Abstract
In vitro culture techniques are recognized as efficient strategies for large-scale plant production, as well as providing alternatives for plant conservation. In this study the micropropagation of Tarenaya rosea was established using petiole and foliar blade segments cultivated on MS medium with 6-benzyladenine (BA) and/or 6-furfurylaminopurine (KIN). The regeneration rate from explants was evaluated after 30-days in culture, as well as the proliferation rate from explant-derived shoots, reached after four subcultures performed at 30-days in culture. In vitro propagation occurred by both direct (DO) and indirect (IO) organogenesis. The highest regeneration rates by DO (50% to 100%) were reached on media containing only BA, while morphogenic calluses (IO) were mainly formed with BA+KIN. Explants on media with BA showed the presence of small black nodules on their surface, and histological analysis revealed the presence of trichomes with anthocyanin content. Elongation and rooting were reached on growth regulator-free MS. Acclimatization rates around 80% were achieved and the in vitro-regenerated plants were successfully maintained under field conditions. Results show significant morphogenetic potential of T. rosea from leaf explants, mainly when cultivated in the presence of 4.4 µM BA, providing a new alternative source of plant material for biotechnological and in vitro conservation studies.
Key words:
anthocyanin; Cleome rosea; cytokinins; organogenesis
Resumo
As técnicas de cultura in vitro são reconhecidas como eficientes estratégias para a produção de plantas em grande escala, oferecendo também alternativas para a conservação vegetal. Neste trabalho foi estabelecida a micropropagação de Tarenaya rosea utilizando explantes de pecíolo e lâmina foliar cultivados em meio MS contendo 6-benziladenina (BA) e/ou 6-furfurilaminopurina (KIN). Foram avaliadas a taxa de regeneração de brotos obtidos diretamente a partir dos explantes foliares, após 30 dias de cultivo, e a taxa de proliferação após quatro subculturas realizadas a intervalos de 30 dias. A propagação ocorreu tanto por organogênese direta (OD) como indireta (OI). As maiores taxas de regeneração por OD (50% a 100%) foram alcançadas na presença de BA, enquanto que calos morfogênicos (OI) foram formados principalmente em BA+KIN. Explantes cultivados em meio com BA apresentaram a formação de pequenos nódulos enegrecidos na superfície e as análises histológicas revelaram a presença de tricomas glandulares contendo antocianina. O alongamento e o enraizamento dos brotos foram obtidos em meio MS sem suplementação com reguladores de crescimento. As plantas transferidas para condições ex vitro foram aclimatizadas com sucesso, alcançando taxas de 80% de sobrevivência. Os resultados demonstraram o expressivo potencial morfogenético dos explantes foliares de T. rosea, principalmente quando cultivados na presença de 4,4 µM de BA, constituindo nova fonte de material para estudos biotecnológicos e de conservação in vitro.
Palavras-chave:
antocianinas; Cleome rosea; citocininas; organogênese
Introduction
Biotechnological tools have been increasingly used to explore the medicinal potential of natural products. The exploitation of populations of medicinal plants grown in the wild has increased, demonstrating the need to apply in vitro methods to increase the supply of germplasm of interest. Among these methods, the most successful is micropropagation, which involves the mass multiplication of plants in a relatively short time, under axenic conditions, through tissue samples (Kumar & Reedy 2011Kumar N & Reddy MP (2011) In vitro plant propagation: a review. Journal of Forest Science 27: 61-72.). In addition, plant cell culture strategies allow the production of active compounds for the herbal and pharmaceutical industries independent of seasonal factors and under controlled and constant in vitro conditions (Gandhi et al. 2015Gandhi SG, Mahajan V & Bedi YS (2015) Changing trends in biotechnology of secondary metabolism in medicinal and aromatic plants. Planta 241: 303-317.).
In particular, species from the Cleomaceae family have been evaluated for the presence of bioactive compounds owing to their use in traditional medicine (Sivanesan & Begum 2007Sivanesan D & Begum VH (2007) Preventive role of Gynandropsis gynandra L., against aflatoxin B1 induced lipid peroxidation and antioxidant mechanism in rat. Indian Journal of Experimental Biology 45: 299-303.; Abdullah et al. 2016Abdullah W, Elsayed WM, Abdelshafeek KA, Nazif NM & Singab ANB (2016) Chemical constituents and biological activities of Cleome genus: a brief review. International Journal of Pharmacognosy and Phytochemical Research 8: 777-787.; Alamilla-Fonseca et al. 2018Alamilla-Fonseca LN, Delgado-Domínguez J, Zamora-Chimal J, Cervantes-Sarabia RB, Jiménez-Arellanes A, Rivero-Cruz JF & Becker I (2018) Leishmania mexicana cell death achieved by Cleoserrata serrata (Jacq.) Iltis: learning from Maya healers. Journal of Ethnopharmacology 211: 180-187.). The Cleomaceae family comprises 18 genera and about 350 species, mostly annual, but sometimes perennial herbaceous plants and shrubs widely distributed in tropical and subtropical regions (Patchell et al. 2014Patchell MJ, Roalson EH & Hall JC (2014) Resolved phylogeny of Cleomaceae based on all three genomes. Taxon 63: 315-328.).
