71. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Minas Geraes: Campis graminosis, locis siccis, in Serra do Papagaio, Alvaro Silveira, Nov. 1897; no. 2609 in Herb. Comm. Geogr. et Geolog. Minas Geraes (BONN-Nessel 52.5, P, RB 36958).
Urostachys orgaosanusNessel, Arch. Bot. Est. S. Paulo 1: 380. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys christii (Silveira) Nessel var. orgaosanus (Nessel) Nessel, Bärlappgewächse 47. 1939. - Type: Brazil: Rio de Janeiro: Serra dos Orgãos, 1896, Ule s.n. (holotype B n.v., isotype BONN-Nessel 53).
Plants terrestrial, erect or ascending from a decumbent base, soft, usually forming small loose clumps, 10-20(35) cm long. Shoots homophyllous, almost equally thick throughout, 7-18 mm in diam. incl. leaves. Stems excl. leaves (1.5-)2-4 mm thick at base, sometimes tapering to 1-2 mm in diam., ridged by decurrent leaves or almost smooth, sporangiate from ca. 5-20 cm above the base and upward, usually 2-5 times dichotomous. Leaves borne in alternating, often irregular and oblique, whorls of (4-)5(-6), these ca. 1-2.5 mm apart, forming (8-)10(-12) longitudinal ranks, usually loosely appressed or ascending, sometimes spreading to recurved in shaded plants, straight to slightly upward curved, subulate to linear-lanceolate, widest at or just above the base, (4-)5-8(-9) × (1-)1.2-1.8(-2) mm, subcoriaceous or sometimes softly herbaceous, adaxially usually concave, but often convex in spreading leaves, abaxially usually convex and evenly rounded especially near the leaf base, with slightly prominent veinal ridge, or flat to concave in recurved leaves, with flat to slightly involute, sparsely to very densely denticulate or short-ciliolate, often distinctly sclerified margins, amphistomatic. Leaf bases often somewhat decurrent. Sporangia 1.3-1.8 mm wide.
Distribution and habitats: Endemic. Terrestrial in open places (mountain tops, grasslands, road banks) in the states of Minas Gerais, Espírito Santo, Rio de Janeiro, Paraná, Santa Catarina, Rio Grande do Sul, alt. 800-2,500 m.
Phlegmariurus christii is related to P. reflexus (Lam.) B. Øllg., but differs by its wider, usually abaxially convex, usually appressed leaves. Phlegmariurus christii generally belongs to a higher altitude range than does P. reflexus, but the two species are often found growing together.
The leaf margin characters of Phlegmariurus christii are somewhat variable. Both in slender and more robust individuals the leaf margin teeth may be remote to very dense. The type of Urostachys orgaosanus has few teeth, while the type of P. christii has dense teeth. See Phlegmariurus rostrifolius (Silveira) B. Øllg. for a discussion of its affinity with the present species.
Reference specimens (14 collections studied): BRAZIL. ESPÍRITO SANTO: Ibitirama, P.N. Caparaó, Pico do Calçado, 2,842 m, Souza et al. 1279 (BHCB, photo AAU). MINAS GERAIS: Serra do Caparaó, 2,100 m, Windisch et al. 4966 (AAU, HB). Camanducaia, Vila Monte Verde, Serra da Mantiqueira, 1,950 m, Windisch et al. 6070 (AAU, HB). Serra do Picú, 1,800 m, Schwacke et al. 5293 (BHCB, P, RB). Serra de Ibitipoca, Schwacke 12309 (BHCB, P). PARANÁ: between Quatro Barras and Morretes, Serra do Mar, 800 m, Windisch et al. 4892 (AAU, HB). RIO DE JANEIRO: Resende, P.N. Itatiaia, at origin of rio Aiuruoca, 2,370 m, Salino 12441 (BHCB). Serra do Itatiaia, 2,300 m, IX.1913, Brade 6550 (HB, NY, S, SP). Serra dos Orgãos, Pedra do Sino, Campo, 2,200 m, 31.VII.1940, Brade 16526.2 (AAU, CESJ, RB). Corcovado, 700 m, v. Luetzelburg 13422 (M). Teresópolis, Pedra do Sino, 2,000 m, 8.9.1929, Brade 9632A (R); Brade 9632B (R). RIO GRANDE DO SUL: Cambará, campos de cima da Serra, entre rochas, Windisch 9672 (ICN). SANTA CATARINA: Campo Alegre, Morro do Iquererim, 1,300 m, Reitz & Klein 6440 (HBR, US). São Francisco do Sul, Morro do Campo Alegre, 1,200 m, Reitz & Klein 10494 (HBR). SÃO PAULO: Campos do Jordão, road to São José dos Alpes, ca. 10 km from Horto Florestal, 1,800 m, Windisch et al. 4996 (AAU, HB). Serra do Mar, road Cunha-Parati, 1,450 m, Windisch et al. 5007 (AAU).
Phlegmariurus comans (Nessel) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 2d-f
Urostachys comansNessel, Arch. Bot. Est. S. Paulo 1: 400. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535., as a nom. nov. for Lycopodium comansChrist, in Schwacke, Pl. Nov. Mineiras 40. 1900Christ H (1900) Spicilegium Pteridologicum Austro-Brasilense. In: Schwacke W (ed.) Citade de Minas (Ouro Preto), Minas Geraes. Plantas Novas Mineiras 2: 12-42, t. 1-4., non Hook. f. 1844. - Urostachys pollucisHerter, Index Lyc. 76. 1949Herter W (1949) Index Lycopodiorum, Montevideo. Pp. 1-120., nom. superfl. - Huperzia comans (Nessel) B. Øllg. & P.G. Windisch, Bradea 5: 8. 1987. - Type: Serra do Itatiaia, 2,300 m, Ule (255) 3537 (holotype P, isotype HBG). Christ in the reprinted version of the original publication (Bull. Herb. Boiss. ser. 2, 2: 703. 1902) added one further collection (Glaziou 5225), which is not a type.
Lycopodium verticillatum L. f. var. apertumFée, Crypt. Vasc. Brésil 222. 1869Fée ALA (1869) Cryptogames vasculaires (fougères, lycopodiacèes, hidropteridèes, equisetacèes) du Brésil. I. Partie. J.B. Baillière et. Fils: V. Masson et Fils, Paris. 346p.. - Type: Rio de Janeiro: Serre des Orgues, Glaziou 3312 (BR, P, RB, S).
Plants epiphytic or rupestral, slender, pendulous, or scrambling-hanging over rocks, often with strongly diverging dichotomies, at least to 60 cm long. Shoots homophyllous, or gradually heterophyllous, 3-8(-12) mm in diam. incl. the leaves in proximal divisions, sometimes tapering to 2-6 mm in distal divisions. Stems excl. leaves 0.8-1 mm thick at the base, tapering to ca. 0.4-0.7 mm upward, largely concealed by the leaves, pale greenish to stramineous, at least to 10 times dichotomous, usually densely omnilaterally sporangiate in repeated, short, periodically produced zones of the distal divisions from 5-15 cm above the base and upward. Leaves gradually slightly modified along the stems. Leaves of proximal divisions densely crowded, borne in irregular alternating whorls of 5-6, these 0.5-2 mm apart, forming 10-12 indistinct longitudinal ranks, ascending to closely imbricate or somewhat secund, acicular to linear-subulate, narrowly and prominently decurrent, 4-7 × (0.3-)0.4-0.6 mm wide just above the widened leaf base, firmly herbaceous to coriaceous, somewhat lustrous, convex below, canaliculate above. Vegetative leaves of distal constricted divisions borne in irregular whorls of 3-4, forming 6-8 indistinct longitudinal ranks, essentially conform, but usually slightly shorter, 3-5 mm long. Sporangiate leaves shorter and wider, or sometimes conform, usually lanceolate to linear-lanceolate and long acuminate, patent to appressed, (2-)3-6 × 0.5-0.8(-1) mm, abaxially convex. Sporangia ca. 1-1.3 mm in diam.
Distribution and habitats: Endemic. Epiphytic and rupestral in high altitude montane and cloud forest in the states of Minas Gerais, Rio de Janeiro (Serra da Mantiqueira, and Serra dos Orgãos), São Paulo, and Paraná, alt. ca. 1,200-2,400 m, the lower altitudes in the southern states.
More compact and shorter, but otherwise approximately twice as large in all parts as Phlegmariurus acerosus in the same area. In the southern states some of the material has thinner leaves and slightly thinner stems. The type material of Lycopodium verticillatum var. apertum represents a shaded, lax, epiphytic form of the species.
Reference specimens (14 collections studied): BRAZIL. MINAS GERAIS: Passa Quatro, Pico do Muro, 1,800 m, Brade 18996 (RB). Serra do Papagaio, Silveira 2608 (P). PARANÁ: Campina Grande do Sul, Pico Paraná, 1,500-1,700 m, Hatschbach 19510 (MBM). Monte Alegre, Serra do Mar, ca. 1,200 m, Dusén 3378 (BONN, P). Quatro Barras, Morro Mãe Catira, Hatschbach 15076 (MBM). RIO DE JANEIRO: Serra dos Orgãos, Morro Assu, Luetzelburg 6020 (M, S, US). Serra do Itatiaia, 2,400 m, Brade 6509 (HB, S, US). Planalto do Itatiaia, between Prateleiras and Pedra Assentada, 2,300 m, Tryon & Tryon 6699 (HB, F, GH). Itatiaia, Pedra Assentada, 2,300 m, Brade 15564 (AAU). Resende, S face of Mt. Itatiaia, above Macieiras, 2,020 m, Eiten & Eiten 7610 (K, SP, UB, US). Serra dos Orgãos, Pedra Assú, 2,000 m, Brade 16509 (AAU, RB). SÃO PAULO: Campos do Jordão, Mattos 15907 (SP).
Phlegmariurus deminuens (Herter) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 7a,b
Lycopodium deminuensHerter, Bot. Jahrb. 43: Beibl. 98: 44. 1909Herter W (1909) Ein neuer beitrag zur Kenntnis der Gattung Lycopodium. Hedwigia 49: 88-92, t. 3.. - Urostachys deminuens (Herter) Nessel, Arch. Bot. Est. S. Paulo 1: 384. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia deminuens (Herter) B. Øllg., Opera Bot. 92: 169. 1987. - Type: Minas Geraes, A. de St. Hilaire, Catal. D. n. 248 (holotype P, isotype Herb. Nessel BONN).
Urostachys luederwaldtiiNessel, Repert. Sp. Nov. Regni Veg. 36: 178, t. 170. 1934Nessel H (1934) Neue Lycopodien, die von allen schon bekannten Arten durch ihren Habitus ganz besonders abweichend und auffallend sind. Repertorium Specierum Novarum Regni Vegetabili 36: 177-193, t. 170-177.. - Huperzia luederwaldtii (Nessel) Holub, Folia Geobot. Phytotax. 20: 74. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Minas Geraes, Serra do Itataiaya, Agulhas Negras, 1.V.1906, Luederwaldt s.n. (holotype BONN-Nessel 41, isotype SP 17990).
Plants terrestrial, stiffly erect, to ca. 30 cm tall, to 4(-6) times dichotomous, sometimes with densely aggregated shoots from the base, but without prostrate basal, juvenating shoots. Shoots homophyllous, or with gradually shorter leaves upward, 10-15 mm in diam. incl. leaves at the base, usually tapering to 5-8 mm upward, sporangiate from ca. 10 cm above the base and upward. Stem excl. leaves 3-5 mm thick at the base, upward tapering to ca. 1-3 mm, completely concealed by leaves. Leaves densely crowded and usually closely appressed, borne in alternating whorls of 6-ca. 8, these ca. 1 mm apart, forming ca. 12-ca. 16 indistinct longitudinal ranks, straight to slightly upward curved, linear-subulate to linear-lanceolate, 8-14 × 1-1.3 mm, adaxially concave with slightly prominent vein, abaxially convex and rounded with indistinct to slightly prominent vein, soft-herbaceous to subcoriaceous, dull to lustrous, with smooth margins. Leaves of upper, densely sporangiate divisions borne in alternating whorls of 6-7, these ca. 1 mm apart, forming 12-14 longitudinal ranks, linear-lanceolate to lanceolate, 4-7 × ca. 1 mm, otherwise conform. Sporangia ca. 1.5-2 mm wide.
Distribution and habitats: Endemic. Rare, high altitude campos in the states of Minas Gerais and Rio de Janeiro, alt. 1,000-1,800 m.
With erect fingerlike distal divisions, in which the leaves are appressed, and strongly convex abaxially. Related to Phlegmariurus treitubensis (Silveira) B. Øllg. and P. regnellii (Maxon) B. Øllg., but softer and more slender in most parts, and apparently a more extreme adaptation to exposed growth conditions than those. Several of the collections of this species have been badly damaged by insects.
Reference specimens (14 collections studied): BRAZIL. MINAS GERAIS: Serra de Ibitipoca, Pico do Pião, Arenito da Serie da Lavras, SW, 1,580-1,600 m, Sucre & Krieger 6766 (AAU, RB). Serra do Picú, in campis elevatis, 1,800 m, Schwacke 5293 (P). Lima Duarte, P.E. Ibitipoca, Pico do Pião, 1,664 m, Almeida et al. 1154 (BHCB). RIO DE JANEIRO: Itatiaia, Pedra do Altar. 2,400 m, Brade 15562 (RB).
Phlegmariurus dichotomus (Jacq.) W.H. Wagner, Novon 3: 305. 1993Wagner WH (1993) A new combination for a North American Lycopod. Novon 3: 305. . Fig. 6a,b
Lycopodium dichotomum Jacq., Enum. Stirp. Vind. 314. 1762. - Huperzia dichotoma (Jacq.) Trevis., Atti Soc. Ital. Sci. Nat. 17: 248. 1874. - Urostachys dichotomus (Jacq.) Herter, Beih. Bot. Centralbl. 39: 249. 1922Herter W (1922) Itinera Herteriana III. Heteropteridophyta austroamericana. (Equisetales Lycopodiales Selaginellales Isoëtales austroamericanae.) Beihefte Botanisches Centralblatt 39: 248-256.. - Type: Fide Proctor, in Howard, Fl. Less. Antilles 2: 28. 1977Proctor GR (1977) Pteridophyta. In: Howard RA (ed.). Flora of the Lesser Antilles, Leeward and Windward Islands 2: 1-414.: Jacq., Hort. Bot. Vind. 3: t. 45. 1766, lectotype, an authentic specimen is in BM (Exemplar, quod collegi in insula Martinica, et quod iconi inservitt mihi olim).
Lycopodium gramineumSpring, Mém. Acad. Roy. Sci. Belgique 24 [Mon. Lyc. 2]: 19. 1849Spring A (1849) Monographie de la famille des Lycopodiacées, seconde partie. Mémoires de l’Académie Royale des Sciences, Lettres et Beaux Arts de Belgique 24: 1-358.. - Urostachys gramineus (Spring) Herter, Index Lyc. 63. 1949Herter W (1949) Index Lycopodiorum, Montevideo. Pp. 1-120.. - Type: Ecuador, prov. Guayaquil, Jameson s.n. (holotype K).
Lycopodium barbatumChrist, Bull. Herb. Boiss. II, 5: 254. 1905Christ H (1905) Primitiae Florae Costaricensis. Filices et Lycopodiaceae. III. Bulletin de l’Herbier Boissier, sér. 2, 5: 248-260., non Kaulf. 1824. - Urostachys barbatus (Christ) Nessel, Bärlappgewächse 30. 1939Nessel H (1939) Die Bärlappgewächse (Lycopodiaceae). G. Fischer, Jena. 404p.. - Type: Costa Rica, Werclé s.n. in herb. Christ (holotype P; isotype fragment BONN-Nessel 7).
Lycopodium chamaepeuceHerter, Bot. Jahrb. 43: Beibl. 98: 50. 1909Herter W (1909) Ein neuer beitrag zur Kenntnis der Gattung Lycopodium. Hedwigia 49: 88-92, t. 3.. - Urostachys chamaepeuce (Herter) Herter, Repert. Spec. Nov. Regni Veg. 19: 164. 1923. - Type: None designated.
Urostachys chamaepeuce (Herter) Herter var. urbanianus Herter, Repert. Sp. Nov. Regni Veg. 19: 164. 1923Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.. - Type: Grenada, Annandale, II.1906, Wideway 3457 (B n.v.; isosyntype NY), 3458b (B n.v.).
Huperzia lindeneri (Nessel) Holub, Folia Geobot. Phytotax. 20: 74. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Urostachys lindeneri Herter ex Nessel, [Bärlappgewächse 153. 1939, nom. inval.]; Revista Sudamer. Bot. 6: 164, excl. fig. 1940. - Type: Chilion, Mazatenango, Guatemala, Bernoulli & Cario s.n. (holotype BONN-Nessel 350).
Huperzia schlechtendalii (Nessel) Holub, Folia Geobot. Phytotax. 20: 76. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Urostachys schlechtendalii Herter ex Nessel, [Bärlappgewächse 1939, nom. inval.]; Revista Sudamer. Bot. 6: 164, t. 11, f. 51. 1940. - Type: “Guaveyma” Mexico, Wagner s.n. (holotype BONN-Nessel 349 p. p.).
Huperzia mortonii (Herter) Holub, Folia Geobot. Phytotax. 20: 75. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. -Urostachys mortonii Herter, in Morton, Repert. Sp. Nov. Regni Veg. 28: 108. 1930. - Syntypes from Guatemala, (B, n.v.).
Published illustrations: Lellinger, 1989Lellinger DB (1989) The ferns and fern-allies of Costa Rica, Panama, and the Chocó. (Part 1: Psilotaceae through Dicksoniaceae). Pteridologia 2A: 1-364.: fig. 23; Øllgaard, 1988Øllgaard B (1988) Lycopodiaceae. In: Harling G & Andersson L (eds.) Flora of Ecuador 33: 1-155.: p. 95 fig. 19 D.
Plants usually epiphytic, pendulous or recurved from an erect base, lax to subrigid, sparsely to densely and spreadingly branched, usually to 25(-50) cm long. Shoots homophyllous, almost equally thick throughout, 20-25 mm in diam. incl. leaves near the base, usually tapering to (10-)15 mm in densely sporangiate distal divisions. Stem excl. leaves 2-3 mm thick at the base, sometimes tapering to 1.5-2 mm, usually almost concealed by leaves, ridged by decurrent leaf bases, sporangiate from 5-15 cm above the base and upward, usually 3-5 times dichotomous. Leaves borne in alternating whorls of 5-6, these 1-2 mm apart, forming 10-12 indistinct longitudinal ranks, spreading to ascending, usually somewhat obliquely falcate, in distal divisions ascending to somewhat appressed, or appressed throughout, linear-subulate, gradually tapering from the base, (8-)10-15(-17) × 0.7-1 mm, almost flat, with slightly prominent vein above, with flat or slightly revolute margins, abaxially with slightly to sharply prominent vein, soft-herbaceous, usually twisted at the base. Leaf bases long and prominently decurrent, green, as wide as, or wider than the lamina, with sharply prominent vein. Sporangia 1-1.5 mm in diam.
Distribution and habitats: Northern Tropical America. Florida, West Indies, Central America, Northern South America, Galapagos Islands, Brazil (Roraima, Amapá, Acre, Mato Grosso, Ceará, Pará, Rondonia, Roraima), amazonian Bolivia.
Epiphytic in riverine, lowland and premontane forests, 0-1,400 m.
The number of synonyms of this species reflects its variability throughout its range. Closer study of the material referred to this species may show the presence of more than one taxon, especially in the northern part of the range, because there is considerable variation in the thickness of stems, leaf length and direction, and compactness of the plants. However, the correlation of the variation with growth conditions is poorly known at this point.
Reference specimens (18 collections studied): BRAZIL. AMAPÁ: E of Vila Breu, in branch of Rio Breu, Igapó forest, 5.XI.79, Austin 7318 (NY, US). Rio Araguari, at mouth of Anicahy, 8.X.1961, Pires et al. 51574 (NY). Rio Oiapoque, 52º39’W, 2º18’N, Irwin et al. 48337 (NY p.p.). CEARÁ: Schwacke 956 (RB). MATO GROSSO: Capão Seco, near Santa Ana de Chapada, Capão, 14.I.1894, Lindman A2705 (S, US). Serra da Chapada, Burití, 3.VI.1894, Malme 1664 (P, S, US). Rio Teles Pires, RPPN Lote Cristalino, 240 m, Henicka et al. 82 (INPA). PARÁ: Belém, São Jerônimo, VIII.1938, Chermont (F). Tucuruí, área de desmatamento, Ramos 854 (INPA). Canaã dos Carajás, Floresta Nacional de Carajás, Serra Sul, corpo A, 681 m, Almeida et al. 2196 (BHCB, photo AAU); Souza 1121 (BHCB, photo AAU). Rio Curua, 54º92’W, 0º95’S, sea level, Strudwick & Sobel 4059, 4400 (NY). RONDONIA: Porto Velho-Cuiabá road, near Santa Barbara, E of km 117, Prance & Ramos 6947 (NY, US). Vilhena, Mata de transição floresta/cerrado, relevo plano, Craziela et al. 167A (RB). RORAIMA: Ilha de Maracá, SEMA Ecological Reserve, Milliken 84 (AAU).
Phlegmariurus erythrocaulos (Fée) B. Øllg., Rodriguésia 63(2): 480. 2012 [as erythrocaulon]. Fig. 5d-f
Lycopodium erythrocaulon Fée, Crypt. Vasc. Brésil 2: 95, t. 106, f. 2. 1872-73. - Urostachys erythrocaulon (Fée) Nessel, Arch. Bot. Est. S. Paulo 1: 421. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia erythrocaulon (Fée) Holub, Folia Geobot. Phytotax. 20: 72. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Brasilia fluminensi, Glaziou 5221 (B, C, K, P, S).
Rupestral, scrambling to hanging, slender, to 25 cm long. Shoots heterophyllous. Proximal divisions, to 20 cm from the base, ca. 10-20 mm in diam. incl. the expanded leaves. Distal divisions abruptly constricted to (1.3-)1.5-2 mm in diam. incl. the imbricate, small leaves, to 12 cm long, subterete. Stems excl. leaves 0.8-1 mm thick at the base, upward tapering to ca. 0.6-0.8 mm, greenish to bright red, at least to 10 times dichotomous. Expanded leaves of proximal divisions, usually decussate or subdecussate to alternate, sometimes borne in alternating whorls of 3, the pairs or whorls 1.5-5 mm apart, usually widely spaced in alternate-leaved stem portions, perpendicular to falcately ascending, lanceolate to linear-lanceolate, widest just above the leaf base or to the middle, 6-15 × 1.3-2.3 mm, soft- herbaceous to subcoriaceous, with flat to slightly revolute margins, the lamina sometimes twisted to vertical position from the base. Leaves of constricted distal divisions decussate, or subdecussate, or often borne in irregular, alternating whorls of 3, the leaf pairs or whorls 1-2 mm apart, usually sporangiate throughout, appressed and clasping with their margins, abaxially rounded to apically carinate, widely lanceolate to widely ovate or suborbicular, acute to acuminate, 1-2(-3) × 1-1.5 mm, equalling to more than twice as long as the sporangia. Sporangia ca. 1 mm in diam.
Distribution and habitats: Endemic. Bahia, Minas Gerais, Rio de Janeiro.
Closely related to Phlegmariurus biformis, P. phylicifolius (Desv. ex Poir.) B. Øllg., and other members of the P. myrsinites group, and apparently adapted and restricted to open epiphytic, or more commonly rupestral or terrestrial habitats in “campo de altitude” on Mount Itatiaia and Passa Quatro, Campo do Muro and Itaguará (Minas Gerais), alt. 1,850-2,400 m. It is more robust, more divaricately branched, slightly more coriaceous-leaved, and more red-colored than Phlegmariurus biformis.
Reference specimens (15 collections studied): BRAZIL. BAHIA: Rio de Contas, Pico das Almas, 1,850 m, Matos 1052 (photo <http://www.fernsoftheworld.com/2014/01/17/phlegmariurus-erythrocaulon>). MINAS GERAIS: Passa Quatro, Campo do Muro, 1,900 m, Brade 19102 (RB). Catas Altas, RPPN Santuário do Caraça, Pico do Sol, 1,865 m, Oliveira 515 (BHCB, photo AAU). RIO DE JANEIRO: Itatiaia, vicinity of Agulhas Negras, near Pedra Altar, 2,300 m, Tryon & Tryon 6686 (AAU, F, GH). Resende, Itatiaia, Base of Pedra Assentada, 2,300 m, Eiten & Eiten 6600 (K, SP, UB, US).
Phlegmariurus flexibilis (Fée) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 8a-d
Lycopodium flexibile Fée, Crypt. Vasc. Brésil 2: 94, t. 105, f. 3. 1872-73. - Urostachys linifolius (L.) Herter var. flexibilis (Fée) Nessel, Arch. Bot. Est. S. Paulo 1: 416. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys flexibilis (Fée) Herter, Index. Lyc. 61. 1949. - Huperzia flexibilis (Fée) B. Øllg., Opera Bot. 92: 169. 1987. - Type: Brasilia fluminensi, Glaziou 5220 (holotype P, isotype C).
Lycopodium linifolium L. var. sanguineumSpring, Mém. Acad. Roy. Sci. Belgique 15: 31. 1842Spring A (1842) Monographie de la famille des Lycopodiacées, premiére partie. Mémoires de l’Académie Royale des Sciences, Lettres et Beaux Arts de Belgique 15: 1-110. . - Urostachys linifolius (L.) Herter var. sanguineus (Spring) Nessel, Arch. Bot. S. Paulo 1: 416. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Syntypes (according to protologue): Sellow, Beyrich (B); ad Parà, Martius (M); in prov. Ceara, fr. Aug.-Nov.: Gardner (G-Deless.).
Lycopodium linifolium L. var. subaristatumChrist, in Schwacke, Pl. Nov. Mineiras 41. 1900Christ H (1900) Spicilegium Pteridologicum Austro-Brasilense. In: Schwacke W (ed.) Citade de Minas (Ouro Preto), Minas Geraes. Plantas Novas Mineiras 2: 12-42, t. 1-4. [Bull. Herb. Boiss. 2 ser., 2: 705. 1902]. - Syntypes: S. Francisco, Ule 77 (P); Blumenau, Moeller 74 (P).
Published illustrations: Fée 1872-73: t. 105, fig. 3.
Plants epiphytic, pendulous, usually with flaccidly hanging divisions, at least to 70 cm long, the distal divisions often aggregated in fasciculate clusters. Shoots homophyllous or gradually heterophyllous, equally thick throughout, 15-35 mm in diam. incl. leaves, or gradually tapering to 6-15 mm in diam. in distal, densely sporangiate divisions. Stems excl. leaves 0.4-0.8 mm thick at the base, slightly tapering upward, slightly to strongly flexuous, making a sharp bend at each leaf attachment, pale greenish to red tinged, sporangiate from 5-30 cm above the base and upward, at least to 10 times dichotomous. Leaves of proximal divisions singly inserted, or occasionally in few opposite pairs, not producing evident longitudinal ranks, subdistant, 1-5 mm apart, soft-herbaceous, spreading to ascending, straight to slightly falcately ascending, usually with the lamina vertical due to a twist of the lamina base, linear-lanceolate to lanceolate, widest in the basal third or quarter, distinctly narrowed into a petiole-like, twisted, usually perpendicular or ascending lamina base, 12-21 × 1-2.5 mm, flat, or with slightly revolute, smooth margins. Leaves of middle and distal divisions spirally arranged, paired or borne in irregular alternating whorls of 3, conform, or usually narrower, 5-15 × 0.8-1.5 mm, often with a long, narrowly subulate, somewhat convolute or subcarinate apex from a subauriculate, non-twisted lamina base. Sporangia ca. 1.5 mm in diam.
Distribution and habitats: Endemic. Epiphytic, especially in montane forest in the states of Espírito Santo, Minas Gerais, Rio de Janeiro, São Paulo, Paraná, and Santa Catarina, alt. 30-1,350 m, the lower altitudes in the southern part of the range.
A close relative of Phlegmariurus linifolius (L.) B. Øllg., from which it is easily distinguished by the reddish, thin, usually strongly flexuous stem. In Atlantic Brazil Phlegmariurus flexibilis is replaced by P. linifolius ssp. jenmanii (Underw. & F. E. Lloyd) B. Øllg. & P.G. Windisch, in Northern Espírito Santo.
The syntypes of Lycopodium linifolium L. var. sanguineum Spring include material belonging to Phlegmariurus flexibilis, but no specimens annotated by Spring were seen.
Nessel applies the combination Urostachys schwendeneri (Herter) Herter var. subaristatus (Christ) Nessel, Bärlappgewächse 167. 1939, to Lycopodium linifolium L. var. subaristatum Christ 1900[1902], not Christ, Bull. Soc. Bot. Genève, ser. 2, 1: 236. 1909 (type from Costa Rica), although the reference for Brazil is cited for the basionym. Explicitly excluding the Brazilian material in his discussion, Nessel thus invalidates his own combination.
Reference specimens (43 collections studied): BRAZIL. MINAS GERAIS: Aiuroca, 1878, Wittig in Schwacke 955 (RB). Felicio dos Santos, APA Felicio, Mata do Isidoro, near Pico Dois Irmãos, 1,150-1,350 m, Salino 9967 (BHCB, photo AAU). ESPÍRITO SANTO: Cachoeira do Itapemirim, Vargem Alta, Corrego d’Ouro, 23 May 1949, Brade 19883 (RB). PARANÁ: Antonina, Rio Pequeno, 31.X.1973, Hatschbach 32989 (MBM). Campina Grande do Sul, Rio Pardinho, 22.I.1961, Hatschbach 7818 (US). Morretes, Rio Sagrado de Cima, 100-300 m, Hatschbach 19586 (C, MBM). Porto de Cima, 29.III.1912, Dusén 14013 (S). RIO DE JANEIRO: Barreira de Teresópolis, Rio Soberbo, Duarte 5739 (HB). Magdalena, Mata de Rifa, Emygdio Mello 1194 (R). Santo Antonio de Imbé, Agulha, Brade & Santos Lima 11661 (R). SANTA CATARINA: Azambuja-Brusque, alt. 35 m, 7.IX.1948, Reitz 2204 (RB). Joinville, 30 m, Pirabeiraba, Schmalz 153 (NY, S). Morro do Antão, Ilha Santa Catarina, 250 m, Sehnem 813 (HBR). Governador Celso Ramos, Vargem do Macário, 5 m, Klein & Bresolin 9954 (HBR). Santo Amaro da Imperatriz, Pilões, 300 m, Reitz & Klein 3556 (HBR). Azambuja-Brusque, 100 m, Reitz C1802 (RB, S). SÃO PAULO: Iguape, Morro das Pedras, 1,920 m, Brade 8500 (HB); Rio Peroupava, Boa Vista, 1916, Brade 8499 (HB). Campo Grande, 800 m, 1.II.14, Brade 6928 (HB, S). Butantan, 3.VI.1917, Hoehne (SP 281).
Phlegmariurus fontinaloides (Spring) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 9e-h
Lycopodium fontinaloidesSpring, in Mart., Fl. Bras. 1(2): 112, t. 5, II. 1840Spring A (1840) Lycopodium. In: Martius (ed.) Flora brasiliensis l: 109-117, t. 5. . - Lycopodium fontinaloides Spring var. brasiliense Spring, Mém. Acad. Roy. Sci. Belgique 15: 49. 1842. [Mon. Lyc. 1: 49]. - Huperzia fontinaloides (Spring) Trevis., Atti Soc. Ital. Sci. Nat. 17: 248. 1874. - Urostachys fontinaloides (Spring) Nessel, Arch. Bot. Est. S. Paulo 1: 401. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Type: Sellow (B, GL, HBG, K, L, P).
Lycopodium serpyllifoliumFée, Crypt. Vasc. Brésil 1: 222, t. 73, fig. 3. 1869. - Urostachys fontinaloides (Spring) Nessel var. serpyllifolius (Fée) Nessel, Arch. Bot. Est. S. Paulo 1: 404. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys serpyllifolius (Fée) Herter, Index Lyc. 81. 1949Herter W (1949) Index Lycopodiorum, Montevideo. Pp. 1-120.. - Type: Rio de Janeiro: Haut des Orgues, 7 IX 68, Glaziou 2793 (holotype P, isotypes BR, C).
Urostachys gehrtiiNessel, Repert. Sp. Nov. Regni Veg. 36: 189-190, t. 177. 1934Nessel H (1934) Neue Lycopodien, die von allen schon bekannten Arten durch ihren Habitus ganz besonders abweichend und auffallend sind. Repertorium Specierum Novarum Regni Vegetabili 36: 177-193, t. 170-177.. - Huperzia gehrtii (Nessel) Holub, Folia Geobot. Phytotax. 20: 73. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Paraty, St. Catarina, 25.X.1928, Hoehne s.n. (holotype SP 23178, isotype NY).
Published illustrations: Øllgaard 1992Øllgaard B (1992) Neotropical Lycopodiaceae - an overview. Annals of the Missouri Botanical Garden 79: 687-717.: p. 704, fig. 11.
Plants epiphytic, lax and pendulous, at least to 70 cm long, at least to 10 times dichotomous. Shoots very slender, usually covered by small imbricate leaves throughout, usually homophyllous or with gradually smaller leaves in distal divisions, but sometimes with longer and wider expanded leaves in the proximal divisions; sporangiate in separate, seasonally produced zones or continuously sporangiate from 10-50 cm above the base and upward. Constricted shoots incl. leaves terete throughout, 0.7-1.5 mm in diam. incl. leaves at the base, toward the apex sometimes slightly thickening, 1-2 mm in diam. Stem excl. leaves 0.7-1(-1.3) mm thick at the base, usually bright red, tapering to 0.3-0.5 mm in distal divisions. Expanded leaves, if present, decussate, closely situated and usually continuously overlapping, elliptic to obovate with acute to acuminate or mucronulate apex and cuneate leaf base, usually 4-5(-6) × 2-2.5(-3) mm, flat, ascending to appressed, with smooth to minutely denticulate-verruculate, often reddish margins. Leaves of proximal and upper vegetative constricted divisions decussate (rarely a few whorls of 3 leaves present in approx. 1 cm of the stem base), the leaf pairs (1-)1.5-2(-4) mm apart, usually appressed or with slightly diverging apex, lanceolate-ovate to widely ovate, clasping, with short-decurrent leaf-base margins, acute to subobtuse, often with pale margins and apex, abaxially evenly rounded, softly to firmly herbaceous, dull to lustrous, 1.5-2.5 × 1-1.3(-1.5) mm, with smooth to minutely denticulate-verruculate margins. Sporangiate leaves usually as long as wide, 1-1.5 mm long and wide, equalling or slightly exceeding the sporangia. Sporangia ca. 1 mm wide.
Distribution and habitats: Endemic. Delicate pendulous epiphytes in montane and nebular forest, and Araucaria formations, alt. 500-2,400 m. States of Minas Gerais, Espírito Santo, Rio de Janeiro, São Paulo, Paraná, Santa Catarina, and Rio Grande do Sul.
Perhaps related to Phlegmariurus hexastichus (B. Øllg. & P.G. Windisch) B. Øllg. and P. quadrifariatus (Bory) B. Øllg., but much more delicate with thin, red stems, and small, decussate, dorsally rounded, imbricate leaves throughout in the wholly constricted individuals. The vegetative and sporangiate leaves essentially similar. Some collections with only expanded leaves may reflect strongly shaded growth conditions.
Reference specimens (60 collections studied): BRAZIL. ESPÍRITO SANTO: Castelo, Braço do Sul, Brade 19302 (RB). MINAS GERAIS: Aiuruoca, RPPN do Matutu, Vale do Matutu, region of Macieira, Salino & Almeida 10439 (BHCB, photo AAU). Alagoa, P.E. Serra do Papagaio, Serra do Agostinho no Garrafão, Salimena 3311 (AAU). Serra do Caparaó, 1,900 m, Brade 17093 (AAU, GH, HB, US). Delfim Moreira, Fazenda Córrego Alegre, Kuhlmann & Gehrt (US2690480, SP40076). Lima Duarte, Baixada do Pião-Serra da Ibitipoca, Magalhães 503 (HB). PARANÁ: Campina Grande do Sul, Serra do Espia, 1,000-1,100 m, Hatschbach 9231 (MBM). Garatuva, cloud forest, Lindemann & Haas 5788 (Z). RIO DE JANEIRO: Macaé, Alto Macaé, 1,800 m, 12.III.1870, Glaziou 4471 (K, P). Nova Friburgo, Serra do Macahé, 1,500 m, Ule 4974 (HBG). Serra dos Orgãos, 1,800 m, X.1896, Ule (GH, RB 13485). Rio de Janeiro, Pico de Papagaio, forest of Tijuca, 900 m, Brade 8615 (AAU, HB, K, M, RB). Teresópolis, Krieger 2663 (AAU). RIO GRANDE DO SUL: Cambará do Sul, Fortaleza, s.col. s.d. (HAS 79989); Canion de Fortaleza, Silveira (HAS 79970). São Francisco de Paula, Alpes de São Francisco, ca. 900 m alt. Windisch 11065 (ICN). SANTA CATARINA: Blumenau, Spitzkopf, Viereck 111 (M). Itajaí, Morro do Baú, 850 m, Reitz 2991 (HBR, US). Joinville, 300-700 m, Schmalz 207 (F); Estrada Dona Francisca, 500 m, Reitz & Klein 4230 (HBR). Fachinal-Biguassú, 500 m, Reitz C943 (RB). Queimados, 900 m, Schmalz 154.8 (F, NY). Ilha Santa Catarina, Saco Grande, 400 m, Klein & Bresolin 8314 (HBR). Rio do Sul, Alto Matador, 800 m, Reitz & Klein 8894 (HBR). São Bento, 16.XII.1919, Fischer (SP 3739). SÃO PAULO: Campo Grande, 900 m, Brade 5850 (HB). Serra da Bocaina, 1,700 m, Brade 20651 (AAU, RB). Near Rio Grande at São Paulo Railway, 800 m, VI.1901, von Wettstein & Schiffner (P, Z). Campos do Jordão, 25.I.1935, Kuhlmann (SP 32258). Alto da Serra, Wacket in Rosenstock exs. 341 (GB, K, L). Iguape, Löfgren & Edwall 653 (SP). Rio Claro, Capuera, 20.X.1901, Löfgren (SP 17991).
Phlegmariurus friburgensis (Nessel) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 10a-c
Urostachys friburgensisNessel, Arch. Bot. Est. S. Paulo 1: 391. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys reflexus (Lam.) Herter var. friburgensis (Nessel) Nessel, Bärlappgewächse 114. 1939. - Lycopodium friburgense (Nessel) Rolleri, Revista Mus. La Plata, n. ser. 13 (Bot. 78): 191. 1984Rolleri C (1984) Notas nomenclaturales y taxonomicas en la sección Crassistachys Herter del género Lycopodium (Lycopodiaceae). Revista del Museo de La Plata, N.S. Bot. 13: 189-196.. - Huperzia friburgensis (Nessel) B. Øllg. & P.G. Windisch, Bradea 5: 10. 1987. - Type: Ao Norte do Rio de Janeiro (Alto Macahé), Glaziou 4476 (“RJ” (?=RB) lectotype, not seen, chosen by Rolleri (1984)Rolleri C (1984) Notas nomenclaturales y taxonomicas en la sección Crassistachys Herter del género Lycopodium (Lycopodiaceae). Revista del Museo de La Plata, N.S. Bot. 13: 189-196., BONN herb. Nessel no 217, P isotypes). See Rolleri (1984: 191-193) for discussion.
Plants terrestrial, erect or ascending from a decumbent base, soft, usually forming small loose clumps, 10-30(-40) cm tall. Shoots homophyllous, almost equally thick throughout, 7-13 mm in diam. incl. leaves. Stems excl. leaves (1-)2-2.5 mm thick at base, sometimes tapering to 1-1.5 mm in diam., ridged by decurrent leaves or almost smooth, sporangiate from 5-30 cm above the base and upward, usually 2-4 times dichotomous. Leaves borne in alternating, often irregular and oblique, whorls of 6(-7), these ca. 1-2 mm apart, forming 12(-14) longitudinal ranks, ascending to spreading or sharply reflexed, straight to strongly recurved, linear-subulate to linear-lanceolate, widest at the base to below the middle, 4.5-6 × 0.5-0.8 mm, subcoriaceous, or soft-herbaceous in shaded individuals, adaxially convex and usually somewhat lustrous, abaxially slightly concave to convex, with obscure to widely prominent vein, the vein usually shrunk (sulcate) when dried, with somewhat thick, flat to revolute, entirely smooth to very sparsely denticulate margins. Leaf bases often somewhat decurrent. Sporangia 1-1.5 mm in diam.
Distribution and habitats: Endemic. Terrestrial, on moist banks, rocks and boggy places in the upper montane forest zone. Altitudinal limits uncertain, due to incomplete label information. Rare, known from the states of Minas Gerais, Rio de Janeiro, and Paraná.
Phlegmariurus friburgensis is closely related to P. reflexus (Lam.) B. Øllg., especially the small form of that species, of which it may represent a high-altitude form. It differs from P. reflexus mainly in its more slender stems and more coriaceous leaves with most often entirely smooth margins.
Specimens studied: BRAZIL. ESPÍRITO SANTO: Castelo, Forno Grande, 1,600 m, Brade 19850 (AAU, F, GH, MO, NY, RB, SP, US). MINAS GERAIS: Serra do Caparaó, 1,800 m, Duarte 13961 (AAU, HB). Alto Caparaó, P.N. Caparaó, Cachoeira Bonita, 1,828 m, Almeida 3377 (BHCB, photo AAU). Serra de Ibitipoca, Krieger 8387 (AAU). PARANÁ: Sengés, Fda. Morungava, Rio do Funil, Hatschbach & Lange 5320 (MBM). RIO DE JANEIRO: Monts des Orgues, Glaziou 1790 (P). Alto Macahé, Glaziou 4476 (P). Rio de Janeiro, Estrada do Redentor, Ponte do Inferno, Duarte 4788 (AAU, RB).
Phlegmariurus hemleri (Nessel) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 11a,b
Urostachys hemleriNessel, Arch. Bot. Est. S. Paulo 1: 389, t. 9. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia hemleri (Nessel) B. Øllg., Opera Bot. 92: 169. 1987. - Lectotype: Rio de Janeiro: Arredores da Capital Federal, V.1905, Hemler s.n. (lectotype BONN-Nessel 167, designated by Øllgaard, Biol. Skr. Dan. Vid. Selsk. 34: 92, 1989Øllgaard B (1989) Index of the Lycopodiaceae. Biologiske Skrifter Kongelige Danske Videnskabernes Selskab 34: 1-135., isotype R). Syntype: Matto Grosso: Cuyabá, em logares humidos, barrancas sombrias, VIII.1909, Hartmann 106 (not seen).
Plants terrestrial, ascending to erect from a decumbent base, to ca. 35 cm tall or to ca. 60 cm long, sparsely branched, to 4 times dichotomous. Shoots homophyllous, equally thick throughout, ca.15-25 mm in diam. incl. leaves (depending on leaf direction), sporangiate from ca. 20-40 cm above the stem base and upward, often in separate, seasonally produced zones. Stems excl. leaves 2-4 mm thick at the base, sometimes tapering to ca. 1.5 mm upward, pale greenish white. Leaves borne in more or less regular, often oblique, alternating whorls of 4-5(-6), these 2-4 mm apart, forming 8-10(-12) indistinct longitudinal ranks, spreading to sometimes sharply reflexed, linear-lanceolate, widest just below the middle, 8-11 × 1.5-2 mm, not twisted at base, prominently decurrent, adaxially flat to somewhat convex (dried), or slightly downward folded along the vein, abaxially with slightly prominent vein, with pale, irregularly denticulate margins. Sporangia 1.5-2 mm wide.
Distribution and habitats: Endemic. Rare, at higher elevations (1,450-1,700 m) in the states of Mato Grosso (?), Minas Gerais and Rio de Janeiro (Serra dos Orgãos, Nova Friburgo), in montane forest and campo vegetation. One specimen, Ule 4666 (P) indicates the state of Santa Catarina as origin, but the same number from HBG indicates Nova Friburgo. The same sheet (HBG) has material apparently from Serra do Ouro Preto (leg. Schwacke). Hartmann 106 cited in the protologue, from Mato Grosso, Cuyabá, 1909, was not seen. It is probably a mislabelled specimen.
Phlegmariurus hemleri superficially resembles P. sellowianus (Herter) B. Øllg., but is easily distinguished by the denticulate leaf margins. The spores are well developed. Presumably belongs in shaded high-altitude habitats in forest.
Specimens studied: BRAZIL. MINAS GERAIS: Aiuruoca, Vale do Matutu, RPPN do Matutu, Cachoeira do Indio, 1,430-1,480 m, Salino 9763 (BHCB, photo AAU). Araponga, Parque Estadual da Serra do Brigadeiro, trail to Pico do Boné, Salino 5473 (BHCB, photo AAU). RIO DE JANEIRO: Nova Friburgo, Pedra do Conico, Ule 4666 (HBG, P). Petrópolis, Cortico, Spannagel 558 (BONN-herb. Nessel 143, S). Organ Mountains, between Soberbo and Guapy, 100-900 m, Smith 1524 (GH). Santa Maria Magdalena, Parque Estadual do Desengano, Pedra do Desengano, 1,600-1,700 m, Leitmann et al. 324 (AAU). Alto Macahé, Mendonça 1411 (BONN-herb. Nessel nº. 167). Teresópolis, Parque Nacional da Serra dos Orgãos, trail to Pedra do Sino, Sylvestre et al. 1925 (RB).
Phlegmariurus heterocarpos (Fée) B. Øllg., Rodriguésia 63(2): 480. 2012 [as heterocarpon]. Fig. 12d-f
Lycopodium heterocarpon Fée, Crypt. Vasc. Brésil, 2: 93. 1872-73. - Urostachys heterocarpon (Fée) Herter, Repert. Sp. Nov. Regni Veg. 19: 164. 1923Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.. - Huperzia heterocarpon (Fée) Holub, Folia Geobot. Phytotax. 20: 73. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Brasilia fluminensi, Rio de Janeiro, Tijuca, 18.V.1872, Glaziou 5636 (holotype P).
Lycopodium longearistatumChrist, in Schwacke, Pl. Nov. Mineiras 2: 40. 1900Christ H (1900) Spicilegium Pteridologicum Austro-Brasilense. In: Schwacke W (ed.) Citade de Minas (Ouro Preto), Minas Geraes. Plantas Novas Mineiras 2: 12-42, t. 1-4.. - Urostachys longearistatus (Christ) Nessel, Arch. Bot. Est. S. Paulo 1: 414. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys heterocarpon (Fée) Herter var. longearistatus (Christ) Nessel, Bärlappgewächse 156. 1939. - Huperzia longearistata (Christ) Holub, Folia Geobot. Phytotax. 20: 74. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Syntypes: Montagne du Signal de l’Ile St. Catarina, Ule 203 (P); S. Francisco, Ule 78 (P); Santa Catarina, Minas, Ule 322 (P) (probably = 2322 (HBG); Blumenau, Viereck 124 (M, P).
Urostachys spegazziniiNessel, Arch. Bot. Est. S. Paulo 1: 412. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys dichotomus (Jacq.) Herter var. spegazzinii (Nessel) Nessel, in: Hoehne, Fl. Brasilica 2, 2: 74. 1955. - Lectotype: Argentina: Missiones, La Platou, 28.III.1867, Spegazzini 2.420 (lectotype BONN-herb. Nessel, designated by Øllgaard 1989Øllgaard B (1989) Index of the Lycopodiaceae. Biologiske Skrifter Kongelige Danske Videnskabernes Selskab 34: 1-135. p. 101. - Syntypes: Rio de Janeiro: Binot no. 1.907, Norte de Petropolis, sobre árvores revestidas de musgos. - Procedencia incerta: Goldschmidt 96-A, em 9 de Março de 1869, registrado como var. II do Lyc. dichotomum Jacq.
Published illustrations: Øllgaard 1992Øllgaard B (1992) Neotropical Lycopodiaceae - an overview. Annals of the Missouri Botanical Garden 79: 687-717.: p. 698, fig. 7.
Epiphytic, lax and pendulous to spreading, or sometimes recurved from an erect and somewhat rigid stem base, at least to 60 cm long. Shoots more or less abruptly tapering from ca. 20-35 mm in diam. incl. leaves in proximal divisions, to (4-)5-15 mm in diam. in distal divisions, sometimes not, or only slightly tapering (juvenile or tardily sporangiate individuals); homophyllous or gradually heterophyllous, sporangiate from ca. 15-30 cm above the base and upward. Stems excl. leaves 1.5-2 mm thick at the base, tapering to 0.5-1.5 mm upward, prominently ridged by decurrent leaf bases, pale greenish to brownish, at least to 8 times dichotomous. Leaves usually reduced and modified upward, borne in alternating whorls or irregular low spirals of 3 or 4, forming 6-8 indistinct longitudinal ranks, the whorls 1.5-3 mm apart in proximal divisions, upward sometimes 1-2 mm apart. Leaves of proximal divisions spreading to ascending, usually slightly falcately upward curved, the lamina twisted to a vertical position from the base, linear to narrowly linear-lanceolate, widest at or near the base, evenly tapering, widely joined, somewhat clasping and seemingly adnate-decurrent on the stem, with pale leaf base center and long-decurrent green leaf base margins, soft-herbaceous to subcoriaceous, 10-20 × 1-1.5 mm, slightly concave to canaliculate adaxially, with flat or slightly involute, smooth margins, abaxially convex and evenly rounded, with evident to somewhat prominent vein. Sporangiate leaves of middle and distal divisions borne in alternating irregular whorls of 3-4, rarely decussate, conform, or gradually shorter, with a short, strongly widened, abaxially rounded (rarely subcarinate), clasping base, with a prominent vein, and usually an abruptly narrowed lamina beyond the base; lamina variable, conform to leaves of proximal divisions, to highly reduced to a short, narrow, involute cusp, 3.5-15 × 1.5-2 mm. Sporangia 1.5-2.2 mm wide.
Distribution and habitats: Pendulous epiphyte, sometimes epilithic; montane forest, and Araucaria formations in the states of Minas Gerais, Rio de Janeiro, São Paulo, Paraná, Santa Catarina, Rio Grande do Sul, and in Argentina (Missiones), alt. 500-1,900 m, the lower altitudes in the southern parts of the range.
Phlegmariurus heterocarpos appears to be related to P. loefgrenianus (Silveira) B. Øllg. and P. silveirae (Nessel) B. Øllg.. In some characters Phlegmariurus loefgrenianus seems intermediate between P. heterocarpos and species in the P. quadrifariatus group. And P. silveirae appears intermediate between P. heterocarpos and P. taxifolius (Sw.) A.Löve & D.Löve.