Tarenaya rosea (Vahl ex DC.) Soares Neto & Roalson, formely named Cleome rosea (Soares Neto et al. 2018Soares Neto RL, Thomas WW, Barbosa MRV & Roalson EH (2018) New combinations and taxonomic notes for Tarenaya (Cleomaceae). Acta Botanica Brasilica 32: 540-545.), is a native Brazilian herbaceous species occurring in coastal sandy plains (“restingas”). These ecosystems are now undergoing intense anthropogenic impact (Rocha et al. 2007Rocha CFD, Bergallo HG, Van Sluys M, Alves MAS & Jamel CE (2007) The remnants of restinga habitats in the Brazilian Atlantic Forest of Rio de Janeiro state, Brazil: habitat loss and risk of disappearance. Brazilian Journal of Biology 67: 263-273.). This species has been evaluated for its medicinal potential (Simões et al. 2006Simões C, Mattos JCP, Sabino KCC, Caldeira-de-Araújo A, Coelho MGP, Albarello N & Figueiredo SFL (2006) Medicinal potential from in vivo and acclimatized plants of Cleome rosea Vahl ex DC. (Capparaceae). Fitoterapia 77: 94-99., 2010aSimões C, Castro TC, Cordeiro LS, Albarello N, Mansur E & Romanos MTV (2010a) Antiviral activity of Cleome rosea extracts from field-grown plants and tissue culture-derived materials against acyclovir-resistant Herpes simplex viruses type 1 (ACVr-HSV-1) and type 2 (ACVr-HSV-2). World Journal of Microbiology and Biotechnology 26: 93-99.; Simões-Gurgel et al. 2012aSimões-Gurgel C, Rocha AS, Cordeiro LS, Gayer CRM, Castro TC, Coelho MGP, Albarello N, Mansur E & Rosa ACP (2012a) Antibacterial activity of field-grown plants, in vitro propagated plants, callus and cell suspension cultures of Cleome rosea Vahl. Journal of Pharmacy Research 5: 3304-3308.), and protocols aiming at its in vitro production and conservation have already been established, including in vitro germination (Castro et al. 2014Castro TC, Simões-Gurgel C, Ribeiro IG, Garcia MC & Albarello N (2014) Morphological aspects of fruits, seeds, seedlings and in vivo and in vitro germination of species of the genus Cleome. Journal of Seed Science 36: 326-335.), callogenesis (Simões et al. 2009aSimões C, Bizarri CHB, Cordeiro LS, Castro TC, Coutada LCM, Silva AJR, Albarello N & Mansur E (2009a) Anthocyanin production in callus cultures of Cleome rosea: modulation by culture conditions and characterization of pigments by means of HPLC-DAD/ESIMS. Plant Physiology and Biochemistry 47: 895-903.), cell suspension culture (Simões-Gurgel et al. 2011Simões-Gurgel C, Cordeiro LS, Castro TC, Callado CH, Albarello N & Mansur E (2011) Establishment of anthocyanin-producing cell suspension cultures of Cleome rosea Vahl ex DC. (Capparaceae). Plant Cell Tissue and Organ Culture 106: 537-545.) and cryopreservation (Cordeiro et al. 2015aCordeiro LS, Simões-Gurgel C & Albarello N (2015a) Multiplication and cryopreservation of adventitious roots of Cleome rosea Vahl. In vitro Cellular and Developmental Biology-Plant 51: 249-257.,bCordeiro LS, Simões-Gurgel C, Albarello N & Engelmann F (2015b) Cryopreservation of in vitro-grown shoot tips of Cleome rosea Vahl (Cleomaceae) using the V cryo-plate technique. In vitro Cellular and Developmental Biology-Plant 51: 688-695., 2016Cordeiro LS, Simões-Gurgel C & Albarello N (2016) Cryopreservation of adventitious roots of Cleome rosea Vahl (Cleomaceae) using a vitrification technique and assessment of genetic stability. Cryoletters 37: 231-242., 2017Cordeiro LS, Simões-Gurgel C, Albarello N & Engelmann F (2017) Cleomaceae (Cleome rosea Vahl ex DC.), shoot tips, V-cryoplate method. In: Niino T, Matsumoto T, Yamamoto S-I, Maki S, Tanaka D & Engelmann F (eds.) Manual of cryopreservation methods using cryo-plate, 1st ed. Vol 1. Plant Tissue Culture and Cryopreservation Group (PTTCCryoG), Jalisco. Pp 62-63., 2020Cordeiro LS, Collin M, Callado CH, Simões-Gurgel C, Albarello N & Engelmann F (2020) Long-term conservation of Tarenaya rosea (Cleomaceae) root cultures: histological and histochemical analyses during cryopreservation using the encapsulation-vitrification technique. Protoplasma 257: 1-13.). With respect to in vitro propagation studies (Simões et al. 2004Simões C, Santos AS, Albarello N & Figueiredo SFL (2004) Shoot organogenesis and plantlet regeneration from stem explants of Cleome rosea Vahl (Capparaceae). Journal of Plant Biotechnology 6: 199-204., 2009bSimões C, Albarello N, Callado CH, Castro TC & Mansur E (2009b) New approaches for shoot production and establishment of in vitro root cultures of Cleome rosea Vahl. Plant Cell Tissue and Organ Culture 98: 79-86., 2010bSimões C, Albarello N, Callado CH, Castro TC & Mansur E (2010b) Somatic embryogenesis and plant regeneration from Callus cultures of Cleome rosea Vahl. Brazilian Archives of Biology and Technology 53: 679-686.), the morphogenetic potential of leaves, an important source of explants has not been explored so far. Many studies reported the use of leaves as a source of explants owing to the possibility of obtaining a high number of explants per donor plant (Sreedhar et al. 2008Sreedhar RV, Venkatachalam L, Thimmaraju R, Bhagyalakshmi N, Narayan MS & Ravishankar GA (2008) Direct organogenesis from leaf explants of Stevia rebaudiana and cultivation in bioreactor. Biologia Plantarum 52: 355-360.; Naing et al. 2016Naing AH, Park KI, Chung MY, Lim KB & Kim CK (2016) Optimization of factors affecting efficient shoot regeneration in chrysanthemum cv. Shinma. Brazilian Journal of Botany 39: 975-984.). Leaves of T. rosea have 3 to 5 leaflets and long petioles, which provide large and suitable amounts of plant material for in vitro cultures. Therefore, the present work aimed to evaluate the in vitro morphogenetic capacity from petiole and foliar blade explants, as well as from explant-derived shoots over time in culture, in order to establish new and efficient protocol for large-scale plant production.
Material and Methods
Plant material
The fruits of Tarenaya rosea were collected between February and March 2006 from populations located at Maricá, Rio de Janeiro, Brazil (22º58’01”S, 42º58’36”W). The authenticity of the species was previously confirmed, and a voucher was deposited in the Herbarium of the Rio the Janeiro State University, Rio de Janeiro, Brazil (HRJ7185). Seeds were inoculated in a mixture of soil and sand (1:1) and maintained at 28 ± 2º C under a 12-h photoperiod. Two-month-old seedlings were used as the source of explants. They have leaves formed by 3 to 5 leaflets and long petioles (5-7 cm). Plants were washed under running tap water, roots were removed, and the remaining aerial parts were immersed in 0.5% sodium hypochlorite and 0.05% Tween 80 (v/v) for 10 min, with agitation, and rinsed three times (5 min each) in distilled water.
Bud induction and shoot proliferation
Petiole (1 cm length) and foliar blade (1 cm2) segments were inoculated on Murashige & Skoog (1962)Murashige T & Skoog FA (1962) A revised medium for rapid growth and biossays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497. medium (MS) containing 30 g.L-1 sucrose, solidified with 8 g.L-1 agar (Merck) and supplemented with different concentrations of 6-benzyladenine (BA) (1.1; 2.2; 4.4 µM) or 6-furfurylaminopurine (KIN) (1.2; 2.3; 4.6 µM) used alone or in combination. The pH of all media was adjusted to 5.8 prior to autoclaving at 121 ºC for 15 min. Petiole explants were inoculated vertically, and foliar blades were placed with the abaxial surface in contact with the culture media. Four explants were inoculated per glass flask (60 × 80 mm) containing 30 mL of culture medium, and the flasks were closed with polypropylene caps. The flasks were maintained in a growth chamber at 26 ± 2º C under a 16 h photoperiod provided by cool white fluorescent tubes (45 µmol.m-2.s-1). Five flasks were used per treatment, and each experiment was repeated twice. The percentage of explants that induced shoots (regeneration rate) and the mean number of shoots per explant obtained by direct (DO) or indirect (IO) organogenesis were evaluated after 30 days of culture.
Primary explant-derived shoot cultures
Shoots developed in petiole and laminar foliar explants after 30-day in culture were isolated and subcultured onto fresh medium of the same composition used to the primary explants cultures. The shoot cultures were performed at 30-day intervals during four subcultures. The percentage of explant derived-shoots with multiplication capacity (proliferation rate) and the mean number of shoots produced by DO or IO were evaluated after each subculture and represented in the results by the average (mean ± standard deviation) of the four subcultures.
Rooting and acclimatization
In vitro propagated shoots (> 0.5 cm) were transferred to flasks containing 30 mL of solid MS medium devoid of growth regulators (MS0) to induce rooting. The flasks were maintained for 30 days under the same physical conditions as those described above. After this period the percentage of rooting was evaluated. Fifteen flasks with two shoots each were used and the experiment was repeated twice.
Whole plants were transferred to plastic pots (7.5 × 7 cm) containing a mixture of garden soil and sand (2:1). The pots were placed into glass chambers (80 cm × 40 cm × 40 cm) at 28 ± 2º C under a 12-h photoperiod for 30 days. In order to reduce relative humidity inside the chambers, covers were gradually opened after the second week and completely removed 30 days after transplanting. Ten pots with two plants each were used, and the experiment was repeated twice. Ex vitro establishment was assessed through the percentage of acclimatized plants surviving three months after planting.
Histological analysis
Petiole and foliar blade explants were fixed in CRAF III (Sass 1958Sass JE (1958) Elements of botanical microtechnique. McGraw-Hill, New York. 222p.) and dedhydrated through a graduated series of ethyl alcohol (ethanol) and distilled water. After that, the ethanol was replaced by xylol and the material was gradually infiltrated by solutions of xylol and paraffin wax 56 oC. Finally, the material was embedded in paraffin and allowed to solidify in block form at room temperature (Johansen 1940Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York. 52p.). Serial sections (8-10 µm) were obtained with Leica rotary microtome (Model RM2025), deparaffinized in xylol and double-stained in astra blue and basic fuchsin (Roeser 1972Roeser KR (1972) Die Nadel der Schwarzkiefer - Massenproduckt und Kunstwerk der Natur. Mikrokosmos 61: 33-36.). Slides were mounted with synthetic resin and then analyzed under an Olympus BX41-BF-I-20 microscope. Images of histological sections were obtained with a Q Color R3 video camera and Image-Pro Express 6.0 software.
Pigment identification
To determine the chemical nature of pigment produced in the explants surface, the material was extract using an acidified methanol solution (0.1% hydrochloric acid). After that, a few drops of the alkaline solution sodium hydroxide (0.1%) were dripped into the extraction solution and then a few drops of the acidic solution hydrochloric acid (0,1%) were used. The change in color as a function of pH was followed to determine the nature of the pigment produced (Vankar & Bajpai 2015Vankar PS & Bajpai D (2015) Rose amthocyanins as acid base indicators. EJEAFChe 9: 875-884.).
Statistical analysis
The experiments were organized in a completely randomized design and were repeated twice. BA and KIN treatments (isolated or in combination) were evaluated at the same time (10 treatments × 5 flasks × 2 replications) and the percentage values were subjected to arcsine transformation prior to analysis. The data were submitted to analysis of variance (ANOVA), and the means were compared by Tukey test. The statistical analysis was performed at the 5% level of significance using the GraphPad Prism 5 statistical software package.
Results
Petiole and foliar blade cultures
The micropropagation of T. rosea was efficiently induced when leaf explants were cultivated on MS medium supplemented with cytokinins (Fig. 1). Explants from petioles and foliar blades showed the development of adventitious buds after 5 to 9 days in culture. At the end of the first week, petiole explants showed swelling in the region directly in contact with the culture medium, followed by shoot formation by indirect organogenesis (IO). In addition, direct organogenesis (DO) was observed in the apical region of the explants (Fig. 1a).
a-f. Micropropagation of Tarenaya rosea from leaf explants cultivated on MS medium supplemented with 4.4 µM BA - a. shoot regeneration on petiole explants during the second week in culture by direct (black arrow) and indirect (red arrow) organogenesis; b. shoot regeneration on foliar blade explant during the second week in culture by direct (black arrow) and indirect (red arrow) organogenesis; c. direct (black arrow) and indirect (red arrow) shoot multiplication from petiole-derived shoot during the third subculture; d. fasciated shoot developed from foliar blade explant-derived shoot during the fourth subculture; e. in vitro root system developed after 30 days in culture on MS medium devoid of growth regulators; f. three month-old acclimatized plants. Bars: a = 0.58 cm; b = 0.20 cm; c = 0.28 cm; d = 0.44 cm; e = 0.87 cm; f = 4.90 cm.
The morphogenic response of foliar blades was first observed by DO at the cut ends of the explants (Fig. 1b) with no differences in terms of regeneration capacity between apical and basal explant regions. During the second week in culture, calluses were produced near the cut ends, followed by the development of shoots through IO (Fig. 1b). Shoots were induced by DO and IO along the period of culture from both the abaxial and adaxial surfaces.
Supplementation with phytoregulators was essential to induce morphogenic response since explants cultivated on medium devoid of growth regulators were nonresponsive. Media supplemented with BA were the most efficient to induce organogenesis from both types of explants, reaching regeneration rates up to 100% (Tab. 1).
Effect of BA and KIN on the regeneration rate and mean number of shoots produced from petiole and foliar blade explants of Tarenaya rosea after 30 days in culture.
Petiole explants cultivated on media supplemented with only BA reached the highest regeneration rates by DO, while the combination of BA + KIN was more suitable to the induction of regeneration by IO (Tab. 1). In cultures of foliar blade, the combination of BA + KIN induced the highest regeneration rates by both DO and IO.
Cultures established on medium supplemented with 4.4 µM BA displayed the highest number of shoots per explant, both from petiole (4.40 ± 0.52) and foliar blade (8.50 ± 1.65) explants. On the other hand, morphogenic calluses were mainly produced on media supplemented with the combination of 2.2 µM BA + 2.3 µM KIN (5.40 ± 0.75/petiole and 4.27 ± 1.32/foliar blade) (Tab. 1).
The supplementation of culture medium with only KIN could not efficiently induce morphogenesis. The lowest concentration tested (1.2 µM) was nonresponsive for both types of explants, while the highest concentration (4.6 µM) resulted in regeneration rates up to 30% (Tab. 1). Furthermore, the main response of foliar blade explants maintained on media supplemented with KIN was an enlargement in size.
Primary explant-derived shoot cultures
Newly developed shoots obtained from petiole and foliar blade explants showed propagation capacity by both DO and IO (Fig. 1c) during four subcultures. Shoots derived from petiole explants reached higher proliferation rates when compared to the regeneration rates obtained directly from the explants, especially considering propagation by DO. The maximum number of shoots per explant was achieved through DO on medium supplemented with 4.4 µM BA (5.03 ± 0.65) (Tabs. 1; 2).
Effect of BA and KIN on the proliferation rate and mean number of shoots produced from primary explant-derived shoots of Tarenaya rosea after four subcultures.