Reference specimens (more than 90 collections studied): BRAZIL. ESPÍRITO SANTO: Santa Teresa, Reserva Biológica de Santa Lucia, 647 m, Salino 8313 (BHCB, photo AAU). Divino de São Lourenço, Parque Nacional do Caparaó, Rio do Veado, Pedra Escorada, 1,190 m, Souza et al. 1447 (BHCB, photo AAU). MINAS GERAIS: Delfim Moreira, Fazenda, Córrego Alegre, 21.IV.1939, Kuhlmann & Gehrt (SP 40249, HB, K, M, US). Ouro Fino, Hoehne (SP 19568). Parque Florestal Estadual de Ibitipoca, Canion, Oliveira & Pita 401 (AAU). Caldas, 1869, Regnell III 1500 (BR). Carrancas, Serra do Perdizes, 1,560 m, Viana et al. 3329 (BHCB, photo AAU). PARANÁ: Campina Grande do Sul, Cerne, Hatschbach 7542 (MBM). Morretes, Pilão de Pedra, Hatschbach 7852 (L, MBM, US). Ipiranga, Serra do Mar, Dusén 6673 (E, NY, S); desvio Ipiranga, Dusén 6738 (K, US, Z). Morretes, Alto da Serra, 48º40’W, 25º28’S, 600 m, Windisch 4885 (AAU). Guaratuba, Rio Itararé, 950 m, 6.VI.1968, Hatschbach 4822 (US). Serra do Mar, upper Rio Corvo S of old road to Morretes, ca. 25 km E of Curitiba, 900-1,100 m, Lindeman & Haas 4072 (Z). RIO DE JANEIRO: Rio de Janeiro, Tijuca, Papagaio, Glaziou 2047 (BR, P). Serra dos Orgãos, Rio Paquequer, 1,000 m, 17.VII.1940, Brade 16411 (AAU, RB). Pico da Tijuca, 1,000 m, 2.VI.1929, Brade 21457 (HB). Itatiaia, bank of Rio Maromba, Campos Porto 1105 (AAU). RIO GRANDE DO SUL: Rio Pardo, Fazenda Soledade, Juergens in Rosenst., Fil. austrobras. exsicc. 225 (GH, M, P, US). Canela, 900 m, Richter RB 10244 (HB). São Francisco de Paula, near Passo do Inferno, Mansan (HAS 36635). SANTA CATARINA: Itajai, Morro Baú, 850 m, Reitz C2067 (RB, S). Fachinal-Biguaçú, 500 m, 18.I.1945, Reitz C925 (RB, S, US). Rio do Sul, Alto Matador, 700 m, 1.VIII.1958, Reitz & Klein 6850 (US). Blumenau, Spitzkopf, 50-997 m, 49º6’W, 26º53’S, 20.III.1952, Smith & Reitz 6257 (US). Itajaí, Morro do Baú, 850 m, Reitz 2990 (GH, HBR, P); Reitz C2067 (RB, S). Palhoça, Campo do Massiambu, 5 m, Reitz & Klein 589 (HBR). Vidal Ramos, Sabiá, 750 m, Reitz & Klein 4352 (HBR). Papanduva, Serra do Espigão, 1,000 m, Reitz & Klein 13065 (HBR). Lajes, Alto da Serra, Encruzilhada, 900 m, Reitz & Klein 13267, 13290 (HBR). Joinvile, Estrada Dona Francisca, 500 m, Reitz & Klein 4455 (HBR). Ilha da Santa Catarina, Morro Costa da Lagoa, 400 m, Klein 7847 (HBR). Rio dos Cedros, Represa Rio dos Cedros, 550 m, Reitz & Klein 3529 (HBR). Araranguá, Serra da Pedra, 700 m, Reitz C323 (RB). SÃO PAULO: Alto da Serra, 800-900 m, Smith 1919 (GH). Campos do Jordão, Fazenda São Francisco, 1,500 m, Windisch et al. 4997, 4998 (AAU, HB). Iguape, Ribeira, Brade 5143 (HB, S). São Paulo, Morro Jaraguá, 1,000 m, 11.VIII.1912, Brade 5282 (AAU, HB, NY). Campo Grande, Serra do Mar, Brade 6927 (AAU, HB, NY). Bananal, Serra da Bocaina, Sertão do Rio Vermelho, Brade 15209 (RB).
Phlegmariurus hexastichus (B. Øllg. & P.G. Windisch) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 13a-e
Huperzia hexasticha B. Øllg. & P.G. Windisch, Bradea 5: 11, fig. 2. 1987. - Type: BRAZIL: Estado de São Paulo: Campos do Jordão, estrada a São José dos Alpes, a ca. 12 km do Horto Florestal, matinha nebular, ca. 1,850 m alt., Windisch et al. 4995 (holotype HB, isotypes AAU, GH, SP).
Lycopodium fontinaloides var. adpressa Fée, Crypt. Vasc. Brésil, IIe partie: 95, 1872-73. - Type: Rio de Janeiro, Glaziou 3600 (isotypes P).
Published illustrations: Øllgaard & Windisch 1987Øllgaard B (1987) A revised classification of the Lycopodiaceae sensu lato. Opera Botanica 92: 153-178., fig. 2.
Plants epiphytic, initially recurved from an erect base, ultimately lax and pendulous, at least to 70 cm long, at least to 8 times dichotomous, sometimes forming large, heavy clumps. Shoots narrow, usually covered by small imbricate leaves throughout, usually homophyllous or with gradually smaller leaves in distal divisions, but sometimes with long expanded leaves in short portions of the proximal divisions, sporangiate in separate, seasonally produced zones from (7-)20-50 cm above the base and upward. Constricted shoots incl. leaves terete or bluntly angular at base, 1.5-2.5(-3) mm in diam. incl. leaves, distally becoming bluntly to sharply quadrangular, 1-2 mm in diam. Stem excl. leaves 1.5-2 mm thick at the base, not red, tapering to 0.5-1 mm in distal divisions. Expanded leaves (if present), linear to oblanceolate, 5-15 × 1.5-2.5 mm, with short-acute to obtuse apex, flat, with slightly revolute margins, spreading to ascending; transitional leaves to imbricate leaves intermediate, loosely appressed. Leaves of proximal constricted divisions usually borne in alternating whorls of 3, these 1-3 mm apart, forming 6 longitudinal ranks, or sometimes decussate, usually closely appressed, sometimes ascending, ovate to widely triangular-ovate, acute or subacute, abaxially rounded or with a blunt, prominent veinal ridge or carina, sometimes with sunken vein in the leaf base, widely and prominently long-decurrent, coriaceous, dull to lustrous, 1.5-2(-3) × 1.5-2 mm, with smooth to somewhat unevenly erose margins. Vegetative leaves of distal divisions decussate, smaller, 1.5-2 × 1-1.5 mm, often more sharply carinate, otherwise conform. Sporangiate leaves of densely sporangiate divisions often wider than long, 1-1.5 × 1-1.3 mm, equalling or slightly exceeding the sporangia. Sporangia ca. 1-1.3 mm wide.
Distribution and habitats: Endemic. Epiphytic in nebular forests of Southeastern Brazil, alt. 630-2,400 m. Minas Gerais, Espírito Santo, Rio de Janeiro, São Paulo, Paraná, Santa Catarina, and Rio Grande do Sul.
The closest relatives of this species are Phlegmariurus quadrifariatus, and P. fontinaloides. Phlegmariurus hexastichus differs from the former of these by the terete or bluntly angular proximal divisions, with the leaves usually 6-seriate. The proximal divisions of P. quadrifariatus are sharply quadrangular. Phlegmariurus fontinaloides differs from both by the very thin, usually red stems of proximal divisions, with small decussate leaves. Phlegmariurus hexastichus seems to be as common or more common than its nearest relatives.
Reference specimens (65 collections studied): BRAZIL. ESPÍRITO SANTO: Santa Teresa, 630 m, Stehmann 4117 (BHCB, photo AAU). MINAS GERAIS: Serra do Caparaó, 1,900 m, Brade 17092 (AAU, F, GH, NY, RB, S, US). Baependi, P.E. da Serra do Papagaio, banks of Rio do Charco, near Centro da Apoio ao Pesquisador, Furtado 66 (AAU). Passa Quatro, Sertão dos Martins, 1,400 m, Brade 19037 (AAU, RB); Pico do Muro, 1,800 m, Brade 18972 (RB, S). Camanducaia, Monte Verde, Pico do Bispo, 1,890 m, Melo et al. 62 (BHCB, photo AAU). Santa Maria do Salto, distrito de Talismã, Fazenda Duas Barras, 820 m, Salino 9446 (BHCB, photo AAU). PARANÁ: Campina Grande do Sul, Serra do Capivari Grande, 1,800 m, Hatschbach 8187 (MBM, US). Guaratuva, cloud forest on top, Lindeman & Haas 5788 (Z). Morretes, Serra Marumbí, Crista do Gigante, Curial 541 (RB, SI). RIO DE JANEIRO: Itatiaia, Pinheiral, 2,100 m, Brade 15563 (AAU, HB, MO, RB). Serra dos Orgãos, Pedra do Frade, 1,800 m, Brade 16565 (AAU, F, GH, RB, S). Fazenda do Taquaral, en montant aux Campos Brejos, Glaziou 7494 (C, F, K, P). RIO GRANDE DO SUL: Bom Jesus da Serra, Aparado da Rocinha, p. Bom Jesus, Rambo 45489 (S). SANTA CATARINA: Serra Geral, Serra do Oratório, Ule 302, 2302 (HBG, P). Bom Retiro, Campo dos Padres, 1,900 m, Reitz (US); 2,000 m, Reitz 3490 (S); 1,650 m, Smith et al. 7716 (HBR, US). Campo Alegre, Morro do Iquererim, 1,300 m, Reitz & Klein 6117 (HBR, US). Urubici, Morro da Igreja, 1,600 m, Reitz 2944 (RB). São José, Serra da Boa Vista, 1,000 m, Reitz & Klein 9860 (HBR). São Joaquim, Bom Jardim, Curral Falso, 1,500 m, Reitz & Klein 8399 (HBR). Urupema, Fazenda Farofa, 1,535 m, Salino et al. 11975 (BHCB, photo AAU). Bom Retiro, Campo dos Padres, 1,700 m, Sehnem 6948 (HBR); Sehnem 1269, 1271 (HBR). SÃO PAULO: Serra da Bocaina, 1,650 m, Brade 20828 (AAU, RB). Campos do Jordão, Umuarama, Kuhlmann (BONN-Nessel 287, SP32261, SP32263); Leite 3465 (AAU, GH, MO, US). São José do Barreiro, Serra da Bocaina, 1,770 m, Handro 793 (SP).
Phlegmariurus hippurideus (Christ) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 14a,b
Lycopodium hippurideumChrist, in: Pittier, Primit. Fl. Costar. 3 (1): 56. 1901Christ H (1901) Filices, Equisetaceae, Lycopodiaceae, Selaginellaceae, Rhizocarpaceae. Pittier Primitiae Florae Costaricensis 3: 1-67.. - Urostachys hippurideus (Christ) Nessel, Bärlappgewächse 88. 1939Nessel H (1939) Die Bärlappgewächse (Lycopodiaceae). G. Fischer, Jena. 404p.. - Huperzia hippuridea (Christ) Holub, Folia Geobot. Phytotax. 20: 73. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Costa Rica, El Páramo, 3,000 m, massif de Buena Vista, 1897, Pittier 10619 (holotype, P; isotype US).
Urostachys poseidonis Herter, Revista Sudamer. Bot. 10: 122-123. 1953. - Lycopodium poseidonis (Herter) C.V.Morton, Amer. Fern J. 54: 42. 1964Morton CV (1964) New combinations in Lycopodium. American Fern Journal 54: 71-73.. - Type: Ecuador, [prov. Chimborazo] In regione arborea superiore prope pagum Penipe, 3,400 m, XII. 1921, Rose, in Mille 35 (holotype US, isotypes GH, NY).
Lycopodium bolivianum Rosenst., Repert. Spec. Nov. Regni Veg. 11: 59. 1912. - Urostachys bolivianus (Rosenst.) Nessel, Arch. Bot. São Paulo 1: 396. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia boliviana (Rosenstock) Rolleri & Deferrari, Notas Mus. La Plata, Bot. 21 (100): 155. 1988. -Type: Unduavi, Bolivia, Buchtien 2632 (L, Z, isotype).
Published illustrations: Øllgaard 1988Øllgaard B (1988) Lycopodiaceae. In: Harling G & Andersson L (eds.) Flora of Ecuador 33: 1-155.: Fig. 1A, 2A.; Lellinger, 1989Lellinger DB (1989) The ferns and fern-allies of Costa Rica, Panama, and the Chocó. (Part 1: Psilotaceae through Dicksoniaceae). Pteridologia 2A: 1-364.: fig. 27; Mickel & Beitel, 1988Mickel JT & Beitel JM (1988) Pteridophyte Flora of Oaxaca, Mexico. Memoirs of the New York Botanical Garden 46: 1-568.: fig. 2E.
Plants terrestrial, ascending to stiffly erect from a decumbent base, to 60 cm tall, sparsely branched, to 3(-5) times dichotomous. Shoots homophyllous, equally thick throughout, 10-35 mm in diam. incl. leaves, sporangiate from ca 20 cm above the ground, often in separate zones. Stems excl. leaves 2.5-4 mm thick at the base, tapering to ca 2-3 mm upward. Leaves borne in more or less regular, often oblique, alternating whorls of 5-8, these 2-5 mm apart, forming 10-16 indistinct longitudinal ranks, spreading to reflexed, sometimes sharply reflexed and appressed to the stem, linear to linear-subulate, evenly tapering from the base or the middle, (10-)11-19 × 0.8-1.3 mm, not or rarely, twisted at base, broadly decurrent, adaxially slightly convex-slightly concave or canaliculate, with slightly prominent vein, abaxially with slightly prominent vein, with smooth, sometimes slightly revolute margins, with indistinctly to prominently and broadly decurrent leaf bases. Sporangia 1.5-2 mm wide.
Distribution and habitats: Central America, Greater Antilles, Andes from Venezuela south to Bolivia, and the Venezuelan and Brazilian Roraima Formation.
Upper montane forest, especially near the forest limit, usually on the forest floor, in semishade.
Phlegmariurus hippurideus belongs to a group of closely related taxa of montane forests in tropical America. Phlegmariurus nudus (Nessel) B. Øllg. is the closest relative in Brazil, and and differs most conspicuously in its much smaller size. Only one Brazilian collection studied.
Specimen studied: BRAZIL. RORAIMA: encosta sul do Monte Roraima, 1,500 m, Sette 14 (INPA).
Phlegmariurus huberi (B. Øllg.) B. Øllg., Phytotaxa 57: 16. 2012. Fig. 15a-c
Huperzia huberi B. Øllg., Amer. Fern J. 79: 153, Fig. 1 E-G. 1989. - Type: Venezuela, Estado Bolívar, Distr. Piar, Macizo de Chimantá, altiplanicie en la base meridional de los farallones superiores del Apacará-tepui, sector Norte del Macizo, 5º20’N, 62º12’W, ± 2,200 m, 30 Jan.-1 Feb. 1983, Steyermark et al. 128412 (holotype AAU).
Plants terrestrial, erect or ascending to erect from a decumbent base, to ca. 40 cm tall, sparsely branched, to 4 times dichotomous. Shoots homophyllous, almost equally thick throughout, ca. 15-28 mm in diam. incl. leaves (depending on leaf direction), sporangiate from approx. 15-30 cm above the stem base and upward, often in separate, seasonally produced zones. Stems excl. leaves 3-5 mm thick (dried) at the base, sometimes tapering to ca. 1.5 mm upward. Leaves borne in more or less regular, often oblique, alternating whorls of 6-8, these 1-2 mm apart, forming 12-16 indistinct longitudinal ranks, spreading to ascending, slightly to strongly recurved from an ascending leaf base, linear to linear-lanceolate, broadest near the base, 8-14 × (1-)1.2-1.6(-2) mm, adaxially flat to somewhat concave (dried), abaxially with slightly tumid vein, usually somewhat lustrous, hypostomatic, with entirely smooth margins. Sporangia 1.5-2 mm wide.
Distribution and habitats: This species occurs in open situations on sandstone mesetas, and in swampy savannas, at 2,000-2,300 m elev. Endemic for the Venezuelan and Brazilian Roraima formation.
This seems related to Phlegmariurus recurvifolius (Rolleri) B. Øllg. and P. hippurideus (Christ) B.Øllg.). From the former it is recognized by its broader leaves that taper gradually into an acute apex. In P. recurvifolius the apex is protracted into a narrow, twisted, often yellowish or transparent, whip-like tip. From P. hippurideus is is distinguished by the broader, more densely crowded leaves, which are recurved from an ascending leaf base, and not reflexed from the very base. Only one Brazilian collection studied.
Specimen studied: BRAZIL. RORAIMA: Vira Onça, Monte Roraima, acampamento do Coati, 2,702 m, Mota 1243 (BHCB).
Phlegmariurus intermedius (Trevis.) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 16a,b
Lycopodium intermediumSpring, in Martius, Fl. bras. I, 2: 111. 1840Spring A (1840) Lycopodium. In: Martius (ed.) Flora brasiliensis l: 109-117, t. 5. , non Blume 1828. - Urostachys intermedius (Spring) Herter, Repert. Sp. Nov. Regni Veg. 19: 163. 1923Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.. - Urostachys commutatus Herter, Index Lyc. 57. 1949. - Huperzia intermedia Trevis., Atti Soc. Ital. Sci. Nat. 17: 248. 1874. - Syntypes: Prope Rio de Janeiro: Freyreiss (BR), Sellow (B, K, L).
Plants terrestrial or rupestral, erect or ascending from a decumbent base, or spreading-scrambling over the ground with erect shoot apices, to 40 cm tall or to 80 cm long, at least to 10 times dichotomous, scrambling individuals usually with strongly divergent dichotomies. Shoots homophyllous, equally thick throughout, (4-)5-7(-9) mm in diam. incl. leaves, sporangiate from ca. 10-30 cm above the base, often in separate periodically produced zones. Stems excl. leaves 1-1.5 mm thick at the base, tapering to ca. 0.5-1 mm upward. Leaves borne in usually regular, alternating whorls of 5-7, these 0.5-1.5(-2) mm apart, forming 10-14 indistinct longitudinal ranks, usually uniformly strongly recurved and hook-like from an initially appressed leaf base, sometimes slightly recurved to perpendicular, linear-subulate to linear-lanceolate, widest at or just above the base, 4-6 × 0.5-0.7 mm, not twisted, adaxially slightly concave to slightly convex, with indistinct to slightly prominent vein, abaxially with strongly and widely prominent and long-decurrent vein, with smooth margins, firmly herbaceous to subcoriaceous, usually lustrous, with distinctly prominent decurrent leaf bases. Sporangia 1-1.3 (-1.5) mm wide.
Distribution and habitats: Guadeloupe, Venezuela, Brazil (Bahia, Minas Gerais, Espírito Santo). Rupestral, rock outcrops in mountains, alt. 1,400-2,050 m.
Resembles Phlegmariurus friburgensis in size and shape of the leaves, but deviates by the high number and strongly divergent dichotomies, and the hook-like leaves.
Reference specimens (18 collections studied): BRAZIL. BAHIA: Abaíra, Campo do Cigano, 1,800 m, Stannard et al. H51190 (AAU); 1,700-1,800 m, Sano & Læssøe 52340 (AAU). Serra dos Lençois, Serra da Larguinha, ca. 2 km NE of Caeté-Açu (Capão Grande), 1,000-1,400 m, Harley et al. 22599 (AAU, K). MINAS GERAIS: Caraça, Morro da Carapuça, Glaziou 15800 (K, P). Alto de Itacolomi, 1897, Damazio (RB). Serra do Ouro Preto, Ule 2358 (HBG). Serra da Piedade, Riedel 584 (GH, P, S, US). Serra do Cipó, Silveira. 186 (P). Camarinhas, VII.1938, Badini (AAU, OUPR 22802). Serra da Gavião, Pico do Itambé, 1,800 m, Wels-Windisch & Gillany 191 (HB). São Gonçalo do Rio Preto, Parque Estadual Rio Rio Preto, 1,780 m, Mota & Viana 47 (BHCB, photo AAU). Serra dos Lençois, Serra da Larguinha, ca. 2 km NE of Caeté-Açu (Capão Grande), 41º29’W, 12º36’S, 1,000-1,400 m, Harley et al. 22599 (AAU, K). Serra dos Lençois, Middle and upper slopes of Pai Inácio ca. 15 km NW of Lençois, just N of main Seabra-Itaberaba road, Mun. Palmeiras, 41º28’W, 12º27’S, 900-1,100 m, Harley 22529 (AAU, K).
Phlegmariurus itambensis (B. Øllg. & P.G.Windisch) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 4d-f
Huperzia itambensis B. Øllg. & P.G.Windisch, Bradea 5: 12, f. 1B. 1987. - Type: Minas Gerais: Serra do Espinhaço, Summit of Pico do Itambé; 2,250 m, in clumps on rocks, shrubby vegetation, mostly to 1 m or less, with mossy ground cover in organic soil overlying sandy soil on sandstone rocks, 10.II.1972, Anderson et al. 35783 (holotype SP, isotypes C, F, UB, US, Z).
Published illustrations: Øllgaard & Windisch 1987Øllgaard B (1987) A revised classification of the Lycopodiaceae sensu lato. Opera Botanica 92: 153-178., fig. 1B.
Terrestrial, stiffly erect, forming clumps, to 28 cm tall, to 6 times dichotomous. Shoots homophyllous, or gradually with shorter leaves upward, equally thick throughout, or tapering and with fewer leaf ranks upward, 5-8 mm in diam. at the base incl. leaves, sometimes tapering to 3-4 mm upward, sporangiate from ca. 15-20 cm above the base and upward. Stem excl. leaves 2-3 mm thick at the base, upward tapering to ca. 1 mm, almost completely concealed by leaves. Leaves of proximal divisions crowded and closely imbricate, borne in alternating whorls of 4-5, these 2-3 mm apart, forming 8-10 regular longitudinal ranks, straight, lanceolate to widely triangular-lanceolate, evenly tapering, 4-5 × 1.5-2.5 mm, evenly tapering, slightly to strongly carinate, coriaceous, somewhat lustrous, with smooth or uneven to shallowly erose margins. Leaves of upper, densely sporangiate divisions borne in alternating whorls of (2-)3-4, these 1.5-2 mm apart, forming (4-)6-8 regular longitudinal ranks, ovate-lanceolate to widely triangular-ovate, acuminate, with an abaxially rounded and somewhat swollen (massive) leaf base, and usually carinate apex, or sometimes carinate throughout, 2-3 × 1.5-2 mm, coriaceous, with smooth to shallowly erose margins. Sporangia ca. 1.5 mm wide.
Distribution and habitats: Endemic. Minas Gerais. A very distinct species, which seems most closely related to Phlegmariurus mooreanus (Baker) B. Øllg., and Phlegmariurus ruber (Cham. & Schlecht.) B. Øllg., all of which occur on rather isolated high mountains in Southern and Eastern Brazil, 1,200-2,250 m. Personal observation by Ashley Field (BRI) “P. itambensis is an extraordinary epilith growing in some of the driest harshest quartzitic rock conditions on Itambé”.
Reference specimens (6 collections studied): BRAZIL. MINAS GERAIS: Serra do Espinhaço, summit of Pico do Itambé, 2,250 m, Anderson et al. 35783 (C, F, SP, UB, US). Bocaiúvas, Parque Nacional das Sempre Vivas, 1,297 m, Almeida 900 (BHCB). São Gonçalo do Rio Preto, Pico Dois Imãos, 1,800-1,850 m, Salino 9356 (BHCB, photo AAU).
Phlegmariurus linifolius (L.) B. Øllg. var. jenmannii (Underw. & F.E.Lloyd) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 17a-c
Lycopodium jenmanii Underw. & F.E.Lloyd, Bull. Torrey Bot. Club 33: 112. 1906. - Urostachys jenmanii (Underw. & F.E.Lloyd) Nessel. Bärlappgewächse 158. 1939Nessel H (1939) Die Bärlappgewächse (Lycopodiaceae). G. Fischer, Jena. 404p.. - Huperzia jenmanii (Underw. & F.E.Lloyd) Holub, Folia Geobot. Phytotax. 20: 74. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Huperzia linifolia (L.) Trevisan var. jenmanii (Underw. & F.E.Lloyd) B. Øllg. & P.G. Windisch, Bradea 5: 13. 1987. - Type: British Guyana, Moruca River, Jenman s.n. (holotype NY, isotypes BONN-Nessel 388, E).
Published illustrations: Øllgaard 1988Øllgaard B (1988) Lycopodiaceae. In: Harling G & Andersson L (eds.) Flora of Ecuador 33: 1-155.: p. 91 fig. 18A.
Plants epiphytic, pendulous, usually with flaccidly hanging divisions, the distal divisions often aggregated in fasciculate clusters, to 60 cm long. Shoots homophyllous or gradually heterophyllous, equally thick throughout, 20-30 (-45) mm in diam. incl. leaves, or gradually tapering to 10-15 mm in diam. in distal, densely sporangiate divisions. Stems excl. leaves 0.8-1 mm thick at the base, slightly tapering upward, almost straight to somewhat sinuous, but not sharply flexuous, pale transparently stramineous, the vascular tissue usually visible through the cortex, sporangiate from 3-30 cm above the base and upward, to 6 times dichotomous. Leaves of proximal divisions spirally arranged, single, or in occasional pairs or whorls of 3, 0.5-3 mm apart, not predominantly whorled, forming ca. 6 indistinct longitudinal ranks, subdistant, soft-herbaceous, perpendicular to spreading-ascending, straight to slightly falcate, usually with the lamina vertical due to a twist of the lamina base, linear-lanceolate, widest in the basal third or quarter, distinctly narrowed into a petiole-like, twisted, usually perpendicular or deflexed lamina base, 13-25 × 1-2 mm, flat, or with slightly revolute, smooth margins. Leaves of middle and distal divisions spirally arranged, paired or borne in irregular, alternating whorls of 3, spreading to perpendicular, conform, or usually narrower, (5-)7-15(-20) × 0.7-1.5 mm, transparently to brownish green, or rarely reddish tinged. Sporangia ca. 1.5 mm in diam.