Shoots derived from foliar blade explants reached higher proliferation rates when compared to the regeneration rates by IO alone. However, the maximum number of shoots after four subcultures was achieved through DO on medium supplemented with 4.4 µM BA + 4.6 µM KIN (5.40 ± 1.74) (Tab. 2).
As observed in cultures initiated with primary explants, explant-derived shoots showed low proliferation capacity on media supplemented with only KIN. Cultures maintained in these conditions achieved proliferation rates up to 30% and low production of shoots per explant (Tab. 2).
The development of shoots with abnormal morphology was observed along the subcultures in the presence of BA alone or in combination with KIN. These shoots were fasciated with a wide stem, and they exhibited undulations resembling the fusion of stem axis of several shoots, albeit slightly compressed to a flattened state (Fig. 1d). The percentage of fasciated shoots was low and they were not considered a factor in determining the mean number of shoots per treatment.
Pigment identification
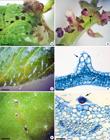
During the third week of culture on media supplemented with BA alone or in combination with KIN, small black nodules were formed on the surface of explants. These nodules were observed mainly at the top of the ribs on the adaxial surface of foliar blades (Fig. 2a), but they also occurred in the petiole explants (Fig. 2b). Initially, the nodules resembled buds, once buds are also known to present a dark color at the beginning of their development. However, unlike buds, these nodules were not attached to the explants and could be easily removed without visual tissue damage. The chemical composition of these nodules was identified as anthocyanin after the extraction of pigments in the presence of acidified methanol and a typical color change induced by pH variation (Delpech 2000Delpech R (2000) The importance of red pigments to plant life: experiments with anthocyanins. Journal of Biological Education 34: 206-210.).
a-f. Leaf surface of Tarenaya rosea - a. detail of anthocyanin secretion (arrow) on the adaxial surface of foliar blade explant; b. detail of anthocyanin secretion (arrow) in petiole explant; c. emergences on the adaxial leaf surface; d. histological section of an emergence showing epidermal (arrow) and subepidermal (asterisk) tissues; e. glandular trichomes at the top of the ribs on the adaxial leaf surface; f. microscopic view of a glandular trichome (arrow). Bars: a = 0.12 cm; b = 0.10 cm; c = 0.05 cm; d = 30 µm; e = 0.05 cm; f = 30 µm.
The histological analysis of petiole and foliar blade explants revealed the absence of any internal structures that could be associated with pigment production or storage. Leaf surface analysis showed the presence of emergences and trichomes (Fig. 2c,e). The emergences were non-glandular and constituted by epidermal and subepidermal tissues (chlorenchyma cells) (Fig. 2d), while the trichomes were glandular, formed just by the epidermal layer and occurring only on the adaxial surface at the top of the ribs (Fig. 2f).
Rooting and acclimatization
The shoots transferred to MS medium devoid of growth regulators (MS0) reached an average length of about 2 cm and presented rooting rates higher than 90%. The root development started after 3 to 5 days in culture and after 30 days, the plants showed long root systems (Fig. 1e). The plants transferred to ex vitro conditions developed new leaves and three months after the beginning of the process, they reached acclimatization rates around 80%. The in vitro-regenerated plants were phenotypically normal and were successfully maintained under ex vitro conditions (Fig. 1f).
Discussion
Supplementation with BA alone, or in combination with KIN, resulted in shoot formation from petiole and foliar blade explants. Cultures established on medium supplemented with 4.4 µM BA achieved the highest number of shoots per explant by DO, while morphogenic calluses were mainly produced on media supplemented with the combination of 2.2 µM BA + 2.3 µM KIN. Previous work of in vitro propagation of T. rosea using stem explants also reached the largest number of shoots regenerated by DO on medium containing 4.4 µM BA, although the combination of 4.4 µM BA + 4.6 µM KIN resulted in the highest production of shoots by IO (Simões et al. 2004Simões C, Santos AS, Albarello N & Figueiredo SFL (2004) Shoot organogenesis and plantlet regeneration from stem explants of Cleome rosea Vahl (Capparaceae). Journal of Plant Biotechnology 6: 199-204.). Organogenesis from leaf explants has also been evaluated in other Cleomaceae species. Indirect shoots were obtained from Cleome viscosa L. explants cultivated on MS medium supplemented with a combination of BA and 1-Naphthaleneacetic acid (NAA) (Naseem & Jha 1994Naseem M & Jha KK (1994) Differentiation and regeneration in Cleome leaves culture in vitro. Egypt Journal of Botany 1: 37-49.). On the other hand, leaf explants of Cleome spinosa Jacq. failed to present any morphogenic response when cultivated on MS medium supplemented with different concentrations of BA and KIN alone or in combination (Albarello et al. 2006Albarello N, Simões C, Rosas PFG, Castro TC, Gianfaldoni MG, Callado CH & Mansur E (2006) In vitro propagation of Cleome spinosa (Capparaceae) using explants from nursery-grown seedlings and axenic plants. In vitro Cellular and Developmental Biology-Plant 42: 601-606.).
Shoots were induced from both the abaxial and adaxial surfaces of foliar blades devoid of petioles. Similar results were achieved in another species (Vij & Pathak 1990Vij SP & Pathak P (1990) Micropropagation of orchids through leaf segments. Journal of the Orchid Society of India 4: 69-88.; Kaur & Vij 2000Kaur S & Vij SP (2000) Regeneration potential of Saccolabium papillosum leaf segments. The Journal of the Orchid Society of India 14: 67-73.), although some studies showed the production of shoots on only one of the surfaces (Nayak et al. 1997Nayak NR, Patnaik S & Rath SP (1997) Direct shoot regeneration from leaf explants of epiphytic orchids Acampe praemorsa (Roxb.). Plant Cell Report 16: 583-586.; Kaur & Bhutani 2009Kaur S & Bhutani KK (2009) In vitro propagation of Vanda testacea (Lindl.) Reichb.F. - a rare Orchid of high medicinal value. Plant Tissue Culture and Biotechnology 19: 1-7.). Shoot induction from foliar blades of T. rosea was more intense near the petiole region, suggesting the occurrence of a physiological gradient of phytohormones and other metabolites leading to differences in shoot regeneration (Karam & Al-Majathoup 2000Karam NS & Al-Majathoup M (2000) Direct shoot regeneration and microtuberization in wild Cyclamen persicum Mill. using seedling tissue. Scientia Horticulturae 86: 235-236.; Sreedhar et al. 2008Sreedhar RV, Venkatachalam L, Thimmaraju R, Bhagyalakshmi N, Narayan MS & Ravishankar GA (2008) Direct organogenesis from leaf explants of Stevia rebaudiana and cultivation in bioreactor. Biologia Plantarum 52: 355-360.). The presence of the petiole was also essential to induce regeneration in other species (Corredoira et al. 2008Corredoira E, Ballester A & Vieitez AM (2008) Thidiazuron-induced high-frequency plant regeneration from leaf explants of Paulownia tomentosa mature trees. Plant Cell Tissue and Organ Culture 95: 197-208.; Jain et al. 2011Jain R, Sinha A, Jain D, Kachhwaha S & Kothari SL (2011) Adventitious shoot regeneration and in vitro biosynthesis of steroidal lactones in Withania coagulans (Stocks) Dunal. Plant Cell Tissue and Organ Culture 105: 135-140.).