Distribution and habitats: Often in riverine forest. Throughout the Amazonian lowland region of Venezuela, Colombia, Ecuador, Peru and Bolivia, the Guayanas and Northern coastal Brazil to Bahia, Amapá, Amazonas, Pará, Rondonia, Mato Grosso, São Paulo.
Reference specimens (26 collections studied): BRAZIL. AMAPÁ: Rio Araguari, Pires et al. 51304 (NY). Rio Oiapoque, ca. 2 km SW of mouth of Ingarari, Irwin et al. 48337 (NY, US). AMAZONAS: Manaus, Cachoeira Alta do Tarumã, 28.III.1955, s.col. (HB, INPA915, MG21223). Nova Japurá, Vila Bittencourt, Río Apaporís, Cid & Lima 3734 (INPA, NY). Rio Negro, between Manaus and São Gabriel, along Rio Marié, Maruná, Alencar 478 (NY, US). Rio Negro, between Manaus and São Gabriel, NW of San Gabriel, near mouth of the Rio Uaupes, igapó, Poole 2101 (NY). BAHIA: Salvador, Sucre 11197 (RB). Ilhéus, Road Ilhéus-Itacaré, near Praia do Norte at ca. 4 km N of river bridge, Edwards 2353 (AAU, K). Coastal Zone near Maraú, Harley et al. 18548 (K). MATO GROSSO: Juruena, Hoehne 5592 (R). PARÁ: Rio Una, Planalto de Santarém, Fróes 32004 (HB). Cachoeira do Rio Arua, Pires & Silva 4214 (INPA, US). Mojú, Igarapé, Marachimbé, 15.III.1975, Bouças et al. (NY). Marajó, W of the island, Pires & Silva 6635 (US) RONDONIA: Rio Pacáas Novos, 28.III.1978, Santos et al. 290 (F, GH, NY, US).
Phlegmariurus loefgrenianus (Silveira) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 8e,f
Lycopodium loefgrenianumSilveira, Flora e Serras Mineiras 78, t. 30. 1908Silveira A (1908) Flora e Serras Mineiras. Imprensa Oficial, Belo Horizonte. 282p.. - Urostachys loefgrenianus (Silveira) Herter, Index Lyc. 68. 1949Herter W (1949) Index Lycopodiorum, Montevideo. Pp. 1-120.. - Huperzia loefgreniana (Silveira) B. Øllg. & P.G. Windisch, Bradea 5: 13. 1987. - Type: S. Paulo: In silvis de arboribus pendens prope Bocaina; Löfgren, IV. 1894, n. 197 in Herb. Silveira (holotype R 154768, type fragment BONN-herb. Nessel no. 368a).
Urostachys leitziiNessel, Repert. Sp. Nov. Regni Veg. 36: 184, t. 173. 1934Nessel H (1934) Neue Lycopodien, die von allen schon bekannten Arten durch ihren Habitus ganz besonders abweichend und auffallend sind. Repertorium Specierum Novarum Regni Vegetabili 36: 177-193, t. 170-177.. - Type: Cidade Jardin, São Paulo, 30.XI.1930, Gehrt (holotype BONN-herb. Nessel no. 368a, isotypes SP 27049, NY, US 1619557, isotypes).
Plants epiphytic, lax and pendulous, to 80 cm long. Shoots tapering from ca. 6-15 mm in diam. incl. leaves in proximal divisions, to 2-6 mm in diam in densely sporangiate divisions of fully developed plants, sometimes not, or only slightly tapering (juvenile or tardily sporangiate individuals), homophyllous or gradually heterophyllous, sporangiate from ca. 15-45 cm above the base and upward. Stems excl. leaves 1-2 mm thick at the base, tapering to 0.5-1 mm upward, prominently ridged by decurrent leaf bases, pale greenish to brownish, at least to 5 times dichotomous. Leaves usually reduced and modified upward, borne in alternating whorls or irregular low spirals of 3(-4?), forming 6(-8?) indistinct longitudinal ranks, or subdecussate, the whorls or leaf pairs 1.5-2 mm apart in proximal divisions, upward sometimes 1-2 mm apart. Leaves of proximal divisions spreading to ascending, slightly falcately upward curved to slightly recurved, the lamina twisted or not, linear to narrowly linear-lanceolate, widest at or near the base, evenly tapering, widely joined, somewhat clasping and seemingly adnate-decurrent on the stem, with pale leaf base center and long-decurrent green leaf base margins, soft-herbaceous to subcoriaceous, (3-)6-13 × 1-1.5 mm, sightly concave to canaliculate adaxially, with flat or slightly involute, smooth margins, abaxially usually subcarinate to carinate. Sporangiate leaves of middle and distal divisions subdecussate or borne in alternating irregular whorls of 3, conform, or gradually shorter, with a short, widened, abaxially subcarinate or carinate, clasping base, evenly tapering or with a short, abruptly narrowed involute cusp, 2-6 × 1.5-1.8 mm. Sporangia 1.2-1.8 mm wide.
Distribution and habitats: Endemic. Epiphytic or epilithic. Montane regions, one collection from cloud forest at 1,200 m. States of Minas Gerais, Rio de Janeiro, São Paulo, Paraná, Santa Catarina, Rio Grande do Sul.
A somewhat heterogenous assemblage of plants, related to Phlegmariurus heterocarpos and apparently intermediate to members of the P. quadrifariatus group, exhibiting strong reduction of the leaves in the squarish distal divisions, and short leaves also in the proximal divisions of the stems. Sylvestre 1854 (RB) from Itatiaia was found together with Phlegmariurus heterocarpos (Sylvestre 1853, RB). Plants earlier referred to Urostachys leitzii are the forms resembling Phlegmariurus heterocarpos the most.
Reference specimens (11 collections studied): BRAZIL. MINAS GERAIS: Delfim Moreira, Kuhlmann (SP 44445). PARANÁ: Quatro Marcos, Morro Mae Catira, 1,200 m, Hatschbach 16486 (MBM). Jaguaraiva, 735 m, Dusén 17061 (F, GH, MO, S). São José dos Pinhãis, Col. Sto. Andrade, Hatschbach 14848 (MBM). RIO DE JANEIRO: Pico da Tijuca, Glaziou 6403 (P). Itatiaia, trail from Hotel Simon to Tres Picos, 1,200 m, Sylvestre 1854 (RB). RIO GRANDE DO SUL: São Francisco de Paula, Passo do Inferno, Fazenda Tres Cachoeiras, Mansan 11 (HAS). Cambará do Sul, P.N. da Serra Geral, Canyon Fortaleza, Mynssen et al. 1058 (RB), SANTA CATARINA: Ilha Santa Catarina, Flaggenberg, (Estreito near São José), Ule 202 (P), 2202 (HBG). SÃO PAULO: Campos do Jordão, Parque Estadual, trail to Sapucai, Salino 1368 (BHCB, photo AAU).
Phlegmariurus mandiocanus (Raddi) B. Øllg., Rodriguésia 63(2): 480. 2012. Fig. 14c,d
Lycopodium mandiocanumRaddi, Opusc. Sci. Bolon. 3: 280. 1819Raddi J (1819) Synopsis Filicum Brasiliensium. Opusculi Scientifici Bologna 3: 279-297.. - Huperzia mandiocana (Raddi) Trevis., Atti Soc. Ital. Sci. Nat. 17: 248. 1874. - Urostachys mandiocanus (Raddi) Herter, Repert. Sp. Nov. Regni Veg. 19: 164. 1923Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.. - Lycopodium mandiocanum Raddi var. brasilienseSpring, Mém. Acad. Roy. Sci. Belgique 15: 45, [Mon. Lyc. 1] 1842Spring A (1842) Monographie de la famille des Lycopodiacées, premiére partie. Mémoires de l’Académie Royale des Sciences, Lettres et Beaux Arts de Belgique 15: 1-110. . - Lycopodium dichotomum Jacq. var. mandiocanum (Raddi) Rosenst., Hedwigia 46: 165. 1907. - Urostachys mandiocanus (Raddi) Nessel var. brasiliensis (Spring) Nessel, Arch. Bot. Est. S. Paulo 1: 410. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Type: In saltibus umbrosis et humidis apud Mandioca, in provincia di Rio Janeiro, BRAZIL, Raddi (holotype PI).
Lycopodium pseudo-mandiocanumHerter, Bot. Jahrb. 43: Beibl. 98: 49. 1909Herter W (1909) Ein neuer beitrag zur Kenntnis der Gattung Lycopodium. Hedwigia 49: 88-92, t. 3.. - Urostachys pseudo-mandiocanus (Herter) Nessel, Arch. Bot. Est. S. Paulo 1: 411. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Type: None designated; the protologue indicates the following area: Amazonas, Minas Gerais, Rio de Janeiro, Santa Catarina, Rio Grande do Sul. Several specimens annotated seen with Herter’s annotation.
Published illustrations: Øllgaard, 1988Øllgaard B (1988) Lycopodiaceae. In: Harling G & Andersson L (eds.) Flora of Ecuador 33: 1-155.: p. 17 fig. 1B.
Plants epiphytic, erect and to ca. 20 cm tall, or recurved to pendulous, to 60 cm long, to 7 times dichotomous. Shoots homophyllous or with gradually smaller leaves in distal divisions, equally thick throughout, 20-45 mm in diam. incl. leaves, or tapering to ca. 10-20 mm upward, sporangiate in separate, seasonally produced zones from 10-30 cm above the base and upward, or continuously sporangiate in distal branches. Stems excl. leaves (1.5-)3-4(-5) mm thick at the base, upward tapering to (0.5-)1.5-3 mm, usually almost covered by the wide, bright red, decurrent leaf bases. Leaves borne in alternating whorls of 5-8, these 1-2 mm apart, forming 10-16 indistinct longitudinal ranks in proximal divisions, upward in whorls of (4-)5-7, ca. 1 mm apart, spreading to reflexed, straight or upward curved, sometimes with aproximal twist and falcately upward curved, 12-25 mm long in proximal divisions, upward sometimes reduced to (7-)10-15 mm, (0.5-)0.6-0.8(-1) mm wide at the base, evenly tapering, linear to filiform, bisulcate, with prominently tumid margins and vein, the vein prominent to sunken beneath (in small juvenile individuals often only the margins somewhat tumid). Leaf bases with prominently decurrent margins and median veinal ridge, widening to ca. 1-1.5 mm below the insertion in proximal divisions, usually bright red, at least in the center and the base of the vein. Sporangia ca. 1.5-2 mm wide.
Distribution and habitats: Paraguay, Northern Argentina, Brazil (Ceará, Bahia, Mato Grosso do Sul, Minas Gerais, Espírito Santo, Rio de Janeiro, São Paulo, Paraná, Santa Catarina, Rio Grande do Sul).
Epiphytic in forests, alt. 50-1,000 m, the lower altitudes in the southern part of its range.
Phlegmariurus mandiocanus is closely related to P. pithyoides (Schlecht. & Cham.) B. Øllg. (Central America, Greater Antilles and Venezuela), from which it is distinguished by its smaller dimensions.
In the protologue Raddi indicates that only one individual was found, and that this was shared with Langsdorff and Gaudichaud, who accompanied Raddi in Mandioca. (Pichi Sermolli & Pizzarri 2005Pichi Sermolli REG & Pizzarri MP (2005) A Revision of Raddi’s pteridological collection from Brazil (1817-1818). Webbia 60: I-VII, 1-393.). There is a Langsdorff specimen (isotype) from Mandioca in P.
Reference specimens (80 collections studied): BRAZIL. BAHIA: Itanagra, road Itanagra-Subaúma, km 8, alt. ca. 50 m, Boom & Mori 967 (NY). Belem, 10 km S of Eunápolis, Pinheiro & Pinheiro 2641 (UB). Itabepí, Pinheiro 356 (NY). CEARÁ: Freire Allemão (R 154769). ESPÍRITO SANTO: Lagoa Bonita, Linhares region, 21.VI.1971, Duarte 13982 (AAU). MATO GROSSO DO SUL: Corumbá, 994 m, Pott et al. 7480 (BHCB, photo AAU). MINAS GERAIS: Serra do Henrique, near Rio Novo, 600 m, Schwacke 11802 (P, RB). Caldas, 25.I.1869, Regnell III 1500xx(M, S). PARANÁ: Laranjeiras do Sul, Alto Santiago, Usina, Hatschbach 36622 (AAU, MBM, MO). Serra do Mar, between Morretes and Alto da Serra, 350-600 m, Windisch et al. 4884 (AAU). Guaratuba, Pedra Branca da Araraquara, 15.VII.1961, Hatschbach 8154 (US). Paranaguá, Ilha do Mel, 5 m, Hatschbach 25639 (US). Tibagi, Faz. Monte Alegre, Antas, Hatschbach. & Duarte 7171 (US). RIO DE JANEIRO: Mandioca, Langsdorff (P). Itatiaia, Rio Campo Belo, Brade 20188 (RB). Tijuca, Glaziou 1878 (P). Santa Maria Magdalena, Serra da Grama, Santos Lima 259 (RB). Serra dos Orgãos, Barreira, Valley of Rio Soberbo, 1,000 m, Markgraf 10124 (RB). RIO GRANDE DO SUL: Novo Hamburgo, XI.1959, Silfredo (HB 18181). Torres, Itapeva, Camargo 102 (HAS). Morinhos do Sul, Morro da Queimada, Waechter 2628 (HAS). Serra João Rodriguez, Rosenst. Fil. Austrobras. (US 600626). Porto Alegre, Gloria, Sehnem 749 (B). SANTA CATARINA: São Bento, 16.XII.1919, Fischer (SP3742). Tubarão, Capivari, VI.1889, Ule 312, 2312 (HBG). Blumenau, Parque das Nascentes, Sobral & Jarenkow 9133 (HAS). Brusque Azambuja, 35-135 m, Brusque, 90 m, Smith & Reitz 6132 (US). Anita Garibaldi, Passo do Rio Canoas, 600 m, Reitz & Klein 15386 (HBR). Garuva, Tres Barras, 200 m, Reitz & Klein 4691 (HBR, L). Palhoça, Campo do Massiambu, 5 m, Reitz & Klein 1286 (HBR). Porto União, 800 m, Reitz & Klein 11636 (HBR). SÃO PAULO: Iguape, Morro das Pedras, Brade 8497 (AAU, BONN-Nessel 336, HB, M). Serra da Paranapiacaba, 30.VI.1936, Handro (US 2690475). Baurú, XI.1904, Edwall (SP 17996). Santa Rita de Passa Quatro, Hemmendorff 220 (S). Ilha de Cardoso, Silva 80 (SP). Ubatuba, near Base Norte, Válio 115 (SP).
Phlegmariurus martii (Wawra) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 12a-c
Lycopodium martiiWawra, Oesterr. Bot. Zeitschr. 13: 218. 1863Wawra H (1863) In: Wawra H & Maly F (eds.) Neue Pflanzenarten gesammelt auf der transatlantischen Expedition Sr. k. Hoheit des durchlauchtigsten Herrn Erzherzogs Ferdinand Maximilian. Oesterreichische Botanische Zeitschrift 13: 218-227.. -Urostachys martii (Wawra) Nessel, Arch. Bot. Est. S. Paulo 1: 385. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia martii (Wawra) Holub, Folia Geobot. Phytotax. 20: 74. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Brazil: Bahia: Ilheos, Wawra & Maly 339 (holotype W, isotypes BR, HBG, BR).
Lycopodium cipoenseDamazio, Bull. Soc. Bot. Genève, ser. 2, 7: 119, fig. 1-8. 1915. - Urostachys cipoensis (Damazio) Nessel, Bärlappgewächse 169. 1939Nessel H (1939) Die Bärlappgewächse (Lycopodiaceae). G. Fischer, Jena. 404p.. - Urostachys passerinoides (Kunth in H. B. K.) Nessel var. cipoensis (Damazio) Nessel, in Hoehne, Flora Bras. Fasc. 11: vol. II: 85. 1955. - Type: Minas Geraes, Serra do Cipó, mense Junii 1908, L. Damazio (Herb. Damazio no 2001, not located, isotypes RB, BONN-herb. Nessel no. 415).
Published illustrations: Øllgaard 1992Øllgaard B (1992) Neotropical Lycopodiaceae - an overview. Annals of the Missouri Botanical Garden 79: 687-717.: p. 698, fig. 6.
Plants epiphytic, lax and pendulous to spreading, at least to 40 cm long. Shoots more or less abruptly tapering from ca. 7-20 mm in diam. incl. leaves in proximal divisions, to 1.2-3 mm in diam in distal constricted divisions, sometimes not, or only slightly tapering (juvenile or tardily sporangiate individuals), usually gradually heterophyllous, sporangiate from ca. 15-30 cm above the base and upward. Stems excl. leaves 1.5-2 mm thick at the base, tapering to 0.5-1 mm upward, prominently ridged by decurrent leaf bases, pale greenish to brownish, at least to 8 times dichotomous. Leaves usually reduced and strongly modified upward, borne in alternating whorls or irregular low spirals of 4-6 in proximal divisions, forming 8-12 indistinct longitudinal ranks, densely crowded, the whorls 1-2 mm apart, upward usually 1 mm apart. Leaves of proximal divisions spreading to ascending, usually with falcately upward curved tip, the lamina twisted to a vertical position from the base, linear, widest at the base, tapering from ca. the middle, widely joined, somewhat clasping and seemingly adnate-decurrent on the stem, soft-herbaceous to subcoriaceous, 6-12 × 0.7-1 mm, slightly concave to slightly convex adaxially, with slightly involute to revolute, smooth margins, abaxially slightly convex to slightly concave, with evident to somewhat prominent vein. Sporangiate leaves subdecussate to decussate, or borne in alternating irregular whorls of 3(-4), gradually shorter; transitional leaves lanceolate to ovate-lanceolate, with a widened, abaxially rounded, clasping base and elongate tapering slightly involute apex; sporangiate leaves of ultimate divisions strongly reduced and imbricate, clasping, rhombic-ovate to rhombic-lanceolate, usually somewhat cuspidate, with a prominent vein, sometimes subcarinate, 1.5-3 × 1-1.3 mm. Sporangia ca. 1 mm wide.
Distribution and habitats: Endemic. Epiphytic in forest. Known only from the states of Bahia, Espírito Santo, and Minas Gerais. 600-1,350 m.
Distinguished by the densely crowded, rather short and narrow proximal leaves and the very strongly reduced leaves of the distal, fertile divisions.
Specimens studied: BRAZIL. BAHIA: Serra do Sincorá, Barra da Estiva on the Capão da Volta road, 1,300 m, Harley et al. 20708 (AAU, K). ESPÍRITO SANTO: Castelo, Braço do Sul, 17.VIII.1948, Brade 19311 (AAU, F, GH, MO, NY, S, SP). Cachoeira do Itapemerim, Vargem Alta, 31.V.1949, 650 m, Brade 19934 (AAU, RB). Santa Teresa, Reserva Biologica Santa Lucia, 647 m, Salino et al. 8313 (BHCB, photo AAU). MINAS GERAIS: Serra do Cipó, km 149 estrada Conceição-Capão, Barreto 8560 (GH). Santa Maria do Salto, distrito de Talismã, Fazenda Duas Barras, 750-850 m, Salino 9203 (BHCB, photo AAU). Carrancas, Serra da Carrancas, Serra das Broas na Chapada dos Perdizes, 1,270-1,350 m, Salino 12282 (BHCB, photo AAU).
Phlegmariurus mollicomus (Spring) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 17d-f
Lycopodium dichotomum Jacq. ssp. mollicomum Martius exSpring, Flora 1: 162. 1838Spring A (1838) Beiträge zur Kenntniss der Lycopodien. Flora 21: 145-158; 161-175, 177-191; 193-205; 209-224.. - Lycopodium mollicomum Spring, in: Martius, Flora bras. 1(2): 113. 1840. - Lycopodium setaceum Buch.-Ham. var. brasiliense Spring, Mém. Acad. Roy. Sci. Belgique 15 [Mon. Lyc. 1]: 43. 1842. - Huperzia setacea (Buch.-Ham.) Trevis. var. mollicoma (Spring) Trevis., Atti Soc. Ital. Sci. Nat. 17: 248. 1874. - Urostachys mollicomus (Spring) Nessel, Arch. Bot. Est. S. Paulo 1: 407. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys setaceus (Buch.-Ham) Nessel var. brasiliensis (Spring) Nessel, Arch. Bot. Est. S. Paulo 1: 408. 1927. - Huperzia mollicoma (Spring) Holub, Folia Geobot. Phytotax. 20: 75(1985)Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: BRAZIL, Pará (“Prov. Paraënsis”) L. mollicomum. subulifol. et pulcherrimo proximum. Sylvis, Paraënsis. Dr. Martius. Iter Brazil Apr. Martius (holotype M)
Lycopodium flaccidum Fée, Crypt. Vasc. Brésil 2: 92, t. 106, fig. 1, 1872-73, non Bory 1833. - Urostachys flaccidus (Fée) Herter, Repert. Spec. Nov. Regni Veg. 19: 164. 1923Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.. - Huperzia flaccida (Fée) Holub, Folia Geobot. Phytotax. 20: 72, 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Urostachys neptuni Herter, Index Lyc. 72. 1949. - Type: Brazilia fluminensi, Alto Macahe, Glaziou 4677 (holotype P, isotypes B, C, RB).
Lycopodium williamsii Underw. & F.E.Lloyd, Bull. Torrey Bot. Club 33: 112. 1906. - Urostachys williamsii (Underw. & F.E.Lloyd) Nessel, Bärlappgewächse 164. 1939Nessel H (1939) Die Bärlappgewächse (Lycopodiaceae). G. Fischer, Jena. 404p.. - Type: Bolivia: New Brazil, 5,500 ft., Williams 1393 (holotype NY, isotypes AAU, GH, US).
Published illustrations: Lellinger, 1989Lellinger DB (1989) The ferns and fern-allies of Costa Rica, Panama, and the Chocó. (Part 1: Psilotaceae through Dicksoniaceae). Pteridologia 2A: 1-364.: fig. 31; Øllgaard, 1988Øllgaard B (1988) Lycopodiaceae. In: Harling G & Andersson L (eds.) Flora of Ecuador 33: 1-155.: p. 91 fig. 18 B.
Plants epiphytic, pendulous, usually with flaccidly hanging divisions, to 30 cm long. Shoots homophyllous or almost so, almost equally thick throughout, 7-15 mm in diam. incl. the leaves, or gradually tapering to ca. 5 mm in diam. Stems excl. leaves 0.5-1 mm thick at the base, often tapering to 0.3 mm, prominently to sharply ridged by decurrent leaf veins, discontinuously or ultimately continuously sporangiate from 6-15 cm above the base and upward, to 6(-8) times dichotomous. Leaves borne in alternating, irregular, often oblique whorls of 4-5 in proximal divisions, or of 3-4 in distal divisions, the whorls 1-2 mm apart, forming 6-10 obscure longitudinal ranks, in proximal divisions ascending to patent, upward more appressed, rarely slightly twisted at the base, straight to slightly upward curved, linear to linear-subulate. Leaves of proximal divisions (7-)10-13 × 0.5-0.7 mm, not or very slightly widened at the base, adaxially flat or convex, usually somewhat revolute (dried), abaxially with sharply prominent vein at least in the basal half. Leaves of distal sporangiate divisions 7-11 mm long, usually loosely appressed, otherwise conform, or almost flat, with less prominent vein, often slightly widened at the base (not auriculate), rarely tinged with red on the margins near the base. Decurrent leaf bases not wider than lamina. Sporangia ca. 1 mm in diam.
Distribution and habitats: Mexico (Oaxaca), Hispaniola, Costa Rica, Panamá, Colombia, Venezuela, Ecuador, Bolivia, central and southeastern Brazil, Minas Gerais, Espírito Santo, Rio de Janeiro, São Paulo and one collection labelled as from Pará (Martius s.n. at M). Epiphytic in lower montane forest, 800-2,400 m.
Superficially resembling the Phlegmariurus sarmentosus (Spring) B. Øllg. and P. watsonianus (Maxon) B. Øllg. in size and growth habit, but differing by the more appressed, nonauriculate leaves and the prominent vein on the leaf undersides.
Reference specimens (25 collections studied): BRASIL. ESPÍRITO SANTO: Magdalena, Pedra da Republica, Santos Lima 6 (RB). Castelo, Braço do Sul, Brade 19199 (RB). Serra do Caparaó, 1,550-2,460 m, Mexia 4062a (UC). MINAS GERAIS: Caraça, Ule 2357 (HBG, P). Serra do Cipó, Caravelli (HB 51408). Itamarandiba, P.E. Serra Negra, 1,570-1,600 m, Salino et al. 11273 (BHCB, photo AAU). Alagoa, Parque Estadual da Serra do Papagaio, ascent to Pico do Garrafão, 2,000 m, Salino 12930 (BHCB, photo AAU). RIO DE JANEIRO: Petrópolis, Road Fazenda Inglesa-Pati dos Alferes, 1,180 m, Plowman & Martinelli 10128 (AAU, F). Itatiaia, Rio Campo Belo, 1,000 m, Brade 17500 (RB). SÃO PAULO: Iguape, Serra do Paranapiacaba, Brade 8491 (HB). Serra do Cantareira, Urwald, 1,000 m, Brade 6544 (AAU, HB). Campo Grande, 800 m, Brade 8498 (HB). Mogi das Cruzes, limite com Salesópolis, Estrada Usaka, 800 m, Salino 5391 (BHCB, photo AAU).