Supplementation with KIN alone did not sufficiently induce shoot propagation. Similar results were also reached in cultures of stem explant of T. rosea cultivated on solid MS medium (Simões et al. 2004Simões C, Santos AS, Albarello N & Figueiredo SFL (2004) Shoot organogenesis and plantlet regeneration from stem explants of Cleome rosea Vahl (Capparaceae). Journal of Plant Biotechnology 6: 199-204.). However, when stem explants were cultivated in liquid MS medium supplemented with KIN alone, high regeneration rates were achieved, both by DO and IO (Simões et al. 2009bSimões C, Albarello N, Callado CH, Castro TC & Mansur E (2009b) New approaches for shoot production and establishment of in vitro root cultures of Cleome rosea Vahl. Plant Cell Tissue and Organ Culture 98: 79-86.). These responses reflect a significant difference between culture conditions on solid and in liquid media. The higher propagation capacity verified in liquid media could be explained by the better contact of the tissues with nutrients and growth regulators (Suthar et al. 2011Suthar RK, Habibi N & Purohit SD (2011) Influence of agar concentration and liquid medium on in vitro propagation of Boswellia serrata Roxb. Indian Journal of Biotechnology 10: 224-227.).
Although the supplementation with KIN alone was not efficient to shoot propagation in T. rosea, the foliar blade explants cultivated in the presence of this phytoregulator showed a significant increase in size. The ability of cytokinins to promote cell enlargement is well known, especially in leaf disks (van Staden & Zazimalova 2008van Staden J & Zazimalova E (2008) Plant growth regulators II: Cytokinins, their Analogues and Antagonists. In: George EF, Hall MA & De Klerk G-J (eds.) Plant Propagation by Tissue Culture, Dordrecht. Pp. 205-226.). Studies report the enlargement of foliar blade on media supplemented with cytokinins, as observed for C. spinosa on media with KIN (Albarello et al. 2006Albarello N, Simões C, Rosas PFG, Castro TC, Gianfaldoni MG, Callado CH & Mansur E (2006) In vitro propagation of Cleome spinosa (Capparaceae) using explants from nursery-grown seedlings and axenic plants. In vitro Cellular and Developmental Biology-Plant 42: 601-606.) and Stevia rebaudiana (Bertoni) Bertoni (Sreedhar et al. 2008Sreedhar RV, Venkatachalam L, Thimmaraju R, Bhagyalakshmi N, Narayan MS & Ravishankar GA (2008) Direct organogenesis from leaf explants of Stevia rebaudiana and cultivation in bioreactor. Biologia Plantarum 52: 355-360.) on media containing BA and KIN in various combinations.
The distinct proliferation capacity observed between petiole and foliar blade explants, as well as between explants and primary explant-derived shoots, reflects not only anatomical and physiological features, but also the differential endogenous hormone balance in these materials. Moreover, this response may be related to distinct distribution of protein receptors for the phytoregulators used exogenously and the differential acquisition of morphogenetic competence (Almeida et al. 2015Almeida M, Graner EM, Brondani GE, Oliveira LS, Artioli FA, Almeida LV, Leone GF, Baccarin FJB, Antonelli PO, Cordeiro GM, Oberschelp GPJ & Batagin-Piotto KD (2015) Plant morphogenesis: theorical bases. Advances in Forestry Science 2: 13-22.). Other studies also reported differential morphogenetic responses from leaf explants, as in Kalanchoe blossfeldiana, Poelln. the foliar blade explants of which were responsive to in vitro propagation, but not the petiole (Thomé et al. 2004Thomé GCH, Gressler PD & Santos G (2004) In vitro propagation of Kalanchoe blossfeldiana Poelln. by organogenesis. Revista Brasileira de Agrociência 10: 197-202.).
Explants cultivated on media supplemented with BA alone or in combination with KIN developed small black nodules containing anthocyanin pigments. Secretion of anthocyanins was also observed in the leaflets of in vitro shoots of Hypericum perforatum L., and histological analysis showed that the pigment was stored in glands localized in the mesophyll and formed by a small cluster of cells (Mulinacci et al. 2008Mulinacci N, Giaccherini C, Santamaria AR, Caniato R, Ferrari F, Valletta A, Vincieri FF & Pasqua G (2008) Anthocyanins and xanthones in the calli and regenerated shoots of Hypericum perforatum var. angustifolium (sin. Fröhlich) Borkh. Plant Physiology and Biochemistry 46: 414-420.). In T. rosea, the presence of specialized internal structures to store the pigment was not observed. However, some glandular trichomes that accumulated anthocyanin pigments were observed mainly at the top of the ribs. The presence of glandular trichomes has been frequently reported in the literature, and the importance of these structures has been highlighted in taxonomic, pharmacognostic and/or phytochemical studies (Metcalfe & Chalk 1957Metcalfe CR & Chalk L (1957) Anatomy of the dicotyledons. Vol. 1. Claredon Press, Oxford/London. Pp. 87-95.; Williams et al. 2003Williams LAD, Vasques E, Reid W, Porter R & Kraus W (2003) Biological activities of an extract from Cleome viscosa L. (Capparaceae). Naturwissenschaften 90: 468-72.; Edeoga et al. 2009Edeoga HO, Omosun G, Osuagwu GGE, Mbaebie BO & Madu BA (2009) Micromorphological characters of the vegetative and floral organs of some Cleome species from Nigeria. American-Eurasian Journal of Scientific Research 4: 124-127.; Gupta & Rao 2012Gupta PC & Rao CV (2012) Pharmacognostical studies of Cleome viscosa Linn. Indian Journal of Natural Products and Resources 3: 527-534.; Khuntia et al. 2013Khuntia A, Mohanty SK & Bose A (2013) Pharmacognostical & preliminary phytochemical investigation of Cleome rutidosperma aerial parts. International Journal of Research in Pharmacy and Science 3: 67-77.).
Because of the commercial importance of anthocyanins, enhanced production of these pigments from plant cell and tissue culture strategies has been extensively explored (Simões et al. 2012bSimões C, Albarello N, Castro TC & Mansur E (2012b) Production of anthocyanins by plant cell and tissue culture strategies. In: Orhan IE (ed.) Biotechnological production of plant secondary metabolites. Bentham Science Publishers, Sharjah. Pp. 67-86.). The induction of anthocyanins was previously reported in callus (Simões et al. 2009aSimões C, Bizarri CHB, Cordeiro LS, Castro TC, Coutada LCM, Silva AJR, Albarello N & Mansur E (2009a) Anthocyanin production in callus cultures of Cleome rosea: modulation by culture conditions and characterization of pigments by means of HPLC-DAD/ESIMS. Plant Physiology and Biochemistry 47: 895-903.) and cell suspension cultures (Simões-Gurgel et al. 2011Simões-Gurgel C, Cordeiro LS, Castro TC, Callado CH, Albarello N & Mansur E (2011) Establishment of anthocyanin-producing cell suspension cultures of Cleome rosea Vahl ex DC. (Capparaceae). Plant Cell Tissue and Organ Culture 106: 537-545.) of T. rosea. In addition, some shoots propagated from stem explants took on a transitory violaceus coloration after the subcultures (Simões et al. 2004Simões C, Santos AS, Albarello N & Figueiredo SFL (2004) Shoot organogenesis and plantlet regeneration from stem explants of Cleome rosea Vahl (Capparaceae). Journal of Plant Biotechnology 6: 199-204.). However, the presence of anthocyanins in trichomes is reported here for the first time for the species. These glandular trichomes could be the origin of the small black nodules observed in the leaf surface. The mechanical and hydric stresses suffered by plant material during in vitro manipulation could have contributed to the induction of these pigments.
The development of fasciated shoots was also observed along the subcultures of explant-derived shoots. A similar response was verified from observation of in vitro shoots originated from stem explants of T. rosea (Simões et al. 2004Simões C, Santos AS, Albarello N & Figueiredo SFL (2004) Shoot organogenesis and plantlet regeneration from stem explants of Cleome rosea Vahl (Capparaceae). Journal of Plant Biotechnology 6: 199-204.). Numerous studies have reported on the appearance of fasciated plants in natural environmental conditions (Stange et al. 1996Stange RR, Jeffares D, Young C, Scott DB, Eason JR & Jameson PE (1996) PCR amplification of the fas-1 gene for the detection of virulent strains of Rhodococus fascians. Plant Pathology 45: 407-417.; Reboredo & Silvares 2007Reboredo F & Silvares C (2007) Fasciation phenomena and mineral balance in Spartium junceum L. Phyton, International Journal of Experimental Botany 47: 123-132.). However, little experimental evidence can confirm the effect of a particular environmental factor or treatment that causes fasciation (Iliev & Kitin 2011Iliev I & Kitin P (2011) Origin, morphology, and anatomy of fasciation in plants cultured in vivo and in vitro. Plant Growth Regulators 63: 115-129.). Several studies also reported the development of in vitro fasciated shoots induced by exogenously applied cytokinins (Iliev & Kitin 2011Iliev I & Kitin P (2011) Origin, morphology, and anatomy of fasciation in plants cultured in vivo and in vitro. Plant Growth Regulators 63: 115-129.; Kitin et al. 2005Kitin P, Iliev I, Scaltsoyiannes A, Nellas C, Rubos A & Funada R (2005) A comparative histological study between normal and fasciated shoots of Prunus avium generated in vitro. Plant Cell Tissue and Organ Culture 82: 141-150.; Mitras et al. 2009Mitras D, Kitin P, Iliev I, Dancheva D, Scaltsoyiannes A, Tsaktsira M, Nellas C & Rohr R (2009) In vitro propagation of Fraxinus excelsior L. by epicotyls. Journal of Biological Research 11: 37-48.). In the present work, the number of fasciated shoots was directly related to the increase in phytoregulator concentrations. Similar to T. rosea, media supplementation with BA induced fasciation in cultures of Prunus avium (L.) L. (Kitin et al. 2005Kitin P, Iliev I, Scaltsoyiannes A, Nellas C, Rubos A & Funada R (2005) A comparative histological study between normal and fasciated shoots of Prunus avium generated in vitro. Plant Cell Tissue and Organ Culture 82: 141-150.) and Fraxinus excelsior L. (Mitras et al. 2009Mitras D, Kitin P, Iliev I, Dancheva D, Scaltsoyiannes A, Tsaktsira M, Nellas C & Rohr R (2009) In vitro propagation of Fraxinus excelsior L. by epicotyls. Journal of Biological Research 11: 37-48.). The presence of the cytokinin-like compound thidiazuron also was reported to cause fasciation in some woody species (Bosela & Michler 2008Bosela MJ & Michler CH (2008) Media effects on black walnut (Juglans nigra L.) shoot culture growth in vitro: evaluation of multiple nutrient formulations and cytokinin types. In vitro Cellular and Developmental Biology-Plant 44: 316-329.; Durkovic 2008Durkovic J (2008) Micropropagation of mature Cornus mas ‘Macrocarpa’. Trees 22: 597-602.). Under in vitro conditions, Iliev & Kitin (2011)Iliev I & Kitin P (2011) Origin, morphology, and anatomy of fasciation in plants cultured in vivo and in vitro. Plant Growth Regulators 63: 115-129. reported that shoot fasciation is a direct result of abnormally enlarged shoot apical meristems and changes in the developmental control of meristematic cells.
Shoots of T. rosea achieved high rooting rates on culture medium devoid of growth regulators. The efficiency of in vitro rooting and elongation on MS0 was also observed in micropropagation protocols previously established from stem and root explants of the same species (Simões et al. 2004Simões C, Santos AS, Albarello N & Figueiredo SFL (2004) Shoot organogenesis and plantlet regeneration from stem explants of Cleome rosea Vahl (Capparaceae). Journal of Plant Biotechnology 6: 199-204., 2009bSimões C, Albarello N, Callado CH, Castro TC & Mansur E (2009b) New approaches for shoot production and establishment of in vitro root cultures of Cleome rosea Vahl. Plant Cell Tissue and Organ Culture 98: 79-86.). Similar results were achieved with C. spinosa (Albarello et al. 2006Albarello N, Simões C, Rosas PFG, Castro TC, Gianfaldoni MG, Callado CH & Mansur E (2006) In vitro propagation of Cleome spinosa (Capparaceae) using explants from nursery-grown seedlings and axenic plants. In vitro Cellular and Developmental Biology-Plant 42: 601-606.). On the other hand, supplementation with auxins was necessary for root regeneration in C. viscosa (Naseem & Jha 1994Naseem M & Jha KK (1994) Differentiation and regeneration in Cleome leaves culture in vitro. Egypt Journal of Botany 1: 37-49.) and C. gynandra L. (Naseem & Jha 1997Naseem M & Jha KK (1997) Rapid clonal multiplication of Cleome gynandra DC. through tissue culture. Phytomorphology 47: 405-411.).
Rooted plants reached acclimatization rates around 80%. Similar rates were obtained for plants propagated from stem explants (Simões et al. 2004Simões C, Santos AS, Albarello N & Figueiredo SFL (2004) Shoot organogenesis and plantlet regeneration from stem explants of Cleome rosea Vahl (Capparaceae). Journal of Plant Biotechnology 6: 199-204.), results which demonstrate the effectiveness of the micropropagation process using petiole and foliar blade explants.