Phlegmariurus mooreanus (Baker) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 18a,b
Lycopodium mooreanumBaker, Gard. Chron., ser. 3, 12: 582. 1892Baker JG (1892) New or noteworthy plants: Lycopodium mooreanum Hort. Sander. Garden’s Chronicle 12: 582.. - Urostachys mooreanus (Baker) Herter, Index Lyc. 70. 1949Herter W (1949) Index Lycopodiorum, Montevideo. Pp. 1-120.. - Huperzia mooreana (Baker) Holub, Folia Geobot. Phytotax. 20: 75. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Brazil: Bahia, Forget (holotype K).
Lycopodium sydowiorumHerter, Repert. Sp. Nov. Regni Veg. 13: 296. 1914Herter W (1914) Lycopodium Sydowiorum spec. nov. Repertorium Specierum Novarum Regni Vegetabili 13: 296.. - Urostachys sydowiorum (Herter) Nessel, Arch. Bot. Est. S. Paulo 1: 390. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia sydowiorum (Herter) Rolleri & Deferrari, Notas Mus. La Plata, Bot. 21 (100): 157. 1988. - Type: Bahia, Serra do Sincorá, auf Felsen, XI.1906, alt. 1,400 m, Ule 7296 (holotype B, isotype HBG).
Lycopodium luetzelburgii Rosenst., Repert. Sp. Nov. Regni Veg. 20: 95. 1924. Type: Bahia: Gipfel der Serra das Almas, ca. 1,800 m, 10.VIII.1913, von Luetzelburg, Ph. 18871 (holotype M).
Plants terrestrial, stiffly erect, to ca. 30 cm tall, to 6 times dichotomous. Shoots homophyllous, equally thick throughout, 6-10 mm in diam. incl. leaves, or tapering to 4-6 mm upward, sporangiate from ca. 10-25 cm above the stem base. Stems excl. leaves 2.5-6 mm thick at the base, sometimes tapering to ca. 1.5-2 mm upward, usually completely concealed by decurrent leaf bases. Leaves of proximal divisions borne in densely crowded, more or less regular, alternating whorls of 8-10, these 1-1.5 mm apart, forming 16-20 indistinct longitudinal ranks, uniformly strongly recurved and hook-like from an initially appressed leaf base, subulate to lanceolate, evenly tapering from the base, or protracted into a long, pale pungent apex, 4-7 × 1-1.5 mm, not twisted, prominently long-decurrent, adaxially flat or slightly convex, sometimes with slightly prominent vein, abaxially with strongly prominent vein, with pale, strongly sclerified, smooth margins. Leaves of upper, densely sporangiate divisions essentially conform, but sometimes smaller, borne in alternating whorls of 5-7, 3-4 mm long, with ovate to rhombic-ovate, not decurrent, somewhat raised leaf bases. Sporangia ca. 1.5 mm wide.
Distribution and habitats: Endemic. - Known only from the higher parts of the Serra do Sincorá and Serra Nova (Minas Gerais), in moist to marshy, exposed, rupestral vegetation. Bahia, Minas Gerais. 1,000-1,900 m.
According to a note on the holotype by Raymond M. Harley the type locality is uncertain, but probably either Andaraí, Serra do Sincorá, or Serra de Lençois.
Reference specimens (17 collections studied): BRAZIL. BAHIA: Abaíra, Serra S of Riacho da Taquara, Harley et al. 51252a (AAU); Harley et al. 51253 (AAU). Serra do Rio de Contas, upper caldeira, on slopes of the Pico das Almas, ca. 25 km NNW of the town of the Rio de Contas, 1,600 m, Harley 15433 (K, MO, NY, P, U, US). Palmeiras, Pai Inacio, BR-242, W of Lençois at km 232, Boom & Mori 1136 (K, NY). Pico das Almas, campo rupestre, 1,600-1,850 m, Mori et al. 12459 (K, NY, US). Serra do Sincorá, 1,460 m, Ule 7296 (HBG). Serras dos Lençois, Serra da Larguinha, ca. 2 km NE of Caeté-Açu (Capão Grande), Harley et al. 22600 (AAU, K); 1,400 m, Mello Silva et al. CFCR 7192 (AAU, SPF). Barra da Estiva, NW face of Serra de Ouro, ca. 9 km S of Barra da Estiva, 1,300-1,500 m, Harley et al. 20874 (AAU, K). Mucugê, Furlan et al. CFCR 441 (AAU, SPF). MINAS GERAIS: Serra Nova, Parque Nacional da Serra Nova, 1,335 m, Leite 2779 (BHCB, photo AAU).
Phlegmariurus myrsinites (Lam.) B. Øllg., Phytotaxa 57: 17. 2012. Fig. 23a,b
Lycopodium myrsinites Lam., Encycl. Méth. Bot. 3: 654. 1789. - Plananthus myrsinites (Lam.) P.Beauv., Prodr. Aeth. 111. 1805. - Urostachys myrsinites (Lam.) Herter, Repert. Spec. Nov. Regni Veg. 19: 166. 1923Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.. - Huperzia myrsinites (Lam.) Trevis., Atti Soc. Ital. Sci. Nat. 17: 249. 1874. - Type: S. Domingue, Comm. Joseph Martin s.n. (P-Lam., holotype).
Lycopodium patens Willd. ex Spreng., Syst. Veg. (ed. 16) 4: 12. 1827. - Urostachys patens (Spreng.) Nessel, Bärlappgewächse 245. 1939Nessel H (1939) Die Bärlappgewächse (Lycopodiaceae). G. Fischer, Jena. 404p.. - Type: s.c. s.n. (B-Willd. 19342, holotype).
Lycopodium roraimense Underw. & F.E.Lloyd, Bull. Torrey Bot. Club 33: 115. 1906. - Urostachys roraimensis (Underw. & F.E.Lloyd) Nessel, Bärlappgewächse 246. 1939Nessel H (1939) Die Bärlappgewächse (Lycopodiaceae). G. Fischer, Jena. 404p.. - Type: British Guiana, forest slopes near Roraima, Herb. Jenman (NY holotype).
Lycopodium skutchiiMaxon, Proc. Biol. Soc. Washington 46: 159. 1933Maxon WR (1933) A new Lycopodium from Western Guatemala. Proc. Biol. Soc. Washington 46: 159-160. . - Type: Guatemala, Chimaltenango, Chichavac, alt. 2,400-2,700 m, Skutch 243 (US 1.494.904 holotype).
Published Illustrations: Smith (1981: fig. 82 a, b); Lellinger (1989: fig. 32); Mickel & Beitel (1988: fig. 3A).
Plants epiphytic, pendulous, at least to 65 cm long. Shoots heterophyllous, usually not all sharply differentiated in the basal divisions, 10-18 mm in diameter including the expanded leaves, upward gradually, within a short transition, or abruptly constricted to 1.5-3 mm thick including the reduced, imbricate leaves in the terminal divisions. Stem excluding leaves ca. 1 mm thick at the base, tapering to ca. 0.5 mm, pale greenish, to 7 times dichotomous. Expanded leaves of basal divisions decussate or subdecussate, often irregularly shaped, subdistant to densely crowded and somewhat overlapping, the leaf pairs 1.5-6 mm apart, ascending to perpendicular, ovate-lanceolate or narrowly so, acute, usually the widest ones with a rounded base, 6-11 × 1.5-3 mm, usually flat, straight to somewhat recurved, the lamina twisted to a vertical position. Leaves of terminal constricted divisions highly variable, often with complete reduction series, and with recurrent series to expanded shape, decussate or subdecussate, continuously or discontinuously sporangiate. Transitional leaves with widely ovate base, and short to long acuminate apex, appressed and clasping, with the wide base abaxially rounded to bluntly carinate, with straight to recurved apex, 2.5-5 × 1.5-2 mm. Shortest leaves with base conform, but with straight to falcate apex, bluntly to sharply carinate, scarcely exceeding the sporangia, ca. 2 mm long. Sporangia 1-1.3 mm wide.
Distribution and habitats: Hispaniola, S. Mexico to Costa Rica, Trinidad, Hispaniola, British Guiana, Venezuela to Bolivia, Brazilian Roraima.
Reported by Barbosa-Silva et al. (2016)Barbosa-Silva RG, Labiak PH, Bragança Gil AS, Goldenberg R, Michelangeli FA, Martinelli G, Nadruz Coelho MA, Zappi DC & Forzza RC (2016) Over the hills and far away: new plant records for the Guayana Shield in Brazil. Brittonia 68: 397. from Brazilian Roraima: Serra do Aracá by Tavares 146 (not seen).
Phlegmariurus nudus (Nessel) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 10d,e
Urostachys nudus Christ exNessel, Arch. Bot. Est. S. Paulo 1: 396, t. 14. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys nudiusculusHerter, Index Lyc. 72. 1949Herter W (1949) Index Lycopodiorum, Montevideo. Pp. 1-120.; nom. superfl. - Huperzia nuda (Nessel) B. Øllg. & P.G. Windisch, Bradea 5: 14. 1987. - Lectotype: Ilha de Santo Amaro, perto de Santos, alt. 5-50 m, em VI.1901, medrando na agua; Campos da Itatiaya, V.1906, Luederwaldt s.n. (lectotype SP18.082 chosen by Øllgaard & Windisch, Bradea 5: 15 (1987)Øllgaard B (1987) A revised classification of the Lycopodiaceae sensu lato. Opera Botanica 92: 153-178., isotype NY).
Plants terrestrial, ascending to erect from a decumbent base, to ca. 25 cm tall or to 50 cm long, sparsely branched, to 3(-6) times dichotomous. Shoots homophyllous, equally thick throughout, 8-24 mm in diam. incl. leaves (depending on leaf direction), sporangiate from ca. 10-30 cm above the stem base, often in separate, seasonally produced zones. Stems excl. leaves 1.3-2 mm thick at the base, sometimes tapering to ca. 1.3 mm upward, pale greenish white. Leaves borne in more or less regular, often oblique, alternating whorls of 6, these 1.5-3 mm apart, forming 12 indistinct longitudinal ranks, spreading to sometimes sharply reflexed and appressed to the stem, linear, evenly tapering from the base or the middle, 6-13 × 0.6-0.8(-1) mm, not twisted at base, prominently decurrent, adaxially flat, with slightly prominent vein, abaxially with slightly prominent vein, with smooth margins. Sporangia 1.5-2 mm wide.
Distribution and habitats: Endemic. Restricted to nebular forest and moist rock outcrops, moist road banks, in grasslands and river margins, alt. ca. 1,000-2,150 m in SE Brazil, in the states of Espírito Santo, Minas Gerais, Rio de Janeiro (Itatiaia, Serra dos Orgãos, Alto Macahé), and São Paulo (Serra do Mar).
The two localities cited in the protologue are confused, the first, without collector and herbarium indication, most likely refers to the type of Lycopodium reflexum var. nudum Christ (= Mayaca, Mayacaceae). The second half of the citation applies to the lectotype. Nessel indicated Brade as collector of the type, but Brade arrived in Brazil for the first time in 1910.
Hoehne added a note to the protologue, indicating that he did not consider Urostachys nudus a good species, but rather an aquatic form of Lycopodium reflexum and recommended to treat it as a variety of the latter. This, however, does not invalidate the name Urostachys nudus, as Hoehne is not its author.
Phlegmariurus nudus as lectotypified here is a member of the P. hippurideus group. Nessel referred other material of P. nudus to Urostachys bolivianus (Rosenst.) Nessel, which represents southern Andean populations (varieties) of P. hippurideus. Phlegmariurus nudus differs from the Andean material in the smaller proportions in all organs.
Reference specimens (21 collections studied): BRAZIL. ESPÍRITO SANTO: Castelo, road to Forminho, P.E. Forno Grande, 1,650 m, Salino 13745 (BHCB, photo AAU). MINAS GERAIS: Espera Feliz, Parque Nacional de Caparaó, Pedra Menina 1,200-1,800 m, Salino 11504 (HBCB, photo AAU). Itamonte, Parque Nacional do Itatiaia, road to Abrigo Rebouças, 2,125 m, Salino 12362 (BHCB, photo AAU). Alagoa, P.E. Serra do Papagaio, ascent to Pico do Garrafão, 2,150 m, Salino 12929 (BHCB, photo AAU). Serra do Papagaio, Magalhaes 5778 (BHCB, photo AAU). Araponga, Parque Estadual Serra do Brigadeiro, Salino 5524 (BHCB, photo AAU). RIO DE JANEIRO: Serra dos Orgãos, Haut des Orgues, coté de Sto. Antonio avec la comm. Belge, Glaziou 6405 (C, P). Itatiaia, Retiro, 2,100 m, Dusén 651 (AAU, BONN-Nessel 153, GH, P, S, US). SÃO PAULO: Serra da Bocaina, estrada de acesso aos Campos da Bocaina, 1,450 m, Windisch et al. 4984 (AAU, HB, SP). Serra do Mar, estrada Cunha-Parati, ca. 1,450 m, Windisch et al. 5005 (AAU, HB).
Phlegmariurus pungentifolius (Silveira) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 16c,d
Lycopodium pungentifoliumSilveira, Bol. Com. Geogr. Geol. Minas Geraes 2, 5: 119, t. 4. 1898Silveira A (1898) Novae species Lycopodiacearum civitatis Minas Geraes (Brazil). Boletim da Commissão Geográfica e Geológica do Estado Minas Geraes 5: 117-145, t. 1-12. . - Urostachys pungentifolius (Silveira) Nessel, Arch. Bot. Est. S. Paulo 1: 394. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys reflexus (Lam.) Herter var. pungentifolius (Silveira) Nessel, Bärlappgewächse 115. 1939. - Huperzia pungentifolia (Silveira) B. Øllg., Opera Bot. 92: 169. 1987. - Type: In cerrados in Serra Negra, inter Lima Duarte et Rio Preto, civ. Minas Geraes, VII.1898, Henrique Magalhães no. 2917 in herb. Com. Geog. et Geolog. civ. Minas Geraes (holotype P).
Lycopodium ouropretanumChrist, in Schwacke, Pl. Nov. Mineiras 39, t. 4. 1900Christ H (1900) Spicilegium Pteridologicum Austro-Brasilense. In: Schwacke W (ed.) Citade de Minas (Ouro Preto), Minas Geraes. Plantas Novas Mineiras 2: 12-42, t. 1-4. [seors. Bull. Herb. Boiss., ser. 2, 2: 702. 1902]. - Syntypes: Ouro Preto, Schwacke 11995 (P), 12737 (P); Serra Negra près Rio Preto, Magalhaes Gomes 2917 (P: identical to the type collection of Lycopodium pungentifolium; Santa Catarina, Ule 4663 (herbarium unknown).
Urostachys hennebergorumNessel, Repert. Sp. Nov. Regni Veg. 48: 169, t. 316, f. 1. 1940Nessel H (1940) Beiträge zur Kenntniss der Lycopodiaceen. Revista Sudamericana de Botanica 6: 156-175, t. 7-19.. - Type: Haïti, environs Bayeux, O. B. 10. Juillet 1912 (ex herb. E. Miethe, holotype BONN herb. Nessel 351). The type is probably mislabelled. No material from the Greater Antilles matches the type, which on the other hand is indistinguishable from Phlegmariurus pungentifolius.
Plants terrestrial or rupestral, erect or ascending from a decumbent base, or scrambling over the ground with erect shoot apices, to 30 cm tall or to 150 cm long, at least to 10 times dichotomous, often with strongly divergent dichotomies. Shoots homophyllous, equally thick throughout or slightly tapering upward, (7-)10-20 mm in diam. incl. leaves, sporangiate from ca. 10-20 cm above the ground, often in separate periodically produced zones. Stems excl. leaves 3-4(-5) mm thick at the base, tapering to ca. 1-2 mm upward. Leaves borne in more or less regular, often oblique, alternating whorls of 6, sometimes 5 in distal divisions, sometimes 7 in proximal divisions, the whorls 1-2.5 mm apart, forming 10-14 indistinct longitudinal ranks, perpendicular to reflexed, sometimes sharply reflexed, linear to linear-lanceolate, in apical, densely sporangiate divisions usually lanceolate, (4-)5-9(-15) × (0.8-)1-1.3(-1.5) mm, not twisted, adaxially flat to evenly rounded and convex, sometimes with prominent vein, rarely concave or canaliculate, abaxially with widely prominent vein, with smooth margins, firmly herbaceous to coriaceous, usually lustrous above, with indistinctly and shallowly decurrent leaf bases. Sporangia 1.5-2 mm wide.
Distribution and habitats: Endemic: Minas Gerais, Rio de Janeiro.
Terrestrial, grasslands and shrublands, campo rupestre, among sandstone rocks, and in rock crevices in the mountains, alt. ca. 1,200-2,000 m.
In Nessel (1927)Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535. also as Urostachys myrtuosus (Spring) Nessel and in 1955 as Urostachys limidus nom. illeg.
Resembling Phlegmariurus recurvifolius (Rolleri) B. Øllg., but with shorter, more coriaceous leaves, and more divaricately branched, often quite shrub-like and scrambling over the ground. Superficially resembling P. reflexus (Lam.) B. Øllg., but with entire-margined, coriaceous leaves, and a more open branching pattern.
Reference specimens (33 collections studied): BRAZIL. MINAS GERAIS: Serra de Antonio Pereira, Baeta 472 (P, R). Barão de Cocais, Serra do Garimpo, 10 km by road NW of Barão de Cocais,1,200-1,300 m, Hensold 770 (AAU). Serra do Cipó, 15.IV.1935, Barreto & Brade 14403 (RB). Alto da Serra da Caraça, 1,907 m, Beato (RB 35964). Catas Altas, Serra do Caraça, Pico de Inficinonado, ca. 1,900 m, Prado 1003 (AAU). Serra do Espinhaço, Serra da Caraça, 1,750-1,950 m, 26.I.1971, Irwin et al. 29233 (C, F, K, NY, P, SP, UB, US, Z). Ouro Preto, Itacolomí, 1,450 m, Macedo 2798 (MO, RB, S, US). Parque Nacional do Caparaó, Cachoeira Bonita, Krieger et al. CESJ22656 (AAU). São Gonçalo do Rio Preto, Parque Estadual Rio Preto, Pico Dois Irmãos, 1,800-1,850 m, Salino 9323 (BHCB, photo AAU). Aiuruoca, P.E. Serra do Papagaio, trail to Pico do Papagaio from Vale do Matutu, 1,400-2,020 m, Salino 10470 (BHCB, photo AAU). Lima Duarte, Serra Negra, Fazenda dos Avelinos, 1,500 m, Brügger et al. 25155 (AAU, BHCB). Itamonte, Itatiaia, Brejo da Lapa, 2,116 m, Almeida et al. 1694 (BHCB, photo AAU). RIO DE JANEIRO: Santa Maria Magdalena, Serra da Forquilha, 1,600 m, Santos Lima & Brade 14359 (RB). Serra dos Orgaos, Sommet des Orgues, Glaziou 2797 (BR, C, P p.p.).
Phlegmariurus quadrifariatus (Bory) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 9a-d
Lycopodium quadrifariatum Bory, in: Duperry, Voyage Coquille, Bot. 1: 245. 1828 [as quadriforiatum]. - Urostachys quadrifariatus (Bory) Nessel, Arch. Bot. Est. S. Paulo 1: 405. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia quadrifariata (Bory) Rothm., Feddes Repert. 54: 60. 1944. - Type: île de Ste Catherine, Brésil, 1827, d’Urville (holotype P).
Lycopodium quadrangulareSpring, in Mart., Fl. bras. 1(2): 112-113, t. 5, I. 1840Spring A (1840) Lycopodium. In: Martius (ed.) Flora brasiliensis l: 109-117, t. 5. . - Urostachys quadrangularis (Spring) Nessel, Arch. Bot. Est. S. Paulo 1: 404. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Syntypes: Serra dos Orgaôs, Sellow (GL, K, L, P), Lhotsky s.n. from Brazil (B?, BR?, n. v.)
Lycopodium fontinaloides Spring var. coronata Fée, Crypt. Vasc. Brésil 2: 94-95. 1872-73. - Urostachys fontinaloides (Spring) Nessel var. coronatus (Spring) Nessel, Arch. Bot. Est. S. Paulo 1: 403. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Type: Rio de Janeiro, Glaziou 3219 (isotype P-herb. Cosson).
Lycopodium aschersoniiHerter, Bot. Jahrb. 43: Beibl. 98: 53. 1909Herter W (1909) Ein neuer beitrag zur Kenntnis der Gattung Lycopodium. Hedwigia 49: 88-92, t. 3.. - Urostachys aschersonii (Herter) Nessel, Arch. Bot. Est. S. Paulo 1: 422. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia aschersonii (Herter) Holub, Folia Geobot. Phytotax. 20: 70. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Syntypes: Rio de Janeiro, Tijuca, Morro da Fazenda coté du Pico do Papagaio, 12.XI.1870, Glaziou 5219 and 7194 (P syntypes).
Published illustrations: Øllgaard 1992Øllgaard B (1992) Neotropical Lycopodiaceae - an overview. Annals of the Missouri Botanical Garden 79: 687-717.: p. 704, fig. 10.
Plants epiphytic, lax and pendulous, at least to 90 cm long, at least to 10 times dichotomous. Shoots narrow, usually covered by small imbricate leaves throughout, usually homophyllous or with gradually smaller leaves in distal divisions, but sometimes with long expanded leaves in short portions of the proximal divisions, sporangiate in separate, seasonally produced zones or continuously sporangiate from 10-50 cm above the base and upward. Constricted shoots incl. leaves sharply quadrangular throughout, usually concave-sided (dried), 2-3 mm in diam. incl. leaves at the base, toward the apex sometimes tapering to 1.5-2 mm in diam. Stem excl. leaves 1.5-2 mm thick at the base, not red, tapering to 0.5-1 mm in distal divisions. Expanded leaves, if present, linear to oblanceolate, to 12 × 2-3 mm, with short-acute to obtuse or mucronulate apex, flat, with slightly revolute margins, spreading to ascending. Leaves of proximal and upper vegetative constricted divisions decussate, the leaf pairs 1-2 mm apart, usually appressed with slightly diverging apex, ovate to widely triangular-ovate, acute to acuminate, abaxially sharply carinate and conduplicate, widely and prominently long-decurrent, coriaceous, dull to lustrous, 2-3(-4) × 2-2.5(-3) mm (adding conduplicate faces), with smooth to somewhat uneven-erose margins. Sporangiate leaves often wider than long, 1.5-2 × ca. 2 mm, equalling or slightly exceeding the sporangia. Sporangia ca. 1 mm wide.
Distribution and habitats: Endemic. Pendulous epiphyte in moist montane forest, alt. 100-1300 m. Minas Gerais, Espírito Santo, Rio de Janeiro, São Paulo, Paraná, Santa Catarina.
Relatively robust, differing from Phlegmariurus fontinaloides and P. hexastichus by the sharply carinate leaves throughout in the constricted, and sharply quadrangular divisions. Expanded leaves, when present, are linear-oblong, and resembling those in P. hexastichus.
Reference specimens (20 collections studied): BRAZIL. ESPÍRITO SANTO: Castelo, Braço Sul, Brade 19191 (AAU, GH, RB). MINAS GERAIS: Santa Maria do Salto, Distrito Talisma, Fazenda Duas Barras, 820 m, Salino 9446 (ex BHCB, photo AAU). PARANÁ: Morretes, Rio Sagrado de Cima, 200 m, Hatschbach 19463 (MBM). Jaguaraiva, ca. 740 m, Dusén 16999 (C). RIO DE JANEIRO: Petrópolis, Glaziou 9068 (K, P). Itatiaia, Rio Campo Belo, 900 m, Brade 21455 (AAU, HB). SANTA CATARINA: Orleães, Rio Mirador, 250 m, Reitz 3396 (RB). Joinville, Schmalz 147 (F). São Francisco, VII.1884, Ule (HBG). Blumenau, Parque Nacional Serra do Itajai, 660 m, Cadorin et al. 718 (BHCB, photo AAU). Pilões, Palhoça, 300 m, Reitz & Klein 2430 (HBR). Botuvera, Cinema, 526 m, Korte & Kniess 347 (BHCB, photo AAU). SÃO PAULO: Reserva Florestal da Bocaina, 1,300 m, Sucre 3009 (AAU, RB). Ribeira, Pariquera-assú, Brade 5141 (HB). Apiaí, Puiggari 7 (SP 17983). São Paulo, Cidade Jardim, 30.XI.1930, Gehrt .(SP 27046).
Phlegmariurus recurvifolius (Rolleri) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 19a-c
Huperzia recurvifoliaRolleri, Notas Mus. La Plata 21 (Bot. 103): 210, fig. 1-3 (1989). - TYPE: Brazil, Bahia: Pico das Almas, ca. 25 km WNW of the Vila do Río de Contas, 41º57’W, 13º33’S, 1,600-1,850 m, Harley 19702 (holotype LP; isotypes CEPEC, K, NY).
Plants terrestrial or rupestral, erect or ascending to erect from a decumbent base, to ca. 50(-80) cm tall, sparsely branched, at least to 5 times dichotomous. Shoots homophyllous or gradually slightly reduced in distal divisions, almost equally thick throughout, ca. 13-25 mm in diam. incl. leaves or tapering to 10-15 mm, sporangiate usually from approx. 15-30 cm above the stem base and upward. Stems excl. leaves 2-4 mm thick (dried) at the base, sometimes tapering to ca. 1.5 mm upward. Leaves borne in more or less regular, often oblique, alternating whorls of (5-)6-7, these 1-2 mm apart, forming (10-)12-14 indistinct longitudinal ranks, spreading to ascending, slightly to strongly recurved from an ascending leaf base, linear to linear-lanceolate, widest just above the base, at least those of upper divisions usually terminating in a short, very thin, whip-like, yellowish or brownish, usually strongly curved or twisted tip, (5-)7-14 × (0.8-)1.2 mm, adaxially flat to somewhat concave (dried), abaxially with slightly tumid vein, usually somewhat lustrous, hypostomatic, with smooth margins. Sporangia 1.5-2 mm wide.
Distribution and habitats: Venezuela, Guyana, Brazil (Bahia, Minas Gerais, Espírito Santo). Terrestrial, erect or ascending. Open situations on sandstone mesetas, and marshy areas, 1,150-1,900 m. Information on type label: Sandstone conglomerate metamorphic & quartzitic rock outcrops with associated scrubby vegetation with damp flushes, grass & marshes in some areas. This plant spreading between rocks on summit.
Deviates from Phlegmariurus hippurideus mainly in the softly recurving rather than sharply reflexed leaves, and by the finely protracted, twisted, pale or brownish leaf apices.
Specimens studied: BRAZIL. BAHIA: Serra do Sincora, among rocks, 1,200 m, Ule 7297 (BONN herb. Nessel 220, HBG). Abaíra, Serra S of Riacho da Taquara, 1,890 m, Harley et al. 51252b (AAU). ESPÍRITO SANTO: São Roque do Canaa, 1,130 m, Labiak et al. 4123 (RB). MINAS GERAIS: Jequitinhonha, Res. Biol. da Mata Escura, 1,000 m, Salino 13246 (BHCB, photo AAU).
Phlegmariurus reflexus (Lam.) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 20a-d
Lycopodium reflexum Lam., Encycl. Méthodique Bot. 3: 653. 1789. - Plananthus reflexus (Lam.) P.Beauv., Prodr. Aeth. 100. 1805. - Urostachys reflexus (Lam.) Herter, Beih. Bot. Centralbl. 39: 249. 1922Herter W (1922) Itinera Herteriana III. Heteropteridophyta austroamericana. (Equisetales Lycopodiales Selaginellales Isoëtales austroamericanae.) Beihefte Botanisches Centralblatt 39: 248-256.. - Huperzia reflexa (Lam.) Trevis., Atti Soc. Ital. Sci. Nat. 17: 248. 1874. - Type: Martinique, herb. Lamarck, Comm. Joseph Martin s.n. (holotype P).
Lycopodium bifidum Willd., Sp. Pl. ed. 4. 5: 53. 1810. - Lycopodium reflexum Lam. var. majusSpring, Mém. Acad. Roy. Sci. Belgique 15 [Mon. Lyc. 1]: 26. 1842Spring A (1842) Monographie de la famille des Lycopodiacées, premiére partie. Mémoires de l’Académie Royale des Sciences, Lettres et Beaux Arts de Belgique 15: 1-110. . - Huperzia reflexa (Lam.) Trevis. var. bifida (Willd.) Trevis., Atti Soc. Ital. Sci. Nat. 17: 248. 1874. - Urostachys bifidus (Willd.) Nessel, Bärlappgewächse 110. 1939Nessel H (1939) Die Bärlappgewächse (Lycopodiaceae). G. Fischer, Jena. 404p.. - Huperzia bifida (Willd.) Holub, Folia Geobot. Phytotax. 20: 71. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Venezuela, Cuchilla de Guajana Guajana, Humboldt & Bonpland 474 (holotype B-Willd. 19421, isotype P-Humboldt).
Lycopodium reversum C.Presl, Rel. Haenkeana 1: 82. 1825Presl CB (1825) Lycopodiaceae. Pragae. Reliquiae Haenkeana 1: 77-83. . - Urostachys reversus (Presl) Herter, Index Lyc. 78. 1949Herter W (1949) Index Lycopodiorum, Montevideo. Pp. 1-120.. - Type: Ecuador, Guayaquil, Haenke s.n. (holotype PRC).
Lycopodium reflexum Lam. var. minusSpring, Mém. Acad. Roy. Sci. Belgique 15 [Mon. Lyc. 1]: 26. 1842Spring A (1842) Monographie de la famille des Lycopodiacées, premiére partie. Mémoires de l’Académie Royale des Sciences, Lettres et Beaux Arts de Belgique 15: 1-110. . - Urostachys reflexus (Lam.) Herter var. minor (Spring) Nessel, Arch. Bot. Est. S. Paulo 1: 393. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia reflexa var. minor (Spring) B. Øllg. in Harling and Andersson, Fl. Ecuador 33: 26. 1988. - Phlegmariurus reflexus (Lam.) B. Øllg. var. minor (Spring) B. Øllg., Rodriguésia 63(2): 481. 2012. - Syntypes: BRAZIL, Pr. Rio de Janeiro, Gaudichaud s.n. (P); Langsdorff s.n. (M-Mart.); Brazil, Serra dos Orgãos, fr. Majo, Guillemin s.n. (P); in sylvis prov. Paraënsis, Martius s.n. (M); Brazil, in prov. Minarum, Claussen s.n. (P).
Lycopodium reflexum Lam. var. densifoliumBaker, Handb. Fern-Allies 11. 1887Baker JG (1887) Handbook of the fern-allies. George Bell & Sons, London. 159p.. - Lycopodium densifolium (Baker) Underw. & F.E.Lloyd, Bull. Torrey Bot. Club 33: 106. 1906. - Urostachys reflexus (Lam.) Herter var. densifolius (Baker) Nessel, Arch. Bot. Est. S. Paulo 1: 394. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys densifolius (Underw. & F.E.Lloyd) Herter, Index Lyc. 58. 1949. - Syntypes: Popayan, Hartweg 1480 (G, K); Moritz 2266 (=226b: HBG, K); Glaziou 15797 (K).
Lycopodium brutumHerter, Bot. Jahrb. 43: Beibl. 98: 47. 1909Herter W (1909) Ein neuer beitrag zur Kenntnis der Gattung Lycopodium. Hedwigia 49: 88-92, t. 3.. - Urostachys brutus (Herter) Herter, Repert. Spec. Nov. Regni Veg. 19: 162. 1923. - Urostachys reflexus (Lam.) Herter var. brutus (Herter) Nessel, Bärlappgewächse 116. 1939Nessel H (1939) Die Bärlappgewächse (Lycopodiaceae). G. Fischer, Jena. 404p.. - Type: Trinidad, Hooker ded. 1845 (holotype P; isotype? L). The type matches the small forms, with nearly smooth leaf margins.
Urostachys parvifoliusNessel, Arch. Bot. Est. S. Paulo 1: 395, t. 10, f. 2. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys reflexus (Lam.) Herter var. parvifolius (Nessel) Nessel, Bärlappgewächse 114. 1939. - Syntypes: Rio de Janeiro: Without certain locality, Vidal, 29.VI.922 (RB?, BONN-herb. Nessel no. 219 isotype?; GH, Pico da Tijuca); Matto Grosso: Cuyabá, VIII.1909, Hartmann 133 (not located).
Urostachys intermedius (Spring) Herter var. cipoanusNessel, Arch. Bot. Est. S. Paulo 1 (4): 397, t. 16. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Type: Brazil: Minas Gerais, in Serra do Cipo, IV.1905, Silveira s.n. (holotype BONN-herb. Nessel no. 236, left hand specimen,).
Urostachys jergiiNessel, Repert. Spec. Nov. Regni Veg. 39: 70, t. 194. 1935Nessel H (1935) Beiträge zur Kenntnis der Gattung Lycopodium. Repertorium Specierum Novarum Regni Vegetabili 39: 61-71, t. 189-194.. - Type: Puerto Rico, Sintenis 6345 (holotype BONN-Nessel 229, isotype NY).
Lycopodium mexiae Copel., Univ. California Publ. Bot. 19: 294, pl. 47. 1941. - Huperzia mexiae (Copel.) Rolleri & Deferrari, Notas Mus. La Plata, Bot. 21 (100): 156. 1988. - Type: Peru, Huánuco, Churubamba, trail Cotirarda-Mercedes, 1,875 m, Mexia 8193 a (holotype UC; isotypes F, GH, K, MICH).
Urostachys stellae-polaris Herter, Revista Sudamer. Bot. 10: 121. 1953. - Lycopodium stellae-polaris (Herter) C.V.Morton, Amer. Fern J. 54: 72. 1964Morton CV (1964) New combinations in Lycopodium. American Fern Journal 54: 71-73.. - Type: Colombia, Cundinamarca, Guayabetal to Monte Redondo, SE of Quetame, 1,300-1,500 m, Pennell 1801 (holotype US, isotypes GH, NY).
Published illustrations: Øllgaard 1988Øllgaard B (1988) Lycopodiaceae. In: Harling G & Andersson L (eds.) Flora of Ecuador 33: 1-155.: Fig. 4A. Lellinger, 1989Lellinger DB (1989) The ferns and fern-allies of Costa Rica, Panama, and the Chocó. (Part 1: Psilotaceae through Dicksoniaceae). Pteridologia 2A: 1-364.: fig. 36; Mickel & Beitel, 1988Mickel JT & Beitel JM (1988) Pteridophyte Flora of Oaxaca, Mexico. Memoirs of the New York Botanical Garden 46: 1-568.: fig. 1E, F.
Plants terrestrial, erect or ascending from a decumbent base, soft, usually forming small loose clumps, 10-30(-40) cm tall. Shoots homophyllous, almost equally thick throughout, 8-14(-20) mm in diam. incl. leaves. Stems excl. leaves 1-3(-4) mm thick at base, sometimes tapering to 0.5-1.5 mm in diam., ridged by decurrent leaves or almost smooth, sporangiate from 2-10(-20) cm above the base and upward, usually 2-5 times dichotomous. Leaves borne in alternating, often irregular and oblique, whorls of 5-8(-9), these ca. 0.7-2.5 mm apart, forming 10-14(-18) longitudinal ranks, ascending to spreading or sharply reflexed, straight to strongly recurved, linear-subulate to linear-lanceolate, widest at or just above the base, (3.5-)4-8 × 0.5-1(-1.2) mm, softly herbaceous to subcoriaceous, adaxially convex and usually somewhat lustrous, or concave near the base, abaxially flat, or slightly concave to convex, with obscure to widely prominent vein, with flat to revolute, very sparsely to densely denticulate to short-ciliolate margins. Leaf bases often somewhat decurrent. Sporangia 1-1.5 mm in diam.
Distribution and habitats: Throughout humid mountainous regions of tropical America, south to northern Argentina. Land slides, road banks, and other open or disturbed habitats in montane forest, alt. 300-2,300 m.
Brazil: Amazonas, Pará, Minas Gerais, Rio de Janeiro, São Paulo, Paraná, Santa Catarina, Rio Grande do Sul.
As testified by the number of synonyms this is a variable species, or rather a species aggregate. In earlier treatments (Øllgaard 1988Øllgaard B (1988) Lycopodiaceae. In: Harling G & Andersson L (eds.) Flora of Ecuador 33: 1-155., 1994, 1995, 2012a) Phlegmariurus reflexus var. minor (Spring) B. Øllg. was recognized on the basis of size mainly. However, during the present revision it was found that this criterium was insufficient to maintain such a taxon.
The combination Huperzia parvifolia (Raddi) Rothm., Feddes Repert. 54: 60. 1944 is invalid, based on an unpublished name, and without reference to the publication by Nessel (1927)Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Listed in Nessel 1955, p. 47: Freire & Vidal, Pico da Tijuca, 10.VI.1922 no. 21.682). The same, Gávea, DF, 29.VI.1922 (Herb. Rio de Janeiro (R ? or ? RB?) 21.279), probably type collection (same date) (All in BONN-herb. Nessel no. 218, 2199).
Reference specimens (190 collections studied): BRAZIL. AMAZONAS: São Gabriel do Cachoeira, P.N. Serra da Neblina, trail to Cachoeira do Anta, 2,343 m, Carvalho 379 (BHCB, photo AAU, ICN). MINAS GERAIS: Santa Rita do Jacutinga, Krieger CESJ 21233 (AAU). Serra do Espinhaço, 18 km SW of Diamantina, Steep rocky hillside, sloping down to gallery forest, 1,400 m, Anderson 8474 (K, UB). Barão Cocais, Serra de Boa Vista, Pereira 2507 (RB). Ouro Preto, 6 km SSE of Belo Horizonte, Planalto of Itacolomi, 1,550 m, Tryon & Tryon 6886 (GH). Camanducaia, Vila Monte Verde (Serra da Matiqueira), 1,500 m, Windisch 5900 (AAU, SJRP); 1,950 m, Windisch 6076 (AAU, HSJP). Mariana, distrito Alegria, Mina de Conta Historia, 1,300-1,400 m, Salino 8923 (BHCB, photo AAU). Congonhas, road Ouro Branco-Ouro Preto, km 5, 1,000 m, Windisch et al. 4947a, 4947b, 4947c (AAU). Poços de Caldas, Morro do Ferro, Emmerich 1806 (HB). Carandaí, Duarte 6346 (B, HB, NY). Serra do Caparaó, 1,700 m, Windisch et al. 4974 (AAU, SJRP). Serra do Caraça, entre Trindade e Cachoeira do Campo, Damazio 1684 (RB). Serra do Gongo Socco, 11.I.1921, Hoehne (SP 4889, US). Caldas, Cabo Verde, Regnell I 494 (K, M, S, US). Santa Maria do Salto, distrito Talismã, Fazenda Duas Barras, 850-1,000 m, Salino 10044 (BHCB, photo AAU). Aiuruoca, Vale do Matutu e P.E. Serra do Papagaio, 1,700-2,070 m, Salino 9726 (BHCB, photo AAU). Delfim Moreira, Serra da Mantiqueira, 1,617 m, Fernandes 894 (BHCB, photo AAU). Santo Antonio de Carrancas, Serra de Carrancas, Serra das Broas na Chapada dos Perdizes, 1,300 m, Salino 12287 (BHCB, photo AAU). Baependi, Parque Estadual Serra do Papagaio, 1,761 m, Souza 1020 (AAU). PARÁ: Prov. Paraënsis, in sylvis passim, Martius 1819 (M). RIO DE JANEIRO: Itatiaia, Riberão Campo Belo, 150 km WNW of Rio de Janeiro, Tryon &Tryon 6677 (HB). Serra dos Orgãos, Pedra Chapados, 1,900 m, Brade 16386 (AAU, GH, MO, SP). Itaipava-Theresópolis, km 27, 1,000 m, 28.X.1962, Pabst & Abendroth 7150 (HB). Nova Friburgo, road to Picos da Salina, 1,070 m, Windisch et al. 4977 (AAU, HB). Petrópolis, road Faz. Inglesa-Pati do Alferes, 1,180 m, 22.IV.1980, Plowman & Martinelli 10144 (AAU). Santa Maria Magdalena, Alto de Desengano, Santos Lima 338 (AAU). RIO GRANDE DO SUL: São Leopoldo, Reitz 223 (US). Rio Pardo, Estirão Resende, 1,907 m, Jürgens in Rosenst. Fil. Austrobras. 354 (M, NY, US). Palmeira das Missões, 600 m, Bornmüller 784 (GH). Santa Maria, Malme 1223, 1223a (S). PARANÁ. Ponta Grossa, 900 m, Dusén 2514 (M, P, S). Piraquara, Banhado, Hatschbach 299 (SI, US). São José dos Pinhais, Colonia Santo Andrade, Hatschbach 14846 (MBM). Sengés, Rio do Funil, Hatschbach 5320 (MBM, US). Serra do Mar, between Quatro Barras and Morretes, 800 m, Windisch et al. 4891 (AAU, HB). Balsa Nova, Serra S. Luis, Hatschbach 24464 (NY). SANTA CATARINA: Brusque, Azambuja, 50 m, Reitz & Klein 892 (HBR, US). Lages, 950 m, Sehnem 5535 (HBR). Serra da Boa Vista, Rancho Queimado, 1,300 m, Reitz 5421 (HBR). São Francisco do Sul, Garuva, 900 m, Reitz & Klein 10296 (HBR). Timbé do Sul, Serra da Rocinha (Serra dos Pinheiros), ascent to São José dos Ausentes, ca. 19 km from Timbé, 1,100 m, Windisch 6059 (AAU). Urubici, road to Tubarão, após Morro da Igreja, entrance to Campo dos Padres, at base of mountain, 1,000 m, Windisch 6045 (AAU). Mafra, 750 m, Reitz 5357 (HBR). São Bento do Sul, ca. 20 km SE of São Bento do Sul, on road to Corupá, Pedersen, 13773 (AAU). SÃO PAULO: Campos do Jordão (divisa com Pindamonhangaba), São José dos Alpes, 1,800 m, Windisch 6815 (AAU, SJRP). Campos da Bocaina, Serra do Mar (acesso Campos de Cunha e Macacos), 1,500 m, Windisch 6821 (AAU, SJRP). Atibaia, Pedra Grande, 6.IX.1939, Gehrt (SP 41647). São Paulo, Jardim Botanico, 3.VIII.1939, Handro (SP 47445). Ubatuba, Núcleo Santa Virginia, P.E. Serra do Mar, Rio Ipiranga, Rodrigues & Coral 1294 (BHCB, photo AAU). Anhembí, Rio Piracicaba, Kuhlmann 4575 (SP). Apiaí, road Iporanga-Apiaí, alto da Serra, Windish 6078 (AAU, HSJP). Piquete-Itajubá road, Duarte 750 (HB, RB). Cunha, Serra do Mar, Reserva Florestal de Cunha, 1,000 m, Windisch et al. 5002 (AAU, HSJP). Moji das Cruzes, 840 m, 26.VI.1961, Eiten & Eiten 2489 (GH, NY, SP). Tatuí, Löfgren 106 (SP).
Phlegmariurus regnellii (Maxon) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 22d,e
Lycopodium regnelliiMaxon, Contrib. U.S. Nat. Herb. 17: 424, pl. 23. 1914Maxon WR (1914) Studies of tropical American Ferns, nº. 5. Contributions from the United States National Herbarium 17: 391-425.. - Urostachys regnellii (Maxon) Nessel, Arch. Bot. Est. S. Paulo 1: 3. 81. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia regnellii (Maxon) B. Øllg. & P.G. Windisch, Bradea 5: 16. 1987. - Type: Brazil. Pedra Branca, Caldas, 21.X.1868, Regnell III 1,500 (holotype US 201172, isotypes BONN-Herb. Nessel 54-55, C, S, UC).
Published illustrations: Maxon, 1914Maxon WR (1914) Studies of tropical American Ferns, nº. 5. Contributions from the United States National Herbarium 17: 391-425.: t. 23.
Plants terrestrial or rupestral, erect or ascending from a decumbent base, to ca. 30 cm tall, at least to 6 times dichotomous. Shoots homophyllous or with gradually shorter leaves upward, equally thick throughout or tapering, ca. 15-20 mm in diam. incl. leaves, usually tapering to 5-10 mm, sporangiate from ca. 10-20 cm above the ground. Stems excl. leaves 3-5 mm thick at the base, tapering to ca. 1-2 mm upward. Leaves of proximal divisions borne in more or less regular, often oblique, alternating whorls of 6-7, these 1-3 mm apart, in distal divisions in whorls of 5-6, ca. 1 mm apart, forming 10-14 indistinct longitudinal ranks, perpendicular to ascending, upward sometimes closely appressed, linear to linear-lanceolate, in apical, densely sporangiate divisions usually lanceolate, (4-)5-9(-15) × 1-1.3(-1.5) mm, not twisted, adaxially flat to slightly concave, usually with prominent vein, abaxially flat to distinctly convex, with flat to slightly revolute margins and widely prominent vein, with smooth margins, firmly herbaceous to coriaceous, usually lustrous, with shallowly decurrent leaf bases. Sporangia 1.5-2 mm wide.
Distribution and habitats: Endemic. The information about the ecology of this species is limited. There are few collections. Forest margins, among rocks, 1,000-1,835 m. Minas Gerais, Rio de Janeiro.
Phlegmariurus regnellii is related to P. pungentifolius, but differs in the stiffly erect growth habit, and the reduced and ascending to appressed leaves in the distal divisions. This seems a different reaction to exposed conditions than the reflexed leaves in P. pungentifolius under similar conditions. Personal observation by Ashley Field (CNS, BRI): “Phlegmariurus regnellii grows in humus pockets in crevices or out in the open on the massive rocky outcrop of Pedra Branca, it is softer than P. pungentifolius. By contrast P. pungentifolius (I did not see the two anywhere near each other) I found grew more in grasslands and shrublands like Campo Rupestre”. The cited specimen: Serra dos Orgãos, Glaziou s.n. (RB) is probably mislabelled, like several other Glaziou specimens.
Specimens studied: BRAZIL. MINAS GERAIS: Serra de Caldas, 1,000 m, Mosén 4654 (P, S); Mosén 2015 (BONN-Nessel 54, S). Carangola, Alto da Serra da Gramma, 1,835 m, Kuhlmann 57 (RB). Caldas, distriro de Pocinhos do Rio Verde, Pedra Branca, 1,650 m, Salino 12646 (BHCB, photo AAU). RIO DE JANEIRO: Serra dos Orgãos, Glaziou (RB) probably a wrong locality.
Phlegmariurus rostrifolius (Silveira) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 7c,d
Lycopodium rostrifoliumSilveira, Bol. Comm. Geogr. Geol. Minas Geraes 2, 5: 118, t. 2. 1898Silveira A (1898) Novae species Lycopodiacearum civitatis Minas Geraes (Brazil). Boletim da Commissão Geográfica e Geológica do Estado Minas Geraes 5: 117-145, t. 1-12. . - Urostachys rostrifolius (Silveira) Nessel, Arch. Bot. Est. S. Paulo 1: 379. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia rostrifolia (Silveira) Holub, Folia Geobot. Phytotax. 20: 76. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Minas Geraes: In marginibus rivulorum in Serra da Papagaio, Alvaro Silveira, XI.1897, no. 2605 in Herb. Comm. Geogr. Geolog. Minas Geraes (holotype? P, isotypes HB, RB, possible isotype BONN-Nessel 52 p.p.).
Plants terrestrial, erect or ascending from a decumbent base, soft, usually forming small loose clumps, to ca. 10 cm tall. Shoots homophyllous, almost equally thick throughout, ca. 6-7 mm in diam. incl. leaves. Stems excl. leaves 1.5-2 mm thick, densely covered by leaves, sporangiate from ca. 5 cm above the base and upward, to 4 times dichotomous. Leaves borne in alternating, irregular whorls of 5-6, these ca. 1-1.5 mm apart, forming 10-12 longitudinal ranks, loosely appressed to ascending, upward curved especially at the apex, subulate at the base, to linear-lanceolate in apical divisions, widest just above the base, 3-5 × ca. 1 mm, subcoriaceous, adaxially concave with slightly prominent vein, abaxially convex but with sunken vein (dried), with slightly involute, sparsely and remotely denticulate, indistinctly sclerified margins, often with several teeth near the leaf base, amphistomatic. Sporangia ca. 1.2 mm wide.
Distribution and habitats: Endemic. Only known from the type collection with certainty. Maintained with doubt, possibly not distinct from Phlegmariurus christii, from which it differs chiefly in the smaller, more remotely denticulate leaves. Also related to P. reflexus, from which it differs by the thick, subcoriaceous leaves and fewer teeth on the leaf margins, and abaxially convex leaves. One collection from the state of Santa Catarina may belong here: Reitz & Klein 6440 (US), Morro do Iquererim, Campo Alegre, 1,300 m. This collection seems intermediate between Phlegmariurus rostrifolius and P. christii.
Phlegmariurus ruber (Cham. & Schltdl.) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 18c-e
Lycopodium rubrum Cham. & Schltdl., Linnaea 8: 389. 1833. - Urostachys ruber (Cham. & Schltdl.) Nessel, Arch. Bot. Est. S. Paulo 1: 381. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia rubra (Cham. & Schlecht.) Trevis., Atti Soc. Ital. Sci Nat. 17: 247. 1874. - Type: Brasilia, Sellow s.n. (holotype B, isotypes K, L, P).
Published illustrations: Øllgaard 1992Øllgaard B (1992) Neotropical Lycopodiaceae - an overview. Annals of the Missouri Botanical Garden 79: 687-717.: Fig. 5.
Plants terrestrial, stiffly erect, often from an ascending base, forming dense clumps, to ca. 30 cm tall, at least to 3 times dichotomous. Shoots bright dark red, gradually heterophyllous, with shorter leaves upward, tapering and with fewer leaf ranks upward, 6-10 mm in diam. incl. leaves at the base, tapering to (3-)4-6 mm, sporangiate from ca. 10-20 cm above the base and upward. Stem excl. leaves 3-5 mm thick at the base, upward tapering to ca. 1 mm, completely concealed by leaves. Leaves of proximal divisions densely crowded and closely imbricate, borne in alternating whorls of 4-5, these 1.5-2 mm apart, forming 8-10 longitudinal ranks, straight, or with slightly curved or twisted apices, linear to linear-lanceolate, evenly tapering from the base, 9-12 × 1.5-2 mm, bluntly carinate to almost conduplicate, bright dark red, with smooth margins. Leaves of upper, densely sporangiate divisions borne in alternating whorls of 3-4, these 1-1.5 mm apart, forming 6-8 regular longitudinal ranks, lanceolate to widely ovate, acuminate to long-cuspidate (due to conduplicate apex), with sharply carinate apex, or carinate throughout, (3-)3.5-7 × 1.5-2.5 mm, coriaceous, with smooth margins. Sporangia ca. 2 mm wide.