This work presented a new protocol for assessing the efficacy of micropropagation of T. rosea using leaf explants. The propagation capacity was achieved from both petiole and foliar blade, contributing to large-scale in vitro production of the species and providing an alternative source of plant material for biotechnological studies. The occurrence of propagation by direct organogenesis, the preferential pathway for micropropagation, was more expressive in the presence of 4.4 µm BA for both explants. Moreover, the maintenance of propagation capacity of primary explant-derived shoots over time in culture demonstrates the feasibility of long-term in vitro proliferation of T. rosea.
Acknowledgements
The authors would like to thank Jeanne Teixeira Glória and Matheus da Silva Tirado, for valuable technical assistance. This work was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- Abdullah W, Elsayed WM, Abdelshafeek KA, Nazif NM & Singab ANB (2016) Chemical constituents and biological activities of Cleome genus: a brief review. International Journal of Pharmacognosy and Phytochemical Research 8: 777-787.
- Alamilla-Fonseca LN, Delgado-Domínguez J, Zamora-Chimal J, Cervantes-Sarabia RB, Jiménez-Arellanes A, Rivero-Cruz JF & Becker I (2018) Leishmania mexicana cell death achieved by Cleoserrata serrata (Jacq.) Iltis: learning from Maya healers. Journal of Ethnopharmacology 211: 180-187.
- Albarello N, Simões C, Rosas PFG, Castro TC, Gianfaldoni MG, Callado CH & Mansur E (2006) In vitro propagation of Cleome spinosa (Capparaceae) using explants from nursery-grown seedlings and axenic plants. In vitro Cellular and Developmental Biology-Plant 42: 601-606.
- Almeida M, Graner EM, Brondani GE, Oliveira LS, Artioli FA, Almeida LV, Leone GF, Baccarin FJB, Antonelli PO, Cordeiro GM, Oberschelp GPJ & Batagin-Piotto KD (2015) Plant morphogenesis: theorical bases. Advances in Forestry Science 2: 13-22.
- Bosela MJ & Michler CH (2008) Media effects on black walnut (Juglans nigra L.) shoot culture growth in vitro: evaluation of multiple nutrient formulations and cytokinin types. In vitro Cellular and Developmental Biology-Plant 44: 316-329.
- Castro TC, Simões-Gurgel C, Ribeiro IG, Garcia MC & Albarello N (2014) Morphological aspects of fruits, seeds, seedlings and in vivo and in vitro germination of species of the genus Cleome Journal of Seed Science 36: 326-335.
- Cordeiro LS, Simões-Gurgel C & Albarello N (2015a) Multiplication and cryopreservation of adventitious roots of Cleome rosea Vahl. In vitro Cellular and Developmental Biology-Plant 51: 249-257.
- Cordeiro LS, Simões-Gurgel C, Albarello N & Engelmann F (2015b) Cryopreservation of in vitro-grown shoot tips of Cleome rosea Vahl (Cleomaceae) using the V cryo-plate technique. In vitro Cellular and Developmental Biology-Plant 51: 688-695.
- Cordeiro LS, Simões-Gurgel C & Albarello N (2016) Cryopreservation of adventitious roots of Cleome rosea Vahl (Cleomaceae) using a vitrification technique and assessment of genetic stability. Cryoletters 37: 231-242.
- Cordeiro LS, Simões-Gurgel C, Albarello N & Engelmann F (2017) Cleomaceae (Cleome rosea Vahl ex DC.), shoot tips, V-cryoplate method. In: Niino T, Matsumoto T, Yamamoto S-I, Maki S, Tanaka D & Engelmann F (eds.) Manual of cryopreservation methods using cryo-plate, 1st ed. Vol 1. Plant Tissue Culture and Cryopreservation Group (PTTCCryoG), Jalisco. Pp 62-63.
- Cordeiro LS, Collin M, Callado CH, Simões-Gurgel C, Albarello N & Engelmann F (2020) Long-term conservation of Tarenaya rosea (Cleomaceae) root cultures: histological and histochemical analyses during cryopreservation using the encapsulation-vitrification technique. Protoplasma 257: 1-13.
- Corredoira E, Ballester A & Vieitez AM (2008) Thidiazuron-induced high-frequency plant regeneration from leaf explants of Paulownia tomentosa mature trees. Plant Cell Tissue and Organ Culture 95: 197-208.
- Delpech R (2000) The importance of red pigments to plant life: experiments with anthocyanins. Journal of Biological Education 34: 206-210.
- Durkovic J (2008) Micropropagation of mature Cornus mas ‘Macrocarpa’. Trees 22: 597-602.
- Edeoga HO, Omosun G, Osuagwu GGE, Mbaebie BO & Madu BA (2009) Micromorphological characters of the vegetative and floral organs of some Cleome species from Nigeria. American-Eurasian Journal of Scientific Research 4: 124-127.
- Gandhi SG, Mahajan V & Bedi YS (2015) Changing trends in biotechnology of secondary metabolism in medicinal and aromatic plants. Planta 241: 303-317.
- Gupta PC & Rao CV (2012) Pharmacognostical studies of Cleome viscosa Linn. Indian Journal of Natural Products and Resources 3: 527-534.
- Iliev I & Kitin P (2011) Origin, morphology, and anatomy of fasciation in plants cultured in vivo and in vitro Plant Growth Regulators 63: 115-129.
- Jain R, Sinha A, Jain D, Kachhwaha S & Kothari SL (2011) Adventitious shoot regeneration and in vitro biosynthesis of steroidal lactones in Withania coagulans (Stocks) Dunal. Plant Cell Tissue and Organ Culture 105: 135-140.
- Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York. 52p.
- Karam NS & Al-Majathoup M (2000) Direct shoot regeneration and microtuberization in wild Cyclamen persicum Mill. using seedling tissue. Scientia Horticulturae 86: 235-236.
- Kaur S & Bhutani KK (2009) In vitro propagation of Vanda testacea (Lindl.) Reichb.F. - a rare Orchid of high medicinal value. Plant Tissue Culture and Biotechnology 19: 1-7.
- Kaur S & Vij SP (2000) Regeneration potential of Saccolabium papillosum leaf segments. The Journal of the Orchid Society of India 14: 67-73.
- Khuntia A, Mohanty SK & Bose A (2013) Pharmacognostical & preliminary phytochemical investigation of Cleome rutidosperma aerial parts. International Journal of Research in Pharmacy and Science 3: 67-77.
- Kitin P, Iliev I, Scaltsoyiannes A, Nellas C, Rubos A & Funada R (2005) A comparative histological study between normal and fasciated shoots of Prunus avium generated in vitro Plant Cell Tissue and Organ Culture 82: 141-150.
- Kumar N & Reddy MP (2011) In vitro plant propagation: a review. Journal of Forest Science 27: 61-72.
- Metcalfe CR & Chalk L (1957) Anatomy of the dicotyledons. Vol. 1. Claredon Press, Oxford/London. Pp. 87-95.
- Mitras D, Kitin P, Iliev I, Dancheva D, Scaltsoyiannes A, Tsaktsira M, Nellas C & Rohr R (2009) In vitro propagation of Fraxinus excelsior L. by epicotyls. Journal of Biological Research 11: 37-48.