Distribution and habitats: Endemic. Summits of higher mountains of the Iron Quadrangle in Minas Gerais State, with recent records only in the region of Serra do Caraça. Two Glaziou specimens from Bahia and Rio de Janeiro are probably mislabelled. On rocks and in rock crevices, occurring apparently only at alt. 1,300-2,060 m.
Another highly distinctive high-altitude species. Readily identified by its deep red stems and leaves.
Reference specimens (17 collections studied): BRAZIL. MINAS GERAIS: Serra do Espinhaço, sandstone summit of Serra da Caraça, 1,750-1,950 m, Irwin et al. 29064 (K, P, SP, UB, US). Alto da Serra da Caraça, 1,907 m, ex herb. Damazio (AAU, MO, RB 36989). Serra do Capanema, 1,800 m, herb. Schwacke 11997 (P, RB, SP). Alto da Serra do Itacolumy, Damazio (RB 36989 B, D). Summit of Carapuça, 1,760 m, 11.VI.1884, Glaziou 15801 (K, P). Santa Barbara, Serra do Caraça, Pico do Inficionado, 1,900-2,060 m, Mello-Silva et al. 1380 (AAU, SPF).
Phlegmariurus sellowianus (Herter) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 21a,b
Lycopodium sellowianumHerter, Bot. Jahrb. 43: Beibl. 98: 44. 1909Herter W (1909) Ein neuer beitrag zur Kenntnis der Gattung Lycopodium. Hedwigia 49: 88-92, t. 3.. - Urostachys sellowianus (Herter) Nessel, Arch. Bot. Est. S. Paulo 1: 385. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Huperzia sellowiana (Herter) B. Øllg., Opera Bot. 92: 169. 1987. - Lectotype: Sellow s.n. (B designated here). Syntypes: Rio de Janeiro, Alto Macahé, 12.III.1870, Glaziou 4468 (B not seen, C p. p., P), Alto Macahé, Mendonça 1411 (B not seen). Caldas, Lindberg 674 (B not seen).
Lycopodium brasilianumHerter, Bot. Jahrb. 43: Beibl. 98: 44. 1909Herter W (1909) Ein neuer beitrag zur Kenntnis der Gattung Lycopodium. Hedwigia 49: 88-92, t. 3.. - Urostachys brasilianus (Herter) Nessel, Arch. Bot. Est. S. Paulo 1: 386. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Lectotype: Brazil: Rio de Janeiro, Tijuca, s. Tingua, 6.VI.1877, Glaziou 9066 (P, designated here). Herter (1909) mentioned, but questioned the identity of Glaziou 4470 in P.
Urostachys uleiHerter, Repert. Sp. Nov. Regni Veg. 19. 1923Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.. - Huperzia ulei (Herter) Holub, Folia Geobot. Phytotax. 20: 77. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Rio de Janeiro: Auf einem hohen Berge der Serra Macahé, Nova Friburgo, 1,500 m, II.1900, Ule, herb. Bras. 4973 (holotype B, n. v., isotype HBG).
Plants epiphytic or terrestrial, erect from a hanging or decumbent base, at least to 40 cm tall, or to 70 cm long, sparsely branched, to 3(-4) times dichotomous, often with lax and flexuous stems. Shoots homophyllous, almost equally thick throughout, 20-30 mm in diam. incl. leaves. Stems excl. leaves 2-4 mm thick near the base, 1-3 mm in upper dividions, somewhat ridged by decurrent leaf bases, sporangiate from 5-30 cm above the base and upward. Leaves almost uniform throughout, borne in alternating, often oblique whorls of 3-4, these 1.5-4 mm apart, forming 6-8 indistinct longitudinal ranks, perpendicular-spreading to somewhat reflexed, usually straight, sometimes slightly recurved, not twisted at the base, lanceolate, with long-acute apex, papery to subcoriaceous, (10-)13-20 × 2.5-3.5 mm, almost flat, with prominent vein above, or folded slightly down along the vein, with slightly revolute, smooth to minutely rugose by individually protruding margin cells. Sporangia ca. 2 mm in diam.
Distribution and habitats: Endemic. Terrestrial or epiphytic in forest, alt. (200-)700-2,000 m, the lower altitudes in the southern part of the range. Minas Gerais, Espírito Santo, Rio de Janeiro, São Paulo, Paraná, Santa Catarina.
Phlegmariurus sellowianus is related to P. brongniartii (Spring) B. Øllg. from the Andes, and often has been classified as that species. However, differences of phyllotaxis and a more lax, decumbent to ascending growth habit separates this species form the latter species.
Reference specimens (26 collections studied): BRAZIL. ESPÍRITO SANTO: Divino de São Lourenço, Patrimonio da Penha, Parque Nacional do Caparaó, Facão de Pedra, Kollman et al. 10457 (RB). MINAS GERAIS: Pico do Papagaio, Glaziou 2046 (BR, P). Caparaó, Alto Caparaó, trail Tronqueira-Terreirrão, 1,970 m, Heringer et al. 242 (BHCB, photo AAU). Passa Quatro, Campo do Muro, 1,800 m, Brade et al. 18967 (RB). Alto do Itacolomi, Badini 2451 (AAU, OUPR). Itamonte, P.N. Itatiaia, road to Vargem Grande, 1,850-2,000 m, Salino 12494 (BHCB, photo AAU). PARANÁ: Campina Grande do Sul, Serra do Capivari Grande, 1,800 m, Hatschbach 8188 (HB, MBM, US). RIO DE JANEIRO: Serra dos Orgãos, Picada do Rancho Frio, 1,400 m, Brade 16624 (F, GH, MO, NY, R, S, US). Alto Macahé, chez les Trannin, Glaziou 7276 (P, US). Santa Magdalena, Serra Norte Vermelho, Santos Lima 413 (AAU, RB). SANTA CATARINA: Joinville, 700 m, 16 Schmalz 204 (F, MO, NY, UC). Itajai, Morro do Baú, 850 m, Reitz C2072 (RB, S). Ilha de Santa Catarina, Sertão da Lagôa, 200 m, Rohr 3079 (HB). SÃO PAULO: Alto da Serra, Wald im Campo, Brade 21456 (HB).
Phlegmariurus silveirae (Nessel) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 21c-e
Urostachys silveiraeNessel, Arch. Bot. Est. S. Paulo 1: 386. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys brasilianus (Herter) Nessel var. silveirae (Nessel) Nessel, Bärlappgewächse 84. 1939. - Huperzia silveirae (Nessel) B. Øllg. & P.G. Windisch, Bradea 5: 17. 1987. - Type: “BRAZIL: Minas Gerais, Serra do Bispo”, leg. Silveira s.n. (holotype BONN-herb. Nessel no. 144).
Urostachys schwackeanusNessel, Arch. Bot. Est. S. Paulo 1: 388. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys brasilianus (Herter) Nessel var. schwackeanus (Nessel) Nessel, Bärlappgewächse 85. 1939. - Lectotype: Silveira “no. 393”, with Silveira’s original label: Lycop. myrtuosum Spring. -In pratis arenosis in Serra do Cipó, Minas: Silveira, IV.1905; and with an extra label by Nessel with Serra do Papagaio crossed out, (BONN-herb. Nessel no. 145, designated by Øllgaard 1989Øllgaard B (1989) Index of the Lycopodiaceae. Biologiske Skrifter Kongelige Danske Videnskabernes Selskab 34: 1-135. p. 100). - Syntype: Glaziou s/ind., no. 9,066 (P); this mixed collection includes material of typical Phlegmariurus sellowianus and a type of Lycopodium brasilianum Herter in addition to material identical to Urostacys schwackeanus.
Urostachys portoanusNessel, Repert. Sp. Nov. Regni Veg. 39: 70, t. 194 b. 1935Nessel H (1935) Beiträge zur Kenntnis der Gattung Lycopodium. Repertorium Specierum Novarum Regni Vegetabili 39: 61-71, t. 189-194.. - Lectotype: São Paulo, Serra do Paranapiacaba, Iguape, XI.1925, Brade 8495 (BONN-herb. Nessel in no. 400, illustrated in protologue, designated by Øllgaard, 1989Øllgaard B (1989) Index of the Lycopodiaceae. Biologiske Skrifter Kongelige Danske Videnskabernes Selskab 34: 1-135. p. 98). - Syntype São Paulo: Umuarama, Campos de Jordão, 29.I.1935, Kuhlmann s.n. (SP 32260, BONN-herb. Nessel BONN in no. 400, SP).
Plants epiphytic or rupestral, lax and pendulous to spreading, sometimes recurved from an erect and somewhat rigid stem base, to 40 cm long. Shoots almost equally thick throughout or gradually tapering, ca. 20-30 mm in diam. incl. leaves at the base, sometimes tapering to (5-)7-15 mm in diam. in distal, densely sporangiate divisions of fully developed plants, sporangiate from 10-25 cm above the base and upward, rarely abruptly constricted. Stems excl. leaves 1.5-2 mm thick at the base, tapering to 0.5-1.5 mm upward, prominently ridged by decurrent leaf bases, pale greenish to brownish, usually 3-6(-8) times dichotomous. Leaves borne in alternating whorls or irregular low spirals of 3 or 4, these 1.5-4 mm apart, forming 6-8 indistinct longitudinal ranks, or leaves of distal divisions sometimes indistinctly decussate. Leaves of proximal divisions spreading to somewhat reflexed, rarely ascending, often twisting the lamina to a vertical position from the base, lanceolate to linear-lanceolate, widest from the lower half to the middle, rather narrowly joined to the stem, not clasping, somewhat adnate-decurrent on the stem, with prominently long-decurrent leaf base margins and vein, soft herbaceous to subcoriaceous, 11-20 × 1.2-3 mm, almost flat or somewhat convex adaxially, with flat to strongly revolute, smooth margins, adaxially with prominent vein, with evident to somewhat prominent vein abaxially. Leaves of middle and distal divisions spreading, usually conform but often gradually shorter and narrower, rarely more appressed and abaxially more convex. Leaves of fully sporangiate divisions usually spreading, rarely ascending to slightly appressed, often with slightly widened, somewhat clasping, abaxially convex base, not covering the sporangia, 5-12(-17) × 1.2-2 mm. Sporangia 1.5-2 mm wide.
Distribution and habitats: Endemic. Epiphyte, or occasionally rupestral in high altitude montane forest, 800-2,400 m alt., in the states of Minas Gerais, Paraná, Rio de Janeiro, Santa Catarina, and São Paulo.
With rather wide, and flattened, strongly twisted leaves, approaching Phlegmariurus taxifolius (Sw.) A.Löve & D.Löve.
In the protologue of Urostachys silveirae Nessel refers to the text and illustration of Lycopodium martii in Silveira (Bol. Comm. Geogr. Geolog. Minas Geraes (2) 5: 142, t, 5. 1898Silveira A (1898) Novae species Lycopodiacearum civitatis Minas Geraes (Brazil). Boletim da Commissão Geográfica e Geológica do Estado Minas Geraes 5: 117-145, t. 1-12. ), and probably meant to base his new species on Silveira’s material. The plate in Silveira (l. c.) illustrates a plant belonging to this taxon.
Reference specimens (35 collections studied): BRAZIL. PARANÁ: Piraquara, Rio Taquari, Hatschbach 317 (MBM). Guaratuba, Serra da Araçatuba, 1,250 m, Hatschbach 6566 (US). Campina Grande do Sul, Serra do Capivari Grande, 1,800 m, Hatschbach 8186 (HB, MBM, US). RIO DE JANEIRO: Corcovado, VII.1887, Ule (HBG). Serra dos Orgãos, pr. Cachoeira do Rancho Frio, 1,400 m, Brade 16623 (AAU, RB). Santa Magdalena, Alto do Desengano, 2,000 m, Santos Lima & Brade 13186 (BONN-herb. Nessel 145a, R, RB). Macaé, Frade do Macaé, Brade 15821 (AAU, RB). Santo Antonio de Imbé, Agulha, Brade & Santos Lima 11656 (BONN-herb. Nessel 409a, R). SANTA CATARINA: Morro do Iquererim, Campo Alegre, 1,400 m, Reitz & Klein 6119 (HBG, US). Botuverá, Morro do Barão, 1,100 m, Reitz & Klein 17980 (HBR). SÃO PAULO: Paranapiacaba, Alto da Serra, Brade 5848 (BONN-herb. Nessel 409, HB, S). Campo Grande, Serra do Mar, 800 m, Brade 6605 (HB, NY, S, UC). Serra da Bocaina, Morro do Matano, 1,900 m, Brade 21096 (AAU, RB). Iguape, Serra do Paranapiacaba, 800 m, Brade 8490 (AAU, HB). Campos do Jordão, Umuarama, Kuhlmann (BONN-herb. Nessel 142).
Phlegmariurus taxifolius (Sw.) A.Löve & D.Löve, Taxon 26: 324 (1977). Fig. 17g-i
Lycopodium taxifolium Sw., Prodromus 138. 1788. - Huperzia taxifolia (Sw.) Trevis., Atti Soc. Ital. Sci. Nat. 17: 248. 1874. - Urostachys taxifolius (Sw.) Herter, Repert. Spec. Nov. Regni Veg. 19: 162. 1923Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.. - Type: A remounted specimen, marked as type, but without original annotation, in the type herbarium in S, is possibly the holotype.
Lycopodium passerinoides Kunth, in: Humb., Bonpl. & Kunth, Nov. Gen. Sp. Pl. 1: 41 quarto ed. 1815 [1816]. - Huperzia passerinoides (Kunth) Trevisan, Atti Soc. Ital. Sci. Nat. 17: 248. 1874. - Urostachys passerinoides (Kunth) Nessel, Arch. Bot. São Paulo 1: 417. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys rubiginosus Nessel, Revista Sudamer. Bot. 6: 162. 1940. - Type: Peru, prope Olleras et Aipate, alt. 747 hexp., Humboldt s.n. (P-Humb., BONN-herb. Nessel 160).
Lycopodium nitens Schlecht. & Cham., Linnaea 5: 623. 1830. - Huperzia passerinoides (Kunth) Trevisan var. nitens (Schlecht. & Cham.) Trevis., Atti Soc. Ital. Sci. Nat. 17: 248. 1874. - Urostachys nitens (Schlecht. & Cham.) Herter, Repert. Spec. Nov. Regni Veg. 19: 164. 1923Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.. - Urostachys passerinoides (Kunth) Nessel var. nitens (Schlecht. & Cham.) Nessel, Arch. Bot. São Paulo 1: 418. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys schwendeneri (Herter) Herter var. nitens (Schlecht. & Cham.) Nessel, Bärlappgewächse 166. 1939. - Type: Mexico, in arboris vetustis prope Jalapam, Schiede & Deppe s.n. (holotype B, isotype BM).
Lycopodium herminieriSpring, Bull. Acad. Roy. Sci. Bruxelles 8: 514. 1841Spring A (1841) Enumeratio Lycopodinearum, quas in ejusdem plantarum ordinis monographia mox edenda descripsit A. Spring. Bulletins de l’Académie Royale des Sciences et Belle-Lettres de Bruxelles 8: 511-523.. - Urostachys herminieri (Spring) Herter, Repert. Spec. Nov. Regni Veg. 19: 162. 1923Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.. - Type: Guadeloupe, L’Herminier s.n. (P).
Lycopodium schwendeneriHerter, Bot. Jahrb. 43: Beibl. 98: 50. 1909Herter W (1909) Ein neuer beitrag zur Kenntnis der Gattung Lycopodium. Hedwigia 49: 88-92, t. 3.- Urostachys schwendeneri (Herter) Herter, Repert. Spec. Nov. Regni Veg. 19: 165. 1923. - Type: Mexico (indicated as locus classicus by Herter, 1923). No type designated.
Urostachys bruelkeiNessel, Repert. Spec. Nov. Regni Veg. 36: 184, t. 173. 1934Nessel H (1934) Neue Lycopodien, die von allen schon bekannten Arten durch ihren Habitus ganz besonders abweichend und auffallend sind. Repertorium Specierum Novarum Regni Vegetabili 36: 177-193, t. 170-177.. - Type: Colombia, 1,600-2,100 m, Killip 11595 (holotype BONN herb. Nessel 399).
Lycopodium brauseanumHerter, in: Urban, Symb. Antill. 7 (2): 165. 1912Herter W (1912) Lycopodiaceae. In: Urban I (ed.) Symbolae antillanae 7: 165-166.. - Urostachys brauseanus (Herter) Herter, Repert. Spec. Nov. Regni Veg. 19: 162. 1923. - Huperzia brauseana (Herter) Rolleri & Deferrari, Notas Mus. La Plata, Bot. 21(100): 155. 1988. - Type: Venezuela, Isla Margarita in San Juan Mt., 600 m, Johnston 156 p.p. (holotype B n. v.; isotypes BONN-Nessel 175, GH, US).
Lycopodium cubanumHerter, Bot. Jahrb. Syst. 43: Beibl. 98: 50. 1909Herter W (1909) Ein neuer beitrag zur Kenntnis der Gattung Lycopodium. Hedwigia 49: 88-92, t. 3.. - Urostachys cubanus (Herter) Herter, Repert. Spec. Nov. Regni Veg. 19: 165. 1923. - Huperzia cubana (Herter) Holub, Folia Geobot. Phytotax. 20: 71. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Type: Cuba: El Gato, St Yago de Cuba, 1844, Linden 2185 (P 2 sheets, both annotated later by Herter).
Published illustrations: Lellinger, 1989Lellinger DB (1989) The ferns and fern-allies of Costa Rica, Panama, and the Chocó. (Part 1: Psilotaceae through Dicksoniaceae). Pteridologia 2A: 1-364.: fig. 39; Mickel & Beitel, 1988: fig. 4A; Øllgaard, 1988Øllgaard B (1988) Lycopodiaceae. In: Harling G & Andersson L (eds.) Flora of Ecuador 33: 1-155.: p. 81 fig. 15A.
Plants epiphytic, lax and pendulous, or sometimes recurved from an erect and somewhat rigid stem base, to 50(-80) cm long. Shoots usually gradually tapering from ca. 15-30 mm in diam. incl. leaves at the base, to 5-15 mm in diam. in distal, densely sporangiate divisions of fully developed plants, sporangiate from 20-50 cm above the base and upward, sometimes not, or only slightly tapering (juvenile or tardily sporangiate individuals), rarely abruptly constricted. Stems excl. leaves 1.5-2.5 mm thick at the base, tapering to 1-1.5 mm upward, somewhat ridged by decurrent leaf bases, pale greenish to brownish, usually to 6 times dichotomous. Leaves usually reduced and modified upward, borne in alternating whorls or irregular low spirals of 3 or 4, these 1.5-4 mm apart, forming 6-8 indistinct longitudinal ranks. Leaves of proximal divisions spreading to ascending or somewhat appressed, often twisting the lamina to a vertical position from the base, narrowly lanceolate, widest in the lower half, widely joined to the stem, firmly herbaceous to subcoriaceous, 11-18 × 1.5-2 mm, almost flat or somewhat concave adaxially, with flat or slightly revolute, smooth margins, with evident to somewhat prominent vein abaxially. Leaves of middle and distal divisions usually gradually shorter, narrower and more appressed, abaxially more convex, often with involute margins. Leaves of fully sporangiate divisions often distinctly 6-ranked, rarely decussate, with strongly widened, clasping base, partly covering the sporangia, often abruptly contracted into a short to long, narrow, involute apex, (3-)5-12 × 1-1.5 mm. Sporangia ca. 1.5 mm wide.
Distribution and habitats: Central America, West Indies, northern South America, south to Peru and in Brazil in coastal forest, alt. 0-1.050 m, in the states of Amapá, Amazonas, Ceará, Rio de Janeiro, and São Paulo, and Paraná.
A variable species of problematic delimitation. Individuals which are morphologically intermediate between Phlegmariurus taxifolius and P. silveirae are frequent.
Specimens studied: BRAZIL. AMAPÁ: Porto Terezinha, Estrada Nova, 100-150 m, Cowan 38291 (NY). CEARÁ: Serra do Baturité, Santa Clara, Eugenio 52 (RB). PARANÁ: Alexandria, Dusén 8320 (S). Jaguaraíva, 710 m, Dusén 15409 (S). Paranaguá, Matinhos, 3-5 m, Hatschbach 761 (MBM, RB); Rio Cachoeirinha, 50-100 m, Hatschbach 19493 (MBM, UC). RIO DE JANEIRO: Rio de Janeiro, Corcovado, prope Paineiras, Mosén 49 (S); 1836, Gardner (P). RORAIMA: near Venezuelan border, Cord. Paracaima, ca. 1,050 m, Rosa & Nascimento 3543 (photo ex INPA at AAU) . SÃO PAULO: Iguape, Est. Serra do Paranapiacaba, 800 m, Brade 8492 (BONN-Nessel 353, HB). Santos, coastal forest, Mosén 3553 (S).
Phlegmariurus treitubensis (Silveira) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 22a-c
Lycopodium treitubenseSilveira, Bol. Comm. Geogr. Geol. Minas Geraes 2, 5: 118, t. 3. 1898Silveira A (1898) Novae species Lycopodiacearum civitatis Minas Geraes (Brazil). Boletim da Commissão Geográfica e Geológica do Estado Minas Geraes 5: 117-145, t. 1-12. . - Urostachys treitubensis (Silveira) Nessel, Arch. Bot. Est. S. Paulo 1: 380. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys christii (Silveira) Nessel var. treitubensis (Silveira) Nessel, Bärlappgewächse 46. 1939. - Huperzia treitubensis (Silveira) B. Øllg., Opera Bot. 92: 170. 1987. - Type: Inter saxa in locis arenosis in Serra da Treituba, Minas Geraes, IV.1897, Silveira 183 in Herb. Com. Geogr. et Geolog. civit. Minas Geraes 2210 (P, R).
Lycopodium inflexumSilveira, Bol. Comm. Geogr. Geol. Minas Geraes 2, 5: 118, t. 2. 1898Silveira A (1898) Novae species Lycopodiacearum civitatis Minas Geraes (Brazil). Boletim da Commissão Geográfica e Geológica do Estado Minas Geraes 5: 117-145, t. 1-12. , non Sw. 1806. - Urostachys inflexus (Silveira) Nessel, Arch. Bot. Est. S. Paulo 1: 384. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Urostachys capriHerter, Index Lyc. 55. 1949Herter W (1949) Index Lycopodiorum, Montevideo. Pp. 1-120., nom superfl. - Type: In cacumine montis Serra da Papagaio, Minas Geraes, inter saxa quartzitosa, in locis siccis: Silveira & Tavares, XI.1897, Herb. Comm. Geogr. et Geolog. civit. Minas Geraes no. 2606 (P).
Urostachys hoehneiNessel, Arch. Bot. Est. S. Paulo 1: 383, t. 6. 1927Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.. - Type: Minas Gerais, Miguel Burnier, campos pedregosos, no tôpo de rochas expostas aos raios do sol, 31.I.1921, Hoehne 5253 (SP, BONN-herb. Nessel no. 40a).
Plants terrestrial, stiffly erect, to 30 cm tall, to 4(-5) times dichotomous. Shoots homophyllous, or with gradually shorter leaves upward, (12-)15-20 mm in diam. incl. leaves at the base, usually tapering to 8-12 mm upward, sporangiate from ca. 10 cm above the base and upward. Stem excl. leaves 5-7 mm thick at the base, upward tapering to ca. 2-3 mm, completely concealed by leaves. Leaves of proximal divisions densely crowded and closely appressed, at the very stem base often aggregated to a thickened somewhat bulb-like shoot base, borne in alternating whorls of ca. 8-10, these ca. 1 mm apart, forming ca. 16-20 indistinct longitudinal ranks, straight, linear-subulate to linear-lanceolate, evenly tapering, 12-16 × 1-1.3 mm, adaxially usually concave (rarely convex) with distinctly prominent vein, abaxially flat to slightly convex, with widely prominent or sunken (dried) vein, subcoriaceous, dull to lustrous, with smooth margins. Leaves of upper, densely sporangiate divisions borne in alternating whorls of 5-8, these ca. 1 mm apart, forming 10-16 longitudinal ranks, linear-lanceolate to lanceolate, 5-9 × 1-1.5 mm, otherwise conform. Sporangia ca. 2 mm wide.
Distribution and habitats: Endemic. Restricted to few high mountains, in open rupestral habitats on sandy soil. Minas Gerais. 1,300-1,900 m.
Leaves of proximal divisions densely crowded, closely appressed, at the stem base often aggregated to a thickened bulblike shoot base.
Specimens studied: BRAZIL. MINAS GERAIS: São Tomé das Letras, Baependi, 1,300 m, Brade 20402 (AAU, F, GH, HB, MO, NY, RB). Pedra Branca, Hoehne (SP 26641). São Roque de Minas, Parque Nacional Serra da Canastra, Salino 3184 (BHCB); P.N. Serra da Canastra, near origin of Rio São Francisco, 1,331 m, Batista et al. 1805a (BHCB, photo AAU). Aiuruoca, P.E. Papagaio, Campo do Retiro dos Pedros, 1,900 m, Viana et al. 3873 (BHCB, photo AAU).
Phlegmariurus wilsonii (Underw. & F.E.Lloyd) B. Øllg., Rodriguésia 63(2): 481. 2012. Fig. 14e,f
Lycopodium wilsonii Underw. & F.E.Lloyd, Bull. Torrey Bot. Club 33: 111. 1906. - Urostachys wilsonii (Underw. & F.E.Lloyd) Herter, Repert. Spec. Nov. Regni Veg. 19: 163. 1923Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.. - Huperzia wilsonii (Underw. & F.E.Lloyd) B. Øllg., Opera Bot. 92: 170. 1987. - Type: Puerto Rico, Luquillo Mountains, Wilson, P. 271 (holotype NY).