- Mulinacci N, Giaccherini C, Santamaria AR, Caniato R, Ferrari F, Valletta A, Vincieri FF & Pasqua G (2008) Anthocyanins and xanthones in the calli and regenerated shoots of Hypericum perforatum var. angustifolium (sin. Fröhlich) Borkh. Plant Physiology and Biochemistry 46: 414-420.
- Murashige T & Skoog FA (1962) A revised medium for rapid growth and biossays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497.
- Naing AH, Park KI, Chung MY, Lim KB & Kim CK (2016) Optimization of factors affecting efficient shoot regeneration in chrysanthemum cv. Shinma. Brazilian Journal of Botany 39: 975-984.
- Naseem M & Jha KK (1994) Differentiation and regeneration in Cleome leaves culture in vitro Egypt Journal of Botany 1: 37-49.
- Naseem M & Jha KK (1997) Rapid clonal multiplication of Cleome gynandra DC. through tissue culture. Phytomorphology 47: 405-411.
- Nayak NR, Patnaik S & Rath SP (1997) Direct shoot regeneration from leaf explants of epiphytic orchids Acampe praemorsa (Roxb.). Plant Cell Report 16: 583-586.
- Patchell MJ, Roalson EH & Hall JC (2014) Resolved phylogeny of Cleomaceae based on all three genomes. Taxon 63: 315-328.
- Reboredo F & Silvares C (2007) Fasciation phenomena and mineral balance in Spartium junceum L. Phyton, International Journal of Experimental Botany 47: 123-132.
- Rocha CFD, Bergallo HG, Van Sluys M, Alves MAS & Jamel CE (2007) The remnants of restinga habitats in the Brazilian Atlantic Forest of Rio de Janeiro state, Brazil: habitat loss and risk of disappearance. Brazilian Journal of Biology 67: 263-273.
- Roeser KR (1972) Die Nadel der Schwarzkiefer - Massenproduckt und Kunstwerk der Natur. Mikrokosmos 61: 33-36.
- Sass JE (1958) Elements of botanical microtechnique. McGraw-Hill, New York. 222p.
- Simões C, Santos AS, Albarello N & Figueiredo SFL (2004) Shoot organogenesis and plantlet regeneration from stem explants of Cleome rosea Vahl (Capparaceae). Journal of Plant Biotechnology 6: 199-204.
- Simões C, Mattos JCP, Sabino KCC, Caldeira-de-Araújo A, Coelho MGP, Albarello N & Figueiredo SFL (2006) Medicinal potential from in vivo and acclimatized plants of Cleome rosea Vahl ex DC. (Capparaceae). Fitoterapia 77: 94-99.
- Simões C, Bizarri CHB, Cordeiro LS, Castro TC, Coutada LCM, Silva AJR, Albarello N & Mansur E (2009a) Anthocyanin production in callus cultures of Cleome rosea: modulation by culture conditions and characterization of pigments by means of HPLC-DAD/ESIMS. Plant Physiology and Biochemistry 47: 895-903.
- Simões C, Albarello N, Callado CH, Castro TC & Mansur E (2009b) New approaches for shoot production and establishment of in vitro root cultures of Cleome rosea Vahl. Plant Cell Tissue and Organ Culture 98: 79-86.
- Simões C, Castro TC, Cordeiro LS, Albarello N, Mansur E & Romanos MTV (2010a) Antiviral activity of Cleome rosea extracts from field-grown plants and tissue culture-derived materials against acyclovir-resistant Herpes simplex viruses type 1 (ACVr-HSV-1) and type 2 (ACVr-HSV-2). World Journal of Microbiology and Biotechnology 26: 93-99.
- Simões C, Albarello N, Callado CH, Castro TC & Mansur E (2010b) Somatic embryogenesis and plant regeneration from Callus cultures of Cleome rosea Vahl. Brazilian Archives of Biology and Technology 53: 679-686.
- Simões-Gurgel C, Cordeiro LS, Castro TC, Callado CH, Albarello N & Mansur E (2011) Establishment of anthocyanin-producing cell suspension cultures of Cleome rosea Vahl ex DC. (Capparaceae). Plant Cell Tissue and Organ Culture 106: 537-545.
- Simões-Gurgel C, Rocha AS, Cordeiro LS, Gayer CRM, Castro TC, Coelho MGP, Albarello N, Mansur E & Rosa ACP (2012a) Antibacterial activity of field-grown plants, in vitro propagated plants, callus and cell suspension cultures of Cleome rosea Vahl. Journal of Pharmacy Research 5: 3304-3308.
- Simões C, Albarello N, Castro TC & Mansur E (2012b) Production of anthocyanins by plant cell and tissue culture strategies. In: Orhan IE (ed.) Biotechnological production of plant secondary metabolites. Bentham Science Publishers, Sharjah. Pp. 67-86.
- Sivanesan D & Begum VH (2007) Preventive role of Gynandropsis gynandra L., against aflatoxin B1 induced lipid peroxidation and antioxidant mechanism in rat. Indian Journal of Experimental Biology 45: 299-303.
- Soares Neto RL, Thomas WW, Barbosa MRV & Roalson EH (2018) New combinations and taxonomic notes for Tarenaya (Cleomaceae). Acta Botanica Brasilica 32: 540-545.
- Sreedhar RV, Venkatachalam L, Thimmaraju R, Bhagyalakshmi N, Narayan MS & Ravishankar GA (2008) Direct organogenesis from leaf explants of Stevia rebaudiana and cultivation in bioreactor. Biologia Plantarum 52: 355-360.
- Stange RR, Jeffares D, Young C, Scott DB, Eason JR & Jameson PE (1996) PCR amplification of the fas-1 gene for the detection of virulent strains of Rhodococus fascians Plant Pathology 45: 407-417.
- Suthar RK, Habibi N & Purohit SD (2011) Influence of agar concentration and liquid medium on in vitro propagation of Boswellia serrata Roxb. Indian Journal of Biotechnology 10: 224-227.
- Vankar PS & Bajpai D (2015) Rose amthocyanins as acid base indicators. EJEAFChe 9: 875-884.
- van Staden J & Zazimalova E (2008) Plant growth regulators II: Cytokinins, their Analogues and Antagonists. In: George EF, Hall MA & De Klerk G-J (eds.) Plant Propagation by Tissue Culture, Dordrecht. Pp. 205-226.
- Thomé GCH, Gressler PD & Santos G (2004) In vitro propagation of Kalanchoe blossfeldiana Poelln. by organogenesis. Revista Brasileira de Agrociência 10: 197-202.
- Vij SP & Pathak P (1990) Micropropagation of orchids through leaf segments. Journal of the Orchid Society of India 4: 69-88.
- Williams LAD, Vasques E, Reid W, Porter R & Kraus W (2003) Biological activities of an extract from Cleome viscosa L. (Capparaceae). Naturwissenschaften 90: 468-72.
Edited by
Publication Dates
-
Publication in this collection
08 Mar 2021 -
Date of issue
2021
History
-
Received
04 May 2019 -
Accepted
20 Feb 2020