Lycopodium andinumHerter, Bot. Jahrb. 43: Beibl. 98: 49. 1909Herter W (1909) Ein neuer beitrag zur Kenntnis der Gattung Lycopodium. Hedwigia 49: 88-92, t. 3., non Rosenstock (1908). - Lycopodium lindavianum Herter, Hedwigia 49: 90. 1909. - Urostachys lindavianus (Herter) Nessel, Bärlappgewächse 146. 1939Nessel H (1939) Die Bärlappgewächse (Lycopodiaceae). G. Fischer, Jena. 404p.. - Huperzia lindaviana (Herter) Holub, Folia Geobot. Phytotax. 20: 74. 1985Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.. - Lectotype: In territorio rei publ. Ecuador, Herb. Drake, Fraser s.n. (P, designated by B. Øllg. in Harling and Anderson, Fl. Ecuador 33: 97. 1988; isotype, G).
Lycopodium trichodendronHerter, Bot. Jahrb. 43: Beibl. 98: 49. 1909Herter W (1909) Ein neuer beitrag zur Kenntnis der Gattung Lycopodium. Hedwigia 49: 88-92, t. 3.. - Urostachys trichodendron (Herter) Herter, Repert. Spec. Nov. Regni Veg. 19: 163 (1909). - Syntypes: Guadeloupe, L’Herminier s.n. (P), Bory 103 (P).
Lycopodium stamineumMaxon, Smithsonian Misc. Coll. 56 (29): 2, pl. 2. 1912Maxon WR (1912) Three new Clubmosses from Panama. Smithsonian Miscellaneous Collection 56: 1-4, t. 1-3.. - Urostachys stamineus (Maxon) Nessel, Bärlappgewächse 148. 1939Nessel H (1939) Die Bärlappgewächse (Lycopodiaceae). G. Fischer, Jena. 404p.. - Types: Panama, Chiriquí, above El Boquete, ca. 1,750 m, Maxon 5636 (holotype US).
Lycopodium arcanumMaxon, in Yuncker, Field. Mus. Publ. Bot. 17: 310, pl. 3. 1938Maxon WR (1938) Pteridophyta [excl. Selaginella] pp. 299-311, in Yuncker. Publications of the Field Museum of Natural History Botanical Series 17: 287-407.. - Type: Honduras, Comayagua, above El Achote, above plains of Siguatepeque, 1,800 m, Yuncker, Dawson & Youse 6149 (holotype US).
Urostachys mandiocanus (Raddi) Herter var. filifolius Herter, Revista Sudamer. Bot. 10: 124. 1953. - Type: Ecuador, Eastern slope of eastern cordillera, bank of Palora river, 1,500 m, Rimbach 125 (holotype US).
Published illustrations: Lellinger, 1989Lellinger DB (1989) The ferns and fern-allies of Costa Rica, Panama, and the Chocó. (Part 1: Psilotaceae through Dicksoniaceae). Pteridologia 2A: 1-364.: fig. 44; Mickel & Beitel, 1988: fig. 4B; Øllgaard, 1988Øllgaard B (1988) Lycopodiaceae. In: Harling G & Andersson L (eds.) Flora of Ecuador 33: 1-155.: p. 17 fig. 1C.
Plants epiphytic, or occasionally terrestrial, erect, arcuate-spreading to pendulous, to 20(-30) cm long. Shoots homophyllous, 1.5-2.5(-3) cm in diam. incl. leaves, equally thick throughout, or in some slender individuals gradually tapering to 1-1.5 cm in distal divisions. Stems excl. leaves (1-)1.5-2(-3) mm thick at the base, often tapering to (0.7-)1 mm, prominently ridged by decurrent leaf bases, pale green to stramineous, often with bright red spots on leaf bases, sporangiate, often in seasonally produced zones, from 5-15 cm above the base and upward, 3-6(-9) times dichotomous. Leaves usually uniform throughout, borne in alternating, often irregularly oblique whorls of 6-7, these 0.5-1.5 mm apart, in proximal divisions, upward often in whorls of 4-5, forming 8-14 indistinct longitudinal ranks, perpendicularly spreading to ascending, straight to upward curved, usually not twisted at the base, linear to filiform, (6-)10-17 × 0.3-0.5 mm, quickly narrowed to ca. 0.2 mm wide due to involution, gradually tapering toward the tip, adaxially canaliculate to involute, often with a prominent vein abaxially near the base. Leaves of distal divisions in old plants sometimes gradually reduced to 6(-4) mm long. Decurrent leaf bases usually not wider than the lamina base, often bright red. Sporangia 1-1.5 mm in diam.
Distribution and habitats: Mexico (Oaxaca) to Panama, West Indies, northern South America south to Bolivia, Brazil: Mato Grosso. Wet mid-altitude forests.
Phlegmariurus wilsonii is related to P. dichotomus, P. pithyoides and P. mandiocanus. With these it shares the bottle-brush-like growth habit, and with the latter two the bright red coloration of the leaf bases. Pendulous individuals of P. wilsonii are rather similar to P. polycarpos (Kunze) B. Øllg. (Costa Rica, Panamá, Colombia to Bolivia), which is very slender and has shorter, more flattened, leaves, and uniformly falcate leaves due to a basal twist, and is apparently always pendulous.
The absence or presence of red colour on the leaf bases seems not to be correlated with other characters, and the intensity and size of the coloration is variable. The direction of leaves is correlated with growth habit. In erect-growing plants the leaves are wide-spreading to almost reflexed, whilst in pendulous plants the leaves are somewhat ascending.
Usually an erect epiphyte, but sometimes becoming pendulous when very large. Only one Brazilian collection studied.
Note: According to the protolog the type of Lycopodium wilsonii is Wilson 271, but Wilson 153 (NY) is annotated by the author as sp. nov. and fits the description best, while Wilson 271 (NY) is Phlegmariurus dichotomous and is not annotated as sp. nov.
Specimen studied: BRAZIL. MATO GROSSO: Fazenda Cachimbo, sub base Projeto Radam, mata baixa, serrado, rupestral, 27.XI.1976, Cordeiro 1215 (NY).
Acknowledgements
This project would not have been possible without the generous collaboration of several herbaria, who provided large loans of Brazilian material: AAU, B, BHCB, BM, BONN, BR, C, CESJ, E, F, FLOR, GH, GUA, HB, HBG, HBR, HRCB, ICN, K, L, M, MBM, MG, MO, NY, P, PACA, PAMG, R, RB, S, SI, SJRP, SP, SPF, U, UB, UC, UNB, US, W.
Numerous excellent photographs of specimens were provided by Vinicius Antonio de Oliveira Dittrich (CESJ), Alexandre Salino (BHCB), and Fernando Matos (NY). They added important data on distribution and morphological variation. Illustrations were made by K. Tind (Figs. 1, 2, 4, 5, 7, 8, 9, 10 , 11, 12, 13, 15, 16, 17a-f, 18, 19, 20, 21, 22) and B. Johnsen (Figs. 6, 14, 17g-i).
The first author is grateful to the staff of the herbarium of the Botanical Garden in Rio de Janeiro, especially Lana Sylvestre and Claudine Mynssen for their help during his stay there, and to the staff of the Botanical Museum of Rio de Janeiro for help during my visit. We are grateful to Thais Almeida for Ashley Field for very useful suggestions for the manuscript, and for sharing their important field observations of these plants with us.
The working facilities and generous assistance, particularly by Finn Borchsenius and Birgitte Bergmann, for this project in Herbarium AAU of the institute of Bioscience, and the Science Museums of Aarhus University are gratefully acknowledged.
References
- Badré F (1983) Antoine Frédéric Spring (1814-1872) et son herbier Lejeunia Nouvelle 109: 1-14.
- Baker JG (1887) Handbook of the fern-allies. George Bell & Sons, London. 159p.
- Baker JG (1892) New or noteworthy plants: Lycopodium mooreanum Hort. Sander. Garden’s Chronicle 12: 582.
- Barbosa-Silva RG, Labiak PH, Bragança Gil AS, Goldenberg R, Michelangeli FA, Martinelli G, Nadruz Coelho MA, Zappi DC & Forzza RC (2016) Over the hills and far away: new plant records for the Guayana Shield in Brazil. Brittonia 68: 397.
- Beitel JM (1979) Clubmosses (Lycopodium) in North America. Fiddlehead Forum 6: 1-8.
- Beitel JM (1984) The Appalachian Firmoss, a new species in the Huperzia selago (Lycopodiaceae) complex in eastern North America. American Journal of Botany 71: 140.
- Bernhardi DII (1801) Tentamen alterum filices in genera redigendi. Journal für die Botanik (Schrader) 1800: 121-136, t. 1-2.
- Bory de Saint-Vincent JBGM (1829) In: Duperrey LJ (ed.) Voyage autour du monde sur la corvette de sa Majesté, La Coquille. 1. Botanique. Cryptogamie i-iii. Atlas, Paris. Pp. 1-301, t. 1-40.
- Chamisso A (1833) Adalbertus de Chamisso Diderico de Schlechtendal Suo S. - Lycopodium rubrum Linnaea 8: 388-389.
- Christ H (1900) Spicilegium Pteridologicum Austro-Brasilense. In: Schwacke W (ed.) Citade de Minas (Ouro Preto), Minas Geraes. Plantas Novas Mineiras 2: 12-42, t. 1-4.
- Christ H (1901) Filices, Equisetaceae, Lycopodiaceae, Selaginellaceae, Rhizocarpaceae. Pittier Primitiae Florae Costaricensis 3: 1-67.
- Christ H (1902) Spicilegium Pteridologicum Austro-Brasiliense. Bulletin de l’Herbier Boissier, ser. 2, 2: 699-708.
- Christ H (1905) Primitiae Florae Costaricensis. Filices et Lycopodiaceae. III. Bulletin de l’Herbier Boissier, sér. 2, 5: 248-260.
- Christ H (1909) Primitiæ Floræ Costaricensis. Filices. VI. Bulletin Societé Botanique, Genève, sér. 2, 1: 216-236.
- Copeland EB (1941) Tropical American Ferns. University of California Publications in Botany 19: 287-340, t. 36-66.
- Damazio L (1915) Un nouveau Lycopodium brésilien. Bulletin Societé Botanique, Genève, ser. 2, 7: 119, fig. 1-8.
- Fée ALA (1866) Histoire des Fougères et des Lycopodiacées des Antilles. Onzième et dernière mémoire sur la famille des fougères, Strasbourg. 164p.
- Fée ALA (1869) Cryptogames vasculaires (fougères, lycopodiacèes, hidropteridèes, equisetacèes) du Brésil. I. Partie. J.B. Baillière et. Fils: V. Masson et Fils, Paris. 346p.
- Fée ALA (1872-1873) Cryptogames vasculaires (fougères, lycopodiacèes, hidropteridèes, equisetacèes) du Brésil. II partie: Supplément et révision. J.B. Baillière, Paris. 115p.
- Field AR, Testo W, Bostock PD & Waycott M (2016) Molecular phylogenetics and the morphology of the Lycopodiaceae subfamily Huperzioideae supports three genera: Huperzia, Phlegmariurus and Phylloglossum. Molecular Phylogenetics and Evolution 94: 635-657.
- Herter W (1909) Beiträge zur Kenntnis der Gattung Lycopodium Studien über die Untergattung Urostachys Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 43 Beiblätter 98: 1-56. f. 1-4.
- Herter W (1909) Ein neuer beitrag zur Kenntnis der Gattung Lycopodium. Hedwigia 49: 88-92, t. 3.
- Herter W (1912) Lycopodiaceae. In: Urban I (ed.) Symbolae antillanae 7: 165-166.
- Herter W (1914) Lycopodium Sydowiorum spec. nov. Repertorium Specierum Novarum Regni Vegetabili 13: 296.
- Herter W (1922) Itinera Herteriana III. Heteropteridophyta austroamericana. (Equisetales Lycopodiales Selaginellales Isoëtales austroamericanae.) Beihefte Botanisches Centralblatt 39: 248-256.
- Herter W (1923) Die Urostachys-Arten der Antillen. Repertorium Specierum Novarum Regni Vegetabili 19: 161-170.
- Herter W (1930) In: F Morton (ed.) Planta nova Florae Guatemalensis. Feddes Repertorium 28: 108-109.
- Herter W (1949) Heteropteridophyta aliquot nova vel critica. Revista Sudamericana de Botanica 8: 19-25.
- Herter W (1949) Index Lycopodiorum, Montevideo. Pp. 1-120.
- Herter W [1953 (Jan. 1954)] Neue und kritische hochandine Urostachys-Arten (Lycopodiaceae). Revista Sudamericana de Botanica 10: 110-129.
- Holub J (1964) Lycopodiella, novy rod rádu Lycopodiales (Lycopodiella, eine neue Gattung der Ordnung Lycopodiales). Preslia 36: 16-22.
- Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on Generic Classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20: 67-80.
- Hooker WJ (1840) Icones Plantarum 3: t. 201-300; (t. 201-250 publ. Jan-Feb. 1839; t. 251-300 publ. 6 Jan.-6 Feb. 1840).
- Jacquin NJ (1762) Enumeratio stirpium plerarumque, quae sponte crescunt in agro vindobonensi. Vindobonae. i-iv, Pp. 1-322, t. 1-9.
- Kunth CS (1816) In: Humboldt, Bonpland & Kunth. Lutetiae Parisiorum. Nova genera et Species plantarum 1: i-lviii, Pp. 1-377, t. 1-96.
- Lamarck JBPA (1789-1792) Monet Chevalier de 1789-1792. Encyclopédie méthodique. Publ. 19 oct. 1789; 361-759 (publ. 13 Feb. 1792). Paris, Liège. Botanique 3: 1-360.
- Lellinger DB (1989) The ferns and fern-allies of Costa Rica, Panama, and the Chocó. (Part 1: Psilotaceae through Dicksoniaceae). Pteridologia 2A: 1-364.
- Linnaeus C (1753) Species Plantarum L. Salvius, Stockholm. 1200p.
- Löve A & Löve D (1977) New combinations in Ferns. Taxon 26: 324-326.
- Maxon WR (1909) Studies of tropical American Ferns nº. 2. Contributions from the United States National Herbarium 13: 1-43, t. 1-9.
- Maxon WR (1912) Three new Clubmosses from Panama. Smithsonian Miscellaneous Collection 56: 1-4, t. 1-3.
- Maxon WR (1913) Studies of tropical American Ferns, nº 4. Contributions from the United States National Herbarium 17: 133-179, t. 1-10.
- Maxon WR (1914) Studies of tropical American Ferns, nº. 5. Contributions from the United States National Herbarium 17: 391-425.
- Maxon WR (1933) A new Lycopodium from Western Guatemala. Proc. Biol. Soc. Washington 46: 159-160.
- Maxon WR (1938) Pteridophyta [excl. Selaginella] pp. 299-311, in Yuncker. Publications of the Field Museum of Natural History Botanical Series 17: 287-407.
- Mickel JT & Beitel JM (1988) Pteridophyte Flora of Oaxaca, Mexico. Memoirs of the New York Botanical Garden 46: 1-568.
- Mirbel CFB (1802) In: Lamarck JBPA, Monet Chevalier & Mirbel CFB (eds.). 1802. Histoire naturelle des vegétaux, classés par familles 3: i-iii, 1-588.
- Morton CV (1964) New combinations in Lycopodium American Fern Journal 54: 71-73.
- Nessel H (1927) As Lycopodiáceas do Brasil. Archivos de Botânica do estado de São Paulo l: 355-535.
- Nessel H (1934) Neue Lycopodien, die von allen schon bekannten Arten durch ihren Habitus ganz besonders abweichend und auffallend sind. Repertorium Specierum Novarum Regni Vegetabili 36: 177-193, t. 170-177.
- Nessel H (1935) Beiträge zur Kenntnis der Gattung Lycopodium Repertorium Specierum Novarum Regni Vegetabili 39: 61-71, t. 189-194.
- Nessel H (1939) Die Bärlappgewächse (Lycopodiaceae). G. Fischer, Jena. 404p.
- Nessel H (1940) Beiträge zur Kenntniss der Lycopodiaceen. Revista Sudamericana de Botanica 6: 156-175, t. 7-19.
- Nessel H (1955) Lycopodiaceae. In: Hoehne FC (ed.) Flora Brasílica II: 1-131.
- Øllgaard B (1987) A revised classification of the Lycopodiaceae sensu lato Opera Botanica 92: 153-178.
- Øllgaard B (1988) Lycopodiaceae. In: Harling G & Andersson L (eds.) Flora of Ecuador 33: 1-155.
- Øllgaard B (1989) New taxa and combinations of Venezuelan Lycopodiaceae. American Fern Journal 79: 151-154.
- Øllgaard B (1989) Index of the Lycopodiaceae. Biologiske Skrifter Kongelige Danske Videnskabernes Selskab 34: 1-135.
- Øllgaard B (1992) Neotropical Lycopodiaceae - an overview. Annals of the Missouri Botanical Garden 79: 687-717.
- Øllgaard B (1994) Lycopodiaceae. In: Tryon RM & Stolze RG (eds.). Pteridophyta of Peru, part VI. Fieldiana Botany, New Series 34: 16-66.
- Øllgaard B (1995) Lycopodiaceae. In: Davidse G, Sousa MS & Knapp S (eds.) Flora Mesoamericana 1: 5-22.
- Øllgaard B (2012a) Nomenclatural changes in Brazilian Lycopodiaceae. Rodriguésia 63: 479-482.
- Øllgaard B (2012b) New combinations in Neotropical Lycopodiaceae. Phytotaxa 57: 10-22.
- Øllgaard B (2014) Six new species and some nomenclatural changes in neotropical Lycopodiaceae. Nordic Journal of Botany 33: 186-196.
- Øllgaard B & Windisch PG (1987) Sinopse das Licopodiáceas do Brasil. Bradea 5: 1-43.
- Øllgaard B & Windisch PG (2014) Lycopodiaceae in Brazil. Conspectus of the family. I. The genera Lycopodium, Austrolycopodium, Diphasium and Diphasiastrum Rodriguésia 65: 261-277.
- Øllgaard B & Windisch PG (2016) Lycopodiaceae in Brazil. Conspectus of the family. II. The genera Lycopodiella s.str., Palhinhaea, and Pseudolycopodiella Rodriguésia 67: 691-719.
- Palisot de Beauvois AMFJ (1805) Prodrome des cinquième et sixième familles de L’Æthéogamie. Les Mousses. Les Lycopodes, Paris. Pp. 1-114.
- Pichi Sermolli REG & Pizzarri MP (2005) A Revision of Raddi’s pteridological collection from Brazil (1817-1818). Webbia 60: I-VII, 1-393.
- Plumier C (1705) Traité des fougères de l’Amérique, Paris. 146p., t. 1-170.
- Presl CB (1825) Lycopodiaceae. Pragae. Reliquiae Haenkeana 1: 77-83.
- Pritzel E (1900) Lycopodiaceae. In: Engler A & Prantl K (eds.) Die Natürlichen Pflanzenfamilien, Teil 1, Abt. 4. W. Engelmann, Leipzig. Pp. 563-606.
- Proctor GR (1977) Pteridophyta. In: Howard RA (ed.). Flora of the Lesser Antilles, Leeward and Windward Islands 2: 1-414.
- Raddi J (1819) Synopsis Filicum Brasiliensium. Opusculi Scientifici Bologna 3: 279-297.
- Rolleri C (1984) Notas nomenclaturales y taxonomicas en la sección Crassistachys Herter del género Lycopodium (Lycopodiaceae). Revista del Museo de La Plata, N.S. Bot. 13: 189-196.
- Rolleri C (1989) Huperzia recurvifolia Rolleri (Huperziaceae Roth., Lycopodiales - Pteridophyta), una nueva especie de Brasil. Notas del Museo de La Plata 21 (Botánica 103): 209-215.
- Rolleri C & Deferrari AM (1988) Nota sobre la transferencia de especies del genero Lycopodium L. (Lycopodiaceae Mirb.) al genero Huperzia Bernh. (Huperziaceae Roth. - Lycopodiales - Pteridophyta). Notas del Museo de La Plata, Botánica 21: 153-157.
- Rosenstock E (1907) Beiträge zur Pteridophytenflora Südbrasiliens II. Hedwigia 46: 57-167.
- Rosenstock E (1912) Filices novae a cl. Dr. O. Buchtien in Bolivia collectae. Repertorium Specierum Novarum Regni Vegetabili 11: 53-60.
- Rosenstock E (1924) Neue Arten und Abarten Brasilianischer Pteridophyten. Repertorium Specierum Novarum Regni Vegetabili 20: 89-95.
- Rothmaler W (1944) Pteridophyten-Studien, I. Feddes Repertorium specierum novarum regni vegetabilis 54: 55-82.
- Schlechtendal DFL & Chamisso A (1830) Plantarum mexicanarum a cel. viris Schiede et Deppe collectarum recensio brevis. Linnaea 5: 554-625.
- Silveira A (1898) Novae species Lycopodiacearum civitatis Minas Geraes (Brazil). Boletim da Commissão Geográfica e Geológica do Estado Minas Geraes 5: 117-145, t. 1-12.
- Silveira A (1908) Flora e Serras Mineiras. Imprensa Oficial, Belo Horizonte. 282p.
- Smith AR (1981) Pteridophytes. In: Breedlove DE (ed.) Flora of Chiapas, Part 2. California Academy of Sciences, San Francisco. Pp. 1-370.
- Sodiro A (1883) Recensio cryptogamarum vascularium provinciæ Quitensis. Republicæ Æquatorianæ. I-VII. Typis curiae ecclesiasticae, Quito. Pp. 1-114.
- Sodiro A (1893) Cryptogamae vasculares quitenses adiectis speciebus in aliis provinciis ditionis ecuadorensis hactenus detectis. i-v. Typis Universitatis, Quito. Pp. 1-656, t. 1-7.
- Sprengel C [1827 (1828)] Caroli Linnaei Systema Vegetabilium. Ed. 16. Vol. IV. Sumtibus Librariae Dieterichianae, Gottingae. 410p.
- Spring A (1838) Beiträge zur Kenntniss der Lycopodien. Flora 21: 145-158; 161-175, 177-191; 193-205; 209-224.
- Spring A (1840) Lycopodium. In: Martius (ed.) Flora brasiliensis l: 109-117, t. 5.
- Spring A (1841) Enumeratio Lycopodinearum, quas in ejusdem plantarum ordinis monographia mox edenda descripsit A. Spring. Bulletins de l’Académie Royale des Sciences et Belle-Lettres de Bruxelles 8: 511-523.
- Spring A (1842) Monographie de la famille des Lycopodiacées, premiére partie. Mémoires de l’Académie Royale des Sciences, Lettres et Beaux Arts de Belgique 15: 1-110.
- Spring A (1849) Monographie de la famille des Lycopodiacées, seconde partie. Mémoires de l’Académie Royale des Sciences, Lettres et Beaux Arts de Belgique 24: 1-358.
- Stevenson DW (1976) Observations on phyllotaxis, stelar morphology, the shoot apex and gemmae of Lycopodium lucidulum Michaux (Lycopodiaceae). Botanical Journal of the Linnean Society 72: 81-100.
- Swartz O (1788) Nova genera & species plantarum seu Prodromus, i-x. Upsaliae & Aboae, Holmiae. Pp. 1-152 (153-158).
- Swartz O (1806) Jo. Jacobi Palmii, Erlangae. Flora Indiae occidentalis 3: 1231-2018.
- Trevisan de Saint-Léon V (1874) Sylloge sporophytarum Italiae. Atti della Società Italiana di Scienze Naturali 17: 242-249.
- Underwood LM & Lloyd FE (1906) The species of Lycopodium of the American tropics. Bulletin of the Torrey Botanical Club 33: 101-124.
- Wagner WH (1993) A new combination for a North American Lycopod. Novon 3: 305.
- Wagner WHJr & Beitel JM (1992) Generic classification of modern North American Lycopodiaceae. Annals Missouri Botanical Garden 79: 676-686.
- Wagner WH, Wagner FS & Beitel JM (1985) Evidence for interspecific hybridisation in pteridophytes with subterranean mycoparasitic gametophytes. Proceedings of the Royal Society of Edinburgh 86B: 273-281.
- Wawra H (1863) In: Wawra H & Maly F (eds.) Neue Pflanzenarten gesammelt auf der transatlantischen Expedition Sr. k. Hoheit des durchlauchtigsten Herrn Erzherzogs Ferdinand Maximilian. Oesterreichische Botanische Zeitschrift 13: 218-227.
- Wikström N, Kenrick P & Chase M (1999) Epiphytism and terrestrialization in tropical Huperzia (Lycopodiaceae). Plant Systematics and Evolution 218: 221-243.
- Wikström N & Kenrick P (2000) Phylogeny of epiphytic Huperzia (Lycopodiaceae): paleotropical and neotropical clades corroborated by rbcl sequences. Nordic Journal of Botany 20: 165-171.
- Wilce JH (1972) Lycopod Spores, I. General Spore Patterns and the Generic Segregates of Lycopodium American Fern Journal 62: 65-79.
- Willdenow KL (1810) Caroli a Linné Species Plantarum. Vol. 5. Ed. Quarta, Berolini. Pp. 1-542.
Edited by
Publication Dates
-
Publication in this collection
25 Apr 2019 -
Date of issue
2019
History
-
Received
16 May 2017 -
Accepted
01 Sept 2017